Abstract
Background
The presence of coronaviruses on surfaces in the patient environment is a potential source of indirect transmission. Manual cleaning and disinfection measures do not always achieve sufficient removal of surface contamination. This increases the importance of automated solutions in the context of final disinfection of rooms in the hospital setting. Ozone is a highly effective disinfectant which, combined with high humidity, is an effective agent against respiratory viruses. Current devices allow continuous nebulization for high room humidity as well as ozone production without any consumables.
Aim
In the following study, the effectiveness of a fully automatic room decontamination system based on ozone was tested against bacteriophage Φ6 (phi 6) and bovine coronavirus L9, as surrogate viruses for the pandemic coronavirus SARS-CoV-2.
Methods
For this purpose, various surfaces (ceramic tile, stainless steel surface and furniture board) were soiled with the surrogate viruses and placed at two different levels in a gas-tight test room. After using the automatic decontamination device according to the manufacturer's instructions, the surrogate viruses were recovered from the surfaces and examined by quantitative cultures. Then, reduction factors were calculated.
Findings
The ozone-based room decontamination device achieved virucidal efficacy (reduction factor >4 log10) against both surrogate organisms regardless of the different surfaces and positions confirming a high activity under the used conditions.
Conclusion
Ozone is highly active against SARS-CoV-2 surrogate organisms. Further investigations are necessary for a safe application and efficacy in practice as well as integration into routine processes.
Keywords: SARS-CoV-2, Bovine coronavirus, Bacteriophage Phi 6, Surrogate virus, Automated room disinfection, Ozone
Introduction
The spread of viruses with pandemic potential due to indirect contact transmission is controversial. Even in the current pandemic situation of Covid-19, the persistence of SARS-CoV-2 on inanimate surfaces and the role of contaminated surfaces as transmission pathway is not clear. A current study showed stability of SARS-CoV-2 on different surface material (copper, cardboard, stainless steel and plastic) for 8–72 h under experimental conditions [1]. Therefore, touching contaminated surfaces might be a potential source of viral transmission [2]. Recent studies conducted in China and Hong Kong during the SARS-CoV-2 pandemic showed viral RNA in the patient environment [3,4]. It therefore seems rational to reduce the microbial load by disinfection. This assumption was supported by investigations that revealed contamination with viral RNA on surfaces even after final cleaning and disinfection of a patient room [5,6]. In addition, several studies demonstrated that environmental cleaning in hospitals is frequently lacking. It has been shown that less than 50% [7] respectively averagely 57% [8] of surfaces were cleaned adequately following patient discharge.
To improve this problem and prevent environment-borne transmission, the usage of automated room disinfection systems could be an additional method of disinfection in hospital settings [5]. Currently aerosolized and vapourized hydrogen peroxide, ozone, chlorine dioxide and ultraviolet radiation are mechanisms used for room decontamination after the discharge of patients [9,10].
Ozone is not a common reagent, because of the need for permanent moisture to achieve effectiveness [11]. Consequently, only a few studies reported using ozone for room decontamination in general but not yet in the hospital setting [10,12,13]. In a current study, Dubuis et al. showed that ozone combined with high relative humidity is an effective disinfectant for respiratory viruses [14]. Because of recent technologies which enable ozone to be generated from atmospheric oxygen in combination with an integrated nebulizer for controlled increase of room humidity, the aim of this study was to evaluate the effectiveness of an automatic room disinfection unit based on ozone combined with high relative humidity against SARS-CoV-2 surrogates.
As a consequence of biosafety concerns and high demands for working with SARS-CoV-2, surrogate viruses were used in this study. Bacteriophages are known as suitable surrogates for human respiratory viruses owing to great similarities in size, shape, surface properties and environmental persistence, however they are non-pathogenic to humans [15]. Due to its lipid envelope, bacteriophage Φ6 (phi 6) from the family of the Cystoviridae has been suggested as a surrogate for coronaviruses [[16], [17], [18], [19]].
Coronaviruses form a large and pleomorphic family that is further divided into groups based on serological findings and phylogenetic analysis [[20], [21], [22]]. The bovine coronavirus (BCoV) from the genus Betacoronavirus is genetically closely related to SARS-CoV, MERS-CoV and the pandemic SARS-CoV-2 viruses and can be handled outside a BSL-3 safety laboratory. Therefore, we used the BCoV and Φ6 as surrogate organisms for the present experiments.
Methods
To evaluate the efficacy of an ozone-based device for automated room disinfection (STERISAFE™ Pro version 1.0, STERISAFE ApS, Ole Maaløe's vej 5, DK – 2200 Copenhagen, Denmark), carriers contaminated with two different surrogate viruses of SARS-CoV-2 were decontaminated in a 6 m³ gas-tight test room furnished with a shelf.
Surrogate virus bacteriophage Φ6 (DSM 21518) and the bacterial host strain Pseudomonas syringae pv. Syringae (DSM 21482) were purchased from Leibniz-Institute DSMZ – Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (Braunschweig, Germany). Initial lysate of bacteriophage Φ6 with a titre of 4 × 1011 plaque forming units (pfu)/mL was produced using a top agar overlay technique as described by the manufacturer. Then, 20 μL of a 1:10 dilution was struck out and dried on ceramic tiles (5 × 5 cm, #3709PN00, Villeroy & Boch, Mettlach, Germany), stainless steel carriers (#0344818, Modulor GmbH, Berlin, Germany) and furniture boards (melamine-coated solid core panels). After each experiment Φ6 from both treated and untreated carriers was recovered by rinsing the surfaces with 1 mL tryptic soy broth (TSB) + 5 mM CaCl2 medium 15 times. A quantitative plaque assay was performed using top agar overlay with tryptic soy agar (TSA) + 5 mM CaCl2 culture media after ten-fold serial dilution (detection limit: <10 pfu/mL). Plates were incubated at 23°C for 24 h.
In the same way, further carriers were contaminated with 50 μL of virus inoculum of BCoV strain L9. BCoV L9 and the host U373 cells (passage 8) were obtained from G. Zimmer, Institute of Virology, School of Veterinary Medicine, Hannover, Germany. For preparation of test virus solution, a monolayer of U373 cells were infected with BCoV L9. After an incubation period of 24–48 h, cells were lysed by a rapid freeze/thaw cycle. Cellular debris was removed, and the supernatant was mixed with bovine serum albumin (BSA) (final concentration: 0.3 g/L BSA). After each experiment an endpoint dilution assay was performed. Therefore, the treated and untreated carriers were rinsed with 1 mL medium without fetal calf serum (FCS). Remaining infectivity was determined by transferring 0.1 mL of appropriate 10-fold serial dilutions into eight wells of a microtitre plate with a preformed monolayer of U373 cells (10–15 × 103 cells per well), beginning with the highest dilution. Before addition of virus, cells were washed twice with Eagle's minimum essential medium (EMEM) and incubated for 3 h with 100 μL EMEM with trypsin. Microtitre plates were incubated at 37°C in a 5% CO2-atmosphere. The cytopathic effect was read using an inverted microscope after five days and the infective dose TCID50/mL was calculated.
For the decontamination experiments, contaminated carriers were placed horizontally at two different heights on the shelf to represent the efficacy at high and low room levels. Three prepared carriers of each material and surrogate virus were positioned at the high (1.69 m) and two at the low (0.07 m) positions. For both surrogate organisms in each experiment, two contaminated control carriers were placed in a room without treatment. For bacteriophage Φ6, additional control experiments at 90% relative humidity and 22°C were performed in a climate chamber.
The disinfection process using the STERISAFE™ Pro system was investigated in two independent experiments for each organism. According to the manufacturer's instructions, the decontamination time was 60 min with a target ozone concentration of 80 ppm and a target relative humidity of 90% generated with the integrated humidifier and ozone generator [23,24]. Ozone concentration and relative humidity were continuously measured by integrated instruments and displayed on a mobile tablet computer outside of the room, as well as being recorded by the instrument (Supplementary Figure S1) [24]. After completion of the disinfection process, the ozone was converted back into pure oxygen (Supplementary Figure S1) and by-products were removed in an air-purification phase. When the process was displayed as finished on the tablet computer, the room could be entered again immediately [24]. The ozone concentration in the treated room then complied to usual limit values of 0.1 ppm (exposure limit for 8 h per day carrying out light work) set by Occupational Safety and Health Administration (OSHA) or The National Institute for Occupational Safety and Health (NIOSH) [25]. Both surrogate viruses were investigated together in two independent experiments and reduction factors were calculated by subtracting log10 of untreated and treated samples. As defined elsewhere, virucidal efficacy was suggested if the mean reduction factor was >4 log10 [26].
Results
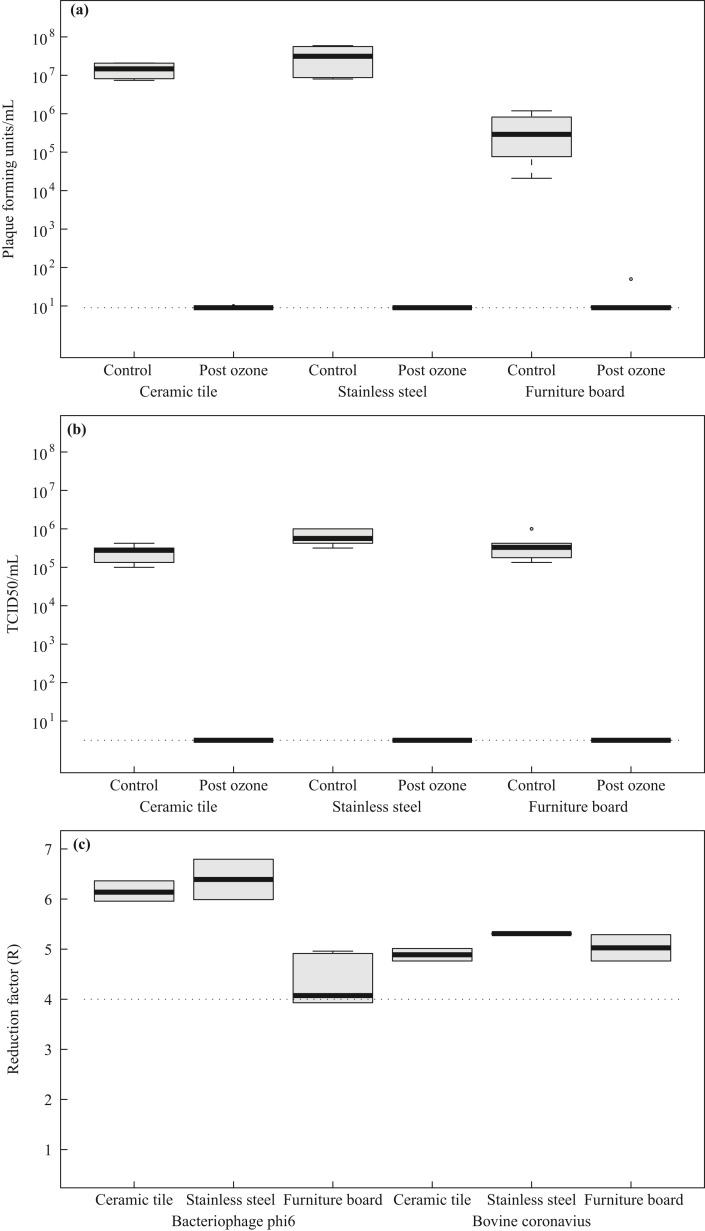
The aim of the present study was to evaluate the virus-inactivating properties of ozone in the presence of high relative humidity against surrogate BCoV and bacteriophage Φ6 in a setting of room disinfection. Initial desiccation of bacteriophage Φ6 resulted in mean concentrations of 1.4 × 107, 3.2 × 107 and 4.5 × 105 pfu/mL on ceramic tiles, stainless steel and furniture board, respectively. Initial desiccation of BCoV resulted in mean concentrations of 2.5 × 105, 4.0 × 105, and 6.4 × 105 TCID50/mL on ceramic tiles, stainless steel and furniture board, respectively. The stability of both surrogate organisms in the desiccation phase allowed further investigations to determine virucidal activity.
After the decontamination process with STERISAFE™ Pro, independent of the carrier material used or the room height, no plaque forming units of bacteriophage Φ6 could be recovered from the surfaces (Figure 1 a). The STERISAFE™ Pro achieved mean log10 reduction factors of 6.15 on ceramic tiles, 4.29 on furniture board and 5.31 on stainless steel surfaces for the surrogate virus bacteriophage Φ6 (Figure 1c). Control experiments with high humidity without additional ozone as disinfectant revealed a minor decrease of viral activity (Supplementary Figure S2), indicating that the observed virucidal activity can only be reached by a combination of ozone and humidity.
Figure 1.
Microbial load of bacteriophage Φ6 (a) and bovine coronavirus (CoV) (b) on different surfaces before and post ozone decontamination and comparison of the reduction factors achieved (c). The boxplots represent the variation of contamination with bacteriophage Φ6 (plaque forming units/mL) on ceramic tile, stainless steel and furniture board examined before and after automated room decontamination (a). The control boxplots result from four samples of each material, whereas post ozone boxplots include 10 values per material. Likewise, variation of viral load on surfaces contaminated with bovine CoV (TCID50/mL) were determined (b). The boxplots result from six (control) and 10 (post ozone) samples for each surface material. All results were calculated from two independent experiments. The dashed lines (a, b) display the detection limits resulting from the method used. Moreover, reduction factor (R) of bacteriophage Φ6 and bovine CoV determined for different surfaces is displayed (c). The dashed line (c) represents the log10 reduction factor of four, which means virucidal effectiveness.
For BCoV, post ozone application, no residual virus could be detected independent of the carrier material used or the position in the room (corresponding to 3.16 TCID50/mL) (Figure 1b). For the BCoV, mean log10 reduction factors of 4.88 on ceramic tiles, 5.03 on furniture board and 5.31 on stainless steel surfaces could be determined (Figure 1c). STERISAFE™ Pro showed virucidal efficacy (reduction factor >4 log10) for both surrogate organisms on all investigated surfaces.
Discussion
Previous studies have shown the distribution and transmission of nosocomial pathogens due to surface contamination [11,27]. A common reason seems to be inadequate manual cleaning and disinfection, which fail to remove surface bioburden [9,11,27]. To improve the effectiveness of surface disinfection and to increase patient and occupational safety, automated room disinfection systems could be a useful method. Based on previous studies showing the efficacy of ozone against respiratory viruses, the aim of the present study was to test the efficacy of an ozone-based automatic room decontamination device against surrogate viruses of the pandemic coronavirus SARS-CoV-2 [14].
The present results indicate a virucidal effectiveness (reduction factor >4 log10) of ozone in combination with high relative humidity for both tested surrogate viruses (bacteriophage Φ6 and BCoV), independent from the surface material. The virucidal effect could be detected at different levels in the test room. Therefore, a distribution of ozone and humidity can be assumed as sufficient for successful decontamination. Interestingly, on the furniture board, for bacteriophage Φ6, the calculated extent of the reduction was lower than on the other materials tested. Differences in the reduction of bacteriophage Φ6 mainly are due to reduced recovery of phages after initial contamination of control surfaces, which probably results from random fluctuation or specific surface conditions.
Recent studies have already shown that surface stability and survival time of SARS-CoV-2 was influenced by environmental conditions in particular temperature and relative humidity [[28], [29], [30]]. Higher humidity and temperature decrease virus survival time on surfaces [28]. However, for bacteriophage Φ6 we observed only a low decrease of viral activity under humid conditions without the application of ozone. Therefore, it can be assumed that only the combination of ozone with high relative humidity achieves full virucidal efficacy.
Because bacteriophage Φ6 is a small-enveloped virus, it shares similarities with coronavirus. However, it is considered to be more stable than coronavirus because it has a double-stranded RNA genome [31]. In contrast, BCoV belongs to the same family (Coronaviridae) and the same genus Betacoronavirus and subgenus Sarbecovirus as SARS-CoV-2. Both viruses are likely to have similar properties and can be considered as surrogate viruses for SARS-CoV-2. Therefore, it is assumed that ozone is also effective against SARS-CoV-2. This assumption is also supported by current literature reviews and initial results from laboratory experiments that were able to show an efficacy of ozone against SARS-CoV-2 [[32], [33], [34]].
The tested ozone room disinfection system represents a safe and useful additional disinfection method that can be implemented after the discharge of patients infected with contagious and environmentally resistant pathogens such as SARS-CoV-2. However, due to toxicity of ozone, doors and ventilation diffusers must be strictly sealed to prevent unintentional dissemination [24], resulting in an additional workload for the operating person. Additionally, due to the generated water aerosol, smoke detectors must also be covered to avoid unwanted alarms. During the disinfection cycle, a concept is needed to prevent unauthorized room entrance during the disinfection process.
Our study has several limitations. Firstly, clean conditions were used for the experiments on solid surfaces. It has been demonstrated that organic loading could have an inhibitory effect on the efficacy of disinfection methods [35,36]. Further experiments using test soiling for dirty conditions (bovine albumin 3.0 g/L + sheep erythrocytes 3 mL/L [26]) as well as experiments with absorbent items need to be performed to evaluate the virucidal effect for applications where insufficient cleaning prior the disinfection process is expected. Secondly, the experiments were conducted in a small room with a simple room structure and only a few furnishings. However, in a recent study, effectiveness against environmental resistant Enterococcus faecium was analysed within complex room conditions. A position-independent bactericidal effectiveness could be shown, confirming a sufficient distribution of ozone and humidity even in a furnished room with anteroom and bathroom [37]. Furthermore, in order to achieve conditions that are as close to reality as possible, we did not use standardized but realistic room conditions for the untreated control panels that prevailed at the time of the test. Spontaneous reductions that could be caused by temperature and humidity fluctuations will therefore not be excluded and assessed. Finally, before the general implementation of such an ozone-generating device can be recommended, further studies are needed to ensure the safe operation in the hospital environment. The oxidizing properties of ozone can lead to damage of many materials and thus to a shortening of the life cycle of products [38]. Elastomers and surface coatings in particular can be damaged [34]. The compatibility of ozone in connection with electronic medical devices should be clarified with the manufacturers, as is the case for all airborne disinfection processes. Thus, further experiments are necessary to ensure compatibility with common furnishing and medical device materials in hospitals [11,39]. To verify safety operation and efficacy, logging of process data independent from disinfection device should be recommended for practical application.
In summary, we found that ozone in combination with high humidity as generated by an automated room decontamination system has a high activity against SARS-CoV-2 surrogate viruses bacteriophage Φ6 and BCoV on different solid surfaces in the hospital environment, confirming the process as a virucidal disinfection. Future work is needed to study compatibility with different surface materials to ensure safe operation of automated room decontamination in the hospital setting.
Acknowledgements
We thank Tatjana Kostenko for excellent technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhin.2021.04.007.
Conflict of interest statement
B.K. and J.K. received travel grants from Infuser Deutschland GmbH, Mannheim, Germany.
All other authors have no conflicts of interest to declare.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fathizadeh H., Maroufi P., Momen-Heravi M., Dao S., Köse Ş., Ganbarov K. Protection and disinfection policies against SARS-CoV-2 (COVID-19) Infez Med. 2020;28(2):185–191. [PubMed] [Google Scholar]
- 3.Cheng V.C.C., Wong S.-C., Chen J.H.K., Yip C.C.Y., Chuang V.W.M., Tsang O.T.Y. Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong. Infect Control Hosp Epidemiol. 2020;41(5):493–498. doi: 10.1017/ice.2020.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otter J.A., Donskey C., Yezli S., Douthwaite S., Goldenberg S.D., Weber D.J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92(3):235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macias A.E., La Torre A de, Moreno-Espinosa S., Leal P.E., Bourlon M.T., Ruiz-Palacios G.M. Controlling the novel A (H1N1) influenza virus: don’t touch your face! J Hosp Infect. 2009;73(3):280–281. doi: 10.1016/j.jhin.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 7.Carling P.C., Parry M.F., Beheren SM von. Identifying opportunities to enhance environmental cleaning in 23 acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(1):1–7. doi: 10.1086/524329. [DOI] [PubMed] [Google Scholar]
- 8.Carling P.C., Beheren S von, Kim P., Woods C. Intensive care unit environmental cleaning: an evaluation in sixteen hospitals using a novel assessment tool. J Hosp Infect. 2008;68(1):39–44. doi: 10.1016/j.jhin.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Boyce J.M. Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrob Resist Infect Control. 2016;5:10. doi: 10.1186/s13756-016-0111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dancer S.J. Controlling hospital-acquired infection: focus on the role of the environment and new technologies for decontamination. Clin Microbiol Rev. 2014;27(4):665–690. doi: 10.1128/CMR.00020-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otter J.A., Yezli S., Perl T.M., Barbut F., French G.L. The role of ‘no-touch’ automated room disinfection systems in infection prevention and control. J Hosp Infect. 2013;83(1):1–13. doi: 10.1016/j.jhin.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Sharma M., Hudson J.B. Ozone gas is an effective and practical antibacterial agent. Am J Infect Control. 2008;36(8):559–563. doi: 10.1016/j.ajic.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Moat J., Cargill J., Shone J., Upton M. Application of a novel decontamination process using gaseous ozone. Can J Microbiol. 2009;55(8):928–933. doi: 10.1139/w09-046. [DOI] [PubMed] [Google Scholar]
- 14.Dubuis M.-E., Dumont-Leblond N., Laliberté C., Veillette M., Turgeon N., Jean J. Ozone efficacy for the control of airborne viruses: bacteriophage and norovirus models. PLoS ONE. 2020;15(4) doi: 10.1371/journal.pone.0231164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallandat K., Lantagne D. Selection of a Biosafety Level 1 (BSL-1) surrogate to evaluate surface disinfection efficacy in Ebola outbreaks: comparison of four bacteriophages. PLoS One. 2017;12(5) doi: 10.1371/journal.pone.0177943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prussin A.J., Schwake D.O., Lin K., Gallagher D.L., Buttling L., Marr L.C. Survival of the enveloped virus Phi 6 in droplets as a function of relative humidity, absolute humidity, and temperature. Appl Environ Microbiol. 2018;84 doi: 10.1128/AEM.00551-18. e00551-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casanova L.M., Weaver S.R. Evaluation of eluents for the recovery of an enveloped virus from hands by whole-hand sampling. J Appl Microbiol. 2015;118(5):1210–1216. doi: 10.1111/jam.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cadnum J.L., Li D.F., Redmond S.N., John A.R., Pearlmutter B., Donskey C.J. Effectiveness of ultraviolet-C light and a high-level disinfection cabinet for decontamination of N95 respirators. Pathog Immun. 2020;5(1):52–67. doi: 10.20411/pai.v5i1.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitworth C., Mu Y., Houston H., Martinez-Smith M., Noble-Wang J., Coulliette-Salmond A. Persistence of bacteriophage Phi 6 on porous and nonporous surfaces and the potential for its use as an Ebola virus or coronavirus surrogate. Appl Environ Microbiol. 2020;86 doi: 10.1128/AEM.01482-20. e01482-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss S.R., Leibowitz J.L. Coronavirus pathogenesis. Adv Virus Res. 2011;81:85–164. doi: 10.1016/B978-0-12-385885-6.00009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vijgen L., Keyaerts E., Moës E., Thoelen I., Wollants E., Lemey P. Complete genomic sequence of human coronavirus OC43: molecular clock analysis suggests a relatively recent zoonotic coronavirus transmission event. J Virol. 2005;79(3):1595–1604. doi: 10.1128/JVI.79.3.1595-1604.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sterisafe A.P.S. Datasheet sterisafe Pro V1.1. https://sterisafe.eu/wp-content/uploads/2019/10/Datasheet_STERISAFE_PRO-1.pdf Available at: [last accessed March 2021]
- 24.INFUSER ApS . March 21, 2021. Sterisafe TM Pro V.1.1 manual: German version. [Google Scholar]
- 25.Occupational Safety and Health Administration OSHA Occupational Chemical Database – Ozone. https://www.osha.gov/chemicaldata/chemResult.html?recNo=9 Available from: [last accessed March 2021]
- 26.European Committee for Standardization. Chemical disinfectants and antiseptics - application of European Standards for chemical disinfectants and antiseptics; German version (DIN EN 14885:2019-10). Berlin: Beuth Verlag GmbH.
- 27.Rutala W.A., Weber D.J. Disinfectants used for environmental disinfection and new room decontamination technology. Am J Infect Control. 2013;41(5 Suppl):S36–S41. doi: 10.1016/j.ajic.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Biryukov J., Boydston J.A., Dunning R.A., Yeager J.J., Wood S., Reese A.L. Increasing temperature and relative humidity accelerates inactivation of SARS-CoV-2 on surfaces. mSphere. 2020;5 doi: 10.1128/mSphere.00441-20. e00441-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aboubakr H.A., Sharafeldin T.A., Goyal S.M. Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: A review. Transbound Emerg Dis. 2020:1–17. doi: 10.1111/tbed.13707. 00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris D.H., Yinda K.C., Gamble A., Rossine F.W., Huang Q., Bushmaker T. The effect of temperature and humidity on the stability of SARS-CoV-2 and other enveloped viruses. bioRxiv. 2020 doi: 10.7554/eLife.65902. 10.16.341883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aquino de Carvalho N., Stachler E.N., Cimabue N., Bibby K. Evaluation of Phi 6 persistence and suitability as an enveloped virus surrogate. Environ Sci Technol. 2017;51(15):8692–8700. doi: 10.1021/acs.est.7b01296. [DOI] [PubMed] [Google Scholar]
- 32.Yano H., Nakano R., Suzuki Y., Nakano A., Kasahara K., Hosoi H. Inactivation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by gaseous ozone treatment. J Hosp Infect. 2020;106(4):837–838. doi: 10.1016/j.jhin.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bayarri B., Cruz-Alcalde A., López-Vinent N., Micó M.M., Sans C. Can ozone inactivate SARS-CoV-2? A review of mechanisms and performance on viruses. J Hazard Mater. 2021;415:125658. doi: 10.1016/j.jhazmat.2021.125658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grignani E., Mansi A., Cabella R., Castellano P., Tirabasso A., Sisto R. Safe and effective use of ozone as air and surface disinfectant in the conjuncture of Covid-19. Gases. 2021;1(1):19–32. [Google Scholar]
- 35.Abreu A.C., Tavares R.R., Borges A., Mergulhão F., Simões M. Current and emergent strategies for disinfection of hospital environments. J Antimicrob Chemother. 2013;68(12):2718–2732. doi: 10.1093/jac/dkt281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rutala W.A., Weber D.J. 2008. Healthcare infection control practices advisory committee. Guideline for disinfection and sterilization in healthcare facilities.https://www.cdc.gov/infectioncontrol/pdf/guidelines/disinfection-guidelines-H.pdf Available at: [last accessed December 2020] [Google Scholar]
- 37.Knobling B., Franke G., Klupp E.M., Belmar Campos C., Knobloch J.K. Evaluation of the effectiveness of two automated room decontamination devices under real-life conditions. Front Public Health. 2021;9:618263. doi: 10.3389/fpubh.2021.618263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee D.S., Holland M.R., Falla N. The potential impact of ozone on materials in the U.K. Atmospheric Environment. 1996;30(7):1053–1065. [Google Scholar]
- 39.Davies A., Pottage T., Bennett A., Walker J. Gaseous and air decontamination technologies for Clostridium difficile in the healthcare environment. J Hosp Infect. 2011;77(3):199–203. doi: 10.1016/j.jhin.2010.08.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.