Abstract
Background/Aims
Growth hormone (GH) is the main regulator of somatic growth, metabolism, and gender dimorphism in the liver. GH receptor (GHR) signaling in cancer is derived from a large body of evidence, although the GHR signaling pathway involved in the prognosis of hepatocellular carcinoma (HCC) in patients with hepatitis C virus (HCV)-related HCC, remains unclear. We aimed to explore the expression of GHR and analyze its association with clinicopathologic features and prognosis of patients with chronic hepatitis C and HCC.
Methods
The expression of GHR mRNA was investigated by quantitative real-time polymerase chain reaction in paired tumors and adjacent non-tumorous (ANT) liver tissues of 200 patients with chronic hepatitis C and HCC. Western blotting and immunofluorescence assays using the HCV-infected Huh7.5.1 cell model was performed.
Results
GHR mRNA was significantly lower in HCV-HCC tissues than in corresponding ANT liver tissues. GHR mRNA and protein levels also decreased in the HCV-infected Huh7.5.1 cell model. Notably, lower GHR expression was associated with age of >60 years (P=0.0111) and worse clinicopathologic characteristics, including alpha-fetoprotein >100 ng/mL (P=0.0403), cirrhosis (P=0.0075), vascular invasion (P=0.0052), pathological stage II–IV (P=0.0002), and albumin ≤4.0 g/dL (P=0.0055), which were linked with poor prognosis of HCC. Most importantly, the high incidence of recurrence and poor survival rates in patients with a low ratio of tumor/ANT GHR (≤0.1) were observed, indicating that low expression levels of GHR had great risk for development of HCC in patients with chronic hepatitis C.
Conclusions
Our study demonstrates a significant down-regulation of GHR expression as a new unfavorable independent prognostic factor in patients with chronic hepatitis C and HCC.
Keywords: Receptors, Somatotropin; Carcinoma, Hepatocellular; Hepacivirus; Recurrence; Prognosis
Graphical Abstract
INTRODUCTION
Around 2–3% of the world population is chronically infected with hepatitis C virus (HCV), which is one of the leading causes of end-stage liver diseases such as liver cirrhosis (LC), hepatic decompensation, and hepatocellular carcinoma (HCC) [1,2]. HCC is one of the most prevalent cancers in human beings, ranking 5th worldwide and 2nd in Taiwan [3], and is also the third leading cause of cancer mortality [4].
HCV-induced HCC development is a multi-step process that could take 20–40 years, and each step in the process could be a target for the prevention of HCC [5]. Viral, host, and environmental factors have been associated with an increased risk of HCV-related HCC, including HCV genotypes, severity of liver fibrosis, genetics variants, serum levels of Major Histocompatibility Complex class I chain-related A, and alcohol intake [6-10]. Successful antiviral therapy has been reported to greatly reduce the incidence of HCC [7]; however, the risk of HCC remains in patients even after the eradication of HCV, especially in the elderly with advanced liver diseases, high baseline gamma-glutamyl transferase (rGT), and diabetes [11-13]. Furthermore, the development of HCC has been observed in mice expressing HCV transgenes in the absence of appreciable hepatic inflammation and fibrosis, suggesting that HCV infection is likely to have direct and unique cancer-promoting effects [14]. The first choice in the management of HCC in the early stage is surgical resection [15]; nevertheless, the 5-year recurrence rate remains as high as 60% even after curative resection [16]. Factors associated with HCC recurrence include tumor size [17], fibrosis [18], vascular invasion [19], satellite lesions [20], serum alpha-fetoprotein (AFP), etiologies [21], and serum albumin levels [22]. Understanding the factors and mechanisms associated with HCV-related HCC development and recurrence could provide potential targets and promising strategies to improve cancer survival [23].
Growth hormone (GH) is the main regulator of somatic growth, metabolism, and gender dimorphism in the liver [24]. The GH receptor (GHR) was first identified and characterized in the liver [25], a predominant target organ for GH [26]. GH binds its cognate receptor GHR, activating Janus kinase 2 (JAK2), which in turn phosphorylates the signal transducer and activator of transcription 5 (STAT5) and facilitates the expression of several GH downstream genes, including insulin-like growth factor-1 (IGF-1), an important effector of GH action [27,28]. The effect of the GH/IGF-1 system on cancer progression has recently been garnering much interest. GH/IGF-1 axis dysregulation enhances the synergistic effect of GH and IGF-1 on the promotion of uncontrolled cell proliferation, cell movement, and angiogenesis, and also increases neoplasia risk [29]. Importantly, disruption of GH-JAK2-STAT5-IGF-1 signaling is associated with hepatic metabolic changes and the pathogenesis of fatty liver, fibrosis, LC, and HCC [24], while STAT5 activation is correlated with the progression of human HCC [30]. In contrast, the loss of STAT5 in mice causes liver steatosis and fibrosis, and promotes chemically-induced HCC by altering the expression of cell cycle pathway regulators [31]. A recent animal study showed that hepatic GH signaling is crucial for the maintenance of lipid homeostasis, and that the impairment of this signaling causes severe metabolic liver disease predisposing to HCC [32]. In humans, low levels of circulating IGF-1 and high levels of GH, indicative of GH resistance, were commonly found in patients with hepatic fibrosis and cirrhosis [33] and have been implicated in the development of HCC [34]. However, whether the GH-STAT5-IGF-1 signaling pathway is involved in the prognosis of HCV-related HCC remains unclear.
In the present study, we firstly identified that GHR was highly down-regulated in HCC tissues in patients with chronic hepatitis C as compared to adjacent non-tumor tissues (ANT). Then, the effects of HCV infection on the GH-STAT5-IGF-1 signaling pathway were evaluated in HCV infectious clones. Finally, the prognostic role of the GH-STAT5-IGF-1 signaling pathway in a cohort of HCC patients with chronic hepatitis C who received curative resection for HCC was validated by determining the expression levels of GHR and its downstream genes in HCC tumor and ANT tissues. We innovatively revealed that there are interplays between virus and host through dysregulation of GHR and its downstream genes that were correlated to poor prognosis and low survival rate of HCC patients with chronic hepatitis C who received curative resection for HCC.
MATERIALS AND METHODS
Study population
For empirical validations, HCC tissues with paired ANT liver tissues and associated clinical information of 200 chronic hepatitis C patients who underwent surgical resection of HCC were provided by the Taiwan Liver Cancer Network (TLCN). Tumor specimens and paired ANT liver tissue were obtained immediately after surgical resection. This study was approved by the Institutional Review Board of Kaohsiung Medical University Hospital (ethic approval number: KMUHIRB-20140118) and the TLCN User Committee.
Cell culture and generation of HCVcc stocks and experimental infection
In this study, Huh7.5.1 cells were obtained from American Type Culture Collection. Huh7.5.1 cells were maintained in DMEM (Gibco) with 10% fetal bovine serum (Gibco). The cultural medium also contained penicillin (100 units/mL), streptomycin (100 μg/mL), L-glutamine (2 mmol/L), and sodium pyruvate (1 mmol/L) (Gibco) at 37°C in a humidified incubator containing 5% CO2. For the generation of HCVcc stocks, Huh7.5.1 cells were transfected with Chimeric J6/JFH genome genotype 2a HCV cDNA from Dr. Lan Keng-Hsin. Transfected cells were incubated for 6 days, and virus (HCVcc)-containing supernatants were collected. For infection, Huh7.5.1 cells were seeded at a density of 1×104 or 1×105 in 12- or 6-well dishes and infected with HCVcc at a multiplicity of infection of 0.5, respectively. At 24 hours post-infection, the HCV-infected Huh7.5.1 cells were treated with 5 pM daclatasvir (DCV) for 3 days. The supernatant was replaced with fresh media 6 hours post-infection. At 2, 3, and 4 days post-infection, cells were harvested for RNA isolation, immunostaining, and Western blotting analysis.
RNA isolation and cDNA synthesis
Total RNA extraction was performed using Quick-RNATM MiniPrep Kits for cells according to the manufacturer’s instructions (Zymo Research, Irvine, CA, USA). Total RNA was extracted from HCV-related HCC tumors and ANT tissues obtained from the tissue bank of TLCN. The cDNA was synthesized from 1 μg of total RNA incubated in a 20 μL volume reaction using the Superscript III kit for RT-PCR (Invitrogen, Life Technologies, Carlsbad, CA, USA) with random primers.
Quantitative real-time reverse transcription-PCR
The relative abundances of GHR, IGF-I, CDKN1A, 5’UTR, and NS5B mRNA in the HCV-infected Huh7.5.1 cells and the liver biosamples were detected using quantitative real-time polymerase chain reaction (RT-qPCR). The real-time PCRs were performed using the ABI PRISM 7900 system (applied Biosystems, Foster, CA, USA) and SYBR GreenERTM qPCR SuperMix (Invitrogen, Life Technologies, Carlsbad, CA, USA) with cycling conditions of 20 seconds at 95°C and 40 cycles of 1 second at 95°C and 20 seconds at 60°C. The ΔCt (threshold cycle) method was used to calculate the relative abundance of mRNA, and expressed as a tumor ΔCt/non-tumor ΔCt (T/NT) ratio in the liver bio-samples. The changes in gene expression were analyzed by the ΔΔCt method, using glyceraldehyde 3-phosphate dehydrogenase as an endogenous control. All samples were analyzed in triplicate. All primers are listed in Supplementary Table 1.
Immunofluorescence staining and confocal microscopy
For immunofluorescent labeling, the Huh7.5.1 cells were grown on glass coverslips at a density of 1×105 cells in 12-well dishes overnight. After infection, cell cultures were rinsed several times with PBS and fixed in 4% paraformaldehyde for 5 minutes, and permeabilized with 0.5% Triton X-100 in phosphate buffered saline (PBS) for 5 minutes. The fixed cells were rinsed in PBS. Nonspecific binding was blocked with 5% normal goat serum/1% bovine serum albumin in PBS pH 7.4 for at least 30 minutes at 37°C. After a brief wash, cells were incubated for 45 minutes at 37°C with the primary antibodies (e.g., anti-HCV core protein [Millipore, Temecula, CA, USA] and anti-GHR [Abcam, Cambridge, UK]). After extensive washes with PBS, the cultures were then incubated with the appropriate secondary antibody conjugated to either Alexa 488 or Alexa 568 (Molecular Probes, Waltham, MA, USA) for 45 minutes at 37°C. Finally, the slides were incubated for 5 minutes with DAPI (Molecular Probes) before mounting with Prolong Gold antifade reagent (Molecular Probes). Confocal images were obtained using a ZEISS LSM700 microscope, controlled by ZEN software (Carl Zeiss Group, Hartford, CT, USA). All images were imported to Adobe Photoshop v7.0 (Adobe Inc., San Jose, CA, USA) for contrast manipulation.
Western blotting
The cellular lysate was prepared using the radioimmunoprecipitation assay buffer, and the protein content was determined by a Bio-Rad Protein Assay system. Proteins were separated on 10% or 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto the polyvinylidene fluoride membrane. Then, the membrane was incubated with GHR (Abcam), anti-phospho Stat5 and anti-total STAT5 (cell signalling), anti-HCV core protein (Millipore), or anti-β-actin (Sigma-Aldrich, St. Louis, MO, USA). The secondary antibody used was goat anti-mouse or anti-rabbit IgG conjugated to horseradish peroxidase, and the enhanced chemiluminescence reagents (Fisher Scientific, Amersham, UK) were used for immunodetection. The chemiluminescent signal was captured by an Image Quant™ LAS 4000 mini system (GE Healthcare Life Sciences, Marlborough, MA, USA).
Statistical analysis
All categorical and continuous variables were analyzed by chi-squared test, and the group means (presented as the mean± standard deviation) were compared using analysis of variance and Student’s t-test. For continuous variables with outliers, nonparametric Mann-Whitney tests were used. The area under the curve by using receiver-operating characteristics (AUROC) was performed, and the cut-off point tumor/ANT GHR ratio was determined to best distinguish the risk of HCC recurrence of HCC in patients with chronic hepatitis C who underwent surgical resection. Kaplan-Meier analysis and log-rank test were performed by comparing the differences in the cumulative incidence of HCC among patients with tumor/ANT GHR ratio >0.1 and ≤0.1. The risk factors independently associated with HCC development were evaluated using Cox regression analysis. Statistical analyses were performed using the JMP software (version 9; SAS, Cary, NC, USA). All statistical analyses were based on two-sided hypothesis tests with a significance level of P<0.05.
RESULTS
GHR expression was down-regulated in HCV-related HCC
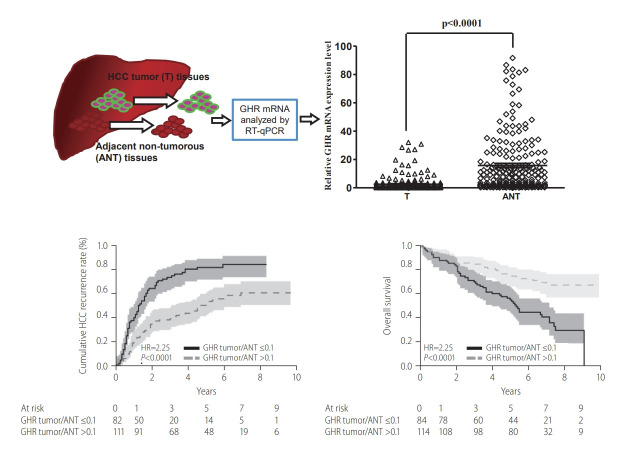
To examine the differential expressions of host genes between tumor and ANT tissues in patients with HCV-related HCC, we employed microarray analysis to reveal the gene profiles from three HCV-related HCC liver tissue samples. Our exploratory gene array data showed that there were 69 up-regulated and 165 down-regulated genes with at least a two-fold change (Supplementary Tables 2, 3, and Supplementary Fig. 1). GHR was one of the down-regulated genes in a subset of these samples. As mention above, GH-STAT5-IGF-1 signaling was associated with the pathogenesis of liver diseases and HCC [24,34]. However, the effects of HCV on GHR expression and the molecular mechanisms in HCC have not been elucidated. To further validate these findings, we first detected the expression level of GHR in 200 paired HCV-related HCC tissue specimens from the TLCN by RT-qPCR. We observed that GHR expression level decreased in 89% (178 of 200 cases) and increased in 11% of HCV-related HCC cases (22 of 200 cases). The biochemical and clinicopathological characteristics of the study population are outlined in Table 1. The relative expression of GHR mRNA in HCV-related HCC tissues was significantly lower than that in ANT tissues (2.557±5.341 vs. 15.780±20.591, P<0.0001) (Supplementary Fig. 2A, Supplementary Table 4). We further examined the IGF-1 and CDKN1A expression levels, which were downstream genes of the GH-STAT5-IGF-1 signaling pathway. Our data showed that the expression levels of IGF-1 and CDKN1A also decreased in HCV-related HCC tissues compared to ANT tissues (Supplementary Fig. 2B, C and Supplementary Table 4). These findings revealed that GHR and its downstream genes, IGF1 and CDKN1A, were significantly down-regulated in HCV-related HCC tissues.
Table 1.
Clinicopathological information of HCV-related HCC patients obtained from the TLCN
| Variable | No. of patients (n=200) |
|---|---|
| Gender | |
| Male | 128 (64.0) |
| Female | 72 (36.0) |
| Age (years) | |
| >60 | 141 (70.5) |
| ≤60 | 59 (29.5) |
| Tumor size (cm) | |
| >5 | 52 (26.0) |
| ≤5 | 148 (74.0) |
| AFP (ng/mL) | |
| >100 | 72 (36.2) |
| ≤100 | 127 (63.8) |
| HCV | |
| Genotype | 116 (58.0) |
| Non-genotype 1 | 84 (42.0) |
| History of HCV treatment | |
| Yes | 34 (17.0) |
| No | 166 (83.0) |
| Cirrhosis | |
| Absence | 94 (47.0) |
| Presence | 106 (53.0) |
| HCC | |
| Recurrence | 135 (69.9) |
| Non-recurrence | 58 (30.1) |
| Vascular invasion | |
| Absence | 105 (52.5) |
| Presence | 95 (47.5) |
| Pathological stage | |
| I | 82 (41.0) |
| II–IV | 118 (59.0) |
| AST (IU/L) | |
| >40 | 135 (67.5) |
| ≤40 | 65 (32.5) |
| ALT (IU/L) | |
| >60 | 91 (45.5) |
| ≤60 | 109 (54.5) |
| BMI (kg/m2) | |
| >25 | 64 (32.0) |
| ≤25 | 136 (68.0) |
| Albumin (g/L) | |
| >4.0 | 86 (43.0) |
| ≤4.0 | 114 (57.0) |
| HCV viral loads (×106 IU/mL) | 0.75±1.58 |
| Follow-up period (months) | 61.8±29.6 |
Values are presented as mean±standard deviation or number (%).
Pathological stage according to AJCC and UICC, 7th ed. [49]
HCV, hepatitis C virus; TLCN, Taiwan Liver Cancer Network; AFP, alphafetoprotein; HCV, hepatitis C virus; HCC, hepatocellular carcinoma; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI, body mass index.
Down-regulation of GHR in the HCV-infected Huh7.5.1 cell model
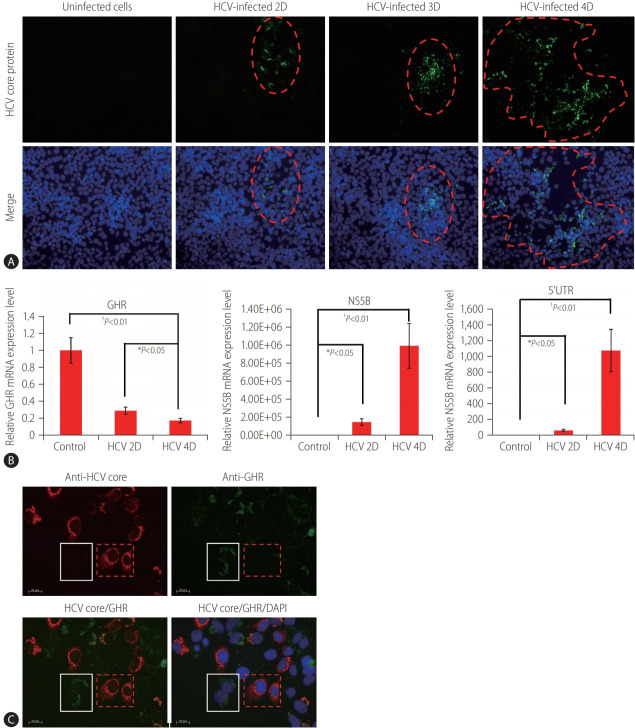
To examine whether HCV infection regulates GHR expression, we performed the cell experiments using the HCV (J6/JFH)-infected Huh7.5.1 cell model. Our data showed that the production of infectious HCV gradually increased with time. This simulated the gradual increase of infected cells over time under physiological conditions. The infected cells grossly appeared to cluster at Day 2 and Day 3, and then spread out on Day 4 (Fig. 1A). GHR expressions were measured by RT-qPCR in HCV-infected cells. The expression of HCV 5’UTR and NS5B increased concomitantly with decreased expression of GHR over time during HCV infection (Fig. 1B). Moreover, we used an antibody to detect the expression level of GHR in HCV-infected cells by immunostaining. GHR expression was evenly scattered in the cytoplasm and membrane in nonHCV-infected cells, but it was significantly decreased in HCV-infected cells (Fig. 1C). Our data suggested that GHR levels were negatively correlated with HCV viral load in the HCV-infected cells.
Figure 1.
GHR expression in the HCV-infected Huh7.5.1 cell model. (A) The efficient production of infectious HCV gradually increased at different time points (2D, 3D, and 4D), as detected by immunostaining. Red dash line ranges indicate the HCV-infected cells. Anti-HCV core protein (green) and nuclei (DAPI, blue). (B) The expressions of HCV 5’UTR, NS5B, and GHR detected at different time points (2D, 3D, and 4D) in the HCV-infected Huh7.5.1 cells by RT-qPCR. The values indicate the mean±standard deviation for three separate experiments. (C) GHR was highly decreased in HCV-infected cells (red dash line squares), and white line squares indicate non-infected cells. Anti-HCV core protein (red), anti-GHR (green), and nuclei (DAPI, blue). HCV, hepatitis C virus; 2D, Day 2; 3D, Day 3; 4D, Day 4; GHR, growth hormone receptor; NS5B, nonstructural protein 5B; 5’UTR, 5’ untranslated region; DAPI, 4’,6-diamidino-2-phenylindole; RT-qPCR, quantitative real-time polymerase chain reaction. *P<0.05. †P<0.01.
Effect of HCV on the GH-STAT5 signaling pathway in the HCV-infected Huh7.5.1 cell model
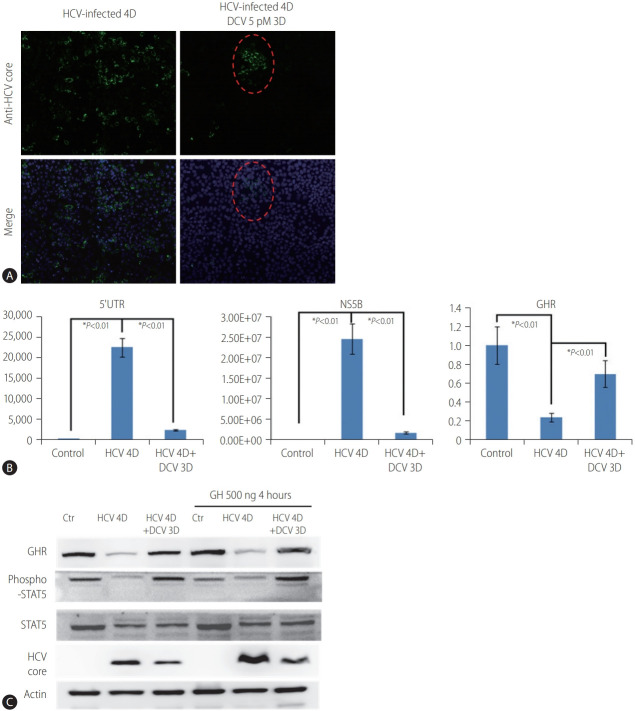
The GH binds its cognate receptor GHR, which results in the activation of the GH-STAT5 signaling pathway [24]. We further investigated whether the GHR expression and activation of the GHSTAT5 signaling pathway were regulated by HCV-infection. HCVinfected cells were treated with DCV, a potent direct-acting antiviral agent, to inhibit HCV replication. We demonstrated that HCV replication was robustly inhibited after 3-day daclatasvir treatment in HCV-infected cells (Fig. 2A, B) concomitantly with a moderate restoration of GHR expression (Fig. 2B, C; lane 2 compared to lane 3). This finding suggested that GHR is negatively regulated by HCV infection, and can be restored by inhibiting HCV replication. We further examined the status of STAT5 involved in the GH-STAT5 signaling pathway. Our data showed that the phospho-STAT5 (activated form) was moderately reduced in HCV-infected cells (Fig. 2C; lane 2 compared to lanes 1 and 3). Furthermore, we used GHs to stimulate the GH-STAT5 signaling pathway during HCV infection. The phospho-STAT5 (activated form) significantly increased in the control and daclatasvir treatment groups, but not in the HCV-infected group, upon GH administration (Fig. 2C; lanes 4 and 6 compared to lane 5). Taken together, these findings implicate that GHR was negatively regulated by HCV infection, and affected the GH-STAT5 signaling pathway in the HCV-infected Huh7.5.1 cell model.
Figure 2.
Effects of HCV on GHR expression and GH/JAK2/STAT5/IGF-1 signaling in the HCV-infected Huh7.5.1 cell model. (A) Immunostaining detected the effect of DCV on HCV replication. Twenty-four hours after HCV infection, DCV treatment continued for 3 days. Red dash line ranges indicate the HCV-infected cells. Anti-HCV core protein (green) and nuclei (DAPI, blue). (B) The expression levels of 5’UTR, NS5B, and GHR were detected on 4D in the HCV-infected Huh7.5.1 cells by RT-qPCR. The values indicate the mean±standard deviation for three separate experiments. (C) Western blot analyses for GHR, STAT5, p-STAT5, and HCV core protein in the HCV-infected Huh7.5.1 cells, respectively. β-actin was used as a loading control. The data are representative of three independent experiments. HCV, hepatitis C virus; 4D, Day 4; 3D, Day 3; DCV, daclatasvir; 5’UTR, 5’ untranslated region; NS5B, nonstructural protein 5B; GHR, growth hormone receptor; GH, growth hormone; STAT5, signal transducer and activator of transcription 5; JAK2, Janus kinase 2; IGF-1, insulin-like growth factor-1; DAPI, 4’,6-diamidino-2-phenylindole; RT-qPCR, quantitative real-time polymerase chain reaction. *P<0.01.
Correlations between GHR expression and clinical characteristics of HCC patients with chronic hepatitis C
The correlation between GHR and the clinicopathological characteristics in HCC patients with chronic hepatitis C was further evaluated by using the integer-fittest value with the AUROC curve for tumor/ANT GHR ratio to predict HCC recurrence. Our analysis excluded seven out of the 200 HCC patients who developed recurrence after receiving operations. Therefore, during the follow-up period, all samples are confirmed post-operative for recurrence. We observed that tumor/ANT GHR ratio ≤0.1 was the best cutoff value for predicting HCC recurrence after surgical resection with sensitivity, specificity, and AUROC of 49.6%, 79.0%, and 0.657, respectively. We further stratified the patients into two groups depending on the tumor/ANT GHR ratio >0.1 or ≤0.1 to evaluate the role of tumor/ANT GHR expression ratio in the clinical characteristics of HCC patients with chronic hepatitis C. Compared to the HCC patients with chronic hepatitis C with higher tumor/ANT GHR ratio (>0.1), those with lower tumor/ANT GHR ratio had significantly higher proportion of patients with age >60 years (81.2% vs. 62.6%, P=0.0051), AFP >100 ng/mL (47.1% vs. 28.1%, P=0.0047), vascular invasion (60.7% vs. 38.3%, P=0.0023), advanced pathological stage II–IV of HCC (68.2% vs. 52.2%, P=0.0167), and higher risk of HCC recurrence (85% vs. 59.8%, P=0.0002). However, lower tumor/ANT GHR ratio did not correlate with gender, tumor size, HCV genotype, history of HCV treatment, viral loads, cirrhosis status, as well as the levels of AST, ALT, albumin, and body mass index (all P>0.05, Table 2). We also observed that the tumor/ANT ratio of IGF and CDKN1A was significantly lower in patients with lower tumor/ANT GHR ratio compared to those with higher tumor/ANT GHR ratio (both P<0.0001, Table 2).
Table 2.
Clinical characteristics of HCC patients with chronic hepatitis C according to the ratio of GHR expression (tumor/ANT)
| Table | Tumor/ANT GHR >0.1 (n=115) | Tumor/ANT GHR ≤0.1 (n=85) | χ2 | P-value |
|---|---|---|---|---|
| Gender | 0.000 | 0.9928 | ||
| Male | 74 (64.3) | 54 (63.5) | ||
| Female | 41 (35.7) | 31 (36.5) | ||
| Age (years) | 7.831 | 0.0051* | ||
| >60 | 72 (62.6) | 69 (81.2) | ||
| ≤60 | 43 (37.4) | 16 (18.8) | ||
| Tumor size (cm) | 1.345 | 0.2462 | ||
| >5 | 82 (71.3) | 66 (77.6) | ||
| ≤5 | 33 (28.7) | 19 (22.4) | ||
| AFP (ng /mL) | ||||
| >100 | 32 (28.1) | 40 (47.1) | ||
| ≤100 | 82 (71.9) | 45 (52.9) | ||
| HCV | 0.781 | 0.3770 | ||
| Genotype 1 | 64 (55.7) | 52 (61.2) | ||
| Non-genotype 1 | 51 (44.3) | 33 (38.8) | ||
| History of HCV treatment | 1.767 | 0.1838 | ||
| Yes | 23 (67.7) | 11 (32.3) | ||
| No | 92 (55.4) | 74 (44.6) | ||
| Cirrhosis | 0.877 | 0.3489 | ||
| Yes | 58 (50.4) | 48 (56.5) | ||
| No | 57 (49.6) | 37 (43.5) | ||
| Vascular invasion | 9.879 | 0.0017* | ||
| Yes | 44 (38.3) | 51 (60.7) | ||
| No | 71 (61.7) | 33 (39.3) | ||
| Pathological stage I | 55 (47.8) | 27 (31.8) | 5.726 | 0.0167* |
| Pathological stage II–IV | 60 (52.2) | 58 (68.2) | ||
| Recurrence | 14.173 | 0.0002* | ||
| Yes | 67 (59.3) | 68 (85.0) | ||
| No | 46 (40.7) | 12 (15.0) | ||
| AST (IU/L) | 2.407 | 0.3001 | ||
| >40 | 76 (66.9) | 59 (70.2) | ||
| ≤40 | 37 (33.1) | 25 (29.8) | ||
| ALT (IU/L) | 0.556 | 0.4560 | ||
| >60 | 50 (43.5) | 41 (48.8) | ||
| ≤60 | 65 (56.5) | 43 (51.2) | ||
| BMI (kg/m2) | 2.081 | 0.3533 | ||
| >25 | 54 (47.3) | 22 (27.9) | ||
| ≤25 | 60 (52.7) | 59 (72.1) | ||
| Albumin (g/dL) | 2.092 | 0.3513 | ||
| >4.0 | 51 (38.9) | 32 (39.3) | ||
| ≤4.0 | 81 (61.1) | 50 (60.7) | ||
| HCV viral loads (×106 IU/mL) | 0.86±0.15 | 0.61±0.17 | 0.2820 | |
| Ratio of tumor/ANT IGF-1 | 1.17±3.22 | 0.40±0.55 | <0.0001* | |
| Ratio of tumor/ANT CDKN1A | 1.54±3.09 | 0.51±0.65 | <0.0001* |
Values are presented as mean±standard deviation or number (%).
Pathological stage according to AJCC and UICC, 7th ed. [49]
HCV, hepatitis C virus; HCC, hepatocellular carcinoma; GHR, growth hormone receptor; ANT, adjacent non-tumor tissues; AFP, alpha-fetoprotein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI, body mass index; IGF-1, insulin-like growth factor-1.
P-value <0.05 is considered significant.
Relationship between the tumor/ANT GHR expression ratio and the prognosis of HCC patients with chronic hepatitis C after surgical resection
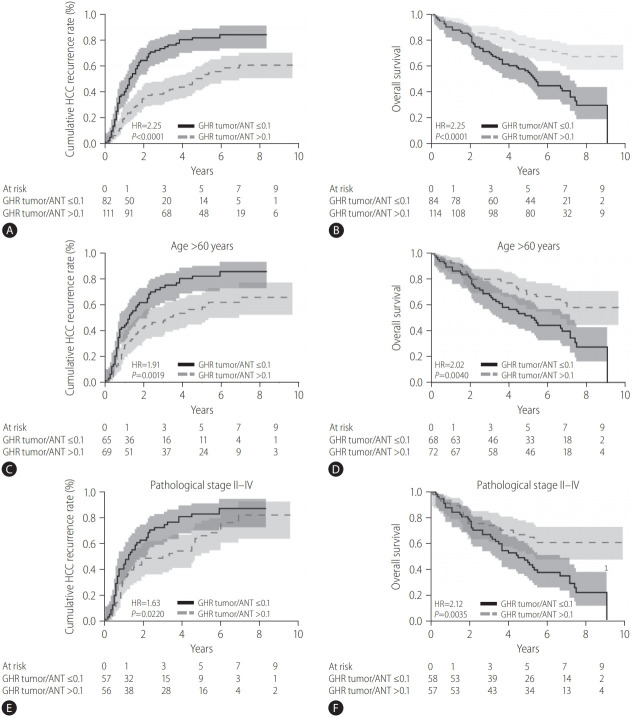
With a median follow-up period of 65.0 months (range, 1–119; total 231.6 person-years), after surgical resection, 128 (64.0%) of 200 HCC patients with chronic hepatitis C after resection experienced HCC recurrence and 86 (43%) patients had expired, with an annual incidence of HCC recurrence of 1.81% and annual death of 5.15%. The 1-, 3-, and 5-year cumulative HCC recurrence rates were 29.2%, 53.8%, and 64.3%, respectively. Using Kaplan-Meier and log-rank survival tests, we demonstrated that the 1-, 3-, and 5-year cumulative rates of HCC recurrence were 40.4%, 73.9%, and 82.1%, respectively, for patients with tumor/ANT GHR ratio ≤0.1, compared to 21.1%, 39.4%, and 51.7%, respectively, for patients with tumor/ANT GHR ratio >0.1 (hazard ratio [HR], 2.25, 95% confidence interval [CI], 1.59–3.22; P<0.001; Fig. 3A). For overall survival, the 1-, 3-, and 5-year cumulative rates of survival after HCC resection in HCC patients with chronic hepatitis C with tumor/ANT GHR ratio ≤0.1 were 88.1%, 70.2%, and 55.3%, respectively, which were significantly lower than 93.8%, 85.0%, and 75.0%, respectively, among those with tumor/ANT GHR ratio >0.1 (HR, 2.57; 95% CI, 1.67–4.02; P<0.001; Fig. 3B). We further evaluated the predictive value of GHR in HCC recurrence and overall survival in specific subgroups of HCV-related HCC patients. Among patients aged >60 years, those with a lower tumor/ANT GHR expression ratio (≤0.1) remained at significantly higher risk of HCC recurrence (P=0.0019) and mortality (P=0.004) (Fig. 3C, D). Similarly, among those with advanced pathological stages II–IV, patients with lower tumor/ANT GHR expression ratio (≤0.1) still had significantly higher rate of HCC recurrence (P=0.022) and shorter overall survival (P=0.0035) (Fig. 3E, F).
Figure 3.
The comparison of tumor recurrence and OS rates after surgery in patients with tumor/ANT GHR ratio ≤0.1 and tumor/ANT GHR ratio >0.1. (A) The recurrent rate of patients with tumor/ANT GHR ratio ≤0.1 was significantly higher than that of patients with tumor/ANT GHR ratio >0.1 (P<0.0001). (B) The OS of patients with tumor/ANT GHR ratio ≤0.1 was significantly shorter than that if patients with tumor/ANT GHR ratio >0.1 (P<0.0001). (C, D) Tumor/ANT GHR ratio ≤0.1 in HCC patients with chronic hepatitis C aged >60 years showed apparent prognostic value in predicting the higher recurrent rate and poorer OS. (E, F) Tumor/ANT GHR ratio ≤0.1 in HCC patients with chronic hepatitis C with pathological stages II–IV was significantly correlated with higher recurrence rate and shorter OS. Solid line represents patients with a tumor/ANT GHR ratio of ≤0.1. Dotted line represents patients with tumor/ANT GHR ratio >0.1. HCC, hepatocellular carcinoma; HR, hazard ratio; GHR, growth hormone receptor; ANT, adjacent non-tumor tissues; OS, overall survival; HCV, hepatitis C virus.
Factors associated with HCC recurrence and overall survival in HCC patients with chronic hepatitis C after resection
Univariate analysis
Univariate analysis by Cox Hazard model showed that lower tumor/ANT GHR ratio (≤0.1) (P= 0.0014), presence of cirrhosis (P=0.0167), and advanced pathological stage II–IV (P=0.0049) were significantly associated with shorter time to HCC recurrence after surgical resection in HCC patients with chronic hepatitis C (Table 3). For overall survival, lower tumor/ANT GHR ratio (≤0.1) (P<0.0001), age >60 years (P= 0.0075), vascular invasion (P=0.0061), and advanced pathological stage II–IV (P=0.0001) were significantly associated with worse overall survival in HCC patients with chronic hepatitis C after resection (Table 4).
Table 3.
Univariate and multivariate analyses of factors related to HCC recurrence in HCC patients with chronic hepatitis C
| Variable | Univariate |
Multivariate |
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Tumor/ANT GHR ratio, ≤0.1 vs. >0.1 | 2.68 (1.46–5.07) | 0.0014* | 2.40 (1.26–4.54) | 0.0075* |
| Gender, male vs. female | 0.75 (0.41–1.39) | 0.3708 | ||
| Age, >60 vs. ≤60 (years) | 1.63 (0.87–3.03) | 0.1256 | ||
| Tumor size, >5 vs. ≤5 (cm) | 1.32 (0.67–2.59) | 0.4258 | ||
| AFP, >100 vs. ≤100 (ng/mL) | 1.74 (0.93–3.26) | 0.0814 | ||
| HCV genotype, GT1 vs. non-GT1 | 1.53 (0.85–2.73) | 0.1564 | ||
| HCV treatment, yes vs. no | 0.66 (0.31–1.40) | 0.2811 | ||
| Cirrhosis, yes vs. no | 2.07 (1.14–3.68) | 0.0167* | 2.05 (1.11–3.78) | 0.0214* |
| Vascular invasion, yes vs. no | 1.73 (0.96–3.10) | 0.0685 | ||
| Pathological stage, II–IV vs. I | 2.37 (1.29-4.22) | 0.0049* | 2.14 (1.15–3.96) | 0.0153* |
| AST, >40 vs. ≤40 (IU/L) | 1.29 (0.70–2.38) | 0.4140 | ||
| ALT, >60 vs. ≤60 (IU/L) | 1.39 (0.78–2.50) | 0.2667 | ||
| BMI, >25 vs. ≤25 (kg/m2) | 0.56 (0.30–1.03) | 0.0611 | ||
| Albumin, >4.0 vs. ≤4.0 (g/dL) | 0.59 (0.33–1.05) | 0.0733 | ||
Pathological stage according to AJCC and UICC, 7th ed. [49]
HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HR, hazard ratio; CI, confidence interval; ANT, adjacent non-tumor tissues; AFP, alpha-fetoprotein; HCV, hepatitis C virus; GT1, genotype 1; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI, body mass index.
P-value <0.05 is considered significant.
Table 4.
Univariate and multivariate analyses of factors related to the mortality in HCC patients with chronic hepatitis C
| Variable | Univariate |
Multivariate |
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Tumor/ANT GHR ratio, ≤0.1 vs. >0.1 | 3.60 (1.99–6.50) | <0.0001* | 3.17 (1.68–5.98) | 0.0004* |
| Gender, male vs. female | 0.61 (0.34–1.09) | 0.0925 | ||
| Age, >60 vs. ≤60 (years) | 2.44 (1.27–4.68) | 0.0075* | 2.12 (1.04–4.30) | 0.0376* |
| Tumor size, >5 vs. ≤5 (cm) | 1.67 (0.88–3.16) | 0.1168 | ||
| AFP, >100 vs. ≤100 (ng/mL) | 1.36 (0.76–2.44) | 0.2954 | ||
| HCV genotype, GT1 vs. non-GT1 | 1.35 (0.76–2.38) | 0.3068 | ||
| HCV treatment, yes vs. no | 0.59 (0.43–1.21) | 0.2793 | ||
| Cirrhosis, yes vs. no | 1.38 (0.78–2.41) | 0.2668 | ||
| Vascular invasion, yes vs. no | 2.22 (1.26–3.93) | 0.0061* | 0.48 (0.17–1.39) | 0.1560 |
| Pathological stage, II–IV vs. I | 3.34 (1.82–6.14) | 0.0001* | 5.52 (1.93–15.74) | 0.0014* |
| AST, >40 vs. ≤40 (IU/L) | 1.13 (0.62–2.05) | 0.6979 | ||
| ALT, >60 vs. ≤60 (IU/L) | 1.12 (0.64–1.97) | 0.6853 | ||
| BMI, >25 vs. ≤25 (kg/m2) | 0.84 (0.46–1.54) | 0.5739 | ||
| Albumin, >4.0 vs. ≤4.0 (g/dL) | 0.34 (0.36–1.13) | 0.1200 | ||
Pathological stage according to AJCC and UICC, 7th ed. [49]
HCV, hepatitis C virus; HCC, hepatocellular carcinoma; HR, hazard ratio; CI, confidence interval; ANT, adjacent non-tumor tissues; AFP, alpha-fetoprotein; HCV, hepatitis C virus; GT1, genotype 1; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI, body mass index.
P-value <0.05 is considered significant.
Multivariate analysis
Subsequently, multivariate Cox’s proportional hazard regression analysis was performed on three or four prognostic factors that showed statistical significance for HCC recurrence and/or mortality in univariate analysis (P<0.05). Lower tumor/ANT GHR ratio (≤0.1) (HR, 2.40; 95% CI, 1.26–4.54; P=0.0075), presence of cirrhosis (HR, 2.05; 95% CI, 1.11–3.78; P=0.0214), and advanced pathological stage II–IV (HR, 2.14; 95% CI,1.15–3.96; P=0.0153) were independent risk factors for HCC recurrence after surgical resection for HCC patients with chronic hepatitis C (Table 3); while lower tumor/ANT GHR ratio (≤0.1) (HR, 3.14; 95% CI, 1.68–5.98; P=0.0004), age >60 years (HR, 2.12; 95% CI, 1.04–4.30; P=0.0376), and advanced pathological stage II–IV (HR, 5.52; 95% CI, 1.93–15.74; P=0.0014) were independent predictors of mortality (Table 4). Our data suggested that a lower tumor/ANT GHR ratio (≤0.1) was an important risk factor associated with HCC recurrence and mortality in HCV-related HCC patients after surgical resection.
DISCUSSION
In the current study, we firstly validated and revealed that the GHR expression level was significantly lower in human HCV-related HCC tumor tissues than in corresponding ANT liver tissues. We also observed that HCV infection down-regulated the mRNA and protein levels of GHR in the cell model. Notably, lower tumor/ANT GHR expression ratio was associated with old age (>60 years) and worse clinicopathologic characteristics of HCC, including high AFP levels, vascular invasion, and advanced pathological stage of HCC, which were linked with poorer prognosis in HCC. Most importantly, we found that a lower tumor/ANT GHR ratio of ≤0.1 was associated with poor prognosis of HCV-HCC patients after tumor resection, in terms of higher incidence of HCC recurrence and poorer survival.
Several studies have reported that both expressions of GHR mRNA and protein were elevated in human cancers [35-38]. ConwayCampbell et al. [36] reported that nuclear localization of GHR induces dysregulated cell cycle progression and tumorigenesis. Basu et al. [37] also demonstrated that siRNA-mediated GHR knock-down attenuated tumor proliferation, migration, and invasion in human melanoma cells. All of these studies demonstrated that GH binds to aberrantly expressed GHR and activates the JAK2 kinase, driving epithelial-mesenchymal transition and promoting tumor progression. García-Caballero et al. [39] revealed that GHR showed high expression in HCC than in the normal liver by immunohistochemistry staining. On the contrary, a lower GHR level has led to the development of GH resistance, and was associated with more advanced liver disease and fibrosis progression [40]. To our knowledge, the role of GHR in the development of HCC has never been explored in the HCV cohort.
In our study, we showed that GHR and its downstream genes, IGF-1 and CDKN1A, were significantly down-regulated in tumor tissue in HCC patients with chronic hepatitis C. More importantly, the suppression of GHR was highly associated with HCC recurrence and poorer survival rates in HCV-related HCC patients. It may be due to various reasons. Firstly, HCV infection has often several effects on inflammation, oxidative stress, insulin resistance, steatosis, and progressive fibrosis in the liver [41]. At the same time, HCV also dysregulates the cell cycle causing cell proliferation [42-44]. The multiple incidents induced by HCV infection probably modulate GHR expression. Secondly, human GHR is expressed abundantly in the liver tissue compared to other tissues. The down-regulation of GHR might lead to interference in the GH signaling pathway in the liver. Besides, our data showed that CDKN1A, one of the key cell cycle inhibitors, declined in HCV-related HCC specimens. This result suggested that the down-regulation of CDKN1A might promote cell cycle progression in HCC patients with chronic hepatitis C. Our data was consistent with a previous report that the GH-mediated STAT5 negatively regulated cell proliferation through the activation of CDKN2B and CDKN1A expressions [31]. Finally, other signaling pathways, such as the Ras/extracellular signal-regulated kinase and PI3-kinase/AKT, are also activated by GHR [45]. Dysregulation of the GH-IGF-1 axis could promote uncontrolled cell proliferation and angiogenesis, and suppress apoptosis to increase the risk of neoplasia [46]. Our findings indicated the potential significance of the GH-STAT5 signaling pathway in HCV-mediated hepatocarcinogenesis.
In the current study, we found that a lower tumor/ANT GHR expression ratio was associated with worse clinicopathologic characteristics, including older age, higher AFP levels, cirrhosis, vascular invasion, advanced stage, and lower albumin level, which are related to a poor prognosis in HCC, suggesting that GHR might be closely related to the progress of hepatocarcinogenesis. Our data indicated that the down-regulation of GHR might lead to cell cycle defects and further promote the progression of HCC related to chronic hepatitis C.
Previous studies have shown that old age was more than just an unfavorable factor for HCV outcomes [47]. This study confirmed that old age is a negative predictor of 5-year recurrence-free survival, and the overall survival of patients with low GHR expression aged ≤60 years was significantly higher than that in patients aged >60 years. This indicated that age is an important factor in post-treatment recurrence and patient survival results. Nevertheless, we observed that lower tumor/ANT GHR ratio still played an important role in poorer long-term outcomes among older HCC patients with chronic hepatitis C after surgical resection. Similar results were also observed where a lower tumor/ANT GHR ratio remained a negative predictor of long-term outcomes among HCV patients who had advanced pathological HCC stage after surgical resection.
Many studies have shown that the size and number of tumors are important factors for post-treatment recurrence and survival [16,17]. However, in our cohort, tumor size and number had no significant effect on the prognosis by multivariate analysis. This indicated that the prognosis of HCC patients with chronic hepatitis C was related to multiple factors, and comprehensive information should be evaluated. Given that GHR was strikingly down-regulated in tumor tissue in HCC related to chronic hepatitis C, which was closely associated with age and the advanced pathological stage II–IV, we suggest that GHR might serve as a novel biomarker to predict the prognosis in HCC patients with chronic hepatitis C. Taken together, our data suggest that GHR is a sensitive clinical parameter predicting the HCC recurrence and survival of HCC patients with chronic hepatitis C, and could serve as a useful prognostic molecular marker for various subgroups among HCC patients with chronic hepatitis C.
This study had some limitations. Firstly, the study design was retrospective. To confirm our findings, a prospective study with a longer follow-up period should be conducted. Secondly, our research showed that the effect of HCV infection would lead to the down-regulation of GHR, causing a decrease in IGF-1. It should be noted that IGF-1 also has a negative effect on regulating GH secretion, and patients with low IGF-1 levels might be the cause of excessive GH secretion [48]. To date, good animal model of HCV infection has not been established, and it is difficult to measure the GH levels in serum for verification. Thirdly, it is very interesting to compare the expression of GHR and its downstream pathway among patients with chronic hepatitis C, patients with HCV-related cirrhosis, and HCC patients with chronic hepatitis C. However, in this study, the HCV-related HCC tumors and ANT tissues were obtained from the patients with HCC resection, as we were not able to obtain the liver tissues from patients with chronic hepatitis C and those with HCV-related cirrhosis. Therefore, we could not check the expressions of GHR and its downstream pathway between patients with chronic hepatitis C and those with HCV-related cirrhosis. Finally, the relationship between HCV-related downregulation of GHR expression and HCC development should be further investigated.
In this study, we revealed that GHR is down-regulated in tumor tissue in HCC patients with chronic hepatitis C and HCV-infected Huh7.5.1 cell models, which might contribute to HCC recurrence and survival. A lower tumor/ANT GHR expression ratio was associated with a higher risk of HCC recurrence and mortality among HCC patients with chronic hepatitis C after surgical resection, indicating that GHR might be a promising biomarker and a potential therapeutic target for HCC patients with chronic hepatitis C.
Acknowledgments
This work was financially supported by the Ministry of Science and Technology of Taiwan (MOST 104-2314-B-037-077-MY3, MOST 107-2314-B-037-084-MY2, MOST 108-2314-B-037-066-MY3), and Kaohsiung Medical University Research Center Grant Center for Cancer Research KMU-TC108A04 -3, Center for Liquid Biopsy KMU-TC108B06 and Cohort Research Center KMU-TC108B07, KMU-DK109002, and Kaohsiung Medical University Hospital (KMUH108-8R05, KMUH108-8R07). We thank the Center for Research Resources and Development (CRRD) in Kaohsiung Medical University for the assistance in confocal image analysis. We are grateful to Taiwan Liver Cancer Network (TLCN) for providing hepatoma tissue samples and related clinical data (anonymous) for our research.
Abbreviations
- AFP
alpha-fetoprotein
- ANT
adjacent non-tumor tissues
- AUROC
area under the curve by using receiver-operating characteristics
- CI
confidence interval
- DCV
daclatasvir
- GH
growth hormone
- GHR
growth hormone receptor
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HR
hazard ratio
- IGF-1
insulinlike growth factor-1
- JAK2
Janus kinase 2
- LC
liver cirrhosis
- PBS
phosphate buffered saline
- RT-qPCR
quantitative real-time polymerase chain reaction
- STAT5
signal transducer and activator of transcription 5
- TLCN
Taiwan Liver Cancer Network
Study Highlights
• The GHR expression level was significantly lower in human HCV-related HCC tumor tissues than in corresponding ANT liver tissues.
• A lower tumor/ANT GHR ratio of <0.1 was associated with poor prognosis of patients with chronic hepatitis C and HCC after tumor resection, in terms of higher incidence of HCC recurrence and poorer survival.
• A significant down-regulation of GHR expression was shown to be a new unfavorable independent prognostic factor in patients with chronic hepatitis C and HCC.
Footnotes
Authors’ contribution
All authors contributed to the study conception and design. C-C Lin, W-L Chuang, C-Y Dai, and M-L Yu conceived and designed the study. C-C Lin, T-W Liu, C-Y Dai, M-L Yu contributed to conducting the experiments. T-W Liu, M-L Yeh, Y-S Tsai, P-C Tsai, C-F Huang, J-F Huang, C-Y Dai, W-L Chuang, M-L Yu contributed to analyzing and interpreting the data. C-C Lin drafted the manuscript. C-Y Dai, and M-L Yu critically revised the article. W-L Chuang, C-Y Dai, and M-L Yu supervised the research. All authors reviewed the results and approved the final version of the manuscript.
Conflicts of Interest: The authors have no conflicts to disclose.
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
List of primers used for real-time PCR
Differentially expressed genes in comparisons between tumor and ANT tissues in patients with HCV-related HCC down-regulated genes
Differentially expressed genes in comparisons between tumor and ANT tissues in patients with HCV-related HCC up-regulated genes
Summary of candidate genes expression level analyzed by real-time PCR in HCV-HCC patients
Differential expression profile of host genes between tumor and ANT tissues in patients with HCV-related HCC. Clustering was performed to visualize the correlations among the replicates and varying sample conditions. Up- and down-regulated genes (fold change ≥2, P<0.05) are represented in red and green colors, respectively. ANT, adjacent non-tumor tissues; HCV, hepatitis C virus; HCC, hepatocellular carcinoma.
The expression levels of GHR, IGF-1, and CDKN1A in paired T and ANT in HCC patients with chronic hepatitis C, analyzed b RT-qPCR. The mRNA levels of (A) GHR, (B) IGF-1, and (C) CDKN1A in 200 paired T and ANT in HCC patients with chronic hepatitis C were determined by RT-qPCR. GHR, growth hormone receptor; T, tumor tissues; ANT, adjacent non-tumor tissues; IGF-1, insulin-like growth factor-1; HCV, hepatitis C virus; HCC, hepatocellular carcinoma; RT-qPCR, quantitative real-time polymerase chain reaction. *P<0.0001.
REFERENCES
- 1.Global Burden Of Hepatitis C Working Group Global burden of disease (GBD) for hepatitis C. J Clin Pharmacol. 2004;44:20–29. doi: 10.1177/0091270003258669. [DOI] [PubMed] [Google Scholar]
- 2.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.Bertuccio P, Turati F, Carioli G, Rodriguez T, La Vecchia C, Malvezzi M, et al. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol. 2017;67:302–309. doi: 10.1016/j.jhep.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Hoshida Y, Fuchs BC, Bardeesy N, Baumert TF, Chung RT. Pathogenesis and prevention of hepatitis C virus-induced hepatocellular carcinoma. J Hepatol. 2014;61(1 Suppl):S79–S90. doi: 10.1016/j.jhep.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(5 Suppl 1):S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Yu ML, Lin SM, Lee CM, Dai CY, Chang WY, Chen SC, et al. A simple noninvasive index for predicting long-term outcome of chronic hepatitis C after interferon-based therapy. Hepatology. 2006;44:1086–1097. doi: 10.1002/hep.21363. [DOI] [PubMed] [Google Scholar]
- 8.Lin CW, Lin CC, Mo LR, Chang CY, Perng DS, Hsu CC, et al. Heavy alcohol consumption increases the incidence of hepatocellular carcinoma in hepatitis B virus-related cirrhosis. J Hepatol. 2013;58:730–735. doi: 10.1016/j.jhep.2012.11.045. [DOI] [PubMed] [Google Scholar]
- 9.Huang CF, Huang CY, Yeh ML, Wang SC, Chen KY, Ko YM, et al. Genetics variants and serum levels of MHC class I chain-related A in predicting hepatocellular carcinoma development in chronic hepatitis C patients post antiviral treatment. EBioMedicine. 2017;15:81–89. doi: 10.1016/j.ebiom.2016.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu MC, Yuan JM. Environmental factors and risk for hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S72–S78. doi: 10.1016/j.gastro.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Yu ML, Huang CF, Yeh ML, Tsai PC, Huang CI, Hsieh MH, et al. Time-degenerative factors and the risk of hepatocellular carcinoma after antiviral therapy among hepatitis C virus patients: a model for prioritization of treatment. Clin Cancer Res. 2017;23:1690–1697. doi: 10.1158/1078-0432.CCR-16-0921. [DOI] [PubMed] [Google Scholar]
- 12.Huang CF, Yeh ML, Tsai PC, Hsieh MH, Yang HL, Hsieh MY, et al. Baseline gamma-glutamyl transferase levels strongly correlate with hepatocellular carcinoma development in non-cirrhotic patients with successful hepatitis C virus eradication. J Hepatol. 2014;61:67–74. doi: 10.1016/j.jhep.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 13.Huang CF, Yeh ML, Huang CY, Tsai PC, Ko YM, Chen KY, et al. Pretreatment glucose status determines HCC development in HCV patients with mild liver disease after curative antiviral therapy. Medicine (Baltimore) 2016;95:e4157. doi: 10.1097/MD.0000000000004157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Branda M, Wands JR. Signal transduction cascades and hepatitis B and C related hepatocellular carcinoma. Hepatology. 2006;43:891–902. doi: 10.1002/hep.21196. [DOI] [PubMed] [Google Scholar]
- 15.Lu WP, Dong JH. Hepatectomy for hepatocellular carcinoma in the era of liver transplantation. World J Gastroenterol. 2014;20:9237–9244. doi: 10.3748/wjg.v20.i28.9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roayaie S, Obeidat K, Sposito C, Mariani L, Bhoori S, Pellegrinelli A, et al. Resection of hepatocellular cancer ≤2 cm: results from two Western centers. Hepatology. 2013;57:1426–1435. doi: 10.1002/hep.25832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai CY, Lin CY, Tsai PC, Lin PY, Yeh ML, Huang CF, et al. Impact of tumor size on the prognosis of hepatocellular carcinoma in patients who underwent liver resection. J Chin Med Assoc. 2018;81:155–163. doi: 10.1016/j.jcma.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 18.Ko S, Kanehiro H, Hisanaga M, Nagao M, Ikeda N, Nakajima Y. Liver fibrosis increases the risk of intrahepatic recurrence after hepatectomy for hepatocellular carcinoma. Br J Surg. 2002;89:57–62. doi: 10.1046/j.0007-1323.2001.01969.x. [DOI] [PubMed] [Google Scholar]
- 19.Bruix J, Sherman M, American Association for the Study of Liver Diseases Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–207. doi: 10.1016/s0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 21.Yeh ML, Huang CF, Huang CI, Hsieh MY, Hou NJ, Lin IH, et al. The prognostic factors between different viral etiologies among advanced hepatocellular carcinoma patients receiving sorafenib treatment. Kaohsiung J Med Sci. 2019;35:624–632. doi: 10.1002/kjm2.12105. [DOI] [PubMed] [Google Scholar]
- 22.Kaibori M, Matsui Y, Yanagida H, Yokoigawa N, Kwon AH, Kamiyama Y. Positive status of alpha-fetoprotein and des-gamma-carboxy prothrombin: important prognostic factor for recurrent hepatocellular carcinoma. World J Surg. 2004;28:702–707. doi: 10.1007/s00268-004-7205-y. [DOI] [PubMed] [Google Scholar]
- 23.Lin ZY, Wang JH, Yeh ML, Huang CI, Kee KM, Yen YH, et al. Primary cultures of aspiration residual specimens predict outcomes of hepatocellular carcinoma patients receiving curative treatment. Kaohsiung J Med Sci. 2020;36:750–756. doi: 10.1002/kjm2.12221. [DOI] [PubMed] [Google Scholar]
- 24.Baik M, Yu JH, Hennighausen L. Growth hormone-STAT5 regulation of growth, hepatocellular carcinoma, and liver metabolism. Ann N Y Acad Sci. 2011;1229:29–37. doi: 10.1111/j.1749-6632.2011.06100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boutin JM, Jolicoeur C, Okamura H, Gagnon J, Edery M, Shirota M, et al. Cloning and expression of the rat prolactin receptor, a member of the growth hormone/prolactin receptor gene family. Cell. 1988;53:69–77. doi: 10.1016/0092-8674(88)90488-6. [DOI] [PubMed] [Google Scholar]
- 26.Husman B, Andersson G, Norstedt G, Gustafsson JA. Characterization and subcellular distribution of somatogenic receptor in rat liver. Endocrinology. 1985;116:2605–2611. doi: 10.1210/endo-116-6-2605. [DOI] [PubMed] [Google Scholar]
- 27.Davey HW, Xie T, McLachlan MJ, Wilkins RJ, Waxman DJ, Grattan DR. STAT5b is required for GH-induced liver Igf-I gene expression. Endocrinology. 2001;142:3836–3841. doi: 10.1210/endo.142.9.8400. [DOI] [PubMed] [Google Scholar]
- 28.Waxman DJ, O’Connor C. Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol. 2006;20:2613–2629. doi: 10.1210/me.2006-0007. [DOI] [PubMed] [Google Scholar]
- 29.Dehkhoda F, Lee CMM, Medina J, Brooks AJ. The growth hormone receptor: mechanism of receptor activation, cell signaling, and physiological aspects. Front Endocrinol (Lausanne) 2018;9:35. doi: 10.3389/fendo.2018.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee TK, Man K, Poon RT, Lo CM, Yuen AP, Ng IO, et al. Signal transducers and activators of transcription 5b activation enhances hepatocellular carcinoma aggressiveness through induction of epithelialmesenchymal transition. Cancer Res. 2006;66:9948–9956. doi: 10.1158/0008-5472.CAN-06-1092. [DOI] [PubMed] [Google Scholar]
- 31.Yu JH, Zhu BM, Wickre M, Riedlinger G, Chen W, Hosui A, et al. The transcription factors signal transducer and activator of transcription 5A (STAT5A) and STAT5B negatively regulate cell proliferation through the activation of cyclin-dependent kinase inhibitor 2b (Cdkn2b) and Cdkn1a expression. Hepatology. 2010;52:1808–1818. doi: 10.1002/hep.23882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mueller KM, Kornfeld JW, Friedbichler K, Blaas L, Egger G, Esterbauer H, et al. Impairment of hepatic growth hormone and glucocorticoid receptor signaling causes steatosis and hepatocellular carcinoma in mice. Hepatology. 2011;54:1398–1409. doi: 10.1002/hep.24509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donaghy A, Ross R, Gimson A, Hughes SC, Holly J, Williams R. Growth hormone, insulinlike growth factor-1, and insulinlike growth factor binding proteins 1 and 3 in chronic liver disease. Hepatology. 1995;21:680–688. [PubMed] [Google Scholar]
- 34.Chang TC, Lin JJ, Yu SC, Chang TJ. Absence of growth-hormone receptor in hepatocellular carcinoma and cirrhotic liver. Hepatology. 1990;11:123–126. doi: 10.1002/hep.1840110121. [DOI] [PubMed] [Google Scholar]
- 35.Brunet-Dunand SE, Vouyovitch C, Araneda S, Pandey V, Vidal LJ, Print C, et al. Autocrine human growth hormone promotes tumor angiogenesis in mammary carcinoma. Endocrinology. 2009;150:1341–1352. doi: 10.1210/en.2008-0608. [DOI] [PubMed] [Google Scholar]
- 36.Conway-Campbell BL, Wooh JW, Brooks AJ, Gordon D, Brown RJ, Lichanska AM, et al. Nuclear targeting of the growth hormone receptor results in dysregulation of cell proliferation and tumorigenesis. Proc Natl Acad Sci U S A. 2007;104:13331–13336. doi: 10.1073/pnas.0600181104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basu R, Wu S, Kopchick JJ. Targeting growth hormone receptor in human melanoma cells attenuates tumor progression and epithelial mesenchymal transition via suppression of multiple oncogenic pathways. Oncotarget. 2017;8:21579–21598. doi: 10.18632/oncotarget.15375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subramani R, Lopez-Valdez R, Salcido A, Boopalan T, Arumugam A, Nandy S, et al. Growth hormone receptor inhibition decreases the growth and metastasis of pancreatic ductal adenocarcinoma. Exp Mol Med. 2014;46:e117. doi: 10.1038/emm.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.García-Caballero T, Mertani HM, Lambert A, Gallego R, Fraga M, Pintos E, et al. Increased expression of growth hormone and prolactin receptors in hepatocellular carcinomas. Endocrine. 2000;12:265–271. doi: 10.1385/ENDO:12:3:265. [DOI] [PubMed] [Google Scholar]
- 40.Carotti S, Guarino MPL, Valentini F, Porzio S, Vespasiani-Gentilucci U, Perrone G, et al. Impairment of GH/IGF-1 axis in the liver of patients with HCV-related chronic hepatitis. Horm Metab Res. 2018;50:145–151. doi: 10.1055/s-0043-118911. [DOI] [PubMed] [Google Scholar]
- 41.Goossens N, Hoshida Y. Hepatitis C virus-induced hepatocellular carcinoma. Clin Mol Hepatol. 2015;21:105–114. doi: 10.3350/cmh.2015.21.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen H, Mudryj M, Guadalupe M, Dandekar S. Hepatitis C virus core protein expression leads to biphasic regulation of the p21 cdk inhibitor and modulation of hepatocyte cell cycle. Virology. 2003;312:245–253. doi: 10.1016/s0042-6822(03)00209-5. [DOI] [PubMed] [Google Scholar]
- 43.Hassan M, Ghozlan H, Abdel-Kader O. Activation of RB/E2F signaling pathway is required for the modulation of hepatitis C virus core protein-induced cell growth in liver and non-liver cells. Cell Signal. 2004;16:1375–1385. doi: 10.1016/j.cellsig.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Massagué J. G1 cell-cycle control and cancer. Nature. 2004;432:298–306. doi: 10.1038/nature03094. [DOI] [PubMed] [Google Scholar]
- 45.Brooks AJ, Waters MJ. The growth hormone receptor: mechanism of activation and clinical implications. Nat Rev Endocrinol. 2010;6:515–525. doi: 10.1038/nrendo.2010.123. [DOI] [PubMed] [Google Scholar]
- 46.Chhabra Y, Waters MJ, Brooks AJ. Role of the growth hormoneIGF-1 axis in cancer. Expert Rev Endocrinol Metab. 2011;6:71–84. doi: 10.1586/eem.10.73. [DOI] [PubMed] [Google Scholar]
- 47.Huang JF, Yeh ML, Yu ML, Dai CY, Huang CF, Huang CI, et al. The tertiary prevention of hepatocellular carcinoma in chronic hepatitis C patients. J Gastroenterol Hepatol. 2015;30:1768–1774. doi: 10.1111/jgh.13012. [DOI] [PubMed] [Google Scholar]
- 48.Chishima S, Kogiso T, Matsushita N, Hashimoto E, Tokushige K. The relationship between the growth hormone/insulin-like growth factor system and the histological features of nonalcoholic fatty liver disease. Intern Med. 2017;56:473–480. doi: 10.2169/internalmedicine.56.7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wittekind C, Bertolini J. TNM-System 2010. Onkologe. 2010;16:175–180. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of primers used for real-time PCR
Differentially expressed genes in comparisons between tumor and ANT tissues in patients with HCV-related HCC down-regulated genes
Differentially expressed genes in comparisons between tumor and ANT tissues in patients with HCV-related HCC up-regulated genes
Summary of candidate genes expression level analyzed by real-time PCR in HCV-HCC patients
Differential expression profile of host genes between tumor and ANT tissues in patients with HCV-related HCC. Clustering was performed to visualize the correlations among the replicates and varying sample conditions. Up- and down-regulated genes (fold change ≥2, P<0.05) are represented in red and green colors, respectively. ANT, adjacent non-tumor tissues; HCV, hepatitis C virus; HCC, hepatocellular carcinoma.
The expression levels of GHR, IGF-1, and CDKN1A in paired T and ANT in HCC patients with chronic hepatitis C, analyzed b RT-qPCR. The mRNA levels of (A) GHR, (B) IGF-1, and (C) CDKN1A in 200 paired T and ANT in HCC patients with chronic hepatitis C were determined by RT-qPCR. GHR, growth hormone receptor; T, tumor tissues; ANT, adjacent non-tumor tissues; IGF-1, insulin-like growth factor-1; HCV, hepatitis C virus; HCC, hepatocellular carcinoma; RT-qPCR, quantitative real-time polymerase chain reaction. *P<0.0001.