Abstract
Purpose
To determine whether the cornea remodeling-related genes aldehyde dehydrogenase 3A1 (ALDH3A1), lysyl oxidase (LOX), and secreted protein acidic and rich in cysteine (SPARC) were potential susceptibility candidate genes for keratoconus in Korean patients, we investigated the associations of single nucleotide polymorphisms (SNPs) in these three genes in Korean patients with keratoconus.
Methods
Genomic DNA was extracted from blood samples of unrelated patients with keratoconus and healthy control individuals. For screening of genetic variations, all exons from the entire coding regions of the ALDH3A1, LOX, and SPARC genes were directly sequenced to determine the presence of mutations. Control individuals were selected from the general population without keratoconus.
Results
In this study, we detected nine SNPs in ALDH3A1, four SNPs in LOX, and 18 SNPs in SPARC. rs116992290, IVS3-62c>t, rs116962241, and rs2228100 in ALDH3A1 and rs2956540 and rs1800449 in LOX were significantly different between patient and control groups. In the SPARC gene, the distribution of the *G allele of EX10+225 T>G (p = 0.018; odds ratio, 1.869) was strongly associated with the risk of keratoconus in the Korean population. In haplotype analysis, C-G of rs2956540-rs2288393 in LOX (p = 0.046) and C-C-G and G-G-G of rs60610024-rs2228100-rs57555435 (p = 0.021 and p < 0.001), G-A of IVS3-62 a>g - rs116962241 in ALDH3A1 (p = 0.048) predisposed significantly to keratoconus. After cross-validation consistency and permutation tests, two locus model was the best SNP variations interaction pattern.
Conclusions
Our results suggested that genetic variations in ALDH3A1, LOX, and SPARC genes were associated with a predisposition for keratoconus in Korean individuals. Moreover, variations in ALDH3A1and LOX may serve as strong biomarkers for keratoconus.
Keywords: ALDH3A1, Keratoconus, LOX, Multifactor dimensional reduction, SPARC
Keratoconus is the clinical diagnosis of corneal thinning and protrusion, which results in corneal steepening, altered refractive power, and reduced vision [1]. The manifestations of keraconus include noninflammatory stromal thinning, corneal protrusion, Fleischer’s ring, Vogt’s striae, increased nerve fiber visibility, and rupture of Bowman’s layer [2]. This disease is an asymmetric, bilateral disease that starts in early adolescence and progresses over 10 to 20 years. The visual outcome varies from mild irregular astigmatism to corneal scarring requiring keratoplasty [1,3].
The pathogenesis of keratoconus is not fully understood; however, the progression of disease is known to be associated with a decrease in the biomechanical strength of the cornea, which is composed of collagen and keratocytes [4–6]. Both genetic predisposition and environmental factors, such as contact lens wearing and eye rubbing, are involved in the pathogenesis of keratoconus [1,7,8]. Histological studies have demonstrated that corneal epithelial cells, stromal keratocytes, and extracellular matrix (ECM) are affected in keratoconus corneas [9–11]. Assuming that all layers and tissues are involved in the pathogenesis of keratoconus [12–16], genes related to corneal remodeling may be potential susceptibility candidate genes in patients with keratoconus.
Therefore, in this study, we evaluated the association of single nucleotide polymorphisms (SNPs) in the aldehyde dehydrogenase 3A1 (ALDH3A1), lysyl oxidase (LOX), and secreted protein acidic and rich in cysteine (SPARC) genes in Korean patients with keratoconus.
Materials and Methods
The study sample included 220 patients with unrelated keratoconus and 150 healthy controls. Written informed consent was obtained from all participants, and study was approved by the Medical Ethics Committee of the Catholic University of Korea (KC14TISI0593). The patients were diagnosed with keratoconus based on the following criteria: (1) symptoms of keratoconus, including the Munson sign, protrusion, Vogt’s striae, corneal thickness, scarring, the Fleischer ring, photokeratoscopy, videokeratography, and refractive errors; and (2) medical history, including age, sex, contact lens use, eye rubbing behavior, systemic disease, atopy, and connective tissue disease. One hundred fifty age-matched control individuals with no history of keratoconus were also enrolled from the Korea Eye Tissue and Gene Bank related to Blindness.
Genomic DNA was extracted from peripheral blood samples using a QIAamp DNA blood kit (Qiagen, Valencia, CA, USA). Polymerase chain reaction (PCR) was performed with 25 ng of genomic DNA as a template in a mixture of PCR buffer, 2.5 mM MgCl2, 200 nM dNTPs, 0.4 pmol of each primer, and 0.75 units of h-Taq polymerase (Enzynomics, Seoul, Korea) (Table 1). For DNA sequencing, amplified DNA was purified using a QIAquick PCR purification kit (Qiagen) and sequenced directly using a BigDye Terminator cycle sequencing kit (Applied Biosystems, Foster City, CA, USA), according to the manufacturer’s instructions.
Table 1.
Primers for single nucleotide polymophisms analysis
| Primers | bp | |
|---|---|---|
| ALDH3A1_4F | cctctctcccctttctgctt | 750 |
| ALDH3A1_4R | gagagggcagctgctaagaa | |
| ALDH3A1_6F2 | cagaacccgtatctgcacct | 871 |
| ALDH3A1_6R2 | tagctcactgcagcctcaaa | |
| ALDH3A1_7F2 | ttgagaccagcctgggtaac | 994 |
| ALDH3A1_7R2 | gacccgagatctgtctccag | |
| ALDH3A1_8F | cccgagtttgtcaaggagac | 656 |
| ALDH3A1_8R | ctcattcagtgccctcaggt | |
| ALDH3A1_10F | cctatttcatggaggcctga | 761 |
| ALDH3A1_10R | AAGGGGTGGAGACTTGGAAT | |
| LOX_Exon 1-1 F1 | ccccagattaagccagtgtg | 995 |
| LOX_Exon 1-1 R1 | ACTGAGCGCAGGAACTTCTC | |
| LOX Exon 1-2 F | CCGTCACTGGTTCCAAGCTG | 336 |
| LOX Exon 1-2 R | ACGTCGAGAAGCCACATAGC | |
| LOX Exon 2 F | CCAGCTATGTGGCTTCTCGAC | 543 |
| LOX Exon 2 R | ACTTCCCAGCTCTTGTCCC | |
| LOX Exon 3 F1 | tgcatcactcacaccattga | 911 |
| LOX Exon 3 R1 | tgggcttcagattttccatt | |
| LOX Exon 4 F | ATTTGGTCTCAATTTTAATGTG | 358 |
| LOX Exon 4 R | ATGCTATTTAATGCTAACTAACGG | |
| LOX IVS 4 F-1 | tgatggcttgatgatccaaa | 964 |
| LOX IVS 4 R-1 | gggggaaccagaagtgctat | |
| LOX IVS 4 F1 | ctgctcttcccaaatcaagc | 693 |
| LOX IVS 4 R1 | tgtggcaggaacaatcgtaa | |
| LOX Exon 5 F1 | ttacgattgttcctgccaca | 936 |
| LOX Exon 5 R1 | atcaagcagggaagggattt | |
| LOX Exon 6 F | AACGTCTCCAGAGTTTAACCA | 388 |
| LOX Exon 6 R | GCATACCATTTTCTGCCTTTG | |
| SPARC 2F | ggatttctggtaggggtggt | 556 |
| SPARC 2R | accacccctaccagaaatcc | |
| SPARC 3F | cagtgtcatcccctctggat | 617 |
| SPARC 3R | gaaggtttgggaagcattca | |
| SPARC 4F | ctttccctaacacccctggt | 567 |
| SPARC 4R | cagggcaaagagctatgagg | |
| SPARC 5F | ttcaatggagacccaggaac | 679 |
| SPARC 5R | ggaacctgatggtgctgttt | |
| SPARC 6F | gactcagtcatgcctctgct | 601 |
| SPARC 6R | ttccctgatgttgaccttcc | |
| SPARC 7F | agcttcaaacacctgccagt | 637 |
| SPARC 7R | ctccaaaggcaggaagagaa | |
| SPARC 8F | cttcgccaggtgattttgat | 561 |
| SPARC 8R | tttctttgtcccaggtccac | |
| SPARC 9F | atggccatctcctcctcttt | 616 |
| SPARC 9R | gtgctaacgcttgaggaagg | |
| SPARC 10F | ggcagcgtgtgtaagagaca | 629 |
| SPARC 10R | GCCAAGACCCTGAAATGAAA |
ALDH3A1 = aldehyde dehydrogenase 3A1; LOX = lysyl oxidase; SPARC = secretory protein acidic and rich in cysteine.
In SNP selection and genotyping, we searched the public domain of the National Center for Biotechnology Information Single Nucleotide Polymorphisms database (NCBI dbSNP) at http://www.ncbi.nlm.nih.gov/snp to identify potentially functional polymorphisms in cell remodeling-related genes. Primers were designed according to the published nucleotide sequence in the ENSEMBL database using Primer3 software for LOX, ALDH3A1, and SPARC (Table 1).
To determine statistically significant differences between the groups by genotyping of SNPs, we used chi-square tests and 2 × 2 and 2 × m Fisher exact tests for the contingency table file. The 2 × 2 contingency tables for each individual allele and the 2 × m contingency tables for each locus were used, where m refers to the number of marker alleles detected in the population. Results with p-values of less than 0.05 were considered statistically significant. The strength of the association was estimated by odds ratio (OR) of risk and 95% confidence intervals (CIs) (JavaStat, http://members.aol.com/johnp71/ctab2x2.html). Haplotype frequencies and linkage disequilibrium measures were estimated using the Haploview package ver. 4.0 [17]. Haplotype frequencies and associations were calculated with Haploview ver. 4.0 (http://www.broadinstitute.org/haploview/haploview), which uses the expectation maximization algorithm. Haplotype distributions were evaluated by permutation tests on the basis of 10,000 replications to obtain empirical significance.
Potential locus-locus interactions were evaluated using nonparametric MDR software ver. 2.0 alpha (https://www.multifactordimensionalityreduction.org) with risk alleles. Briefly, the multilocus genotypes were pooled into high-risk and low-risk groups, effectively reducing the genotype predictors to one dimension. The new, one-dimensional multilocus-genotype variable was then evaluated for its ability to classify and predict disease status through cross-validation and permutation testing. A detailed explanation on the MDR method has been described elsewhere [18].
Results
The mean ages of patients with keratoconus and normal controls were 28.00 ± 7.75 and 26.83 ± 11.47 years, respectively. The percentages of men were 64.0% in patients with keratoconus and 65.8% in controls. We analyzed nine SNPs in ALDH3A1, four SNPs in LOX1, and 18 SNPs in SPARC (Table 2). Statistically significant genotype and allele frequencies of ALDH3A1, LOX, and SPARC gene variants in patients with keratoconus are listed in Table 3.
Table 2.
Observed SNPs in ALDH3A1, LOX1, and SPARC genes
| Position | Nucleotide | Amino acid | dbSNPs | |
|---|---|---|---|---|
| ALDH3A1 (9 SNPs) | Exon 4 | IVS3-193g>a | rs116992290 | |
| IVS3-170c>t | rs887240 | |||
| IVS3-62c>t | ||||
| IVS3-43g>t | rs116962241 | |||
| TCA>GCA | S134A | rs887241 | ||
| Exon 8 | IVS7-41 g>t | |||
| IVS7-29 g>a | rs60610024 | |||
| CCG>GCG | P329A | rs2228100 | ||
| Exon 10 | TAC>TAT | Y413Y | rs57555435 | |
| LOX1 (4 SNPs) | Exon 1 | CGG>CAG | R158Q | rs1800449 |
| Intron 1 | g>c | rs2288393 | ||
| Intron 4 | G>C | rs2956540 | ||
| g>a | rs10519694 | |||
| SPARC (18 SNPs) | Exon 3 | EX3+9A>G | E22E | rs2304052 |
| Intron 3 | IVS3+36 t>g | |||
| IVS3+42 t>c | ||||
| Intron 4 | IVS4+31c>t | rs1978707 | ||
| IVS4+127 a>g | ||||
| IVS4+143 g>a | ||||
| IVS4+153 g>c | ||||
| Intron 5 | IVS4-234 a>c | |||
| IVS4-228 t>c | ||||
| Exon 5 | EX5+30 G>A | G80C | ||
| Exon 8 | EX8+48 C>T | H211H | ||
| Intron 8 | IVS8+26 c>t | |||
| Intron 9 | IVS8-35 a>g | |||
| IVS8-27 g>a | ||||
| 3′ UTR | IVS9-53 c>t | |||
| EX10+58 C>G | ||||
| EX10+212 G>A | ||||
| EX10+225 T>G |
SNP = single nucleotide polymorphism; ALDH3A1 = aldehyde dehydrogenase 3A1; LOX = lysyl oxidase; SPARC = secretory protein acidic and rich in cysteine.
Table 3.
Genotype and allele frequencies of ALDH3A1, LOX, and SPARC genes variants in keratoconus patients
| Gene | Lead SNP | Genotypes/alleles | KTCN (%) | CNT (%) | p-value | OR | 95% CI |
|---|---|---|---|---|---|---|---|
| ALDH3A1 | rs116992290 | g/g | 77.6 | 93.4 | 0.007 | 0.25 | 0.075<<0.748 |
| g/a | 20.0 | 6.6 | 0.020 | 3.55 | 1.145<<11.715 | ||
| 2.4 | 0.0 | 0.500 | 18.31 | 0.204<<2526 | |||
| g | 0.876 | 0.967 | 0.003 | 0.24 | 0.077<<0.700 | ||
| a | 0.124 | 0.033 | 0.003 | 4.14 | 1.428<<12.912 | ||
| IVS3-62c>t | c/c | 1.2 | 0.9 | 0.702 | 1.55 | 0.160<<15.115 | |
| c/t | 16.5 | 8.0 | 0.028 | 2.30 | 1.003<<4.925 | ||
| t/t | 82.2 | 91.2 | 0.028 | 0.45 | 0.217<<0.931 | ||
| c | 0.095 | 0.049 | 0.069 | 1.83 | 0.947<<3.528 | ||
| t | 0.905 | 0.951 | 0.069 | 0.55 | 0.283<<1.056 | ||
| rs116962241 | g/g | 82.6 | 91.2 | 0.035 | 0.46 | 0.223<<0.959 | |
| g/t | 16.1 | 7.1 | 0.020 | 2.51 | 1.132<<5.571 | ||
| t/t | 1.2 | 1.8 | 0.779 | 0.77 | 0.127<<4.696 | ||
| g | 0.907 | 0.947 | 0.069 | 0.55 | 0.283<<1.056 | ||
| t | 0.093 | 0.053 | 0.069 | 1.83 | 0.947<<3.528 | ||
| rs2228100 | C/C | 44.8 | 28.0 | 0.002 | 2.09 | 1.297<<3.361 | |
| C/G | 49.0 | 55.1 | 0.019 | 0.56 | 0.339<<0.910 | ||
| G/G | 6.3 | 16.9 | <0.001 | 0.23 | 0.107<<0.502 | ||
| C | 0.682 | 0.555 | <0.001 | 1.81 | 1.308<<2.490 | ||
| G | 0.308 | 0.445 | <0.001 | 0.55 | 0.402<<0.765 | ||
| LOX | rs1800449 | GG | 64.0 | 59.2 | 0.375 | 1.23 | 0.781<<1.924 |
| GA | 34.7 | 33.3 | 0.879 | 0.96 | 0.601<<1.545 | ||
| AA | 1.3 | 7.5 | 0.002 | 0.16 | 0.041<<0.597 | ||
| G | 0.814 | 0.758 | 0.084 | 1.39 | 0.955<<2.024 | ||
| A | 0.186 | 0.242 | 0.084 | 0.72 | 0.494<<1.047 | ||
| rs2956540 | gg | 56.7 | 49.0 | 0.189 | 1.36 | 0.858<<12.167 | |
| gc | 39.6 | 37.3 | 0.615 | 0.88 | 0.536<<1.447 | ||
| cc | 3.7 | 13.7 | 0.001 | 0.23 | 0.094<<0.567 | ||
| g | 0.778 | 0.676 | 0.011 | 1.56 | 1.088<<2.236 | ||
| c | 0.239 | 0.324 | 0.011 | 0.63 | 0.436<<0.898 | ||
| SPARC | EX10+225 T>G | TT | 36.7 | 60.3 | 0.003 | 0.38 | 0.195<<0.746 |
| TG | 63.3 | 39.7 | 0.003 | 2.62 | 1.340<<5.140 | ||
| GG | 0.0 | 0.0 | |||||
| T | 0.683 | 0.762 | 0.018 | 0.54 | 0.313<<0.911 | ||
| G | 0.317 | 0.189 | 0.018 | 1.87 | 1.097<<3.191 |
ALDH3A1 = aldehyde dehydrogenase 3A1; LOX = lysyl oxidase; SPARC = secretory protein acidic and rich in cysteine; SNP = single nucleotide polymorphisms; KTCM = keratoconus; CNT = control; OR = odds ratio; CI = confidence interval.
Four of nine SNPs in ALDH3A1 were significantly different in the patient and control groups; for rs116992290 (IVS3-193G>a), the frequency of the *g/*g genotype was lower in patients with keratoconus (77.6%) than in the control group (93.4%; p = 0.07; OR, 0.25; 95% CI, 0.075–0.748). The frequency of the *g/*a genotype of rs116992290 was higher in patients with keratoconus (20.0%) than in normal controls (6.6%; p = 0.020; OR, 3.55; 95% CI, 1.145–11.715). The *g allele frequency at rs116992290 was lower in patients with keratoconus (87.6%) than in the control group (96.7%; p = 0.003; OR, 0.24; 95% CI, 0.077–0.700). For IVS3-62c>t, the frequency of the *t/*t genotype was lower in patients with keratoconus (82.2%) than in the control group (91.2%; p = 0.028; OR, 0.45; 95% CI, 0.217–0.931). The *t/*c genotype frequency at IVS3-62c>t was higher in patients with keratoconus (16.5%) than in controls (8.0%; p = 0.028; OR, 2.30; 95% CI, 1.003–4.925). For rs116962241 (IVS3-43g>t), the frequency of the *g/*g genotype was lower in patients with keratoconus (82.6%) than in controls (91.2%; p = 0.035; OR, 0.46; 95% CI, 0.223–0.959), and the frequency of *g/*t genotype was higher in patients with keratoconus (16.1%) than in controls (7.1%; p = 0.020; OR, 2.51; 95% CI, 1.132–5.571). The frequency of the *C/*C genotype of rs2228100 (P329A) was higher in patients with keratoconus (44.8%) than in controls (28.0%; p = 0.002; OR, 2.09; 95% CI, 1.297–3.361). The frequency of the *G/*G genotype was lower in patients with keratoconus (6.3%) than in controls (16.9%; p < 0.001; OR, 0.23; 95% CI, 0.107–0.502), and the frequency of *C/*G genotype was lower in patients with keratoconus (49.0%) than in controls (55.1%; p = 0.019; OR, 0.56; 95% CI, 0.339–0.901). Finally, the *C allele frequency of rs2228100 was higher in patients with keratoconus (68.2%) than in controls (59.2%; p < 0.001; OR, 1.81; 95% CI, 1.308–2.490).
Two of three SNPs in LOX were significantly different between keratoconus and normal controls; for rs1800449 (R158Q), the frequency of *A/*A genotype had a lower frequency in patients with keratoconus (1.3%) than in normal controls (7.5%; p = 0.002; OR, 0.16; 95% CI, 0.041–0.597). For rs2956540, the frequency of *c/*c genotype showed a lower frequency in patients with keratoconus (3.7%) than in controls (13.7%; p = 0.001; OR, 0.23; 95% CI, 0.094–0.567), and the *g allele frequency was higher in patients with keratoconus (77.8%) than in controls (60.3%; p = 0.011; OR, 1.56; 95% CI, 1.088–2.236).
One of 19 SNPs in SPARC was significantly different between patients with keratoconus and normal controls; for EX10+225 T>G, the *T/*T genotype frequency was lower in patients with keratoconus (36.7%) than in controls (60.3%; p = 0.003; OR, 0.34; 95% CI, 0.195–0.746), and the *T/*G genotype was higher in patients with keratoconus (63.3%) than in normal controls (39.7%; p = 0.003; OR, 2.62; 95% CI, 1.340–5.140). The *T allele frequency of EX10+225 T>G was lower in patients with keratoconus (68.3%) than in controls (76.2%; p = 0.018; OR, 0.54; 95% CI, 0.313–0.911).
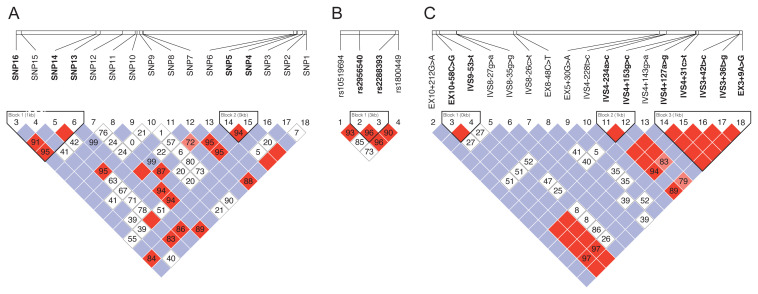
In haplotype analysis, we identified rs60610024-rs2228100-rs57555435 and IVS3-62 a>g-rs116962241 for ALDH3A1, rs2956540-rs2288393 for LOX, and EX10+58C>G-IVS9-53c>t, IVS4-234a>c-IVS4+153g>c and IVS4+127a>g-IVS4+31c>t-IVS3+42t>c-IVS3+36t>g-EX3+9A>G for SPARC (Fig. 1). The G-C (LOX H2) and G-G (LOX H3) haplotypes in LOX (rs2956540-rs2288393) were less frequent in patients with keratoconus than in controls (p = 0.360 and p = 0.058). In ALDH3A1, rs60610024-rs2228100-rs57555435 haplotype (ALDH3A1 H1: C-C-G) was more prevalent in patients with keratoconus than in the control group (p = 0.021), and the C-G-G (ALDH1A1 H2) haplotype was less frequent in patients with keratoconus than in controls (p < 0.001), The IVS3-62 a>g-rs116962241 (ALDH3A1 H5 : C-G) haplotype was more prevalent in patients with keratoconus than in the control group (p = 0.046). In SPARC, no significant results were observed among haplotypes (Table 4). Interaction between LOX, ALDH3A1, and SPARC variations in relation to the risk of keratoconus was evaluated by non-parametric MDR method. Table 5 shows the results of cross validation consistency (CVC), accuracy and OR (95% CI) obtained from MDR analysis. LOX_rs2956540/ALDH3A1_rs2228100 was the best model of SNP interaction for Keratoconus risk (CVC; 10/10, accuracy 0.634, p ≤ 0.001).
Fig. 1.
Haplotype structure of single nucleotide polymorphisms (SNPs) in (A) aldehyde dehydrogenase 3A1 (ALDH3A1), (B) lysyl oxidase (LOX), and (C) secreted protein acidic and rich in cysteine (SPARC). We estimated the pairwise linkage disequilibrium by calculating pairwise D′ and r2 (D′ > 0.70, r2 > 0.80). The images were generated with the Haploview software pack.
Table 4.
Haplotype analysis of LOX, ALDH3A1, and SPARC genes in Korean keratoconus patients
| Gene | Haplotype | Case | Control | Chi-square | p-value |
|---|---|---|---|---|---|
| LOX | rs2956540-rs2288393 | ||||
| LOX_H1 : C-G | 0.762 | 0.693 | 3.999 | 0.046 | |
| LOX_H2 : G-C | 0.133 | 0.158 | 0.838 | 0.360 | |
| LOX_H3 : G-G | 0.101 | 0.148 | 3.597 | 0.058 | |
| ALDH3A1 | rs60610024-rs2228100-rs57555435 | ||||
| ALDH3A1_H1 : C-C-G | 0.569 | 0.477 | 5.33 | 0.021 | |
| ALDH3A1_H2 : C-G-G | 0.303 | 0.441 | 13.187 | <0.001 | |
| ALDH3A1_H3 : A-C-T | 0.089 | 0.055 | 2.633 | 0.105 | |
| ALDH3A1_H4 : A-C-G | 0.031 | 0.026 | 0.144 | 0.704 | |
| IVS3-62 a>g - rs116962241 | |||||
| ALDH3A1_H5 : G-A | 0.9 | 0.945 | 3.922 | 0.048 | |
| ALDH3A1_H6 : A-C | 0.089 | 0.05 | 3.27 | 0.071 | |
| SPARC | EX10+212G>A_EX10+58C>G | ||||
| SPARC H1 : C-C | 0.651 | 0.654 | 0.003 | 0.959 | |
| SPARC H2 : G-T | 0.349 | 0.346 | 0.003 | 0.959 | |
| IVS4-234a>c_IVS4+153g>c | |||||
| SPARC H3 : C-G | 0.904 | 0.926 | 0.499 | 0.479 | |
| SPARC H14 : G-C | 0.077 | 0.074 | 0.013 | 0.908 | |
| IVS4+127a>g_IVS4+31c>t_IVS3+42t> c_IVS3+36t>g_EX3+9A>G | |||||
| SPARC H5 : A-C-T-T-A | 0.512 | 0.506 | 0.01 | 0.919 | |
| SPARC H6 : G-T-T-G-A | 0.359 | 0.327 | 0.369 | 0.544 | |
| SPARC H7 : G-T-C-T-G | 0.088 | 0.093 | 0.019 | 0.889 | |
| SPARC H8 : G-T-T-T-A | 0.035 | 0.074 | 2.433 | 0.119 |
LOX = lysyl oxidase; ALDH3A1 = aldehyde dehydrogenase 3A1; SPARC = secretory protein acidic and rich in cysteine.
Table 5.
LOX, ALDH3A1, and SPARC genes interactions with overall keratoconus risk based on MDR analysis
| Model | Bal.Acc.CV training | Bal.Acc.CV testing | CVC | p-value* | Testing OR (95% CI) |
|---|---|---|---|---|---|
| ALDH3A1_rs2228100 | 0.575 | 0.538 | 8/10 | 0.018 | 1.895 (1.113 to 3.229) |
| LOX_ rs2956540/ALDH3A1_rs2228100 | 0.634 | 0.633 | 10/10 | <0.001 | 3.164 (1.881 to 5.324) |
| LOX_rs2956540/ALDH3A1_rs116962241/ALDH3A1_rs2228100 | 0.649 | 0.581 | 5/10 | <0.001 | 3.541 (2.103 to 5.962) |
| LOX_rs1800449/ALDH3A1_rs116992290/ALDH3A1_rs3744694/ALDH3A1_rs2228100 | 0.672 | 0.658 | 9/10 | <0.001 | 4.563 (2.680 to 7.769) |
| LOX_rs1800449/LOX_rs2956540/ALDH3A1_rs116962241/ALDH3A1_rs3744694/ALDH3A1_rs2228100 | 0.684 | 0.613 | 9/10 | <0.001 | 5.054 (2.956 to 8.642) |
LOX = lysyl oxidase; ALDH3A1 = aldehyde dehydrogenase 3A1; SPARC = secretory protein acidic and rich in cysteine; CVC = cross-validation consistency; OR = odds ratio; CI = confidence interval.
1000-fold permutation test.
Discussion
Keratoconus is multifactorial disease with complex etiology, and some genetic conditions including inflammatory bowel disease, familial Mediterranean fever and Down syndrome, are known to be associated with keratoconus [19–21]. However, isolated keratoconus without any genetic association is far more frequent, and previous studies have focused on the identification of genes related to this type of keratoconus [1,2,22]. In the present study, we investigated the impact of corneal remodeling genes, including ALDH3A1, LOX, and SPARC polymorphisms, on the risk of keratoconus in a sample Korean population. LOX and SPARC are localized on chromosomes 5q23.2 and 5q31.3–q32, respectively [23,24]. Because this region shows possible linkage in familial keratoconus, both genes were assumed to be candidate genes in keratoconus.
LOX is one of the most extensively studied genes in the field of keratoconus genetic analysis [25–27]. LOX is expressed in the cornea, vitreous, iris/ciliary body, lens, choroid/retinal pigment epithelium, and retina and initiates the crosslinking of two basic components of the ECM, which includes collagens and elastin, by catalyzing oxidative deamination of the epsilon-amino group in certain lysine and hydroxylysine residues [26,28]. Moreover, LOX protein affects the assembly, tensile strength, and mechanical stability of collagen fibrils. Previous genotyping studies have confirmed the effects of the SNP rs2956540 in LOX in European, Chinese, and Iranian populations and in a meta-analysis [26,29–31]. Our study showed the rs2956540 of LOX had an exceptionally high odds ratio in patients with keratoconus and also could be a genetic biomarker in unrelated Korean patients with keratoconus.
SPARC encodes secreted protein acidic and rich in cysteine/osteonectin/BM40, a matrix-associated protein that elicits changes in cell shape, inhibits cell-cycle progression, and influences the synthesis of the ECM [25,32–34]. SPARC is found in the ECM and is predominantly expressed during embryogenesis and in adult tissues undergoing remodeling or repair. SPARC plays a role in cell-cell and cell-matrix interactions, differentiation, ECM production and organization, wound healing, and angiogenesis [34]. The ECM-related function of SPARC and the observation of a region near to LOX suggested that SPARC may have a role in the pathogenesis of keratoconus [25]. Previous findings have shown various outcomes related to SPARC in genotyping in patients with keratoconus, suggesting that such polymorphisms are rare rather than strong candidates for unrelated keratoconus-susceptibility genes [23,25,35].
ALDH3A1 encodes aldehyde dehydrogenase 3 family, member A1, which is localized on chromosome 17p11.2 [36]. ALDH3A1 is a nuclear protein expressed in the corneal epithelium and stroma that has roles in cell cycle regulation and corneal homeostasis by modulating proliferative and differentiation programs [36–38]. ALDH3A1 also has a protective effect on cells during environmental stressors, and previous studies have found that ALDH3A1 is upregulated in keratoconus corneal stroma, as identified by two-dimensional-difference gel electrophoresis. We found four SNPs in ALDH3A1 in patients with keratoconus, including rs116992290 (IVS3-193g>a), IVS3-62c>t, rs116962241 (IVS3-43g>t), and rs2228100 (P329A). In the human cornea, ALDH3A1 was found in the epithelium and stroma, but not in the endothelium [36–38]. Additionally, high expression of ALDH3A1 in mouse epithelium was altered after light exposure, suggesting that ALDH3A1 may play a role in the constantly exposed and changing cornea to maintain corneal homeostasis [36].
MDR analysis detected significant high order interactions for Keratoconus in our study. The most significant finding was the association between the LOX_rs2956540/ALDH3A1_rs2228100 and Keratoconus-locus by MDR analysis. And compared to the single SNP effect or the haplotype combined effect of LOX and ALDH3A1, a greater odds ratio for the best model with two-locus indicated that a synergistic interaction between the two SNPs was more strongly associated with keratoconus. And 4 SNPs (LOX_rs1800449/ALDH3A1_rs116992290/ALDH3A1_rs3744694/ALDH3A1_rs2228100; CVC 9/10; p < 0.001; OR, 4.563) and 5 SNPs (LOX_rs1800449/LOX_rs2956540/ALDH3A1_rs116962241/ALDH3A1_rs3744694/ALDH3A1_rs2228100; CVC 9/10; p < 0.001; OR, 5.054) including rs2956540 and rs2228100 were associated with a significantly increased risk in Keratoconus. It suggests that it will help to better understand the complex genetic basis of keratoconus. Our results all indicate that a combination of biomarkers provides a better prediction of the risk of keratoconus.
In conclusion, our study results supported that genetic variations in ALDH3A1, LOX, and SPARC genes may be associated with a predisposition for keratoconus in Koreans. Additionally, we demonstrated that rs2228100 of ALDH3A1 gene and rs2956540 in the LOX gene may serve as a genetic biomarker for keratoconus. Further investigations of ALDH3A1, LOX, and SPARC polymorphisms are needed in individuals of different ethnicities and from different countries. Also further study seems necessary to elucidate the role of genetic factors in the development of Keratoconus disease.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2012R1A1A3012219).
Footnotes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 2.Rabinowitz YS. The genetics of keratoconus. Ophthalmol Clin North Am. 2003;16:607–20. doi: 10.1016/s0896-1549(03)00099-3. [DOI] [PubMed] [Google Scholar]
- 3.Henein C, Nanavaty MA. Systematic review comparing penetrating keratoplasty and deep anterior lamellar keratoplasty for management of keratoconus. Cont Lens Anterior Eye. 2017;40:3–14. doi: 10.1016/j.clae.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Vitart V, Bencic G, Hayward C, et al. New loci associated with central cornea thickness include COL5A1, AKAP13 and AVGR8. Hum Mol Genet. 2010;19:4304–11. doi: 10.1093/hmg/ddq349. [DOI] [PubMed] [Google Scholar]
- 5.Vithana EN, Aung T, Khor CC, et al. Collagen-related genes influence the glaucoma risk factor, central corneal thickness. Hum Mol Genet. 2011;20:649–58. doi: 10.1093/hmg/ddq511. [DOI] [PubMed] [Google Scholar]
- 6.Lu Y, Dimasi DP, Hysi PG, et al. Common genetic variants near the Brittle Cornea Syndrome locus ZNF469 influence the blinding disease risk factor central corneal thickness. PLoS Genet. 2010;6:e1000947. doi: 10.1371/journal.pgen.1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bawazeer AM, Hodge WG, Lorimer B. Atopy and keratoconus: a multivariate analysis. Br J Ophthalmol. 2000;84:834–6. doi: 10.1136/bjo.84.8.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macsai MS, Varley GA, Krachmer JH. Development of keratoconus after contact lens wear. Patient characteristics. Arch Ophthalmol. 1990;108:534–8. doi: 10.1001/archopht.1990.01070060082054. [DOI] [PubMed] [Google Scholar]
- 9.Jongebloed WL, Worst JF. The keratoconus epithelium studied by SEM. Doc Ophthalmol. 1987;67:171–81. doi: 10.1007/BF00142711. [DOI] [PubMed] [Google Scholar]
- 10.Kim WJ, Rabinowitz YS, Meisler DM, Wilson SE. Keratocyte apoptosis associated with keratoconus. Exp Eye Res. 1999;69:475–81. doi: 10.1006/exer.1999.0719. [DOI] [PubMed] [Google Scholar]
- 11.Wilson SE, Kim WJ. Keratocyte apoptosis: implications on corneal wound healing, tissue organization, and disease. Invest Ophthalmol Vis Sci. 1998;39:220–6. [PubMed] [Google Scholar]
- 12.Kaldawy RM, Wagner J, Ching S, Seigel GM. Evidence of apoptotic cell death in keratoconus. Cornea. 2002;21:206–9. doi: 10.1097/00003226-200203000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Ku JY, Niederer RL, Patel DV, et al. Laser scanning in vivo confocal analysis of keratocyte density in keratoconus. Ophthalmology. 2008;115:845–50. doi: 10.1016/j.ophtha.2007.04.067. [DOI] [PubMed] [Google Scholar]
- 14.Patel S, McLaughlin JM. Effects of central corneal thickness on measurement of intra-ocular pressure in keratoconus and post-keratoplasty. Ophthalmic Physiol Opt. 1999;19:236–41. doi: 10.1046/j.1475-1313.1999.00420.x. [DOI] [PubMed] [Google Scholar]
- 15.Simo Mannion L, Tromans C, O’Donnell C. An evaluation of corneal nerve morphology and function in moderate keratoconus. Cont Lens Anterior Eye. 2005;28:185–92. doi: 10.1016/j.clae.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Kenney MC, Nesburn AB, Burgeson RE, et al. Abnormalities of the extracellular matrix in keratoconus corneas. Cornea. 1997;16:345–51. [PubMed] [Google Scholar]
- 17.Nakamura Y, Sotozono C, Kinoshita S. Inflammatory cytokines in normal human tears. Curr Eye Res. 1998;17:673–6. [PubMed] [Google Scholar]
- 18.Tuominen IS, Tervo TM, Teppo AM, et al. Human tear fluid PDGF-BB, TNF-alpha and TGF-beta1 vs corneal haze and regeneration of corneal epithelium and subbasal nerve plexus after PRK. Exp Eye Res. 2001;72:631–41. doi: 10.1006/exer.2001.0999. [DOI] [PubMed] [Google Scholar]
- 19.Trechot F, Angioi K, Latarche C, et al. Keratoconus in inflammatory bowel disease patients: a cross-sectional study. J Crohns Colitis. 2015;9:1108–12. doi: 10.1093/ecco-jcc/jjv151. [DOI] [PubMed] [Google Scholar]
- 20.Kosker M, Arslan N, Alp MY, et al. Association between keratoconus and familial mediterranean fever in Turkey. Cornea. 2016;35:77–80. doi: 10.1097/ICO.0000000000000662. [DOI] [PubMed] [Google Scholar]
- 21.Papoulidis I, Papageorgiou E, Siomou E, et al. A patient with partial trisomy 21 and 7q deletion expresses mild Down syndrome phenotype. Gene. 2014;536:441–3. doi: 10.1016/j.gene.2013.11.078. [DOI] [PubMed] [Google Scholar]
- 22.Bykhovskaya Y, Margines B, Rabinowitz YS. Genetics in Keratoconus: where are we? Eye Vis (Lond) 2016;3:16. doi: 10.1186/s40662-016-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bisceglia L, De Bonis P, Pizzicoli C, et al. Linkage analysis in keratoconus: replication of locus 5q21.2 and identification of other suggestive Loci. Invest Ophthalmol Vis Sci. 2009;50:1081–6. doi: 10.1167/iovs.08-2382. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Rabinowitz YS, Tang YG, et al. Two-stage genome-wide linkage scan in keratoconus sib pair families. Invest Ophthalmol Vis Sci. 2006;47:3791–5. doi: 10.1167/iovs.06-0214. [DOI] [PubMed] [Google Scholar]
- 25.De Bonis P, Laborante A, Pizzicoli C, et al. Mutational screening of VSX1, SPARC, SOD1, LOX, and TIMP3 in keratoconus. Mol Vis. 2011;17:2482–94. [PMC free article] [PubMed] [Google Scholar]
- 26.Dudakova L, Palos M, Jirsova K, et al. Validation of rs2956540:G>C and rs3735520:G>A association with keratoconus in a population of European descent. Eur J Hum Genet. 2015;23:1581–3. doi: 10.1038/ejhg.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bykhovskaya Y, Li X, Epifantseva I, et al. Variation in the lysyl oxidase (LOX) gene is associated with keratoconus in family-based and case-control studies. Invest Ophthalmol Vis Sci. 2012;53:4152–7. doi: 10.1167/iovs.11-9268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dudakova L, Liskova P, Trojek T, et al. Changes in lysyl oxidase (LOX) distribution and its decreased activity in keratoconus corneas. Exp Eye Res. 2012;104:74–81. doi: 10.1016/j.exer.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Hasanian-Langroudi F, Saravani R, Validad MH, et al. Association of lysyl oxidase (LOX) polymorphisms with the risk of keratoconus in an Iranian population. Ophthalmic Genet. 2015;36:309–14. doi: 10.3109/13816810.2014.881507. [DOI] [PubMed] [Google Scholar]
- 30.Hao XD, Chen P, Chen ZL, et al. Evaluating the association between keratoconus and reported genetic loci in a Han Chinese population. Ophthalmic Genet. 2015;36:132–6. doi: 10.3109/13816810.2015.1005317. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Zhang L, Hong J, et al. Association of common variants in LOX with keratoconus: a meta-analysis. PLoS One. 2015;10:e0145815. doi: 10.1371/journal.pone.0145815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lane TF, Sage EH. The biology of SPARC, a protein that modulates cell-matrix interactions. FASEB J. 1994;8:163–73. [PubMed] [Google Scholar]
- 33.Sage EH, Bornstein P. Extracellular proteins that modulate cell-matrix interactions. SPARC, tenascin, and thrombospondin. J Biol Chem. 1991;266:14831–4. [PubMed] [Google Scholar]
- 34.Bradshaw AD, Reed MJ, Sage EH. SPARC-null mice exhibit accelerated cutaneous wound closure. J Histochem Cytochem. 2002;50:1–10. doi: 10.1177/002215540205000101. [DOI] [PubMed] [Google Scholar]
- 35.Bradshaw AD, Graves DC, Motamed K, Sage EH. SPARC-null mice exhibit increased adiposity without significant differences in overall body weight. Proc Natl Acad Sci U S A. 2003;100:6045–50. doi: 10.1073/pnas.1030790100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koppaka V, Chen Y, Mehta G, et al. ALDH3A1 plays a functional role in maintenance of corneal epithelial homeostasis. PLoS One. 2016;11:e0146433. doi: 10.1371/journal.pone.0146433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pappa A, Brown D, Koutalos Y, et al. Human aldehyde dehydrogenase 3A1 inhibits proliferation and promotes survival of human corneal epithelial cells. J Biol Chem. 2005;280:27998–8006. doi: 10.1074/jbc.M503698200. [DOI] [PubMed] [Google Scholar]
- 38.Joseph R, Srivastava OP, Pfister RR. Differential epithelial and stromal protein profiles in keratoconus and normal human corneas. Exp Eye Res. 2011;92:282–98. doi: 10.1016/j.exer.2011.01.008. [DOI] [PubMed] [Google Scholar]