Abstract
Objectives/Hypothesis:
Head and neck cancer (HNC) is the fifth most common malignancy in sub-Saharan Africa, a region with hyperendemic human immunodeficiency virus (HIV)-infection. HIV patients have higher rates of HNC, yet the effect of HIV-infection on oncologic outcomes and treatment toxicity is poorly characterized.
Study Design:
Prospective observational cohort study.
Methods:
HNC patients attending a government-funded oncology clinic in Botswana were prospectively enrolled in an observational cohort registry from 2015 to 2019. Clinical characteristics were analyzed via Cox proportional hazards and logistic regression followed by secondary analysis by HIV-status. Overall survival (OS) was evaluated via Kaplan-Meier.
Results:
The study enrolled 149 patients with a median follow-up of 23 months. Patients presented with advanced disease (60% with T4-primaries), received limited treatment (19% chemotherapy, 8% surgery, 29% definitive radiation [RT]), and had delayed care (median time from diagnosis to RT of 2.5 months). Median OS was 36.2 months. Anemia was associated with worse survival (HR 2.74, P = .001). Grade ≥ 3 toxicity rate with RT was 30% and associated with mucosal subsite (OR 4.04, P = .03) and BMI < 20 kg/m2 (OR 6.04, P = .012). Forty percent of patients (n = 59) were HIV-infected; most (85%) were on antiretroviral therapy, had suppressed viral loads (90% with ≤400 copies/mL), and had immunocompetent CD4 counts (median 400 cells/mm3). HIV-status was not associated with decreased receipt or delays of definitive RT, worse survival, or increased toxicity.
Conclusions:
Despite access to government-funded care, HNC patients in Botswana present late and have delays in care, which likely contributes to suboptimal survival outcomes. While a disproportionate number has comorbid HIV infection, HIV-status does not adversely affect outcomes.
Keywords: Head and neck neoplasms, HIV-related neoplasms, Radiotherapy, Global oncology, HPV-related malignancies
Editor’s Note:
This Manuscript was accepted for publication on October 13, 2020
INTRODUCTION
The burden of cancer is increasing worldwide. In 2018, there were an estimated 9.6 million cancer-related deaths, an increase of >60% since 1990.1 Already, cancer-related deaths are disproportionately concentrated in low and middle-income countries (LMIC)1; and the cancer burden in LMICs are estimated to double by 2040.2 Many LMIC are at the early stages of optimizing oncologic care: cancer awareness is still limited, and access to specialized oncologic management is scarce.3,4 As such, many patients present with advanced-stage malignancies with limited treatment options at the time of delayed diagnosis.5,6
Head and neck cancer (HNC), in particular, is the fifth most common cancer in Sub-Saharan Africa (SSA).1 In 2018, approximately 30% of the 1.5 million newly diagnosed HNC cases worldwide occurred in LMICs.1 Comprehensive work-up of HNCs often entails fiberoptic nasopharyngolaryngoscopy, high-quality axial diagnostic imaging (e.g. CT Neck with contrast), and human papillomavirus (HPV) staining/testing on pathology for staging purposes. While early-stage HNCs may be readily managed with curative intent (sometimes via monotherapy), advanced-stage disease imparts poorer outcomes. For such patients, definitive management entails multimodality combinations of surgery, chemotherapy, and/or radiation therapy (RT). These aggressive courses are accompanied by significant toxicities, warranting supportive measures such as dental care, pain management, nutrition, speech pathology, and/or percutaneous feeding tube (PEG) placement. However, access to many of these diagnostic, specialized oncologic, and supportive care resources remain limited in LMICs.
The incidence of squamous cell carcinoma (SCC) of the head and neck is also increased by two- to three-fold among human immunodeficiency virus (HIV) -positive patients7–9: a pertinent demographic in SSA, where HIV infection remains endemic.10 Botswana, for example, is a middle-income SSA country with a population of 2.3 million affected by one of the most severe HIV epidemics worldwide (22.2% of the adult population [age 15+] were living with HIV in 2019).11 Robust efforts, including universal healthcare and access to antiretroviral therapy (ART), have fortunately begun to decrease HIV prevalence and mortality in Botswana.11 Yet as HIV-infected patients are now living longer, they remain susceptible to late comorbidities, including HIV-related malignancies.12
The management of non-communicable diseases such as cancer is thus an increasingly important medical concern.1 However, data are limited regarding the treatment of locally advanced HNC among HIV-infected patients. The objective of this study was to prospectively evaluate patterns of oncologic care and outcomes for HNC patients with or without HIV infection in Botswana.
METHODS AND MATERIALS
Study Setting
Healthcare in Botswana entails a tiered system with first entry at the primary care level across decentralized local health posts. If cancer is suspected, patients are subsequently referred to tertiary hospitals where oncologic diagnosis and treatment are available. Nearly all oncologic care in Botswana is provided at 1) Princess Marina Hospital (PMH; a tertiary public hospital in Gaborone), 2) Nyangabwe Hospital (a tertiary public hospital in Francistown), and/or 3) Gaborone Private Hospital (GPH; a private hospital in Gaborone, with the only RT facility in the entire country. Together, PMH and GPH provide oncologic care for approximately 1.3 million (65%) of Botswana’s population. In terms of RT access, the only linear accelerator in the entire country is located at GPH and treats 45 to 65 patients daily (sometimes up to 6 days per week if necessary).13 Many of these patients are referred from the public sector, with all citizens approved for RT (at no out-of-pocket cost) through government subsidy.
Patient Selection and Data Collection
This study is an analysis of HNC patients consecutively enrolled in the Botswana Prospective Cancer Cohort (BPCC), a prospective observational cohort of patients receiving cancer treatment in Botswana, from February 2015 to June 2019. All biopsy proven HNC patients presenting to an oncology clinic at PMH or GPH were approached for enrollment. Detailed methods regarding the BPCC have been previously described.14,15 Briefly, if a patient consented to enrollment, data were collected by a research assistant at initial consultation, on treatment visits for those getting RT, and during follow up. Patients were all followed up every 3 months indefinitely to determine vital status and any signs of recurrence. Data are collected via pre-designed electronic forms and database management tools (Research Electronic Data Capture [REDCap], hosted at the University of Pennsylvania).16 This study was reviewed and approved by the institutional review board at the University of Pennsylvania and by the Ministry of Health in Botswana.
At initial consultation and each follow-up, comprehensive patient data are collected and updated, including: demographic information, distance from treatment facility, marital status, cancer screening history and risk factors, initial presenting and currently active symptoms, HIV history, treatment prescribed versus received, Karnofsky Performance Status (KPS) score, and other relevant medical record data. Staging work-up is frequently limited by lack of fiberoptic nasopharyngolaryngoscopy, high-quality axial diagnostic imaging (e.g. CT Neck with contrast), and/or HPV staining/testing on pathology. Dates of symptom onset, diagnosis, and treatment initiation are recorded, with time-to-treatment defined from pathologic diagnosis to treatment start. With respect to oncologic management, chemotherapy cycles are recorded during weekly treatment visits, and RT summary data (such as dose and duration) are collected at the end of treatment, with any delays or pauses noted.
Antiretroviral Treatment
Although the incidence of new HIV infections has decreased by 36% since 2010, the HIV prevalence in Botswana remains the second highest in the world (at approximately 22.2% of the adult population [age 15+] in 2019).11 The Botswana National ART program provides ART free-of-charge to all citizens. At study inception (2015), only patients with CD4 counts ≤350 cells/μl or with World Health Organization HIV stage three or four conditions17 were eligible for ART, with efavirenz-tenofovir disoproxil fumarate (TDF) - emtricitabine (FTC) offered as standard first-line therapy. However, starting June 2016, all HIV-infected patients (regardless of viral load or CD4 count) became eligible for immediate ART initiation through a Universal Test and Treat Strategy,18 and standard first-line ART transitioned to dolutegravir-TDF-FTC.18 As a result of these measures, in 2019, 82% of HIV-infected patients in Botswana were on ART, and 79% had achieved viral load suppression.11 All study participants with negative or unknown HIV-status were tested for HIV as confirmation prior to initiation of cancer treatment. Note that in Botswana, HIV status does not directly factor into resource allocation of oncologic care.
Oncologic Management and Limitations
Chemo-RT with concurrent cisplatin was considered for any suitable patient deemed fit enough to tolerate definitive therapy; yet many patients were not eligible for aggressive treatment, given their delayed presentations with advanced disease. Common contraindications to definitive chemo-RT included poor performance status, distant metastases, and renal dysfunction or significant cytopenias precluding chemotherapy. Additional limitations include a relative paucity of ancillary services (e.g. dental care, pain management, registered dieticians, speech pathology) and supportive therapies (e.g. PEG-tube placement for enteral feeding) helpful for managing severe treatment-related complications. After consideration of these factors, many patients were ultimately dispositioned to palliative therapy. All treatment plans were subject to peer-review and discussion among the center providers (in the absence of a robust HNC-specialized multidisciplinary forum).
With respect to RT, all patients underwent CT-Simulation for planning purposes, and treatments were delivered via 3D-conformal radiation technique after volumetric target delineation on a VERSA-HD linear accelerator (Elekta, Stockholm, Sweden). During the period of the study, one–two radiation oncologists and one physicist were available at GPH. Following physician contours, plans were developed by dosimetry on Monaco software (Elekta, Stockholm, Sweden), with normal tissue constraints and target parameters accounted for in the dose volume histogram. All plans underwent physics quality assurance prior to treatment delivery. Regarding prescriptions, definitive cases were conventionally fractionated (e.g. 2 Gy per fraction) to a median cumulative dose of 70 Gy in 35 daily treatments. These courses started with larger fields for elective coverage of regional nodal basins (to an initial dose of 50 Gy in 25 fractions), followed by a sequential cone-down (for another 20 Gy in 10 fractions) to the high-risk clinical treatment volume (which also encompassed all gross disease). In contrast, non-definitive cases received a median dose of 37.5 Gy (IQR: 35–45) in 15 daily fractions (IQR: 14–20) without sequential boost. For palliative cases, hypo-fractionated regimens (≥ 2.5 Gy per fraction) - such as 30 Gy in 10 fractions, 20 Gy in 5 fractions, 16 Gy in 4 fractions (BID), and 6 Gy in 1 fraction - were commonly utilized.
Outcomes and Statistical Analysis
Patient demographic and clinical characteristics were also compared within the following patient sub-groups: 1) HIV-infected versus HIV-uninfected patients; and 2) patients who did versus did not receive definitive doses of RT, defined here in this study as an equivalent dose in 2-Gy fractions (EQD2) of ≥60 Gy, assuming an α/β ratio of 10. Continuous variables were compared with the Mann-Whitney test, while categorical variables were compared via χ2 tests, as appropriate. All statistical analyses were performed with SPSS Version 24 (IBM Corp, Armonk, NY). For all analyses, the threshold for statistical significance was P < .05.
Actuarial rates of overall survival (OS) were calculated via Kaplan-Meier method, computed from the date of pathologic diagnosis, with patients censored at last contact or healthcare facility visit. Log-rank tests were used to evaluate potential differences between groups. To minimize follow-up loss, vital status was confirmed through verifying medical records or contacting next-of-kin via phone, for selected cases. Patient and treatment factors were assessed for associations with OS via Cox proportional hazards modeling, with hazard ratios (HRs) calculated for univariate and multivariable analyses.
Acute (<3 months from RT) and late (≥3 months from RT) toxicities were prospectively assessed and graded according to the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. Documented events specific to HNC included: xerostomia, odynophagia, dysphagia, fatigue, weight loss, pain, and dermatitis. Logistic regression analysis was employed to identify potential associations between clinical or treatment variables and toxicity events, with odds ratios (ORs) and 95% confidence intervals (CIs) calculated.
RESULTS
Patient and Disease Characteristics
A total of 149 pathologically confirmed HNC patients were identified, with a median follow-up time of 23 months (Table I). All patients were citizens of Botswana currently residing within the country. The median distance of patient resident district or sub district to treating facility was 197 kilometers (IQR: 67–441 kilometers). Median age was 53 years (interquartile range [IQR]: 42–61), and 60% of patients were male. Most patients (74%) denied active tobacco-use. Roughly half (53%) had good or excellent performance status (KPS 90–100), yet 46% were anemic (Hgb < 12 g/dL) and 57% had low body mass index (BMI) (<20 kg/m2) at presentation. Many patients were also symptomatic with either pain (50%) or bleeding (17%).
TABLE I.
Relationship of clinical and treatment factors of study patients with head-and-neck cancers in Botswana to survival (n = 149).
| Variable | Patients n (%) | Overall Survival, UVA | Overall Survival, MVA | ||
|---|---|---|---|---|---|
| HR (95% CI) | P Value* | HR (95% CI) | P Value* | ||
| HIV status | |||||
| Positive | 59 (40) | 1.14 (.69–1.89) | .606 | ||
| Negative | 90 (60) | ||||
| Sex | |||||
| Male | 89 (60) | 1.18 (.70–1.98) | .54 | ||
| Female | 60 (40) | ||||
| Age (years) | |||||
| Median (IQR) | 53 (42–61) | ||||
| >50 | 81 (54) | 1.17 (.71–1.93) | .548 | ||
| ≤50 | 68 (46) | ||||
| BMI (kg/m2)† | |||||
| Median (IQR) | 19 (16–23) | ||||
| <20 | 48 (57) | 1.74 (.85–3.55) | .129 | ||
| ≥ 20 | 36 (43) | ||||
| Hemoglobin† | |||||
| Median (IQR) | 12 (10–13) | ||||
| <12 (g/dL) | 52 (46) | 2.73 (1.56–4.77) | <.001 | 2.74 (1.53–4.91) | .001 |
| ≥12 (g/dL) | 62 (54) | ||||
| Weight (kg)† | |||||
| Median (IQR) | 54 (46–63) | ||||
| <50 | 40 (42) | 1.49 (.82–2.70) | .187 | ||
| ≥50 | 56 (58) | ||||
| KPS | |||||
| Median (range) | 90 (50–100) | ||||
| 50–80 | 70 (47) | .99 (.60–1.66) | .997 | ||
| 90–100 | 79 (53) | ||||
| Primary stage‡ | |||||
| T4 | 41 (60) | 1.32 (.57–3.03) | .518 | ||
| Non-T4 | 27 (40) | ||||
| Stage (AJCC VII)‡ | |||||
| IV | 38 (66) | .86 (.37–2.02) | .73 | ||
| Non-IV | 20 (34) | ||||
| Nodal disease‡ | |||||
| Positive | 55 (37) | .79 (.47–1.35) | .395 | ||
| Negative | 94 (63) | ||||
| Histology | |||||
| SCC | 107 (72) | 1.46 (.80–2.66) | .213 | 1.90 (.92–3.90) | .081 |
| Non-SCC | 42 (28) | ||||
| RT Indication | |||||
| Definitive | 43 (29) | 0.76 (.43–1.33) | .335 | ||
| Non-definitive | 58 (39) | ||||
| None | 48 (32) | ||||
| RT course length‡ | |||||
| Median, days (IQR) | 57 (46–62) | ||||
| >8 weeks | 25 (58) | .60 (.23–1.57) | .299 | ||
| ≤8 weeks | 18 (42) | ||||
| Definitive EQD2‡ | |||||
| Median, Gy (IQR) | 70 (66–70) | ||||
| ≥70 Gy | 28 (65) | .78 (.30–2.06) | .617 | ||
| <70 Gy | 15 (35) | ||||
| Time from diagnosis to RT† | |||||
| Median, Mos (IQR) | 2.5 (1.6–5.4) | ||||
| ≥3 months | 18 (42) | 1.50 (.58–3.90) | .405 | ||
| <3 months | 25 (58) | ||||
| Surgery | |||||
| Yes | 12 (8) | .17 (.02–1.20) | .075 | .29 (.04–2.15) | .226 |
| No | 137 (92) | ||||
| Chemotherapy | |||||
| Yes | 29 (19) | 1.04 (.56–1.92) | .899 | ||
| No | 120 (81) | ||||
| Tuberculosis | |||||
| Yes | 17 (11) | 1.10 (.52–2.32) | .796 | ||
| No | 132 (89) | ||||
| Married | |||||
| Yes | 33 (22) | .91 (.49–1.68) | .766 | ||
| No | 116 (78) | ||||
| Tobacco use | |||||
| Yes | 39 (26) | .70 (.37–1.31) | .264 | .67 (.33–1.40) | .288 |
| No | 110 (74) | ||||
| Alcohol use | |||||
| Yes | 31 (21) | .98 (.52–1.84) | .953 | ||
| No | 118 (79) | ||||
| Prior malignancy | |||||
| Yes | 9 (6) | .82 (.30–2.27) | .706 | ||
| No | 140 (94) | ||||
| Painful symptoms | |||||
| Yes | 75 (50) | 1.29 (.78–2.12) | .322 | 1.21 (.68–2.15) | .513 |
| No | 74 (50) | ||||
| Bleeding symptoms | |||||
| Yes | 25 (17) | .84 (.44–1.61) | .6 | ||
| No | 124 (83) | ||||
| Treatment year | |||||
| 2017–2019 | 79 (53) | 1.12 (.67–1.87) | .663 | ||
| 2015–2016 | 70 (47) | ||||
| H&N Sub-Site | Patients (%) | Median OS (95% CI) | 2-Year OS (% ± SE) |
|---|---|---|---|
| All patients | 149 | 36.2 months (20.6–51.8) | 58.2% (±4.4%) |
| Oral cavity | 55 (37) | 36.2 (N/A) | 52.9% (±7.1%) |
| Larynx | 14 (9) | 25.6 (16.0–35.2) | 51.9% (±15.8%) |
| Salivary | 14 (9) | N/A | 84.4% (±10.2%) |
| Oropharynx | 13 (9) | 25.2 (13.3–37.2) | 51.6% (±16.4%) |
| Nasopharynx | 13 (9) | 34.2 (8.0–60.5) | 57.7% (±14.7%) |
| Orbit | 13 (9) | 47.0 (N/A) | 61.5% (±13.5%) |
| Skin | 9 (6) | N/A | 75.0% (±15.3%) |
| Sinonasal | 7 (5) | 13.0 (0.0–26.1) | 38.1% (±19.9%) |
| Unknown (neck) | 7 (5) | 20.4 (19.1–21.6) | 41.7% (±22.2%) |
| Thyroid | 4 (3) | N/A | N/A |
Cox proportional hazards analysis.
Data not available for all patients.
Staging limited by lack of comprehensive work-up, including fiberoptic nasopharyngolaryngoscopy and/or axial diagnostic imaging for all patients.
BMI = body mass index; CI = confidence interval; HR = hazard ratio; IQR = interquartile range; MVA = multivariable; SCC = squamous cell carcinoma; UVA = univariate.
By anatomic subsite, these HNC cases included 55 oral cavity, 14 laryngeal, 14 salivary, 13 oropharyngeal, 13 nasopharyngeal, 13 orbital, 9 skin, 7 sinonasal, 7 unknown primaries (presenting with nodal disease of the neck), and four thyroid malignancies. Orbital tumors included pre-orbital, conjunctival, and/or eyelid tumors. Squamous cell carcinoma was the histology among 72% of cases. All orbital tumors with known histology were SCCs. Among the subset of patients for whom staging data were available (n = 68), 41 (60%) presented with T4 disease (per American Joint Committee on Cancer [AJCC] 7th Edition). While axial diagnostic imaging (e.g. CT Neck with contrast) was not available for many patients, 37% presented with clinically apparent nodal disease; however, chest X-rays were available to assess lung involvement, with only one patient noted to have metastatic disease.
Treatment Characteristics and Delays
Most patients failed to receive evidence-supported curative management. Only 8% underwent surgical resection for the following subsites: salivary gland (n = 5), oral cavity (n = 3), thyroid (n = 3), and orbit (n = 2). While 101 patients received RT (including one post-op case), 58 (39%) were treated with non-definitive doses (EQD2 < 60 Gy) versus 43 (29%) treated definitively– with only 28 (65%) of the latter prescribed ≥70 Gy. Furthermore, definitive RT was associated with extended treatment durations (median course length: 57 days [IQR: 46–62]), with 58% treated over >8 calendar weeks; as well as significant treatment delays (median time from diagnosis-to-RT: 2.5 months [IQR: 1.6–5.4]), with 42% delayed ≥3 months.
Nineteen percent of patients received chemotherapy as part of their treatment (all concurrent with RT) for the following subsites: oral cavity (n = 13), nasopharynx (n = 7), larynx (n = 4), oropharynx (n = 3), salivary gland (n = 1), and orbit (n = 1). Patients receiving definitive RT were also more likely to receive chemotherapy as part of their treatment, versus those not receiving definitive RT doses (49% vs. 7.5%; P < .001). While treatments were analyzed by what was actually received, 13 patients did not fully complete their intended treatment course due to toxicity limitations: nine did not finish their prescribed RT fractions, and six missed one or more courses of chemotherapy.
Patient and Treatment Characteristics by HIV Status
Of the 149 study patients, 59 (40%) were HIV-infected (Table II); most of whom were on ART (85%) and had suppressed viral loads (90% with ≤400 copies/mL). The median CD4 count was 400 cells/mm3 (IQR: 237–584). Most clinical factors were similar among HIV-infected and non-infected patients (Table II). However, anatomic subsite distributions varied by HIV status: as compared to their HIV-negative counterparts, the HIV-positive patients had a higher proportion of orbital tumors (17% vs. 3%), but were less likely to present with oropharyngeal (3% vs. 13%) and nasopharyngeal (3% vs. 12%) primaries (P = .012). HIV status did not impact receipt of therapy (surgery, chemotherapy, or RT) or time-to-initiation of definitive RT (P = .440).
TABLE II.
Differences Among HIV-Positive (n = 59) Versus HIV-Negative Patients (n = 90).
| Variable | HIV-Positive (n = 59) No. Patients (%) | HIV-Negative (n = 90) No. Patients (%) | P Value |
|---|---|---|---|
| On ART? | |||
| Yes | 50 (85) | ||
| No | 9 (15) | ||
| Viral load* | |||
| >400 | 3 (10) | ||
| ≤400 | 36 (90) | ||
| CD4 count* | |||
| Median (IQR) | 400 (237–584) | ||
| Age (years) | |||
| Median, (IQR) | 49 (41–56) | 55 (41–64) | .084† |
| Sub-site | |||
| Oral cavity | 24 (41) | 31 (34) | .002‡ |
| Larynx | 7 (12) | 7 (8) | |
| Salivary | 6 (10) | 8 (9) | |
| Oropharynx | 2 (3) | 11 (12) | |
| Nasopharynx | 2 (3) | 11 (12) | |
| Orbit | 11 (19) | 2 (2) | |
| Skin | 1 (2) | 8 (9) | |
| Sinonasal | 1 (2) | 6 (7) | |
| Unknown (neck) | 4 (7) | 3 (3) | |
| Thyroid | 1 (2) | 3 (3) | |
| Histology | |||
| SCC | 45 (76) | 62 (69) | .327§ |
| Non-SCC | 14 (24) | 28 (31) | |
| RT dose | |||
| Definitive‖ | 13 (22) | 30 (33) | .137§ |
| Non-definitive/none | 46 (78) | 60 (67) | |
| Time to RT (months) | |||
| Median (IQR) | 4.1 (1.8–7.4) | 2.3 (1.6–3.8) | .440† |
| Surgery | |||
| Yes | 6 (10) | 6 (7) | .542§ |
| No | 53 (90) | 84 (93) | |
| KPS* | |||
| 50–80 | 30 (55.5) | 40 (45.5) | .290§ |
| 90–100 | 25 (45.5) | 48 (54.5) | |
| Primary stage¶ | |||
| T4 | 12 (60) | 29 (60) | .974§ |
| Non-T4 | 8 (40) | 19 (40) | |
| Stage (AJCC VII)¶ | |||
| IV | 13 (72) | 25 (62.5) | .471§ |
| Non-IV | 5 (28) | 15 (37.5) | |
| Nodal disease¶ | |||
| Positive | 21 (36) | 34 (38) | .787§ |
| Negative | 38 (64) | 56 (62) | |
| Chemotherapy | |||
| Yes | 9 (15) | 20 (22) | .293§ |
| No | 50 (85) | 70 (78) | |
| Prior malignancy | |||
| Yes | 6 (10) | 3 (3) | .088§ |
| No | 53 (90) | 87 (97) | |
Data not available for all patients.
Continuous variables were compared with the Mann-Whitney test.
Categorical variables were compared via Fisher’s exact test.
Categorical variables were compared via χ2 tests.
Defined as EQD2 ≥60 Gy.
Staging limited by lack of comprehensive work-up, including fiberoptic nasopharyngolaryngoscopy and/or axial diagnostic imaging for all patients.
Radiation Toxicity
Among the 101 patients treated with RT, the rates of acute grade ≥ 2, acute grade ≥ 3, and any late toxicities were 44%, 30%, and 11%, respectively (Table III). Grade ≥ 3 toxicities were associated with mucosal subsite (OR 4.04, P = .03) and lower BMI (<20 kg/m2) [OR 6.04, P = .012]; and lower RT dose (EQD2 < 60 Gy) was associated with decreased likelihood of acute grade ≥ 2 events (OR 0.35, P = .012). Notably, toxicities were not found to be associated with HIV-status, anemia (Hgb < 12 g/dL), higher KPS (90–100), low body weight (<50 kg), or receipt of chemotherapy among this study population. Acute grade ≥ 2 and acute grade ≥ 3 were significantly lower in more recently treated patients (OR 0.20, P < .001 and OR 0.12, P < .001).
TABLE III.
Details of Patients who Received Radiation Therapy and Associations with Toxicity (n = 101).
| Variable | Acute, Grade ≥ 2 OR (P value)* | Acute, Grade ≥ 3 OR (P value)* | Any Late Event OR (P value)* |
|---|---|---|---|
| Patients (%) | 44 (44%) | 30 (30%) | 11 (11%) |
| Primary sub-site | |||
| Mucosal† | 2.44 (.075) | 4.04 (.034) | 1.54 (.595) |
| Non-mucosal | |||
| HIV status | |||
| Positive | 1.60 (.279) | 0.96 (.922) | 0.83 (.795) |
| Negative | |||
| RT length (days) | |||
| >8 weeks | 1.77 (.166) | 1.95 (.135) | 2.40 (.175) |
| ≤8 weeks | |||
| EQD2 Dose | |||
| <60 Gy | 0.35 (.012) | 0.44 (.065) | 0.38 (.145) |
| ≥60 Gy | |||
| BMI (kg/m2)‡ | |||
| <20 | 3.37 (.032) | 6.04 (.012) | 1.09 (.919) |
| ≥20 | |||
| Hemoglobin‡ | |||
| <12 (g/dL) | 0.45 (.087) | 0.74 (.527) | 0.47 (.314) |
| ≥12 (g/dL) | |||
| Weight (kg)‡ | |||
| <50 | 1.40 (.503) | 1.61 (.344) | 0.44 (.333) |
| ≥50 | |||
| KPS‡ | |||
| 50–80 | 0.81 (.601) | 0.85 (.710) | 0.34 (.131) |
| 90–100 | |||
| Primary stage§ | |||
| T4 | 0.81 (.697) | 0.73 (.577) | 0.20 (.064) |
| Non-T4 | |||
| Stage (AJCC VII)§ | |||
| IV | 0.39 (.142) | 0.32 (.084) | 1.00 (.999) |
| Non-IV | |||
| Nodal disease§ | |||
| Positive | 0.69 (.378) | 1.00 (.996) | 0.99 (.984) |
| Negative | |||
| Chemotherapy | |||
| Yes | 1.93 (.138) | 2.12 (.107) | 1.49 (.554) |
| No | |||
| Patient age | |||
| >50 years | 1.24 (.598) | 1.34 (.515) | 1.28 (.710) |
| ≤50 years | |||
| Treatment year | |||
| 2017–2019 | 0.20 (<.001) | 0.12 (<.001) | 0.254 (.091) |
| 2015–2016 |
Logistic regression analysis.
Defined as oral cavity, larynx, oropharynx, nasopharynx, or sinonasal tumors.
Data not available for all patients.
Staging limited by lack of comprehensive work-up, including fiberoptic nasopharyngolaryngoscopy and/or axial diagnostic imaging for all patients.
OR = odds ratio; RT = radiation therapy.
OS
The median OS for the entire study cohort was 36.2 months (95% CI: 20.6 – 51.8), with a 2-year OS rate of 58% (Fig. 1). On multivariable analysis, anemia (Hgb < 12 g/dL) was associated with worse survival on multivariable analysis (HR 2.74, P = .001). Notably, HIV status was not demonstrated to be associated with OS (Fig. 2), with no appreciable difference noted between HIV-infected versus HIV-uninfected patients (3-year OS: 51.8% vs. 51.2%, P = .606).
Fig. 1.
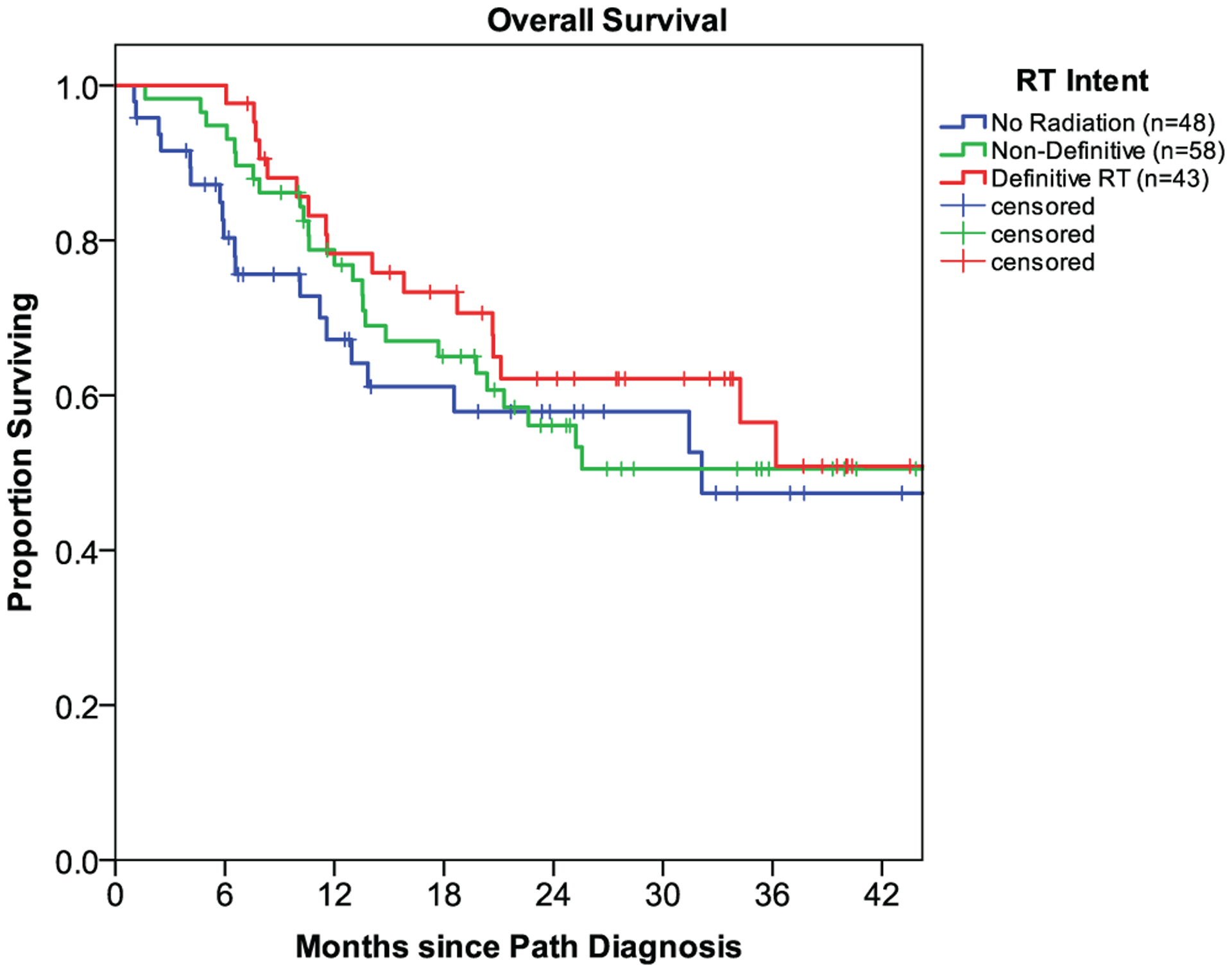
Survival outcomes of head-and-neck cancer patients in Botswana by radiation therapy intent: no radiation (n = 48), non-definitive RT (n = 58), and definitive RT (n = 43).a Abbreviations: RT, radiation therapy; Path, pathological.
Fig. 2.
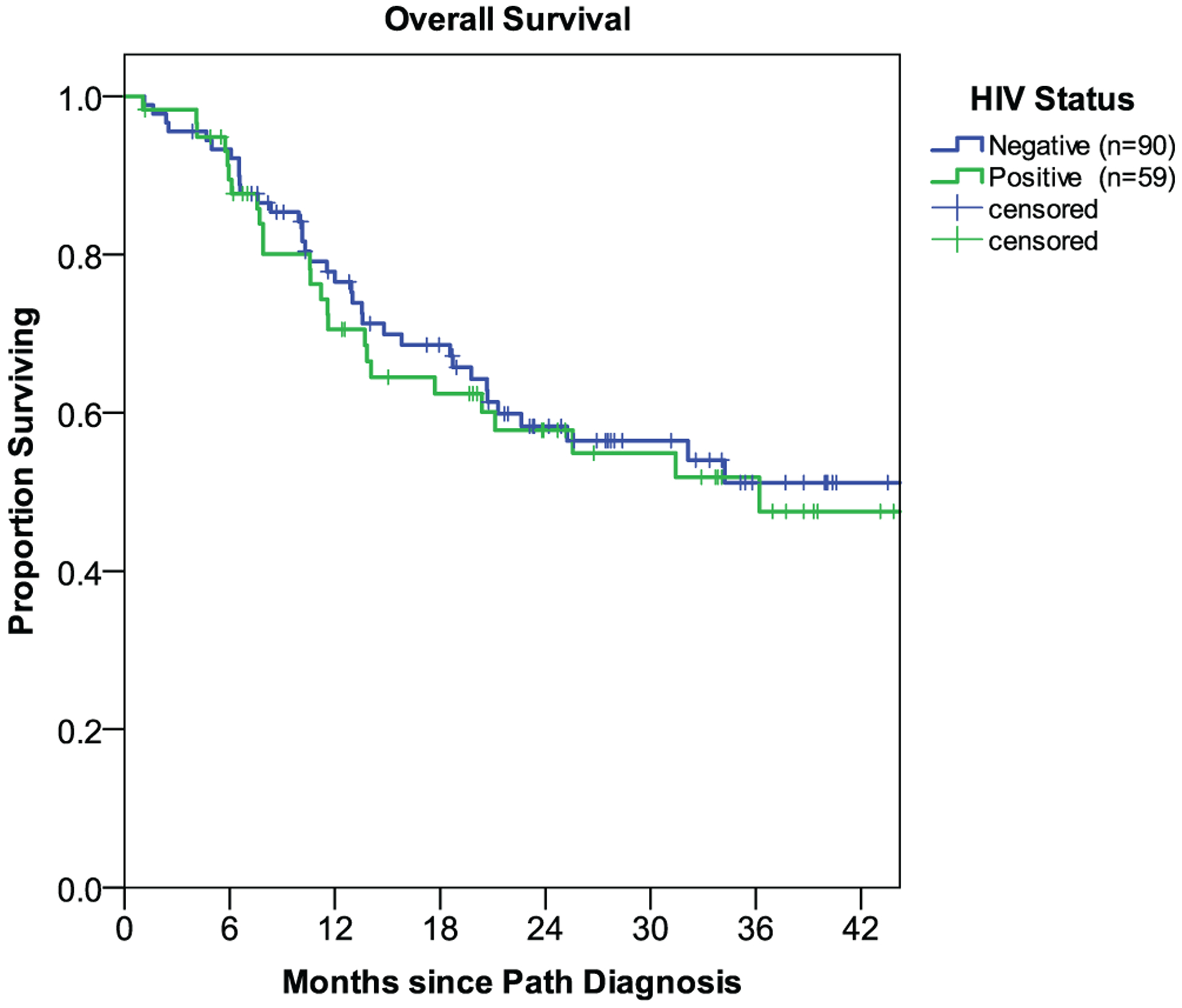
Survival outcomes by HIV status: infected (n = 59) versus uninfected (n = 90).
DISCUSSION
HNCs are one of the most common malignancies worldwide, associated with 650,000 new diagnoses and 330,000 deaths per year, with 30% of newly diagnosed cases occurring in LMICs1,19–26; however, there is a paucity of data regarding the management and outcomes of HNCs within these settings.27 Furthermore, data are limited regarding the treatment of HNC specifically among HIV-infected patients, who comprise a growing demographic among cancer patients in the modern era of ART. Simultaneously addressing these literature voids, our study is the first prospective evaluation of treatment patterns and survival outcomes for HNC patients within: 1) a resource-limited SSA country, and 2) a population with high HIV-burden, providing novel insights for both uncharacterized scenarios.
One of the most important prognostic factors in HNC treatment is clinical stage at presentation, which directs evidence-based stage-specific oncologic management. Unfortunately, comprehensive staging work-up, including HPV status, was not available for most study patients. Within the minority of patients (46%) for whom staging data were available, 60% presented with advanced disease (T4 primaries). The late presentations of this study population are also supported by high proportions of anemic (57%), underweight (57%), and actively symptomatic (50%) patients. Their comorbidities and poor performance statuses preclude aggressive curative treatment, particularly without access to ancillary services to address disease- and treatment-related complications (e.g. percutaneous endoscopic gastrostomy, enteral feeding, nutritional support, speech pathology, and tracheostomy).
Unfortunately, these data are concordant with prior reports indicating that 54% of all cancer patients in Botswana present with advanced-stage disease,3 despite the fact that 90% of the population has ready access to government-sponsored healthcare.28 This does not account for the estimated 45% of patients in Botswana who receive no cancer-directed therapy.29 Barriers to timely oncologic care in Botswana have been previously described,6,30 including limited cancer awareness, lack of diagnostic urgency, logistical hurdles to specialty care access, physical distance to specialized care facilities, schedule uncertainty, and limited provider availability. Similar barriers resulting in delayed presentation have been described among other LMICs in Africa31–33. Based on a recent systematic analysis by Beaudoin et al., additional barriers, which may influence this population but have not been previously described include level of education and use of alternative medicine.27 Taken together, these data advocate for educational and infrastructural interventions to promote early diagnosis, timely referrals, comprehensive staging work-up, and evidence-based multidisciplinary care for these patients.
Given the late presentation of many cases, the relatively poor survival among this HNC population is not surprising. The majority patients also appeared to lack 1) comprehensive staging work-up for appropriate risk and treatment stratification, and/or 2) multimodality oncologic treatment. Surgery and chemotherapy are common cornerstones in definitive HNC management; however, only 8% of patients underwent surgery (due to a paucity of oncologically-specialized otolaryngologists), while only 19% received chemotherapy (as a result of toxicity concerns). Of those who received RT, the majority (57%) were treated with non-definitive doses (defined here as an EQD2 < 60 Gy). Even among those treated definitively, significant treatment delays were noted (2.5 months to RT start) and 35% received doses less than 70 Gy - the literature-supported dose for many HNC subsites. Taken together, these discrepancies contributed to the poor outcomes and lack of benefit noted among RT subgroups.
Aside from access-related concerns, our data provide some reassurance for the HIV-infected population, as HIV-status did not appear to affect survival or toxicity outcomes among this study population. Interestingly, the rate of HIV infection among our cohort (40%) was higher than that of the general Botswana adult population aged 15 years and older (22.2% in 2019).11 This discrepancy is potentially attributable to an increased prevalence of HNC among HIV-infected patients ([~2–3 fold).34–37 However, the high rate of ART utilization mirrored that of the general Botswana population (85%),11 and HIV-infected patients in the studied cohort had similar KPS and disease characteristics as their HIV-uninfected counterparts. Regarding treatment, HIV patients were no more-or-less likely to receive definitive RT or experience treatment-related toxicity than those not infected with HIV. Taken together, these findings appear to indicate that HIV-status should not preclude definitive management of patients with HNC.
The primary limitation of this study is the relatively modest sample size and follow-up, which may have affected the ability to detect survival differences among groups. The specific cause of death for these patients was not captured, although most if not all are assumed to result directly from tumor-related complications or progression, given the advanced presentations and suboptimal treatment paradigms among this population. The heterogeneity in HNC primaries and lack of comprehensive staging work-up for many patients also limited granular comparisons across/within various subsites and tumor stages, and likely contributed to difficulties in optimally tailoring treatments. In addition, the lack of HPV testing, an important prognostic indicator in HNC that has been associated with HIV infection in other populations,38,39 limits exploration of any potential role of HPV infection in this cohort. Furthermore, the poor survival among this population may conceal the potential impact of HIV on outcomes. Nevertheless, these prospectively collected data offer a unique perspective into practice patterns, survival, and toxicity among HNC patients in a resource-limited setting with a high prevalence of HIV-infection.
CONCLUSION
These findings highlight the late presentation, delayed treatment, and suboptimal outcomes among HNC patients in Botswana despite government-sponsored care-alarming figures likely echoed across other LMIC and SSA settings. Educational and infrastructural interventions are warranted to promote earlier detection, timely referrals, access to comprehensive staging work-up, and evidence-based multidisciplinary care for these patients. Notably, a disproportionately high number of our patients were infected with HIV, but HIV-status did not appear to affect survival or toxicity outcomes among our patients and should thus not preclude definitive management for this HNC population.
Acknowledgments
The Mentored Patient Oriented Career Research Development Award (1-K08CA230170-01A1), Department of Radiation Oncology, University of Pennsylvania, and Sub-Saharan African Collaborative HIV and Cancer Consortia-U54 (1U54 CA190158-01) funded this effort.
Footnotes
AL has no conflicts of interest regarding the material in this manuscript, but does report being on an advisory board for Ion Beam applications (paid honorarium) and conducting education for Provision Healthcare (paid honorarium).
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Portions of this study will be presented in oral form at the 2020 American Society of Radiation Oncologists annual meeting (Virtual, October 2020).
BIBLIOGRAPHY
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Ervik M, Lam F, et al. Cancer today. Global cancer observatory. Accessed January 26, 2020. https://gco.iarc.fr/today [Google Scholar]
- 3.Brown CA, Suneja G, Tapela N, et al. Predictors of timely access of oncology services and advanced-stage cancer in an HIV-endemic setting. Oncologist 2016;21:731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atun R, Jaffray DA, Barton MB, et al. Expanding global access to radiotherapy. Lancet Oncol 2015;16:1153–1186. [DOI] [PubMed] [Google Scholar]
- 5.Fidler MM, Bray F, Soerjomataram I. The global cancer burden and human development: a review. Scand J Public Health 2018;46:27–36. [DOI] [PubMed] [Google Scholar]
- 6.Anakwenze C, Bhatia R, Rate W, et al. Factors related to advanced stage of cancer presentation in Botswana. J Glob Oncol 2018;4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deeken JF, Tjen-A-Looi A, Rudek MA, et al. The rising challenge of non-AIDS-defining cancers in HIV-infected patients. Clin Infect Dis 2012;55: 1228–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007;370:59–67. [DOI] [PubMed] [Google Scholar]
- 9.Powles T, Robinson D, Stebbing J, et al. Highly active antiretroviral therapy and the incidence of non-AIDS-defining cancers in people with HIV infection. J Clin Oncol 2009;27:884–890. [DOI] [PubMed] [Google Scholar]
- 10.Botswana - Botswana AIDS Impact Survey IV 2013 - Overview. Accessed December 9, 2019. http://botswana.microdatahub.com/index.php/catalog/14
- 11.AIDSinfo 2019. UNAIDS. Accessed September 18, 2020. https://aidsinfo.unaids.org/
- 12.Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al. Cancer incidence following expansion of HIV treatment in Botswana. PLoS One 2015;10:e0135602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Efstathiou JA, Heunis M, Karumekayi T, et al. Establishing and delivering quality radiation therapy in resource-constrained settings: the story of Botswana. J Clin Oncol 2016;34:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grover S, MacDuffie EC, Wang Q, et al. HIV infection is not associated with the initiation of curative treatment in women with cervical cancer in Botswana. Cancer 2019;125:1645–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grover S, Bvochora-Nsingo M, Yeager A, et al. Impact of human immunodeficiency virus infection on survival and acute toxicities from Chemoradiation therapy for cervical cancer patients in a limited-resource setting. Int J Radiat Oncol Biol Phys 2018;101:201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization, Department of HIV/AIDS. Interim WHO Clinical Staging of HVI/AIDS and HIV/AIDS Case Definitions for Surveillance: African Region. Geneva: World Health Organization; 2005. https://apps.who.int/iris/bitstream/handle/10665/69058/WHO_HIV_2005.02.pdf [Google Scholar]
- 18.Makhema J, Wirth KE, Pretorius Holme M, et al. Universal testing, expanded treatment, and incidence of HIV infection in Botswana. N Engl J Med 2019;381:230–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.da Lilly-Tariah OB, Somefun AO, Adeyemo WL. Current evidence on the burden of head and neck cancers in Nigeria. Head Neck Oncol 2009;1:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amusa YB, Olabanji JK, Akinpelu VO, et al. Pattern of head and neck malignant tumours in a Nigerian teaching hospital-A ten year review. West Afr J Med 2004;23:280–285. [DOI] [PubMed] [Google Scholar]
- 21.Iseh KR, Malami SA. Pattern of head and neck cancer in Sokoto. Niger J Otorhinolaryngol 2006;3:77–83. [Google Scholar]
- 22.Ahmad BM, Pindiga UH. Malignant neoplasms of the ear, nose and throat in north eastern Nigeria. Highl Med Res J 2004;2:45–48. [Google Scholar]
- 23.Onotai LO, Nwogbo AC. Primary head and neck malignant tumours in Port Harcourt, Nigeria: a revisit. J Med Med Sci 2012;3:122–125. [Google Scholar]
- 24.Kanu OO, Nnoli MA, Asoegwu CA. Prevalence of head and neck tumours in Calabar, south eastern Nigeria. Asian J Med Sci 2016;7:123–126. [Google Scholar]
- 25.Arotiba JT, Olusanya AA, Lawal AO, Akinmoladun VI. Trends of oral cancer in university college hospital, Ibadan, Nigeria. Studies 1983;11:15. [Google Scholar]
- 26.Okoh DS, Orikpete EV, Omoregie OF, Ojo MA. A study of the clinicopathologic patterns of Orofacial carcinomas in a Nigerian population. Afr J Oral Maxillofac Pathol Med 2015;1:10–17. [Google Scholar]
- 27.Beaudoin P-L, Anchouche S, Gaffar R, Guadagno E, Ayad T, Poenaru D. Barriers in access to Care for Patients with Head and Neck Cancer in resource-limited settings: A systematic review. JAMA Otolaryngol Head Neck Surg 2020;146:291–297. 10.1001/jamaoto.2019.4311. [DOI] [PubMed] [Google Scholar]
- 28.Suneja G, Ramogola-Masire D, Medhin HG, Dryden-Peterson S, Bekelman JE. Cancer in Botswana: resources and opportunities. Lancet Oncol 2013;14:e290–e291. [DOI] [PubMed] [Google Scholar]
- 29.Dryden-Peterson S, Botebele K, Iyer H, Mmalane M, Lockman S, Tapela N. Estimating the Full Cancer Burden: Quantifying Cancer Cases Not Receiving Treatment in Botswana. Presented at the: AORTIC 2017; 7–10 November 2017; Kigali, Rwanda. http://aorticconference.org/wp-content/uploads/2017/10/2017-AORTIC-Abstracts.pdf [Google Scholar]
- 30.Brown CA, Kohler RE, John O, et al. Multilevel factors affecting time to cancer diagnosis and care quality in Botswana. Oncologist 2018;23:1453–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Onyango JF, Macharia IM. Delays in diagnosis, referral and management of head and neck cancer presenting at Kenyatta National Hospital, Nairobi. East Afr Med J 2006;83:85–91. [DOI] [PubMed] [Google Scholar]
- 32.Adeyi A, Olugbenga S. The challenges of managing malignant head and neck tumors in a tropical tertiary health center in Nigeria. Pan Afr Med J 2011;10:31. [PMC free article] [PubMed] [Google Scholar]
- 33.Larsen-Reindorf R, Owusu-Afriyie O, Acheampong AO, Boakye I, Awuah B. Others. A six-year review of head and neck cancers at the Komfo Anokye teaching hospital, Kumasi, Ghana. Int J Otolaryngol Head Neck Surg 2014; 3:271. [Google Scholar]
- 34.Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst 2000;92:1500–1510. [DOI] [PubMed] [Google Scholar]
- 35.Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst 2009;101:1120–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engsig FN, Gerstoft J, Kronborg G, et al. Head and neck cancer in HIV patients and their parents: a Danish cohort study. Clin Epidemiol 2011;3: 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nwaorgu O, Kokong D, Onakoya P, Adoga S, Ibekwe T. Prevalence of human immunodeficiency virus seropositivity in head and neck malignancies in sub-Saharan Africa. Acta Otolaryngol 2007;127:1218–1221. [DOI] [PubMed] [Google Scholar]
- 38.Beachler DC, Weber KM, Margolick JB, et al. Risk factors for oral HPV infection among a high prevalence population of HIV-positive and at-risk HIV-negative adults. Cancer Epidemiol Biomarkers Prev 2012;21:122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kreimer AR, Alberg AJ, Daniel R, et al. Oral human papillomavirus infection in adults is associated with sexual behavior and HIV serostatus. J Infect Dis 2004;189:686–698. [DOI] [PubMed] [Google Scholar]


