Abstract
Respiratory syncytial virus (RSV) infection is a major cause of respiratory tract disease in young children and throughout life. Infant infection is also associated with later respiratory morbidity including asthma. With a prospective birth cohort study of RSV and asthma, we evaluated the performance of an RSV antibody enzyme-linked immunoassay (EIA) for detecting prior infant RSV infection. Infant RSV infection was determined by biweekly respiratory illness surveillance plus RSV polymerase chain reaction (PCR) testing in their first RSV season and serum RSV antibodies after the season at approximately 1 year of age. RSV antibodies were detected by RSV A and B lysate EIA. Antibody and PCR results on 1707 children included 327 RSV PCR positive (PCR+) and 1380 not RSV+. Of 327 PCR+ children, 314 (96%) were lysate EIA positive and 583 out of 1380 (42%) children not PCR+ were positive. We compared the lysate EIA to RSV F, group A G (Ga), and group B G (Gb) protein antibody EIAs in a subset of 226 sera, 118 PCR+ children (97 group A and 21 group B) and 108 not PCR+. In this subset, 117 out of 118 (99%) RSV PCR+ children were positive by both the F and lysate EIAs and 103 out of 118 (87%) were positive by the Ga and/or Gb EIAs. Comparison of the two G EIAs indicated the infecting group correctly in 100 out of 118 (86%) and incorrectly in 1 out of 118 (1%). The lysate and F EIAs are sensitive for detecting infant infection and the two G EIAs can indicate the group of an earlier primary infection.
Keywords: antibodies, infant, infection, respiratory syncytial virus
1 |. INTRODUCTION
Human respiratory syncytial virus (RSV) belonging to the genus Orthopneumovirus of the family Paramyxoviridae is a leading cause of serious respiratory tract infection in young children worldwide.1–3 Primary RSV infection often occurs during an infant’s first seasonal RSV exposure, and a high percentage of children are infected by 2 years of age.4–8 RSV then causes repeat infections throughout life with especially severe disease in those with compromising cardiac, pulmonary or immune conditions and the elderly.9 RSV infection in young children has also been associated with later respiratory morbidity and the development of asthma.10–12
Primary RSV infections are usually detected during the acute illness by antigen detection or polymerase chain reaction (PCR) assays. An infection can also be detected with a rise in titer of RSV antibodies when acute- and convalescent-phase serum are available.13–25 RSV antibody assays include functional assays, such as neutralization and fusion inhibition, binding assays to detect protein-specific antibodies, and blocking assays to detect epitope- or antigenic-site-specific antibodies.
In a prospective study of the role of infant RSV infection on later development of asthma,26 we determined infant infection during their first RSV season using a combination of passive and active surveillance with viral identification during winter virus season using RSV PCR on nasal wash specimens collected for acute respiratory illnesses and by testing for RSV antibodies in serum specimens collected after the first RSV season at approximately 1 year of life. This study provided the opportunity to determine the performance of the study enzyme immunoassay (EIA) for detecting an earlier primary RSV infection.
We used an RSV tissue culture lysate antigen EIA to detect RSV antibodies in this study. Lysate antigen contains the range of proteins produced by RSV-infected HEp-2 cells including the F, G, N, and P proteins.27,28 Additionally, since RSV has two major antigenic groups, A and B,29 we included lysate antigens from both groups. We also assessed the performance of protein-specific EIAs for the F protein, group A G protein (Ga), and group B G protein (Gb) in a subset of specimens. We chose EIAs for the F and G proteins because of the 11 proteins encoded by the RSV genome, they are the only ones that induce neutralizing antibodies and protective immunity in animal studies and, therefore, are important to understanding RSV immunity.30,31
2 |. MATERIALS AND METHODS
2.1 |. Patients and specimens
Healthy term infants from urban, suburban and rural areas in Middle Tennessee who visited a participating pediatrician’s office between June 1 and December 31 of 2012 and 2013 and whose parents consented were enrolled in the Infant Susceptibility to Pulmonary Infections and Asthma Following RSV Exposure study cohort.26 The infants were born during the six-month period June through December, usually enrolled within 60 days of birth and were on average less than 6 months of age during their first RSV season. Study infants were queried for respiratory illness biweekly from November 1 to March 31 (includes the RSV season in this community) and, if they met prespecified criteria for an acute respiratory illness, had a nasal wash specimen collected. The nasal wash specimen was tested for RSV, human rhinovirus, and enterovirus RNA by PCR as previously described.32 A subset of RSV PCR+ specimens were sequenced and RSV group and genotype determined as described.33 Blood was collected from the infants by venipuncture or finger or toe stick at their first follow-up annual visit, usually within 6 months after their first RSV season. Plasma was separated from the blood specimens, divided into aliquots for storage at −80°C, shipped to Emory on dry ice, and tested by EIA for RSV antibodies.
2.2 |. Lysate EIA antigen
Since there are two antigenic groups of RSV strains, A and B, we used HEp-2 cell (ATCC CCL23 HEp-2 cells) culture lysates infected with a group A strain (A2, ATCC catalog number VR 1540) or a group B strain (B1, ATCC catalog number VR 1400) at a multiplicity of infection of 0.1 TCID50 per cell. Viral cultures were incubated for 2 days under minimum essential medium with 5% fetal bovine serum and then under serum-free medium until harvest when the cytopathic effect reached 3+ to 4+, that is, Day 3 or 4 postinfection. At harvest, cells were scrapped from the flask, the cells and media centrifuged at 3000g, and the supernatant transferred to 50 ml tubes. The cell pellets were pooled, disrupted in lysis buffer (Cell Signaling Technology) on ice and sonicated. The sonicated cell pellet was combined with supernatants, frozen and thawed three times, and centrifuged at 3000g for 30 min. Supernatant containing the RSV lysate antigen was collected and Halt Protease inhibitor (Thermo Fisher Scientific) was added to the clarified supernatants. The lysate was divided into aliquots (A2 or B1) and stored at −80°C. The lysate antigen titer was determined by adsorbing serial two-fold dilutions in carbonate buffer, beginning at 1:2, on enzyme-linked immunosorbent assay plates followed by a goat-anti-RSV antibody (Millipore Inc.), and peroxidase-conjugated anti-goat antibody. Mock-infected HEp2 cells were prepared in a similar fashion for the control antigen. The RSV A2 and B1 antigens were used separately in two EIAs for the first study year and combined in one EIA for the last study year. The respective optimal dilution (highest dilution that maintained near maximum absorbance signal in the antigen titration) was used to coat the EIA plates.
2.3 |. F, Ga, and Gb antibody EIA antigens
The antigens for the F, Ga and Gb EIAs were produced by expressing human codon optimized RSV F, Ga or Gb genes encoding carboxyl terminus 6X histidine tags in 293F cells. The genes were cloned into puromycin-resistant inducible plasmid pCDNA5/TO vectors with an engineered secretory signal to release expressed protein into the medium and woodchuck hepatitis virus posttranscriptional regulatory element to increase expression. The cloned F, Ga, or Gb pCDNA5/TO vector plasmid constructs were transfected into the blasticidin-resistant 293F/Bla cell line. Seventy-two hours post-transfection, the medium was changed to selection medium containing both blasticidin and puromycin to develop stably transfected cell lines. The surviving cells, stably transfected with the plasmid construct, were stored in liquid nitrogen. Desired antigen was produced by growing the stably transfected cells in suspension, inducing with doxycycline for 48–60 h in serum-free medium, harvesting the supernatant, and clarifying the supernatant by centrifugation at 3000 rpm for 30 min. Halt Protease inhibitor (Thermo Fidher Scientific) was added to the clarified supernatant and aliquots were stored at −80°C. Control antigen was produced in a similar fashion with 293F cells stably transfected with the empty vector.
Expression was confirmed by Western blot with the human monoclonal antibody (mAb) Motavizumab (MedImmune, LLC) to detect F and the human mAb 3D3 (kindly provided by Trellis Bioscience; LLC) to detect Ga and Gb. Histidine tag on the F, Ga and Gb proteins was detected with an anti-histidine mAb (Cat no. OB05; Sigma Inc.).
The titer of antigen was determined by adsorbing serial 2-fold dilutions of the preparations in carbonate buffer (pH 9.6) beginning with a 1:25 dilution and detecting the protein by EIA with the BEI high titer anti-RSV antibody serum (Catalog number BEI NR-4021; BEI Resources) and a peroxidase conjugated goat antihuman immunoglobulin G (IgG) antibody (Millipore). The optimal dilution (highest antigen dilution that maintained high absorbance signal) of the F, Ga or Gb antigens was used to coat the plates for the EIAs.
2.4 |. EIAs
The lysate, F protein, Ga protein and Gb protein EIAs were performed using a similar procedure. EIA plates (Maxisorb, Nunc) were coated with predetermined dilutions of the RSV group A plus group B lysate, or F, Ga, or Gb protein or respective control antigens in sodium carbonate bicarbonate coating buffer (pH 9.6) for 2 h at 37°C followed by overnight at 4°C. The antigen-coated plates were washed two times with PBS, blocked with 200 ul of blocking buffer (0.33% fish skin gelatin, 0.33% skimmed milk, and 0.33% bovine casein [GMC buffer]) for 2 h at 37°C and washed two times with PBS-Tween (0.05%). Reference standard specimens and patient specimens were diluted in GMC buffer supplemented with 0.15% Tween 20 (GMC-T). All specimens were tested in triplicate wells against control antigen and the RSV antigen at a 1:200 dilution. A reference standard curve for each run was generated with two-fold serial dilutions of the BEI NR 4021 serum beginning at a 1:100 dilution for Ga and Gb and 1:200 dilution for F and RSV lysate EIAs. To assess the quality and consistency of EIA results, low, medium, and negative RSV antibody control sera were included on each plate. Specimens were incubated at 37°C for 110 min and washed five times with PBS-Tween (0.05%). Horseradish peroxidase-conjugated goat antihuman IgG antibody diluted in GMC-T (1:5000 dilution) was added for 70 min (37°C). After washing the plates with PBS-T, color was developed with OPD/H2O2. The reaction was stopped after 30 min with 4 N H2SO4 and absorbance was read at 490 nm. A specimen was considered positive if the mean of the three RSV antigen wells (P) was greater than the mean plus 3 SD of three control antigen wells (N), P-N was greater than the mean of the no antibody control wells, and P-N for serum was greater than the analogous value for IgG depleted serum. The antibody titer was estimated by referring the P-N value to a 4PL model of the reference standard curve generated from two-fold serial dilutions of the BEI high RSV antibody titer serum (BEI NR 4021) (Figure 1). The 4PL model was generated with SAS version 9.4 (Cary).
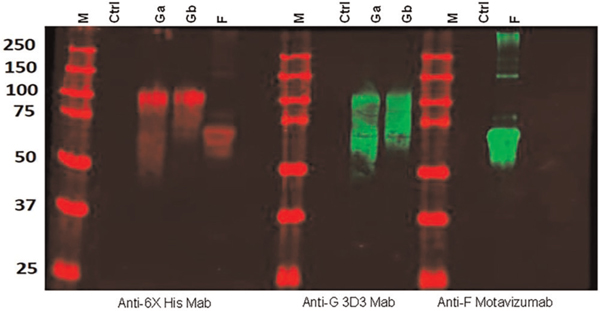
FIGURE 1.
Western blot analysis of the supernatant from 293 cells expressing secreted forms of the respiratory syncytial virus (RSV) F, group A G protein (Ga), and group B G protein (Gb). M indicates lane with molecular weight markers. Ctrl indicates lane from 293 cells transfected with empty plasmid. Anti-6X His Mab is an anti-hexahistidine tag monoclonal antibody. Anti-G 3D3 Mab is a human Mab from Trellis Bioscience. Anti-F Motavizumab is a human Mab from MedImmune
3 |. RESULTS
3.1 |. EIA antigens
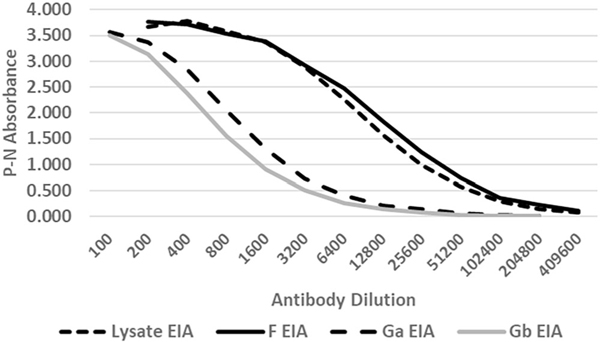
The RSV A and B virus lysate, F, Ga, and Gb antigens served quite satisfactorily for RSV antibody detection. The expressed proteins gave the appropriately sized bands by Western blot (Figure 1), and the optimal dilutions of antigen were 1:16–1:32 for the lysate EIA and between 1:50 and 1:100 for the F, Ga, and Gb EIAs. All four assays gave high absorbance readings at low concentrations of BEI high RSV antibody titer serum (Figure 2). The antibody titer for the BEI NR 4021 serum was highest for the lysate and F antibody EIAs (median titer of 267,000 and 252,000, respectively). The median titer for the Ga and Gb EIA was 43,000 and 44,000, respectively.
FIGURE 2.
Examples of reference standard curves used to estimate specimen titers for the lysate, F, Ga, and Gb enzyme-linked immunoassays (EIAs). The curves were generated from serial two-fold dilutions of the BEI high titer RSV serum (BEI NR 4021). P is the mean absorbance for three wells against the respective antigen and N is the mean absorbance for three wells against the corresponding negative control antigen. RSV, respiratory syncytial virus
3.2 |. Lysate EIA results
We tested 1707 blood specimens collected from study children at approximately one year of age (median 12 months with 90% between 9 and 14 months of age) including 327 children with an RSV PCR+ illness and 1380 children with no positive RSV PCR (not PCR +). Among serum specimens from children with an RSV PCR+ illness, 314 (96%) had a positive lysate EIA,that is, an estimated titer ≥200, and among specimens from children not PCR+, 583 (42%) had a positive lysate EIA suggesting they had an RSV infection missed by surveillance (Table 1). Thus, 897 out of 1707 (53%) of children had an RSV PCR+ result and/or RSV antibodies in serum collected after the RSV season, suggesting they had been infected.
TABLE 1.
Lysate antibodies in study infants
| RSV PCR+ Number in age group (percent) |
Not RSV PCR+ Number in age group (percent) |
|||||||
|---|---|---|---|---|---|---|---|---|
| All Ages | <10 monthsa | >10–12 months | >12 months | All ages | <10 months | >10–12 months | >12 months | |
| Total | 327 | 124 | 148 | 55 | 1380b | 568 | 586 | 223 |
| Lysate+ (% total) | 314 (96%) | 120 | 142 | 52 | 583 (42%)b | 249 | 217 | 114 |
| Titer-200–500 | 24/314 (8%) | 11/120 (9%) | 11/124 (8%) | 2/52 (4%) | 177/580 (31%) | 104/249 (42%) | 53/217 (24%) | 20/114 (18%) |
| Titer-501–1000 | 39/314 (12%) | 10/120 (8%) | 20/142 (14%) | 9/52 (17%) | 70/580 (12%) | 36/249 (14%) | 24/217 (11%) | 10/114 (9%) |
| Titer >1000 | 251/314 (80%) | 99/120 (82%) | 111/142 (78%) | 41/52 (79%) | 333/580 (57%) | 109/249(44%) | 140/217 (65%) | 84/114 (74%) |
Abbreviations: PCR, polymerase chain reaction; RSV, respiratory syncytial virus.
Age at blood draw.
This includes three patients lysate+ without age at blood draw available not included in age-specific data.
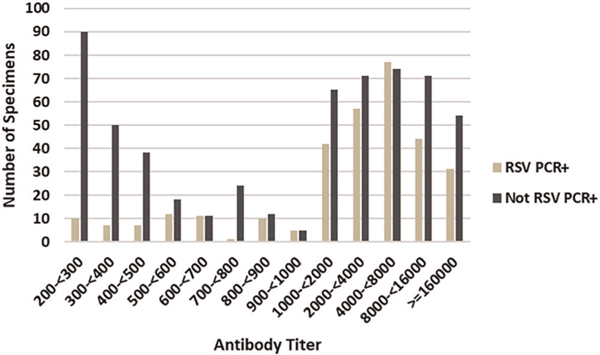
The overrepresentation of low-titer antibodies in children not PCR+ compared to PCR+ children (Figure 3) raised the possibility that maternal antibody and not past infection explains antibody positivity in some children. To explore this possibility, we looked at the percent of low titers, that is, between 200 and 500, by the child’s age at the time of blood collection. We found the percent of low titer antibody in children not PCR+ decreased with increasing age but did not decrease with age in the PCR+ children (Table 1).
FIGURE 3.
Distribution of RSV antibody titers for 314 children who were RSV PCR+ and 583 who were not RSV PCR+. A titer >200 is consider positive for RSV antibodies. Sera were collected between the child’s first and second RSV season at approximately 1 year of age. RSV antibodies were detected by an EIA with tissue culture lysate from group A and group B infected cells as the antigen. The titer was estimated from a standard curve. Note the marked difference in number of specimens with low antibody titers, for example, less than 500, between children with RSV PCR+ infection (RSV PCR+) and children with no RSV PCR+ infection (Not RSV PCR+). EIA, enzyme-linked immunoassay; PCR, polymerase chain reaction; RSV, respiratory syncytial virus
3.3 |. Comparison of lysate, F, Ga and Gb antibody EIAs
In the subset of 118 specimens from PCR+ children in the comparison study, sequence studies determined that 97 contained a group A strain and 21 a group B strain. As expected, the F and lysate EIAs consistently detected antibodies in nearly all RSV PCR+ infections regardless of the virus being from group A or B, 117 out of 118 (99%) (Table 1). The one serologically negative specimen came from an RSV PCR+ child infected with a group A strain. In the 226 sub study sera, the F and lysate EIAs gave concordant positive or negative results for 92% and the titers had a Spearman’s correlation coefficient of 0.916. For 15 out of 17 specimens with discordant EIA results, the titer for the positive EIA was low, between the cutoff titer of 200 and 500. The median titer for F EIA was 6717 and for the lysate EIA was 3636. Ga antibodies were detected in 88 out of 118 (75%) of all infections, 86 out of 97 (89%) of group A infections and 2 out of 21 (10%) of group B infections. Gb antibodies were detected in 51 out of 118 (43%) of all infections, 34 out of 97 (35%) of group A infections, and 17 out of 21 (81%) of group B infections. At least one of the G EIAs was positive in 103 (87%) of 118 PCR-documented infections. In 67 out of 118 (57%) of serum specimens, the Ga was positive and Gb negative or vice versa, and the positive result correctly indicated the RSV group of the detected virus. For 36 out of 118 (31%) specimens both Ga and Gb EIAs were positive with 33 of these having a titer ratio of Ga/Gb or Gb/Ga > 2.5 that correctly indicated a group A infection (32 specimens) or group B infection (1 specimen). For two specimens both ratios were less than 2.5 and for one group B infection the titer ratio for Ga/Gb > 2.5. Thus, for 100 of the 118 (85%) PCR+ specimens, Ga and Gb antibody results correctly indicated the RSV group. In one subject (1%), the relative the Ga and Gb antibody titers indicated the incorrect group. For two, the relative Ga and Gb EIA titers did not indicate the RSV group and for 15 neither G EIA was positive (Table 2).
TABLE 2.
Summary of Lysate, F, Ga, and Gb antibody EIA positivity in RSV PCR+ infants
| EIA | Antibody positive in RSV PCR+ infantsa |
|||
|---|---|---|---|---|
| No+/no tested | Median titer of positive sera | Gp A infection no+/no tested | Gp B infection no+/no tested | |
| Lysate | 117/118 | 5661 | 96/97 | 21/21 |
| F | 117/118 | 13,403 | 96/97 | 21/21 |
| Ga | 88/118 | 1166 | 86/97 | 2/21 |
| Gb | 51/118 | 360 | 34/97 | 17/21 |
| Ga and Gb | 36/118 | 34/97 | 2/21 | |
| Ga or Gb | 103/118 | 86/97 | 17/21 | |
Abbreviations: EIA, enzyme-linked immunoassay; PCR, polymerase chain reaction; RSV, respiratory syncytial virus.
These sera from118 PCR+ plus 108 from not PCR+ children were used for a sub study comparison of the RSV group A and B lysate antibody EIA to an RSV F, group RSV A G, and RSV group B G protein EIAs. The results for the RSV PCR+ group show the relative sensitivity for detecting infant infection with each of these EIAs.
The antibody titers for all assays decreased over time as indicated by titers from specimens collected less than 7 months compared to those collected ≥7 months after an RSV PCR+ infection, that is, a median titer of 7222 compared to a median titer of 4978 for the lysate EIA (p < .01, Wilcoxon rank sum test). However, for the other three assays, the difference in titers for serum collected less than 7 months after RSV PCR+ infection (F EIA median titer of 13,634, Ga EIA median titer of 1488, and Gb EIA median titer of 1038) was not significantly different (p > .05) from titers for serum collected greater than 7 months after the infection (F EIA median titer of 12,675, Ga EIA median titer of 1166, and Gb EIA median titer of 633). Among children not PCR+, 62 out of 108 (57%) had a positive lysate or F EIA with median titers of 1650 and 1562, respectively.
4 |. DISCUSSION
In this study, we focused on performance of the lysate EIA antibody assay for detecting past primary infection in young children and, then, compared it to three other assays: F, Ga, and Gb antibody EIAs. The lysate EIA proved sensitive for detecting antibodies after a primary RSV infection in infants as indicated by 96% of serum from 327 PCR+ infants having a positive lysate EIA antibody test. In a subset of specimens, lysate and F EIAs had a similar level of sensitivity while Ga and Gb antigen EIAs were less sensitive for detecting past infection. Together, however, the Ga and Gb antibody EIAs were usually able to correctly indicate whether the primary infection was caused by a group A or group B strain. The high sensitivity of the lysate and F antibody EIAs is consistent with other studies showing that antibodies against the protein antigens in these assays (F, G, N, P, etc.) are frequently detected in humans.13–5,18,21,22,24,27,28,34 It is also likely that the antigen preparation enhanced the quality of the antigen and, thus, assay sensitivity. First, use of serum-free media in antigen preparation should reduce non-RSV antigens in the preparation and commensurately increase RSV antigens coating the plates. Second, we used lysis buffer to release RSV proteins in from adherent cells and to prevent their removal during the low speed clarification centrifugation. This process presumably increased the concentration of RSV antigen and amount adhering to the plate. For the F, Ga, and Gb antigens we used a secreted form of the protein with serum-free media to produce antigen with minimal non-RSV contaminants. The yield of all four antigens was good and purity sufficient for direct use without further processing.
To streamline testing, we extrapolated RSV antibody titers from an assay standard curve rather than performing serial dilutions. We estimated the titer from the specimen’s absorbance and dilution using a four PL model of a standard curve generated with serial two-fold dilutions of a reference serum (BEI high RSV titer serum, NR-4021). The titer estimates were reproducible (<2-fold within and between run variation for a given specimen) and similar to those obtained by serial dilution studies (<2-fold difference from the serial dilution titer). This approach allowed us to detect a decrease in titer with increasing time after the RSV PCR+ illness as indicated by higher mean titers in serum collected ≤7 months compared to those collected greater than 7 months after the date of infection. A decline in RSV antibody titer after infection has been noted previously.35
The serum titers also suggested the need to consider the possibility that residual maternal antibody and not past infection could be responsible for low-titer antibody detected in some specimens from young children. We reasoned that infection-induced antibody would substantially exceed maternal antibody, resulting in similar antibody levels among RSV-infected children irrespective of residual passively acquired antibody. Among not PCR+ children with detectable antibody, low-titer antibody, however, was more frequent compared to PCR+ children; 31% and 8%, respectively, had titers between 200 and 500. This result is consistent with detection of maternal antibody in not PCR+ subjects who had not had an earlier RSV infection. The distribution of higher titers was similar for children not PCR+ and PCR+ children. Given high sensitivity of lysate and F EIAs, it would not be surprising to detect maternal antibody in some young children. With a serum antibody half-life of approximately 1 month,36–39 it will take 10 or more months for serum antibody titers greater than 200,000 to drop below the positivity threshold (i.e., a titer <200). We regularly detect serum antibody titers greater than 200,000 in adults and cord blood titers are similar to those in maternal serum. We did not, however, have a way to confirm a maternal source of antibody in the children’s sera.
Although known RSV antibody-negative sera were unavailable to calculate assay specificity, the ability of the G assays to correctly distinguish the infecting RSV group based on sequence studies of RSV PCR+ specimens supports the specificity of these assays. Data from another study support specificity of the lysate EIA. We detected a ≥2-fold rise in RSV lysate EIA antibody between acute- and convalescent-phase sera from 9 out of 16 adults hospitalized during the RSV season with an RSV PCR + acute cardiorespiratory illness and 1 out of 31 patients with a non-RSV + cardiorespiratory illness in which influenza virus, an endemic coronavirus, human metapneumovirus, or human parainfluenza was detected by PCR (Anderson, LJ and Anderson, EJ. unpublished data, 2020). Positive and negative result concordance and titer correlation suggest the F EIA performs similarly to the lysate EIA.
In summary, the lysate EIA with RSV A and B antigens is sensitive for detecting RSV antibodies indicative of a past infection in serum collected after a child’s first RSV season. Low titers of antibody in some children could, however, indicate residual maternal antibody and not past infection. The F EIA had similar sensitivity to the lysate assay. The Ga and Gb antibody EIAs were less sensitive but they can be used to indicate whether a group A or B strain caused an earlier primary infection. The lysate and F assays are well suited to determine which children have had a primary RSV infection for vaccine, antiviral drug, epidemiologic, and clinical studies.
ACKNOWLEDGMENTS
Research funding was through U19 AI 095227 grant by National Institute of Health, Bethesda, MD, USA. Emory University, Atlanta, GA provided the laboratory research facilities.
Funding information
National Institutes of Health, Grant/Award Number: U19 AI 095227
Dr. Anderson has done paid consultancies on RSV vaccines for Bavarian Nordic; Novavax; and ClearPath Vaccines Company; and Phizer. Our laboratory is currently receiving funding through Emory University from Phizer and Advac for laboratory studies for RSV surveillance and vaccine studies in adults. Dr. Anderson is coinventor on several CDC patents on the RSV G protein and its CX3C chemokine motif relative to immune therapy and vaccine development and a patent filing for use of RSV platform VLPs with the F and G proteins for vaccines.
Footnotes
CONFLICT OF INTERESTS
The authors do not have conflicts related to the contents of this manuscript.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The laboratory studies were conducted at Emory University and the clinical and epidemiologic studies at Vanderbilt University.
REFERENCES
- 1.Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017; 390(10098):946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stockman LJ, Curns AT, Anderson LJ, Fischer-Langley G. Respiratory syncytial virus-associated hospitalizations among infants and young children in the United States, 1997–2006. Pediatr Infect Dis J. 2012; 31(1):5–9. [DOI] [PubMed] [Google Scholar]
- 3.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6): 588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu G, Gonzalez R, Guo L, et al. Large-scale seroprevalence analysis of human metapneumovirus and human respiratory syncytial virus infections in Beijing, China. Virol J. 2011;8:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebihara T, Endo R, Kikuta H, Ishiguro N, Ishiko H, Kobayashi K. Comparison of the seroprevalence of human metapneumovirus and human respiratory syncytial virus. J Med Virol. 2004;72(2):304–306. [DOI] [PubMed] [Google Scholar]
- 6.Sastre P, Ruiz T, Schildgen O, Schildgen V, Vela C, Rueda P. Seroprevalence of human respiratory syncytial virus and human metapneumovirus in healthy population analyzed by recombinant fusion protein-based enzyme linked immunosorbent assay. Virol J. 2012;9:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glezen WP, Paredes A, Allison JE, Taber LH, Frank AL. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr. 1981;98(5):708–715. [DOI] [PubMed] [Google Scholar]
- 8.Henderson FW, Collier AM, Clyde WA Jr., Denny FW. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Engl J Med. 1979;300(10): 530–534. [DOI] [PubMed] [Google Scholar]
- 9.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749–1759. [DOI] [PubMed] [Google Scholar]
- 10.Wu P, Hartert TV. Evidence for a causal relationship between respiratory syncytial virus infection and asthma. Expert Rev Anti Infect Ther. 2011;9(9):731–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sigurs N, Aljassim F, Kjellman B, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65(12):1045–1052. [DOI] [PubMed] [Google Scholar]
- 12.Abreo A, Wu P, Donovan BM, et al. Infant respiratory syncytial virus bronchiolitis and subsequent risk of pneumonia, otitis media, and antibiotic utilization. Clin Infect Dis. 2019;71:211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capella C, Chaiwatpongsakorn S, Gorrell E, et al. Prefusion F, postfusion F, G antibodies, and disease severity in infants and young children with acute respiratory syncytial virus infection. J Infect Dis. 2017;216(11):1398–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maifeld SV, Ro B, Mok H, et al. Development of electro-chemiluminescent serology assays to measure the humoral response to antigens of respiratory syncytial virus. PLOS One. 2016;11(4):e0153019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh EE, Falsey AR. Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J Infect Dis. 2004;190(2):373–378. [DOI] [PubMed] [Google Scholar]
- 16.Siber GR, Leszczynski J, Pena-Cruz V, et al. Protective activity of a human respiratory syncytial virus immune globulin prepared from donors screened by microneutralization assay. J Infect Dis. 1992; 165:456–463. [DOI] [PubMed] [Google Scholar]
- 17.Rossey I, McLellan JS, Saelens X, Schepens B. Clinical potential of prefusion RSV F-specific antibodies. Trends Microbiol. 2018;26(3):209–219. [DOI] [PubMed] [Google Scholar]
- 18.Trento A, Rodríguez-Fernández R, González-Sánchez MI, et al. The complexity of antibody responses elicited against the respiratory syncytial virus glycoproteins in hospitalized children younger than 2 years. Front Microbiol. 2017;8:2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green CA, Sande CJ, de Lara C, et al. Humoral and cellular immunity to RSV in infants, children and adults. Vaccine. 2018;36(41):6183–6190. [DOI] [PubMed] [Google Scholar]
- 20.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986; 140:543–546. [DOI] [PubMed] [Google Scholar]
- 21.Murphy BR, Graham BS, Prince GA, et al. Serum and nasal-wash immunoglobulin G and A antibody response of infants and children to respiratory syncytial virus F and G glycoproteins following primary infection. J Clin Microbiol. 1986;23(6):1009–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jounai N, Yoshioka M, Tozuka M, et al. Age-specific profiles of antibody responses against respiratory syncytial virus infection. EBioMedicine. 2017;16:124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roca A, Quintó L, Abacassamo F, et al. Antibody response after RSV infection in children younger than 1 year of age living in a rural area of Mozambique. J Med Virol. 2003;69(4):579–587. [DOI] [PubMed] [Google Scholar]
- 24.Walsh EE, Wang L, Falsey AR, et al. Virus-specific antibody, viral load, and disease severity in respiratory syncytial virus infection. J Infect Dis. 2018;218(2):208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shinoff JJ, O’Brien KL, Thumar B, et al. Young infants can develop protective levels of neutralizing antibody after infection with respiratory syncytial virus. J Infect Dis. 2008;198(7):1007–1015. [DOI] [PubMed] [Google Scholar]
- 26.Larkin EK, Gebretsadik T, Moore ML, et al. Objectives, design, and enrollment results from the Infant susceptibility to pulmonary infections and asthma following RSV exposure study (INSPIRE). BMC Pulm Med. 2015;15:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erdman DD, Anderson LJ. Monoclonal antibody-based capture enzyme immunoassays for specific serum immunoglobulin G (IgG), IgA, and IgM antibodies to respiratory syncytial virus. J Clin Microbiol. 1990;28(12):2744–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vainionpaa R, Meurman O, Sarkkinen H. Antibody response to respiratory syncytial virus structural proteins in children with acute respiratory syncytial virus infection. J Virol. 1985;53(3):976–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collins PL, Melero JA. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus Res. 2011;162(1-2):80–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stott EJ, Taylor G, Ball LA, et al. Immune and histopathological responses in animals vaccinated with recombinant vaccinia viruses that express individual genes of human respiratory syncytial virus. J Virol. 1987;61(12):3855–3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Connors M, Collins PL, Firestone C-Y, Murphy BR. Respiratory syncytial virus (RSV) F, G, M2 (22K), and N proteins each induce resistance to RSV Challenge, but resistance induced by M2 and N proteins is relatively short-lived. J Virol. 1991;65:1634–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kodani M, Yang G, Conklin LM, et al. Application of TaqMan low-density arrays for simultaneous detection of multiple respiratory pathogens. J Clin Microbiol. 2011;49(6):2175–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schobel SA, Stucker KM, Moore ML, et al. Respiratory syncytial virus whole-genome sequencing identifies convergent evolution of sequence duplication in the C-terminus of the G gene. Sci Rep. 2016;6:26311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schepp RM, de Haan CAM, Wilkins D, et al. Development and standardization of a high-throughput multiplex Immunoassay for the simultaneous quantification of specific antibodies to five respiratory syncytial virus proteins. mSphere. 2019;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Welliver RC, Kaul TN, Putnam TI, Sun M, Riddlesberger K, Ogra PL. The antibody response to primary and secondary infection with respiratory syncytial virus: kinetics of class-specific responses. J Pediatr. 1980;96:808–813. [DOI] [PubMed] [Google Scholar]
- 36.Brandenburg AH, Groen J, Steensel-Moll HA, et al. Respiratory syncytial virus specific serum antibodies in infants under six months of age: limited serological response upon infection. J Med Virol. 1997;52(1):97–104. [DOI] [PubMed] [Google Scholar]
- 37.Chu HY, Steinhoff MC, Magaret A, et al. Respiratory syncytial virus transplacental antibody transfer and kinetics in mother-infant pairs in Bangladesh. J Infect Dis. 2014;210(10):1582–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ochola R, Sande C, Fegan G, et al. The level and duration of RSV-specific maternal IgG in infants in Kilifi Kenya. PLOS One. 2009; 4(12):e8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groothuis JR, Levin MJ, Rodriguez W, et al. Use of intravenous gamma globulin to passively immunize high-risk children against respiratory syncytial virus: safety and pharmacokinetics. The RSVIG study group. Antimicrob Agents Chemother. 1991;35(7):1469–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]