Abstract
Purpose:
Previously our randomized Phase III trial demonstrated that immunotherapy including dinutuximab, a chimeric anti-GD2 monoclonal antibody, granulocyte macrophage-colony stimulating factor (GM-CSF), and interleukin-2 (IL2) improved survival for children with high-risk neuroblastoma that had responded to induction and consolidation therapy. These results served as the basis for FDA approval of dinutuximab. We now present long-term follow-up results and evaluation of predictive biomarkers.
Patients and Methods:
Patients recieved 6 cycles of isotretinoin with or without 5 cycles of immunotherapy which consists of dinutuximab with GM-CSF alternating with IL2. Accrual was discontinued early due to meeting the protocol-defined stopping rule for efficacy, as assessed by 2-year event-free survival (EFS). Plasma levels of dinutuximab, soluble IL2 receptor (sIL2R) and human anti-chimeric antibody (HACA) were assessed by ELISA. Fcγ receptor 2A and 3A genotypes were determined by PCR and direct sequencing.
Results:
For 226 eligible randomized patients, 5-year EFS was 56.6±4.7% for patients randomized to immunotherapy (n=114) versus 46.1±5.1% for those randomized to isotretinoin only (n=112) (p=0.042). Five-year overall survival (OS) was 73.2±4.2% versus 56.6±5.1% for immunotherapy and isotretinoin only patients, respectively (p=0.045). Thirteen of 122 patients receiving dinutuximab developed HACA. Plasma levels of dinutuximab, HACA, and sIL2R did not correlate with EFS/OS, or clinically significant toxicity. Fcγ receptor 2A and 3A genotypes did not correlate with EFS/OS.
Conclusions:
Immunotherapy with dinutuximab improved outcome for patients with high-risk neuroblastoma. Early stoppage for efficacy resulted in a smaller sample size than originally planned, yet clinically significant long-term differences in survival were observed.
Keywords: Anti-GD2, immunotherapy, neuroblastoma, HACA
INTRODUCTION
Survival from neuroblastoma, the most common extracranial solid tumor of childhood, has improved with multi-modality therapy. However, relapse is common for patients with high-risk disease(1). Dinutuximab is a chimeric monoclonal antibody that binds the disialoganglioside GD2 and activates complement (2). GD2 is a glycolipid that is strongly expressed on the surface of neuroblastoma cells with little intra- or inter-tumor heterogeneity in expression, while normal tissue expression is restricted to neurons, melanocytes, and peripheral pain fibers. Dinutuximab mediates antibody-dependent cellular cytotoxicity (ADCC) by neutrophils and natural killer (NK) cells. These effector cells bind antibody via cell surface Fcγ receptors (FcγRs), a family of cell surface glycoproteins mediating clearance and phagocytosis of immune complexes, ADCC and the release of inflammatory cytokines. The FcγR genes display polymorphisms that greatly influence the affinity of FcγR for IgG (3-5), which may contribute to the action of cytotoxic antibodies. Granulocyte-macrophage colony-stimulating factor (GM-CSF) enhances ADCC (6). Interleukin-2 (IL2) augments ADCC of neuroblastoma cells by NK cells (7).
Phase I studies of dinutuximab and GM-CSF (8), and dinutuximab plus GM-CSF alternating with IL2 and isotretinoin (9), led to a randomized Phase III trial of dinutuximab combined with alternating cycles of GM-CSF and IL2 followed by isotrentinoin in children with high-risk neuroblastoma [Children’s Oncology Group (COG) study ANBL0032]. This trial tested the hypothesis that anti-GD2 immunotherapy would eradicate minimal residual neuroblastoma that persisted following multi-agent induction chemotherapy, primary tumor resection, myeloablative chemotherapy, external beam radiotherapy, and isotretinoin. The initial results of this trial demonstrated that immunotherapy plus isotretinoin improved both EFS and OS (2), leading to FDA approval of ch14.18, renamed dinutuximab (Unituxin, United Therapeutics, Silver Spring, MD) in 2015. Here we report a long-term clinical update of this Phase III trial and the results of immune correlative analyses.
METHODS
Patient Enrollment:
This study (NCT00026312) opened in October 2001 and enrollment to the randomized portion ended in January 2009. All patients had high-risk neuroblastoma. Eligibility criteria included age <31 years, completion of intensive therapy including induction chemotherapy, high-dose chemotherapy followed by autologous stem cell transplant (ASCT), local radiotherapy, and at least partial remission without a history of progressive disease. Time from stem cell infusion to enrollment was recommended to be <100 days; time from start of induction therapy had to be ≤9 months. Patients were also required to have a total absolute phagocyte count (WBC x % [segs + bands + monos]) >1,000/μL following ASCT, performance score ≥50, life expectancy ≥2 months, and adequate organ function. Written informed consent was obtained from parents or guardians before the initiation of any study-related treatment or procedures, and the protocol was approved by the Ethics Committees from all participating institutions. The study was conducted in accordance with Good Clinical Practice principles, and the Declaration of Helsinki.
Study Design:
The trial involved a 1:1 randomization to 6 cycles of isotretinoin with or without 5 concomitant cycles of immunotherapy. Randomization occurred at the time of enrollment and was stratified on the basis of factors thought to potentially affect the post-transplantation outcome: response before ASCT, induction therapy protocol, number of ASCT cycles, and use of purged versus non-purged stem cells (10). Patients with biopsy-proven persistent disease after autologous stem-cell transplantation and radiotherapy were nonrandomly assigned to the immunotherapy group and were excluded from the primary outcome analyses. As previously reported (2), isotretinoin 160mg/m2/day was given orally for 14 days every 28 days for 6 cycles for patients assigned to isotretinoin-alone. The immunotherapy arm consisted of dinutuximab (25mg/m2/day x 4 days) given every 28 days in combination with GM-CSF (Leukine, Berlex/Bayer) (250mcg/m2/day x 14 days) in cycles 1, 3, and 5 or IL2 (Proleukin, Chiron) at 3x106 IU/m2/24 hours x 96 hours during the week prior to dinutuximab and at 4.5x106 IU/m2/24 hours x 96 hours during the dinutuximab. Dinutuximab (provided by the National Cancer Institute) was infused over 5-20 hours each day based on tolerability. Note, 25mg of dinutuximab provided as an experimental agent by the NCI was later determined to be equivalent to 17.5mg of the FDA-approved formulation of dinutuximab (Unituxin, United Therapeutics) (11).
Blood sampling for immune monitoring:
Fresh heparinized blood was obtained from patients receiving immunotherapy at the following time-points: pretreatment (day 1); day 6 (prior to 4th dinutuximab dose during cycle 1, corresponding to a “peak” dinutuximab level); day 80 (prior to first dose of IL2 during cycle 4, corresponding to a “trough” dinutuximab level); day 90 (prior to 4th daily dinutuximab dose during cycle 4, corresponding to a “peak” dinutuximab level); day 111 (prior to initial dose of GM-CSF during cycle 5, corresponding to a “trough” dinutuximab level); day 118 (prior to 4th daily dinutuximab dose during cycle 5, corresponding to a “peak” dinutuximab level). These time-points and their relationship to protocol therapy are summarized in Supplemental Table 1. Blood samples were collected from patients randomized to isotrentinoin alone prior to the 4th cycle and end of therapy. Sample size varies depending on the number of specimens available at each time point.
Dinutuximab levels:
Dinutuximab levels were measured in plasma samples by ELISA as previously described (8,9).
Human anti-chimeric antibodies (HACA) reactivity:
HACA levels were estimated in plasma samples by the ELISA “bridging assay” as published previously (8,9,12) for all patients entered into this trial that received dinutuximab and provided plasma samples. Briefly, for these assays OD values <0.3 were considered negative; OD values ≥0.3 but <1.0 were considered intermediate and OD values ≥1.0 were considered strong.
Soluble IL2 Receptor-alpha (sIL2R) levels:
sIL2R levels in plasma were measured as reported previously (8,9,13).
Fc gamma receptor polymorphisms:
The region surrounding the polymorphic codon 158 of FcγRIIIA (FCGR3A, rs396991) (5) and codon 131 of FcγRIIA (FCGR2A, rs1801274) (14) were selectively amplified, purified and genotyped by direct sequence analysis, as shown below:
A). FcγR3A (NG_009066.1):
FcγRIIIA was selectively amplified over the highly homologous FcγRIIIB (AH003573) using a FcγRIIIA specific antisense primer in combination with a common sense primer (4983F). The genotype of codon 158 was assessed by direct sequence analysis with primers 5128F and 5390R. Specific amplification of FcγRIIIA over FcγRIIIB is validated by assessing the sequence of polymorphic codons 129, 140, 148, which differ between the two genes.
Primers for FcγR3A
3A-X5R-C 5′-CCT TCC AGT CTC TTG TTG AGC TTC G -3′
4983F 5′-CTC AGG ATC TGG GTG GTA CG -3′
5128F 5′-TGA GGT GTC ACA GCT GGA AG -3′
5390R 5′-AGT GTG ATT GCA GGT TCC ACT -3′
B). FcγR2A (AH003095.2):
FcγRIIA was selectively amplified over the relatively dissimilar isoforms FcγRIIB (AH005422.2) or FcγRIIC (AH002832.2) using FcγRIIA selective primers Fc2A-JF and FC2A-JR. The genotype of codon 131 was assessed by direct sequence analysis with FcγRIIA selective primer FC2A-JiR. Specific amplification of FcγRIIA was validated by assessing the sequence of the multiple polymorphic codons unique to FcγRIIA.
Primers for FcγR2A
Fc2A-JF 5′-GGA AAA TCC cAG AAA TTc TCG C -3′
Fc2A-JR 5′-CAA CAG CCT GAC TAC CTA TTA CGC GGG -3′
Fc2A-JiR 5′-AGC TCT GGC CCC TAC TTG TT -3
C). Amplification conditions:
Amplifications were performed using 50 ng genomic DNA extracted from MNCs or granulocytes using the Gentra DNA isolation kit (Qiagen). Fc receptors were amplified in a 50 μl reaction mixture consisting of 2 mM MgSO4, 100 μM 4dNTP, 10 pmol each sense and antisense primer, 1.25 units Platinum Taq DNA Polymerase High Fidelity (Invitrogen) in 1X Platinum Taq DNA Polymerase High Fidelity buffer. Reactions were denatured at 94°C for 2 min, then cycled 35X at 94°C (30 sec), [64°C (15 sec) for FcγRIIIA, 55°C (60 sec) FcγRIIA] and 68°C (120 sec).
PCR products were purified using Qiagen QiaQuick or Zymo DNA Clean and Concentrate DNA purification columns and sequenced either at the Moores-UCSD Cancer Center shared sequencing resource or at Retrogen Inc. (San Diego).
Fcγ genotypes were correlated with EFS/OS of patients based on actual treatment received using a log-rank test.
Statistical analysis:
The primary analysis was an intention-to-treat comparison of EFS between the two randomized treatment groups (2). The study was designed to enroll 386 randomized patients, with 80% power using a two-sided log-rank test with alpha=0.05 (or one-sided test with alpha=0.025) to detect an absolute difference of 15% in 3-year EFS between the two randomized groups.
Sequential monitoring of the intention-to-treat population was performed as previously reported. The study met criteria for early stopping owing to efficacy, and randomization was halted (2). Patients on the isotretinoin-alone arm were permitted to cross-over to receive antibody after randomization was stopped. Patients with biopsy-proven persistent disease after ASCT and radiotherapy were non-randomly assigned to the immunotherapy group and were excluded from the primary outcome analyses.
EFS time was defined as the time from study enrollment until first occurrence of relapse, progressive disease, secondary cancer, or death or, if none occurred, until last contact. OS time (secondary endpoint) was defined as the time from study enrollment until death, or last contact if death did not occur. Kaplan–Meier survival curves (15) [reported as point estimates ± standard error (SE)] were compared by treatment group using a one-sided log-rank test; all other survival comparisons were two-sided. Cross-over patients from the isotretinoin-alone arm who received antibody were censored at the start of antibody therapy in survival analyses. Patient characteristics at baseline were compared between randomized groups using a chi-square test. P-values <0.05 were considered statistically significant.
Wilcoxon signed-rank tests were performed to test for differences in median levels of dinutuximab and sIL2R between timepoints. The data tables for these plasma assays indicate the number of patients with evaluable samples at the specified timepoints. Cox proportional hazards models were used to test for association between EFS and dinutuximab levels at each timepoint. Wilcoxon rank-sum tests compared levels of dinutuximab or sIL2R at each timepoint between a) patients in the randomized cohort that received dinutuximab, with vs. without occurrence of a dose-limiting toxicity (DLT) and, b) between HACA positive vs. negative patients. DLT was defined as ≥Grade 4 allergic reaction, vascular leak syndrome, or persistent neuropathic pain ≥4 days after completing ch14.18 infusion, or ≥Grade 3 motor neuropathy for ≥2 weeks. Log-rank tests were used to compare EFS and OS between HACA positive vs. negative patients. Associations between occurrence of a DLT at any time during treatment and HACA positivity were tested with a Fisher’s exact test. Comparisons were two-sided, except for tests of HACA versus dinutuximab levels which were one-sided based on findings (12) that strong anti-drug antibody responses (like HACA) can neutralize detection of plasma levels of the therapeutic drug, resulting in lower dinutuximab levels in HACA positive patients.
Except as otherwise stated, analyses were performed using SAS version 9.4.
RESULTS
Characteristics of Randomized Patients:
Two hundred and fifty-seven patients were enrolled; enrollment was stopped prior to enrolling the planned 386 patients due to statistical evidence of efficacy (2). Four patients were deemed ineligible and 27 with persistent disease were non-randomly assigned to immunotherapy. These 31 patients were excluded from all analyses, resulting in an analytic cohort of 226 patients randomized to receive isotrentinoin and immunotherapy (n=114) or isotrentinoin-alone (n=112). There were no significant differences in baseline characteristics between randomized cohorts (Table 1). Four patients were permitted to cross-over to the immunotherapy arm after the randomization was stopped. Six patients randomized to immunotherapy refused protocol therapy and received isotrentinoin only; seven patients randomized to isotrentinoin-alone received immunotherapy.
Table 1.
Characteristics and tests of association for 226 study participants at baseline, according to randomized treatment group
| Characteristic | Baseline Comparability | Outcomes | ||||||
|---|---|---|---|---|---|---|---|---|
| Isotretinoin Only (N=112) |
Immunotherapy (N=114) |
P-value& | No. of Patients (N=226) |
5-year EFS ± SE |
P- value&,* |
5-year OS ± SE |
P- value&,* |
|
| n (%)& | n (%)& | % | % | |||||
| Treatment Arm | ||||||||
| Immunotherapy | 114 (50.4) | 56.6 ± 4.7 | 0.042 | 73.2 ± 4.2 | 0.045 | |||
| Isotretinoin Only | 112 (49.6) | 46.1 ± 5.1 | 56.6 ± 5.1 | |||||
| Age at Diagnosis | ||||||||
| <18 months | 21 (18.8) | 13 (11.4) | 0.12 | 34 (15.0) | 73.5 ± 7.6 | 0.0087 | 73.5 ± 7.6 | 0.099 |
| ≥18 months | 91 (81.3) | 101 (88.6) | 192 (85.0) | 47.3 ± 3.8 | 63.6 ± 3.7 | |||
| INSS* | ||||||||
| 2 | 0 (0.0) | 4 (3.8) | 0.80 | 31 (14.5) | 83.4 ± 7.1 | 0.0009 | 86.8 ± 6.4 | 0.021 |
| 3 | 15 (13.9) | 10 (9.4) | ||||||
| 4S | 0 (0.0) | 2 (1.9) | ||||||
| 4 | 93 (86.1) | 90 (84.9) | 183 (85.5) | 46.7 ± 3.8 | 62.1 ± 3.8 | |||
| Unknown | 4 | 8 | ||||||
| MYCN | ||||||||
| Not Amplified | 52 (54.7) | 53 (59.6) | 0.51 | 105 (57.1) | 55.3 ± 5.1 | 0.31 | 72.3 ± 4.6 | 0.19 |
| Amplified | 43 (45.3) | 36 (40.5) | 79 (42.9) | 49.1 ± 5.8 | 60.3 ± 5.7 | |||
| Unknown | 17 | 25 | ||||||
| Histology | ||||||||
| Favorable | 5 (6.0) | 4 (5.1) | 1.00 | 9 (5.6) | 77.8 ± 13.9 | 0.15 | 77.8 ± 13.9 | 0.33 |
| Unfavorable | 79 (94.1) | 74 (94.9) | 153 (94.4) | 53.5 ± 4.2 | 68.2 ± 4.0 | |||
| Unknown | 28 | 36 | ||||||
| Ploidy | ||||||||
| Hyperdiploid | 49 (52.7) | 49 (57.7) | 0.51 | 98 (55.1) | 54.7 ± 5.2 | 0.3235 | 70.8 ± 4.8 | 0.06 |
| Diploid | 44 (47.3) | 36 (42.4) | 80 (44.9) | 46.1 ± 5.8 | 55.8 ± 5.8 | |||
| Unknown | 19 | 29 | ||||||
| Pre-ASCT Response^ | ||||||||
| CR | 37 (33.0) | 41 (36.0) | 0.94 | 174 (77.0) | 56.1 ± 3.9 | 0.0055 | 69.5 ± 3.7 | 0.0026 |
| VGPR | 49 (43.8) | 47 (41.2) | ||||||
| PR | 26 (23.2) | 26 (22.8) | 52 (23.0) | 35.6 ± 6.7 | 51.1 ± 7.2 | |||
| No. of ASCTs | ||||||||
| 1 | 104 (92.9) | 107 (93.9) | 0.76 | 211 (93.4) | 50.0 ± 3.6 | 0.13 | 64.9 ± 3.4 | 0.39 |
| 2 | 8 (7.1) | 7 (6.1) | 15 (6.6) | 72.2 ± 13.5 | 69.3 ± 13.6 | |||
| No. of Purged Infusions | ||||||||
| 1 | 28 (32.6) | 28 (31.5) | 0.88 | 56 (32.0) | 58.1 ± 6.9 | 0.24 | 70.1 ± 6.4 | 0.83 |
| 0 | 58 (67.4) | 61 (68.5) | 119 (68.0) | 50.4 ± 4.6 | 66.4 ± 4.4 | |||
| Unknown | 26 | 25 | ||||||
All p-values for INSS are reported for stage 4 vs. stage 2, 3, or 4S.
All p-values for pre-ASCT response are reported for CR or VGPR vs. PR.
Percentages and P-values calculated on the basis of subjects with known data for each characteristic (with patients with “Unknown” status not included).
Two-sided log-rank test p-values are reported except for comparison by treatment arm, which is one-sided.
Event-free and Overall Survival:
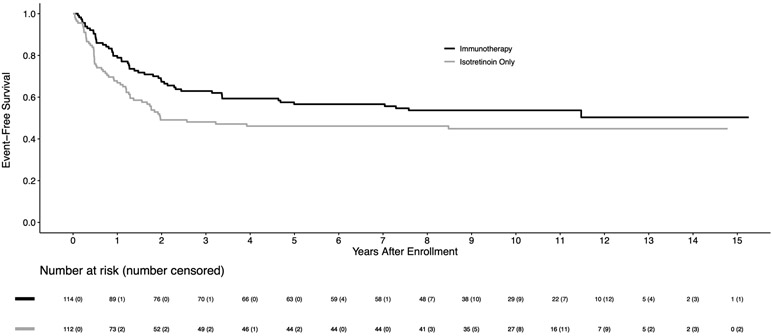
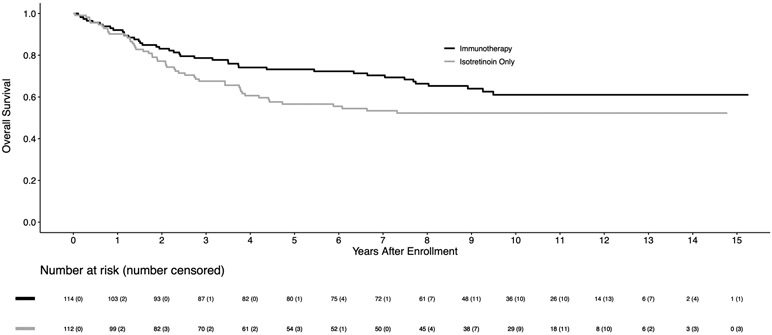
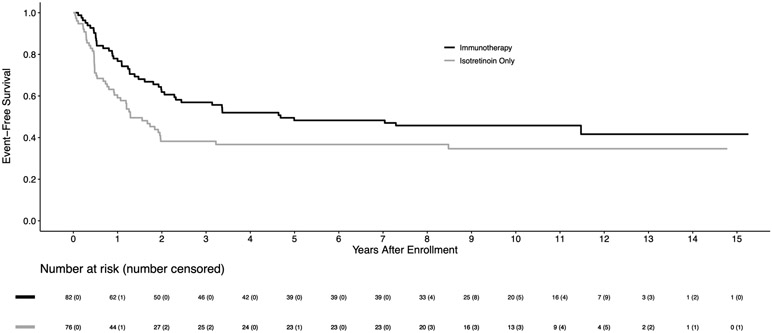
For 226 eligible randomized patients, the 5-year EFS and OS estimates were 51.4±3.5% and 65.2±3.3%, respectively. The median follow-up time in the 113 patients alive without an event was 9.97 (range 0.7–15.3) years. Among baseline characteristics, only age at diagnosis, disease stage, and pre-ASCT response had significant impact on the clinical outcomes (Table 1). The EFS for 183 stage 4 patients was significantly worse than for non-stage 4 patients (5-year EFS: 46.7±3.8% vs. 83.4±7.1%; p=0.0009). For 174 patients in CR/VGPR pre-ASCT, EFS was superior to those in PR (5-year EFS: 56.1±3.9% vs. 35.6±6.7%; p=0.0055). For the randomized comparison, the 5-year EFS was 56.6±4.7% for immunotherapy versus 46.1±5.1% for isotretinoin only (p=0.042; Figure 1A). The 5-year OS was 73.2±4.2% for immunotherapy vs. 56.6±5.1% for isotretinoin only (p=0.045; Figure 1B). As shown in Figure 1A, early relapses occurred more frequently in the isotretinoin group than in the immunotherapy group, with 93.3% vs. 69.8% of events, respectively, occurring within the initial 2 years. After 2 years, there were 4 and 16 events in the isotretinoin and immunotherapy arms, respectively. All events were due to relapse/progression, except for one patient in each group with a secondary malignancy. For 158 stage 4, ≥18 months old patients, EFS (5-year: 48.3±5.6% vs. 36.7±6.1%; p=0.022) and OS (70.1±5.2% vs. 49.1±6.3%; p=0.025) were statistically significantly higher in patients assigned to immunotherapy compared to those assigned to isotretinoin (Figures 2A, 2B).
Figure 1A:
Kaplan-Meier curves of EFS by randomized treatment arm (n=226), with four patients censored at crossover, on COG study ANBL0032
Figure 1B:
Kaplan-Meier curves of OS by randomized treatment arm (n=226), with four patients censored at crossover, on COG study ANBL0032
Figure 2A:
Kaplan-Meier curves of EFS for patients who are ≥18 months old at diagnosis with INSS stage 4 disease (n=158), by randomized treatment arm on COG study ANBL0032
Figure 2B:
Kaplan-Meier curves of OS for patients who are ≥18 months old at diagnosis with INSS stage 4 disease (n=158), by randomized treatment arm on COG study ANBL0032
Laboratory studies:
Dinutuximab levels:
Table 2 shows trough (days 80, 111) and peak (days 6, 90, 118) dinutuximab levels obtained from the 6 timepoints (Supplemental Table 1). Formal pharmacokinetic parameters could not be assessed from these available peak and trough values. Dinutuximab trough levels were higher than the pre-treatment level (p<0.0001), indicating the persistence of dinutuximab for 2.5 weeks following the prior dinutuximab dose and consistent with previously published detailed PK evaluations (16). Peak levels of dinutuximab during GM-CSF-containing cycles (1 and 5) were similar, but the peak value on D90 during the IL2-containing cycle (cycle 4) was lower (p<0.005). There was no significant correlation of either peak or trough dinutuximab levels with DLT or EFS (Supplemental Table 2).
Table 2.
Dinutuximab and sIL2R plasma levels over time
| Dinutuximab Plasma Levels | ||||||
|---|---|---|---|---|---|---|
| Day of plasma collection | ||||||
| Pre- treatment |
D6 (peak) |
D80 (trough) |
D90 (peak) |
D111 (trough) |
D118 (peak) |
|
| N | 127 | 117 | 104 | 96 | 88 | 91 |
| Mean ± SD (mcg/ml) | 0.094±0.41 | 7.9±2.8 | 1.0±0.76 | 6.9±2.9 | 1.1±0.72 | 8.8±3.6 |
| Median (mcg/ml) | 0.010* | 7.6^ | 0.98& | 6.9^ | 1.0& | 9.2^ |
| Range (mcg/ml) | 0 – 4.2 | 0 – 18.5 | 0 – 5.5 | 0 – 14.7 | 0 – 4.1 | 0 – 16.8 |
| sIL2R Plasma Levels | ||||||
| Day of plasma collection | ||||||
| Pre- treatment |
D6 (peak) |
D80 (trough) |
D90 (peak) |
D111 (trough) |
D118 (peak) |
|
| N | 126 | 122 | 105 | 101 | 88 | 91 |
| Mean ± SD (ng/ml) | 2.6 ± 2.3 | 8.0 ± 4.0 | 6.2 ± 5.3 | 20.0 ± 10.1 | 5.3 ± 4.6 | 8.7 ± 5.9 |
| Median (ng/ml) | 2.2$ | 7.2 | 5.1 | 19.4@ | 4.6 | 7.5 |
| Range (ng/ml) | 0.8-25.0 | 0.3-25.3 | 0.08 – 41.8 | 1.9 – 46.1 | 1.1-43.3 | 1.5-47.4 |
The dinutuximab pre-treatment value is lower than all other time points (p<0.0001 for each comparison)
Median dinutuximab peak levels (just after 3 of 4 daily dinutuximab doses; see Suppl. Table-1 for clarification of peak): D90 < D6 (p<0.005); D90 < D118 (p<0.005); D6 < D118 (p<0.01).
Median dinutuximab trough levels (just before the subsequent cycle of dinutuximab; see Suppl. Table-1 for clarification of trough): D80 and D111 are not significantly different (p=0.042).
Median sIL2R pretreatment level is lower than all other time points (p<0.0001)
Median sIL2R D90 level is higher than all other time points (p<0.0001)
sIL2R levels:
sIL2R is released by IL2 responsive cells when activated by IL2. The sIL2R levels were significantly greater during IL2 containing cycles (day 90) compared to the 3 GM-CSF containing cycles and a pre-IL2 level (day 80; Table 2). The sIL2R values for day 6 (GM-CSF containing) were significantly greater than pretreatment levels, consistent with the ability of GM-CSF to induce endogenous IL2 (13). The trough levels following either a GM-CSF or IL2 containing cycle (day 80 and day 111) also remained greater than the pretreatment levels (p<0.0001), indicating sustained and sufficient activation of the endogenous IL2 system to cause continued release of sIL2R. There was no correlation between sIL2R level and DLT status at any time.
HACA:
Detectible HACA responses were rare; 8 and 5 patients developed strong and intermediate responses, respectively (Supplemental Table 3). No HACA reactivity was detected during cycle 1 (day 6) while HACA was detected in 6/105 (5.7%) by cycle 3 (day 80) and 9/91 (9.9%) by cycle 5 (day 118). The presence of HACA was associated with significantly lower trough and peak levels of dinutuximab during cycle 4 (days 80, 90) and 5 (days 111 and 118) (Supplemental Table 4). There was no association of HACA reactivity with EFS or OS (p≥0.41, Supplemental Figure 1), or with occurrence of DLT (Supplemental Table 5). When the characteristics shown in Table 1 were compared in the patients who did or did not develop HACA, no statistically significant differences were found. Following the detection of HACA, the peak plasma levels of dinutuximab in cycle 4 (day 90) and cycle 5 (day 118) of 6 HACA positive patients were <10% of the expected value; namely the peak dinutuximab level in cycle 1 (day 6) for the same patient (Supplemental Table 6) demonstrated that the HACA response, once developed, could neutralize detectible dinutuximab in vivo. Detection of a HACA response at any time after starting treatment was associated with higher levels of sIL2R on day 6 (p=0.0022) and day 90 (p=0.010) (Supplemental Table 7).
Fc gamma receptor polymorphisms:
Polymorphisms of the FCGR2A at codon 131 and FCGR3A at codon 158 are summarized in Table 3. The frequency of the FCGR2A and FCGR3A genotypes was similar in the immunotherapy and isotrentinoin only groups, consistent with that reported in the literature (17). No association between FCGR2A or FCGR3A genotype with survival was identified in either treatment group. Similarly, categorization of patients with heterozygous genotypes as high or low affinity had no association with outcome (data not shown). Patients were also categorized as having a high affinity profile if they did not harbor a homozygous low affinity genotype of either receptor (18) or if they had a homozygous high affinity FCGR2A or FCGR3A genotype, regardless of the low allele genotype (Tables 4 and 5). While slightly different distribution profiles were observed using the two criteria, neither profile was associated with outcome (data not shown).
Table 3:
SNPs of the FCGR2A (CD32) and FCGR3A (CD16) and their distribution relative to treatment stratification.
| FCGR2A (CD32) Genotype Frequency | ||||||
|---|---|---|---|---|---|---|
| FCGR2A genotype H131R (rs1801274) |
Codon 131 |
Predicted Receptor Affinity (14) |
Frequency | |||
| Literature (17) | Isotretinoin only (N=88)* |
Immunotherapy (N=86)* |
||||
| GG | Arg/Arg | CGT/CGT | Low | 26% | 21 (24%) | 20 (23%) |
| AG | His/Arg | CAT/CGT | Mixed | 48% | 43 (49%) | 38 (44%) |
| AA | His/His | CAT/CAT | High | 26% | 24 (27%) | 28 (33%) |
| FCGR3A (CD16) Genotype Frequency | ||||||
| FCGR3A genotype V158F (rs396991) |
Codon 158 |
Predicted Receptor Affinity (14) |
Frequency | |||
| Literature (17) | Isotreinoin only (N=87)* |
Immunotherapy (N=87)* |
||||
| TT | Phe/Phe | TTT/TTT | Low | 46% | 36 (41%) | 39 (45%) |
| TG | Phe/Val | GTT/TTT | Mixed | 40% | 45 (52%) | 36 (41%) |
| GG | Val/Val | GTT/GTT | High | 14% | 6 (7%) | 12 (14%) |
Number of patients with available specimens;
Table 4:
Sorting criteria for combining FCGR32A (CD32) + FcGR3A (CD16) genotype status.
| Fc2a codon 131 | ||||
|---|---|---|---|---|
| Siebert sort criteria (18) |
AA (High) His/His |
AG (His/Arg) |
GG (Low) Arg/Arg |
|
| Fc3A codon 158 | G/G (high) Val/Val | H | H | L |
| T/G Phe/Val | H | H | L | |
| T/T (low) Phe/Phe | L | L | L | |
| Fc2a codon 131 | ||||
| Yu et al. sort criteria (this manuscript) |
AA (High) His/His |
AG (His/Arg) |
GG (Low) Arg/Arg |
|
| Fc3A codon 158 | G/G (high) Val/Val | H | H | H |
| T/G Phe/Val | H | L | L | |
| T/T (low) Phe/Phe | H | L | L | |
FCGR32A (CD32) and FcGR3A (CD16) genotype distribution were sorted based on the genotype of both receptors to create high vs. low affinity “profiles”. As defined by Siebert et al (18), a patient harbors a high affinity (H) profile as long as they do not contain either homozygous low affinity (L) alleles. As defined in this manuscript, a patient is high affinity as long as at least one of the two receptors is homozygous high affinity receptor.
Table 5:
FCGR2A (CD32) + FCGR3A (CD16) Genotype Frequency
| Affinity Profile | Frequency | |
|---|---|---|
| Isotreinoin only n=85^ n (%) |
Immunotherapy n=83^ n (%) |
|
| Siebert et al. (18) High Affinity | 40 (47%) | 42 (51%) |
| Siebert et al. (18) Low Affinity | 45 (53%) | 41 (49%) |
| Yu et al. Sort High Affinity | 28 (33%) | 32 (39%) |
| Yu et al. Sort Low Affinity | 57 (67%) | 51 (61%) |
The sorting criteria in Table 4 result in slightly different patient profile distributions.
number of samples with both FCGR2A (CD32) + FCGR3A (CD16) data.
DISCUSSION
The results of our randomized phase III trial of immunotherapy with dinutuximab, IL2, and GM-CSF have changed the standard of care for patients with high-risk neuroblastoma (2) and led to FDA approval of dinutuximab. Our long-term follow-up data confirm superior EFS and OS for newly diagnosed high-risk neuroblastoma patients randomized to immunotherapy compared to those randomized to isotretinoin-alone, including the cohort of stage 4 patients. It is noteworthy that 13 of 226 (6%) randomized patients did not adhere to the randomized treatment they were assigned. This should be considered when evaluating the intent-to-treat results reported here. Nontheless, our findings attest to the important role of dinutuximab-based immunotherapy in high-risk neuroblastoma.
With longer follow-up, the improved EFS and OS associated with immunotherapy reported are decreased compared to our original 2010 report, due to late relapses in the immunotherapy group. Delayed relapses following immunotherapy with dinutuximab were also observed in a German study (19) in which the EFS declined from 50% at 2 years to 41% at 9 years. Downregulation of GD2 in bone marrow reported in a minority of patients may account for the relpases. Strategies to reduce late relapse following dinutuximab-based immunotherapy include increasing the number of immunotherapy cycles or delivering higher doses of an anti-GD2 antibody, but this could expose patients to more dinutuximab-associated toxicities (2,11). However, hu14.18K322A, a humanized ch14.18 with a point mutation (K322A) that reduces complement-dependent lysis and allodynia (20), is dosed approximately 3-fold higher than the dose of dinutuximab that was administered in our trial and promising preliminary efficacy data in patients with relapsed or refractory neuroblastoma have been reported (20). Alternatively, addition of agents that may act synergistically with anti-GD2 immunotherapy should be considered. A recent COG study combining dinutuximab with chemotherapy led to an impressive 41.5% response rate in 53 patients with recurrent/refractory neuroblastoma (21), a finding consistent with the response rate observed when chemotherapy was combined with hu14.18K322A (22). High levels of polyamines and ornithine decarboxylase (ODC) activity are found in many human cancers including neuroblastoma. Eflornithine α-Difluoromethylornithine (DFMO), an inhibitor of ODC, was shown to inhibit neuroblastoma initiation and progression in mice (23). DFMO also ameliorates anti-GD2 antibody-induced allodynia (24), making combination of dinutuximab and DFMO an attractive strategy. This approach is being studied in an ongoing COG trial (NCT03794349). Separate preclinical studies have also shown synergism between anti-GD2 antibodies in combination with anti-PD1 checkpoint blockade (25) further supporting future combinatorial therapy trials designed to enhance the efficacy of GD2-directed immunotherapy and reduce toxicities.
We found that sIL2R levels were significantly elevated over baseline levels during GM-CSF containing cycles, suggesting that dinutuximab + GM-CSF treatment induced an endogenous IL2 response. This was reported previously with administration of GM-CSF alone (13) in patients with refractory cancers and in other clinical settings (26). As IL2 is known to activate NK cells to mediate ADCC in vitro (7), the induction of sIL2R in the absence of IL2 administration suggests that some of the desired effects from IL2 administration might also be induced in vivo by the dinutuximab + GM-CSF combination. Furthermore, toxicity associated with IL2 containing cycles is significantly worse than toxicity associated with GM-CSF containing cycles (2). In addition, clinical data from two randomized European trials (27,28) showed no added benefit when IL2 was added to dintuximab beta therapy. Although dinutuximab beta is produced in Chinese hamster ovary (CHO) cells and differs slightly in glycosylation compared to dinutuximab used in our trial (made with the same antibody plasmid construct but in murine hybridoma cells), the data still suggest that the addition of exogenous IL2 to dinutuximab in this setting may not be necessary. Data from a series of non-randomized single-arm trials of a murine anti-GD2 antibody, 3F8, suggest that GM-CSF may augment the activity of immunotherapy in children with high-risk neuroblastoma (29). Taken together, our trial results and those of other investigators have prompted COG to eliminate IL2 from dinutuximab-based post-consolidation therapy, but retain GM-CSF as a way to augment the activity of the GD2-directed antibody.
Although large increases in anti-GD2 antibody-based dosing can slightly augment the response to in vivo ADCC in preclinical mouse models, clinical studies have not consistently shown a dose effect in anti-GD2 antibody trials (20). In our trial, neither peak nor trough dinutuximab levels correlated with progression or toxicity in this cohort. Trough dinutuximab levels remained detectable (~100-fold the concentration needed to induce ADCC in vitro) nearly 2.5 weeks after the last dinutuximab infusion in the prior cycle (Table 2). It is possible that the variations in dinutuximab levels between patients may not be large enough to detectably influence outcome. Alternatively, greater plasma dinutuximab levels may be associated with improved outcome in other settings. For example, we have recently shown that in patients with relapsed/refractory neuroblastoma receiving dinutuximab in combination with irinotecan/temozolomide, those with complete or partial responses had significantly higher trough dinutuximab levels than non-responders (21).
In this study, neither trough nor peak concentrations of dinutuximab correlated with the incidence of antibody-related toxicities. The etiology of dinutuximab-related toxicity is likely multifactorial. While pain is related to complement activation upon binding of dinutuximab to GD2 on nerve fibers (30), interpatient variability in the level of GD2 expression on nerve fibers, differences in downstream pain signaling pathways and variation in levels of complement components may also affect the magnitude of pain that is experienced. In addition, some of the immunotherapy-related toxicities are attributable to the effects of IL2 and GM-CSF. Polymorphisms in the genes encoding receptors for these cytokines or components of their downstream signaling pathways may influence the frequency and severity of the toxicities experienced by different patients. Taken together, these factors may impact toxicity to a greater degree than does the level of dinutuximab, resulting in the absence of a detectable association between dinutuximab level and regimen-related toxicity. Despite the known toxicities of dinutuximab, long-term follow-up analyses indicate that all toxicities appear to resolve with time (30,31).
Patients treated with murine monoclonal antibodies often generate neutralizing human anti-mouse antibodies (HAMA) against the therapeutic antibody. Chimeric or humanized monoclonal-antibodies have been used to decrease the likelihood of developing a neutralizing antibody and mitigate the effects of anti-drug-antibodies by minimizing the number of xenogeneic antigenic epitopes recognizable on the monoclonal-antibody. The incidence of detectible HACA response in this study was relatively low, which may be due to the chimeric nature of dinutuximab. It may also reflect the effects of immunosuppressive therapy as part of induction and consolidation therapy received prior to dinutuximab administration. The rarity of HACA detected here is in contrast to the 33% rate of HACA development in adult melanoma patients following treatment with dinutuximab without prior chemotherapy exposure (12), and the 19% HACA rate in relapsed neuroblastoma patients receiving a genetically similar anti-GD2 antibody (dinutuximab-beta) that was produced in CHO cells (32). While the rate of HACA was low in our study, it was associated with diminished dinutuximab plasma levels, suggesting that the HACA response can neutralize dinutuximab in vivo. In analyses of neuroblastoma patients receiving the murine 3F8 mAb, a substantial fraction (75%) developed a HAMA response (33). Interestingly, even though strong HAMA responses lead to discontinuation of murine anti-GD2 therapy, patients that develop a HAMA response have better survival outcomes than those who do not. This may reflect induction of an anti-idiotype network response with resultant generation of endogenous “antibody-3”, which is cross-reactively able to recognize GD2 (33). In the current study, we saw no difference in outcome based on HACA status; however the number of HACA positive patients may be too small (Supplemental Figure 1) to detect a correlation. Separate analyses suggest the HACA response may be blunted when anti-GD2 is given concurrently with chemotherapy (21,22).
Polymorphisms of Fc receptors can influence antibody-binding affinity and ADCC mediated by NK cells and granulocytes, which can in turn impact therapeutic efficacy of antibody therapy (34). In patients with high-risk neuroblastoma treated with the murine 3F8, those with homozygous high affinity FcR2A genotypes had significantly longer PFS than those with the homozygous low affinity genotypes, but no such association was identified with respect to FcR3A genotype (35). A more recent trial demonstrated that high-risk neuroblastoma patients harboring a high-affinity FcR2A+FcR3A genotype “profile” had a better EFS compared to patients with low FcR2A+FcR3A genotype profile when treated with dinutuximab-beta (18). However, we did not find any relationship between Fc receptor genotypes and outcome in the current study. This is consistent with previous data showing that Fc receptor genotype had little consistent association with patient outcome even when the same antibody was used in different studies (34).
Genotype analyses from this trial did not show an association of inherited Killer Immunoglobulin-Like Receptors (KIR) genes and their KIR-ligands with outcome among patients that received immunotherapy. However these association analyses did suggest that this immunotherapy regimen may provide greater benefit for individuals with a certain combination of KIR/KIR-ligands than those without the potentially “favorable” genotype (36); these KIR and their ligands are known to influence NK function and ADCC. Work is ongoing to validate whether the association of KIR/KIR-ligand genotype and benefit from this immunotherapy regimen may extend to a separate non-randomized cohort of neuroblastoma patients treated with this same dinutuximab-based regimen on the same protocol, following cessation of randomization in 2009. Separately, analyses of additional factors/biomarkers (including evaluation of the tumor micro-environment, and of gene expression ) that impact these immunotherapeutic processes are also in progress.
Summary
This long-term follow-up study demonstrates that immunotherapy with dinutuximab in combination with cytokines is associated with an improvement in survival in children with high-risk neuroblastoma. However, the magnitude of the survival benefit for patients randomized to immunotherapy vs. isotretinoin has decreased over time due to late relapses. Further correlative biological studies may help to identify subsets of patients most likely to benefit from treatment with immunotherapy. Newer approaches to increase the antitumor efficacy and decrease the toxicity of anti-GD2-based immunotherapy have been identified with clinical trials underway.
Supplementary Material
Statement of Translational Relevance.
The primary objective of this extended follow-up of the randomized Phase III trial COG ANBL0032 is to provide important insights into the long-term benefits of immunotherapy with dinutuximab combined with cytokines. Previously, this randomized trial demonstrated that immunotherapy significantly improved 2 year event-free survival (EFS) and overall survival (OS) for children with high-risk neuroblastoma that had responded to induction and consolidation therapy. These results served as the basis for FDA approval of dinutuximab. With a median follow-up of 9.97 years, this randomized cohort of 226 patients confirmed better EFS and OS for those who received immunotherapy compared with those treated with isotrentinoin only. Although the differences in survival remain significant, the magnitude of survival benefit was smaller due to late relapses. Newer approaches to increase the antitumor efficacy and decrease the toxicities of anti-GD2-based immunotherapy, and identification of biomarkers for patients most likely to benefit from immunotherapy are being pursued.
Acknowledgement:
The authors thank Drs. Ralph Reisfeld and Stephen Gillies for their pioneering work in the development of ch14.18, facilitating its translation to clinical testing and regulatory approval as dinutuximab. We thank Biopharmaceutical Development Program of the NCI for producing ch14.18 for this clinical trial; Bayer for providing the GM-CSF. Thanks also to the COG operations office, COG treatment sites involved in this trial and patients and families that paraticipated in this trial. This work was supported by NIH/NCI Grants U10CA180899 (NCTN Statistics and Data Center), U10CA98543 (Children’s Oncology Group Chair’s Grant), U10CA180886 (NCTN Operations Center Grant) and the St. Baldrick’s Foundation, Alex's Lemonade Stand Foundation, Padres Foundation, the Lou Bridgeman Memorial Fund (AL Yu, MB Diccianni), grants R35 CA197078 and support from the Midwest Athletes against Childhood Cancer to the University of Wisconsin (PM Sondel), NCI R35 CA220500 (JM Maris).
Footnotes
Other information
The authors have not been paid to write this manuscript. The corresponding author had full access to all of the data and had final responsibility for the decision to submit for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The full protocol for this trial entitled “Isotretinoin With or Without Dinutuximab, Aldesleukin, and Sargramostim Following Stem Cell Transplant in Treating Patients With Neuroblastoma.” can be accessed by COG members at www.cogmembers.org.
Disclosure of Potential Conflicts of Interest:
A.L.Yu reports grant support from United Therapeutics for immune correlative studies during the conduct of the study. No potential conflicts of interest were disclosed by the other authors.
REFERENCES
- 1.Pinto NR, Applebaum MA, Volchenboum SL, Matthay KK, London WB, Ambros PF, et al. Advances in Risk Classification and Treatment Strategies for Neuroblastoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015;33(27):3008–17 doi 10.1200/JCO.2014.59.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. The New England journal of medicine 2010;363(14):1324–34 doi 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravetch JV, Perussia B. Alternative membrane forms of Fc gamma RIII(CD16) on human natural killer cells and neutrophils. Cell type-specific expression of two genes that differ in single nucleotide substitutions. The Journal of experimental medicine 1989;170(2):481–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu J, Edberg JC, Redecha PB, Bansal V, Guyre PM, Coleman K, et al. A novel polymorphism of FcgammaRIIIa (CD16) alters receptor function and predisposes to autoimmune disease. The Journal of clinical investigation 1997;100(5):1059–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koene HR, Kleijer M, Algra J, Roos D, von dem Borne AE, de Haas M. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood 1997;90(3):1109–14. [PubMed] [Google Scholar]
- 6.Barker E, Mueller BM, Handgretinger R, Herter M, Yu AL, Reisfeld RA. Effect of a chimeric anti-ganglioside GD2 antibody on cell-mediated lysis of human neuroblastoma cells. Cancer research 1991;51(1):144–9. [PubMed] [Google Scholar]
- 7.Hank JA, Robinson RR, Surfus J, Mueller BM, Reisfeld RA, Cheung NK, et al. Augmentation of antibody dependent cell mediated cytotoxicity following in vivo therapy with recombinant interleukin 2. Cancer research 1990;50(17):5234–9. [PubMed] [Google Scholar]
- 8.Ozkaynak MF, Sondel PM, Krailo MD, Gan J, Javorsky B, Reisfeld RA, et al. Phase I study of chimeric human/murine anti-ganglioside G(D2) monoclonal antibody (ch14.18) with granulocyte-macrophage colony-stimulating factor in children with neuroblastoma immediately after hematopoietic stem-cell transplantation: a Children's Cancer Group Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2000;18(24):4077–85 doi 10.1200/JCO.2000.18.24.4077. [DOI] [PubMed] [Google Scholar]
- 9.Gilman AL, Ozkaynak MF, Matthay KK, Krailo M, Yu AL, Gan J, et al. Phase I study of ch14.18 with granulocyte-macrophage colony-stimulating factor and interleukin-2 in children with neuroblastoma after autologous bone marrow transplantation or stem-cell rescue: a report from the Children's Oncology Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2009;27(1):85–91 doi 10.1200/JCO.2006.10.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreissman SG, Seeger RC, Matthay KK, London WB, Sposto R, Grupp SA, et al. Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): a randomised phase 3 trial. The Lancet Oncology 2013;14(10):999–1008 doi 10.1016/s1470-2045(13)70309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozkaynak MF, Gilman AL, London WB, Naranjo A, Diccianni MB, Tenney SC, et al. A Comprehensive Safety Trial of Chimeric Antibody 14.18 With GM-CSF, IL-2, and Isotretinoin in High-Risk Neuroblastoma Patients Following Myeloablative Therapy: Children's Oncology Group Study ANBL0931. Frontiers in immunology 2018;9(1355):1355 doi 10.3389/fimmu.2018.01355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albertini MR, Gan J, Jaeger P, Hank JA, Storer B, Schell K, et al. Systemic interleukin-2 modulates the anti-idiotypic response to chimeric anti-GD2 antibody in patients with melanoma. Journal of immunotherapy with emphasis on tumor immunology : official journal of the Society for Biological Therapy 1996;19(4):278–95 doi 10.1097/00002371-199607000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Schiller JH, Hank JA, Khorsand M, Storer B, Borchert A, Huseby-Moore K, et al. Clinical and immunological effects of granulocyte-macrophage colony-stimulating factor coadministered with interleukin 2: a phase IB study. Clinical cancer research : an official journal of the American Association for Cancer Research 1996;2(2):319–30. [PubMed] [Google Scholar]
- 14.Li X, Gibson AW, Kimberly RP. Human FcR polymorphism and disease. Current topics in microbiology and immunology 2014;382:275–302 doi 10.1007/978-3-319-07911-0_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan EL MP. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:451–8. [Google Scholar]
- 16.Marachelian A, Desai A, Balis F, Katzenstein H, Qayed M, Armstrong M, et al. Comparative pharmacokinetics, safety, and tolerability of two sources of ch14.18 in pediatric patients with high-risk neuroblastoma following myeloablative therapy. Cancer chemotherapy and pharmacology 2016;77(2):405–12 doi 10.1007/s00280-015-2955-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang W, Gordon M, Schultheis AM, Yang DY, Nagashima F, Azuma M, et al. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2007;25(24):3712–8 doi 25/24/3712 [pii] 10.1200/JCO.2006.08.8021. [DOI] [PubMed] [Google Scholar]
- 18.Siebert N, Jensen C, Troschke-Meurer S, Zumpe M, Juttner M, Ehlert K, et al. Neuroblastoma patients with high-affinity FCGR2A, -3A and stimulatory KIR 2DS2 treated by long-term infusion of anti-GD2 antibody ch14.18/CHO show higher ADCC levels and improved event-free survival. Oncoimmunology 2016;5(11):e1235108 doi 10.1080/2162402x.2016.1235108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simon T, Hero B, Faldum A, Handgretinger R, Schrappe M, Klingebiel T, et al. Long term outcome of high-risk neuroblastoma patients after immunotherapy with antibody ch14.18 or oral metronomic chemotherapy. BMC cancer 2011;11:21 doi 10.1186/1471-2407-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navid F, Sondel PM, Barfield R, Shulkin BL, Kaufman RA, Allay JA, et al. Phase I trial of a novel anti-GD2 monoclonal antibody, Hu14.18K322A, designed to decrease toxicity in children with refractory or recurrent neuroblastoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2014;32(14):1445–52 doi 10.1200/jco.2013.50.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mody R, Yu AL, Naranjo A, Zhang FF, London WB, Shulkin BL, et al. Irinotecan, Temozolomide, and Dinutuximab With GM-CSF in Children With Refractory or Relapsed Neuroblastoma: A Report From the Children's Oncology Group. J Clin Oncol 2020;38(19):2160–9 doi 10.1200/JCO.20.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Federico SM, McCarville MB, Shulkin BL, Sondel PM, Hank JA, Hutson P, et al. A Pilot Trial of Humanized Anti-GD2 Monoclonal Antibody (hu14.18K322A) with Chemotherapy and Natural Killer Cells in Children with Recurrent/Refractory Neuroblastoma. Clinical cancer research : an official journal of the American Association for Cancer Research 2017;23(21):6441–9 doi 10.1158/1078-0432.Ccr-17-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bassiri H, Benavides A, Haber M, Gilmour SK, Norris MD, Hogarty MD. Translational development of difluoromethylornithine (DFMO) for the treatment of neuroblastoma. Translational pediatrics 2015;4(3):226–38 doi 10.3978/j.issn.2224-4336.2015.04.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diccianni MB, Kempińska K, Gangoti JA, Yu AL, Sorkin LS. Anti-GD2 induced allodynia in rats can be reduced by pretreatment with DFMO. PloS one 2020;15(7):e0236115 doi 10.1371/journal.pone.0236115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siebert N, Zumpe M, Juttner M, Troschke-Meurer S, Lode HN. PD-1 blockade augments anti-neuroblastoma immune response induced by anti-GD2 antibody ch14.18/CHO. Oncoimmunology 2017;6(10):e1343775 doi 10.1080/2162402x.2017.1343775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teodorczyk-Injeyan JA, Sparkes BG, Mills GB, Peters WJ. Soluble interleukin 2-receptor alpha secretion is related to altered interleukin 2 production in thermally injured patients. Burns : journal of the International Society for Burn Injuries 1991;17(4):290–5. [DOI] [PubMed] [Google Scholar]
- 27.Ladenstein R, Potschger U, Valteau-Couanet D, Luksch R, Castel V, Yaniv I, et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): a multicentre, randomised, phase 3 trial. The Lancet Oncology 2018;19(12):1617–29 doi 10.1016/s1470-2045(18)30578-3. [DOI] [PubMed] [Google Scholar]
- 28.Lode HN, Valteau-Couanet D, Gray J, Luksch R, Wieczorek A, Castel V, et al. Randomized use of anti-GD2 antibody dinutuximab beta (DB) long-term infusion with and without subcutaneous interleukin-2 (scIL-2) in high-risk neuroblastoma patients with relapsed and refractory disease: Results from the SIOPEN LTI-trial. 2019. p 10014-. [Google Scholar]
- 29.Cheung NK, Cheung IY, Kushner BH, Ostrovnaya I, Chamberlain E, Kramer K, et al. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2012;30(26):3264–70 doi 10.1200/jco.2011.41.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding YY, Panzer J, Maris JM, Castaneda A, Gomez-Chiari M, Mora J. Transverse myelitis as an unexpected complication following treatment with dinutuximab in pediatric patients with high-risk neuroblastoma: A case series. Pediatr Blood Cancer 2018;65(1) doi 10.1002/pbc.26732. [DOI] [PubMed] [Google Scholar]
- 31.Tse BC, Navid F, Billups CA, O'Donnell T, Hoehn ME. Ocular abnormalities in patients treated with a novel anti-GD2 monoclonal antibody, hu14.18K322A. J AAPOS 2015;19(2):112–5 doi 10.1016/j.jaapos.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siebert N, Eger C, Seidel D, Juttner M, Zumpe M, Wegner D, et al. Pharmacokinetics and pharmacodynamics of ch14.18/CHO in relapsed/refractory high-risk neuroblastoma patients treated by long-term infusion in combination with IL-2. mAbs 2016;8(3):604–16 doi 10.1080/19420862.2015.1130196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kushner BH, Ostrovnaya I, Cheung IY, Kuk D, Kramer K, Modak S, et al. Prolonged progression-free survival after consolidating second or later remissions of neuroblastoma with Anti-GD2 immunotherapy and isotretinoin: a prospective Phase II study. Oncoimmunology 2015;4(7):e1016704 doi 10.1080/2162402x.2015.1016704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mellor JD, Brown MP, Irving HR, Zalcberg JR, Dobrovic A. A critical review of the role of Fc gamma receptor polymorphisms in the response to monoclonal antibodies in cancer. Journal of hematology & oncology 2013;6:1 doi 10.1186/1756-8722-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheung NK, Sowers R, Vickers AJ, Cheung IY, Kushner BH, Gorlick R. FCGR2A polymorphism is correlated with clinical outcome after immunotherapy of neuroblastoma with anti-GD2 antibody and granulocyte macrophage colony-stimulating factor. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2006;24(18):2885–90 doi JCO.2005.04.6011 [pii] 10.1200/JCO.2005.04.6011. [DOI] [PubMed] [Google Scholar]
- 36.Erbe AK, Wang W, Carmichael L, Kim K, Mendonca EA, Song Y, et al. Neuroblastoma Patients' KIR and KIR-Ligand Genotypes Influence Clinical Outcome for Dinutuximab-based Immunotherapy: A Report from the Children's Oncology Group. Clinical cancer research : an official journal of the American Association for Cancer Research 2018;24(1):189–96 doi 10.1158/1078-0432.CCR-17-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.