Abstract
Background:
Essential tremor involves the cerebellum, yet quantitative analysis of dentate nucleus neurons has not been conducted.
Objectives:
To quantitatively compare neuronal density or neuronal number in the dentate nucleus of essential tremor versus age-matched controls.
Methods:
Using a 7-μm thick Luxol fast blue hematoxylin and eosin-stained paraffin section, dentate nucleus neuronal density (neurons/mm2) was determined in 25 essential tremor cases and 25 controls. We also applied a stereological approach in a subset of four essential tremor cases and four controls to estimate total dentate nucleus neuronal number.
Results:
Dentate nucleus neuronal density did not differ between essential tremor cases and controls (P = 0.44). Total dentate nucleus neuronal number correlated with neuronal density (P = 0.007) and did not differ between essential tremor cases and controls (P = 0.95).
Conclusions:
Neuronal loss, observed in the Purkinje cell population in essential tremor, did not seem to similarly involve the dentate nucleus in essential tremor.
Keywords: essential tremor, cerebellum, dentate neuron, stereology, pathology
Introduction
Essential tremor (ET) is a chronic, progressive, and highly prevalent neurological disease1,2 whose pathophysiology is not completely understood. Numerous studies indicate that the cerebellum is an important contributor to the pathogenesis of ET3–6; however, the exact mechanisms of dysfunction are still poorly defined. The majority of ET experimentation has focused on cerebellar cortex, where unique pathological changes, such as Purkinje cell (PC) loss7,8 and increased PC axonal changes,7,9 have been observed in ET brains relative to age-matched control brains. Other postmortem features associated with ET include reduced PC dendritic arborization and spine density,10 increased number of heterotopic PCs,11 basket cell axonal alterations around the PC body and initial axon segment,12–14 abnormal arrangement of climbing fiber–PC synapses along the PC dendritic arbor,15,16 changes in dentate γ-aminobutyric acid (GABA) receptors17 and accumulation of cerebellar β-amyloid.18
In contrast, less post-mortem research has been conducted on the dentate nucleus (DN) in ET. The DN is the largest of four cerebellar nuclei and is functionally responsible for crucial neuronal outputs from the cerebellar neocortex. The DN contains anatomically separate and functionally different motor and non-motor regions, which are dorsally and ventrally located, respectively.19 These distinct processing regions undergo task-dependent activation and are associated with separate thalamocortical projections to motor and prefrontal regions.20 The DN receives dense inhibitory input from PCs and excitatory input from collateral branches of mossy fiber and climbing fiber collaterals.21 In relation to ET, a recent study found defective GABA receptors in the DN of ET cases in comparison to Parkinson’s disease (PD) cases and controls.17 A case report documented extensive degenerative changes (ie, neuronal loss and reduced number of efferent fibers) in the DN of one ET patient22 and similar DN changes were noted in a second ET patient.7
In this study, we evaluated whether quantitative neuronal loss occurs in the cerebellar DN in ET. Using a 7-μm paraffin section from the neocerebellum, we first determined the DN neuronal density in 25 ET cases and 25 age-matched controls. Then, we validated this non-stereological method by using an optical fractionator stereological approach in a subset of ET cases and controls to obtain an unbiased estimate of the total number of neurons in the DN.
Methods
We selected 25 ET and 25 non-diseased control brains from the Essential Tremor Centralized Brain Repository (ETCBR). We broadly sampled ET cases to represent cases harvested during each year (from inception of the ETCBR-2015) while also taking into consideration the need to age-match cases with available controls. All brains underwent a complete neuropathological assessment by a senior neuropathologist.7
A single 7-μm thick paraffin section from a standard cerebellar block from 25 ET cases and 25 controls was stained with LH&E and used for neuronal density quantification. For a subset of four ET and four control brains, we applied an optical fractionator stereological approach to obtain an unbiased estimate of the total neuron number in the DN.
Parametric tests were used to analyze the DN neuronal density and DN neuronal counts. Spearman’s correlation coefficient (rSpearman) was used to assess the association between total DN neuronal density and total DN neuronal number. We also used multivariate linear regression analysis to assess potential confounding variables.
All procedures are described in detail (Supplementary Appendix S1).
Results
ET cases and controls had similar age at death, gender, brain weight, Braak Alzheimer’s disease (AD) stage, and Consortium to establish a Registry for AD (CERAD) plaque score, but ET cases had a longer post mortem interval (PMI). Consistent with our previous studies, ET cases had lower PC counts and higher torpedo counts than controls (Table 1). None had PD or progressive supranuclear palsy.
TABLE 1.
Clinical and pathological data on controls and ET cases
| Controls | ET cases | P value | |
|---|---|---|---|
| n | 25 | 25 | |
| Age at death (yr) | 81.7 ± 9.2 | 84.2 ± 6.2 | 0.27a |
| Gender | 0.56b | ||
| Men | 14 (56.0) | 17 (68.0) | |
| Women | 11 (44.0) | 8 (32.0) | |
| ET medications | β-blocker 21 (84.0) | ||
| Primidone: 17 (68.0) | |||
| Other ET medication: 12 (48.0) | |||
| Ethanol (no. drinks/day) | 0.8 ± 0.4 | ||
| Median = 0.9 | |||
| Total tremor score (range = 0–36) | 26.4 ± 5.3 | ||
| Median = 25.0 | |||
| Minimum = 18 | |||
| Maximum = 36 | |||
| Brain weight (g) | 1203.4 ± 143.8 | 1220.3 ± 136.2 | 0.67a |
| Postmortem interval (h) | 16.2 ± 10.9 | 26.4 ± 8.1 | <0.001c |
| Median = 13.9 | Median =27.5 | ||
| Braak AD staged | 0.22e | ||
| 0 | 4 (16.0) | 0 (0.0) | |
| I-II | 15 (60.0) | 12 (50.0) | |
| III-IV | 5 (20.0) | 10 (41.7) | |
| V-VI | 1 (4.0) | 2 (8.3) | |
| CERAD plaque scored | 0.32e | ||
| 0 | 11 (45.8) | 11 (45.8) | |
| A | 10 (41.7) | 7 (29.2) | |
| B | 3 (12.5) | 3 (12.5) | |
| C | 0 (0.0) | 3 (12.5) | |
| Purkinje cell countf,g | 11.6 ± 1.8 | 8.6 ± 1.4 | <0.001a |
| Torpedoesh | 6.2 ± 7.6 | 14.3 ± 12.3 | 0.003c |
| Median = 4.0 | Median = 9 | ||
| Total DN neuronal density (neurons/mm2) | 27.1 ± 6.20 | 28.8 ± 8.47 | 0.44a |
| Ventral DN neuronal density (neurons/mm2) | 27.1 ± 7.01 | 29.3 ± 8.75 | 0.35a |
| Dorsal DN neuronal density (neurons/mm2) | 27.3 ± 5.78 | 28.3 ± 8.63 | 0.62a |
| Total DN neuron number (×106) | 2.11 ± 0.35 | 2.09 ± 0.31 | 0.95a |
| Median = 2.14 | Median = 1.99 |
Values represent mean ± SD or number (%).
For variables that were not normally distributed, the median is reported as well.
Student t test.
Fisher’s exact test.
Mann-Whitney test.
Data available on <50 brains.
χ2 test.
Mean number of Purkinje cells per 100× microscopic field, among 15 sampled fields.
For the eight controls versus five ET cases not reported in Louis et al.7 or Choe et al.,8 mean ± SD Purkinje cell counts were 12.7 ± 1.8 versus 9.2 ± 0.6 (given the small number, these were not subjected to statistical testing).
For the eight controls versus five ET cases not reported in Louis et al.7 or Choe et al.,8 median torpedo counts were 8.5 versus 20 (given the small number, these were not subjected to statistical testing).
Abbreviations: AD, Alzheimer’s disease; CERAD, the Consortium to establish a Registry for Alzheimer’s disease; DN, dentate nucleus; SD, standard deviation.
To determine DN neuronal density, we divided the DN in a 7-μm LH&E-stained section into outlined dorsal and ventral contours, and counted neurons with a visible nucleolus (Supplementary Figure S1). Total DN neuronal density (dorsal + ventral) was similar in ET cases and controls (Fig. 1A, Table 1). After dividing the DN into dorsal and ventral areas, we did not find a difference between ET cases and controls. Additionally, DN neuronal density was highly similar in dorsal and ventral regions (Table 1).
FIG. 1.
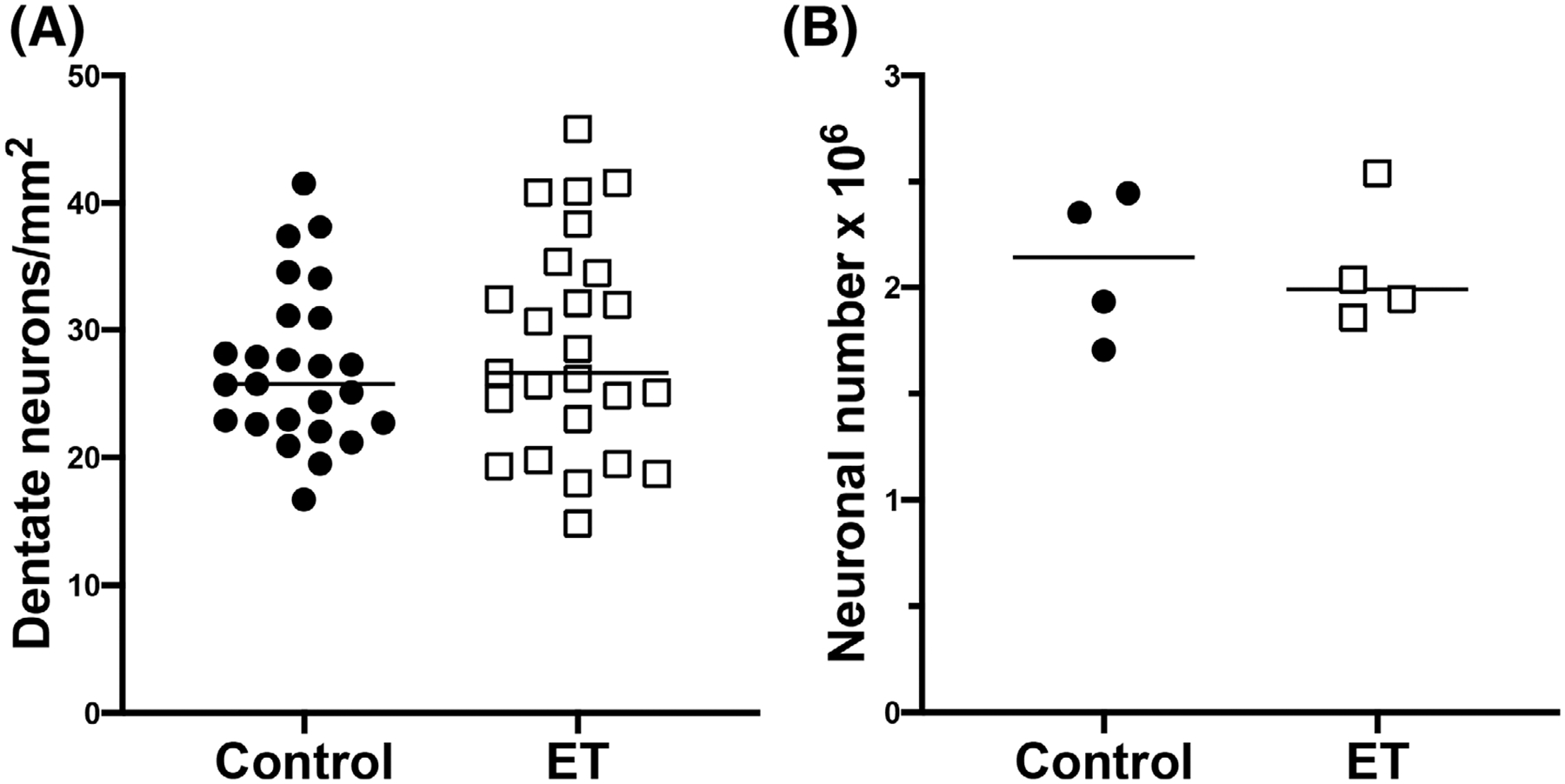
Dentate nucleus neuronal density and total neuronal number. Scatterplots of the dentate neuronal density determined in paraffin sections (A) and total dentate neuronal number determined by unbiased stereology analysis (B). The neuronal density and total neuronal number do not differ between ET cases and controls. The central bar in each graph indicates the median of the data set.
Total DN neuronal density of the entire sample was not associated with gender (females, 29.3 ± 7.3 neurons/mm2; males, 26.3 ± 7.3 neurons/mm2, P = 0.15), PMI (rSpearman = −0.05, P = 0.74), brain weight (rPearson = 0.20, P = 0.16), Braak AD stage (rSpearman = 0.08, P = 0.58), or CERAD plaque score (rSpearman = 0.00, P = 0.99). Older age was associated with higher DN neuronal density (rPearson = 0.31, P = 0.03). DN neuronal density did not correlate with total tremor scores in ET cases (Table 1; rPearson = 0.26, P = 0.22). To assess potential effects of confounding variables, we performed a multivariate linear regression analysis. In this analysis, the dependent variable was total DN neuronal density, the independent variable was diagnosis, and additional variables were age and PMI. In this adjusted analysis, there was no association between diagnosis and total DN neuronal density (β = −2.31, P = 0.38). Therefore, we report a null finding even when potential confounding effects are taken into consideration.
For the stereological component of the study, the four ET cases and four controls (both with mean age = 85 years) did not differ with respect to the mean or median total neuron number in the DN (Fig. 1B and Supplementary Table S1). Individual estimates for all eight subjects had a coefficient of error in the range of 0.03–0.07, demonstrating an adequate precision for our optical fractionator sampling scheme (Supplementary Table S1).
We examined the correlation between the stereological and non-stereological aspects of our study. There was a strong correlation between total DN neuronal number (stereology) and total DN neuronal density (measured in single sections) (rSpearman = 0. 88, P = 0.007).
Discussion
To further investigate the extent of structural abnormalities in ET brains, we examined the DN, a crucial area of the cerebellar system, in a quantitative and systematic way. This is the only study to do so. We found no difference in DN neuronal density in ET cases compared to age-matched controls, indicating that extensive cell loss in the DN does not seem to be a feature of ET pathogenesis. Likewise, in a supplemental stereology study, we found no ET case–control difference in DN neuronal number. The stereological data strongly correlated with the DN neuronal density data, which, in turn, validates our single section density analysis approach. Overall, our null finding aligns with the hypothesis that the cerebellar cortex may be the source of dysfunction in ET. There may also be subtle anatomic changes, other than DN cell death, in the cerebellar network that contribute to ET.
Previous post-mortem studies of cerebellar abnormalities in ET reported that only 2 of 33 (6.1%) ET patients exhibited extensive neurodegenerative changes in the DN, as visualized in LH&E stained sections.7,22 However, clear evidence of neuronal loss in the cerebellar nuclei has not been quantitatively established in a larger sample of ET cases, or in comparison to controls. In one study, defective GABA receptors were reported in the DN of ET cases in comparison to PD cases and controls.17 Positron emission tomography (PET) scans of tremor-related networks in ET cases and controls revealed decreased GABAA receptor binding patterns in the ET DN.23 These observations introduce the possibility of localized GABAergic dysfunction in the DN or receptor upregulation because of abnormal function in the cerebellum. We also previously quantified protein levels of the excitatory amino acid transporter 2 (EAAT2), the major glial glutamate transporter, in ET cerebellum versus controls, and demonstrated enhanced EAAT2 expression in the ET DN.24 These findings raise the possibility that lower levels of inhibition in DN, because of PC loss in ET, could trigger a compensatory increase in EAAT2 to reduce the effects of glutamatergic synaptic inputs to the DN and maintain excitation-inhibition balance in cerebellar nuclei. Despite the above-mentioned alterations in the DN, the changes do not seem to result in significant neuronal loss in the ET DN.
There are only two prior stereological studies of neuronal number in human DN25,26 and none in ET. Using the optical fractionator approach, we obtained an unbiased estimate of neuron number without the need to investigate the exact volume or area of the DN, which is important given concerns of volume reduction following formalin fixation and tissue preparation. Our stereological approach also allowed us to validate the null findings of our DN neuronal density data set. One issue is that stereology studies required representation of the entire DN in a hemi-cerebellum, which is not a feature of most brain banking protocols. Hence, our stereological analyses were able to draw on only a limited number of brains. Regardless, the sample size we were able to obtain for these analyses (4 brains/group) is common in other human stereology studies (ie, ≤5/group),25,27–30 probably for similar reasons. Neuronal density and neuronal number can provide information about the physical population of cells in the DN, but these metrics in postmortem tissue do not necessarily contribute to functional knowledge about DN activity. Nonetheless, our study is the first to address the question of whether cell loss in the DN is a feature of ET pathogenesis. We separately examined both dorsal and ventral regions of the DN, representing distinct compartments having motor and non-motor functions, respectively. We had a well-powered study, with 25 in each group, to determine whether DN density differed by disease, and we evaluated a large number of potential confounding factors to rule out the presence of other causal relationships. Additionally, our samples were age-matched to minimize case–control differences.
Several limitations should be considered. First, our study was restricted to the DN and similar data for other cerebellar nuclei would be of value. Second, data on the structural integrity of this nucleus aids in but not does fully encompass all that is needed to decode abnormalities in neuronal oscillatory networks in ET. Third, more extensive use of stereological analysis would have allowed for more definitive conclusions.
In sum, cerebellar changes are a feature of ET, but the current study found no evidence for significant neuronal loss in the DN of ET brains. We investigated a possible pathological aspect of ET by quantifying the DN neuronal density and DN neuronal number in a large, age-matched group of ET cases and controls. Our results contribute to a greater understanding of the DN in the context of ET and the disease’s associated structural abnormalities.
Supplementary Material
Acknowledgments:
This work was supported by the National Institutes of Health (NINDS R01 NS088257).
Funding agencies:
This work was supported by the National Institutes of Health (NINDS R01 NS088257).
Financial Disclosures
S.H.K. has received funding from the National Institutes of Health: NINDS R01 NS104423 (principal investigator), NINDS R03 NS114871 (principal investigator), the Louis V. Gerstner Jr. Scholar Award, Parkinson’s Foundation, and International Essential Tremor Foundation. A.J.D. has received funding from the National Institutes of Health: NIMH/FIC 1R56 MH117769 (principal investigator) and NIA R01AG066162 (co-investigator), Lyme Disease Biobank Foundation (principal investigator), the US Veterans Affairs: 1I01BX003794, and 5I01BX002876 (co-investigator on both). E.D.L. has received research support from the National Institutes of Health: NINDS R01 NS094607 (principal investigator), NINDS R01 NS085136 (principal investigator), NINDS R01 NS073872 (principal investigator), and NINDS R01 NS088257 (principal investigator). He has also received support from the Claire O’Neil Essential Tremor Research Fund (Yale University). P.L.F. has received funding from the National Institutes of Health: NINDS R01 NS085136 (principal investigator), NINDS RO1 NS088257 (principal investigator), and NINDS R01 NS086736 (co-investigator).
Relevant conflicts of interest/financial disclosures:
There are no conflicts of interest or competing financial interests.
Footnotes
Supporting Data
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
References
- 1.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord 2010;25:534–541. [DOI] [PubMed] [Google Scholar]
- 2.Benito-León J, Bermejo-Pareja F, Morales JM, Vega S, Molina JA. Prevalence of essential tremor in three elderly populations of central Spain. Mov Disord 2003;18:389–394. [DOI] [PubMed] [Google Scholar]
- 3.Louis ED. Re-thinking the biology of essential tremor: from models to morphology. Parkinsonism Relat Disord 2014;20(Suppl 1): S88–S93. [DOI] [PubMed] [Google Scholar]
- 4.Benito-León J, Labiano-Fontcuberta A. Linking essential tremor to the cerebellum: clinical evidence. Cerebellum 2016;15:253–262. [DOI] [PubMed] [Google Scholar]
- 5.Marin-Lahoz J, Gironell A. Linking essential tremor to the cerebellum: neurochemical evidence. Cerebellum 2016;15:243–252. [DOI] [PubMed] [Google Scholar]
- 6.Filip P, Lungu OV, Manto MU, Bareš M. Linking essential tremor to the cerebellum: physiological evidence. Cerebellum 2016;15: 774–780. [DOI] [PubMed] [Google Scholar]
- 7.Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain 2007; 130:3297–3307. [DOI] [PubMed] [Google Scholar]
- 8.Choe M, Cortés E, Vonsattel JP, Kuo SH, Faust PL, Louis ED. Purkinje cell loss in essential tremor: random sampling quantification and nearest neighbor analysis. Mov Disord 2016;31:393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babij R, Lee M, Cortés E, Vonsattel JP, Faust PL, Louis ED. Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control brains. Brain 2013;136:3051–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Louis ED, Lee M, Babij R, et al. Reduced Purkinje cell dendritic arborization and loss of dendritic spines in essential tremor. Brain 2014;137:3142–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louis ED, Kuo SH, Tate WJ, et al. Heterotopic Purkinje cells: a comparative postmortem study of essential tremor and spinocerebellar ataxias 1, 2, 3, and 6. Cerebellum 2018;17:104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erickson-Davis CR, Faust PL, Vonsattel JP, Gupta S, Honig LS, Louis ED. “Hairy baskets” associated with degenerative Purkinje cell changes in essential tremor. J Neuropathol Exp Neurol 2010;69: 262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuo SH, Tang G, Louis ED, et al. Lingo-1 expression is increased in essential tremor cerebellum and is present in the basket cell pinceau. Acta Neuropathol 2013;125:879–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delay C, Tremblay C, Brochu E, et al. Increased LINGO1 in the cerebellum of essential tremor patients. Mov Disord 2014;29: 1637–1647. [DOI] [PubMed] [Google Scholar]
- 15.Lin CY, Louis ED, Faust PL, Koeppen AH, Vonsattel JP, Kuo SH. Abnormal climbing fibre-Purkinje cell synaptic connections in the essential tremor cerebellum. Brain 2014;137:3149–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louis ED, Kerridge CA, Chatterjee D, et al. Contextualizing the pathology in the essential tremor cerebellar cortex: a patholog-omics approach. Acta Neuropathol 2019;138:859–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paris-Robidas S, Brochu E, Sintes M, et al. Defective dentate nucleus GABA receptors in essential tremor. Brain 2012;135:105–116. [DOI] [PubMed] [Google Scholar]
- 18.Beliveau E, Tremblay C, Aubry-Lafontaine E, et al. Accumulation of amyloid-beta in the cerebellar cortex of essential tremor patients. Neurobiol Dis 2015;82:397–408. [DOI] [PubMed] [Google Scholar]
- 19.Steele CJ, Anwander A, Bazin PL, et al. Human cerebellar sub-millimeter diffusion imaging reveals the motor and non-motor topography of the dentate nucleus. Cereb Cortex 2017;27: 4537–4548. [DOI] [PubMed] [Google Scholar]
- 20.Bernard JA, Peltier SJ, Benson BL, et al. Dissociable functional networks of the human dentate nucleus. Cereb Cortex 2014;24: 2151–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng N, Raman IM. Synaptic inhibition, excitation, and plasticity in neurons of the cerebellar nuclei. Cerebellum 2010;9:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louis ED, Vonsattel JG, Honig LS, Lawton A, Moskowitz C, Ford B, Frucht S. Essential tremor associated with pathologic changes in the cerebellum. Arch Neurol 2006;63:1189–1193. [DOI] [PubMed] [Google Scholar]
- 23.Boecker H, Weindl A, Brooks DJ, et al. GABAergic dysfunction in essential tremor: an 11C-flumazenil PET study. J Nucl Med 2010; 51:1030–1035. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Kelly GC, Tate WJ, et al. Excitatory amino acid transporter expression in the essential tremor dentate nucleus and cerebellar cortex: a postmortem study. Parkinsonism Relat Disord 2016;32: 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersen BB, Korbo L, Pakkenberg B. A quantitative study of the human cerebellum with unbiased stereological techniques. J Comp Neurol 1992;326:549–560. [DOI] [PubMed] [Google Scholar]
- 26.Andersen BB, Fabricius K, Gundersen HJG, Jelsing J, Stark AK. No change in neuron numbers in the dentate nucleus of patients with schizophrenia estimated with a new stereological method–the smooth fractionator. J Anat 2004;205:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perl DP, Good PF, Bussière T, Morrison JH, Erwin JM, Hof PR. Practical approaches to stereology in the setting of aging- and disease-related brain banks. J Chem Neuroanat 2000;20:7–19. [DOI] [PubMed] [Google Scholar]
- 28.Andersen BB, Gundersen HJ, Pakkenberg B. Aging of the human cerebellum: a stereological study. J Comp Neurol 2003;466: 356–365. [DOI] [PubMed] [Google Scholar]
- 29.Agashiwala RM, Louis ED, Hof PR, Perl DP. A novel approach to non-biased systematic random sampling: a stereologic estimate of Purkinje cells in the human cerebellum. Brain Res 2008;1236: 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitney ER, Kemper TL, Bauman ML, Rosene DL, Blatt GJ. Cerebellar Purkinje cells are reduced in a subpopulation of autistic brains: a stereological experiment using calbindin-D28k. Cerebellum 2008;7:406–416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


