Abstract
BACKGROUND
Aneurysmal subarachnoid hemorrhage (aSAH) is associated with a high mortality and poor neurologic outcomes. The biologic underpinnings of the morbidity and mortality associated with aSAH remain poorly understood.
OBJECTIVE
To ascertain potential insights into pathological mechanisms of injury after aSAH using an approach of metabolomics coupled with machine learning methods.
METHODS
Using cerebrospinal fluid (CSF) samples from 81 aSAH enrolled in a retrospective cohort biorepository, samples collected during the peak of delayed cerebral ischemia were analyzed using liquid chromatography-tandem mass spectrometry. A total of 138 metabolites were measured and quantified in each sample. Data were analyzed using elastic net (EN) machine learning and orthogonal partial least squares-discriminant analysis (OPLS-DA) to identify the leading CSF metabolites associated with poor outcome, as determined by the modified Rankin Scale (mRS) at discharge and at 90 d. Repeated measures analysis determined the effect size for each metabolite on poor outcome.
RESULTS
EN machine learning and OPLS-DA analysis identified 8 and 10 metabolites, respectively, that predicted poor mRS (mRS 3-6) at discharge and at 90 d. Of these candidates, symmetric dimethylarginine (SDMA), dimethylguanidine valeric acid (DMGV), and ornithine were consistent markers, with an association with poor mRS at discharge (P = .0005, .002, and .0001, respectively) and at 90 d (P = .0036, .0001, and .004, respectively). SDMA also demonstrated a significantly elevated CSF concentration compared with nonaneurysmal subarachnoid hemorrhage controls (P = .0087).
CONCLUSION
SDMA, DMGV, and ornithine are vasoactive molecules linked to the nitric oxide pathway that predicts poor outcome after severe aSAH. Further study of dimethylarginine metabolites in brain injury after aSAH is warranted.
Keywords: Aneurysm, Biomarker, Machine learning, Metabolites, Metabolomics, Subarachnoid hemorrhage, Cerebrospinal fluid
Graphical Abstract
Graphical Abstract.
ABBREVIATIONS
- ADMA
asymmetric dimethylarginine
- aSAH
aneurysmal subarachnoid hemorrhage
- AUC
area under the curve
- CI
confidence interval
- CSF
cerebrospinal fluid
- DCI
delayed cerebral ischemia
- DMGV
dimethylguanidine valeric acid
- EN
elastic net
- EVD
external ventricular drain
- HPLC
high-performance liquid chromatography
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- mRS
modified Rankin Scale
- NO
nitric oxide
- OPLS-DA
orthogonal partial least squares-discriminant analysis
- OR
odds ratio
- QQQ MS
triple-quadrupole mass spectrometry
- SDMA
symmetric dimethylarginine
- TCD
transcranial doppler ultrasonography
- VIP
variable importance in the projection
- WFNS
World Federation of Neurological Surgeons
Aneurysmal subarachnoid hemorrhage (aSAH) is a devastating form of acute central nervous system injury with an occurrence of approximately 1:10 000 patients per year and results in ∼15% mortality prior to receiving medical attention.1,2 Of those able to reach medical attention, 20% have a poor neurologic outcome.3 This relates to both the primary brain injury sustained during aneurysmal rupture as well as secondary injury from the sequelae of the hemorrhage. Despite extensive research, the pathophysiology underlying patient outcomes remain poorly understood.4-6
Clinical and demographic patient factors have a limited ability to predict neurological outcome. Initial neurologic examination, transcranial doppler ultrasonography (TCD), and electroencephalography can be used to augment prediction and may also inform the decision to pursue intervention and cerebral angiography.7-10 Metabolomics is an established method to better understand the pathophysiological and metabolic state in disease, and to inform prognosis.11 Prior research demonstrated the utility of this method combined with machine learning in uncovering putative markers in aSAH patient plasma samples.12,13 Given that the blood-brain barrier may lead to differing changes in the cerebrospinal fluid (CSF) compared to plasma, we sought to evaluate the relationship of CSF metabolites with clinical outcome in a cohort of severe aSAH patients.
METHODS
Cohort
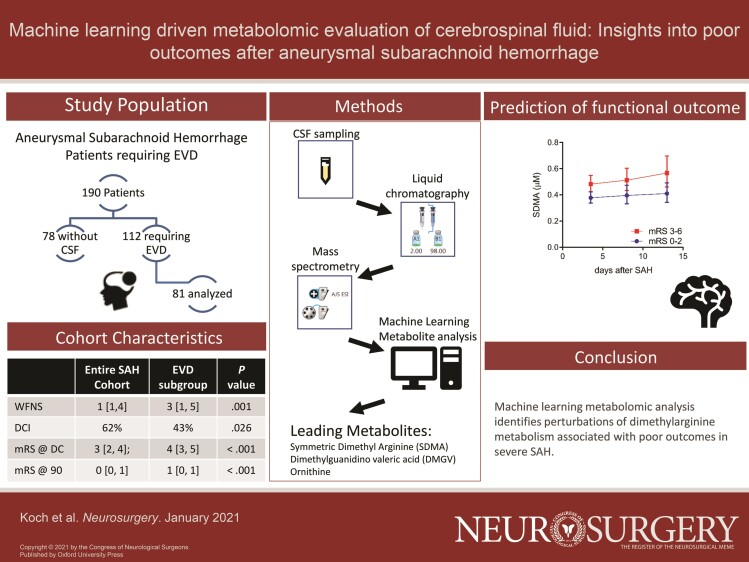
With institutional review board approval, we prospectively enrolled 190 consecutive adult patients with aSAH, greater than 18 yr old, who consented to participation in the neuroscience intensive care unit biospecimen collection at a single institution. In patients who had an external ventricular drain (EVD) placed (n = 112), CSF samples were collected during acute hospitalization, at 3 time epochs during hospitalization (early: day 0-5 after aSAH, mid: day 6-10, and late: day 11-15), and 81 were available for analysis. Clinical information was collected at the time of entry into the repository as well as at 90-d follow-up. CSF was only collected passively (from the burette) when the EVD was open and draining. Patients with clamped EVDs did not have samples drawn. A total of 154 CSF samples from 81 aSAH patients with external ventricular drains were available. Patients were excluded if their subarachnoid hemorrhage (SAH) was from an alternate etiology. We also collected a separate cohort of nonruptured cerebral aneurysm patients prospectively, which served as a control population. In this cohort after preprocedural consent, CSF was obtained at the time of open cerebral aneurysm surgery from either a lumbar drain or intracranial cistern (n = 16 patients).
Patient demographic and clinical characteristics including World Federation of Neurological Surgeons (WFNS) grade, modified Fischer score, means of treatment, and need for permanent CSF diversion were collected. Discharge and 90-d outcome were assessed using the modified Rankin Scale (mRS) score. Vasospasm was evaluated on TCD, a peak systolic velocity >200 cm/s was defined as elevated; delayed cerebral ischemia (DCI) was evaluated based on the consensus definition of Vergouwen et al.14
Metabolite Profiling
CSF samples were collected after <1 h accumulation in the burette of a ventriculostomy system, centrifuged at 2000 × g for 15 min to remove cellular material, and aliquots of supernatant frozen and stored at −80°C. Samples were treated with acetonitrile/methanol (3:1) with citrulline-d8. CSF samples were separated using a high-performance liquid chromatography (HPLC) system with hydrophilic interaction chromatography on a 2.1 × 100 mm, 3.5-μm XBridge Amide column (Waters, Milford, Massachusetts), followed by targeted triple-quadrupole mass spectrometry (QQQ MS) detection in positive and negative polarity modes, using our previously established method.13,15 Briefly, the chromatography system consisted of a 1290 Infinity autosampler and 2 1290 HLPC binary pumps, connected to 649S QQQ MS (Agilent). Mobile phase A was 95:5 (v:v) water:acetonitrile with 20 mM ammonium acetate and 20 mM ammonium hydroxide (pH 9.5). Mobile phase B was acetonitrile. Ammonium acetate, ammonium hydroxide, and HPLC grade solvents were obtained from Fisher Scientific (Hampton, New Hampshire).
Peak integration for metabolite quantification was carried out using MassHunter QQQ MS Quantitative Analysis software (Agilent). Peaks quality was checked and normalized to human pooled CSF samples that were interspersed at regular intervals every 10 injections, using standard procedures.15,16 A total of 138 metabolites were measured and quantified in each sample.
The metabolites of interest, as identified with the screening method (described below), were re-analyzed and quantitated. This assay achieved chromatographic separation of asymmetric dimethylarginine (ADMA) from symmetric dimethylarginine (SDMA) and, thus, allowing quantitation of these arginine metabolites. In addition to ADMA and SDMA, citrulline, arginine, monomethylarginine, methionine, S-adenosyl methionine and S-adenosyl-L-homocysteine were also quantitated.
The collected CSF samples, calibration standards, and quality control samples were defrosted on wet ice and vortex-mixed thoroughly before extraction by protein precipitation. A total of 30 μl of each sample was aliquoted and 20 μL of internal standard intermediate solution (20 μM citrulline-d7 and 20 μM methionine-d4) in acetonitrile:water (50:50) was added to each sample before mixing for 5 min. Protein precipitation was carried out by adding 150 μL of acetonitrile:methanol:formic acid (75:25:0.2) to each sample well. Sample plates were sealed and vortex-mixed for 5 min before centrifugation (∼3500 × g, 5 min at 4˚C). For chromatographic separation, an Atlantis Silica HILIC 3 μm, 2.1 mm × 100 mm (waters) was used. Mobile phase A was ammonium formate 10 mM (aq):formic acid (100:0.2), mobile phase B was acetonitrile:formic acid (100:0.2). A shallow gradient from 80% B to 60% B over 4.6 min was run at 1 mL/min before re-equilibration. Duplicate calibration curves, with ascending line at the start of the batch and a descending line at the end of the batch, were run to establish linearity. Peak integration was carried out using MassHunter QQQ MS Quantitative Analysis software (Agilent) and internal standards were used for data normalization.
Machine Learning Analysis
We used a previously established method, elastic net (EN) machine learning, to identify putative metabolites of interest.13,17,18 EN is a selection method and extension of the linear regression model. EN is able to automatically select the best features linked with the outcome from the dataset and applies a penalty function that provides a sparse solution. In order to get unbiased results, 75% of the samples were randomly divided into a training set with the remaining 25% used as a validation set. Subsequent 10-fold cross-validation and 100 iterations optimized the penalty parameters, which allowed a subsequent combinatorial analysis of the EN to rank the metabolites according to their predictive value of mRS at discharge and at 90 d.19 The stronger the penalty (close to 1), the smaller number of variables are selected, whereas if the penalty is weaker (close to 0), higher numbers of variables are selected. We focused our analysis on the time period when secondary brain injury is most likely to occur (6-10 d after aSAH ictus), reasoning that metabolites changes during this time frame would be most informative. These selected metabolite features were further modeled using logistic regression and area under the curve (AUC) calculations. We produced 2 AUC distributions. One is from random label sampling, ie, randomizing the sample labels in each iteration and averaged over 100 iterations and displayed as a “random AUC.” The other AUC is based on the true bootstrapped samples and considered as true distributions of AUC.
We also performed an additional machine learning approach using orthogonal partial least squares-discriminant analysis (OPLS-DA) regression.20 The variable importance in the projection (VIP) parameter ranks the importance of each metabolite in its contribution to the discriminatory model with a VIP value of >1.5 considered as significant. We leveraged EN machine learning and OPLS-DA to identify common metabolites in both methods.
Statistical Analysis
These metabolites were then further evaluated using 2-tailed student t-test and categorical variables using Fischer's exact test. A linear mixed effects repeated measures model was performed to evaluate for significant change in metabolite levels maintained over time. Analyses were performed using R statistical computing environment (R Version 3.6.0). A P-value < .05 was considered statistically significant.
RESULTS
From the starting cohort of 190 patients, 112 patients presented with SAH that required placement of an EVD. Of those, 81 patients had 140 CSF samples available for analysis, which constituted the EVD study cohort. The clinical characteristics of the study cohort are presented in Table 1 and are compared to the entire cohort of SAH patients. Patients with EVDs demonstrated worse neurological function on presentation and on follow-up. Compared to the starting cohort, the EVD cohort had a higher WFNS grade (3 [1, 5] vs 1 [1, 4]; P < .001), a higher rate of DCI (62% vs 43%; P = .026), worse outcome at discharge (mRS 4 [3, 5] vs 3 [2, 4]; P < .001), and worse outcome at 90 d (mRS 1 [0, 1] vs 0 [0, 1]; P < .001). No procedure related rehemorrhage or ischemia was observed in our metabolomics cohort.
TABLE 1.
Clinical Characteristics of the CSF Cohort Compared to the Entire Cohort
| Entire cohort | Study cohort | ||
|---|---|---|---|
| (n = 190) | (n = 81) | P | |
| Age, mean ± SD | 57.3 ± 12.0 | 57.7 ± 12.4 | .78 |
| Female, n (%) | 117 (62%) | 49 (60%) | .97 |
| WFNS, median (interquartile range) | 1 [1, 4] | 4 [1, 5] | <.001 |
| Clip, n (%) | 113 (61%) | 45 (57%) | .61 |
| DCI, n (%)a | 61 (43%) | 29 (62%) | .026 |
| Vasospasm, n (%)b | 43 (48%) | 24 (58%) | .28 |
| VP shunt, n (%) | 17 (9%) | 12 (15%) | .20 |
| mRS at discharge | 3 [2, 4] | 4 [3, 5] | <.001 |
| mRS at 90 d | 0 [0, 1] | 1 [0, 1] | .001 |
aDCI was available in 142 patients.
bVasospasm was available in 89 patients.
VP, ventriculoperitoneal.
Within the EVD cohort, clinical and demographic factors associated with worse discharge outcome included older age (odds ratio [OR] 1.15, 95% CI 1.06-1.24, P < .001) and WFNS score 4 to 5 (OR 3.71, 95% CI 1.01-13.7, P = .048), but not sex (OR 1.46, 95% CI 0.41-5.24, P = .56). Age was also associated with worse mRS at 90 d (OR 1.06, 95% CI 1.01-1.12, P = .011).
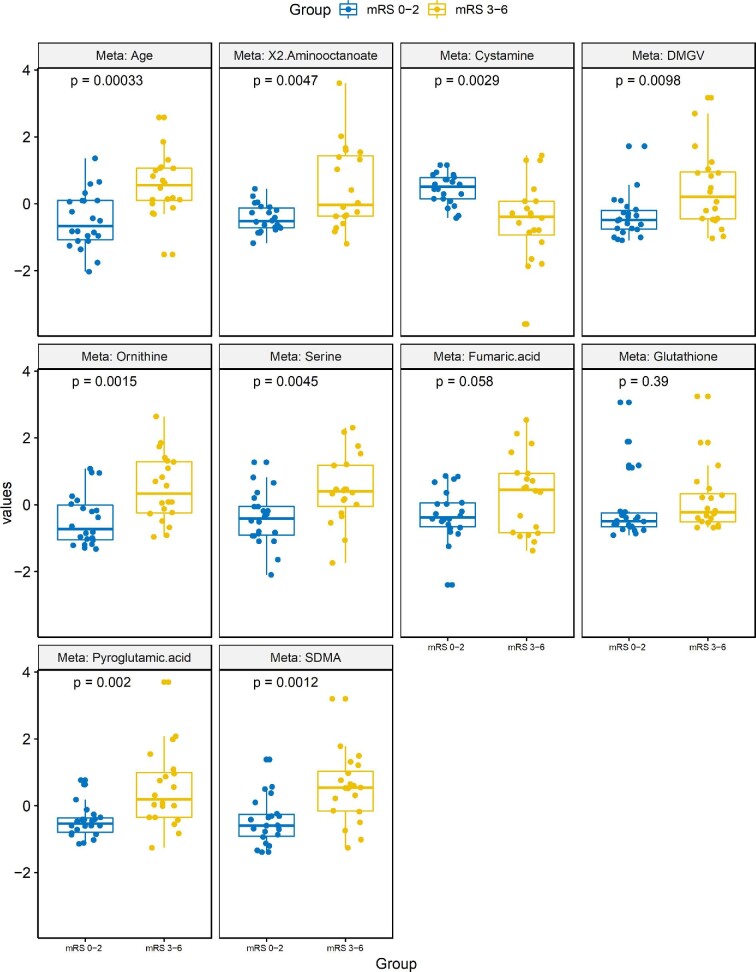
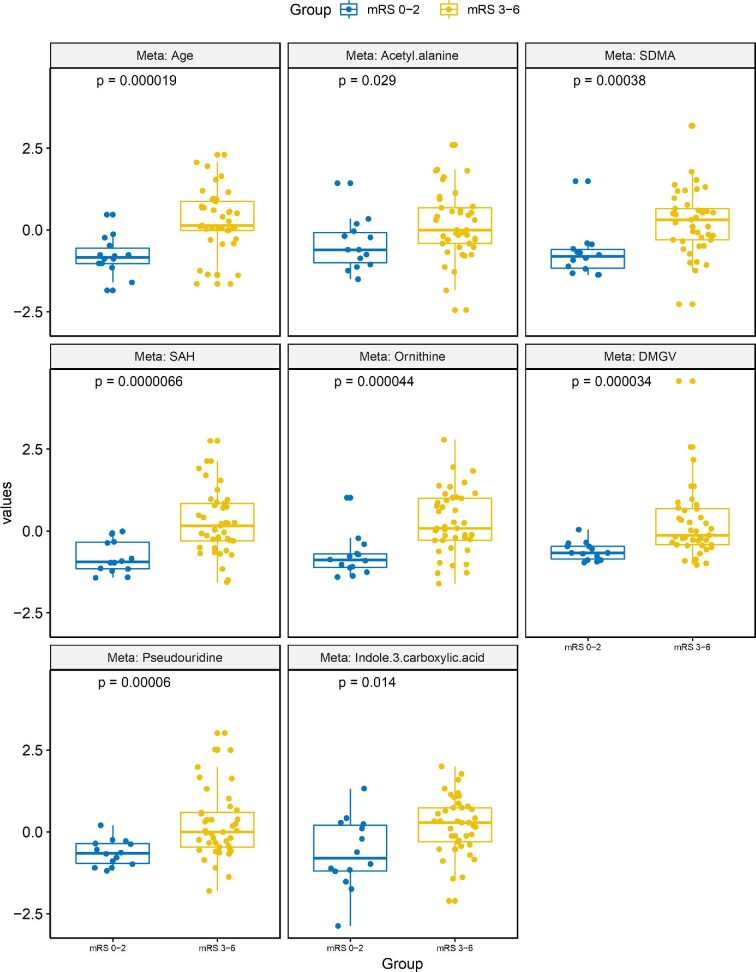
To examine the CSF metabolites linked to discharge and 90-d mRS, we used EN machine learning method to select metabolites automatically. Figure 1 demonstrates boxplots of the leading metabolites identified in the EN model that predicted discharge mRS. These included acetyl-alanine (P = .029), SDMA (P = .00038), and dimethylguanidine valeric acid (DMGV) (P = 3.4 × 10−5). Figure 2 demonstrates boxplots of the top features in the EN model that predicted 90-d mRS outcome. Common variables that predicted both discharge and 90-d mRS included ornithine (P = .0015), DMGV (P = .0098), and SDMA (P = .0012). The AUC using those metabolites after 100 bootstrapping resulted 0.8 (Figure, Supplemental Digital Content).
FIGURE 1.
Top metabolites that predict discharge outcome identified by the EN machine learning model. Individual boxplots with associated P-values are shown for each of the top metabolites found to predict discharge outcome (mRS 0-2 vs 3-6).
FIGURE 2.
Top metabolites that predict 90-d outcome identified by the EN machine learning model. Individual boxplots with associated P-values are shown for each of the top metabolites found to predict outcome at 90 d (mRS 0-2 vs 3-6).
We next employed a second method of machine learning, OPLS-DA, which prioritizes features that can best distinguish between 2 classes (eg, good vs poor outcome).21 The leading metabolites that contributed to distinguishing both discharge and 90-d mRS, as defined by the VIP score > 1.5 are shown in Table 2. Between the 2 methodologies at both time points, only 2 metabolites were significant in all analysis: SDMA and ornithine. SDMA appeared within each subset as associated with poor outcomes, and given its prior investigations in SAH, was thus investigated further as it related to patient outcomes.22,23
TABLE 2.
Top Metabolites That Predict Good vs Poor Outcome in OPLS-DA Model VIP Values
| Discharge | 90 d | |||
|---|---|---|---|---|
| P | VIP | P | VIP | |
| Ornithine | 4.41E-05 | 1.91 | 1.51E-03 | 1.72 |
| Phenylalanine | 4.85E-05 | 1.67 | 1.01E-02 | 1.52 |
| Indole lactate | 4.73E-04 | 1.63 | 1.12E-02 | 1.56 |
| Kynurenic acid | 5.44E-05 | 1.79 | 1.73E-02 | 1.57 |
| Pantothenic acid | 7.02E-05 | 1.66 | 7.89E-03 | 1.50 |
| Pseudouridine | 5.96E-05 | 1.60 | 5.88E-03 | 1.66 |
| Pyroglutamic acid | 3.88E-05 | 1.79 | 1.99E-03 | 1.69 |
| SAH | 3.12E-06 | 1.93 | 2.95E-03 | 1.74 |
| SAM | 9.38E-04 | 1.68 | 1.35E-02 | 1.63 |
| SDMA | 5.05E-04 | 1.90 | 3.66E-03 | 1.81 |
SAH, s-adenosylhomocysteine; SAM, s-adenosylmethionine.
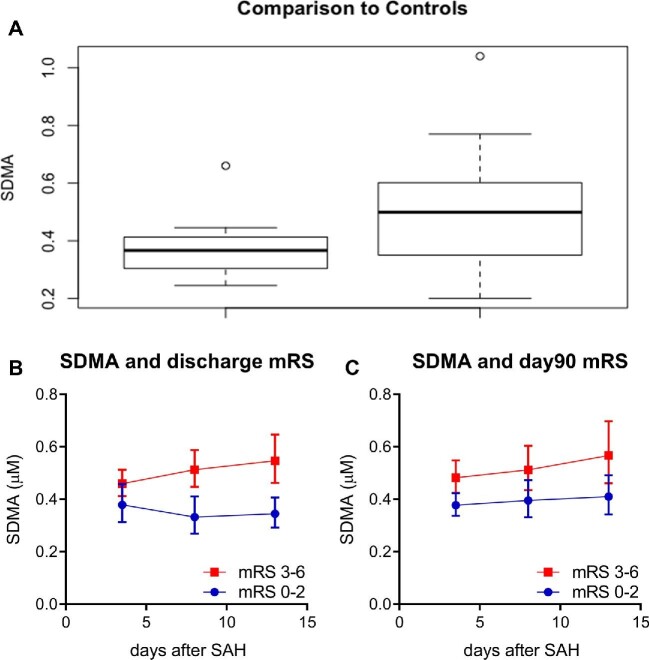
To further corroborate our findings, we compared CSF concentrations of SDMA in SAH patients vs those undergoing elective aneurysm clipping. SDMA concentrations in control patients were significantly lower than that of patients with poor outcomes (Figure 3A; P = .009). There was no difference in CSF SDMA level between aSAH patients with good outcome and elective aneurysm clipping patients (P = .9).
FIGURE 3.
CSF SDMA concentrations over time repeated measures analysis and relative to control patients. A, Comparison of CSF SDMA concentrations between elective aneurysm patients and SAH patients (P = .009). B, Average SDMA concentrations of poor and good outcome at discharge with associated repeated measures analysis and associated p value, P = .002. C, Average SDMA concentrations based on good and poor outcomes at 90 d with associated P-value of repeated measures analysis, P = .001.
In our initial liquid chromatography-tandem mass spectrometry (LC-MS/MS) metabolomics method, the dimethylarginine enantiomers (SDMA and ADMA) were not distinguishable from each other. We therefore developed a follow-up dimethylarginine-targeted LC-MS/MS method that differentiated SDMA from ADMA, as well as detected additional related metabolites, including methylmalonic acid, s-adenosylhomocysteine and s-adenosylmethionine. Examination of these metabolites revealed that SDMA predicted outcome at discharge and at 90 d (epoch1 P = .0005 and .0036) but not with radiographic vasospasm (OR 0.12, 95% CI 0.001-13.9, P = .38). Because SDMA measurements were available at serial timepoints, we next constructed linear mixed effects models, which confirmed that SDMA was elevated across time points in patients with poor outcome at discharge (Figure 3B; β = 0.19, P = .002) and at 90 d (Figure 3C; β = 0.18, P = .001). Additional metabolites within the pathway that were significant included s-adenosylhomocysteine (β = 0.01, P = .017 at discharge; β = 0.02, P = .006 at 90 d), ADMA (β = 0.17, P = .04 at discharge; β = 0.19, P = .014 at 90 d), DMGV (β = 0.32, P = .002 at discharge; β = 0.46, P < .0001 at 90 d), and ornithine (β = 0.53, P < .0001 at discharge; β = 0.28, P = .004 at 90 d), indicating a perturbation in dimethylarginine metabolism at several points in the pathway.
DISCUSSION
Using complementary machine learning approaches, we noted elevated CSF concentrations of dimethylarginines were associated with poor outcomes at both discharge and 90-d follow-up after severe aSAH.17 Further, when compared to CSF of control patients undergoing elective aneurysm clipping, severe aSAH patients demonstrated a significantly higher concentration of SDMA in CSF, which was most notable in patients with poor outcomes.
Dimethylarginines play a known role in cardiovascular disease and renal dysfunction.22,24-27 Known breakdown product of proteins containing methylarginines, dimethylarginines play a critical role in regulating the synthesis of nitric oxide (NO). ADMA (an enantiomer of SDMA) has been shown to alter the synthesis of NO as a competitive inhibitor. Multiple studies have demonstrated that high serum concentrations of ADMA are associated with poor outcomes in the critically ill patients. Additionally, primate models have demonstrated an association of ADMA with cerebral vasospasm.23 We found that both SDMA and transamination byproducts, DMGV and ornithine, were associated with poor outcome.28 Moreover, SDMA was not originally thought to have a direct effect on the NO synthase pathway; however, recent studies have found that high density lipoprotein in combination with SDMA, but not ADMA, inhibit NO synthase via innate immunity pathways.29 Taken together, our study found that CSF SDMA, ornithine, and DMGV, related metabolites that alter the NO pathway, were significantly associated with outcomes.
Prior studies demonstrated a putative role of both SDMA and ADMA in neurovascular disease.30 SDMA and ADMA are implicated as markers and putative mediators of poor outcomes based on an association with DCI and vasospasm.22,23 These papers, although lower powered than our study (n = 34 and 13, respectively) specifically analyzed the cerebral spinal fluid for SDMA without evaluating for multiple other markers. Our study reaches a similar conclusion that the CSF concentration of SDMA is associated with poor outcomes with the additional demonstration of association with other metabolites in dimethylarginine metabolism, DMGV, and ornithine.
As a retrospective study, we relied on the availability of CSF for analysis, thus our cohort was biased towards higher grade SAH patients. Our findings did not correlate metabolite levels with vasospasm. Similarly, although statistically robust, the odds ratio of 1.27 and 1.5 of SDMA concentrations with poor outcome at discharge and 90 d, respectively, demonstrated a moderate effect size. These effects may be a byproduct of exclusively studying sicker patients. A future study with a more diverse cohort can clarify and these findings. Vasospasm does not correlate with outcomes in larger cohorts and the lack of correlation with SDMA in our cohort may add further support to the pathophysiology occurring at the microvascular level.31,32 Additionally, although the patient samples were obtained and processed in less than an hour, this time may introduce a change in the metabolite profile that we are unable to control for in our study.
With a limited number of therapeutic options beyond supportive care for aSAH, our study adds further support of the role of SDMA in SAH pathophysiology. The secondary injury associated with aSAH is an important therapeutic target. Studies of serum are limited to those metabolites in the peripheral circulation, whereas CSF provides a unique look more proximate to the site of injury. Whether these significant metabolites are a marker of damage or a causative agent and point for intervention remains a question. Future work will explore the origin of differential concentrations of SDMA, DMGV, and ornithine in aSAH.
CONCLUSION
Using a combination of metabolomics and machine learning, we have demonstrated an association of poor clinical outcomes with SDMA, DMGV, and ornithine concentrations in the CSF of severe aSAH patients. These findings are a platform for future investigations into the pathologic underpinnings of secondary injury from aSAH.
Funding
This study did not receive any funding or financial support.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article. Dr Kimberly has received grant support from the NIH (R01 NS099209) and the American Heart Association (17CSA 33440004), grant support and consulting fees from Biogen Inc and from NControl Therapeutics Inc, and serves as a scientific advisory board member for Biogen Inc and NControl Therapeutics Inc. Dr Acharjee was supported by the National Institute for Health Research (NIHR) Surgical Reconstruction and Microbiology Research Centre (SRMRC), Birmingham, United Kingdom. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, the Medical Research Council, or the Department of Health, United Kingdom. Dr Bevers is supported by grants from the American Academy of Neurology (AI18-0000000062), National Institute of Neurologic Disorders and Stroke (K23NS112474), and Andrew David Heitman Neurovascular Fund. Dr Bevers receives grants and personal fees from Biogen Inc, unrelated to the current work. Dr Patel acts as a consultant and proctor for MicroVention, Medtronic, and Penumbra.
Supplementary Material
Contributor Information
Matthew Koch, Department of Neurosurgery, Massachusetts General Hospital, Boston, Massachusetts.
Animesh Acharjee, College of Medical and Dental Sciences, Institute of Cancer and Genomic Sciences, Centre for Computational Biology and NIHR Surgical Reconstruction and Microbiology Research Centre, University Hospital Birmingham, Birmingham, United Kingdom.
Zsuzsanna Ament, Division of Neurocritical Care and Center for Genomic Medicine, Department of Neurology, Massachusetts General Hospital, Boston, Massachusetts.
Riana Schleicher, Division of Neurocritical Care and Center for Genomic Medicine, Department of Neurology, Massachusetts General Hospital, Boston, Massachusetts.
Matthew Bevers, Divisions of Stroke, Cerebrovascular and Critical Care Neurology, Brigham and Women's Hospital, Boston, Massachusetts.
Christopher Stapleton, Department of Neurosurgery, Massachusetts General Hospital, Boston, Massachusetts.
Aman Patel, Department of Neurosurgery, Massachusetts General Hospital, Boston, Massachusetts.
W Taylor Kimberly, Division of Neurocritical Care and Center for Genomic Medicine, Department of Neurology, Massachusetts General Hospital, Boston, Massachusetts.
Supplemental Digital Content. Figure. AUC curve of generalized linear model multiple features. EN AUC analysis with 1000 iterations.
COMMENTS
In the current study, the authors present a machine learning-based analysis of CSF metabolites in severe aneurysmal subarachnoid hemorrhage (aSAH), with the stated goal of identifying candidate biomarkers for clinical prognostication. Their assessment is a novel, timely, and potentially impactful application of machine learning technologies to a patient population with an unambiguous need for innovative approaches to their clinical care. The key findings pertain to dimethylarginine metabolites including SDMA, DMGV, and Ornithine, which were observed at significantly higher concentrations in the CSF of patients with severe aSAH and poor outcomes, as compared to those patients who reached a more favorable outcome after severe aSAH. This highlights an important link between an excess of metabolites that alter the nitric oxide pathway and poor aSAH outcomes; however, the study is also limited by a small cohort size, poor generalizability due to the specific focus on higher-grade hemorrhages in patients with EVDs, and marked risk of systematic error, including selection bias. Further, as has been described in several preceding critiques of machine learning as applied to small, heterogeneous, potentially confounded cohorts, the risk of a spurious statistical outcome is considerable–particularly when employing a 0.05 alpha threshold. Nevertheless, the authors present their findings with appropriate caution and reserve, and their interesting work should be interpreted as a promising exercise in hypothesis generation, which may guide more impactful work in this critical clinical space.
Christopher S. Graffeo
Rochester, Minnesota
In this study of 81 patients with aneurysmal subarachnoid hemorrhage (aSAH), the authors assessed metabolomic markers within cerebrospinal fluid (CSF) and used machine learning techniques to associate CSF markers with functional outcomes at discharge and 90-day follow up. In conclusion, elevated CSF levels of symmetric dimethylarginine (SDMA), dimethylguanidino valeric acid (DMGV), and ornithine were associated with poorer outcomes after aSAH.
As machine learning techniques advance, the identification of disease biomarkers is simultaneously enhanced. Metabolomics specifically addresses the end results of cellular processes, with metabolic products sequentially following gene expression and protein translation from DNA. Given the complexity of neuro-metabolomics, this search for biomarkers amongst a broad cohort of metabolic markers is appropriate for machine learning strategies. In this study, biomarker identification has implications on prognostication; however, other biomarkers may be used to guide treatment decisions or even identify possible pharmacologic targets.
In light of the authors' results, it is reasonable to conclude that SDMA, DMGV, and ornithine are markers of poor outcome after severe aSAH. However, a critique of the study design is the patient cohort, which is made up of aSAH patients with external ventricular drains (EVDs) and non-aSAH patients undergoing elective surgery for aneurysm treatment. One major population that is excluded is good grade aSAH patients who did not require an EVD. While the results do indicate that biomarker discovery is reasonable using machine learning, it is important to note this absence of a major patient cohort and its potential implications for bias. While obtaining CSF specimen in this group may be difficult, this is certainly a space for further research in the future.
Kurt Yaeger
J. Mocco
New York, New York
REFERENCES
- 1.Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 2011;10(7):626-636. [DOI] [PubMed] [Google Scholar]
- 2.Schievink WI, Wijdicks EFM, Parisi JE, Piepgras DG, Whisnant JP. Sudden death from aneurysmal subarachnoid hemorrhage. Neurology. 1995;45(5):871-874. [DOI] [PubMed] [Google Scholar]
- 3.Springer MV, Schmidt JM, Wartenberg KE, Frontera JA, Badjatia N, Mayer SA. Predictors of global cognitive impairment 1 year after subarachnoid hemorrhage. Neurosurgery. 2009;65(6):1043-1050; discussion 1050-1041. [DOI] [PubMed] [Google Scholar]
- 4.Barker FG, Ogilvy CS. Efficacy of prophylactic nimodipine for delayed ischemic deficit after subarachnoid hemorrhage: a metaanalysis. J Neurosurg. 1996;84(3):405-414. [DOI] [PubMed] [Google Scholar]
- 5.Molyneux AJ, Kerr RS, Yu LM et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366(9488):809-817. [DOI] [PubMed] [Google Scholar]
- 6.Spetzler RF, McDougall CG, Zabramski JM et al. The Barrow Ruptured Aneurysm Trial: 6-year results. J Neurosurg. 2015;123(3):609-617. [DOI] [PubMed] [Google Scholar]
- 7.Velat GJ, Kimball MM, Mocco JD, Hoh BL. Vasospasm after aneurysmal subarachnoid hemorrhage: review of randomized controlled trials and meta-analyses in the literature. World Neurosurg. 2011;76(5):446-454. [DOI] [PubMed] [Google Scholar]
- 8.Frontera JA, Claassen J, Schmidt JM et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified Fisher scale. Neurosurgery. 2006;59(1):21-27; discussion 21-27. [DOI] [PubMed] [Google Scholar]
- 9.Vespa PM, Nuwer MR, Juhász C et al. Early detection of vasospasm after acute subarachnoid hemorrhage using continuous EEG ICU monitoring. Electroencephalogr Clin Neurophysiol. 1997;103(6):607-615. [DOI] [PubMed] [Google Scholar]
- 10.Rosenthal ES, Biswal S, Zafar SF et al. Continuous electroencephalography predicts delayed cerebral ischemia after subarachnoid hemorrhage: a prospective study of diagnostic accuracy. Ann Neurol. 2018;83(5):958-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis GD, Gerszten RE. Toward metabolomic signatures of cardiovascular disease. Circ Cardiovasc Genet. 2010;3(2):119-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis GD, Asnani A, Gerszten RE. Application of metabolomics to cardiovascular biomarker and pathway discovery. J Am Coll Cardiol. 2008;52(2):117-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stapleton CJ, Acharjee A, Irvine HJ, Wolcott ZC, Patel AB, Kimberly WT. High-throughput metabolite profiling: identification of plasma taurine as a potential biomarker of functional outcome after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2019:1-8 (doi:10.3171/2019.9.JNS191346). [DOI] [PubMed] [Google Scholar]
- 14.Vergouwen MD, Vermeulen M, van Gijn J et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41(10):2391-2395. [DOI] [PubMed] [Google Scholar]
- 15.Kimberly WT, O’Sullivan JF, Nath AK et al. Metabolite profiling identifies anandamide as a biomarker of nonalcoholic steatohepatitis. JCI Insight. 2017;2(9):e92989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunn WB, Broadhurst D, Begley P et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat Protoc. 2011;6(7):1060-1083. [DOI] [PubMed] [Google Scholar]
- 17.Bravo-Merodio L, Williams JA, Gkoutos GV, Acharjee A. -Omics biomarker identification pipeline for translational medicine. J Transl Med. 2019;17(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc Series B Stat Methodol. 2005;67(2):301-320. [Google Scholar]
- 19.Bravo-Merodio L, Acharjee A, Hazeldine J et al. Machine learning for the detection of early immunological markers as predictors of multi-organ dysfunction. Sci Data. 2019;6(1):328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trygg J, Wold S. Orthogonal projections to latent structures (O-PLS). J Chemom. 2002;16(3):119-128. [Google Scholar]
- 21.Worley B, Powers R. Multivariate analysis in metabolomics. Curr Metabolomics. 2013;1(1):92-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung CS, Oldfield EH, Harvey-White J et al. Association of an endogenous inhibitor of nitric oxide synthase with cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2007;107(5):945-950. [DOI] [PubMed] [Google Scholar]
- 23.Appel D, Seeberger M, Schwedhelm E et al. Asymmetric and symmetric dimethylarginines are markers of delayed cerebral ischemia and neurological outcome in patients with subarachnoid hemorrhage. Neurocritical Care. 2018;29(1):84-93. [DOI] [PubMed] [Google Scholar]
- 24.Brinkmann SJ, de Boer MC, Buijs N, van Leeuwen PA. Asymmetric dimethylarginine and critical illness. Curr Opin Clin Nutr Metab Care. 2014;17(1):90-97. [DOI] [PubMed] [Google Scholar]
- 25.Bode-Böger SM, Scalera F, Kielstein JT et al. Symmetrical dimethylarginine: a new combined parameter for renal function and extent of coronary artery disease. J Am Soc Nephrol. 2006;17(4):1128-1134. [DOI] [PubMed] [Google Scholar]
- 26.Kielstein JT, Salpeter SR, Bode-Boeger SM, Cooke JP, Fliser D. Symmetric dimethylarginine (SDMA) as endogenous marker of renal function—a meta-analysis. Nephrol Dial Transplant. 2006;21(9):2446-2451. [DOI] [PubMed] [Google Scholar]
- 27.Schulze F, Carter AM, Schwedhelm E et al. Symmetric dimethylarginine predicts all-cause mortality following ischemic stroke. Atherosclerosis. 2010;208(2):518-523. [DOI] [PubMed] [Google Scholar]
- 28.O’Sullivan JF, Morningstar JE, Yang Q et al. Dimethylguanidino valeric acid is a marker of liver fat and predicts diabetes. J Clin Invest. 2017;127(12):4394-4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Speer T, Rohrer L, Blyszczuk P et al. Abnormal high-density lipoprotein induces endothelial dysfunction via activation of toll-like receptor-2. Immunity. 2013;38(4):754-768. [DOI] [PubMed] [Google Scholar]
- 30.Ormstad H, Aass HCD, Lund-Sørensen N, Amthor K-F, Sandvik L. Serum levels of cytokines and C-reactive protein in acute ischemic stroke patients, and their relationship to stroke lateralization, type, and infarct volume. J Neurol. 2011;258(4):677-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rooij NKd, Rinkel GJE, Dankbaar JW, Frijns CJM. Delayed cerebral ischemia after subarachnoid hemorrhage. Stroke. 2013;44(1):43-54. [DOI] [PubMed] [Google Scholar]
- 32.Vergouwen MDI, Ilodigwe D, Macdonald RL. Cerebral infarction after subarachnoid hemorrhage contributes to poor outcome by vasospasm-dependent and -Independent effects. Stroke. 2011;42(4):924-929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.