Abstract
Background:
Food deserts are neighborhoods with low access to healthy foods and are associated with poor health metrics. We investigated association of food desert residence and cancer outcomes.
Methods:
In this population-based study, data from the 2000–2012 California Cancer Registry was used to identify patients with stage II/III breast or colorectal cancer. Patient residence at time of diagnosis was linked by census tract to food desert using the USDA Food Access Research Atlas. Treatment and outcomes were compared by food desert residential status.
Results:
Among 64,987 female breast cancer patients identified, 66.8% were <65 years old, and 5.7% resided in food deserts. Five-year survival for food desert residents was 78% compared with 80% for non-desert residents (p<0.0001). Among 48,666 colorectal cancer patients identified, 50.4% were female, 39% were <65 years old, and 6.4% resided in food deserts. Five-year survival for food desert residents was 60% compared with 64% for non-desert residents (p <0.001).
Living in food deserts was significantly associated with diabetes, tobacco use, poor insurance coverage, and low socioeconomic status (p<0.05) for both cancers. There was no significant difference in rates of surgery or chemotherapy by food desert residential status for either diagnosis. Multivariable analyses showed that food desert residence was associated with mortality on different models examined.
Conclusion:
Survival, despite treatment for stage II/III breast and colorectal cancers was worse for those living in food deserts. This association remained significant absent treatment differences, suggesting factors other than differential care access may link food desert residence and cancer outcomes.
Keywords: cancer outcomes, colon cancer, disparity, insulin resistance, rectal cancer, surgical outcomes
INTRODUCTION
Health care disparities continue to be a major issue in the US despite advances in overall health care strategies and environment. Studies have shown relationships between race, neighborhood segregation, socioeconomic status, and poor cancer outcomes.1 Although the concept of a food desert has existed since the 1990s,2 and has been investigated as a possible contributor to health and wellness outcomes,1,3,4 few studies have investigated the potential associations between food deserts and cancer outcomes.
Those who live greater distances from supermarkets tend to have less healthy diets compared with residents of neighborhoods with supermarket access. Supermarket access is associated with improved compliance with the US Department of Agriculture (USDA) recommended fruit and vegetable intake.5,6 Additionally, food deserts, low socioeconomic status (SES) neighborhoods, and long distances to grocery stores are associated with higher BMI.5,7–9 A pre-diagnosis diet high in green vegetables and fiber is associated with improved outcomes in cancer,10,11 indicating that these dietary elements play a role in cancer outcomes.
In the current study, we investigated this relationship by analyzing the cause of differences in health outcomes by economic status, location, race, sex, and other metrics.
METHODS
Definition of Food Deserts
The CDC defines food deserts as “areas that lack access to affordable fruits, vegetables, whole grains, low-fat milk, and other foods that make up a full and healthy diet”.12 Although there is no standard definition, the USDA defines areas of low access as those where the populace is at least 500 people, and/or at least 1/3rd of the census tract lives > 1 mile in urban communities, or > 10 miles in rural communities from a grocery store.13 What is known is that food deserts are not randomly dispersed, but are clustered in low income and often minority neighborhoods.3 Though a causal link between food availability and health outcomes has not been definitively proven, food deserts have been associated with many poor health outcomes14,15.
Adults age 18 or older diagnosed with stage II or III breast or colorectal cancers between 2000–2012 were identified from the California Cancer Registry (CCR).16 The CCR data set includes patient demographic (age, sex, race, insurance, socioeconomic status), clinical (site, histopathology, stage), and treatment information (surgery, chemotherapy). Additionally, CCR records include patient vital status, with December 31, 2015, as the latest date available for follow-up among those diagnosed between 2000 to 2012. CCR unique and anonymized patient identification numbers (PINs) were linked to corresponding PINs in the inpatient discharge records available from the California Office of Statewide Health Planning and Development (OSHPD). This linkage permitted collection of patient-specific risk factors by using ICD-9-CM diagnosis codes associated with diabetes, hypertension, obesity, and tobacco smoking. CCR cases were 2linked by the residential census tract to the USDA food desert data set. Compared with zip code level measures, census tract level measures of inequality are more reliably associated with cancer mortality risk,17 and represent the smallest patient-linked residential area available for analysis at the population level. CCR cases without linked inpatient discharge data or matching food desert census tracts were excluded. Males were included in the cohort of breast cancer cases. The study focused on onstage II or III cancer as these are cases most likely to receive curative-intent surgical intervention. Outcome of stage 4 disease is much more dependent on systemic therapies and access to clinical trials, and involves many issues of access to trials not easily deciphered from retrospective databases.
The California State Committee for the Protection of Human Subjects approved our use of this CCR-OSHPD data set for health care disparities research.
Statistics
Statistical analysis was performed using commercially available software (Stata v. 14; StataCorp, College Station, TX). Univariate mortality outcomes were compared by Kaplan-Meier analysis and frequencies compared by Pearson chi-squared analysis. For survival analyses, cases who remained alive up to 5-years or longer or who were lost to follow-up were treated as censored, and the patients who died within the initial 5-years of follow-up were uncensored in the standard manner. Cases with missing information were included in the analysis but treated as a separate category labeled “missing” or “unknown”. Univariate Cox proportional hazards analyses were performed to identify potential covariates to include with food desert status in models of multivariable survival analyses. An alpha of p < 0.05 was the criterion applied in tests of statistical significance.
RESULTS
Demographics
Of the 64,987 breast cancer patients identified, 99% were female, 66.8% were <65 years of age, and 5.7% resided in food deserts. Compared to the cohort not residing in the food desert, patients residing in a food desert were more likely to have diabetes, hypertension, obesity, be a smoker, have Medicare/ Medicaid/ uninsured insurance status (Table 1). The incidence of surgery or chemotherapy was not different between patients living in food desert and those not living in food deserts (Table 1).
Table 1.
Characteristics of patients with Stage II, III breast cancer.
| Parameter | Not desert | Desert | p | |
|---|---|---|---|---|
| Age | <65 | 67% | 65% | 0.02 |
| >65 | 33% | 35% | ||
| Stage | II | 77% | 76% | 0.05 |
| III | 23% | 24% | ||
| Surgery | No | 3% | 3% | 0.4 |
| Yes | 97% | 97% | ||
| Chemotherapy | No | 37% | 37% | 0.2 |
| Yes | 63% | 63% | ||
| Diabetes | No | 95% | 93% | 0.001 |
| Yes | 5% | 7% | ||
| Hypertension | No | 71% | 68% | 0.001 |
| Yes | 29% | 32% | ||
| Obesity | No | 93% | 91% | 0.001 |
| Yes | 7% | 9% | ||
| Smoker | No | 89% | 85% | 0.001 |
| Yes | 11% | 15% | ||
| Insurance | Private/PPO | 46% | 39% | 0.001 |
| HMO | 17% | 13% | ||
| Medicare | 23% | 28% | ||
| Medicaid/Uninsured | 11% | 17% | ||
| Other | 3% | 4% | ||
| Socio-Economic Status | Very low | 11% | 31% | 0.001 |
| Low | 15% | 31% | ||
| Middle | 19% | 21% | ||
| High | 22% | 7% | ||
| Very high | 25% | 2% | ||
| Missing | 10% | 8% | ||
| Race | White | 60% | 62% | 0.001 |
| Black | 7% | 7% | ||
| Hispanic | 18% | 24% | ||
| Asian/ Pac Islander | 12% | 5% | ||
| Other | 3% | 2% |
Of the 48,666 colorectal cancer patients identified 50.4% were female, 39% were <65 years of age, and 6.4% resided in a food desert. Compared to the cohort not residing in the food desert, patients residing in a food desert were more likely to have diabetes, obesity, be a smoker, have Medicare/ Medicaid/ uninsured insurance status (Table 2). The incidence of surgery or chemotherapy was not different between patients living in food desert and those not living in food deserts (Table 2).
Table 2.
Characteristics of patients with Stage II, III colorectal cancer.
| Parameter | Not desert | Desert | p | |
|---|---|---|---|---|
| Age | <65 | 39% | 38% | 0.5 |
| >65 | 61% | 62% | ||
| Gender | Male | 50% | 50% | 0.3 |
| Female | 50% | 50% | ||
| Stage | II | 50% | 51% | 0.2 |
| III | 50% | 49% | ||
| Anatomic Site | Colon | 74% | 73% | 0.2 |
| Rectum | 26% | 27% | ||
| Surgery | No | 2% | 3% | 0.3 |
| Yes | 98% | 97% | ||
| Chemotherapy | No | 53% | 55% | 0.08 |
| Yes | 43% | 41% | ||
| Unknown | 3% | 3% | ||
| Diabetes | No | 89% | 87% | 0.005 |
| Yes | 11% | 13% | ||
| Hypertension | No | 59% | 59% | 0.5 |
| Yes | 41% | 41% | ||
| Obesity | No | 92% | 92% | 0.2 |
| Yes | 8% | 8% | ||
| Smoker | No | 82% | 79% | 0.001 |
| Yes | 18% | 21% | ||
| Insurance | Private/PPO | 36% | 31% | 0.001 |
| HMO | 14% | 11% | ||
| Medicare | 40% | 45% | ||
| Medicaid/Uninsured | 8% | 11% | ||
| Other | 2% | 2% | ||
| Socio-Economic Status | Very low | 12% | 32% | 0.001 |
| Low | 16% | 32% | ||
| Middle | 20% | 19% | ||
| High | 21% | 3% | ||
| Very high | 23% | 2% | ||
| Missing | 9% | 9% | ||
| Race | White | 61% | 65% | 0.001 |
| Black | 6% | 7% | ||
| Hispanic | 16% | 21% | ||
| Asian/ Pac Islander | 14% | 5% | ||
| Other | 3% | 2% |
Survival Analysis
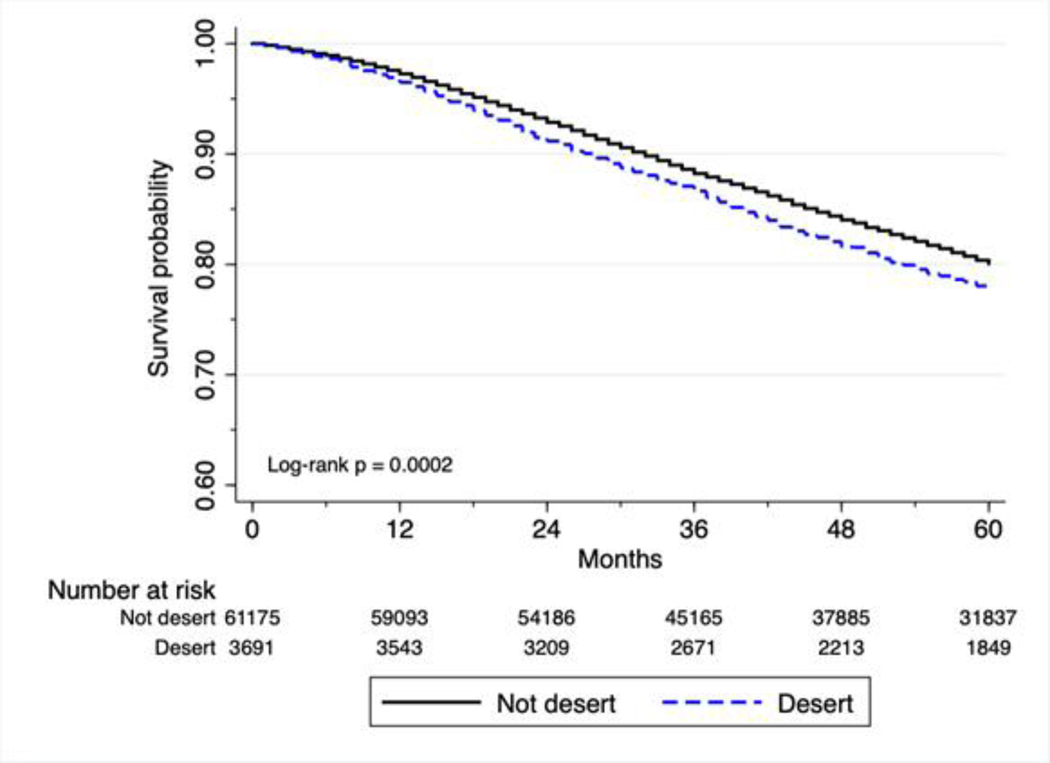
For breast cancer patients living in food deserts, 5-year survival was 78%, compared with 80% for non-desert residents (p < 0.0001).
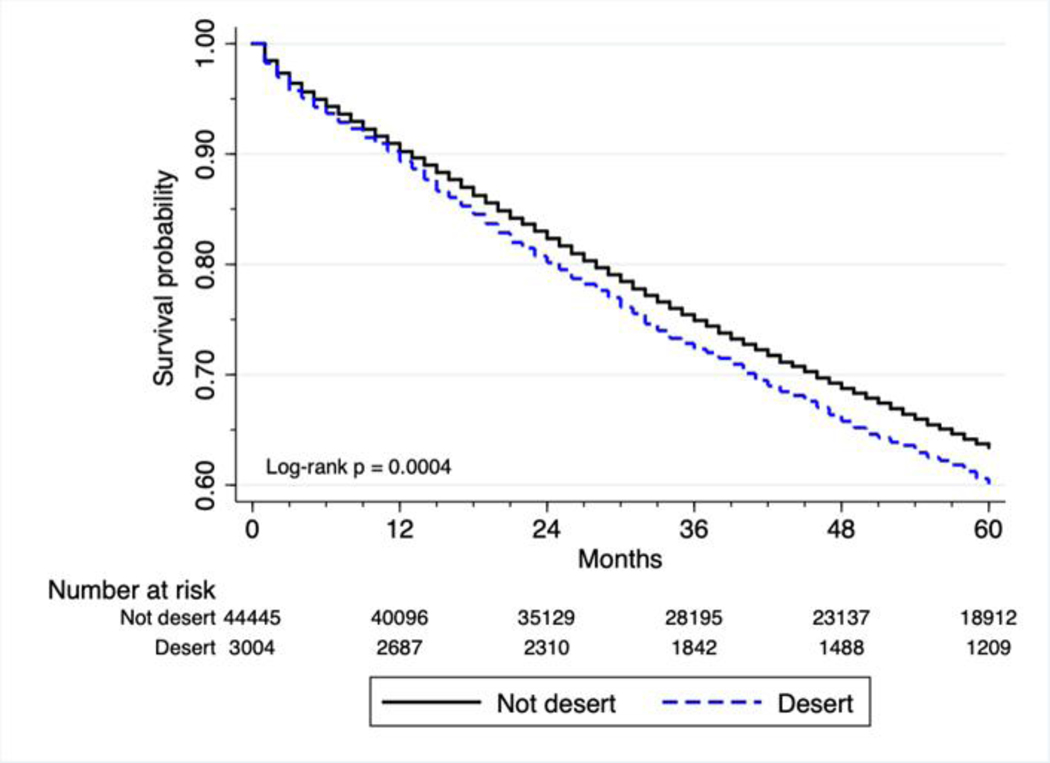
Among the colorectal cancer patients living in food deserts, 5-year survival was 60%, compared with 64% for non-desert residents (p < 0.001).
Kaplan-Meier analyses indicate greater mortality for food desert residents with stage II/III breast cancer (log-rank p < 0.001, Figure 1), and colorectal cancer (log-rank p < 0.001, Figure 2).
Figure 1. Survival of patients with surgically treatable stage II and III breast cancer.
Kaplan-Meier plot showing the survival probability of breast cancer patients with surgically treatable stage II and III breast cancer. (Hazard ratio [HR] = 1.15, p < 0.001, 95% CI = 1.07–1.24) p-value obtained by the log-rank test. Hazard ratio and 95% CI obtained by Cox proportional-hazards analysis.
Figure 2. Survival of patients with surgically treatable stage II and III colorectal cancer.
Kaplan-Meier plot showing the survival probability of colorectal cancer patients with surgically treatable stage II and III colorectal cancer. (Hazard ratio [HR] = 1.12, p < 0.001, 95% CI = 1.05–1.19). p-value obtained by the log-rank test. Hazard ratio and 95% CI obtained by Cox proportional-hazards analysis.
Univariate Analysis
Univariate Cox proportional hazard analyses indicated that breast cancer patients (Hazard ratio [HR] = 1.15, p < 0.001, 95% CI = 1.07 – 1.24) and colorectal cancer patients (HR = 1.12, p < 0.001, 95% CI = 1.05 – 1.19) residing in food deserts have greater 5-year mortality risk compared with non-desert residents (Table 3, 4).
Table 3.
Univariate analysis for predictors of survival in stage II and III breast cancer.
| Variables | Category | n (%) | Survival at 5 years | P-value (log-rank) | HR on 5-year survival (95% CI, p-value) |
|---|---|---|---|---|---|
| Stage | Stage II | 50,189 (77.2) | 84% | <0.0001 | reference |
| Stage III | 14,798 (22.3) | 66% | 2.45 (2.36 – 2.55, <0.001) | ||
| Age | Age <65 | 43,147 (66.8) | 86% | <0.0001 | reference |
| Age 65+ | 21,570 (31.2) | 69% | 2.40 (2.31 – 2.49, <0.001) | ||
| Gender | Female | 64,415 (99.1) | 80% | <0.0001 | reference |
| Male | 572 (0.9) | 72% | 1.45 (1.22 – 1.72, <0.001) | ||
| Surgery at Primary Site | No | 1,849 (2.8) | 47% | <0.0001 | reference |
| Yes | 63,138 (97.2) | 81% | 0.26 (0.24 – 0.28, <0.001) | ||
| Chemotherapy | No | 23,749 (36.5) | 74% | <0.0001 | reference |
| Yes | 39,734 (61.2) | 83% | 0.61 (0.59 – 0.63, <0.001) | ||
| unknown | 1,486 (2.3) | 83% | 0.66 (0.58 – 0.75, <0.001) | ||
| Diabetes | No | 61,751 (95.0) | 81% | <0.0001 | reference |
| Yes | 3,236 (5.0) | 66% | 2.01 (1.89 – 2.14, <0.001) | ||
| Hypertension | No | 46,073 (70.9) | 82% | <0.0001 | reference |
| Yes | 18,914 (29.1) | 75% | 1.45 (1.39 – 1.50, <0.001) | ||
| Obese | No | 60,250 (92.7) | 80% | 0.2331 | reference |
| Yes | 4,737 (7.3) | 80% | 1.04 (0.97 – 1.12, =0.234) | ||
| Tobacco Use | No | 57,994 (89.2) | 80% | <0.0001 | reference |
| Yes | 6,993 (10.8) | 77% | 1.22 (1.15 – 1.29, <0.001) | ||
| Insurance | Private or PPO | 29,249 (45.0) | 84% | <0.0001 | reference |
| HMO | 10,999 (16.9) | 84% | 1.03 (0.97 – 1.10, =0.321) | ||
| Medicare | 15,143 (23.3) | 69% | 2.33 (2.23 – 2.44, <0.001) | ||
| Medicaid/uninsured | 7,445 (11.5) | 77% | 1.61 (1.15 – 1.71, <0.001) | ||
| Other | 2,151 (3.3) | 83% | 1.12 (1.00 – 1.25, =0.052) | ||
| Socioeconomic Status | Very Low | 7,745 (11.9) | 73% | <0.0001 | reference |
| Low | 10,140 (15.6) | 78% | 0.82 (0.77 – 0.88, <0.001) | ||
| Middle | 12,121 (18.6) | 79% | 0.75 (0.71 – 0.80, <0.001) | ||
| High | 13,455 (20.7) | 81% | 0.68 (0.64 – 0.73, <0.001) | ||
| Very High | 15,389 (23.7) | 85% | 0.53 (0.50 – 0.56, <0.001) | ||
| Missing | 6,137 (9.4) | 82% | 0.64 (0.59 – 0.70, <0.001) | ||
| Race | Non-Hispanic White | 39,211 (60.3) | 80% | <0.0001 | reference |
| Non-Hispanic Black | 4,644 (7.2) | 72% | 1.51 (1.42 – 1.61, <0.001) | ||
| Hispanic | 11,585 (17.8) | 81% | 0.94 (0.89 – 0.99, =0.012) | ||
| Asian/Pacific Islander | 7,734 (11.9) | 86% | 0.66 (0.62 – 0.71, <0.001) | ||
| Other | 1,813 (2.8) | 84% | 0.73 (0.64 – 0.83, <0.001) | ||
| Food desert | No | 61,292 (94.3) | 80% | 0.0002 | reference |
| Yes | 3,695 (5.7) | 78% | 1.15 (1.07 – 1.24, <0.001) | ||
Table 4.
Univariate analysis for predictors of survival in stage II and III colorectal cancer.
| Variables | Category | n (%) | Survival at 5 years | P-value (log-rank) | HR on 5-year survival (95% CI, p-value) |
|---|---|---|---|---|---|
| Stage | Stage II | 24,514 (50.4) | 69% | <0.0001 | reference |
| Stage III | 24,152 (49.6) | 58% | 1.47 (1.43 – 1.52, <0.001) | ||
| Age | Age <65 | 18,961 (39.0) | 76% | <0.0001 | reference |
| Age 65+ | 29,705 (61.0) | 55% | 2.23 (2.15 – 2.31, <0.001) | ||
| Gender | Female | 24,529 (50.4) | 63% | 0.414 | reference |
| Male | 24,137 (49.6) | 63% | 1.01 (0.96 – 1.02, =0.413) | ||
| Surgery at Primary Site | No | 1,114 (2.9) | 28% | <0.0001 | reference |
| Yes | 47,552 (97.7) | 64% | 0.31 (0.29 – 0.33, <0.001) | ||
| Chemotherapy | No | 26,075 (53.6) | 59% | <0.0001 | reference |
| Yes | 21,043 (43.2) | 68% | 0.69 (0.67 – 0.71, <0.001) | ||
| Unknown | 1,548 (3.2) | 64% | 0.84 (0.77 – 0.92, <0.001) | ||
| Diabetes | No | 42,997 (88.4) | 64% | <0.0001 | reference |
| Yes | 5,669 (11.6) | 58% | 1.22 (1.17 – 1.28, <0.001) | ||
| Hypertension | No | 28,787 (59.2) | 65% | <0.0001 | reference |
| Yes | 19,879 (40.8) | 61% | 1.12 (1.08 – 1.15, <0.001) | ||
| Obese | No | 44,926 (92.3) | 63% | <0.0001 | reference |
| Yes | 3,740 (7.7) | 67% | 0.84 (0.79 – 0.90, <0.001) | ||
| Tobacco Use | No | 39,915 (82.0) | 64% | <0.0001 | reference |
| Yes | 8,751 (18.0) | 60% | 1.11 (1.06 – 1.15, <0.001) | ||
| Insurance | Private or PPO | 17,486 (35.9) | 70% | <0.0001 | reference |
| HMO | 6,566 (13.5) | 69% | 1.01 (0.95 – 1.07, =0.794) | ||
| Medicare | 19,684 (40.4) | 55% | 1.75 (1.68 – 1.81, <0.001) | ||
| Medicaid/unins ured | 4,130 (8.5) | 64% | 1.25 (1.18 – 1.33, <0.001) | ||
| Other | 800 (1.6) | 65% | 1.25 (1.10 – 1.41, =0.001) | ||
| Socioeconomic Status | Very Low | 6,278 (12.9) | 59% | <0.0001 | reference |
| Low | 8,305 (17.1) | 60% | 0.95 (0.90 – 1.00, =0.060) | ||
| Middle | 9,550 (19.6) | 62% | 0.91 (0.86 – 0.96, =0.001) | ||
| High | 9,640 (19.8) | 65% | 0.81 (0.77 – 0.86, <0.001) | ||
| Very High | 10,349 (21.3) | 68% | 0.71 (0.67 – 0.75, <0.001) | ||
| Missing | 4,544 (9.3) | 64% | 0.83 (0.77 – 0.90, <0.001) | ||
| Race | Non-Hispanic White | 29,859 (61.3) | 62% | <0.0001 | reference |
| Non-Hispanic Black | 3,050 (6.3) | 58% | 1.12 (1.06 – 1.19, <0.001) | ||
| Hispanic | 7,769 (16.0) | 66% | 0.86 (0.82 – 0.90, <0.001) | ||
| Asian/Pacific Islander | 6,684 (13.7) | 70% | 0.74 (0.71 – 0.78, <0.001) | ||
| Other | 1,304 (2.7) | 71% | 0.70 (0.63 – 0.78, <0.001) | ||
| Food desert | No | 45,563 (93.6) | 64% | 0.0004 | reference |
| Yes | 3,103 (6.4) | 60% | 1.12 (1.05 – 1.19, <0.001) | ||
Other factors associated with lower survival by univariate analysis for both breast cancer (Table 3) and colorectal cancer (Table 4) included age, gender, stage, not receiving surgery, not receiving chemotherapy, insurance type, and SES.
Multivariate Factors Associated with Outcome
Multivariable survival analysis of food desert residential status in the context of potential co-predictors was performed. The analysis included as co-variates age, sex, stage, effects of treatment (surgery and chemotherapy), known health risks (diabetes, hypertension, obesity, and smoking), SES, race, and insurance status. Overall survival at five years was the outcome analyzed.
For breast cancer patients, living in a food desert was associated with greater mortality risk controlling for treatment factors (Table 5, HR = 1.16, 95% CI = 1.07 – 1.25, p <0.001), health risks, SES, race, and insurance status. Similarly, among colorectal patients, food desert residence was associated with greater 5-year mortality risk controlling for treatment (Table 5, HR = 1.18, 95% CI = 1.05 – 1.19, p < 0.001), health risks, SES, race, and insurance status.
Table 5.
Multivariate analysis for breast or colorectal cancer outcomes.
| Breast Cancer | Colorectal Cancer | |||||
|---|---|---|---|---|---|---|
| Components | HR | P-value | 95% CI | HR | P-value | 95% CI |
| Age 65+ | 2.39 | <0.001 | 2.31–2.49 | 2.23 | <0.001 | 2.15–2.31 |
| Gender-female | --- | --- | --- | 1.01 | 0.41 | 0.98–1.05 |
| Stage III | 2.45 | <0.001 | 2.36–2.55 | 1.47 | <0.001 | 1.43–1.52 |
| Surgery receipt | 0.26 | <0.001 | 0.24–0.27 | 0.31 | <0.001 | 0.29–0.33 |
| Chemotherapy receipt | 0.61 | <0.001 | 0.59–0.64 | 0.69 | <0.001 | 0.67–0.71 |
| Diabetes | 2.01 | <0.001 | 1.89–2.14 | 1.22 | <0.001 | 1.17–1.28 |
| Hypertension | 1.45 | <0.001 | 1.39–1.51 | 1.19 | <0.001 | 1.08–1.15 |
| Obesity | 1.05 | 0.19 | 0.98–1.13 | 0.84 | <0.001 | 0.79–0.90 |
| Smoking | 1.22 | <0.001 | 1.15–1.29 | 1.11 | <0.001 | 1.06–1.15 |
| SES-low | 1.21 | <0.001 | 1.20–1.22 | 1.15 | <0.001 | 1.14–1.17 |
| Race-black | 1.51 | <0.001 | 1.42–1.61 | 1.12 | <0.001 | 1.06–1.19 |
| Race-hispanic | 0.94 | 0.017 | 0.89–0.99 | 0.86 | <0.001 | 0.77–0.89 |
| Insurance | ||||||
| Private | reference | reference | ||||
| HMO | 1.02 | 0.51 | 0.96–1.09 | 1.01 | 0.80 | 0.95–1.07 |
| Medicare | 2.33 | <0.001 | 2.23–2.43 | 1.75 | <0.001 | 1.68–1.81 |
| Medicaid/uninsured | 1.61 | <0.001 | 1.52–1.71 | 1.25 | <0.001 | 1.18–1.34 |
| Other insurance | 1.13 | 0.04 | 1.00–1.26 | 1.25 | =0.001 | 1.10–1.41 |
| Food Desert | 1.16 | <0.001 | 1.07–1.25 | 1.12 | <0.001 | 1.05–1.19 |
DISCUSSION
The national rates of breast and colon cancer are high with an estimated 268,600 new cases of invasive breast cancer, and 145,600 new cases of colon cancer estimated to be diagnosed in 2019 (SEER, Cancer Stat Facts).18 Survival rates and estimated deaths from both cancers have decreased over the past decade. New chemotherapy and hormonal agents, as well as new surgical techniques, have played roles in the decreased mortality rates. Nevertheless, improvements in cancer outcomes are not uniform across the United States. Previous studies have shown that socioeconomic factors, poverty, and segregation are associated with worse health care and cancer outcomes.19–24 The driving forces behind the worse outcomes are debated but may be due to differences in access to care. Interestingly, our data show that despite comparable rates of surgery and chemotherapy for patients living in and not living in food deserts for Stage II/III breast cancer or Stage II/III colorectal cancer, patients living in areas classified as food deserts had higher mortality risk compared with non-food desert patients. Results from our third multivariable model, in particular, suggest that food desert residence mortality risk may be independent of access to care since insurance status was included as a co-predictor. While these data demonstrate no difference in access to cancer care, we cannot determine from the current database the quality of care, as data on margins of resection, surgical complications, and duration of chemotherapy are not available.
Univariate analyses showed several predictors independently associated with mortality risk, including age > 65, sex, race, SES, insurance, surgery at primary site, chemotherapy, and health risks including hypertension, diabetes, obesity, and tobacco use. These predictors of mortality are well known, and our findings are also consistent with studies linking socioeconomic status or neighborhood characteristics with worse outcomes for cancer and heart disease.20–24 What is novel is our finding of greater mortality risk for cancer patients living in food deserts in models alternatively controlling for treatment, health risks, or insurance, controlling for age, sex, and stage.
There are at least two theories proposed to explain how neighborhoods may become food deserts. One proposes that food deserts result from competition from larger stores that drive out smaller grocers. A second theory postulates that low SES urban neighborhoods lack large enough market to attract grocers.25 Regardless of the theorized causes, food deserts are a public health issue in California. Based on 2010 data, approximately 1 million people were identified as living in food deserts, and about 45% of food deserts were classified as low income. The majority of food desert census tracts (85%) were located in urban areas.16
A relationship exists among race, neighborhood segregation, socioeconomic status and worse cancer outcomes.26–29 Our results suggest that food desert residential status may be another socio-geographical risk factor associated with survival outcomes. Factors correlated with food desert status, including diet or exposure to pollution, noise, crime, violence, absence of recreational facilities, or other environmental stressors may partially account for the effect of food deserts on mortality. It will be difficult to completely resolve the exact reason for the influence of residency in a food desert on cancer outcome through a retrospective study. Future studies of environmental pollutants, obesity, exercise, insulin resistance and many other factors will be needed to determine the best interventions to eliminate this association. These additional factors warrant further prospective study.
This may be the first study linking food desert residential status with cancer mortality risk, but we are mindful of its limitations. As with most studies based on retrospective data, our results only represent a correlational and not a causal relationship between food desert status and cancer mortality risk. Food desert residential status was based on where patients lived at time of cancer diagnosis and there is no information about how long patients lived at this address. Long-term residents of food deserts would represent greater exposure to the effects of this risk factor compared with short term or recently located residents. Consequently, with these data, it is not possible to compare differences by short- or long-term food desert residency on mortality risk. Additionally, if patients moved after diagnosis date this may have resulted in misclassification of food desert status for an undetermined number of cases. Furthermore, as defined and measured by the USDA data we linked to cancer patients’ food desert status may be confounded with other socioeconomic and race-related variables associated with diet, health, and vital status including access to resources.30
Clearly, access to fresh, healthy foods is an important component of the higher health risk associated with food desert residential status. Therefore, interventions have been tried to change the access to fresh food in an attempt to change health outcomes. The results from these interventional studies have been conflicting. For example, one study found that opening a grocery store in a food desert was not associated with improved health, while other studies found that health improves among people who moved out of food deserts.31 Whether changing access to fresh food may alter cancer outcomes is not currently known. Residency in a food desert may be merely a proxy for poverty and the associated poor outcome in many diseases. However, rigorous studies to examine whether changing access for fresh food may change cancer outcome warrants further investigation. What would also be important is to study potential biomarkers of cancer outcome such as insulin resistance, proteomics, or exosomics to both serve as targets as well as endpoints for interventional studies in the future.
Synopsis:
We investigated the relationship between food desert residential status for breast and colorectal cancer patients, and found greater mortality risk among those living in food deserts. We describe food desert linked risk factors for poor outcomes associated with these cancers.
ACKNOWLEDGMENTS
All work and all figures are original and unpublished.
Yuman Fong, MD had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors would like to thank Supriya Deshpande, Ph.D. for assistance with manuscript editing.
Disclosures/Financial Support: None. Supported by grant P30 CA033572 from the NIH.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Walker RE, Keane CR, Burke JG. Disparities and access to healthy food in the United States: A review of food deserts literature. Health Place. September 2010. 10.1016/j.healthplace.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Cummins S, Macintyre S. “Food deserts”--evidence and assumption in health policy making. BMJ. August 24 2002. 10.1136/bmj.325.7361.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright JDD AM; Gualtieri MC; Strickhouser SM Food Deserts: What is the Problem? What is the Solution? Society 2016. [Google Scholar]
- 4.IOM. The Public Health Effects of Food Deserts: Workshop Summary. The Public Health Effects of Food Deserts. 2009. [Google Scholar]
- 5.Larson NI, Story MT, Nelson MC. Neighborhood environments: disparities in access to healthy foods in the U.S. Am J Prev Med. January 2009. 10.1016/j.amepre.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 6.Moore LV, Diez Roux AV, Nettleton JA, Jacobs DR Jr. Associations of the local food environment with diet quality--a comparison of assessments based on surveys and geographic information systems: the multi-ethnic study of atherosclerosis. Am J Epidemiol. April 15 2008. 10.1093/aje/kwm394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inagami S, Cohen DA, Finch BK, Asch SM. You are where you shop: grocery store locations, weight, and neighborhoods. Am J Prev Med. July 2006. 10.1016/j.amepre.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Morland K, Diez Roux AV, Wing S. Supermarkets, other food stores, and obesity: the atherosclerosis risk in communities study. Am J Prev Med. April 2006. 10.1016/j.amepre.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Dubowitz T, Zenk SN, Ghosh-Dastidar B, et al. Healthy food access for urban food desert residents: examination of the food environment, food purchasing practices, diet and BMI. Public Health Nutr. August 2015. 10.1017/S1368980014002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Playdon MC, Nagle CM, Ibiebele TI, et al. Pre-diagnosis diet and survival after a diagnosis of ovarian cancer. Br J Cancer. June 6 2017. 10.1038/bjc.2017.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolecek TA, McCarthy BJ, Joslin CE, et al. Prediagnosis food patterns are associated with length of survival from epithelial ovarian cancer. J Am Diet Assoc. March 2010. 10.1016/j.jada.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Prevention CfDCa. https://www.cdc.gov/features/fooddeserts/index.html. 2017; https://www.cdc.gov/features/fooddeserts/index.html. [Google Scholar]
- 13.Agriculture USDo. Food Access Research Atlas. 2017; https://www.ers.usda.gov/dataproducts/food-access-research-atlas. [Google Scholar]
- 14.Kelli HM, Kim JH, Samman Tahhan A, et al. Living in Food Deserts and Adverse Cardiovascular Outcomes in Patients With Cardiovascular Disease. J Am Heart Assoc. February 19 2019. 10.1161/JAHA.118.010694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liese AD, Lamichhane AP, Garzia SCA, et al. Neighborhood characteristics, food deserts, rurality, and type 2 diabetes in youth: Findings from a case-control study. Health Place. March 2018. 10.1016/j.healthplace.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin J. California Food Deserts: Nearly 1 Million Live Far from Supermarkets, Grocery Stores. Huffpost 2011. [Google Scholar]
- 17.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter?: the Public Health Disparities Geocoding Project. Am J Epidemiol. September 1 2002. 10.1093/aje/kwf068. [DOI] [PubMed] [Google Scholar]
- 18.Surveillance E, and End Results Program. Cancer Stat Facts. 2019; https://seer.cancer.gov/statfacts/. [Google Scholar]
- 19.Edwards BK, Noone AM, Mariotto AB, et al. Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. May 1 2014. 10.1002/cncr.28509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warner ET, Gomez SL. Impact of neighborhood racial composition and metropolitan residential segregation on disparities in breast cancer stage at diagnosis and survival between black and white women in California. J Community Health. Aug 2010. 10.1007/s10900-010-9265-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landrine H, Corral I, Lee JGL, Efird JT, Hall MB, Bess JJ. Residential Segregation and Racial Cancer Disparities: A Systematic Review. J Racial Ethn Health Disparities. December 2017. 10.1007/s40615-016-0326-9. [DOI] [PubMed] [Google Scholar]
- 22.Russell EF, Kramer MR, Cooper HL, Gabram-Mendola S, Senior-Crosby D, Jacob Arriola KR. Metropolitan area racial residential segregation, neighborhood racial composition, and breast cancer mortality. Cancer Causes Control. Sep 2012. 10.1007/s10552-012-0029-4. [DOI] [PubMed] [Google Scholar]
- 23.Kershaw KN, Osypuk TL, Do DP, De Chavez PJ, Diez Roux AV. Neighborhood-level racial/ethnic residential segregation and incident cardiovascular disease: the multi-ethnic study of atherosclerosis. Circulation. January 13 2015. 10.1161/CIRCULATIONAHA.114.011345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross J, Braswell KV, Madeira da Silva L, et al. Unraveling the etiology of ovarian cancer racial disparity in the deep south: Is it nature or nurture? Gynecol Oncol. May 2017. 10.1016/j.ygyno.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 25.Cummins S, Macintyre S. Food environments and obesity--neighbourhood or nation? Int J Epidemiol. February 2006. 10.1093/ije/dyi276. [DOI] [PubMed] [Google Scholar]
- 26.Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and Ethnic Disparities in Cancer Survival: The Contribution of Tumor, Sociodemographic, Institutional, and Neighborhood Characteristics. J Clin Oncol. January 1 2018. 10.1200/JCO.2017.74.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hines RB, Markossian TW. Differences in late-stage diagnosis, treatment, and colorectal cancer-related death between rural and urban African Americans and whites in Georgia. J Rural Health. Summer 2012. 10.1111/j.1748-0361.2011.00390.x. [DOI] [PubMed] [Google Scholar]
- 28.Sitenga JL, Aird G, Ahmed A, Walters R, Silberstein PT. Socioeconomic status and survival for patients with melanoma in the United States: an NCDB analysis. Int J Dermatol. October 2018. 10.1111/ijd.14026. [DOI] [PubMed] [Google Scholar]
- 29.Terplan M, Temkin S, Tergas A, Lengyel E. Does equal treatment yield equal outcomes? The impact of race on survival in epithelial ovarian cancer. Gynecol Oncol. Nov 2008. 10.1016/j.ygyno.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Susman E. It’s Poverty Not Race that Causes Cancer Mortality Disparity. Oncology Times: Society of Thoracic Surgeons Annual Meeting 2016; https://journals.lww.com/oncology-times. [Google Scholar]
- 31.RIchardson AG-D M.; Beckman R. Can the Introduction of a Full-Service Supermarket in a Food Desert Improve Residents’ Economic Status and Health?. Ann Epidemiol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]