Abstract
BACKGROUND
Historically, symptomatic, benign intradural extramedullary (IDEM) spine tumors have been managed with surgical resection. However, minimal robust data regarding patient-reported outcomes (PROs) following treatment of symptomatic lesions exists. Moreover, there are increasing reports of radiosurgical management of these lesions without robust health-related quality of life data.
OBJECTIVE
To prospectively analyze PROs among patients with benign IDEM spine tumors undergoing surgical resection to define the symptomatic efficacy of surgery.
METHODS
Prospective, single-center observational cohort study of patients with benign IDEM spine tumors undergoing open surgical resection. Pre- and postoperative Brief Pain Index (BPI) and MD Anderson Symptom Inventory (MDASI) questionnaires were used to quantitatively assess their symptom control after surgical intervention. Matched pairs were analyzed with the Wilcoxon signed-rank test.
RESULTS
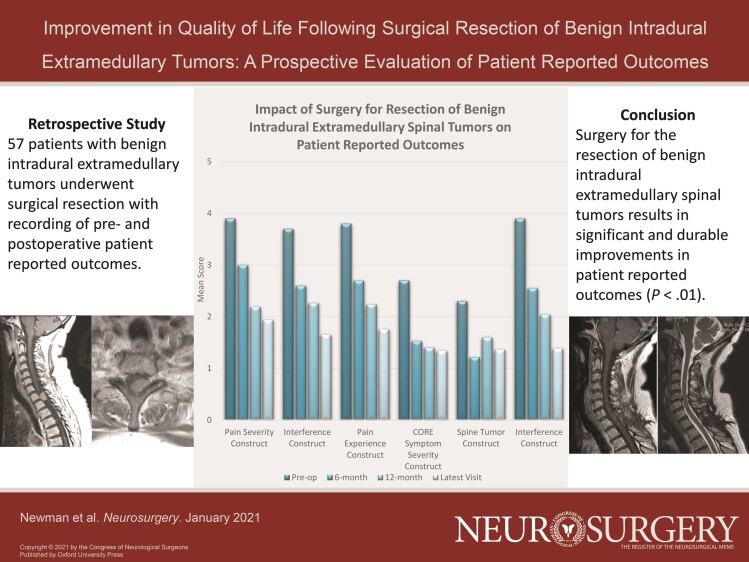
A total of 57 patients met inclusion criteria with both pre- and postoperative PROs. There were 35 schwannomas, 18 meningiomas, 2 neurofibromas, 1 paraganglioma, and 1 mixed schwannoma/neurofibroma. Most patients were American Spinal Injury Association Impairment (ASIA) E (93%) with high-grade spinal cord compression (77%), and underwent either a 2 or 3 level laminectomy (84%). Surgical resection resulted in statistically significant improvement in all 3 composite BPI constructs of pain-severity, pain-interference, and overall patient pain experience (P < .0001). Surgical resection resulted in statistically significant improvements in all composite scores for the MDASI core symptom severity, spine tumor, and disease interference constructs (P < .01). Three patients (5%) had postoperative complications requiring surgical interventions (2 wound revisions and 1 ventriculo-peritoneal shunt).
CONCLUSION
Surgical resection of IDEM spine tumors provides rapid, significant, and durable improvement in PROs.
Keywords: Intradural extramedullary spine tumors, Patient-reported outcomes, Quality of life, Spinal cord compression
Graphical Abstract
Graphical Abstract.
ABBREVIATIONS
- ASIA
American Spinal Injury Association Impairment
- BPI
Brief Pain Index
- ECOG
Eastern Cooperative Oncology Group
- IDEM
intradural extramedullary
- MDASI
MD Anderson Symptom Inventory
- MRC
Medical Research Council
- PRO
Patient-reported outcomes
- SD
Standard deviation
- SRS
stereotactic radiosurgery
Intradural extramedullary (IDEM) tumors of the spine present with significant pain or neurological symptoms due to progressive spinal cord compression. Historically, symptomatic IDEM tumors have been managed with open surgical resection. Evidence has shown that for benign noninfiltrative tumors, gross total resection with microsurgical technique is safe, efficacious, and results in a low likelihood of recurrence.1-4 However, the
success of stereotactic radiosurgery (SRS) in the management of benign intracranial lesions with minimal toxicity5-8 combined with numerous studies showing good local tumor control with SRS for the treatment of spinal metastatic disease9-12 has led some groups to investigate the role of SRS in treating benign IDEM pathologies.13,14
While both the open surgical and radiosurgical literature discuss the importance of symptomatic relief in patient outcomes,1,3,4,13,14 to date neither body of literature has prospectively used quantitative scales such as the Brief Pain Inventory (BPI) questionnaire or the MD Anderson Symptom Inventory (MDASI) questionnaires to assess patient-reported symptomatic outcomes. In this study, we collected BPI and MDASI data on consecutive patients undergoing open surgical resection of IDEM lesions at a tertiary cancer center from April 2015 to December 2018 in order to determine the rate and degree of patient-reported symptomatic improvement following open surgical resection in the largest case series to date.
METHODS
Design
This is a prospective, single-center observational cohort study involving a large tertiary cancer center. The institutional review board approved this study (IRB#16-1263) and informed consent was obtained. Patient-reported outcome (PRO) measures were collected preoperatively, at 3 wk, then 3 to 6 mo intervals until a year postoperatively, and then yearly until follow-up concluded. PROs at the latest post-operative visit were the primary endpoint.
Population
Patients who underwent open surgical resection of IDEM tumors between April 2015 and December 2018 were screened. Inclusion criteria were imaging confirming an IDEM tumor, undergoing open surgical treatment of the lesion, a pathological diagnosis of a benign tumor pathology (ie, meningioma, schwannoma, neurofibroma, etc), and the ability to use patient reporting tools. Seventy patients met inclusion criteria. Thirteen patients who did not have either preoperative or sufficient postoperative PROs were excluded, yielding 57 patients who were included in the final analysis (Figure 1). No patients received prior treatment to the IDEM lesion.
FIGURE 1.
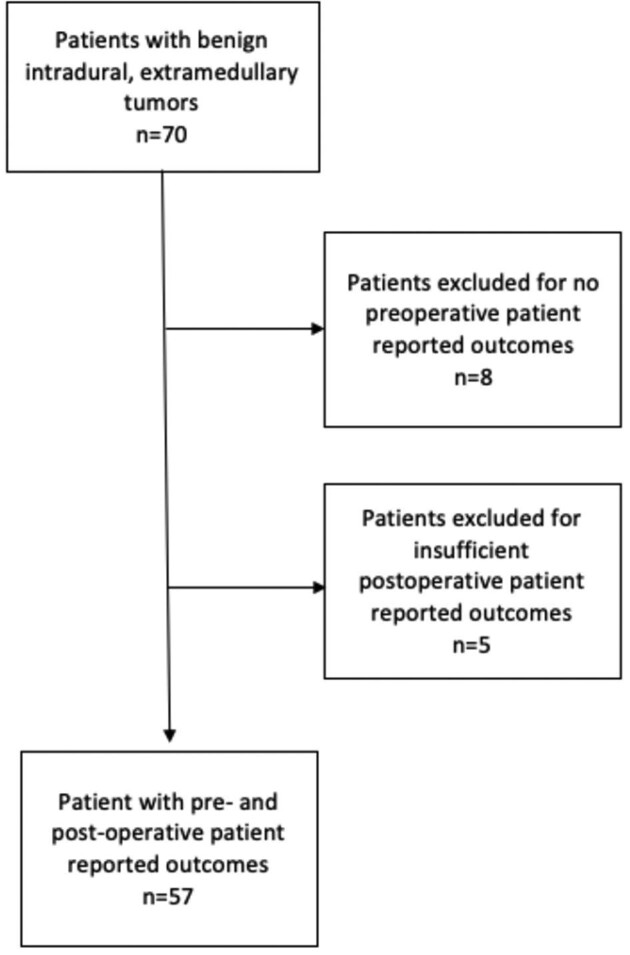
Patient selection flowchart.
Patient-Reported Variables
Data were collected either electronically in clinic, via an electronic link, or by handwritten survey and transferred to the electronic database. All data were kept in accordance with Health Insurance Portability and Accountability Act regulations.
BPI questionnaires included 12 individual items: 4 related to pain, 7 to disease interference, and 1 to relief. Combining the 7 disease interference items generated a BPI interference construct and combining the 4 pain items generated a BPI pain construct. The combination of the BPI pain construct and the interference construct items generated the BPI patient pain experience construct.15
The MDASI questionnaire included 13 core symptoms that we combined to generate the MDASI core symptom construct. The MDASI questionnaires also included 6 disease interference items, which we combined to generate the MDASI disease interference construct. Lastly, we combined the 5 spine tumor-specific items from the MDASI to generate the MDASI spine tumor-specific construct.
Both the MDASI and BPI are validated PRO measures to assess symptomatic outcomes in cancer patients.16-20
Treatment
All patients underwent open surgical resection of their benign IDEM lesion. No patients required posterior fixation. No patients received postoperative radiation treatment.
Statistical Analysis
Descriptive statistics were used to characterize the cohort and included medians, means, frequencies, and standard deviations (SD). Preoperative and postoperative individual items from the BPI (n = 12) and MDASI (n = 24) were compared using the Wilcoxon signed-rank test for matched pairs. Three BPI and three MDASI constructs were composed as described above using mean scores for each individual patient. In order to be counted, a majority of each construct's individual items must have been answered; if not, the mean value of that patient's construct was not calculated. Preoperative and postoperative construct values were compared utilizing the Wilcoxon signed-rank test for matched pairs. All P values were 2-sided with an alpha level of statistical significance set at <.05 for all constructs and <.0014 for the individual items composing the constructs (this is Bonferroni correction of 0.05 divided by the 36 items included in the constructs). The primary endpoint was the comparison of preoperative to postoperative PROs, where the postoperative timepoint was defined as the last known follow-up after surgery. The follow-up time period ranged from approximately 3 wk to 49 mo with an average follow-up of 15 mo. All statistical analyses were done in SAS version 9.4 (SAS Institute, Cary, North Carolina) and were performed as previously described by our group for utilization of the MDASI.15
RESULTS
Preoperative Evaluation
Of the 57 patients included, 39 were female (68%) with a mean age of 57.1 (SD = 15.3). The pathologies treated included 35 schwannomas (61%), 18 meningiomas (32%), 2 neurofibromas (4%), 1 paraganglioma (2%), and 1 mixed schwannoma/neurofibroma (2%). The majority of surgeries included a 2- or 3-level laminectomy (84%) with most involving the thoracic spine (58%) or the lumbar spine (inclusive of the cauda equina and conus medullaris) (30%). There were 55 patients (98%) with lesions causing high-grade spinal cord compression analogous to epidural spinal cord compression grades of 2 or 3 (Figure 2). Preoperatively, most patients were evaluated as “E” (53 patients, 93%) on the American Spinal Injury Association (ASIA) Impairment Scale (normal motor and sensory exam), had a median muscle strength grade of 5 (53 patients, 93%, range, 2-5) on the Medical Research Council (MRC) Muscle Scale (full strength in all muscle groups), and an Eastern Cooperative Oncology Group (ECOG) Performance Status score of 1 (46 patients, 81%) (range 0-3) (Table 1).
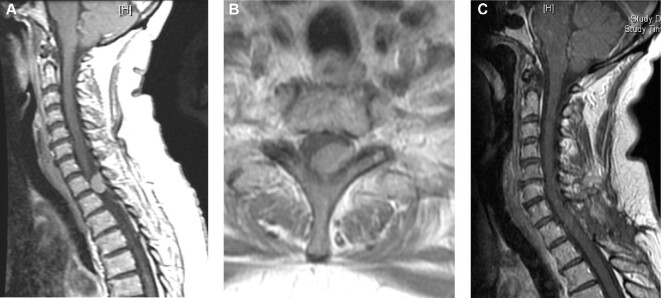
FIGURE 2.
Representative image of a 65-yr-old female who presented with upper thoracic back pain with sagittal A and axial B postcontrast MRI images demonstrating a T1-2 lesion with high-grade spinal cord compression. Postoperative sagittal imaging C demonstrates gross total resection with no residual tumor.
TABLE 1.
Patient Characteristics
| Variable | Category | N | % |
|---|---|---|---|
| Age | – | 57 | 100 |
| Range 21 to 83 | |||
| Mean 57.1 | |||
| Median 59.3 | |||
| Sex | Female | 39 | 68 |
| Male | 18 | 32 | |
| Surgical treatment level | Cervical | 7 | 12 |
| Thoracic | 33 | 58 | |
| Lumbar | 17 | 30 | |
| Histology | Schwannoma | 35 | 61 |
| Schwannoma, neurofibroma | 1 | 2 | |
| Meningioma | 18 | 32 | |
| Paraganglioma | 1 | 2 | |
| Neurofibroma | 2 | 4 | |
| Preoperative ASIA | E | 53 | 93 |
| D | 3 | 5 | |
| C | 1 | 2 | |
| Preoperative ECOG | 0 | 5 | 9 |
| 1 | 46 | 81 | |
| 2 | 2 | 4 | |
| 3 | 4 | 7 | |
| Preoperative MRC | 2 | 1 | 2 |
| 3 | 1 | 2 | |
| 4 | 2 | 4 | |
| 5 | 53 | 93 | |
| Previous surgery | No | 57 | 100 |
| Laminectomy levels | 1 | 7 | 12 |
| 2 | 35 | 61 | |
| 3 | 13 | 23 | |
| 4 | 1 | 2 | |
| 5 | 1 | 2 | |
| Extent of resection | Gross total resection | 52 | 91 |
| Subtotal resection | 5 | 9 |
Brief Pain Inventory
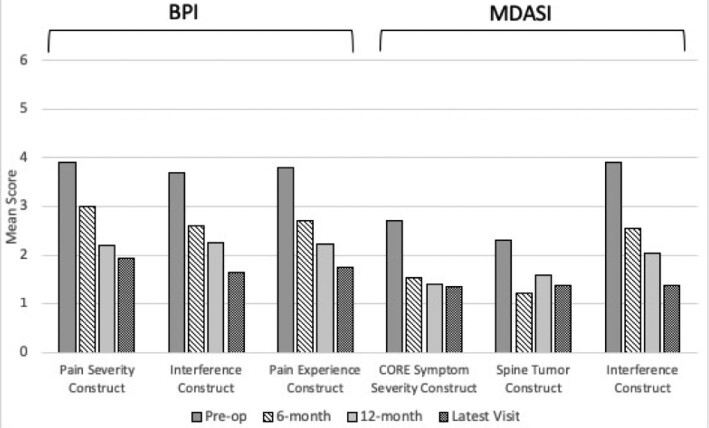
Among BPI individual questionnaire items, worst pain, least pain, average pain, and pain experienced at the time of completing the questionnaire all demonstrated statistically significant decreases (P < .001 for all) (Table 2). Of all patients included in our study, 28 had BPI data at the 6-mo follow-up timepoint, 23 had data at the 12-mo follow-up time-point, and 26 patients had data at a follow-up timepoint greater than 1 yr. Patients experienced a statistically significant improvement in mean score in general activity, mood, walking ability, ability to participate in normal work, quality of relations, quality of sleep, and enjoyment of life (P < .001) (Table 2). The 3 combined BPI constructs (pain severity, pain interference with daily life, and overall patient pain experience) were significantly improved for all patients at all timepoints (P < .0001) (Figure 3). In particular, regarding the BPI pain severity construct, 40/55 patients (73%) had improvement in their pain severity and 10/55 had worsened pain (18%), with 5/55 patients (9%) having no change in pain severity and 2 patients missing a majority of items contributing to the construct. With respect to those patients experiencing worsened pain, most reported no pain or minimal pain on the day of their screening, but there was a wide range of ratings of their worst overall pain. Regarding the BPI interference construct, 43/54 patients (80%) had improvement in their interference, 7 (13%) had worsened pain, 4 patients had no change, and 3 were missing a majority of items contributing to the construct.
TABLE 2.
BPI and MDASI Individual Item and Construct Results at Baseline and Latest Follow-up (Primary End Point)
| Survey | Individual item or construct | Preoperative mean score | Postoperative mean score | Wilcoxon signed-rank test P value | N comparison |
|---|---|---|---|---|---|
| BPI | Worst pain | 5.7 | 2.9 | <.0001 | 54 |
| Least pain | 2.5 | 1.3 | .0002 | 55 | |
| Average pain | 4.2 | 2 | <.0001 | 52 | |
| Right now pain | 3.4 | 1.4 | <.0001 | 55 | |
| General activity | 3.9 | 1.9 | <.0001 | 54 | |
| Mood | 3.3 | 1.8 | .0001 | 54 | |
| Walking ability | 3.1 | 1.7 | .0006 | 54 | |
| Normal work | 3.9 | 1.9 | <.0001 | 54 | |
| Relations | 2.6 | 1.3 | .0003 | 52 | |
| Sleep | 4.6 | 1.8 | <.0001 | 52 | |
| Enjoyment of life | 4.2 | 1.9 | <.0001 | 51 | |
| Relief | 39.1% | 36.5% | .80 | 28 | |
| Pain severity construct | 3.9 | 1.9 | <.0001 | 55 | |
| Interference construct | 3.7 | 1.5 | <.0001 | 54 | |
| Pain experience construct | 3.8 | 1.9 | <.0001 | 53 | |
| MDASI | Pain | 5.7 | 2.8 | <.0001 | 52 |
| Fatigue | 4 | 2.2 | <.0001 | 52 | |
| Nausea | 0.7 | 0.4 | .14 | 54 | |
| Sleep | 5.2 | 1.8 | <.0001 | 55 | |
| Distress | 3.9 | 1.7 | <.0001 | 53 | |
| Shortness of breath | 1.3 | 1.1 | .83 | 53 | |
| Memory | 1.9 | 1.1 | .09 | 53 | |
| Appetite | 1.5 | 0.4 | .004 | 53 | |
| Drowsy | 2.6 | 1.3 | .001 | 55 | |
| Dry mouth | 3.2 | 0.9 | .30 | 53 | |
| Sadness | 2.7 | 1.4 | .001 | 51 | |
| Vomiting | 0.2 | 0 | .25 | 54 | |
| Numbness | 4.4 | 2.5 | .001 | 54 | |
| Spine pain | 4.8 | 2 | <.0001 | 52 | |
| Limb weakness | 3.3 | 2.1 | .03 | 51 | |
| Bowel/bladder control | 0.9 | 0.5 | .15 | 53 | |
| Bowel pattern | 1.4 | 1.1 | .47 | 53 | |
| Sexual function | 1.1 | 1.4 | .72 | 50 | |
| General activity | 4.6 | 1.6 | <.0001 | 53 | |
| Mood | 3.7 | 1.6 | <.0001 | 54 | |
| Work | 3.8 | 1.7 | <.0001 | 53 | |
| Relations | 2.6 | 1.1 | <.0001 | 53 | |
| Walking | 3.9 | 1.6 | <.0001 | 53 | |
| Enjoyment of life | 4.4 | 1.6 | <.0001 | 53 | |
| Core symptom severity construct | 2.7 | 1.4 | <.0001 | 54 | |
| Spine tumor construct | 2.3 | 1.4 | .0015 | 53 | |
| Interference construct | 3.9 | 1.6 | <.0001 | 54 |
FIGURE 3.
Mean preoperative versus postoperative scores for the BPI and MDASI constructs at 6 mo, 12 mo, and latest follow-up visit; * = P < .05.
At each of the 6, 12, and >12-mo follow-up timepoints, all BPI constructs showed statistically significant improvements that remained durable. The only BPI individual item that did not show improvement after surgery was the “relief category” (P = .80).
MD Anderson Symptom Inventory
Analysis of the MDASI questionnaire showed that at the last follow-up, pain, fatigue, sleep, distress, appetite, drowsiness, sadness, numbness, spine pain, and limb weakness had significant improvements after surgical resection (P < .05). Additionally, general activity level, mood, work, relations, walking, and overall enjoyment of life were significantly improved (P < .0001) (Table 2). Of all patients included in our study, 28 had MDASI data at the 6-mo follow-up timepoint, 23 had data at the 12-mo follow-up time-point, and 26 patients had data at a follow-up timepoint greater than 1 yr. Regarding the composite scores of core symptom severity construct, spine tumor construct, and interference construct, all demonstrated statistically significant symptomatic improvements compared to preoperative assessments (P < .01) (Figure 3). Specifically, for the MDASI spine tumor construct, 34/53 patients (64%) experienced improvement, 13/53 (25%) worsened, 6/53 (11%) had no change, and 4 were missing a majority of items contributing to the construct. Regarding the MDASI interference construct, 43/54 patients (80%) improved, 6/54 (11%) had no change, 5/54 (9%) worsened, and 3 were missing data majority of items contributing to the construct. Of the individual items in the MDASI, nausea, shortness of breath, memory, dry mouth, vomiting, bowel/bladder control, bowel patterns, and sexual function did not significantly improve individually after surgery (P > .05 for all).
At each of the 6, 12, and >12-mo follow-up timepoints, all MDASI constructs demonstrated statistically significant improvements that remained durable throughout the study period.
Preoperative Predictors of BPI and MDASI Construct Improvement
The only significant preoperative predictor of improvement in BPI and MDASI construct PROs was tumor location, with cervical location predictive of greater improvement in scores. However, this should be interpreted with caution as there were only 7 patients with cervical tumor location. Otherwise, all other factors, including extent of resection, were not significant predictors of postoperative improvement in PROs at the latest follow-up timepoint.
Postoperative Complications
Of the 57 patients included, 7 (12%) experienced postoperative complications, 3 (5%) of which required surgical interventions. Three patients had cerebrospinal fluid leaks managed with wound oversewing (n = 2) or lumbar drainage (n = 1). Two had wound breakdown requiring a return to the operating room for wound revision. One patient developed a lumbar pseudomeningocele and hydrocephalus requiring ventriculoperitoneal shunt placement. One patient had urinary retention requiring medical management. There were no patient deaths or neurological injuries in this series.
DISCUSSION
Key Results
This prospective study evaluated PROs for pain, disease interference, and other tumor-related symptoms in patients undergoing surgical resection of their benign IDEM spinal cord tumor. Patients experienced statistically significant improvements in most individually rated PROs and in all measured composite symptom constructs. These improvements in composite PROs were statistically significant at all follow-up timepoints.
We had a 91% rate of gross total resection with statistically significant improvements in all BPI and MDASI constructs (P < .05). More specifically, our study shows surgical intervention providing an 82% rate of improved or stable pain severity, an 87% rate of improved or stable interference on activities of daily life, and an 87% rate of core symptom severity improvement or stability at last follow-up. There were only 4 patients presenting with significant preoperative weakness (range 2/5-4/5 strength), all of whom had high-grade spinal cord compression, and all of whom improved to full strength by their last follow-up. These findings are in line with those of other surgical series,1-4 but they provide a more granular understanding of the impact on postoperative quality of life and symptom improvement. This granularity provides a benchmark against which studies discussing the effectiveness of nonopen surgical treatments of these tumors should be compared.
While our data show improvements in most BPI measures, the absence of improvement in the “relief” category is noteworthy. Relief in the BPI is ascertained by asking the patient how much relief have pain treatments provided in the past 24 h. Based on this question, if a patient had significant pain relief from surgery, their answer could be confounded by the fact that they do not experience significant relief from pain with medications because their pain has been ameliorated by surgery. Additionally, in the 13 patients with worsened tumor-specific construct MDASI scores, there was no common pattern. Patients who worsened had different baseline pain scores, neuropathic symptom scores, and distress scores.
Interpretation
The findings of our study are similar to those of other surgical series for benign IDEM tumors that demonstrate significant and durable improvements in patient outcomes after open surgical resection.1-4 Our study adds granularity regarding the subjective patient-reported experience of symptoms and can aid in counseling regarding likely outcomes of surgical intervention. Most importantly, our data demonstrate that the improvements patients experience at the 6-mo follow-up timepoint are durable.
Through granularity, our study establishes a framework against which nonopen surgical interventions for these tumors should be compared. For example, in the study by Gerszten et al,13 in patients reporting preprocedural pain, they reported “significant improvement” or complete resolution of pain at last follow-up visit in 15/19 patients (79%) who underwent SRS. Similarly, Sachdev et al14 demonstrated that of the 60 patients presenting with pain, 91% of them were stable or improved after SRS based on visual analog scale scores. However, whether or not these improvements represented statistically significant changes was not described in either report. Moreover, in each of these studies, there was no detailed information regarding the degree of pain relief or its impact on functional outcomes.
These groups focused on SRS for treating benign IDEM tumors in patients deemed poor surgical candidates due to age, comorbidities, tumor location, or tumor recurrence after open surgical resection.13,14 Their results demonstrate durable tumor control but variable symptom relief that was highly correlated with tumor histology and its impact on radioresponsiveness, a finding not encountered in open surgical literature. An understanding of the patient experience of these symptoms is critical to determining if an intervention is equivalent, inferior, or superior to microsurgical resection.
Limitations
Though our study is the largest analysis of prospectively collected PROs for symptomatic, benign IDEM spine tumors undergoing surgery, there are several important limitations that should be discussed. First, our experience represents that of a single-center tertiary cancer center. Our 57 patients, while the largest to date, represent a small sample size that precludes drawing conclusions regarding the impact, if any, of specific tumor histologies on pain-based outcomes after open surgical intervention. Second, our follow-up period was limited to an average of 15.4 mo. While this is a short follow-up period, due to the immediate decompressive effects of surgical interventions, this likely is an adequate time window and longer follow-up would not have yielded significantly different results in regard to quality of life measures. This notion is reinforced by the high percentage of patients with symptom improvement by their 1-yr follow-up evaluation combined with a 91% gross total resection rate.
Generalizability
Our data are highly generalizable to patients with symptomatic, benign IDEM spinal cord tumors. Our data include patients with compression of the cervical and thoracic spinal cord as well as of the cauda equina and are based upon detailed and validated PROs. While highly generalizable, given our limited sample size, our study cannot be used to determine what if any role tumor histology may have on patient symptom outcomes.
CONCLUSION
The data presented demonstrate that patients with symptomatic, benign IDEM spine tumors undergoing surgical decompression experience significant post-operative improvements in PROs as measured by the BPI and MDASI. These data add granularity beyond that of the visual analog scale or other unidimensional pain assessments. Use of cancer-validated PRO tools such as the BPI and MDASI delineates the true impact of these interventions on the patient's quality of life. Further work is needed to generate comparative data for patients treated with radiosurgery for comparison to surgical outcomes.
Funding
This study was supported in part by the National Institutes of Health/National Cancer Institute Memorial Sloan-Kettering Cancer Center Support Grant P30 CA008748.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Contributor Information
William C Newman, Department of Neurosurgery, Louisiana State University Health Sciences Center, Shreveport, Louisiana.
John Berry-Candelario, UMass Memorial Health Care, Boston, Massachusetts.
Jemma Villavieja, Department of Neurological Surgery, Memorial Sloan Kettering Cancer Center, New York, New York.
Anne S Reiner, Department of Epidemiology and Biostatistics, Memorial Sloan Kettering Cancer Center, New York, New York.
Mark H Bilsky, Department of Neurological Surgery, Memorial Sloan Kettering Cancer Center, New York, New York; Department of Neurological Surgery, Weill Cornell Medical College, New York-Presbyterian Hospital, New York, New York.
Ilya Laufer, Department of Neurological Surgery, Memorial Sloan Kettering Cancer Center, New York, New York; Department of Neurological Surgery, Weill Cornell Medical College, New York-Presbyterian Hospital, New York, New York.
Ori Barzilai, Department of Neurological Surgery, Memorial Sloan Kettering Cancer Center, New York, New York.
REFERENCES
- 1.Angevine PD, Kellner C, Haque RM, McCormick PC. Surgical management of ventral intradural spinal lesions. J Neurosurg Spine. 2011;15(1):28-37. [DOI] [PubMed] [Google Scholar]
- 2.Asazuma T, Toyama Y, Maruiwa H, Fujimura Y, Hirabayashi K. Surgical strategy for cervical dumbbell tumors based on a three-dimensional classification. Spine. 2004;29(1):E10-E14. [DOI] [PubMed] [Google Scholar]
- 3.McCormick PC. Surgical management of dumbbell tumors of the cervical spine. Neurosurgery. 1996;38(2):294-300. [DOI] [PubMed] [Google Scholar]
- 4.Parsa AT, Lee J, Parney IF, Weinstein P, McCormick PC, Ames C. Spinal cord and intradural-extraparenchymal spinal tumors: current best care practices and strategies. J Neurooncol. 2004;69(1-3):291-318. [DOI] [PubMed] [Google Scholar]
- 5.Kondziolka D, Nathoo N, Flickinger JC, Niranjan A, Maitz AH, Lunsford LD. Long-term results after radiosurgery for benign intracranial tumors. Neurosurgery. 2003;53(4):815-822; discussion 821-822. [DOI] [PubMed] [Google Scholar]
- 6.Lunsford LD, Kondziolka D, Flickinger JC et al. Stereotactic radiosurgery for arteriovenous malformations of the brain. J Neurosurg. 1991;75(4):512-524. [DOI] [PubMed] [Google Scholar]
- 7.Park K-J, Kano H, Iyer A et al. Gamma Knife stereotactic radiosurgery for cavernous sinus meningioma: long-term follow-up in 200 patients. published online: July 1, 2018. J Neurosurg. (doi:10.3171/2018.2.JNS172361). [DOI] [PubMed] [Google Scholar]
- 8.Sheehan JP, Kano H, Xu Z et al. Gamma knife radiosurgery for facial nerve schwannomas: a multicenter study. J Neurosurg. 2015;123(2):387-394. [DOI] [PubMed] [Google Scholar]
- 9.Ghia AJ, Chang EL, Bishop AJ et al. Single-fraction versus multifraction spinal stereotactic radiosurgery for spinal metastases from renal cell carcinoma: secondary analysis of phase I/II trials. J Neurosurg Spine. 2016;24(5):829-836. [DOI] [PubMed] [Google Scholar]
- 10.Ho JC, Tang C, Deegan BJ et al. The use of spine stereotactic radiosurgery for oligometastatic disease. J Neurosurg Spine. 2016;25(2):239-247. [DOI] [PubMed] [Google Scholar]
- 11.Moussazadeh N, Lis E, Katsoulakis E et al. Five-year outcomes of high-dose single-fraction spinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2015;93(2):361-367. [DOI] [PubMed] [Google Scholar]
- 12.Thibault I, Al-Omair A, Masucci GL et al. Spine stereotactic body radiotherapy for renal cell cancer spinal metastases: analysis of outcomes and risk of vertebral compression fracture. J Neurosurg Spine. 2014;21(5):711-718. [DOI] [PubMed] [Google Scholar]
- 13.Gerszten PC, Chen S, Quader M, Xu Y, Novotny J, Flickinger JC. Radiosurgery for benign tumors of the spine using the Synergy S with cone-beam computed tomography image guidance. J Neurosurg. 2012;117(Suppl):197-202. [DOI] [PubMed] [Google Scholar]
- 14.Sachdev S, Dodd RL, Chang SD et al. Stereotactic radiosurgery yields long-term control for benign intradural, extramedullary spinal tumors. Neurosurgery. 2011;69(3):533-539; discussion 539. [DOI] [PubMed] [Google Scholar]
- 15.Barzilai O, Amato M-K, McLaughlin L et al. Hybrid surgery-radiosurgery therapy for metastatic epidural spinal cord compression: a prospective evaluation using patient-reported outcomes. Neurooncol Pract. 2018;5(2):104-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armstrong TS, Gning I, Mendoza TR et al. Reliability and validity of the M. D. Anderson Symptom Inventory-Spine Tumor Module. J Neurosurg Spine. 2010;12(4):421-430. [DOI] [PubMed] [Google Scholar]
- 17.Atkinson TM, Rosenfeld BD, Sit L et al. Using confirmatory factor analysis to evaluate construct validity of the Brief Pain Inventory (BPI). J Pain Symptom Manage. 2011;41(3):558-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cleeland CS, Mendoza TR, Wang XS et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634-1646. [DOI] [PubMed] [Google Scholar]
- 19.Dworkin RH, Turk DC, Wyrwich KW et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105-121. [DOI] [PubMed] [Google Scholar]
- 20.Wu JSY, Beaton D, Smith PM, Hagen NA. Patterns of pain and interference in patients with painful bone metastases: a brief pain inventory validation study. J Pain Symptom Manage. 2010;39(2):230-240. [DOI] [PubMed] [Google Scholar]