Abstract
Purpose
The impacts of pre-existing atrial fibrillation (AF) on COVID-19-associated outcomes are unclear. We conducted a systematic review and meta-analysis to investigate the pooled prevalence of pre-existing AF and its short-term mortality risk in COVID-19 patients.
Methods
Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were followed in abstracting data and assessing validity. We searched MEDLINE and Scopus to locate all the articles published up to January 31, 2021, reporting data on pre-existing AF among COVID-19 survivors and non-survivors. The pooled prevalence of pre-existing AF was calculated using a random effects model and presenting the related 95% confidence interval (CI), while the mortality risk was estimated using the Mantel-Haenszel random effects models with odds ratio (OR) and related 95% CI. Statistical heterogeneity was measured using the Higgins I2 statistic.
Results
Twelve studies, enrolling 15.562 COVID-19 patients (mean age 71.6 years), met the inclusion criteria and were included in the final analysis. The pooled prevalence of pre-existing AF was 11.0% of cases (95% CI: 7.8–15.2%, p < 0.0001) with high heterogeneity (I2 = 95.2%). Pre-existing AF was associated with higher risk of short-term death (OR 2.22, 95% CI 1.47–3.36, p < 0.0001), with high heterogeneity (I2 = 79.1%).
Conclusion
Pre-existing AF is present in about 11% of COVID-19 cases but results associated with an increased risk of short-term mortality.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10840-021-00992-2.
Keywords: Atrial fibrillation, COVID-19, Prevalence, Mortality
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and represents an important contributor to morbidity and mortality of general population [1]. Over the latest months, several studies have demonstrated that a history of cardiovascular disease, as well as de novo cardiovascular involvement, during COVID-19 infection is associated with a poor outcome [2–5]. To this regard, AF has been widely investigated in SARS-CoV-2 patients, due its high prevalence in general population, as an independent predictor of mortality, severe disease, or complication of the infection [6–8]. However, comprehensive analyses of these findings as well as data regarding the outcomes of COVID-19 patients with a history of AF are still scant. Aim of the present manuscript is to perform a systematic review and meta-analysis to evaluate the mortality risk associated with an history of AF in COVID-19 patients.
Methods
Data sources and searches
The study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (Supplementary File 1) [9]. PubMed and Scopus databases were systematically searched for articles, published in English language, from inception through January 31, 2021, with the following medical subject heading (MESH) terms: COVID-19 [Title/Abstract] OR SARS-CoV-2 [Title/Abstract] AND “Survivors” [Title/Abstract]. In addition, references from the included studies were screened to potentially identify other investigations meeting the inclusion criteria. The informed consent was not required as the study did not enrol human subjects.
Study selection
Specifically, inclusion criteria were as follows: (i) studies enrolling subjects with a confirmed diagnosis of COVID-19 and (ii) studies stratifying the population as survivors (S) and non-survivors (NS), providing data on the pre-existing prevalence of AF. Conversely, studies presenting the prevalence of AF detected performing ECG at admission, the occurrence of AF as a complication of the infection, case reports, review articles, abstracts, editorials/letters, and case series with less than 10 participants were excluded. Each included article was independently evaluated by two reviewers (MZ, LR); in case of discrepancies, a third author was involved (GR), and final consensus was achieved through discussion.
Data extraction and quality assessment
Data were independently extracted by two reviewers (MZ, GR) using a standardized protocol. Disagreements were resolved. For this meta-analysis, the following data elements were extracted: sample size, number of survivors (S) and non-survivors (NS), mean age, gender, and major comorbidities (hypertension (HT) and diabetes mellitus (DM)) stratified according to the outcome status (S and NS). Moreover, to perform a meta-regression, the following potential baseline confounders were also evaluated: prevalence of coronary artery disease (CAD), cerebrovascular events (CVE), heart failure (HF), and body mass index (BMI). The quality of included studies was graded using the Newcastle-Ottawa quality assessment scale (NOS) [10].
Outcomes
The prevalence of AF was chosen as the primary outcome, while the mortality risk due to a history of AF was selected as the secondary outcome.
Data synthesis and analysis
Continuous variables were expressed as mean ± standard deviation (SD) or as median with corresponding interquartile range (IQR) while categorical variables as counts and percentages. The cumulative prevalence of AF (n/N), defined as the ratio between patients with history of AF (n) and the number of patients enrolled in each study (N), were pooled using a random effects model and presented with the corresponding 95% confidence interval (CI). To estimate the mortality risk, data were pooled using the Mantel-Haenszel random effects models with odds ratio (OR) as the effect measure with 95% CI. Heterogeneity among studies was assessed using Higgins and Thomson I2 statistic where I2 values correspond with the following levels of heterogeneity: low (< 25%), moderate (25–75%), and high (> 75%) [11]. Considering that funnel plots have intrinsic limitations in detecting publication bias, we further carried out the Egger’s regression test [12]. To further appraise the impact of potential baseline confounders, a meta-regression analysis was performed. The following variables were considered: age, BMI, gender, DM, HT, CAD, CVE, and HF. All meta-analyses were conducted using Comprehensive Meta-Analysis software, version 3 (Biostat, USA).
Results
Search results
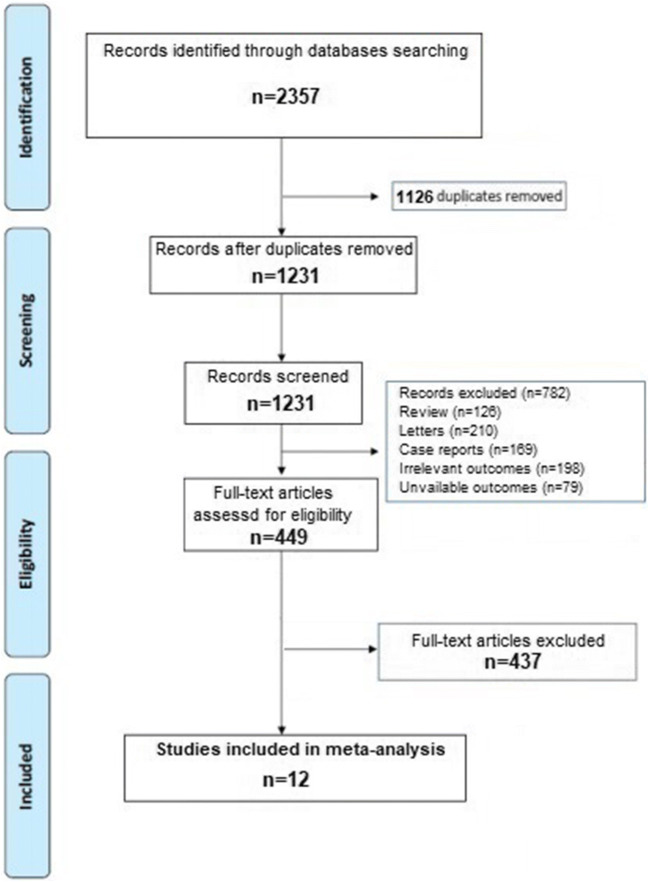
A total of 2357 articles were obtained throughout our search strategy. After excluding duplicates and preliminary screening, 449 full-text articles were assessed for eligibility and 437 studies were excluded for not meeting the inclusion criteria, leaving 12 investigations fulfilling the inclusion criteria [13–24]. A flow diagram of the literature search and related screening process is shown in Fig. 1.
Fig. 1.
PRISMA flow diagram
Study characteristics
Overall, 15,562 COVID-19 patients (mean age 71.6 years) were included in the analysis. The general characteristics of the analysed studies are summarized in Table 1. The mortality rate was 23.1% (95% CI 22.4–23.7). Overall, NS were older (77.9 vs 65.4 ears, p < 0.001), more frequently hypertensive, and diabetic compared to S. Quality assessment showed that all the studies were of moderate-high quality according to the NOS scale.
Table 1.
Characteristics of studies included in the meta-analysis
| Author | Sample size | NS | S | Age (years) [IQR]; (SD) | Males N (%) | DM N (%) | HT N (%) | Stroke N (%) | NOS | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NS | S | NS | S | NS | S | NS | S | NS | S | |||||
| Berrill et al. [13] | 50 | 17 | 33 |
75 (10.9) |
60.2 (21.2)* |
6 (35.3) |
16 (48.5) |
8/16 (50.0) |
9/31 (29.0)** |
12 (70.6) |
15/31 (48.4)* |
NR | 8 | |
| Mendes et al. [14] | 235 | 76 | 159 |
86.9 (6.4) |
86 (6.5) |
48 (63.1) |
54 (33.9)** |
23 (30.3) |
31 (19.5) |
56 (73.7) |
112 (70.4) |
15 (20.3) |
31 (19.6) |
8 |
| Quisi et al. [15] | 349 | 38 | 311 |
69 [60–76] |
55 [4–-61]** |
14 (36.8) |
139 (44.7) |
13 (34.2) |
93 (23.9) |
20 (52.6) |
101 (32.5)* |
2 (5.3) |
5 (1.6) |
7 |
| Rodilla et al. [16] | 12226 | 2630 | 9596 |
79.6 (10.5 |
64.1 (15.7)** |
(61.9) § |
(56.2)** § |
23.2 | 16.6 ** | 70.6 | 45.5** | (14.2)§ | (6.0)** | 8 |
| Cipriani et al. [17] | 109 | 20 | 89 |
86 [77–87] |
69 [57–79] |
10 (50.0) |
60 (71.0) |
6 (30.0) |
21 (24.0) |
16 (80.0) |
52 (58.0) |
5 (25.0) |
12 (14.0) |
7 |
| Knights et al. [18] | 103 | 34 | 69 | 78.9 | 63.8** |
20 (59.0) |
38 (55.0) |
14 (41.0) |
10 (14.0) |
18 (53.0) |
28 (41.0) |
11 (32) |
5 (7)* |
8 |
| Shi et al. [19] | 671 | 62 | 609 |
74 [66–81] |
61 [49–70]** |
35 (56.5) |
287 (67.1)** |
17 (27.4) |
80 (13.1)* |
37 (59.7) |
162 (26.6) |
8 (12.9) |
14 (2.3)** |
7 |
| Rossi et al. [20] | 590 | 334 | 256 |
79.5 [73–84] |
73 [64–80] |
187 (73.0) |
212 (63.5)* |
67 (26.2) |
70 (21.0) |
NR |
8 (2.4) |
13 (5.1) |
7 | |
| Cho et al. [21] | 143 | 36 | 107 |
75.8 (16.8) |
68.5 (17.2) |
20 (55.6) |
68 (63.3) |
9 (25.0) |
41 (38.3) |
16 (44.4) |
63 (58.9) |
NR | 8 | |
| Gomez Antúnez et al. [22] | 746 | 286 | 460 |
79 [74–86] |
75 [66–82] |
274 (86.7) |
365 (79.4) |
72 (25.4) |
119 (26.1) |
125 (75.74) |
298 (65.0) |
NR | 8 | |
| Turgay Yidirim et al.[23] | 139 | 26 | 113 |
71.8 (8.9) |
44.0 (19.2) |
19 (73.1) |
66 (58.4) |
9 (34.6) |
12 (10.6)* |
13 (50.0) |
26 (23.0)* |
NR | 8 | |
| Denegri et al. [24] | 201 | 42 | 159 |
79.9 (10.8) |
65.6 (14.1)** |
59.5 § |
65.4 § |
26.2 § |
16.5 § |
64.3 § |
54.4 § |
NR | 7 | |
NS non-survivors, S survivors, IQR interquartile range, SD standard deviation, DM diabetes mellitus, HT arterial hypertension, NOS Newcastle-Ottawa quality assessment scale. *p < 0.05 between non-survivors and survivors; **p < 0.001 between non-survivors and survivors. §Only percentages reported
Pooled prevalence of AF
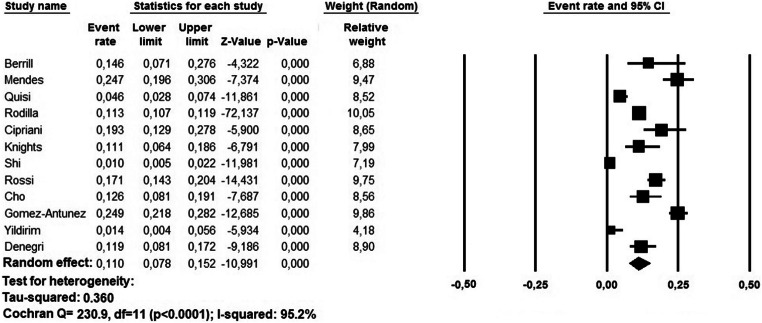
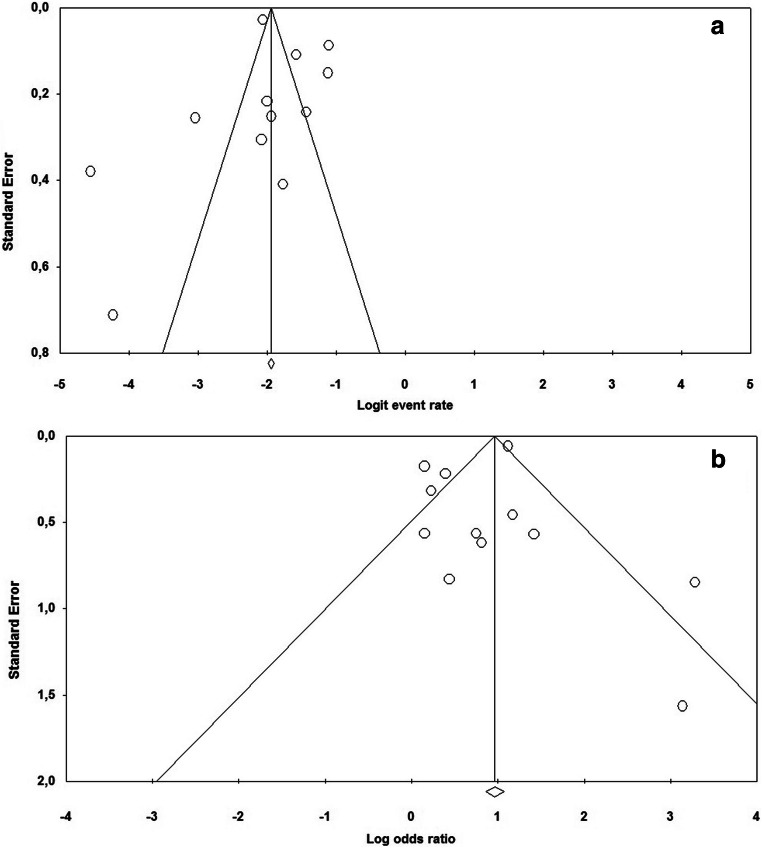
The prevalence of pre-existing AF among COVID-19 patients ranged between 0.5 and 21.8%. A random effect model revealed a pooled prevalence of pre-existing AF in 11.0% of cases (95% CI: 7.8–15.2%, p < 0.0001); heterogeneity was high (I2 = 95.2%) (Fig. 2). The relative funnel plot is presented in Fig. 4a. The Egger’s test did not show publication bias (t = 0.141; p = 0.890).
Fig. 2.
Pooled prevalence of pre-existing atrial fibrillation in COVID-19 patients. CI, confidence interval
Fig. 4.
Funnel plots for a the pooled prevalence of pre-existing atrial fibrillation in COVID-19 patients and b its associated mortality risk
History of AF and mortality risk
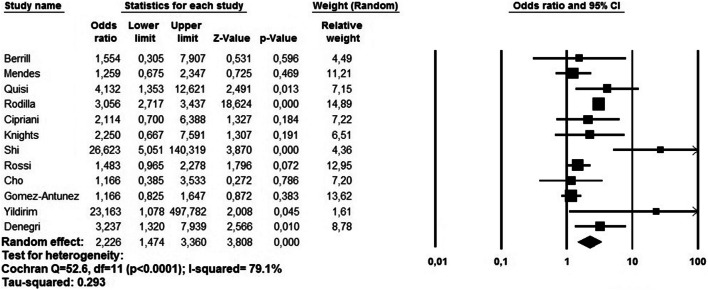
On pooled analysis, pre-existing AF was significantly associated with a higher risk of death in the short-term period (OR 2.22, 95% CI 1.47–3.36, p < 0.0001) (Fig. 3). Again, heterogeneity was high (I2 = 79.1%). Visual inspection of the relative funnel plot (Fig. 4b) did not reveal evidence of publication bias as confirmed by the Egger’s test (t = 0.705, p = 0.496).
Fig. 3.
Forest plot investigating the mortality risk due to pre-existing atrial fibrillation in COVID-19 patients using a random-effect model. CI, confidence interval
Meta-regression
Meta-regression analysis revealed that the association between pre-existing AF and short-term mortality in COVID-19 patients was affected by age (p = 0.011), gender (p = 0.032), HT (p = 0.001), CAD (p = 0.033), and HF (p = 0.0001). Conversely, no association were identified considering BMI, CVE, and DM as moderator variables (Table 2). Multivariable meta-regression including significant covariates in a single analysis showed that age (p = 0.368), HT (p = 0.210), CAD (p = 0.126), and HF (p = 0.421) effect are probably dependent on each other.
Table 2.
Effects of baseline clinical characteristics on the pooled mortality risk due to pre-existing atrial fibrillation in COVID-19 patients assessed by meta-regression analysis
| Variable | Number of studies | Coefficient | SE | 95% CI | p | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Age | 12 | -0.040 | 0.016 | -0.072 | -0.009 | 0.011 |
| Gender (males) | 12 | -0.035 | 0.016 | -0.068 | -0.003 | 0.032 |
| BMI | 4 | 1.167 | 0.866 | -0.530 | 2.864 | 0.177 |
| HT | 7 | -0.055 | 0.017 | -0.096 | -0.021 | 0.001 |
| CAD | 7 | -0.097 | 0.045 | -0.187 | -0.007 | 0.033 |
| CVE | 5 | -0.055 | 0.036 | -0.126 | 0.015 | 0.124 |
| DM | 12 | -0.012 | 0.044 | -0.100 | 0.075 | 0.781 |
| HF | 9 | -0.045 | 0.011 | -0.068 | -0.022 | 0.0001 |
BMI body mass index, HT arterial hypertension; CVE: Cerebrovascular events; CAD; Coronary artery disease; DM: Diabetes Mellitus; HF: Heart Faiulre
Discussion
Our meta-analysis, which enrolled more than 15,000 COVID-19 patients, showed that pre-existing AF is present in about 11% cases and significantly increases the short-term mortality risk. This association was influenced by age, gender, HT, CAD, and HF. All these potential baseline confounders were inversely related with the effect of pre-existing AF on the short-term outcomes. Specifically, the association between pre-existing AF and short-term mortality in COVID-19 patients was higher in younger males’ subjects without HT, CAD, and HF. Reason for such relationship can be found since older patients with such comorbidities may have a more aggressive treatments as both primary and secondary prevention, which partially reduces the risk of AF, especially in those with HF [25]. Our findings confirm the results of several investigations which demonstrated that the clinical outcomes in patients with SARS-CoV-2 infection are closely related to the burden of associated comorbidities [1–5, 26]. Moreover, our results fit well with a recent published meta-analysis evaluating the role of AF in COVID-19 patients in 108,745 patients. In fact, the authors underlined both a severe and worst outcome in COVID-19 patients with AF (OR 1.13, 95% CI 1.02–1.25) [27]. The presented results revealed a higher mortality risk, but our research was based on different study selection criteria, and our study did not focus only on AF patients, but analysed the published studies on COVID-19 patients stratifying their cohort among S and NS; therefore, the studies included in our meta-analysis are different from those considered by Yang et al. [27]. The inclusion of only subjects with pre-existing AF allowed us to limit the study bias since it has been reported that AF could be also a complication of infection. For this reason, investigations defining the presence of AF only based on the first ECG at admission and not considering its anamnestic presence were excluded in order to avoid an overestimation of AF cases [24, 28, 29]. Several potential explanations for the association between COVID-19 infection and AF have been proposed; however, the exact underlying pathophysiology has not yet been clarified. In particular, it has suggested a direct interaction between SARS-CoV2 cardiac cells expressing ACE-2 receptors, as pericytes. To this regard, it has been proposed that SARS-CoV2 infection may interrupt the pericyte-endothelial interaction and cause micro-vascular leakage promoting the release of several biochemical factors in the attempt to restore this reciprocal paracrine crosstalk (i.e., VEGF, basic fibroblast growth factor, Ang1, Ang2). This process would contribute to local tissue inflammation and perturbation of atrial cellular electrophysiology. Other putative mechanisms include the reduction of angiotensin-converting enzyme 2 (ACE2) receptor availability; the atrial structural changes via CD147- and sialic acid-spike proteins; the hypoxemia; which may result in myocardial injury and remodelling; and the activation of sympathetic nervous system, which together with electrolytes and fluids disturbances would lead to atrial fibrillation susceptibility [30].
Unfortunately, among the revised investigations, only Quisi et al. [15] compared the CHA2DS2-VASc score between survivors and non-survivors, reporting higher values among the latter group (p < 0.001). Thus, we were not able to adequately explore the thromboembolic risk in the analysed cohort as well as the contributing role of anticoagulant treatments. Similarly, the considered studies did not systematically describe AF electrocardiographic features, making impossible firm conclusion on the role of AF in COVID-19 patients. In this regard, Cho et al., using continuous telemetry, reported the occurrence of atrial flutter in 3.5% of cases, while new onset AF during the hospitalization was observed in 5.6% of subjects. Moreover, no detail data regarding the type of AF and its duration were provided by the reviewed studies. Considering these elements, our results should be considered preliminary. Further larger and detailed analyses, considering the electrocardiographic and clinical characteristics as well, by the integration of data provided by continuous telemetry monitoring, are needed to better describe the association between AF and COVID-19 infection.
Our meta-regression analysed different potential confounding factors for AF such as gender, age, and pre-existing medical disorders. Age and gender resulted associated with the mortality risk in COVID-19 patients with pre-existing AF and COVID-19 infection. These findings are in accordance with previous analyses performed among general population demonstrating that AF, per se, is associated with aging and gender, as well as HT and CAD [31, 32]. On the other hand, no significant association was observed between BMI and mortality risk, as already suggested in a previous large trial demonstrating that both overweight and obesity in patients with non-valvular AF were associated with a lower risk for the composite outcome of stroke/all-cause death [33]. Despite that pre-existing CVE has been associated with poor outcomes in patients with COVID-19 infection [34], we did not find any correlation in our analysis, probably because not all the revised manuscripts reported data on the occurrence of such comorbidities and therefore our analysis underestimated this relationship. Intriguingly, DM resulted not to be associated with mortality risk. As known, DM represents an independent risk factor for AF. However, as reported by a recent meta-analysis, higher serum glycated haemoglobin levels were significantly associated with incident AF in prospective cohort studies, but not in retrospective case-control studies, as those included in our analysis [35].
Despite that the revised manuscript did not systematically performed multivariate regression analyses, or they did not consider AF as covariate, our findings are in accordance with other recent published investigations suggesting that AF represents an independent risk factor for mortality, irruptively from age and comorbidities such as DM and HT [6, 36]. These investigations have not been included in our analysis since they have not met the inclusion criteria of the present meta-analysis.
Based on our findings, COVID-19 patients with AF constitute a subset with an increased risk for short-term mortality, which may benefit from an early identification and more aggressive surveillance/treatments since the first medical contact after the confirmation of SAR-CoV2- infection.
Limitations
We recognize some limitations to our study. Firstly, the observational and retrospective nature of the reviewed investigations (with their intrinsic and inherited biases) and the potential underestimation of pre-existing AF in the hospitalized patients may be responsible of the high heterogeneity in both the polled prevalence analysis and the mortality risk estimation. Furthermore, the absence of any adjustment for confounding relevant factors such as age, sex, baseline cardiovascular disease, anticoagulant treatments, and previous stroke in the reviewed studies have doubtless influenced the observed heterogeneity levels. Moreover, we could not investigate the effects of persistent AF versus paroxysmal AF on short-term mortality because the reviewed studies did not investigate this issue. Finally, we cannot evaluate the impact of different treatment strategies on the relationships between AF and short-term mortality.
Conclusions
The presence of pre-existing AF in COVID-19 patients is associated with an increased risk of short-term mortality, suggesting the need for closer monitoring and/or more aggressive treatments against the SAR-CoV-2 infection in these subjects.
Supplementary information
PRISMA checklist (DOCX 28 kb)
Authors’ contributions
Zuin M: conception; study design; writing the draft; data collection; data analysis. Rigatelli G: conception; study design; writing the draft; data collection; data analysis. Bilato C: revision; data collection; data analysis. Zanon F: data collection; revision. Zuliani G: data interpretation; critical revision. Roncon L: data interpretation; critical revision; supervision. All authors read and approved the final manuscript.
Data availability
Available upon request.
Declarations
Informed consent
Not applicable.
Conflict of interest
Francesco Zanon reports speaker fees from Abbott, Biotronik, Boston Scientific, Medtronic, and Microport outside the submitted work. The other authors have no conflict of interest to report.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124:2264–2274. doi: 10.1161/CIRCULATIONAHA.111.019893. [DOI] [PubMed] [Google Scholar]
- 2.Lala A, Johnson KW, Januzzi JL. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fried JA, Ramasubbu K, Bhatt R. The variety of cardiovascular presentations of COVID-19. Circulation. 2020;141:1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuin M, Rigatelli G, Zuliani G, Rigatelli A, Mazza A, Roncon L. Arterial hypertension and risk of death in patients with COVID-19 infection: systematic review and meta-analysis. J Infect. 2020;81:e84–e86. doi: 10.1016/j.jinf.2020.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuin M, Rigatelli G, Zuliani G, Bilato C, Zonzin P, Roncon L. Incidence and mortality risk in coronavirus disease 2019 patients complicated by acute cardiac injury: systematic review and meta-analysis. J Cardiovasc Med (Hagerstown). 2020;21(10):759–764. doi: 10.2459/JCM.0000000000001064. [DOI] [PubMed] [Google Scholar]
- 6.Mountantonakis SE, Saleh M, Fishbein J, Gandomi A, Lesser M, Chelico J, Gabriels J, Qiu M, Epstein LM, Northwell COVID-19 Research Consortium Atrial fibrillation is an independent predictor for in-hospital mortality in patients admitted with SARS-CoV-2 infection. Heart Rhythm. 2021;S1547-5271(21):00040-0. doi: 10.1016/j.hrthm.2021.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelesoglu S, Yilmaz Y, Ozkan E, Calapkorur B, Gok M, Dursun ZB, Kilic AU, Demirelli S, Simsek Z, Elcık D. New onset atrial fibrillation and risk factors in COVID-19. J Electrocardiol. 2021;65:76–81. doi: 10.1016/j.jelectrocard.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linschoten M, Peters S, van Smeden M, Jewbali LS, Schaap J, Siebelink HM, Smits PC, Tieleman RG, van der Harst P, van Gilst WH, Asselbergs FW, CAPACITY-COVID collaborative consortium Cardiac complications in patients hospitalised with COVID-19. Eur Heart J Acute Cardiovasc Care. 2020;9:817–823. doi: 10.1177/2048872620974605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses, 2012. http://wwwohrica/programs/clinical_epidemiology/oxfordasp, Accessed February 4, 2020
- 11.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berrill M, Karaj J, Zamfir G, Colman J, Mason R, Akbar S, et al. Chest radiographs may assist in predicting the outcome in the early phase of Covid-19. UK district general hospital experience of Covid-19 first wave. Expert Rev Respir Med. 2020:1–5. 10.1080/17476348.2021.1850278 Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 14.Mendes A, Serratrice C, Herrmann FR, Genton L, Périvier S, Scheffler M, Fassier T, Huber P, Jacques MC, Prendki V, Roux X, Di Silvestro K, Trombert V, Harbarth S, Gold G, Graf CE, Zekry D. Predictors of in-hospital mortality in older patients with COVID-19: the COVID age study. J Am Med Dir Assoc. 2020;21:1546–1554.e3. doi: 10.1016/j.jamda.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quisi A, Alıcı G, Harbalıoğlu H, Genç Ö, Er F, Allahverdiyev S, Yıldırım A, Kurt IH. The CHA2DS2-VASc score and in-hospital mortality in patients with COVID-19: a multicenter retrospective cohort study. Turk Kardiyol Dern Ars. 2020;48:656–663. doi: 10.5543/tkda.2020.03488. [DOI] [PubMed] [Google Scholar]
- 16.Rodilla E, Saura A, Jiménez I, Mendizábal A, Pineda-Cantero A, Lorenzo-Hernández E, Fidalgo-Montero MDP, López-Cuervo JF, Gil-Sánchez R, Rabadán-Pejenaute E, Abella-Vázquez L, Giner-Galvañ V, Solís-Marquínez MN, Boixeda R, Peña-Fernández A, Carrasco-Sánchez FJ, González-Moraleja J, Torres-Peña JD, Guisado-Espartero ME, Escobar-Sevilla J, Guzmán-García M, Martín-Escalante MD, Martínez-González ÁL, Casas-Rojo JM, Gómez-Huelgas R. Association of hypertension with all-cause mortality among hospitalized patients with COVID-19. J Clin Med. 2020;9:3136. doi: 10.3390/jcm9103136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cipriani A, Capone F, Donato F, Molinari L, Ceccato D, Saller A, et al. Cardiac injury and mortality in patients with Coronavirus disease 2019 (COVID-19): insights from a mediation analysis. Intern Emerg Med. 2020:1–9. 10.1007/s11739-020-02495-w Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 18.Knights H, Mayor N, Millar K, Cox M, Bunova E, Hughes M, Baker J, Mathew S, Russell-Jones D, Kotwica A. Characteristics and outcomes of patients with COVID-19 at a district general hospital in Surrey, UK. Clin Med (Lond). 2020;20:e148–e153. doi: 10.7861/clinmed.2020-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi S, Qin M, Cai Y, Liu T, Shen B, Yang F, Cao S, Liu X, Xiang Y, Zhao Q, Huang H, Yang B, Huang C. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41:2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossi L, Malagoli A, Biagi A, Zanni A, Sticozzi C, Comastri G, Pannone L, Gandolfi S, Vergara P, Villani GQ. Renin-angiotensin system inhibitors and mortality in patients with COVID-19. Infection. 2020;49:1–8. doi: 10.1007/s15010-020-01550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho JH, Namazi A, Shelton R, Ramireddy A, Ehdaie A, Shehata M, Wang X, Marbán E, Chugh SS, Cingolani E. Cardiac arrhythmias in hospitalized patients with COVID-19: a prospective observational study in the western United States. PLoS One. 2020;15:e0244533. doi: 10.1371/journal.pone.0244533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gómez Antúnez M, Muiño Míguez A, Bendala Estrada AD, Maestro de la Calle G, Monge Monge D, Boixeda R, Ena J, Mella Pérez C, Anton Santos JM, Lumbreras Bermejo C, SEMI-COVID-19 Network Clinical characteristics and prognosis of COPD patients hospitalized with SARS-CoV-2. Int J Chron Obstruct Pulmon Dis. 2021;15:3433–3445. doi: 10.2147/COPD.S276692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turgay Yıldırım Ö, Kaya Ş. The atherogenic index of plasma as a predictor of mortality in patients with COVID-19. Heart Lung. 2021;50:329–333. doi: 10.1016/j.hrtlng.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denegri A, Pezzuto G, D'Arienzo M, Morelli M, Savorani F, Cappello CG, et al. Clinical and electrocardiographic characteristics at admission of COVID-19/SARS-CoV2 pneumonia infection. Intern Emerg Med. 2021:1–6. 10.1007/s11739-020-02578-8 Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 25.Emdin CA, Callender T, Cao J, Rahimi K. Effect of antihypertensive agents on risk of atrial fibrillation: a meta-analysis of large-scale randomized trials. Europace. 2015;17:701–710. doi: 10.1093/europace/euv021. [DOI] [PubMed] [Google Scholar]
- 26.De Giorgi A, Fabbian F, Greco S, Di Simone E, De Giorgio R, Passaro A, Zuliani G, Manfredini R, OUTcome and COMorbidity Evaluation of INTernal MEDicine COVID19 (OUTCOME-INTMED-COV19) Study Collaborators Prediction of in-hospital mortality of patients with SARS-CoV-2 infection by comorbidity indexes: an Italian internal medicine single center study. Eur Rev Med Pharmacol Sci. 2020;24:10258–10266. doi: 10.26355/eurrev_202010_23250. [DOI] [PubMed] [Google Scholar]
- 27.Yang H, Liang X, Xu J, Hou H, Wang Y. Meta-analysis of atrial fibrillation in patients with COVID-19. Am J Cardiol. 2021;S0002-9149(21):00056–00054. doi: 10.1016/j.amjcard.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pardo Sanz A, Salido Tahoces L, Ortega Pérez R, González Ferrer E, Sanchez Recalde Á, Zamorano Gómez JL. New-onset atrial fibrillation during COVID-19 infection predicts poor prognosis. Cardiol J. 2020. 10.5603/CJ.a2020.0145 Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 29.Iacopino S, Placentino F, Colella J, Pesce F, Pardeo A, Filannino P, Artale P, Desiro D, Sorrenti P, Campagna G, Fabiano G, Peluso G, Giacopelli D, Petretta A. New-onset cardiac arrhythmias during COVID-19 hospitalization. Circ Arrhythm Electrophysiol. 2020;13:e009040. doi: 10.1161/CIRCEP.120.009040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gawałko M, Kapłon-Cieślicka A, Hohl M, Dobrev D, Linz D. COVID-19 associated atrial fibrillation: Incidence, putative mechanisms and potential clinical implications. Int J Cardiol Heart Vasc. 2020;30:100631. doi: 10.1016/j.ijcha.2020.100631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blum S, Meyre P, Aeschbacher S, Berger S, Auberson C, Briel M, Osswald S, Conen D. Incidence and predictors of atrial fibrillation progression: a systematic review and meta-analysis. Heart Rhythm. 2019;16:502–510. doi: 10.1016/j.hrthm.2018.10.022. [DOI] [PubMed] [Google Scholar]
- 32.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 33.Proietti M, Lane DA, Lip GY. Relation of nonvalvular atrial fibrillation to body mass index (from the SPORTIF Trials) Am J Cardiol. 2016;118:72–78. doi: 10.1016/j.amjcard.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Pranata R, Huang I, Lim MA, Wahjoepramono EJ, July J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19-systematic review, meta-analysis, and meta-regression. J Stroke Cerebrovasc Dis. 2020;29:104949. doi: 10.1016/j.jstrokecerebrovasdis.2020.104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang A, Green JB, Halperin JL, Piccini JP., Sr Atrial fibrillation and diabetes mellitus: JACC review topic of the week. J Am Coll Cardiol. 2019;74:1107–1115. doi: 10.1016/j.jacc.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 36.Spinoni EG, Mennuni M, Rognoni A, Grisafi L, Colombo C, Lio V, Renda G, Foglietta M, Petrilli I, D'Ardes D, Sainaghi PP, Aimaretti G, Bellan M, Castello L, Avanzi GC, Corte FD, Krengli M, Pirisi M, Malerba M, Capponi A, Gallina S, Pierdomenico SD, Cipollone F, Patti G, COVID-UPO Clinical Team† Contribution of atrial fibrillation to in-hospital mortality in patients with COVID-19. Circ Arrhythm Electrophysiol. 2021;14:e009375. doi: 10.1161/CIRCEP.120.009375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist (DOCX 28 kb)
Data Availability Statement
Available upon request.