Abstract
Aims
The role of psychological stress in the aetiology of atrial fibrillation (AF) is unclear. The death of a child is one of the most severe sources of stress. We aimed to investigate whether the death of a child is associated with an increased risk of AF.
Methods and results
We studied parents with children born during 1973–2014 included the Swedish Medical Birth Register (n = 3 924 237). Information on death of a child, AF and socioeconomic, lifestyle and health-related covariates was obtained through linkage to nationwide population and health registers. We examined the link between death of a child and AF risk using Poisson regression. Parents who lost a child had a 15% higher risk of AF than unexposed parents [incidence rate ratio (IRR) and 95% confidence intervals (CI): 1.15 (1.10–1.20)]. An increased risk of AF was observed not only if the child died due to cardiovascular causes [IRR (95% CI): 1.35 (1.17–1.56)], but also in case of deaths due to other natural [IRR (95% CI): 1.15 (1.09–1.21)] or unnatural [IRR (95% CI): 1.10 (1.02–1.19)] causes. The risk of AF was highest in the 1st week after the loss [IRR (95% CI): 2.87 (1.44–5.75)] and remained 10–40% elevated on the long term.
Conclusions
Death of a child was associated with a modestly increased risk of AF. Our finding that an increased risk was observed also after loss of a child due to unnatural deaths suggests that stress-related mechanisms may also be implicated in the development of AF.
Keywords: Bereavement, Psychological stress, Death of a child, Atrial fibrillation
See page 1496 for the editorial comment on this article (doi: 10.1093/eurheartj/ehab058)
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia. Its prevalence, currently estimated to 1–2% in the Western world has been increasing along the ageing of the population and is expected to double by 2050.1 Atrial fibrillation is associated with increased risks of cognitive dysfunction, stroke, heart failure, venous thromboembolism, cardiac events, and cardiovascular and total mortality.2 Several risk factors for AF have been identified, e.g. old age, male sex, certain genetic variants, European ancestry, smoking, alcohol consumption, extreme physical activity, obesity, diabetes, obstructive sleep apnoea, hypertension, and certain heart diseases.2 A substantial proportion of AF cases cannot, however, be attributed to these factors, prompting the need to identify additional risk factors.3
Psychological stress has been suggested to play a role in the development and maintenance of AF4 via stress-induced activation of the hypothalamic-pituitary-adrenal axis5 , 6 and the autonomic nervous system,7 adverse changes in health behaviour, endothelial function, blood pressure, inflammatory, metabolic, and thrombotic activity among other potential mechanisms.2 , 4 , 6 , 8 These may induce structural and electrophysiological changes in the atrial substrate, e.g. fibrosis, inflammatory infiltrates, focal ectopic firing from the pulmonary veins, altered atrial refractory periods, and conduction velocity, which in turn may contribute to the stabilisation and the perpetuation of re-entry and of AF.2 , 9 P-wave dispersion, a marker of atrial remodelling and a predictor of AF, has been observed in patients with panic disorder.10 Acute stress and the activation of the autonomic nervous system is common before paroxysmal AF episodes, suggesting a triggering effect.8 , 11 , 12 Only a few prospective studies analysed the association between psychological stress or adverse life events and the risk of AF and their findings have been mixed. Four prospective studies observed an association between job strain13–15 or adverse life events16 and the risk of AF, whereas others found no association.17–19
Death of a child is one of the most extreme sources of stress.20 Parents’ grief following the death of a child is intense and long-lasting21 and may hardly be fully resolved.22 Bereaved parents have an increased risk of psychiatric disorders,23 acute myocardial infarction24 and mortality,25 , 26 compared with unexposed parents. Thus, if stress is involved in the aetiology of AF, we may expect to observe an association between death of a child and AF.
In this large population-based study, we investigated whether the death of a child is associated with an increased risk of incident AF, taking into consideration characteristics of the loss and the time since the death.
Methods
Study population and design
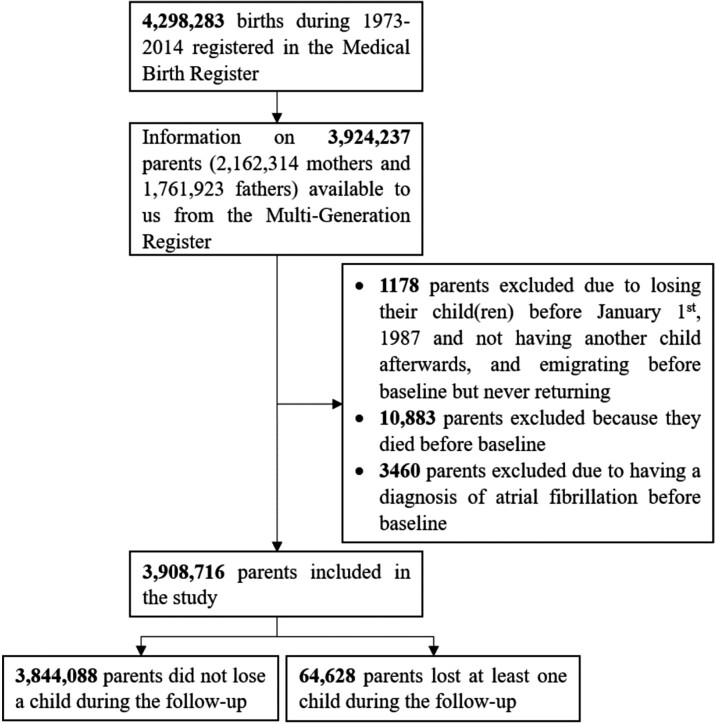
We conducted a population-based cohort study by linking individual-level data from several Swedish population-based registers using the unique personal identification number assigned to all Swedish residents. We defined the study population as parents of the 4 298 283 liveborn children recorded in the Swedish Medical Birth Register (MBR) during 1973–2014. These children were linked to their parents through the Swedish Multi-Generation Register, which includes familial link for individuals born in 1932 onward. Information on mothers was available for virtually all children in the initial cohort; we had information on fathers in 83% of the children, resulting in 3 924 237 parents being included in the parental cohort (Figure 1). Through the Multi-Generation Register, we also identified the parents’ other children who were not included in the MBR, including those born before 1973 when the MBR was launched, and those born outside Sweden and later registered as Swedish residents. Since we aimed to identify AF from the Swedish Patient Register, which achieved nationwide coverage for inpatient care in 1987, we defined the study period as 1987–2014. Follow-up of these parents started on 1 January 1987, birth of the first child, or immigration to Sweden, whichever came later, and ended at the time of the first diagnosis of AF, death, emigration out of Sweden, or 31 December 2014, whichever came first. Parents were included in the study, if they were living in Sweden and were alive at the start of the follow-up, had at least one child alive on 1 January 1987 or later, and did not have a record of AF prior to start of follow-up (n = 3 908 716) (Figure 1).
Figure 1.
The flowchart of the study participants.
The study was approved by the Regional Ethical Review Board in Stockholm.
Measures
Exposure
We defined exposure as the death of a child occurring after the start of follow-up. We obtained information on children’s date and cause of death from the Causes of Death Register. We classified exposed parents according to (i) the deceased child’s cause of death [death due to cardiovascular diseases (CVD), other natural causes, or unnatural causes], (ii) the deceased child’s age (≤1, 2–12, 13–18, or >18 years), (iii) the number of remaining live children at the time of the loss (0, 1–2, or ≥3 children), and (iv) the number of deceased children during the follow-up (0, 1, or ≥2). In case the parent lost several children during the follow-up, we considered the first loss in case of the above first three classifications. The International Statistical Classification of Diseases (ICD) codes used to classify causes of death are shown in Supplementary material online, Table S1.
Outcome
Information on the diagnosis of AF was retrieved from the Patient Register using the ICD codes presented in Supplementary material online, Table S1. The Patient Register was established in 1964 and had covered all inpatient care in the country since 1987; information on specialized outpatient care had been included since 2001. A Swedish validation study reported a positive predictive value of 97% for the diagnoses of AF and atrial flutter in the Patient Register.27
Covariates
Information on parents’ sex, birth year, marital status (married or in registered partnership vs. divorced, widowed, or single), and country of birth (Sweden vs. other countries) was obtained from the Total Population Register. Data on household income were obtained from the Register of Incomes and Taxes; the variable was categorized based on the tertile distribution of each 10-year interval. Information on education was retrieved from the Education Register and was classified as ≤9, 10–14, or ≥15 years; for study participants who entered the cohort before 1991 when the quality of data had been substantially improved, we used the information on education recorded in 1990. We used information on marital status, highest education, and household income from the year before study entry. If information for the corresponding year was lacking, we used data from the closest year with available data in the 5 years before study entry.
Information on study participants’ history of psychiatric disorders and personal and family history (i.e. parents and siblings according to the Multi-Generation Register) of CVD at baseline was obtained from the Patient Register. For women, we also retrieved information from the MBR on hypertensive disorders and diabetes before or during pregnancy (available since 1973) and on smoking (available since 1982) and body mass index (BMI) (available during 1982–1989 and 1992–) in early pregnancy; we used information on these characteristics from the pregnancies women had before baseline. The ICD codes used to retrieve information on the above medical conditions are shown in Supplementary material online, Table S1.
Statistical analyses
We analysed the association between the death of a child, treated as a time-varying exposure, and the risk of incident AF using Poisson regression. An exposed parent contributed person-years to the unexposed group until the date of the death of a child and contributed person-years thereafter to the exposed group. We performed analyses with any loss and with exposure categorized according to (i) the deceased child’s cause of death, (ii) age at death, (iii) the number of remaining live children the parent had at the time of loss, and (iv) the number of deceased children during the follow-up. We included the following potential confounders in our main models: sex, age at follow-up, calendar year at follow-up, country of birth, marital status, household income, highest attained education, history of psychiatric disorders and CVD at baseline. We treated age and calendar year of follow-up as time-dependent variables, i.e. we split the follow-up at every 5 years in case of age (<20, 20–90 by 5 years, and >90 years) and at every 10 years in case of calendar year (≤1989, 1990–1999, 2000–2009, and ≥2010). Given that a large proportion of study participants lacked information on their parents and siblings, we adjusted for family history of CVD in analyses restricted to those with register links to parents. In analyses restricted to mothers, we also adjusted for pre- and gestational hypertension and diabetes, as well as smoking and BMI in early gestation. To analyse the risk of AF in different time periods after bereavement, we performed analysis according to time since the loss (≤7, 8–30 days, 1–6, 6–12 months, 1–5, 5–10, and ≥10 years).
We further performed analyses stratified by parents’ sex, age (<50 vs. ≥50 years), attained education at baseline (<9, 10–14, and ≥15 years), the birth year of the first child prior to or after 1987 (when the coverage of the Patient Register became nationwide), and the start of follow-up before or after 2001 (when information on specialized outpatient care was added to the Patient Register). We also performed a sensitivity analysis by excluding parents who lost a child before 1987. To investigate the importance of conjugal support in bereaved parents, we repeated our main model after classifying bereaved parents based on their marital status at the end of the year that preceded their loss as (i) single, widowed, or divorced before the year preceding the loss, (ii) married or in registered partnership the year preceding the loss and divorced later, and (iii) married or in registered partnership the year preceding the loss and not divorced later.
Analyses were performed using SAS 9.4.
Results
A total of 64 628 (1.7%) of the 3 908 716 parents included in our study lost at least one child between 1987 and 2014 (Figure 1). The bereaved parents were more likely to be older, married or in registered partnership, to have only primary education, lower income, and a family history of CVD than their unexposed counterparts. Bereaved mothers were less likely to be obese and more likely to smoke in early pregnancy than non-bereaved mothers (Supplementary material online, Table S2).
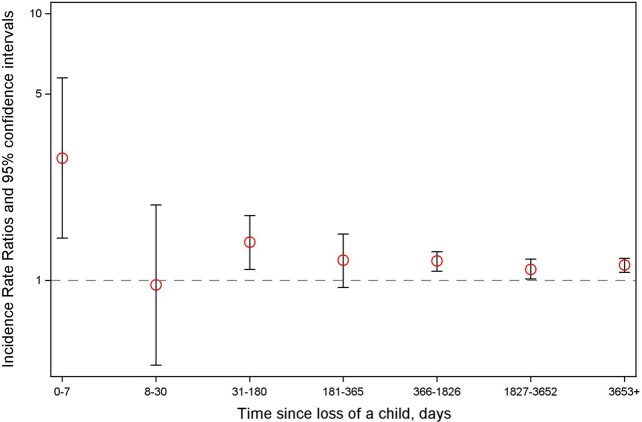
A total of 95 188 parents were diagnosed with AF during the follow-up, leading to an incidence rate of 130.6 per 105 person-years. Death of a child was associated with an increased AF risk [incidence rate ratio (IRR): 1.15, 95% confidence intervals (95% CI): 1.10–1.20]. The association was most pronounced if the child died of CVD but was also present when the child died of other natural or unnatural causes. The association was slightly stronger when the child died at the age of 13–18 years and when the parents had three or more children alive at the time of loss. We found no association between loss of two or more children and AF risk (Table 1). The risk of AF after bereavement was highest in the 1st week after the loss [IRR (95% CI): 2.87 (1.44–5.75)], but an increased risk was present throughout most part of the follow-up (Figure 2).
Table 1.
Adjusted incidence rate ratios and 95% confidence intervals for atrial fibrillation according to death of a child
| Exposure | Events/person-years | Age-adjusted IRR (95% CI) | Multivariable IRRa (95% CI) |
|---|---|---|---|
| Unexposed | 92 983/72 059 900 | 1.00 | 1.00 |
| All deaths | 2205/767 299 | 1.16 (1.11–1.21) | 1.15 (1.10–1.20) |
| Cause of death of the deceased | |||
| Death due to CVD | 187/34 228 | 1.33 (1.15–1.54) | 1.35 (1.17–1.56) |
| Other natural death | 1369/545 081 | 1.14 (1.09–1.21) | 1.15 (1.09–1.21) |
| Unnatural death | 649/187 989 | 1.14 (1.06–1.23) | 1.10 (1.02–1.19) |
| Age of the deceased child at loss (in years) | |||
| ≤1 | 230/281 829 | 1.35 (1.18–1.53) | 1.19 (1.04–1.35) |
| 2–12 | 113/107 717 | 1.01 (0.84–1.22) | 0.95 (0.78–1.14) |
| 13–18 | 222/76 850 | 1.28 (1.13–1.47) | 1.25 (1.10–1.43) |
| >18 | 1640/300 903 | 1.13 (1.08–1.19) | 1.15 (1.09–1.21) |
| Number of remaining live children at loss | |||
| 0 | 166/153 277 | 1.05 (0.90–1.22) | 1.01 (0.87–1.18) |
| 1–2 | 1207/458 317 | 1.12 (1.06–1.19) | 1.11 (1.05–1.18) |
| ≥3 | 832/155 705 | 1.24 (1.16–1.33) | 1.24 (1.16–1.33) |
| Number of deceased children during follow-up | |||
| 1 | 2155/746 967 | 1.16 (1.11-1.21) | 1.16 (1.11-1.21) |
| 2 or more | 50/20 332 | 1.02 (0.77-1.35) | 0.96 (0.72–1.29) |
CI, confidence interval; CVD, cardiovascular diseases; IRR, incidence rate ratio.
Adjusted for sex, age at follow-up, calendar year at follow-up, country of birth, marital status, household income, educational attainment, history of psychiatric disorders, and cardiovascular diseases.
Figure 2.
Incidence rate ratios for atrial fibrillation according to the time since the child’s death.
The association between the death of a child and AF was slightly stronger in mothers than in fathers and among younger (<50 years) than older parents (≥50 years) (Supplementary material online, Table S3 and S4). There was no evidence of effect modification by the parent’s educational attainment, the birth year of the first child, or the calendar year at the start of follow-up (Supplementary material online, Table S4). The point estimates for the association between the death of a child and the risk of AF did not change substantially after (i) excluding study participants who lost a child before 1987, (ii) adjusting for parents’ and siblings’ history of CVD at baseline, or (iii) adjusting for maternal pregestational or gestational diabetes and hypertension, and BMI and smoking in early pregnancy (Supplementary material online, Table S5). The IRR in case of bereaved parents who were single, widowed, or divorced at the end of the year preceding the loss was slightly higher [n = 20 584, IRR (95% CI): 1.30 (1.20–1.40)] than that in case of bereaved parents who remained married after the loss [n = 18 250, IRR (95% CI): 1.17 (1.09–1.25)]. The statistical power was limited to investigate AF risk in bereaved parents who divorced after their loss relative to the unexposed [n = 4065; IRR (95% CI): 0.98 (0.81–1.17)].
Discussion
In this nationwide population-based study, we found that the death of a child was associated with an increased risk of AF. The association was present not only for losses due to CVD, but also for losses due to other natural and unnatural causes. The risk of AF was particularly high in the 1st week after the loss but persisted throughout the follow-up. The association was slightly stronger in mothers than in fathers.
Comparison with earlier studies
Our findings that the loss of a child was associated with a modestly increased risk of AF corroborate the results of several earlier studies in this field reporting a modest association between psychological stress, e.g. job strain or adverse life events,13–16 , 28 negative emotions29 , 30 or mental ill-health,4 , 18 and the risk of AF. In contrast, other investigations found no evidence for an association between perceived stress and AF risk.17–19 Effect sizes in our study were comparable to or slightly lower to those observed in case of well-established risk factors for AF, e.g. hypertension (1.2–1.5), diabetes (1.3), or heavy alcohol consumption (1.3–1.5).31 A number of methodological concerns may limit causal inference in several of the earlier studies regarding the link between stress and AF, primarily the possibility of reverse causation and recall bias,4 small exposure contrasts,17 , 19 and limited statistical power to detect effects of the magnitude we reported.14 , 15 , 17–19 The strengths of our study are noted in several aspects. First, we investigated an objective source of stress that is likely to be emotionally demanding and to induce stress-related physiological changes for most individuals, irrespective of coping resources. Second, we collect information on exposure from a high-quality register before and independently of the outcome. Third, by employing a large population-based cohort with a long follow-up, we could have adequate statistical power to detect even modest associations, to examine short-term and long-term effects, and to investigate several potential effect modifiers. Fourth, linkage to several national population-based registers allowed us to consider a large number of potential confounders.
An important concern in studies regarding the association between bereavement and CVD is the separation of the stress-related effect of the loss from confounding due to genetic and environmental cardiovascular risk factors that cluster in families.26 We, therefore, conducted analyses according to the child’s cause of death. As expected, we found that parents who lost a child due to CVD had the highest risk of AF. However, the fact that an association was present also after adjusting for a large number of sociodemographic, health- and lifestyle-related confounders and also in case of loss due to unnatural deaths, which are less likely to be substantially affected by cardiovascular risk factors shared by family members, may suggest that stress-related mechanisms may also operate. Though several anatomical and electrophysiological differences (e.g. in the left atrial and ventricle size, ventricular wall thickness, structural remodelling, expression of ion channels that influence repolarization and thus refractoriness and re-entry) make men more prone to develop AF,31 , 32 we found a stronger association in mothers than in fathers. This finding, which is in line with several previous studies reporting that bereaved mothers have higher risks of mental illness23 and mortality,25 , 33 than bereaved fathers may be supportive of a causal effect, given that mothers often have a stronger emotional bond with their child than fathers. Though having other children at the time of loss has been suggested to help alleviate grief34 and buffer the impact of the child’s loss on the risk of morbidity and mortality,23 , 25 , 33 we found an increased AF risk in bereaved parents who had other child(ren) at the time of the loss relative to those who lost their single child. These findings are in line with a Danish study concerning the association between the death of a child and the risk of myocardial infarction24 and may reflect higher stress, e.g. due to difficulties in combining personal grief with the daily care and support to the surviving children. The lack of an association between death of two or more children and AF risk is not clear, but we speculate that, besides limited statistical power, the extreme stress that these parents may have experienced is likely to have led to disorganized life patterns, lack of interest in seeking care in case of cardiac symptoms and competing risks due to suicide or premature death of other causes.
Potential linking mechanisms
The mechanisms by which stress may increase AF risk involve the activation of the hypothalamic-pituitary-adrenocortical axis which may trigger paroxysmal AF episodes.11 In addition, chronic stress may result in depression, anxiety, poor lifestyle, dysregulation of the physiological stress systems, hypertension,35 and adverse changes in inflammatory and metabolic activity; these, in turn, may contribute to structural remodelling and electrical changes in the heart36 that increase AF risk.2 , 3 , 37 Our finding that death of a child was associated with an almost three-fold increased risk of AF in the week after the child’s death is supportive of the hypothesis of an acute effect of stress on AF and corroborates the findings of several case reports of paroxysmal AF after acute stress,8 , 12 as well as those of an interview-based study reporting that stress was the most common trigger of paroxysmal AF.38 A Danish study investigating the association between the death of a partner and the risk of AF found a transiently increased risk 8–14 days after the loss, after which the risk gradually declined.16 The reasons for not observing an increased risk of AF in the bereaved group 8–30 days after the child’s death in our study are not clear; we speculate that the parents’ attention was focused primarily on the tasks related to the child’s funeral and that they may have been less observant to the symptoms of AF or they may not have prioritized seeking care for these symptoms during this period.
Limitations
Our study has several limitations. First, though we adjusted for several potential confounders, we cannot exclude the possibility of residual confounding from genetic factors or unmeasured socioeconomic, lifestyle or health-related factors shared by family members. To consider confounding by familial factors, we performed subgroup analyses according to the deceased child’s cause of death and found an increased risk also after unnatural deaths, which are less prone to residual confounding. Second, we cannot exclude the possibility of misclassification of the outcome in our study. The validity of the AF diagnosis has been shown to be high in the Swedish Patient Register with few false positives,27 but we may have missed some cases of AF. These cases are likely to be asymptomatic, paroxysmal, or less severe cases. It is possible that parents who lost their child due to CVD may be more likely to interpret physical symptoms as being cardiac-related, to be more frequently examined and to receive long-term monitoring such as a 24-h electrocardiogram, and thus more likely to receive an AF diagnosis than parents in the other exposure groups. On the other hand, it is also possible that young bereaved parents, e.g. those who lost a child aged 2–12 years in our study, may be less perceptive to cardiac symptoms or less suspected to have AF and to have missed a mild or transient AF. Third, our findings may only be generalized to parents living in countries with low child mortality, with a free universal healthcare system and with a sociocultural context similar to that of Sweden. Fourth, we did not have information on cardiovascular or psychiatric medication or on psychosocial resources such as perceived social support, quality of parental relationships or participation in psychosocial interventions for bereaved parents, which may have influenced their ability to adjust to the loss of a child. Nevertheless, we could perform analyses in which we considered bereaved parents’ marital status before and after the loss. As expected, bereaved parents who were not married or in registered partnership at the time of the loss had a slightly higher AF risk than those who remained married after their loss. Due to the relatively small number of parents who divorced after their loss—a finding in line with those of Finnäs et al.39 reporting a modest, if any association between the death of a child and the risk of parental divorce—we did not have sufficient power to investigate whether parents who divorced after their loss had higher AF risks than those in the unexposed group. Although the death of a child may be accompanied by marital strain for some couples, for others such a traumatic event, and eventually engaging in a new pregnancy, may strengthen the relationship.39
Conclusions
In this large population-based cohort study, we found that the death of a child, one of the most extreme forms of stress, was associated with a modestly increased risk of incident AF. The findings that the association was present also in case of unnatural deaths, which are unlikely to be substantially affected by cardiovascular risk factors shared by family members, may suggest that stress-related mechanisms may contribute to this association. Further studies are needed to continue to investigate whether less severe, but more frequent sources of stress, with potentially more important public health implications, are also associated with AF and to elucidate the underlying mechanisms linking stress to AF.
Supplementary material
Supplementary material is available at European Heart Journal online.
Data availability
The data used in this manuscript were obtained from the National Board of Health and Welfare and from Statistics Sweden and cannot be shared publicly due to the regulations of these authorities and due to ethical considerations. Similar data may be requested from the above data holder authorities for research purposes by researchers that fulfil specific requirements.
Funding
The Swedish Council for Working Life and Social Research (2015-00837), the Karolinska Institutet’s Research Foundation (2018-01924, 2018-01547 and 2020-01600), and the China Scholarship Council (201700260276). J.L. and Y.Y. are supported by the Novo Nordisk Foundation (NNF18OC0052029), the Danish Council for Independent Research (DFF-6110-00019B and 9039-00010B), and the Karen Elise Jensens Fond (2016).
Conflict of interest: R.L. is employed at the Swedish Medical Products Agency, Uppsala, Sweden. The views expressed in this paper do not necessarily represent the views of the Government agency. The remaining authors have nothing to disclose.
Supplementary Material
Contributor Information
Dang Wei, Department of Global Public Health, Karolinska Institutet, Tomtebodavägen 18A, 171 77 Stockholm, Sweden.
Tristan Olofsson, Department of Medicine (Solna), Karolinska Institutet, Karolinska University Hospital D1:04, 171 76 Stockholm, Sweden.
Hua Chen, Department of Global Public Health, Karolinska Institutet, Tomtebodavägen 18A, 171 77 Stockholm, Sweden.
Imre Janszky, Department of Global Public Health, Karolinska Institutet, Tomtebodavägen 18A, 171 77 Stockholm, Sweden; Department of Public Health and Nursing, Norwegian University of Science and Technology, Håkon Jarls gate 11, 7030 Trondheim, Norway.
Fang Fang, Institute of Environmental Medicine, Karolinska Institutet, Nobels väg 13, 171 77 Stockholm, Sweden.
Rickard Ljung, Institute of Environmental Medicine, Karolinska Institutet, Nobels väg 13, 171 77 Stockholm, Sweden.
Yongfu Yu, Department of Clinical Medicine - Department of Clinical Epidemiology, Aarhus University, Olof Palmes Allé 43-45, 8200 Aarhus, Denmark.
Jiong Li, Department of Clinical Medicine - Department of Clinical Epidemiology, Aarhus University, Olof Palmes Allé 43-45, 8200 Aarhus, Denmark.
Krisztina D László, Department of Global Public Health, Karolinska Institutet, Tomtebodavägen 18A, 171 77 Stockholm, Sweden.
References
- 1. Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol 2014;6:213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH. Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circ Res 2017;120:1501–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, Maclehose R, Konety S, Alonso A. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2011;123:1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Galli F, Borghi L, Carugo S, Cavicchioli M, Faioni EM, Negroni MS, Vegni E. Atrial fibrillation and psychological factors: a systematic review. PeerJ 2017;5:e3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buckley T, Stannard A, Bartrop R, McKinley S, Ward C, Mihailidou AS, Morel-Kopp MC, Spinaze M, Tofler G. Effect of early bereavement on heart rate and heart rate variability. Am J Cardiol 2012;110:1378–1383. [DOI] [PubMed] [Google Scholar]
- 6. Uradu A, Wan J, Doytchinova A, Wright KC, Lin AYT, Chen LS, Shen C, Lin SF, Everett THt, Chen PS. Skin sympathetic nerve activity precedes the onset and termination of paroxysmal atrial tachycardia and fibrillation. Heart Rhythm 2017;14:964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen PS, Tan AY. Autonomic nerve activity and atrial fibrillation. Heart Rhythm 2007;4:S61–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ziegelstein RC. Acute emotional stress and cardiac arrhythmias. JAMA 2007;298:324–329. [DOI] [PubMed] [Google Scholar]
- 9. Patel D, Mc Conkey ND, Sohaney R, Mc Neil A, Jedrzejczyk A, Armaganijan L. A systematic review of depression and anxiety in patients with atrial fibrillation: the mind-heart link. Cardiovasc Psychiatry Neurol 2013;2013:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yavuzkir M, Atmaca M, Dagli N, Balin M, Karaca I, Mermi O, Tezcan E, Aslan IN. P-wave dispersion in panic disorder. Psychosom Med 2007;69:344–347. [DOI] [PubMed] [Google Scholar]
- 11. Lombardi F, Tarricone D, Tundo F, Colombo F, Belletti S, Fiorentini C. Autonomic nervous system and paroxysmal atrial fibrillation: a study based on the analysis of RR interval changes before, during and after paroxysmal atrial fibrillation. Eur Heart J 2004;25:1242–1248. [DOI] [PubMed] [Google Scholar]
- 12. Legallois D, Gomes S, Pellissier A, Milliez P. Medical emotional stress-induced atrial fibrillation: my own personal experience. Int J Cardiol 2013;167:e182–e183. [DOI] [PubMed] [Google Scholar]
- 13. Toren K, Schioler L, Soderberg M, Giang KW, Rosengren A. The association between job strain and atrial fibrillation in Swedish men. Occup Environ Med 2015;72:177–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fransson EI, Stadin M, Nordin M, Malm D, Knutsson A, Alfredsson L, Westerholm PJ. The association between job strain and atrial fibrillation: results from the Swedish WOLF study. Biomed Res Int 2015;2015:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fransson EI, Nordin M, Magnusson Hanson LL, Westerlund H. Job strain and atrial fibrillation—results from the Swedish Longitudinal Occupational Survey of Health and meta-analysis of three studies. Eur J Prev Cardiol 2018;25:1142–1149. [DOI] [PubMed] [Google Scholar]
- 16. Graff S, Fenger-Gron M, Christensen B, Pedersen HS, Christensen J, Li J, Vestergaard M. Long-term risk of atrial fibrillation after the death of a partner. Open Heart 2016;3:e000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Graff S, Prior A, Fenger-Gron M, Christensen B, Glumer C, Larsen FB, Vestergaard M. Does perceived stress increase the risk of atrial fibrillation? A population-based cohort study in Denmark. Am Heart J 2017;188:26–34. [DOI] [PubMed] [Google Scholar]
- 18. Garg PK, O'Neal WT, Diez-Roux AV, Alonso A, Soliman EZ, Heckbert S. Negative affect and risk of atrial fibrillation: MESA. J Am Heart Assoc 2019;8:e010603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Svensson T, Kitlinski M, Engstrom G, Melander O. Psychological stress and risk of incident atrial fibrillation in men and women with known atrial fibrillation genetic risk scores. Sci Rep 2017;7:42613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-III-R. Washington DC: APA; 1987. [Google Scholar]
- 21. Rubin SS, Malkinson R, Parental response to child loss across the life cycle: clinical and research perspectives. In: Handbook of Bereavement Research: Consequences, Coping, and Care. Washington, DC: American Psychological Association; 2001. p219–240. [Google Scholar]
- 22. Hendrickson KC. Morbidity, mortality, and parental grief: a review of the literature on the relationship between the death of a child and the subsequent health of parents. Palliat Support Care 2009;7:109–119. [DOI] [PubMed] [Google Scholar]
- 23. Li J, Laursen TM, Precht DH, Olsen J, Mortensen PB. Hospitalization for mental illness among parents after the death of a child. N Engl J Med 2005;352:1190–1196. [DOI] [PubMed] [Google Scholar]
- 24. Li J, Hansen D, Mortensen PB, Olsen J. Myocardial infarction in parents who lost a child: a nationwide prospective cohort study in Denmark. Circulation 2002;106:1634–1639. [DOI] [PubMed] [Google Scholar]
- 25. Li J, Precht DH, Mortensen PB, Olsen J. Mortality in parents after death of a child in Denmark: a nationwide follow-up study. Lancet 2003;361:363–367. [DOI] [PubMed] [Google Scholar]
- 26. Rostila M, Saarela J, Kawachi I. Mortality in parents following the death of a child: a nationwide follow-up study from Sweden. J Epidemiol Community Health 2012;66:927–933. [DOI] [PubMed] [Google Scholar]
- 27. Smith JG, Platonov PG, Hedblad B, Engstrom G, Melander O. Atrial fibrillation in the Malmo Diet and Cancer study: a study of occurrence, risk factors and diagnostic validity. Eur J Epidemiol 2010;25:95–102. [DOI] [PubMed] [Google Scholar]
- 28. O’Neal WT, Qureshi W, Judd SE, Glasser SP, Ghazi L, Pulley L, Howard VJ, Howard G, Soliman EZ. Perceived stress and atrial fibrillation: the REasons for Geographic and Racial Differences in Stroke Study. Ann Behav Med 2015;49:802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eaker ED, Sullivan LM, Kelly-Hayes M, D’Agostino RB, Benjamin EJ. Anger and hostility predict the development of atrial fibrillation in men in the Framingham Offspring Study. Circulation 2004;109:1267–1271. [DOI] [PubMed] [Google Scholar]
- 30. Eaker ED, Sullivan LM, Kelly-Hayes M, D'Agostino RB Sr, Benjamin EJ. Tension and anxiety and the prediction of the 10-year incidence of coronary heart disease, atrial fibrillation, and total mortality: the Framingham Offspring Study. Psychosom Med 2005;67:692–696. [DOI] [PubMed] [Google Scholar]
- 31. Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res 2014;114:1453–1468. [DOI] [PubMed] [Google Scholar]
- 32. Tian XT, Xu YJ, Yang YQ. Gender differences in arrhythmias: focused on atrial fibrillation. J Cardiovasc Transl Res 2020;13:85–96. [DOI] [PubMed] [Google Scholar]
- 33. Schorr L, Burger A, Hochner H, Calderon R, Manor O, Friedlander Y, Lawrence GM, Paltiel O. Mortality, cancer incidence, and survival in parents after bereavement. Ann Epidemiol 2016;26:115–121. [DOI] [PubMed] [Google Scholar]
- 34. Videka-Sherman L. Coping with the death of a child: a study over time. Am J Orthopsychiatry 1982;52:688–698. [DOI] [PubMed] [Google Scholar]
- 35. Buckley T, Mihailidou AS, Bartrop R, McKinley S, Ward C, Morel-Kopp MC, Spinaze M, Tofler GH. Haemodynamic changes during early bereavement: potential contribution to increased cardiovascular risk. Heart Lung Circ 2011;20:91–98. [DOI] [PubMed] [Google Scholar]
- 36. Aldhoon B, Melenovsky V, Peichl P, Kautzner J. New insights into mechanisms of atrial fibrillation. Physiol Res 2010;59:1–12. [DOI] [PubMed] [Google Scholar]
- 37. Brandes A, Smit MD, Nguyen BO, Rienstra M, Van Gelder IC. Risk factor management in atrial fibrillation. Arrhythm Electrophysiol Rev 2018;7:118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hansson A, Madsen-Härdig B, Bertil Olsson S. Arrhythmia-provoking factors and symptoms at the onset of paroxysmal atrial fibrillation: a study based on interviews with 100 patients seeking hospital assistance. BMC Cardiovasc Disord 2004;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Finnäs F, Rostila M, Saarela J. Divorce and parity progression following the death of a child: a register-based study from Finland. Popul Stud (Camb) 2018;72:41–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this manuscript were obtained from the National Board of Health and Welfare and from Statistics Sweden and cannot be shared publicly due to the regulations of these authorities and due to ethical considerations. Similar data may be requested from the above data holder authorities for research purposes by researchers that fulfil specific requirements.