A 55-year-old woman presented with conjunctival congestion, retro-orbital pain, and diplopia. She had received her first vaccine against SARS-CoV-2—ChAdOx1 nCoV-19—10 days before admission. Both on the night after the vaccination and 7 days later, the patient reported marked flu-like symptoms and a fever. She had no medical history of visual problems, autoimmune disorders, stroke, thrombosis, thrombocytopenia, neurological disorders, or arterial disease risk factors—including hypertension, diabetes, or smoking.
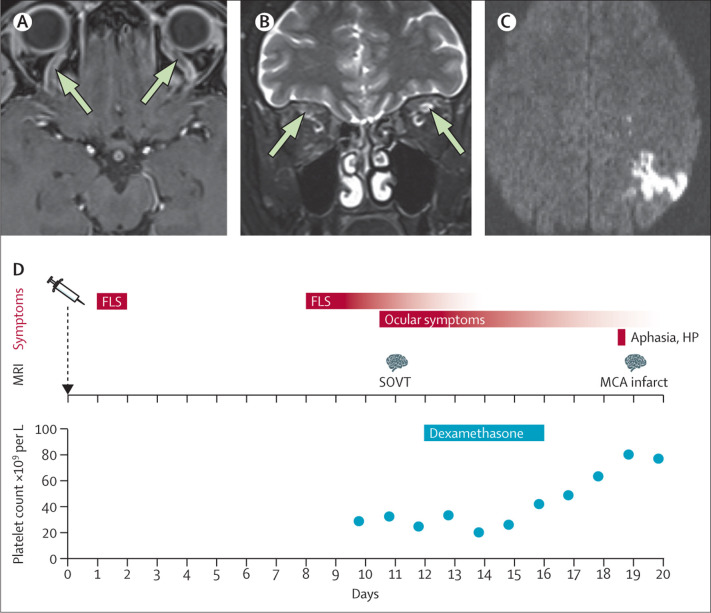
On examination, she had binocular diplopia at vertical and right lateral gaze, and her visual acuity was 0·85 in both eyes. MRI showed superior ophthalmic vein thrombosis (SOVT) with no contrast filling (figure ) and bilateral high T2 signal intensity of the superior ophthalmic vein (figure).
Figure.
SOVT and ischaemic stroke after ChAdOx1 nCoV-19 vaccination
MRI shows SOVT with no contrast filling (arrows; A) and bilateral high T2 signal intensity of the superior ophthalmic vein (arrows; B). MRI shows an ischaemic stroke in the left parietal lobe, MCA territory, with restricted diffusion (C). Diagram shows the timeline of symptoms, MRI findings, dexamethasone treatment, and platelet count (D). FLS=flu-like symptoms. HP=hemiparesis. SOVT=superior ophthalmic vein thrombosis. MCA=middle cerebral artery.
Laboratory investigations on admission showed a marked isolated thrombocytopenia of 30 × 109 per L (figure). IgG antiplatelet antibodies were positive, and IgM antiplatelet antibodies were borderline; a platelet suspension immunofluorescence test and a monoclonal antibody-specific immobilisation of platelet antigens assay were positive—supporting a diagnosis of secondary immune thrombocytopenia (ITP). IgG antibodies against platelet factor 4/polyanion complexes—tested using a lateral flow immunoassay, 4 days after starting heparinisation—were negative. Other possible causes of thrombocytopenia—including antiphospholipid syndrome, thrombotic microangiopathy, and hepatitis B virus and hepatitis C virus, HIV, cytomegalovirus, hantaviruses, and Helicobacter pylori infections—were excluded.
Because we suspected ITP, intravenous dexamethasone 40 mg daily was given for 4 days which resulted in an increasing platelet count (figure). Despite the therapeutic heparinisation, 8 days after admission, the patient developed a transient, mild, right-sided hemiparesis, and aphasia. An MRI showed an ischaemic stroke in the left parietal lobe, middle cerebral artery territory, with restricted diffusion (figure), which had not been detected in the earlier scan. The patient then developed right-sided focal seizures which were controlled with levetiracetam and lacosamide; anticoagulation was switched to phenprocoumon, and 26 days after admission, she was allowed home.
That 8, 10, and 18 days after the ChAdOx1 nCoV-19 vaccination, our previously healthy patient developed marked flu-like symptoms, two rare disorders—namely, bilateral SOVT and ITP, and an ischaemic stroke, may indicate a causal relationship. According to the European Medicines Agency review, March 18, health-care professionals should be on the alert for possible cases of thromboembolism—like cerebral venous sinus thrombosis, pulmonary embolus, and deep vein thrombosis—occurring in people who have recently received the ChAdOx1 nCoV-19 vaccine. The thrombotic events—including bilateral SOVT as seen in our patient—may occur in the context of thrombocytopenia (video).
Declaration of interests
We declare no competing interests.
Contributors
AB, MM, MC, AR, and MN cared for the patient, drafted the manuscript, acquired and interpreted the data, and searched the literature. LB acquired and evaluated the MRI data. We all revised the manuscript. Written consent for publication was obtained from the patient.
Supplementary Material
Neurological problems post vaccine against SARS-CoV-2
Clots and thrombocytopenia post vaccine against SARS-CoV-2
Youtube: https://youtu.be/MkFZODgW63I
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Neurological problems post vaccine against SARS-CoV-2
Clots and thrombocytopenia post vaccine against SARS-CoV-2
Youtube: https://youtu.be/MkFZODgW63I