Abstract
Introduction:
A flatter diurnal cortisol curve has been associated with incident diabetes among older white adults. However, this relationship has not been examined among middle-aged individuals or African Americans [AA]. We analyzed the longitudinal association of baseline diurnal cortisol curve features with incident diabetes over a 10 year period in a cohort of AA and white participants who were, on average, 40 years old.
Methods:
Salivary cortisol was collected immediately post-awakening, then subsequently 45 minutes, 2.5 hours, 8 hours, and 12 hours later, as well as at bedtime. Cortisol curve features included wake-up cortisol; cortisol awakening response (CAR); early, late, and overall decline slopes; bedtime cortisol; and 16-hour area under the curve (AUC). Salivary cortisol (nmol/L) was log-transformed due to positively skewed distributions. Diabetes was defined as fasting plasma glucose ≥ 126 mg/dL or taking diabetes medication. Logistic regression models were used to investigate the association of log-transformed cortisol curve features with incident diabetes. The analysis was stratified by race and adjusted for age, sex, education, depressive symptoms, smoking status, beta-blocker and steroid medication use and BMI.
Results:
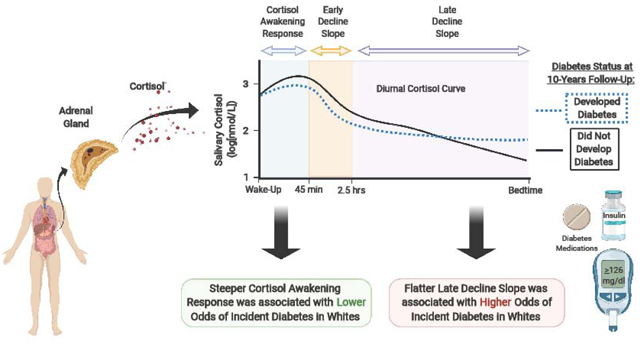
Among 376 AA and 333 white participants (mean age 40 years), 67 incident diabetes cases occurred over 10 years. After full adjustment for additional covariates, a 1-unit log increase in CAR was associated with a 53% lower odds of incident diabetes among whites (Odds Ratio [OR] 0.47, 95% CI: 0.24, 0.90). A 1-SD increase in late decline slope was associated with a 416% higher odds of incident diabetes among whites (OR 5.16, 95% CI: 1.32, 20.20). There were no significant associations in AAs.
Conclusion:
A robust CAR and flatter late decline slope are associated with lower and higher odds of incident diabetes, respectively, among younger to middle-aged whites and may provide a future target for diabetes prevention in this population.
Keywords: Cortisol, Diabetes, African American, hypothalamic-pituitary-adrenal axis
Graphical Abstract
1. Introduction:
Hypothalamic-pituitary-adrenal (HPA)-axis dysregulation, characterized by a flattened diurnal cortisol profile, is cross-sectionally associated with type 2 diabetes (T2D) and with several conditions that promote T2D, including obesity and insulin resistance [1–3]. Cortisol promotes hyperglycemia indirectly through free fatty acids and central adiposity [4]. Cortisol induces a catabolic state by upregulating lipolysis and proteolysis. As a consequence, free fatty acids increase with metabolites (ceramides, diacylglycerols, and long-chain acyl-CoA), promoting hyperglycemia through the disruption of insulin signaling [5]. Glucocorticoids also promote redistribution of adipose tissue from peripheral to central depots with an abundance of glucocorticoid receptors in the visceral adipose tissue of central depots compared to subcutaneous adipose tissue [4]. In addition to its indirect effects on glycemia, cortisol directly: 1) promotes hyperglycemia through induction of hepatic genes responsible for gluconeogenesis [6]; 2) increases skeletal muscle insulin resistance through inhibition of glucose transporter GLUT 4 translocation to the cell surface [6]; and 3) modulates insulin secretion [6]. Cortisol concentrations throughout the day follow a classic circadian pattern. Levels are relatively high at wake-up. In the 30–45 minute period following wake-up, cortisol levels increase dramatically and reach their daily peak (cortisol awakening response [CAR]). Thereafter, levels fall throughout the day (early decline and late decline) and reach their daytime nadir at bedtime [7].
A less dynamic diurnal cortisol curve, characterized by a blunted CAR, flatter decline slopes, and/or higher bedtime cortisol level, has been linked with mental and physical health outcomes [7,8] and has been proposed as an indicator of HPA-axis dysfunction [7–9]. The physical health outcomes include incident diabetes and worse glycemic control among those with diabetes [7–11]. Diurnal cortisol curve profiles are influenced by factors such as age [12,13], sleep duration and quality [14] and psychosocial stress [15,16]. Whether changes in the specific features of the cortisol curve are directly impacting deleterious health outcomes through glucocorticoid receptors or inflammation or are markers of upstream biological factors is an area of investigation [8,17]. Some components of the diurnal cortisol curve, including AUC and the diurnal cortisol decline slope, are hypothesized to represent increased overall cortisol exposure [18,19]. In contrast, other features including the CAR may be more representative of the reactivity or function of the HPA axis [20–22]. Thus, chronobiology, including the level and timing of cortisol exposure, may be critical in understanding the role of cortisol in glucose metabolism.
Although a flattened diurnal curve and higher bedtime cortisol have been associated with dysglycemia and incident diabetes in non-Hispanic whites (whites) [10,23], no study has yet to fully elucidate possible racial differences among whites and AAs in the longitudinal relationship between diurnal cortisol curve features and incident diabetes. Notably, there are significant differences in the diurnal cortisol pattern between African Americans (AAs) and whites. AAs have higher evening salivary cortisol and an overall flatter diurnal curve, independent of socioeconomic status [24,25]. From an environmental perspective, differences in the diurnal cortisol pattern between AAs and whites may be partially explained by shorter sleep duration in AAs [14,26]. The prevalence of short sleep (< 6 hours) among African Americans is greater than 40% and AAs have worse sleep quality compared to whites [27]. Another potential factor is discrimination, a social stressor, wherein repeated exposure to it over time leads to flatter diurnal cortisol slopes among racial/ethnic minorities [16] and in general populations [15]. In the United States, AAs experience discrimination at higher rates than other racial/ethnic groups including whites [28]. Thus, shorter sleep duration and discrimination may be two significant causes of differences in diurnal cortisol patterns in AAs. From a genetic perspective, evidence suggests that heritability of cortisol levels at wake-up and 30 minutes post wake-up is high, whereas evening cortisol levels lack heritability among adults [29]. However, to our knowledge, no study has assessed the role of genetics or epigenetics in diurnal cortisol pattern differences between AAs and whites.
In this study, we aimed to analyze the association of baseline diurnal cortisol curve measures with incident diabetes over 10 years among middle-aged white and AA participants. The primary aims of the study were to examine the association between features of the diurnal cortisol curve with incident diabetes and to assess potential differences in outcomes between white and AA participants by conducting a stratified analysis. We hypothesized that a lower wake-up cortisol, attenuated cortisol awakening response (CAR), flatter decline slopes and higher bedtime cortisol would be associated with incident T2D over a ten-year period among both white and AA participants.
2. Methods:
2.1. Study Population:
The Coronary Artery Risk Development in Young Adults (CARDIA) Study was a longitudinal observational cohort study initiated between 1985 and 1986. CARDIA was designed to investigate the development of cardiovascular disease (CVD) and risk factors associated with its progression among AA and white adults aged 18–30 years of age from Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California [25]. Further details regarding the study design have previously been described [30]. Between 2000 and 2001, participants from the Chicago and Oakland sites living within a 50-mile radius of the participating clinics were recruited into a sub-study examining socioeconomic status and biological risk in the development of CVD, which included the assessment of salivary cortisol. Eight-hundred and ten adults aged 32–51 years were included in this CARDIA ancillary study and underwent baseline examinations between 2000–2001 (Year 15) with subsequent follow-up assessments in 2005–06 (Year 20) and 2010–11 (Year 25). The CARDIA study was approved by institutional review committees at each respective location and all participants gave informed consent.
2.2. Cortisol Measures and the Assessment of Cortisol Curve Features:
The output of cortisol follows a distinct circadian rhythm reaching a peak 30–45 minutes after waking, declining steadily throughout the day and reaching a nadir close to midnight [7]. In the CARDIA study, six salivary cortisol samples were obtained on a single weekday as part of the ancillary study at the Year 15 exam. These samples were collected immediately post-awakening, then 45 minutes, 2.5 hours, 8 hours and 12 hours later and at bedtime. Participants were provided with alarm watches calibrated to their wakeup times and time intervals described above to serve as directives to collect saliva using cotton swabs throughout the day. During sampling, participants were directed to keep the cotton in their mouths for 30 seconds. Once fully saturated with saliva, the samples were placed in salivettes, which were stored at room temperature at participants’ homes and brought to their respective clinics the following day. Samples were then kept frozen until assessed via time-resolved immunoassay with fluorometric end point detection [25,31]. Intra-assay variation was less than 12% and samples containing sub-detectable levels of cortisol were assigned a value of 0.5 nmol/L [25]. The six collections were used to define cortisol curve parameters including wake-up cortisol, the cortisol awakening response (CAR; 0 to 45-min post-awakening rise), early decline slope (45-min to 2.5-hour post awakening decline), late decline slope (2.5 hour to bedtime decline), overall decline slope (wakeup to bedtime; excluding 45-min post awakening), and bedtime cortisol [11,25]. The total area under the curve (AUC), a time-adjusted measure of total cortisol exposure while awake, was defined by the plot of log-transformed cortisol values against the collection times divided by the duration, in hours, between the first and last sample. The AUC measure was computed only for those who had data for at least 4 samples and a minimum of 12 hours between the first and last sample. In this paper, we call this standard AUC the 16-hour AUC because we restricted our samples only up to 16 hours after wakeup.
2.3. Outcomes Assessment:
Fasting plasma glucose (FPG) and insulin were measured with blood drawn by venipuncture and processed at the central laboratory according to a standard protocol. Glucose was assayed using the hexokinase coupled to glucose-6-phosphate dehydrogenase method by Linco Research (St. Louis, MO, USA). Individuals were considered to have impaired fasting glucose (IFG) if FPG was between 100–125 mg/dL. T2D was defined as FPG ≥126 mg/dL or taking diabetes medication. T2D status was assessed at years 20 and 25. Individuals that developed T2D at year 20 were censored and were considered incident at year 25. The radioimmunoassay for insulin required an overnight, equilibrium incubation and used a unique antibody that has less than 0.2% cross-reactivity to human proinsulin and its primary circulating split form Des 31, 32 proinsulin (Linco Research, St. Louis, MO, USA).
2.4. Covariates:
Demographic and socioeconomic factors previously shown to be associated with features of the cortisol curve were collected at Year 15 and subsequently used as covariates in the models [7,25,32]. Information regarding age, sex, education (years of schooling), cigarette smoking (current smoking versus not), current medications (including beta blockers or steroids) and Center for Epidemiologic studies depression scale (CES-D), were obtained during clinical interviews or questionnaires in accordance with the study design [25,30]. Information regarding the development, reliability, and generalizability of the CES-D scale has been described [33]. The overall diurnal profile tends to become flatter with age [12,13]. Men have higher maximum, trough, and total AUC cortisol than women [13,24]. Higher levels of education among whites and blacks is associated with higher wake-up cortisol [34]. Specifically, among whites it is associated with a more robust CAR and among AAs is associated with higher cortisol throughout the day [35]. Current cigarette smoking status is associated with higher total AUC cortisol and an overall flatter diurnal cortisol decline [13,36]. In the Study of Women’s Health Across the Nation (SWAN), higher CES-D scores were associated with flatter diurnal cortisol slopes [37]. In addition to being associated with the diurnal cortisol profile, lower levels of educational attainment, active cigarette smoking and depression are associated with an increased risk of T2D [38–40]. Consistent with a similar analysis, we used categorical BMI to adjust for adiposity [10]. Adiposity measures included both BMI and waist circumference. The former was calculated by weight (in kilograms)/ height^2 (in meters), while the latter was assessed through measurement of the circumference around the umbilicus.
2.5. Statistical Analysis:
Our analysis was restricted to participants with complete diurnal cortisol curve features (exposure) and data on outcomes and important covariates. Following exclusion of those with diabetes at baseline (n=29), missing data on exposures (n=57) and important covariates (smoking status [n=1], BMI [n=3] and CES-D score [n=11]), the final primary analytical cohort consisted of 709 participants (Figure 1, Exclusion Cascade). Differences were observed in the diurnal cortisol curve between individuals that woke up prior to and after 11 A.M. Thus, those that woke up after 11 A.M. (n=18) were excluded in a secondary analytical cohort consisting of 691 participants [25].
Figure 1.
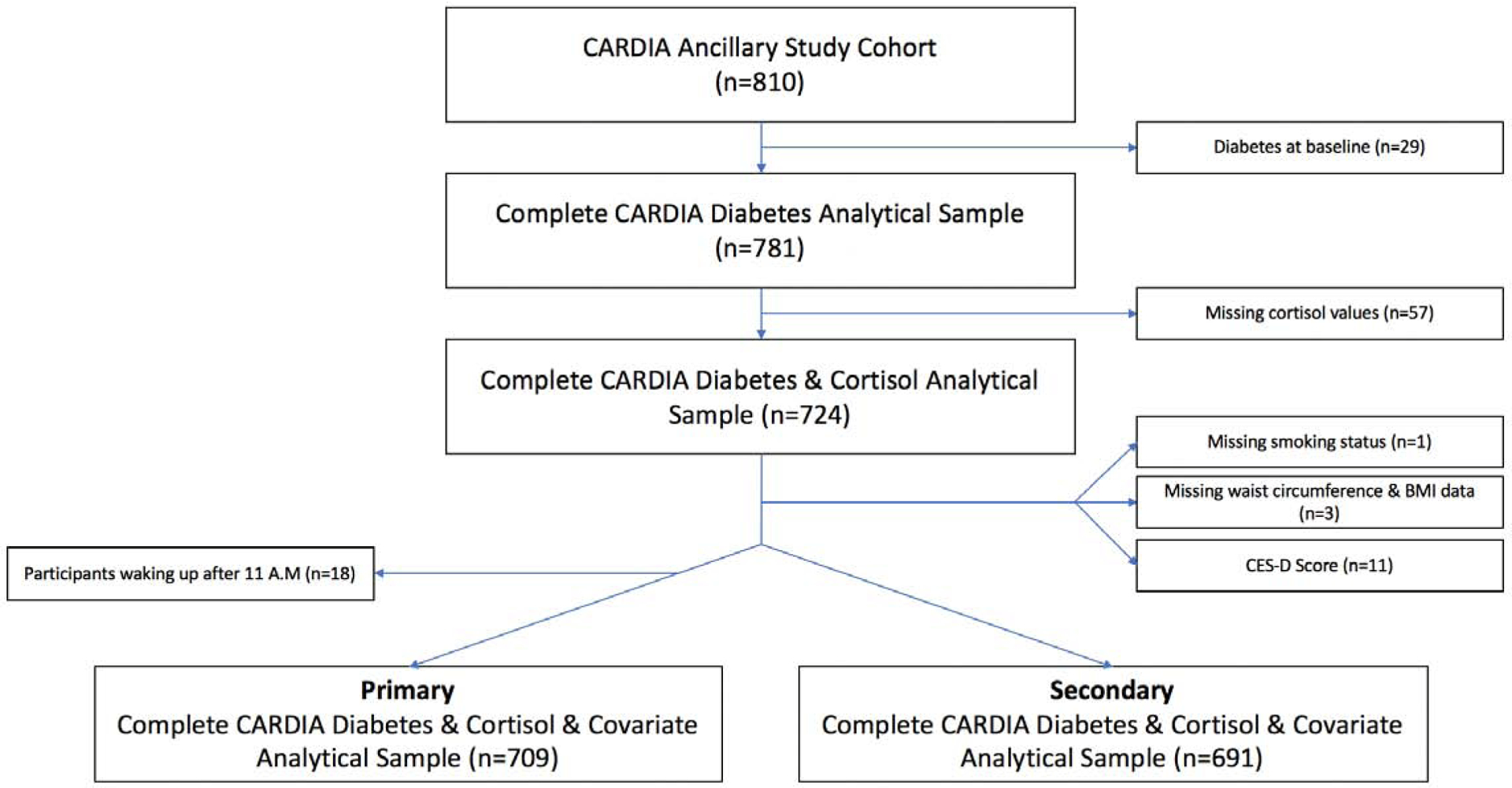
Exclusion Cascade for Participants in this Analysis
The CARDIA ancillary cortisol study cohort included 810 participants. Subjects were excluded on the basis of having diabetes at baseline (n=29) or had missing values for the following variables: diurnal cortisol profile components (n=57), smoking status (n=1), body mass index (BMI) (n=3) and Center for Epidemiologic Studies Depression scale (CES-D) score (n=11). The primary analytical data set included 709 participants. Individuals waking up after 11 A.M were excluded (n=18) for the secondary analysis, which ultimately included 691 individuals.
Baseline characteristics of participants at Year 15 were presented and stratified by race and the development of diabetes during follow-up using chi-square for categorical variables and two-sample t-test for continuous variables. As a result of positively skewed distributions, all cortisol curve values were log-transformed. We used logistic regression models to calculate the odds ratios (OR, 95% confidence interval [CI]) of incident T2D by log-transformed salivary cortisol curve measures among all participants with a race-interaction term in the model to give race specific estimates of the effect of cortisol measures. For the non-slope cortisol curve features, to calculate the OR for a 1-unit increase in log cortisol curve features, we fit a logistic regression model with log cortisol curve features as the predictor. The regression coefficient reflects the change in log-odds for a 1-unit change in log cortisol curve features. Through exponentiation of the regression coefficient, the OR per 1-unit change in log cortisol curve features can be estimated. To calculate the OR for a one standard deviation (SD) change in the early, late and overall decline slopes, we fit a logistic regression with a standardized predictor. Standardizing centers and scales the predictor such that it has a mean of zero and a SD of one. Thus, a 1-unit change in the standardized predictor coincides with a 1-SD change on the original scale. The OR per 1-SD change in the early, late and overall decline slopes was estimated via exponentiation of the regression coefficient. P-values for interaction effects were not adjusted for multiple comparisons and are reported here as preliminary data for descriptive purposes. We tested for effect modification by race using the following equation for the unadjusted models: logit(P(diabetes = Yes)) = β0 + β1 × (Cortisol Curve Feature) + β2 × (Race) + β3 × (Cortisol Curve Feature × Race). We used the likelihood ratio test to assess the statistical significance of the coefficient (β3) on the interaction term. In this equation, β3 is interpreted as the difference between the log-odds ratio corresponding to a change in wake-up cortisol by 1-unit among AAs and the log-odds ratio corresponding to an increase in wake-up cortisol by 1-unit among whites. Statistical significance was defined as two-sided alpha <0.05 in the main analysis. All analyses were performed with the use of SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA).
3. Results:
The 709 participants were majority female (58%) and non-smokers (82%). The average educational attainment was greater than high school (14.96 ± 2.47 years of education) and BMI was 29.25 ± 7.24 kg/m2 (Table 1). The cohort had a relatively even distribution of white and AA participants. White participants were 40.4 years of age compared to 39.6 among AAs with more years of education, and lower current smoking, CES-D scores, BMI, waist circumference and fasting insulin compared to AAs (Table 1). Whites had higher wake-up cortisol, steeper late decline slope, and a lower bedtime cortisol than AAs (p<0.01). Among all participants, those who developed T2D over the 10-year period had significantly higher bedtime cortisol levels (p<0.01) and showed a trend towards having a blunted CAR (p=0.05), as well as flatter late decline slopes (p=0.08) compared to those that did not develop T2D (Supplementary Table 1). Baseline characteristics of the 691 participants included in the secondary analysis are presented in Supplementary Table 2. Individuals that were excluded on the basis of waking up after 11 A.M were less educated with higher BMI and waist circumference (all p<0.05). Furthermore, excluded participants had lower wake-up cortisol (p<0.0001), flatter late and overall decline slopes (p<0.0001 and p=0.0006, respectively), and higher bedtime cortisol levels (p=0.0005). The diurnal cortisol curve of included vs. excluded groups of participants is shown in Figure 2. The figure depicts the plot means with standard error bars of cortisol by 6 time points for those who wake up before 11am vs those who wake up after 11 am. Participants with wake-up time after 11am had a flatter cortisol curve over the course of the 6 measurements compared to participants that woke up prior to 11am (Figure 2).
Table 1:
Baseline Characteristics of Participants by Race/Ethnicity
| Characteristica,b | All | African American | White | p-value |
|---|---|---|---|---|
| Participants - n (%) | 709 (100%) | 376 (53%) | 333 (47%) | |
| Demographic | ||||
| Age - years | 39.96 ± 3.63 | 39.55 ± 3.75 | 40.43 ± 3.44 | 0.0014 |
| Female sex - n (%) | 414 (58%) | 232 (62%) | 182 (55%) | 0.0574 |
| Race/Ethnicity - n (%)c | ||||
| White | 333 (47%) | |||
| African American | 376 (53%) | |||
| Years of Education | 14.96 ± 2.47 | 14.12 ± 2.20 | 15.90 ± 2.41 | <0.0001 |
| Clinical | ||||
| Fasting glucose (mg/dL) | 83.56 ± 9.85 | 84.17 ± 10.33 | 82.87 ± 9.26 | 0.0797 |
| Fasting Insulin (pmol/L) | 14.24 ± 10.73 | 15.84 ± 11.26 | 12.45 ± 9.81 | <0.0001 |
| Body mass indexd | 29.25 ± 7.24 | 31.23 ± 7.54 | 27.02 ± 6.16 | <0.0001 |
| Waist circumference (cm) | 89.11 ± 14.99 | 91.65 ± 14.97 | 86.23 ± 14.49 | <0.0001 |
| Beta-blocker - n (%) | 14 (2%) | 8 (2%) | 6 (2%) | 0.7556 |
| Steroid - n (%) | 34 (5%) | 21 (6%) | 13 (4%) | 0.2957 |
| Current Smoking - n (%) | 125 (18%) | 89 (24%) | 36 (11%) | <0.0001 |
| Center for Epidemiologic Studies Depression Scale Score | 9.19 ± 7.75 | 10.44 ± 8.20 | 7.79 ± 6.96 | <0.0001 |
| Cortisol Featurese | ||||
| Wake-up Cortisol | 2.80 ± 0.62 | 2.74 ± 0.66 | 2.88 ± 0.56 | 0.0035 |
| Cortisol Awakening Response | 0.28 ± 0.67 | 0.29 ± 0.67 | 0.27 ± 0.66 | 0.7132 |
| Early Decline Slope | −0.40 ± 1.71 | −0.46 ± 2.33 | −0.33 ± 0.33 | 0.2801 |
| Late Decline Slope | −0.07 ± 0.20 | −0.05 ± 0.20 | −0.10 ± 0.21 | 0.0002 |
| Overall Decline Slope | −0.10 ± 0.21 | −0.08 ± 0.24 | −0.11 ± 0.18 | 0.0728 |
| Bedtime Cortisol | 1.39 ± 2.36 | 1.64 ±1.77 | 1.11 ± 2.87 | 0.0037 |
| Total Area-Under-the-Curve (16-hours) Cortisol | 1.99 ± 0.83 | 1.98 ± 0.92 | 1.99 ± 0.71 | 0.8553 |
| Dysglycemia | ||||
| Normal - n (%) | 650 (93%) | 335 (91 %) | 315 (96%) | 0.0133 |
| Impaired Fasting Glucose - n (%)f | 47 (7%) | 33 (9%) | 14 (4%) | 0.0133 |
| HOMA-Betag | 4.75 ± 0.52 | 4.84 ± 0.53 | 4.64 ± 0.49 | <0.0001 |
| HOMA-IRg | 0.64 ± 0.60 | 0.75 ± 0.59 | 0.51 ± 0.58 | <0.0001 |
Data are reported as means ± SD or as numbers with percentages.
p-values reported for chi-square tests (categorical variables) and for two-sample t-test (continuous variables)
Race was self-reported.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Cortisol features were calculated using log-transformed cortisol values (unit: log[nmol/L]);
Impaired Fasting Glucose = 100–125 mg/dL
Homeostatic model assessment (HOMA) of β-cell function and insulin resistance (IR) are log-transformed.
Figure 2:
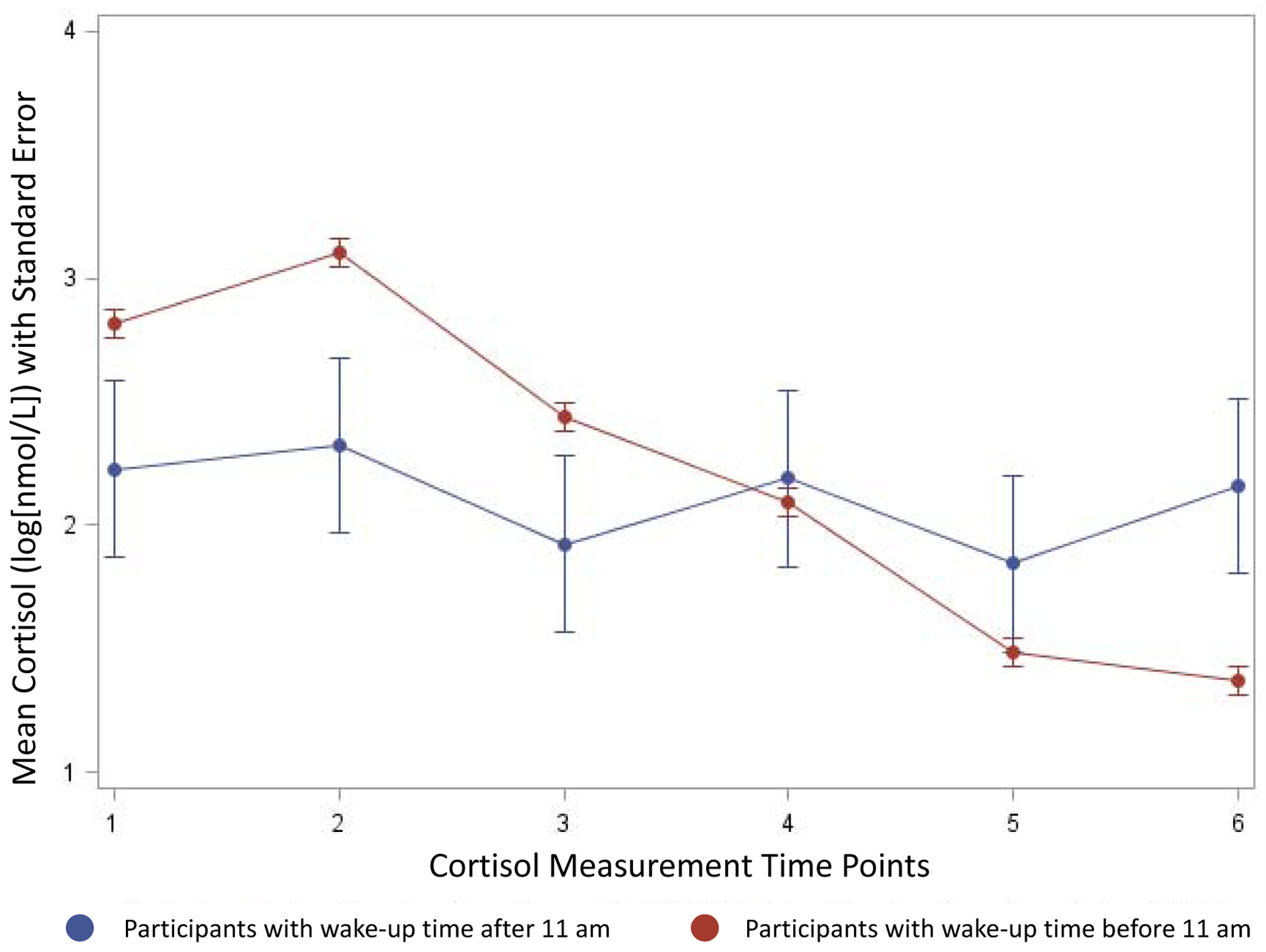
Diurnal Cortisol Profile Based on Wake-Up Time
The figure depicts the plot means with standard error bars of cortisol by 6 time points for those who wake up before 11am vs those who wake up after 11 am. Participants with wake-up time after 11am had a flatter cortisol curve over the course of the 6 measurements compared to participants that woke up prior to 11am.
Time points—1: immediately post-awakening, 2: 45 minutes, 3: 2.5 hours, 4: 8 hours, 5: 12 hours, 6: bedtime
Among whites, we found that a 1 unit increase in log CAR was associated with a 53% lower odds of incident diabetes over a ten-year period after full adjustment for covariates (OR: 0.47, 95% CI: 0.24–0.90). Additionally, a 1-SD flattening of the late decline slope (flattening of the slope) was associated with 5-fold higher odds of T2D among whites in the fully adjusted model (OR: 5.16, 95% CI: 1.32–20.20). There was some evidence of interaction between race and CAR (p=0.04, unadjusted Model 0) and between race and late decline slope (p=0.04, fully adjusted Model). Among AAs, there was no evidence of associations between baseline diurnal cortisol curve features with incident diabetes. The interaction term was non-significant across all models of the remaining diurnal cortisol curve components (Table 2).
Table 2.
Odds Ratios of Incident Diabetes over 10 years in CARDIA
| Variable, Group, Interactiona | Total #, Incident Cases | Model 0 OR (95% CI) | Model 1 OR (95% CI) | Model 2 OR (95% CI) |
|---|---|---|---|---|
| Wake-up Cortisol | ||||
| Whites | 333, 19 | 0.90 (0.40, 2.05) | 0.85 (0.37, 1.95) | 0.82 (0.36, 1.87) |
| African Americans | 376, 48 | 1.12 (0.71, 1.76) | 1.16 (0.73, 1.84) | 1.16 (0.73, 1.84) |
| Interaction | P=0.6548 | P= 0.5242 | P= 0.4793 | |
| Cortisol Awakening Response | ||||
| Whites | 333, 19 | 0.39 (0.21, 0.75) | 0.43 (0.22, 0.82) | 0.47 (0.24, 0.90) |
| African Americans | 376, 48 | 0.90 (0.57, 1.42) | 0.86 (0.54, 1.36) | 0.88 (0.55, 1.40) |
| Interaction | P= 0.0396 | P= 0.0912 | p=0.1238 | |
| Early Decline Slopeb | ||||
| Whites | 333, 19 | 3.98 (0.45, 34.79) | 3.57 (0.41, 31.4) | 3.09 (0.35, 27.67) |
| African Americans | 376, 48 | 0.97 (0.81, 1.15) | 0.96 (0.80, 1.15) | 0.97 (0.81, 1.16) |
| Interaction | P=0.2024 | P=0.2388 | p=0.2998 | |
| Late Decline Slopeb | ||||
| Whites | 333, 19 | 6.94 (1.81, 26.66) | 6.35 (1.58, 25.48) | 5.16 (1.32, 20.20) |
| African Americans | 376, 48 | 1.23 (0.96, 1.58) | 1.23 (0.95, 1.59) | 1.21 (0.93, 1.58) |
| Interaction | P=0.0132 | P=0.0226 | p=0.0414 | |
| Bedtime Cortisol | ||||
| Whites | 333, 19 | 1.43 (0.86, 2.36) | 1.39 (0.83, 2.34) | 1.33 (0.81, 2.20) |
| African Americans | 376, 48 | 1.24 (0.90, 1.71) | 1.23 (0.88, 1.71) | 1.25 (0.89, 1.75) |
| Interaction | P=0.6514 | P=0.6820 | p=0.8376 | |
| Overall Decline Slopeb | ||||
| Whites | 333, 19 | 2.54 (0.78, 8.24) | 2.61 (0.79, 8.63) | 2.47 (0.76, 8) |
| African Americans | 376, 48 | 0.96 (0.77, 1.21) | 0.95 (0.76, 1.19) | 0.96 (0.76, 1.21) |
| Interaction | P=0.1137 | P= 0.1036 | p=0.1203 | |
| Total AUC (16-Hours) Cortisol | ||||
| Whites | 333, 19 | 0.97 (0.52, 1.81) | 0.90 (0.47, 1.71) | 0.91 (0.49, 1.70) |
| African Americans | 376, 48 | 1.07 (0.74, 1.56) | 1.04 (0.72, 1.50) | 1.05 (0.70, 1.57) |
| Interaction | P=0.7845 | P=0.6931 | p=0.7032 |
logistic regression models to calculate the odds ratios
Slopes were standardized using z-scores
Model 0: Odds ratio of cortisol feature with incident diabetes (unadjusted)
Model 1: Odds ratio of cortisol feature with incident diabetes (adjusted for age, sex, race, education, CES-D score, beta-blocker or steroid [inhaled or oral] usage and cigarette smoking).
Model 2: Odds ratio of cortisol feature with incident diabetes (Model 1 + categorical BMI)
Bold = p<0.05
In Table 3, following the exclusion of participants who woke up after 11 A.M, a 1 unit increase in log CAR was associated with a 52% lower odds of incident T2D in the fully adjusted model (OR 0.48: 95% CI 0.25–0.94) among whites. The association of late decline slope with incident diabetes was in the same direction but was non-significant among whites (OR 3.02: 95% CI 0.65, 13.96). No significant associations were found among AA participants and the interaction term for race was non-significant in all models across all components of the diurnal cortisol curve.
Table 3.
Odds Ratios of Incident Diabetes over 10 years in CARDIA excluding Participants with Wake-up Time after 11am
| Variable, Group, Interactiona | Total #, Incident Cases | Model 0 OR (95% CI) | Model 1 OR (95% CI) | Model 2 OR (95% CI) |
|---|---|---|---|---|
| Wake-up Cortisol | ||||
| Whites | 327, 17 | 1.07 (0.45, 2.56) | 1.01 (0.42, 2.46) | 0.96 (0.39, 2.32) |
| African Americans | 364, 48 | 1.07 (0.67, 1.69) | 1.09 (0.68, 1.75) | 1.10 (0.69, 1.75) |
| Interaction | P=0.9907 | P= 0.8829 | P= 0.7859 | |
| Cortisol Awakening Response | ||||
| Whites | 327, 17 | 0.41 (0.21, 0.79) | 0.44 (0.23, 0.86) | 0.48 (0.25, 0.94) |
| African Americans | 364, 48 | 0.89 (0.57, 1.40) | 0.86 (0.54, 1.37) | 0.89 (0.56, 1.42) |
| Interaction | P= 0.0550 | P= 0.1101 | p=0.1377 | |
| Early Decline Slopeb | ||||
| Whites | 327, 17 | 5.71 (0.58, 55.83) | 5.04 (0.51, 49.54) | 4.55 (0.45, 46.47) |
| African Americans | 364, 48 | 0.97 (0.81, 1.16) | 0.97 (0.81, 1.16) | 0.97 (0.81, 1.17) |
| Interaction | P=0.1285 | P=0.1583 | p=0.1937 | |
| Late Decline Slopeb | ||||
| Whites | 327, 17 | 3.88 (0.86, 17.6) | 3.53 (0.74, 16.86) | 3.02 (0.65, 13.96) |
| African Americans | 364, 48 | 1.24 (0.96, 1.6) | 1.23 (0.94, 1.6) | 1.22 (0.93, 1.59) |
| Interaction | P=0.1441 | P=0.1908 | p=0.2492 | |
| Bedtime Cortisol | ||||
| Whites | 327, 17 | 1.24 (0.72, 2.14) | 1.21 (0.70, 2.10) | 1.18 (0.70, 1.98) |
| African Americans | 364, 48 | 1.26 (0.92, 1.74) | 1.25 (0.90, 1.74) | 1.28 (0.92, 1.79) |
| Interaction | P=0.9598 | P=0.9104 | p=0.7894 | |
| Overall Decline Slopeb | ||||
| Whites | 327, 17 | 1.52 (0.44, 5.28) | 1.55 (0.42, 5.72) | 1.57 (0.43, 5.73) |
| African Americans | 364, 48 | 0.97 (0.77, 1.23) | 0.97 (0.76, 1.23) | 0.97 (0.76, 1.25) |
| Interaction | P=0.4915 | P= 0.4843 | p=0.4803 | |
| Total AUC (16-Hours) Cortisol | ||||
| Whites | 327, 17 | 0.97 (0.50, 1.85) | 0.90 (0.46, 1.75) | 0.91 (0.48, 1.74) |
| African Americans | 364, 48 | 1.07 (0.74, 1.56) | 1.05 (0.73, 1.52) | 1.07 (0.71, 1.59) |
| Interaction | P=0.7792 | P=0.6783 | p=0.6885 |
logistic regression models to calculate the odds ratios
Slopes were standardized using z-scores
Model 0: Odds ratio of cortisol feature with incident diabetes (unadjusted)
Model 1: Odds ratio of cortisol feature with incident diabetes (adjusted for age, sex, race, education, CES-D score, beta-blocker or steroid [inhaled or oral] usage and cigarette smoking).
Model 2: Odds ratio of cortisol feature with incident diabetes (Model 1 + categorical BMI)
Bold = p<0.05
4. Discussion:
In CARDIA, a large biracial community-based cohort study with well-characterized data on salivary cortisol and diabetes status, this is the first report of the association of diurnal cortisol curve features with incident diabetes among whites and AAs. In this analysis, among whites a robust CAR at baseline was associated with significantly lower odds of T2D, and a flatter late decline slope was associated with higher risk of incident T2D, which was attenuated after excluding participants that woke-up after 11 A.M. Notably, significant associations between components of the diurnal cortisol curve and incident T2D were not directly evident among AA participants.
To our knowledge, this is the first analysis to report that a robust CAR among whites is associated with lower odds of T2D. The result is directionally congruent with previous cross-sectional investigations revealing that an attenuated CAR is associated with perturbations in glycemic control. A blunted CAR is associated with a trend towards elevated insulin and HOMA-IR [41]. Furthermore, individuals with T2D exhibit blunted CARs compared to those without diabetes, which may be influenced by reductions in hippocampal volume [1,42]. The two previous studies that examined diurnal salivary cortisol curve features and risk of T2D were performed among majority older, white participants in the Longitudinal Aging Study Amsterdam (LASA) and Whitehall II cohorts. In the former, a higher evening salivary cortisol was associated with the development of T2D in women over a period of 6–7.5 years [23]. In the Whitehall II study, elevated evening salivary cortisol levels at baseline were associated with incident T2D over 10 years of follow-up [10]. Furthermore, those with flatter decline slopes and higher bedtime salivary cortisol were more likely to exhibit impaired fasting glucose or develop incident T2D [10]. In conjunction with these previous investigations, our results support the hypothesis that a more dynamic diurnal cortisol curve may be protective of dysglycemia, while a general flattening of the curve is associated with the impairment of glucose metabolism among whites. These prior studies and ours further elucidate the hypothesized temporality of the relationship between changes in the diurnal cortisol profile and diabetes—suggesting that changes to the diurnal cortisol profile precede the development of diabetes.
Our analysis does not support a relationship between diurnal cortisol curve features and the development of T2D among AAs. While we did not observe any strongly significant relationships with respect to the longitudinal development of T2D, cross-sectional findings in the Multi-Ethnic Study of Atherosclerosis (MESA) suggest that a flatter diurnal cortisol curve is associated with impaired glucose control among those who already have T2D in a mixed cohort of whites, AAs and Hispanic Americans [11]. Additionally, morning serum cortisol is cross-sectionally associated with higher FPG, but not HbA1c among AAs without T2D and associated with higher FPG and HbA1c among those with T2D in the Jackson Heart Study [43]. These results indicate that the impact of cortisol may become more pronounced following the development of dysglycemia among AAs.
The direct pathological effects of cortisol and excessive HPA-axis activity on the development of insulin resistance and T2D are well-characterized. HPA-axis stimulation occurs alongside an increase in sympathetic nervous system (SNS) activity, resulting in the release of catecholamine and inflammatory cytokines including TNF-alpha, which promotes insulin resistance in skeletal muscle and dose-dependently upregulates 11β-HSD1 in visceral adipose tissue [44–48]. Cortisol directly impairs insulin signaling in skeletal muscle and liberates free fatty acids, both of which directly leads to insulin resistance [49,50]. Furthermore, cortisol promotes hyperglycemia via increasing gluconeogenesis in the liver and inhibiting insulin secretion in rodent beta-cells [51]. Additionally, the upregulation of 11β-HSD1 activity in visceral fat is significantly correlated with visceral fat cell size [52]. The local activity of cortisol at the adipocyte promotes the redistribution of adipose tissue from the periphery to central depots due to a relative overexpression of glucocorticoid receptors in visceral compared to subcutaneous adipose tissue [4]. Cortisol thus supports the development of visceral obesity, which is strongly associated with metabolic syndrome and T2D [53,54].
The majority of HPA-axis dysregulation likely stems from multifactorial progressive disturbances which accumulate throughout a lifetime. Overall stress burden stemming from various sources may also be linked to improper HPA-axis function, leading to a general flattening of the diurnal cortisol curve across the day [7]. Disruptions in sleep and the circadian rhythm may be another factor contributing to detrimental changes in HPA-axis function. Six consecutive nights of sleep restriction caused significant elevations in bedtime salivary cortisol and SNS activity in healthy men [55]. In addition, glucose tolerance decreases with sleep restriction, a finding which has been replicated in multiple investigations [56–58]. Disruption in circadian rhythms shares many deleterious effects on metabolic health as those seen in states of excessive cortisol production [59]. The molecular circadian pacemaker clock system and glucocorticoids are deeply intertwined.
Glucocorticoids are known to modulate the expression of peripheral clock genes, several of which contain glucocorticoid response elements (GREs) in their regulatory regions. The diurnal rhythm of cortisol thus serves a significant role in mediating synchronization of central and peripheral clocks [8]. Dysregulation of the diurnal rhythm stemming from multiple factors such as shift work [60], shortened sleep [14] or psychosocial stressors [16] may lead to de-coupling of the central and peripheral circadian cycles and ultimately metabolic disturbances. Perturbation of the circadian rhythm may partly explain the additional significant association observed between the late decline slope and incident diabetes when individuals waking up later than 11 A.M were included in the analysis. We show that a flattening of late decline slope is associated with increased odds of T2DM, which remained significant after adjustment for BMI among whites. The differences in associations dependent on the inclusion of those waking up after 11 A.M has implications for the extant literature and future investigations. Previous studies investigating cortisol and incident diabetes do not address inclusion based on wake-up times, which may have impacted the associations found [10,23]. Future studies of the diurnal cortisol features and various outcomes should take into consideration the significant differences in profiles based on wake-up time (Figure 2).
The baseline racial differences in cortisol curve features with AAs having a lower wake-up, flatter late decline slope and higher bedtime cortisol are consistent with the extant literature showing that AAs have flatter slopes than whites [24,25]. Interestingly, although AA participants had flatter late decline slopes, no significant associations between any decline slope features and incident diabetes were apparent among AAs. Higher baseline risk of incident diabetes in AAs may be one source of the non-significant findings in AAs. Compared to whites, AAs had higher fasting insulin, body mass index, and waist circumference with higher rates of smoking and depressive symptoms at baseline. Over the ten-year observational period, AAs had more than double the incidence of diabetes. Additional risk factors, in this case a blunted CAR or flatter diurnal cortisol curve, may impart less risk at the margin for individuals who are at high compared to low baseline risk. Our group has previously shown this phenomenon with respect to the association of modifiable lifestyle risk factors with incident diabetes [61,62]. The lack of significant findings among AAs may also be a result of confounding via unobserved variables such as sleep duration or discrimination. Short sleep duration is associated with both flatter decline slopes and risk of diabetes [14,63]. Discrimination is associated with a flattening of the diurnal cortisol curve and greater risk of incident diabetes [16,64]. Thus, the higher baseline risk of incident diabetes in AAs and unmeasured confounders, including sleep and stress, may have led to the non-significant findings in AAs.
Our study has several strengths. To our knowledge, it is the first to perform a longitudinal analysis examining components of the diurnal cortisol curve and incident diabetes in a middle-aged biracial cohort. Participants were recruited from two different metropolitan areas (Chicago and Oakland), which increases external validity of the findings. The longitudinal nature of the study allows us to make greater inferences regarding the temporality of the relationship observed. The study includes an assessment of diabetes with ascertainment of anti-diabetic medication status and biological assessment of glucose. Furthermore, we were able to control for various confounders that are known to modulate cortisol activity and are associated with T2D. Even with these strengths, our findings should be interpreted in the context of the following limitations. First, the diagnostic criteria for T2D did not include oral glucose tolerance testing or HbA1c, thus we may have misclassified some participants with undiagnosed T2D. Second, all samples of salivary cortisol were collected on a single day and cortisol was not collected throughout the night, thus we are unable to evaluate a full 24-hour diurnal cortisol cycle. Third, the statistical associations were interpreted without adjustment for multiple comparisons, consistent with previous analyses [10,23]. Typical multiple comparison corrections assume that tests are independent and too conservative for correlated hypotheses as we have here. Hence, some caution is warranted in the interpretation of our study results. Fourth, while participants were provided with alarm watches to promote the targeted sampling regimen, it is important to note that the CAR is highly sensitive to sampling time [65]. We cannot rule out the possibility that the CAR was inaccurately measured among a subset of participants. Finally, due to the nature of observational studies, we cannot conclude that a causal relationship exists between perturbations in components of the diurnal cortisol profile and the development of T2DM.
5. Conclusion
In this novel longitudinal analysis examining the association of diurnal cortisol curve features with incident T2D in a middle-aged, biracial cohort, a robust CAR was associated with lower odds of incident T2D among whites. Additionally, a flatter late decline slope was associated with higher odds of T2D among whites when individuals are included independent of wake-up time. In AAs, associations between diurnal cortisol curve features and incident T2D were not apparent. Thus, the diurnal cortisol curve may represent a potential target for diabetes prevention but with potential racial/ethnic variation.
Supplementary Material
Highlights.
Examined the association of the cortisol curve with incident diabetes over 10 years
Cortisol awakening response was inversely associated with diabetes in whites
Late decline slope was positively associated with diabetes in whites
Cortisol features were not associated with diabetes in African Americans
Acknowledgements
The authors thank the other investigators, the staff, and the participants of CARDIA for their valuable contributions. The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201800005I & HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I). This manuscript has been reviewed by CARDIA for scientific content. BK was supported by a research grant from the Endocrine Society Summer Research Fellowship Program. JJJ was supported by K23DK117041 from the National Institute of Diabetes and Digestive and Kidney Diseases (USA) and The Robert Wood Johnson Foundation Harold Amos Medical Faculty Development Program ID# 76236. The funding sources had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication. The graphical abstract was created with BioRender.com.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaismers that apply to the journal pertain.
The authors have no conflicts of interest to declare.
References:
- [1].Champaneri S, Xu X, Carnethon MR, Bertoni AG, Seeman T, Roux AD, et al. Diurnal salivary cortisol and urinary catecholamines are associated with diabetes mellitus: the Multi-Ethnic Study of Atherosclerosis. Metab - Clin Exp 2012;61:986–95. 10.1016/j.metabol.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ranjit N, Young EA, Raghunathan TE, Kaplan GA. Modeling cortisol rhythms in a population-based study. Psychoneuroendocrinology 2005;30:615–24. 10.1016/j.psyneuen.2005.02.003. [DOI] [PubMed] [Google Scholar]
- [3].Ursache A, Wedin W, Tirsi A, Convit A. Preliminary evidence for obesity and elevations in fasting insulin mediating associations between cortisol awakening response and hippocampal volumes and frontal atrophy. Psychoneuroendocrinology 2012;37:1270–6. 10.1016/j.psyneuen.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. The Pathogenetic Role of Cortisol in the Metabolic Syndrome: A Hypothesis. J Clin Endocrinol Metab 2009;94:2692–701. 10.1210/jc.2009-0370. [DOI] [PubMed] [Google Scholar]
- [5].Delarue J, Magnan C. Free fatty acids and insulin resistance. Curr Opin Clin Nutr Metab Care 2007;10:142–148. 10.1097/MCO.0b013e328042ba90. [DOI] [PubMed] [Google Scholar]
- [6].Kuo T, McQueen A, Chen T-C, Wang J-C. Regulation of Glucose Homeostasis by Glucocorticoids. Adv Exp Med Biol 2015;872:99–126. 10.1007/978-1-4939-2895-8_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Joseph JJ, Golden SH. Cortisol dysregulation: the bidirectional link between stress, depression, and type 2 diabetes mellitus. Ann N Y Acad Sci 2017;1391:20–34. 10.1111/nyas.13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Adam EK, Quinn ME, Tavernier R, McQuillan MT, Dahlke KA, Gilbert KE. Diurnal Cortisol Slopes and Mental and Physical Health Outcomes:A Systematic Review and Meta-analysis. Psychoneuroendocrinology 2017;83:25–41. 10.1016/j.psyneuen.2017.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dias JP, Joseph JJ, Kluwe B, Zhao S, Shardell M, Seeman T, et al. The longitudinal association of changes in diurnal cortisol features with fasting glucose: MESA. Psychoneuroendocrinology 2020;119:104698. 10.1016/j.psyneuen.2020.104698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hackett RA, Kivimäki M, Kumari M, Steptoe A. Diurnal Cortisol Patterns, Future Diabetes, and Impaired Glucose Metabolism in the Whitehall II Cohort Study. J Clin Endocrinol Metab 2016;101:619–25. 10.1210/jc.2015-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Joseph JJ, Wang X, Spanakis E, Seeman T, Wand G, Needham B, et al. Diurnal salivary cortisol, glycemia and insulin resistance: The multi-ethnic study of atherosclerosis. Psychoneuroendocrinology 2015;62:327–35. 10.1016/j.psyneuen.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab 1996;81:2468–73. 10.1210/jcem.81.7.8675562. [DOI] [PubMed] [Google Scholar]
- [13].Dmitrieva NO, Almeida DM, Dmitrieva J, Loken E, Pieper CF. A day-centered approach to modeling cortisol: Diurnal cortisol profiles and their associations among U.S. adults. Psychoneuroendocrinology 2013;38:2354–65. 10.1016/j.psyneuen.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Castro-Diehl C, Diez Roux AV, Redline S, Seeman T, Shrager SE, Shea S. Association of Sleep Duration and Quality With Alterations in the Hypothalamic-Pituitary Adrenocortical Axis: The Multi-Ethnic Study of Atherosclerosis (MESA). J Clin Endocrinol Metab 2015;100:3149–58. 10.1210/jc.2015-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Adam EK, Heissel JA, Zeiders KH, Richeson JA, Ross EC, Ehrlich KB, et al. Developmental histories of perceived racial discrimination and diurnal cortisol profiles in adulthood: A 20-year prospective study. Psychoneuroendocrinology 2015;62:279–91. 10.1016/j.psyneuen.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zeiders KH, Hoyt LT, Adam EK. Associations between self-reported discrimination and diurnal cortisol rhythms among young adults: The moderating role of racial-ethnic minority status. Psychoneuroendocrinology 2014;50:280–8. 10.1016/j.psyneuen.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].DeSantis A, DiezRoux A, Hajat A, Aiello A, Golden S, Jenny N, et al. Associations of salivary cortisol levels with inflammatory markers: the Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology 2012;37:1009–18. 10.1016/j.psyneuen.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Edwards S, Clow A, Evans P, Hucklebridge F. Exploration of the awakening cortisol response in relation to diurnal cortisol secretory activity. Life Sci 2001;68:2093–103. 10.1016/S0024-3205(01)00996-1. [DOI] [PubMed] [Google Scholar]
- [19].Golden SH, Sánchez BN, Wu M, Champaneri S, Diez Roux AV, Seeman T, et al. Relationship between the cortisol awakening response and other features of the diurnal cortisol rhythm: The Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology 2013;38. 10.1016/j.psyneuen.2013.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schmidt-Reinwald A, Pruessner JC, Hellhammer DH, Federenko I, Rohleder N, Schürmeyer TH, et al. The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Sci 1999;64:1653–60. 10.1016/S0024-3205(99)00103-4. [DOI] [PubMed] [Google Scholar]
- [21].Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. The cortisol awakening response: More than a measure of HPA axis function. Neurosci Biobehav Rev 2010;35:97–103. 10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- [22].Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: A systematic review and meta-analysis. Biol Psychol 2009;80:265–78. 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- [23].Schoorlemmer RMM, Peeters GMEE, Schoor NMV, Lips P. Relationships between cortisol level, mortality and chronic diseases in older persons. Clin Endocrinol (Oxf) 2009;71:779–86. 10.1111/j.1365-2265.2009.03552.x. [DOI] [PubMed] [Google Scholar]
- [24].Karlamangla AS, Friedman EM, Seeman TE, Stawksi RS, Almeida DM. Daytime Trajectories of Cortisol: Demographic and Socioeconomic Differences. Findings from The National Study of Daily Experiences. Psychoneuroendocrinology 2013;38. 10.1016/j.psyneuen.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic Status, Race, and Diurnal Cortisol Decline in the Coronary Artery Risk Development in Young Adults (cardia) Study. Psychosom Med 2006;68:41–50. 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- [26].Sleep duration partially accounts for race differences in diurnal cortisol dynamics. n.d. https://psycnet.apa.org/fulltext/2017-17131-002.html (accessed August 6, 2020). [DOI] [PMC free article] [PubMed]
- [27].Johnson DA, Jackson CL, Williams NJ, Alcántara C. Are sleep patterns influenced by race/ethnicity - a marker of relative advantage or disadvantage? Evidence to date. Nat Sci Sleep 2019;11:79–95. 10.2147/NSS.S169312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bleich SN, Findling MG, Casey LS, Blendon RJ, Benson JM, SteelFisher GK, et al. Discrimination in the United States: Experiences of black Americans. Health Serv Res 2019;54:1399–408. 10.1111/1475-6773.13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kupper N, de Geus EJC, van den Berg M, Kirschbaum C, Boomsma DI, Willemsen G. Familial influences on basal salivary cortisol in an adult population. Psychoneuroendocrinology 2005;30:857–68. 10.1016/j.psyneuen.2005.04.003. [DOI] [PubMed] [Google Scholar]
- [30].Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, et al. Cardia: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988;41:1105–16. 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- [31].Dressendörfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J Steroid Biochem Mol Biol 1992;43:683–92. 10.1016/0960-0760(92)90294-S. [DOI] [PubMed] [Google Scholar]
- [32].Hajat A, Diez-Roux A, Franklin TG, Seeman T, Shrager S, Ranjit N, et al. Socioeconomic and race/ethnic differences in daily salivary cortisol profiles: The Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology 2010;35:932–43. 10.1016/j.psyneuen.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas 1977;1:385–401. 10.1177/014662167700100306. [DOI] [Google Scholar]
- [34].Dowd JB, Ranjit N, Do DP, Young EA, House JS, Kaplan GA. Education and levels of salivary cortisol over the day in U.S. adults. Ann Behav Med Publ Soc Behav Med 2011;41:13–20. 10.1007/s12160-010-9224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bennett GG, Merritt MM, Wolin KY. Ethnicity, education, and the cortisol response to awakening: a preliminary investigation. Ethn Health 2004;9:337–47. 10.1080/1355785042000285366. [DOI] [PubMed] [Google Scholar]
- [36].Badrick E, Kirschbaum C, Kumari M. The Relationship between Smoking Status and Cortisol Secretion. J Clin Endocrinol Metab 2007;92:819–24. 10.1210/jc.2006-2155. [DOI] [PubMed] [Google Scholar]
- [37].Knight JM, Avery EF, Janssen I, Powell LH. Cortisol and Depressive Symptoms in a Population-Based Cohort of Midlife Women. Psychosom Med 2010;72:855–61. 10.1097/PSY.0b013e3181f4ab87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sacerdote C, Ricceri F, Rolandsson O, Baldi I, Chirlaque M-D, Feskens E, et al. Lower educational level is a predictor of incident type 2 diabetes in European countries: The EPIC-InterAct study. Int J Epidemiol 2012;41:1162–73. 10.1093/ije/dys091. [DOI] [PubMed] [Google Scholar]
- [39].Rimm EB, Chan J, Stampfer MJ, Colditz GA, Willett WC. Prospective study of cigarette smoking, alcohol use, and the risk of diabetes in men. BMJ 1995;310:555. 10.1136/bmj.310.6979.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rotella F, Mannucci E. Depression as a risk factor for diabetes: a meta-analysis of longitudinal studies. J Clin Psychiatry 2013;74:31–7. 10.4088/JCP.12r07922. [DOI] [PubMed] [Google Scholar]
- [41].Lasikiewicz N, Hendrickx H, Talbot D, Dye L. Exploration of basal diurnal salivary cortisol profiles in middle-aged adults: Associations with sleep quality and metabolic parameters. Psychoneuroendocrinology 2008;33:143–51. 10.1016/j.psyneuen.2007.10.013. [DOI] [PubMed] [Google Scholar]
- [42].Bruehl H, Wolf OT, Convit A. A blunted cortisol awakening response and hippocampal atrophy in type 2 diabetes mellitus. Psychoneuroendocrinology 2009;34:815–21. 10.1016/j.psyneuen.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ortiz R, Kluwe B, Odei JB, Echouffo Tcheugui JB, Sims M, Kalyani RR, et al. The association of morning serum cortisol with glucose metabolism and diabetes: The Jackson Heart Study. Psychoneuroendocrinology 2019;103:25–32. 10.1016/j.psyneuen.2018.12.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tomlinson JW, Moore J, Cooper MS, Bujalska I, Shahmanesh M, Burt C, et al. Regulation of Expression of 11β-Hydroxysteroid Dehydrogenase Type 1 in Adipose Tissue: Tissue-Specific Induction by Cytokines. Endocrinology 2001;142:1982–9. 10.1210/endo.142.5.8168. [DOI] [PubMed] [Google Scholar]
- [45].Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK. Tumor Necrosis Factor-α Induces Skeletal Muscle Insulin Resistance in Healthy Human Subjects via Inhibition of Akt Substrate 160 Phosphorylation. Diabetes 2005;54:2939–45. 10.2337/diabetes.54.10.2939. [DOI] [PubMed] [Google Scholar]
- [46].März P, Cheng J-G, Gadient RA, Patterson PH, Stoyan T, Otten U, et al. Sympathetic neurons can produce and respond to interleukin 6. Proc Natl Acad Sci 1998;95:3251–6. 10.1073/pnas.95.6.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Black PH. The inflammatory consequences of psychologic stress: Relationship to insulin resistance, obesity, atherosclerosis and diabetes mellitus, type II. Med Hypotheses 2006;67:879–91. 10.1016/j.mehy.2006.04.008. [DOI] [PubMed] [Google Scholar]
- [48].Esler M, Rumantir M, Wiesner G, Kaye D, Hastings J, Lambert G. Sympathetic Nervous System and Insulin Resistance: From Obesity to Diabetes. Am J Hypertens 2001;14:304S–309S. 10.1016/S0895-7061(01)02236-1. [DOI] [PubMed] [Google Scholar]
- [49].Dimitriadis G, Leighton B, Parry-Billings M, Sasson S, Young M, Krause U, et al. Effects of glucocorticoid excess on the sensitivity of glucose transport and metabolism to insulin in rat skeletal muscle. Biochem J 1997;321:707–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, et al. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest 1996;97:2859–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lambillotte C, Gilon P, Henquin JC. Direct glucocorticoid inhibition of insulin secretion. An in vitro study of dexamethasone effects in mouse islets. J Clin Invest 1997;99:414–23. 10.1172/JCI119175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Michailidou Z, Jensen MD, Dumesic DA, Chapman KE, Seckl JR, Walker BR, et al. Omental 11β-hydroxysteroid Dehydrogenase 1 Correlates with Fat Cell Size Independently of Obesity. Obesity 2007;15:1155–63. 10.1038/oby.2007.618. [DOI] [PubMed] [Google Scholar]
- [53].Fox Caroline S, Massaro Joseph M, Hoffmann Udo, Pou Karla M., Maurovich-Horvat Pal, Liu Chun-Yu, et al. Abdominal Visceral and Subcutaneous Adipose Tissue Compartments. Circulation 2007;116:39–48. 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- [54].Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiol Rev 1994;74:761–811. 10.1152/physrev.1994.74.4.761. [DOI] [PubMed] [Google Scholar]
- [55].Spiegel K, Leproult R, Cauter EV. Impact of sleep debt on metabolic and endocrine function. The Lancet 1999;354:1435–9. 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- [56].González-Ortiz M, Martínez-Abundis E, Balcázar-Muñoz BR, Pascoe-González S. Effect of sleep deprivation on insulin sensitivity and cortisol concentration in healthy subjects. Diabetes Nutr Metab 2000;13:80–3. [PubMed] [Google Scholar]
- [57].Nedeltcheva AV, Kessler L, Imperial J, Penev PD. Exposure to Recurrent Sleep Restriction in the Setting of High Caloric Intake and Physical Inactivity Results in Increased Insulin Resistance and Reduced Glucose Tolerance. J Clin Endocrinol Metab 2009;94:3242–50. 10.1210/jc.2009-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Donga E, van Dijk M, van Dijk JG, Biermasz NR, Lammers G-J, van Kralingen KW, et al. A Single Night of Partial Sleep Deprivation Induces Insulin Resistance in Multiple Metabolic Pathways in Healthy Subjects. J Clin Endocrinol Metab 2010;95:2963–8. 10.1210/jc.2009-2430. [DOI] [PubMed] [Google Scholar]
- [59].Nader N, Chrousos GP, Kino T. Interactions of the circadian CLOCK system and the HPA axis. Trends Endocrinol Metab 2010;21:277–86. 10.1016/j.tem.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Oster H, Challet E, Ott V, Arvat E, de Kloet ER, Dijk D-J, et al. The Functional and Clinical Significance of the 24-Hour Rhythm of Circulating Glucocorticoids. Endocr Rev 2017;38:3–45. 10.1210/er.2015-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Joseph JJ, Echouffo-Tcheugui JB, Talegawkar SA, Effoe VS, Okhomina V, Carnethon MR, et al. Modifiable Lifestyle Risk Factors and Incident Diabetes in African Americans. Am J Prev Med 2017;53:e165–74. 10.1016/j.amepre.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Joseph JJ, Bennett A, Tcheugui JBE, Effoe VS, Odei JB, Hidalgo B, et al. Ideal cardiovascular health, glycaemic status and incident type 2 diabetes mellitus: the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Diabetologia 2019;62:426. 10.1007/s00125-018-4792-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Chattu VK, Chattu SK, Burman D, Spence DW, Pandi-Perumal SR. The Interlinked Rising Epidemic of Insufficient Sleep and Diabetes Mellitus. Healthcare 2019;7. 10.3390/healthcare7010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Whitaker KM, Everson-Rose SA, Pankow JS, Rodriguez CJ, Lewis TT, Kershaw KN, et al. Experiences of Discrimination and Incident Type 2 Diabetes Mellitus: The Multi-Ethnic Study of Atherosclerosis (MESA). Am J Epidemiol 2017;186:445–55. 10.1093/aje/kwx047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kudielka BM, Kirschbaum C. Awakening cortisol responses are influenced by health status and awakening time but not by menstrual cycle phase. Psychoneuroendocrinology 2003;28:35–47. 10.1016/S0306-4530(02)00008-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


