Abstract
Following a decompressive craniectomy, the autologous bone flap is generally considered the reconstructive material of choice in pediatric patients. Replacement of the original bone flap takes advantage of its natural biocompatibility and the associated low risk of rejection, as well as the potential to reintegrate with the adjacent bone and subsequently grow with the patient. However, despite these advantages and unlike adult patients, the replaced calvarial bone is more likely to undergo delayed bone resorption in pediatric patients, ultimately requiring revision surgery. In this review, we describe the materials that are currently available for pediatric cranioplasty, the advantages and disadvantages of autologous calvarial replacement, the incidence and classification of bone resorption, and the clinical risk factors for bone flap resorption that have been identified to date.
Keywords: Craniectomy, Cranioplasty, Pediatric, Resorption, Osteolysis, Aseptic necrosis
ABBREVIATIONS
- CT
computed tomography
- OR
odds ratio
- PEEK
polyetheretherketone
- PMMA
polymethyl methacrylate
- TBI
traumatic brain injury
Decompressive craniectomy has been described in pediatric patients with malignant cerebral edema secondary to a large infarct,1 severe encephalitis,2,3 or severe traumatic brain injury (TBI).4 In the trauma population, a primary decompression is performed early in the patient's hospital course, typically in the context of a mass lesion (such as a subdural hematoma or a contusion) that requires evacuation. In contrast, a secondary decompression is performed in a delayed manner in patients with refractory intracranial hypertension, in whom medical therapies have failed.4-6 A decompressive craniectomy can improve brain compliance and compensatory reserve, resulting in improved cerebral perfusion.7
A number of techniques have been described as a decompressive craniectomy, each with a varying degree of bony removal and/or dural opening.8 A unilateral frontoparietotemporal craniectomy is commonly used, particularly in patients with focal pathology and/or mass lesions.9-12 Other options include a bifrontal craniectomy in patients with diffuse swelling,13-17 bilateral frontoparietotemporal craniectomies,10,11 and bilateral temporal craniectomies.18,19 Once the bony decompression is complete, the options for dural opening include leaving the dura intact, scoring the dura, or opening it with or without an expansile duraplasty.
In 2003, a multidisciplinary task force developed the “Guidelines for the Acute Medical Management of Severe Traumatic Brain Injury in Infants, Children, and Adolescents”;20 the recently published third edition provides a Level III recommendation that “decompressive craniectomy is suggested to treat neurologic deterioration, herniation, or intracranial hypertension refractory to medical management.”21
PEDIATRIC CRANIOPLASTY: AUTOLOGOUS VS SYNTHETIC RECONSTRUCTION
Following a decompressive craniectomy, there is typically a variable period of time during which the cerebral swelling subsides; replacement of the bone flap then becomes necessary via a cranioplasty. Two general categories of reconstructive materials have been described: autologous and synthetic. (Bone allografts are typically not used due to the risk of disease transmission.22) In a systematic review of 24 studies describing 864 pediatric cranioplasties, autologous bone flaps
were used in 56% of the cases, 75% of which utilized the original bone flap.23 Other autologous reconstruction techniques included split thickness calvarial grafts, particulate bone grafts, and rib grafts. In the pediatric population, split thickness calvarial grafts are difficult to obtain due to the immaturity of the diploic space in children under 5 yr of age, though successful techniques for obtaining split thickness grafts in this population have been described.24-26 Furthermore, extracranial donor sites such as rib grafts and iliac crest grafts are complicated by donor site morbidity and contour irregularity at the graft site.27-30
A number of synthetic materials have been used as an alternative to autologous bone flaps, including metals (titanium), acrylics (polymethyl methacrylate [PMMA]), ceramics (calcium phosphate-based cements such as hydroxyapatite), and plastics (porous polyethylene and polyetheretherketone [PEEK]).31-34 Although the ideal cranioplasty material would facilitate osteointegration and be lightweight, cosmetic, durable, physiologically compatible, and cost-effective, none of the materials listed above represents a perfect replacement for autologous bone.8,35 Titanium can be difficult to contour, and generates artifact on a computed tomography (CT) scan.23 PMMA is one of the preferred synthetic materials for adult cranioplasties, but complications associated with PMMA have included infection, extrusion, migration, and thermal sensitivity.30,31,36 Although hydroxyapatite cement has osteoconductive and osteoinductive properties similar to bone, it has been associated with high rates of infection, inflammation, fracture, and fragmentation when exposed to cerebrospinal fluid or blood, and is generally not recommended for full thickness defects.37 Plastic polymers have been used successfully in adults; porous polyethylene theoretically allows for bony ingrowth and revascularization, while PEEK implants are lightweight, durable, and biocompatible, though lacking in osteoconductive or osteoinductive properties.23,30,38,39 However, pediatric data remain sparse.
Autologous bone flaps have several distinct advantages over synthetic materials, including natural biocompatibility, low risk of rejection, and the ability to fit the original defect without the need for additional contouring.23,39-41 Autologous cranioplasty has also been suggested to have a lower risk of infection, though a meta-analysis identified a 3.9% infection rate in the autologous group and a 5.2% infection rate in the synthetic group, a difference that was not statistically significant (P = .56).23 Particularly in pediatric patients, however, an important advantage of autologous bone flaps is the ability to become reintegrated and subsequently grow with the patient.34,42 Consequently, unless there has been gross contamination of the bone, replacement of the original bone flap has typically been advocated for pediatric patients following a decompressive craniectomy.
BONE RESORPTION FOLLOWING PEDIATRIC CRANIOPLASTY
Despite its advantages, the use of autologous bone flaps for pediatric cranioplasty has historically been complicated by high rates of delayed bone resorption (Figure 1). Reintegration of a devascularized calvarial bone flap requires revascularization, osteoconduction, osteoinduction, and osteogenesis, whereby the graft serves as an inert biological scaffold for blood vessels and osteoprogenitor cells from the adjacent viable tissue.34,43,44 Graft remodeling involves a balance between bone resorption and new bone deposition; any interference to this process may result in excessive bone resorption.
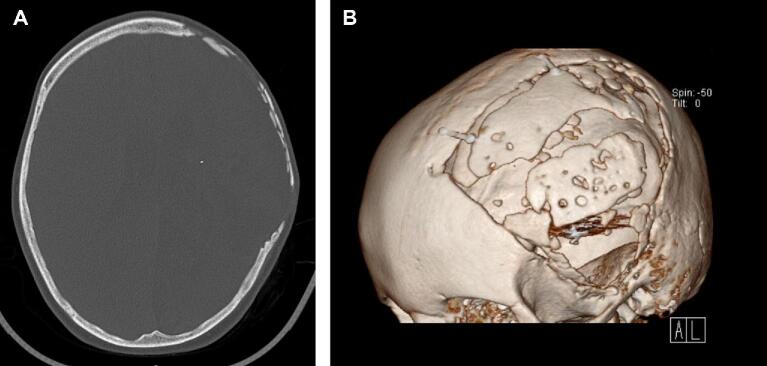
FIGURE 1.
Imaging obtained in a 6-yr-old boy who underwent a decompressive craniectomy for a large intraparenchymal hemorrhage, followed by native bone flap replacement 2 mo later. A, Nonenhanced axial head CT obtained with bone window settings. B, Three-dimensional skull reconstruction showing bone flap resorption 6 mo following the cranioplasty.
The incidence of resorption has varied widely in the literature. Among adults, the quoted incidence ranges from 3% to 23%,45-48 with one meta-analysis finding an overall rate of 20%.49 Among pediatric patients, the incidence of bone resorption is significantly higher, with Piedra et al,50 Grant et al,34 and Bowers et al51 reporting resorption rates of 29.5%, 50%, and 50%, respectively (Table 1). Others have demonstrated even higher rates of resorption, with Beez et al52 reporting an incidence of 76.9% and Martin et al53 documenting resorption in 66.7% of patients. In a multicenter retrospective study, Rocque et al54 found that 21.7% of their patients underwent significant bone resorption requiring revision surgery. Additionally, a systematic review published in 2013 identified an overall rate of resorption of 36%,40 while a more recent systematic review published in 2018 found an overall resorption rate of 16.5%.49 The wide range reported in the literature may be related to (1) differences in how “bone resorption” is defined, (2) confounders such as age, the interval between decompression and cranioplasty, and surgical technique, and/or (3) missing data due to the fact that resorption rates are not reported in many studies.49,52
TABLE 1.
Summary of Existing Pediatric Literature
| Grant et al, 200434 | Piedra et al, 201250 | Bowers et al, 201351 | Martin et al, 201453 | Rocque et al, 201854 | Beez et al, 201952 | ||
|---|---|---|---|---|---|---|---|
| Study design | Single center | Single center | Single center | Single center | Multicenter (13 centers) | Single center | |
| Patients | 40 | 61 | 54 | 27 | 359 | 15 | |
| Defect surface area | 99.4 cm2 (14-147 cm2) | N/A | “Generous frontotemporoparietal decompressive craniectomy” | “As large as possible, covering about two-thirds of the hemisphere” | N/A | 121.6 cm2 (74-159 cm2) | |
| Age | 9.3 yr (6 wk to 19 yr) | 9.5 yr (range not reported) | 6.2 ± 4.7 yr | Group 1: 4.9 yra (1 mo to 14 yr) | Group 2: 17.4 yra (15-17 yr) | 8.4 ± 5.7 yr | 12 yr (1-17 yr)b |
| Interval from craniectomy to cranioplasty | N/A | 2.1 mo (0.2-9.9 mo) | 2.1 mo (0.3-13 mo) | Group 1: 3.0 moa (0.5-3.7 mo) | Group 2: 3.3 moa (2.8-5.1 mo) | N/A | 1.5 mo (1-4 mo) |
| Bone flap resorption | 20 (50%) | 18 (29.5%) | 27 (50%) | Group 1: 12 (66.7%) | Group 2: 0 (0%) | 52 (21.7%)c | 8 patients/10 bone flaps (77%) |
| Infection | 2 (10%) | 4 (6.6%) | 9 (16.7%) | Group 1: 2 (11%) | Group 2: 2 (22%) | 38 (10.5%) | 1 (7.7%) |
| Interval from cranioplasty to resorption | N/A | N/A | 4.8 mo (1-36 mo) | Group 1: 8.4 mo (range not reported) | Group 2: N/A | N/A | 19 mo (4-54 mo) |
| Interval from cranioplasty to revision | 13.3 mo (2-76 mo) | N/A | N/A | Group 1: 8.9 mo (3.2-16.4 mo) | Group 2: N/A | N/A | N/A |
| Follow-up | 57.6 mo (6-72 mo) | 24 mo (2-124 mo) | 37.9 mo (1.5-168 mo) | 83 mo (18-154 mo) | 32 mo (range not reported) | TBI subgroup: 26 mo (2-84 mo)Non-TBI subgroup: 75 mo (8-120 mo) | |
| Risk factors | Craniectomy defect size ≥75 cm2 | Time to cranioplasty ≥6 wk after craniectomy | Age ≤ 2.5 yr, post-traumatic hydrocephalus, comminuted skull fracture, and underlying contusion | Age ≤7 yr | Age (1% decrease in risk of resorption for each month of increasing age), external ventricular drain use, and lumbar shunt | N/A | |
All values represent n (%), mean (range), or mean ± standard deviation unless otherwise specified.
aValues represent medians.
bAge reported for 15 patients undergoing decompressive craniectomy, rather than the subset of 10 patients who ultimately underwent autologous cranioplasty with 13 bone flaps.
cOnly 240 of the total 359 patients were included in the analysis of bone resorption.
Several groups have sought to standardize the definition of bone resorption across studies. Bone resorption was initially categorized by Dunisch et al48 as type I necrosis when there is thinning of the bone flap vs type II necrosis when there is a complete lysis of the inner and outer tables of bone. More recently, software has been used to perform automated segmentation and volume measurements that can be tracked over time. In one study, the percentage of decrease in volume of the bone flap was calculated, and was correlated with a semiquantitative score in which the following radiological features were assigned points: bone suffusion, linear vs jagged bone thinning, loss of differentiation between bone and diploe, central vs peripheral bone loss, and fragment or flap displacement.55 Out of a total potential score of 18, a score ≤6 corresponded to a volume decrease ≤15% and was considered mild (thinning of bone), a score of 7 to 12 corresponded to a volume decrease of 16% to 39% and was considered moderate (loss of bone but with preserved cerebral protection), and a score ≥13 corresponded to a volume decrease ≥40% and was considered severe (loss of bone resulting in loss of cerebral protection). A more straightforward CT-based scoring system was proposed by Korhonen et al.56 The “Oulu resorption score” (Table 2) ranged from 0 to 9 based on the extent (% remaining bone volume), severity (presence and/or size of bicortical perforations), and the number of foci of bone resorption (Figure 2). A score of 0 correlates with no resorption, while scores of 1 to 4 correlate with grade I (nonrelevant) resorption, scores of 5 to 8 correlate with grade II (clinically relevant) resorption, and a score of 9 correlates with grade III resorption (bone flap failure requiring revision).
TABLE 2.
Oulu Resorption Score
| Variable | Score |
|---|---|
| Extent (ie, remaining bone volume) | |
| No BFR/remaining bone volume = 100% | 0 |
| Remaining bone volume 75% to 99.9% | 0 |
| Remaining bone volume 25% to 74.9% | 2 |
| Remaining bone volume <25% | 3 |
| Severity of perforations due to BFR | |
| No BFR or only cancellous bone loss | 0 |
| Nonperforating resorption | 1 |
| A new bicortical perforation of <1.0 cm | 2 |
| A new bicortical perforation of ≥1.0 cm | 3 |
| Focus (ie, total integrity of the bone flap) | |
| No BFR | 0 |
| One focal BFR change | 0 |
| Multiple BFR foci | 2 |
| Diffuse BFR: signs of BFR throughout the flap area | 3 |
BFR = bone flap resorption.
Modified with permission from Korhonen et al69 under the Creative Commons Attribution 4.0 International License.
FIGURE 2.
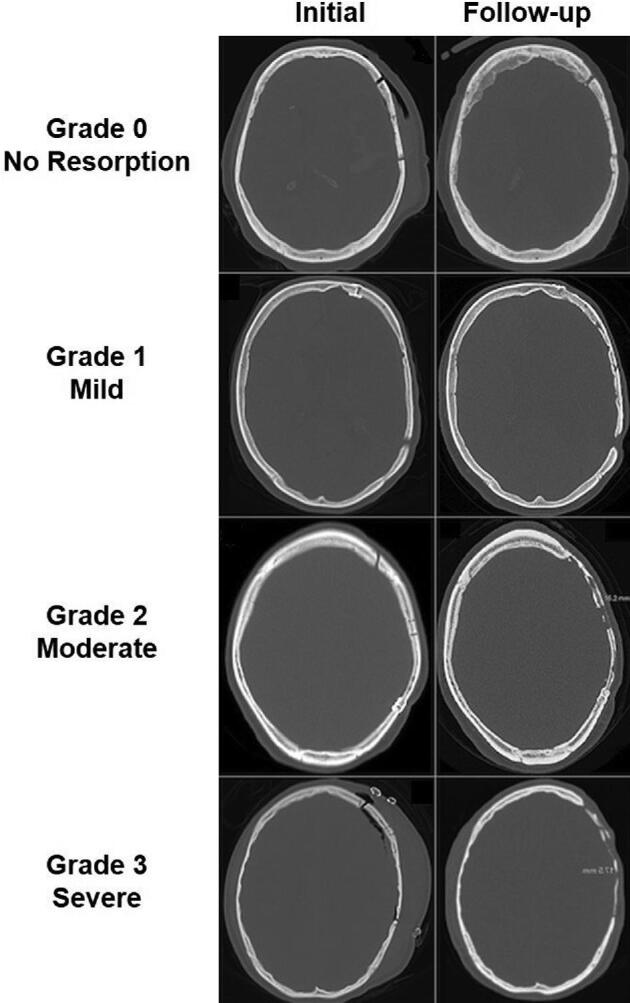
CT slices with bone window settings depicting the initial postoperative and follow-up bone flap status (left and right columns, respectively) of 4 cranioplasty patients with different levels of bone flap resorption (right side of each image). Each row comprises one patient. Modified with permission from Korhonen et al56 under the Creative Commons Attribution 4.0 International License.
Quantitative analyses such as these have demonstrated that some degree of radiological bone resorption is present in a majority of patients following a cranioplasty, but the presence of mild bone resorption does not necessarily signify a progressive process that will ultimately require a revision.43 While routine radiological surveillance is typically not recommended, clinical follow-up is necessary because severe resorption has a number of clinically relevant cosmetic and physiologic effects.54 Bony defects that result in poor cosmesis can impact a patient's quality of life; when large, bony defects can also expose the underlying brain parenchyma to potential injury. Significant bone resorption may even impact the ability of the cranioplasty to improve cerebral blood flow, cerebrospinal fluid hydrodynamics, and neurological function following a decompressive craniectomy.8,57-60 As a result, “significant” bone resorption typically requires a revision of the cranioplasty using either autologous split-thickness bone grafts, exchange cranioplasties, or synthetic materials. This, however, necessitates additional surgery, exposing the patient to further risks (particularly infection), additional hospitalizations, and increased costs.
RISK FACTORS FOR BONE RESORPTION
In an effort to reduce the need for revision surgery, some have sought to mitigate bone resorption itself by identifying the clinical risk factors associated with resorption. Variables that have been studied include patient age, the mode and duration of bone flap storage, the size of the bone flap, the number of bone fragments, and the presence of a shunt, among others (Table 3).
TABLE 3.
Clinical Risk Factors for Bone Resorption in Pediatric Patients
| Grant et al, 200434 | Piedra et al, 201250 | Bowers et al, 201351 | Martin et al, 201453 | Rocque et al, 201854 | |
|---|---|---|---|---|---|
| Age | – | – | ≤ 2.5 yr | ≤ 7 yr | + |
| Reason for craniectomy | – | – | N/A | N/A | – |
| Size of flap | > 75 cm2 | – | – | – | – |
| Fragmentation | – | N/A | + | N/A | N/A |
| Duration of cryopreservation | – | ≥ 6 wk | – | – | N/A |
| Shunt | – | – | + | – | + |
| Underlying contusion | N/A | N/A | + | N/A | N/A |
Patient Age
Grant et al34 and Piedra et al50 did not identify a significant association between patient age and the risk of bone resorption. However, Bowers et al51 found that patients less than or equal to 2.5 yr of age were at increased risk of bone flap resorption (odds ratio [OR] 23.1, P = .01). Additionally, Martin et al reported that bone resorption was significantly more likely to occur in the 0 to 7 yr age range relative to the 8 to 14 yr range (81.8% vs 42%, P < .001). These findings suggest that while bone resorption is fairly common among the general pediatric population, the risk of resorption rises steadily with decreasing age. Indeed, Frassanito et al61 identified a 100% rate of resorption in a subgroup of patients who underwent decompressive craniectomy followed by autologous cranioplasty when under 1 yr of age. In a multivariate analysis, Rocque et al54 found that as the patient's age increases, each additional month decreases the risk of bone resorption by 1% (OR 0.99, P < .001).
Young age may impact the rate of bone resorption in several ways. The thinness of the calvaria has been hypothesized to play a role, as has the naturally high rate of bone turnover in pediatric patients.34,40 Although young children, particularly those less than 2 yr of age, can typically undergo successful calvarial reossification of small calvarial defects, the rapid bone turnover that occurs during this period of rapid head growth is associated with increased metabolic demand on the calvarium and may impact the ability of a large bone flap to create a successful fusion.51,62
Mode and Duration of Bone Flap Storage
Bone that is removed during a decompressive craniectomy is typically either stored in a subcutaneous abdominal pocket or cryopreserved until the patient is ready for a cranioplasty. Subcutaneous storage provides the ability for the bone flap to remain with the patient if he or she transfers to another facility, and has been hypothesized to be a better method of maintaining cell viability relative to cryopreservation.35 In a meta-analysis of 48 studies involving 5346 adult and pediatric patients, there was no significant difference in resorption rates in patients undergoing cranioplasty after subcutaneous storage vs cryopreservation (7.7% vs 9.7%, P = .33).41 However, subcutaneous storage is typically not recommended in pediatric patients, particularly infants and young children, given the large head-to-body ratio and the surgical trauma that would be required to create an adequate abdominal pocket.61
As a result, cryopreservation represents the storage mode of choice for pediatric patients. Some have proposed that cryopreservation impairs cell viability and graft revascularization, and therefore increases the risk of bone resorption.63-65 This is a possible explanation for the finding of Piedra et al50 that bone resorption was 3x more likely among patients whose bone flap was cryopreserved for more than 6 wk (42% of patients undergoing delayed cranioplasty vs 14.3% of patients undergoing early cranioplasty). However, other studies have shown that osteocytes, structural scaffolding, and proteins involved in bone fusion remain intact despite prolonged cryopreservation.46 The freezing method and storage temperature likely have an impact. Fan et al35 suggested that cryopreservation with dimethyl sulfoxide may be a more effective method of preserving osteocyte viability and biological activity by reducing ice crystal formation; the authors also recommended a “slow freezing” method, sequentially transferring the bone flap to colder temperatures until ultimately storing the bone in liquid nitrogen (−196°C). They cautioned against attempting to sterilize the bone using alcohol soaking, autoclaving, or calcination, which devitalizes the bone and destroys the osteocytes.
Bone Flap Size
Grant et al34 reported higher rates of bone resorption with larger skull defects. Although the authors stratified the skull defect area into 6 subgroups based on size, they found that 75 cm2 represented a significant size threshold. Skull defects that were larger than 75 cm2 were associated with a greater than 60% failure rate, while there were no failures among those patients whose skull defect was smaller than 75 cm2. Unfortunately, decompressive craniectomy requires removal of a large region of bone in order to be effective, and it is rarely feasible to modify the decompression in an effort to minimize subsequent resorption. Indeed, Martin et al53 presented a series of patients who all had a decompressive craniectomy that was larger than 75 cm2; this may in part explain the high rate of resorption in their study.
Number of Bone Fragments
Bowers et al51 reported that the presence of a comminuted skull fracture is a risk factor for bone resorption. The presence of multiple bone fragments may increase the amount of cancellous bone that is exposed to circulating osteoclasts in the bloodstream, resulting in bone resorption.66 Alternatively, bone fragmentation may increase the surface area that needs to fuse, and makes it difficult to fixate and minimize the gaps between the fragments. A third hypothesis is that the presence of a comminuted fracture signifies a powerful impact that was dissipated by the skull, resulting in more local damage and ultimately interfering with bone fusion. It is possible that the presence of an underlying contusion is also associated with bone resorption for similar reasons.51
Presence of a Shunt
Bowers et al51 also found post-traumatic hydrocephalus to be a risk factor for bone resorption. Of the 27 patients with bone resorption in their series, 12 patients (44%) required a ventriculoperitoneal shunt placement prior to their cranioplasty. Similarly, Martin et al53 found a trend toward increased bone resorption in pediatric patients with a permanent shunt compared to those without a shunt (75% vs 64%, respectively), though the difference was not significant. Most recently, a multicenter retrospective study found the presence of an external ventricular drain or a lumbar shunt to have significant or nearly significant associations with bone resorption in multivariate models.54
One possibility is that the presence of hydrocephalus serves as a secondary marker of injury severity. Alternatively, the presence of a shunt might interfere with normal intracranial pressure fluctuations, which in turn have been shown to play a dynamic role in skull growth.62 Preclinical data have demonstrated that the growing brain places mechanical, tensile strain on the overlying immature dura, which induces the secretion of growth factors that contribute to osteogenesis, particularly during the first 2 yr of life.61,62,67 By blunting the natural fluctuations in intracranial pressure, a shunt may interfere with the tensile strain on the dura, as well as subsequent cytokine release.
Similarly, an expansile duraplasty may interrupt the physiological interactions between the brain, the native dura, and the overlying bone, and the subsequent lack of growth factor secretion may contribute to bone flap resorption.53 These factors likely become less important with increasing age, as the skull typically attains its final size by 7 yr of age;68 this may, in part, contribute to the increased rate of bone resorption seen in younger patients.30
FUTURE DIRECTIONS
While clinical risk factors for bone resorption have been identified, innovative solutions are required in order to mitigate resorption. Opportunities exist to adapt bone repair strategies that are being developed in the fields of orthopedic surgery and plastic surgery, where active areas of research include novel biomaterial development, tissue engineering, stem cell delivery, and mechanical stimulation.
Biomaterials and Tissue Engineering
Biocompatible and biodegradable scaffolds feature complex, porous, 3-dimensional architectures that promote osteoconduction and vascular ingrowth, and which can be seeded with progenitor cells and/or osteoinductive growth factors.69,70 Cutting-edge developments include the use of magnetic scaffolds that can be guided with an external magnetic field and injectable scaffolds.69,71,72
Stem Cell Delivery
Mesenchymal stem cells and osteoprogenitor cells can be delivered directly to the repair site, either alone or in combination with a cell delivery vehicle. In a pilot study in Finland, adipose-derived stem cells were seeded onto a scaffold material composed of beta-tricalcium phosphate granules.73 Although initially promising, long-term outcomes proved less favorable, with 3 of 5 patients requiring a revision due to graft-related problems.74 Nevertheless, stem cell delivery remains a robust area of research.
Mechanical Stimulation
Mechanical stability and the mechanical loading of bone have both been shown to play a role in bone repair. Shear strain and fluid flow upregulate mechanotransductive molecular pathways that are involved in regulating the formation of fibrous connective tissue, cartilage, and bone.69,75 Noninvasive methods of mechanical stimulation have been explored in the extremities using low-intensity pulsed ultrasound and pulsed electromagnetic fields, and further investigations of their mechanism of action are ongoing.76-78
CONCLUSION
Bone flap resorption remains a significant complication for pediatric patients undergoing an autologous cranioplasty. Despite an increasingly comprehensive understanding of the clinical risk factors associated with bone flap resorption, strategies for enhancing calvarial bone repair remain primarily lab-based. Additional work is necessary to translate bioengineering advances into better outcomes for pediatric patients undergoing cranioplasty.
Funding
This work was supported in part by the National Institutes of Health (R01AR073206 to Dr Khan), a Connecticut Children's Department of Surgery Innovation Grant (to Dr Hersh), and a Thrasher Research Fund Early Career Award (to Dr Hersh).
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Contributor Information
David S Hersh, Division of Neurosurgery, Connecticut Children's, Hartford, Connecticut; Department of Surgery, UConn School of Medicine, Farmington, Connecticut; Department of Pediatrics, UConn School of Medicine, Farmington, Connecticut.
Hanna J Anderson, Connecticut Convergence Institute for Translation in Regenerative Engineering, UConn Health, Farmington, Connecticut; Department of Biomedical Engineering, University of Connecticut School of Engineering, Storrs, Connecticut.
Graeme F Woodworth, Department of Neurosurgery, University of Maryland School of Medicine, Baltimore, Maryland; Marlene and Stewart Greenebaum Comprehensive Cancer Center, University of Maryland School of Medicine, Baltimore, Maryland.
Jonathan E Martin, Division of Neurosurgery, Connecticut Children's, Hartford, Connecticut; Department of Surgery, UConn School of Medicine, Farmington, Connecticut.
Yusuf M Khan, Connecticut Convergence Institute for Translation in Regenerative Engineering, UConn Health, Farmington, Connecticut; Department of Biomedical Engineering, University of Connecticut School of Engineering, Storrs, Connecticut; Department of Orthopedic Surgery, UConn School of Medicine, Farmington, Connecticut.
REFERENCES
- 1. Smith SE, Kirkham FJ, Deveber Get al. . Outcome following decompressive craniectomy for malignant middle cerebral artery infarction in children. Dev Med Child Neurol. 2011;53(1):29-33. [DOI] [PubMed] [Google Scholar]
- 2. Bayram N, Ciftdogan DY, Karapinar Bet al. . A case of herpes simplex encephalitis revealed by decompressive craniectomy. Eur J Pediatr. 2008;167(7):821-822. [DOI] [PubMed] [Google Scholar]
- 3. Singhi P, Saini AG, Sahu JKet al. . Unusual clinical presentation and role of decompressive craniectomy in herpes simplex encephalitis. J Child Neurol. 2015;30(9):1204-1207. [DOI] [PubMed] [Google Scholar]
- 4. Appelboom G, Zoller SD, Piazza MAet al. . Traumatic brain injury in pediatric patients: evidence for the effectiveness of decompressive surgery. Neurosurg Focus. 2011;31(5):E5. [DOI] [PubMed] [Google Scholar]
- 5. Josan VA, Sgouros S.. Early decompressive craniectomy may be effective in the treatment of refractory intracranial hypertension after traumatic brain injury. Childs Nerv Syst. 2006;22(10):1268-1274. [DOI] [PubMed] [Google Scholar]
- 6. Kan P, Amini A, Hansen Ket al. . Outcomes after decompressive craniectomy for severe traumatic brain injury in children. J Neurosurg. 2006;105(5 Suppl):337-342. [DOI] [PubMed] [Google Scholar]
- 7. Young AMH, Kolias AG, Hutchinson PJ. Decompressive craniectomy for traumatic intracranial hypertension: application in children. Childs Nerv Syst. 2017;33(10):1745-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hutchinson PJ, Kolias AG, Tajsic Tet al. . Consensus statement from the International Consensus Meeting on the role of decompressive craniectomy in the management of traumatic brain injury. Acta Neurochir (Wien). 2019;161(7):1261-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coplin WM, Cullen NK, Policherla PNet al. . Safety and feasibility of craniectomy with duraplasty as the initial surgical intervention for severe traumatic brain injury. J Trauma. 2001;50(6):1050-1059. [DOI] [PubMed] [Google Scholar]
- 10. Gaab MR, Rittierodt M, Lorenz M, Heissler HE. Traumatic brain swelling and operative decompression: a prospective investigation. Acta Neurochir Suppl (Wien). 1990;51:326-328. [DOI] [PubMed] [Google Scholar]
- 11. Munch E, Horn P, Schurer L, Piepgras A, Paul T, Schmiedek P. Management of severe traumatic brain injury by decompressive craniectomy. Neurosurgery. 2000;47(2):315-323; discussion 322-313. [DOI] [PubMed] [Google Scholar]
- 12. Carter BS, Ogilvy CS, Candia GJ, Rosas HD, Buonanno F. One-year outcome after decompressive surgery for massive nondominant hemispheric infarction. Neurosurgery. 1997;40(6):1168-1176; discussion 1175-1166. [DOI] [PubMed] [Google Scholar]
- 13. Kjellberg RN, Prieto A Jr.. Bifrontal decompressive craniotomy for massive cerebral edema. J Neurosurg. 1971;34(4):488-493. [DOI] [PubMed] [Google Scholar]
- 14. Venes JL, Collins WF.. Bifrontal decompressive craniectomy in the management of head trauma. J Neurosurg. 1975;42(4):429-433. [DOI] [PubMed] [Google Scholar]
- 15. Polin RS, Shaffrey ME, Bogaev CAet al. . Decompressive bifrontal craniectomy in the treatment of severe refractory posttraumatic cerebral edema. Neurosurgery. 1997;41(1):84-94; discussion 92-84. [DOI] [PubMed] [Google Scholar]
- 16. Whitfield PC, Patel H, Hutchinson PJet al. . Bifrontal decompressive craniectomy in the management of posttraumatic intracranial hypertension. Br J Neurosurg. 2001;15(6):500-507. [DOI] [PubMed] [Google Scholar]
- 17. Yoo DS, Kim DS, Cho KS, Huh PW, Park CK, Kang JK. Ventricular pressure monitoring during bilateral decompression with dural expansion. J Neurosurg. 1999;91(6):953-959. [DOI] [PubMed] [Google Scholar]
- 18. Gower DJ, Lee KS, McWhorter JM. Role of subtemporal decompression in severe closed head injury. Neurosurgery. 1988;23(4):417-422. [DOI] [PubMed] [Google Scholar]
- 19. Taylor A, Butt W, Rosenfeld Jet al. . A randomized trial of very early decompressive craniectomy in children with traumatic brain injury and sustained intracranial hypertension. Childs Nerv Syst. 2001;17(3):154-162. [DOI] [PubMed] [Google Scholar]
- 20. Carney NA, Chesnut R, Kochanek PMet al. . Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Pediatr Crit Care Med. 2003;4(3 Suppl):S1. [DOI] [PubMed] [Google Scholar]
- 21. Kochanek PM, Tasker RC, Carney Net al. . Guidelines for the Management of Pediatric Severe Traumatic Brain Injury, Third Edition: update of the Brain Trauma Foundation Guidelines. Pediatr Crit Care Med. 2019;20(3S Suppl 1):S1-S82. [DOI] [PubMed] [Google Scholar]
- 22. Tomford WW. Transmission of disease through transplantation of musculoskeletal allografts. J Bone Joint Surg Am. 1995;77(11):1742-1754. [DOI] [PubMed] [Google Scholar]
- 23. Klieverik VM, Miller KJ, Singhal A, Han KS, Woerdeman PA. Cranioplasty after craniectomy in pediatric patients—a systematic review. Childs Nerv Syst. 2019;35(9):1481-1490. [DOI] [PubMed] [Google Scholar]
- 24. Steinbok P, Seal SK, Courtemanche DJ. Split calvarial bone grafting in patients less than 1 year of age: technical note and use in craniofacial surgery for craniosynostosis. Childs Nerv Syst. 2011;27(7):1149-1152. [DOI] [PubMed] [Google Scholar]
- 25. Vercler CJ, Sugg KB, Buchman SR. Split cranial bone grafting in children younger than 3 years old: debunking a surgical myth. Plast Reconstr Surg. 2014;133(6):822e-827e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Phillips BZ, Taylor HO, Klinge PM, Sullivan SR. Piezoelectric technology for pediatric autologous cranioplasty. Cleft Palate Craniofac J. 2014;51(3):361-364. [DOI] [PubMed] [Google Scholar]
- 27. Hockley AD, Goldin JH, Wake MJ, Iqbal J. Skull repair in children. Pediatr Neurosurg. 1990;16(4-5):271-275. [DOI] [PubMed] [Google Scholar]
- 28. Williams L, Fan K, Bentley R. Titanium cranioplasty in children and adolescents. J Craniomaxillofac Surg. 2016;44(7):789-794. [DOI] [PubMed] [Google Scholar]
- 29. Koenig WJ, Donovan JM, Pensler JM. Cranial bone grafting in children. Plast Reconstr Surg. 1995;95(1):1-4. [DOI] [PubMed] [Google Scholar]
- 30. Frassanito P, Tamburrini G, Massimi L, Peraio S, Caldarelli M, Di Rocco C. Problems of reconstructive cranioplasty after traumatic brain injury in children. Childs Nerv Syst. 2017;33(10):1759-1768. [DOI] [PubMed] [Google Scholar]
- 31. Goodrich JT, Sandler AL, Tepper O. A review of reconstructive materials for use in craniofacial surgery bone fixation materials, bone substitutes, and distractors. Childs Nerv Syst. 2012;28(9):1577-1588. [DOI] [PubMed] [Google Scholar]
- 32. Feroze AH, Walmsley GG, Choudhri O, Lorenz HP, Grant GA, Edwards MS. Evolution of cranioplasty techniques in neurosurgery: historical review, pediatric considerations, and current trends. J Neurosurg. 2015;123(4):1098-1107. [DOI] [PubMed] [Google Scholar]
- 33. Arnaud E. Advances in cranioplasty with osteoinductive biomaterials: summary of experimental studies and clinical prospects. Childs Nerv Syst. 2000;16(10-11):659-668. [DOI] [PubMed] [Google Scholar]
- 34. Grant GA, Jolley M, Ellenbogen RG, Roberts TS, Gruss JR, Loeser JD. Failure of autologous bone-assisted cranioplasty following decompressive craniectomy in children and adolescents. J Neurosurg. 2004;100(2 Suppl Pediatrics):163-168. [DOI] [PubMed] [Google Scholar]
- 35. Fan MC, Wang QL, Sun Pet al. . Cryopreservation of autologous cranial bone flaps for cranioplasty: a large sample retrospective study. World Neurosurg. 2018;109:e853-e859. [DOI] [PubMed] [Google Scholar]
- 36. Blum KS, Schneider SJ, Rosenthal AD. Methyl methacrylate cranioplasty in children: long-term results. Pediatr Neurosurg. 1997;26(1):33-35. [DOI] [PubMed] [Google Scholar]
- 37. Wong RK, Gandolfi BM, St-Hilaire H, Wise MW, Moses M. Complications of hydroxyapatite bone cement in secondary pediatric craniofacial reconstruction. J Craniofac Surg. 2011;22(1):247-251. [DOI] [PubMed] [Google Scholar]
- 38. Lin AY, Kinsella CR Jr., Rottgers SAet al. . Custom porous polyethylene implants for large-scale pediatric skull reconstruction: early outcomes. J Craniofac Surg. 2012;23(1):67-70. [DOI] [PubMed] [Google Scholar]
- 39. Fu KJ, Barr RM, Kerr MLet al. . An outcomes comparison between autologous and alloplastic cranioplasty in the pediatric population. J Craniofac Surg. 2016;27(3):593-597. [DOI] [PubMed] [Google Scholar]
- 40. Rocque BG, Amancherla K, Lew SM, Lam S. Outcomes of cranioplasty following decompressive craniectomy in the pediatric population. J Neurosurg Pediatr. 2013;12(2):120-125. [DOI] [PubMed] [Google Scholar]
- 41. Corliss B, Gooldy T, Vaziri S, Kubilis P, Murad G, Fargen K. Complications after in vivo and ex vivo autologous bone flap storage for cranioplasty: a comparative analysis of the literature. World Neurosurg. 2016;96:510-515. [DOI] [PubMed] [Google Scholar]
- 42. Lam S, Kuether J, Fong A, Reid R. Cranioplasty for large-sized calvarial defects in the pediatric population: a review. Craniomaxillofac Trauma Reconstr. 2015;8(2):159-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Korhonen TK, Salokorpi N, Niinimaki J, Serlo W, Lehenkari P, Tetri S. Quantitative and qualitative analysis of bone flap resorption in patients undergoing cranioplasty after decompressive craniectomy. J Neurosurg. 2018;130(1):312-321. [DOI] [PubMed] [Google Scholar]
- 44. Gosain AK, Gosain SA, Sweeney WM, Song LS, Amarante MT. Regulation of osteogenesis and survival within bone grafts to the calvaria: the effect of the dura versus the pericranium. Plast Reconstr Surg. 2011;128(1):85-94. [DOI] [PubMed] [Google Scholar]
- 45. Aarabi B, Hesdorffer DC, Ahn ES, Aresco C, Scalea TM, Eisenberg HM. Outcome following decompressive craniectomy for malignant swelling due to severe head injury. J Neurosurg. 2006;104(4):469-479. [DOI] [PubMed] [Google Scholar]
- 46. Iwama T, Yamada J, Imai S, Shinoda J, Funakoshi T, Sakai N. The use of frozen autogenous bone flaps in delayed cranioplasty revisited. Neurosurgery. 2003;52(3):591-596; discussion 595–596. [DOI] [PubMed] [Google Scholar]
- 47. Schuss P, Vatter H, Oszvald Aet al. . Bone flap resorption: risk factors for the development of a long-term complication following cranioplasty after decompressive craniectomy. J Neurotrauma. 2013;30(2):91-95. [DOI] [PubMed] [Google Scholar]
- 48. Dunisch P, Walter J, Sakr Y, Kalff R, Waschke A, Ewald C. Risk factors of aseptic bone resorption: a study after autologous bone flap reinsertion due to decompressive craniotomy. J Neurosurg. 2013;118(5):1141-1147. [DOI] [PubMed] [Google Scholar]
- 49. Malcolm JG, Mahmooth Z, Rindler RSet al. . Autologous cranioplasty is associated with increased reoperation rate: a systematic review and meta-analysis. World Neurosurg. 2018;116:60-68. [DOI] [PubMed] [Google Scholar]
- 50. Piedra MP, Thompson EM, Selden NR, Ragel BT, Guillaume DJ. Optimal timing of autologous cranioplasty after decompressive craniectomy in children. J Neurosurg Pediatr. 2012;10(4):268-272. [DOI] [PubMed] [Google Scholar]
- 51. Bowers CA, Riva-Cambrin J, Hertzler DA 2nd, Walker ML. Risk factors and rates of bone flap resorption in pediatric patients after decompressive craniectomy for traumatic brain injury. J Neurosurg Pediatr. 2013;11(5):526-532. [DOI] [PubMed] [Google Scholar]
- 52. Beez T, Munoz-Bendix C, Ahmadi SA, Steiger HJ, Beseoglu K. From decompressive craniectomy to cranioplasty and beyond-a pediatric neurosurgery perspective. Childs Nerv Syst. 2019;35(9):1517-1524. [DOI] [PubMed] [Google Scholar]
- 53. Martin KD, Franz B, Kirsch Met al. . Autologous bone flap cranioplasty following decompressive craniectomy is combined with a high complication rate in pediatric traumatic brain injury patients. Acta Neurochir (Wien). 2014;156(4):813-824. [DOI] [PubMed] [Google Scholar]
- 54. Rocque BG, Agee BS, Thompson EMet al. . Complications following pediatric cranioplasty after decompressive craniectomy: a multicenter retrospective study. J Neurosurg Pediatr. 2018;22(3):225-232. [DOI] [PubMed] [Google Scholar]
- 55. Barzaghi LR, Parisi V, Gigliotti CRet al. . Bone resorption in autologous cryopreserved cranioplasty: quantitative evaluation, semiquantitative score and clinical significance. Acta Neurochir (Wien). 2019;161(3):483-491. [DOI] [PubMed] [Google Scholar]
- 56. Korhonen TK, Salokorpi N, Ohtonen Pet al. . Classification of bone flap resorption after cranioplasty: a proposal for a computed tomography-based scoring system. Acta Neurochir (Wien). 2019;161(3):473-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fodstad H, Love JA, Ekstedt J, Friden H, Liliequist B. Effect of cranioplasty on cerebrospinal fluid hydrodynamics in patients with the syndrome of the trephined. Acta Neurochir (Wien). 1984;70(1-2):21-30. [DOI] [PubMed] [Google Scholar]
- 58. Winkler PA, Stummer W, Linke R, Krishnan KG, Tatsch K. Influence of cranioplasty on postural blood flow regulation, cerebrovascular reserve capacity, and cerebral glucose metabolism. J Neurosurg. 2000;93(1):53-61. [DOI] [PubMed] [Google Scholar]
- 59. Dujovny M, Fernandez P, Alperin N, Betz W, Misra M, Mafee M. Post-cranioplasty cerebrospinal fluid hydrodynamic changes: magnetic resonance imaging quantitative analysis. Neurol Res. 1997;19(3):311-316. [DOI] [PubMed] [Google Scholar]
- 60. Halani SH, Chu JK, Malcolm JGet al. . Effects of cranioplasty on cerebral blood flow following decompressive craniectomy: a systematic review of the literature. Neurosurgery. 2017;81(2):204-216. [DOI] [PubMed] [Google Scholar]
- 61. Frassanito P, Massimi L, Caldarelli M, Tamburrini G, Di Rocco C. Complications of delayed cranial repair after decompressive craniectomy in children less than 1 year old. Acta Neurochir (Wien). 2012;154(5):927-933. [DOI] [PubMed] [Google Scholar]
- 62. Fong KD, Warren SM, Loboa EGet al. . Mechanical strain affects dura mater biological processes: implications for immature calvarial healing. Plast Reconstr Surg. 2003;112(5):1312-1327. [DOI] [PubMed] [Google Scholar]
- 63. Sultan SM, Davidson EH, Butala Pet al. . Interval cranioplasty: comparison of current standards. Plast Reconstr Surg. 2011;127(5):1855-1864. [DOI] [PubMed] [Google Scholar]
- 64. Bhaskar IP, Yusheng L, Zheng M, Lee GY. Autogenous skull flaps stored frozen for more than 6 months: do they remain viable? J Clin Neurosci. 2011;18(12):1690-1693. [DOI] [PubMed] [Google Scholar]
- 65. Stevenson S, Li XQ, Davy DT, Klein L, Goldberg VM. Critical biological determinants of incorporation of non-vascularized cortical bone grafts. Quantification of a complex process and structure. J Bone Joint Surg Am. 1997;79(1):1-16. [PubMed] [Google Scholar]
- 66. Lee JH, Chough CK, Choi HJet al. . Bone flap changes after cranioplasty using frozen autologous bone flaps: a three-dimensional volumetric reconstruction study. Yonsei Med J. 2019;60(11):1067-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Greenwald JA, Mehrara BJ, Spector JAet al. . Biomolecular mechanisms of calvarial bone induction: immature versus mature dura mater. Plast Reconstr Surg. 2000;105(4):1382-1392. [DOI] [PubMed] [Google Scholar]
- 68. Sgouros S, Goldin JH, Hockley AD, Wake MJ, Natarajan K. Intracranial volume change in childhood. J Neurosurg. 1999;91(4):610-616. [DOI] [PubMed] [Google Scholar]
- 69. Dimitriou R, Jones E, McGonagle D, Giannoudis PV. Bone regeneration: current concepts and future directions. BMC Med. 2011;9:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Villa MM, Wang L, Huang J, Rowe DW, Wei M. Bone tissue engineering with a collagen-hydroxyapatite scaffold and culture expanded bone marrow stromal cells. J Biomed Mater Res B Appl Biomater. 2015;103(2):243-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tampieri A, Landi E, Valentini Fet al. . A conceptually new type of bio-hybrid scaffold for bone regeneration. Nanotechnology. 2011;22(1):015104. [DOI] [PubMed] [Google Scholar]
- 72. Laschke MW, Witt K, Pohlemann T, Menger MD. Injectable nanocrystalline hydroxyapatite paste for bone substitution: in vivo analysis of biocompatibility and vascularization. J Biomed Mater Res B Appl Biomater. 2007;82(2):494-505. [DOI] [PubMed] [Google Scholar]
- 73. Thesleff T, Lehtimaki K, Niskakangas Tet al. . Cranioplasty with adipose-derived stem cells and biomaterial: a novel method for cranial reconstruction. Neurosurgery. 2011;68(6):1535-1540. [DOI] [PubMed] [Google Scholar]
- 74. Thesleff T, Lehtimaki K, Niskakangas Tet al. . Cranioplasty with adipose-derived stem cells, beta-tricalcium phosphate granules and supporting mesh: six-year clinical follow-up results. Stem Cells Transl Med. 2017;6(7):1576-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lacroix D, Prendergast PJ.. A mechano-regulation model for tissue differentiation during fracture healing: analysis of gap size and loading. J Biomech. 2002;35(9):1163-1171. [DOI] [PubMed] [Google Scholar]
- 76. Busse JW, Bhandari M, Kulkarni AV, Tunks E. The effect of low-intensity pulsed ultrasound therapy on time to fracture healing: a meta-analysis. CMAJ. 2002;166(4):437-441. [PMC free article] [PubMed] [Google Scholar]
- 77. Zhang B, Xie Y, Ni Z, Chen L. Effects and mechanisms of exogenous electromagnetic field on bone cells: a review. Bioelectromagnetics. 2020;41(4):263-278. [DOI] [PubMed] [Google Scholar]
- 78. Khan Y, Laurencin CT.. Fracture repair with ultrasound: clinical and cell-based evaluation. J Bone Joint Surg Am. 2008;90(Suppl 1):138-144. [DOI] [PubMed] [Google Scholar]