Abstract
Coronavirus disease 2019(COVID-19) is an ongoing global pandemic with a daily increasing number of affected individuals and a relatively high mortality rate. COVID-19 patients that develop cardiac injury are at increased risk of a worse clinical course with higher rates of mortality. Increasing amounts of evidence suggest that a system-wide inflammatory response and a cytokine storm mediated type syndrome plays a crucial role in disease progression. This systematic review investigates the possible role of hyperinflammation in inducing cardiac injury as one of the severe complications of COVID-19. A systematic literature search was performed using PubMed, Embase and Scopus databases to identify relevant clinical studies that investigated cardiovascular injury manifestations and reported inflammatory and cardiac biomarkers in COVID-19 patients. Only 29 studies met our inclusion criteria and the majority of these studies demonstrated significantly elevated inflammatory and cardiac blood markers. It was evident that underlying cardiovascular diseases may increase the risk of developing cardiac injury. However, many COVID-19 patients included in this review, developed different types of cardiac injury without having any underlying cardiovascular diseases. Furthermore, many of these patients were either children or adolescents. Therefore, age and comorbidities may not always be the two main risk factors that dictate the severity and outcome of COVID-19. Further investigations are required to understand the underlying mechanisms of pathogenicity as an urgent requirement to develop the appropriate treatment and prevention strategies. These strategies may specifically target hyperinflammation as a suspected driving factor for some of the severe complications of COVID-19.
Abbreviations: ARDS, acute respiratory distress syndrome; BNP, brain natriuretic peptide; CI, cardiac injury; CKD, chronic kidney disease; CMR, cardiovascular magnetic resonance imaging; COPD, chronic obstructive pulmonary disease; COVID-19/SARS-COVID-19, severe acute respiratory syndrome coronavirus 2; CRP, C-reactive protein; CT, computed tomography; CVD, cardiovascular disease; DIC, disseminated intravascular coagulation; ECG, electrocardiogram; ECHO, echocardiogram; ECMO, extracorporeal membrane oxygenation; EF, ejection fraction; HCQ, hydroxychloroquine; HFOT, high flow oxygen therapy; HTN, hypertension; HFrEF, heart failure with reduced ejection fraction; IL-1, interleukin 1; IL-6, interleukin 6; IMV, invasive mechanical ventilation; IV fluids, intravenous fluids; IVIg, intravenous immunoglobulin; LA, left atrium; LV, left ventricle; MODS, multiple organ dysfunction syndrome; MRI, magnetic resonance imaging; NIV, non-invasive ventilation; NSAIDs, non-steroidal anti-inflammatory drugs; NT-proBNP, N-terminal (NT)-prohormone brain natriuretic peptide; PCT, procalcitonin; RV, right ventricle; TNF-alpha, tumour necrosis factor-alpha; TPA, tissue plasminogen activator; TTE, transthoracic echocardiography; US, ultrasound
Keywords: Coronavirus, COVID-19, Inflammation, Cardiac injury, Myocarditis
1. Introduction
Over the past year, coronavirus diseases 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been a major public health emergency. On March 11th 2020, the World Health Organisation (WHO) declared it a worldwide pandemic [1]. As of December 7, 2020, the number of new cases continues to increase with over 4.3 million new cases and 74,824 new deaths over a one-week period, leading to a total of over 70 million cases and 1.6 million deaths globally [2]. The disease presents with a very heterogeneous clinical course of varying severity - from asymptomatic carriers to multi-organ failure and death [3]. Due to the significant mortality burden caused by COVID-19, there has been an increased emphasis on identifying the risk factors leading to the severe outcomes of COVID-19 as a means of potentially implementing early interventions to reduce mortality. The symptoms of COVID-19 range from mild to severe and the most common reported symptoms occurring 1–14 days after virus exposure, being fever, dry cough, difficulty breathing, anosmia and dysgeusia [1,4].
Similar to SARS-CoV, SARS-CoV-2 invades the host cells by interacting with angiotensin-converting enzyme 2 (ACE2) which is part of the Renin-Angiotensin-Aldosterone-System (RAAS). ACE2 is expressed in the lung cells as well as other organs [5,6]. The RAAS system comprises several proteins that play multiple roles in regulating blood pressure [7]. ACE, which is expressed by different types of tissues, converts angiotensin I (ATI) to angiotensin II (ATII) which has different functions including a proinflammatory role [8]. ACE2 counteracts this inflammatory effect by breaking down ATII to angiotensin (1–7) [9,10]. In general, ACE2 plays a protective role in downregulating the ATII enzyme, resulting in a decrease in organo-protective efficacy [3,11].
The primary reported cause of death in COVID-19 patients is respiratory failure. However, cardiac dysfunction has been reported as the next cause of mortality [1,4]. Acute cardiac injury, defined as troponin I elevation and electro-cardiac changes, has been reported in 7–17% of hospitalised patients, with increased incidence in more severe cases [1]. Therefore, understanding the underlying mechanisms of the COVID-19 induced cardiac injury (CI) may help to develop and optimise the treatment protocols for those patients. As such, this review was conducted to illustrate and investigate the COVID-19 induced CI and the particular role of hyperinflammation as a possible causative factor.
2. Methods
We conducted a systematic literature search to collect data about any cardiovascular injury related to SARS-CoV-2 infection. A comprehensive online search was performed using PubMed, Embase and Scopus databases between January and July 2020 to identify relevant studies. Combinations of the following search terms were included: severe acute respiratory syndrome coronavirus, severe-acute-respiratory-syndrome-coronavirus-2, 2019-ncov, 2019ncov, covid-19, covid19, covid2019, ncov2019, ncov-2019, hcov19, sars-cov-2, coronavirus, coronaviruses, corona-virus, corona-viruses, covid, hcov, coronavirus [MeSH Terms], heart, cardiac, cardio, coronary, CVD, myocardial, cardiovascular. The systematic review was conducted using Covidence. During the initial phase of screening, all articles in English, that were peer-reviewed and published were included regardless of study design, with no restrictions on country, age or gender of study participants. During the full-text screening, Case reports and case series reporting COVID-19 patients with CI were included only if data for the inflammatory and cardiac blood markers were provided. Similarly, cohort studies reporting the blood inflammatory and cardiac markers data of two populations of COVID-19 patients with or without CI were also included. Cohort studies that did not separate the data of COVID-19 patients with CI from that of COVID-19 patients without CI were excluded as the results were not conclusive for the purpose of this study. Articles that did not contain original patient data, articles written in any language other than English, duplicate articles, review papers, or irrelevant to the topic were excluded from the study. All phases of screening including title and abstract and full-text screening were conducted independently by two different reviewers for each study using Covidence and disagreements were resolved by consensus. Data extraction included collecting the demographic and clinical data of patients reported in each study whenever data were available. These data included age, sex, cardiovascular conditions, presenting condition/symptoms, other comorbidities, imaging, treatment/interventions, and inflammatory and cardiac blood test results. Categorical variables were expressed as percentages while continuous variables were expressed as mean and standard deviation or range of results. Data were extracted from each study by two different reviewers.
3. Results
3.1. Studies selection and their specifications
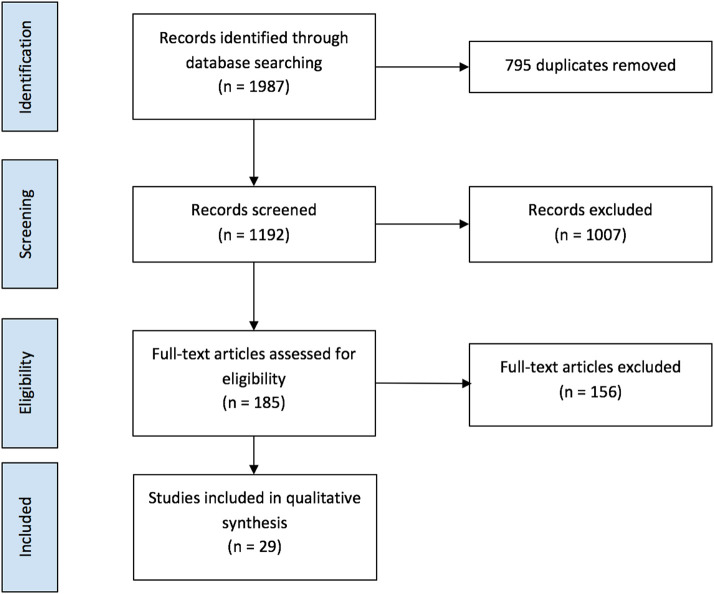
Fig. 1 shows the results of database search and screening. The flow diagram summarizes the details of our protocol. After removing the duplicates, a total of 1192 studies were retrieved, and among those 185 studies were selected for full-text screening. Only 29 studies that met the inclusion criteria were included. A total of 156 studies were excluded as 15 studies were irrelevant to the data of interest, 113 had wrong outcomes or wrong settings, six were not in English, two were duplicates and 20 studies did not have enough data.
Fig. 1.
Flow diagram of article selection protocol.
The results from our search yielded eight cohort studies with control, 20 case series/reports and one cohort study without a control group. Most of the reported cohort studies were conducted in China (six studies), and one each from Turkey and the UK. Seven of the case reports were from the USA, five from Italy, two from China, two from France and one each from India, Luxembourg, Spain, Poland, and Germany.
Most of the cohort studies and case reports/series identified cardiac injury by elevated troponin T or troponin I. However, the majority of case reports/series used different types of scans/tests for diagnosis in addition to measuring the troponin levels which are detailed in the following sections.
3.2. Cohort studies with control
The cohort studies included 2204 COVID-19 patients in total, of which 443 developed cardiac injury (CI) during the course of infection [[12], [13], [14], [15], [16], [17], [18], [19]]. With the exception of Li et al.b, the CI group included patients with an age range from 34 to 95 years while the non-CI group had patients with ages ranging from 21 to 90 years. Li et al.b, reported the age data as the percentage of each group who were below or over 70 years [14]. Out of the two groups, 29.41% and 62.5% of the CI and the non-CI groups were below 70 years, respectively. Barman et al. reported significantly higher rates of acute respiratory distress syndrome (ARDS), intensive care unit (ICU) admission and death in the CI group compared with the non-CI group [12]. Similarly, Guo et al., Shi et al.b and Wei et al. have also reported a significantly higher rate of mortality among COVID-19 patients with cardiac injury compared to those without CI [13,18,19]. Supplementary Table 1 includes the demographic data and the clinical outcome for the CI and non-CI groups.
Supplementary Table 2 summarizes the clinical features of COVID-19 patients with and without CI including comorbidities, treatments, and the blood test results of the inflammatory and cardiac markers in the cohort studies [[12], [13], [14], [15], [16], [17], [18], [19]]. Barman et al. designed their study to exclude any patients with underlying cardiovascular diseases (CVD) [12]. However, the CI group had a significantly higher proportion of patients with hypertension (HTN) and non-CVD comorbidities such as chronic obstructive pulmonary disease (COPD) and chronic kidney disease (CKD). Furthermore, Mahmoud Elsayed et al. reported that unlike the non-CI group, none of the patients in the CI group was reported to have CVD [16]. On the other hand, Guo et al., Li et al.b, Shi et al.a&b and Wei et al., all reported a significantly higher proportions of the CI group with comorbid CVD [13,14,[17], [18], [19]]. In general, the CI groups in five studies were found to have a significantly higher proportion of patients with different underlying comorbidities including diabetes, COPD, HTN, CKD and cerebrovascular disease (CeVD) [12,13,[17], [18], [19]].
Table 1 summarizes some of the demographic and clinical data of the CI group. In seven of the included cohort studies, the COVID-19 patients in the CI group were reported to have myocardial injury which was defined by elevated troponin T or troponin I. Mahmoud Elsayed et al. reported right ventricular (RV) dilation and dysfunction in the CI group identified by transthoracic echocardiography (TTE) [16]. They reported no significant difference in the level of C-reactive protein (CRP) or troponin I between those who had normal vs RV dilation and dysfunction [16]. Conversely, all of the other seven cohort studies included in this review reported at least one inflammatory marker in a significantly higher level in the CI than the non-CI group [[12], [13], [14], [15],[17], [18], [19]]. The inflammatory markers included CRP, procalcitonin (PCT), and ferritin all of which were found to be significantly elevated in the CI group [12]. Seven studies reported significantly higher cardiac markers in the CI group including troponin and brain natriuretic peptide (BNP/NT-proBNP) [[12], [13], [14], [15],[17], [18], [19]].
Table 1.
Types of cardiac abnormalities in 443 COVID-19 patients who developed cardiac injury in the included cohort studies with control.
| Condition | N = 443 | Age/yearsb | Comorbidities | Identification Method | Significantly higher inflammation markers in the CI group | Death (%) | Study |
|---|---|---|---|---|---|---|---|
| Myocardial Injury | 94 | 66 ± 14.5 | HT and other Non-CVDa | Elevated Troponin I | Yes | 37 | Barman et al. [12] |
| 52 | 71.40 ± 9.43 | CVD and Non-CVDa | Elevated Troponin T | Yes | 59.62 | Guo et al. [13] | |
| 39 | 71 (66–83) | NR | Elevated Troponin I | Yes | NR | Li et al. (a) [14] | |
| 34 | 70.59% > 70 | CVD and Non-CVDa | Elevated Troponin I | Yes | 88.23 | Li et al. (b) [15] | |
| 106 | 73 (66–80) | CVD and Non-CVDa | Elevated Troponin I | Yes | NR | Shi et al. (a) [17] | |
| 82 | 74 (34–95) | CVD and Non-CVDa | Elevated Troponin I | Yes | 51.2 | Shi et al. (b) [18] | |
| 16 | 67 (61.0–80.5) | CVD and Non-CVDa | Elevated Troponin T | Yes | 18.8 | Wei et al. [19] | |
| RV Dilation and Dysfunction | 20 | 58 ± 13 | Non-CVDa | TTE | No | 35 | Mahmoud Elsayed et al. [16] |
CVD: Cardiovascular Disease; N: Number of patients; NR: Not reported; RV: Right Ventricle; TTE: Transthoracic Echocardiography.
All comorbidities are listed in Supplementary Table 2.
Values are presented as median (IQR), mean ± SD or % > 70.
3.3. Case reports/series
The characteristics and demographic features of the total of 45 COVID-19 patients with CI included in the selected case reports and case series are demonstrated in Supplementary Table 3 [[20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40]]. Patients' age ranged from 38 days to 74 years and approximately 62% of them were males. Overall, the majority of patients recovered with few complications; when complications occurred, the most common was respiratory distress through ARDS, heart failure or cardiogenic shock. Approximately 11.1% of the patients died and the main causes of death were septic shock, disseminated intravascular coagulation (DIC), cardio-respiratory failure or multiorgan failure. The table also summarizes the clinical features of the COVID-19 patients with CI including comorbidities, treatments, and the blood test results of the inflammatory and cardiac markers.
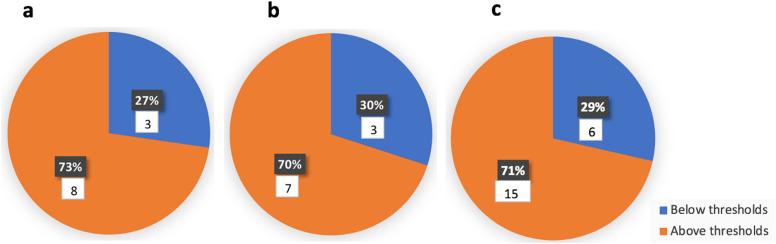
All studies reported at least one elevated inflammation marker. Almost all studies (except Del Barba et al., Latimer et al., and Zeng et al.) reported elevated CRP and eight studies reported CRP levels above 200 mg/L [[20], [21], [22],[26], [27], [28]]. IL-6 was detected in multiple studies at elevated levels ranging from 17.06 to 7305 pg/mL [21,22,24,26,32,33,[36], [37], [38],40]. Ferritin levels ranging from 600 to 7634 ng/mL were reported [21,22,26,[28], [29], [30],[32], [33], [34],36,38]. Supplementary Table 4 includes the reference ranges for the blood markers reported in this review. Fig. 2 shows that 71% of the studies reported at least one inflammation marker above the threshold that may indicate hyperinflammation/cytokine storm syndrome (CSS) in COVID-19 patients. Comparable results were observed when the studies were divided based on the age of patients as children/adolescents or adults (73% and 70% respectively). Hyperinflammation/CSS was defined as CRP > 150 mg/L and/or ferritin > 1500 ng/mL or IL-6 > 80 pg/mL.
Fig. 2.
Number/percentage of case reports/series that reported at least one inflammation marker above the threshold that may indicate hyperinflammation/cytokine storm syndrome (CSS) in children/adolescents (a), adults (b) or children/adolescents and adults (c). Hyperinflammation/CSS was defined as CRP > 150 mg/L and/or ferritin > 1500 ng/mL or IL-6 > 80 pg/mL.
3.4. Types of CI in children/adolescents and adults
CI in the majority of the studies was defined as having an elevated troponin level or having cardiac abnormalities detected by Electrocardiography (ECG), TTE, Cardiac Magnetic Resonance (CMR) or Computed Tomography (CT). All 21 included case studies without control reported elevated levels of troponin and/or brain natriuretic peptide (BNP/ProBNP).
Out of the 21 included case reports/series, 10 studies reported adult patients while 11 studies reported children and adolescents.
Table 2 summarizes the types of COVID-19 associated CI in 10 adults as reported by 10 included case reports. Six patients developed myocarditis out of which four have died. One patient had Takostubo syndrome and three patients developed other types of CI all of which have recovered.
Table 2.
Types of cardiac abnormalities in 10 adult COVID-19 patients who developed cardiac injury in the included case reports/series.
| Condition | Specific/additional complications | N = 10/gender | Age (years) | Comorbidity | Identification method | Treatments | Outcome | Study |
|---|---|---|---|---|---|---|---|---|
| Myocarditis or myopericarditis N = 6 |
Cardiomyopathy, cardiogenic shock | 1 M | 57 | HTN | Elevated Troponin I, ECG and CMR | Aldose reductase inhibitor, anti-gout agents, antibiotics, endotracheal intubation, HCQ, IMV, inotropic vasodilator, oxygen support, plasma exchange, steroids, tocilizumab |
Recovered | Coyle et al. [22] |
| ST elevation, myocardial infarction, arrhythmias | 1 M | 68 | Non-CVDa | Elevated Troponin and ECG | Antiplatelet, aspirin, TPA | Died | Kariyann et al. [28] | |
| Suspected myopericarditis with ventricular dysfunction | 1 M | 73 | HTN, CVD and Non-CVDa | Elevated Troponin T, ECG and TTE | Antiarrhythmic drug, antibiotics, anticoagulants, antivirals, HCQ, IMV, IVIg |
Died | Kazi et al. [29] | |
| Pericardial effusion | 1 M | 79 | Non-CVDa | Elevated Troponin I and CMR | Heart failure treatment | Recovered | Luetkens et al. [31] | |
| Severe necrotizing myocarditis and cardiogenic shock | 1 M | 69 | None | Elevated Troponin I, TTE and endomyocardial biopsy | Adrenaline, ECMO, intra-aortic balloon pump, NIV, noradrenaline |
Died | |Tavazzi et al. [35] | |
| Fulminant myocarditis with MODS | 1 M | 63 | Non-CVDa | Elevated Troponin I, TTE and ECG | Antibiotics, antivirals, CRRT, ECMO, HFOT, interferon alpha-1b, IVIg, steroids | Died | Zeng et al. [40] | |
| Other types of cardiac injury N = 3 |
Intramural hemorrhage | 1 M | 73 | None | Elevated Troponin I and CT | HIV protease inhibitors, HCQ, NIV, paracetamol | Recovered | Terzi et al. [37] |
| Decompensated HFrEF and atrial arrhythmias | 1 M | 68 | HTN, CVD and Non-CVDa | Not specified | Antibiotics, endotracheal intubation, HCQ, supplemental, oxygen, tocilizumab | Recovered | Villanuev et al. [38] | |
| Decreased ejection fraction and left arterial enlargement | 1 M | 74 | HTN, CVD and Non-CVDa | Elevated Troponin T and CMR | Antibiotics, antiviral, magnesium infusion, antiarrhythmic drug, temporary pacemaker | Recovered | Warchol et al. [39] | |
| Takotsubo syndrome N = 1 |
– | 1 M | 52 | HTN and other Non-CVDa | ECG and coronary angiogram | Anti-coagulants, endotracheal intubation, steroids, tocilizumab | Recovered | Taza et al. [36] |
CVD: Cardiovascular diseases; ECMO: Extra Corporeal Membrane Oxygenation; HCQ: Hydroxychloroquine, HFOT: High Flow Oxygen Therapy; r Disease; N: Number of patients; NR: Not reported; CMR: Cardiovascular Magnetic Resonance Imaging; Therapy; CT: Computed Tomography; ECG: Electrocardiography; HFrEF: Heart Failure With Reduced Ejection Fraction; HTN: Hypertension; IMV: Intermittent Mandatory Ventilation; IVIg: Intravenous Immunoglobulin; MODS: Multisystem Organ Dysfunction Syndrome; NIV: Non-Invasive Ventilation; N: Number of patients; NR: Not reported; RV: Right Ventricle; TPA; Tissue Plasminogen Activator; TTE: Transthoracic Echocardiography.
All comorbidities are listed in Supplementary Table 3.
Table 3 summarizes the types of COVID-19 associated CI in 35 children/adolescents as reported by 10 included case reports/series and one cohort study without control. Myocarditis was detected in 29 patients below the age of 16 five of which developed Kawasaki like symptoms. Furthermore, one patient developed Kawasaki like symptoms only and five had other types of CI. All children/adolescents with CI recovered except for one patient who developed multiple organ dysfunction syndrome (MODS).
Table 3.
Types of cardiac abnormalities in 35 children/adolescents with COVID-19 who developed cardiac injury in the included case reports/series and the cohort study without control.
| Condition | Specific/additional complications | N = 35 | Age | Comorbidity | Identification method | Treatment | Outcome | Study |
|---|---|---|---|---|---|---|---|---|
| Myocarditis N = 29 |
– | 1 M | 38 days | None | Elevated Troponin and CMR | NR | Recovered | Del Barba et al. [23] |
| 1 F | 2 months | None | Elevated Troponin T and TTE | Antibiotics, IVg | Recovered | Giacomet et al. [23] | ||
| 1 M | 16 years | None | Elevated Troponin I and ECG | Antiviral, aspirin, HCQ, NSAID | Recovered | Genecchi et al. [25] | ||
| 20 (10 M, 10F) | 2.9–15 years | None | Elevated Troponin, ECG and TTE | Dobutamine, epinephrine, HFOT, IL-1 receptor antagonist, IMV, inotropes, intubation, IVIg, milrinone, NIV, norepinephrine, steroids, tocilizumab | Recovered | Grimaud et al. [27] | ||
| 1 M | 8 years | None | Elevated Troponin T, TTE and CMR | Antibiotics, anticoagulant, dobutamine, IVIg, milrinone, oxygen support, tocilizumab | Recovered | Oberweis et al. [32] | ||
| Kawasaki disease like symptoms | 4 | 6–12 years | None | Elevated Troponin I and/or CMR/TTE | Aspirin, HFOT, IMV, IVIg, steroids, vasoactive agents, volume expanders | Recovered | Blondiaux et al. [20] | |
| 1 M | 5 years | None | TTE | Antibiotics, ACEi, diuretics, inotropic support, IVIg, HFOT, steroids | Recovered | Rauf et al. [34] | ||
| Kawasaki disease like symptoms only N = 1 |
– | 1 F | 11 years | None | Elevated Troponin and TTE | Antivirals, convalescent plasma, enoxaparin, IVIg, milrinone, norepinephrine, vitamin K, steroids, tocilizumab, vitamin K | Recovered | Greene et al. [26] |
| Other types of cardiac Injury N = 5 |
– | 1 M | 2 months | None | Elevated Troponin | IFN inhalation | Recovered | Cai et al. [21] |
| 1 M | 16 years | Non-CVDa | Elevated Troponin T and TTE | Epinephrine, hydrocortisone, HCQ, milrinone, IV fluids | Recovered | Latimer et al. [30] | ||
| MODS | 2 (F,M) | 10, 14 months | Non-CVDa | Not specified | Ribavirin, blood purification IFN inhalation, IMV, IV, IVIg, Methylprednisone, O2 support | 1 Died 1 Recovered |
Cai et al. [21] | |
| RV Failure and cardiogenic shock | 1 | 6 months | Non-CVDa | Elevated Troponin I, ECG and TTE | Antibiotics, anticoagulants, HCQ, IMV, milirinone, norepinephrine, tocilizumab | Recovered | Rodriguez-Gonzalez et al. [33] |
ACEi: angiotensin converting enzyme inhibitors; CVD: Cardiovascular diseases; ECMO: Extra Corporeal Membrane Oxygenation; HCQ: Hydroxychloroquine, HFOT: High Flow Oxygen Therapy; N: Number of patients; NR: Not reported; CMR: Cardiovascular Magnetic Resonance Imaging; Therapy; CT: Computed Tomography; ECG: Electrocardiography; ECHO: echocardiogram; IVIg: Intravenous Immunoglobulin; MODS: Multisystem Organ Dysfunction Syndrome; NIV: Non-Invasive Ventilation; NSAID: Non-Steroidal Anti Inflammatory Drugs; N: Number of patients; NR: Not reported; RV: Right Ventricle; TTE: Transthoracic Echocardiography.
All comorbidities are listed in Supplementary Table 3.
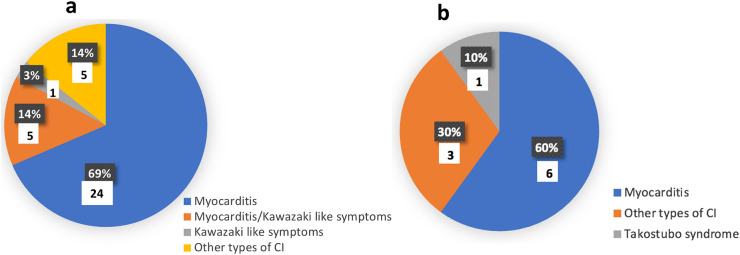
Fig. 3 compares the types of CI that children/adolescents and adults developed secondary to COVID-19.
Fig. 3.
Types of cardiac injury (CI) in the COVID-19 patients included in the case reports/series. Pie chart “a” illustrates that 69% of children/adolescents developed myocarditis, 14% developed myocarditis with Kawasaki like symptoms, 3% developed Kawasaki like symptoms only and 14% developed other types of cardiac injury. Pie chart “b” shows that 60% of adults developed myocarditis, 30% developed other types of cardiac injury while 10% developed Takostubo syndrome.
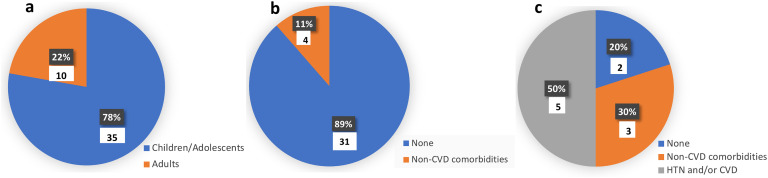
3.5. Age and comorbidities in COVID-19 patients who developed CI
Fig. 4a shows that 78% of the patients included in the case studies without control were children/adolescents while Fig. 4b shows that 89% of them did not have any comorbidities. Furthermore, only 50% of the adult COVID-19 patients who developed CI, did not have any underlying comorbid CVD (Fig. 4c).
Fig. 4.
Age and underlying cardiovascular comorbidities of COVID-19 patients who developed cardiac injury (CI) in the included case reports/series. Pie chart “a” illustrates that 78% of the patients with CI were children/adolescents. Pie chart “b” illustrates that none of children/adolescents who developed CI had any underlying CVD and 89% of them did not have any comorbidities. Pie chart “c” illustrates that only 50% of the adults who developed CI had comorbid HTN and/or CVD.
4. Discussion
It has been well established that CI is one of the complications of COVID-19 that may lead to worse outcomes including mortality. This study evaluated hyperinflammation which is a characteristic feature of COVID-19 as a possible causative agent of the development and progression of CI in COVID-19 patients.
4.1. Hyperinflammation and CI
Increasing amounts of evidence suggest that a system-wide inflammatory response and a cytokine storm type syndrome plays a crucial role in the progression of COVID-19 and it is therefore now established to be one of the disease hallmarks [[41], [42], [43]]. This was attributed to the recruitment of immune cells triggered by a rapid rate of viral replication leading to the release of cytokines. This cycle is perpetuated by high levels of cellular destruction which induces recruitment of immune cells and further dysregulated release of cytokines [42]. These markers are reported to be positively correlated with COVID-19 severity, and current studies are evaluating the use of routine blood tests as a predictor of severity [44].
In order to assess the role of hyperinflammation in developing cardiac injury, studies that enrolled two groups of COVID-19 patients who developed or did not develop CI were included. Seven out of the eight included cohort studies reported at least one inflammatory marker in a significantly higher level than the non-CI group [[12], [13], [14], [15], [16], [17], [18], [19]]. COVID-19 associated CSS refers to the excessive immune response which is characterized by high levels of CRP, IL-6 and ferritin, as well as other features such as lymphopenia and multi-organ failure. Furthermore, IL-6 levels ≥80 pg/mL were reported to predict an increased risk of respiratory failure and death [45]. Reddy et al. and Manson et al. defined hyperinflammation in COVID-19 patients as CRP and ferritin serum levels higher than 150 mg/L and 1500 ng/mL respectively [46,47]. Interestingly, 71% of the case studies without control reported at least one inflammation marker above the threshold that may indicate hyperinflammation or CSS. This indicates that many of the COVID-19 patients enrolled in the case studies had a hyperinflammatory state which may support the involvement of systemic hyperinflammation in triggering CI. This is further supported by the observed significantly higher inflammatory markers in the CI patients compared with the non-CI patients enrolled in the cohort studies. Previous literature demonstrated that IL-6 is an essential mediator in the cytokine release cascade, as it increases permeability leading to organ dysfunction being elevated in nearly two-thirds of hospitalised patients and being nearly three times higher in ICU patients [3,43]. Some studies have shown that IL-6 and granulocyte stimulating factor (GM-CSF) could potentially be secreted by T cells during the infectious period and decreasing levels of IL-6 have been linked to clinical improvement from COVID-19 [40]. IL-6 and ferritin together have been shown to be significantly increased in patients who died compared to survivors [44]. Moreover, CRP has been found to be the most sensitive indicator of an acute inflammatory response, however, there is some controversy about if there is statistical significance in levels between different severities of COVID-19 [42,44]. This may explain the consensus in our collected data which revealed that at least one inflammation marker, CRP, PCT, IL-6 or ferritin, was significantly higher in the CI compared to the non-CI patients while some inflammation markers reached thousands of times higher than normal in the patients of the case reports/series.
4.2. Role of thrombosis in triggering CI in COVID-19 patients
Only four cohort studies with control groups reported the level of D-dimer, three of which reported significantly higher levels in the CI compared with the non-CI group [[12], [13], [14]]. Autopsy studies reported that endothelial inflammation was associated with the attachment of inflammatory immune cells in different organs including the heart in addition to microvascular thrombosis, which is associated with elevated D-dimer levels [48]. Microvascular thrombosis mediated by inflammation and hypercoagulation is another mechanism that instigates CI by compromising coronary microvascular blood flow. This is further supported by the findings of Latimer et al. who reported a 16-year-old male with thrombocytopenia-associated multiple organ failure including myocardial injury [30]. The D-dimer serum level was not reported; however, elevated fibrinogen levels were observed suggesting widespread micro and macro-vascular thrombosis. Furthermore, Terzi et al. reported a COVID-19 patient with characteristics of aortic intramural hematoma supporting macrovascular involvement [37].
4.3. Age and underlying CVD as risk factors for triggering CI in COVID-19 patients
Old age and underlying comorbidities were reported as the main risk factors for cardiac abnormalities in COVID-19 patients. Xu et al. collected data from 102 COVID-19 patients divided into two groups with and without cardiac abnormalities including acute cardiac injury [49]. High proportion of patients who had acute cardiac injury were aged >60 years with underlying comorbidities including hypertension and CVD. Similarly, it was observed in six out of the eight included cohort studies that higher proportions of COVID-19 patients who developed CI had underlying baseline CVD [13,14,[17], [18], [19]]. It was previously reported that pulmonary infections induced hypoxemia, respiratory failure, hypotension or shock may compromise the oxygen supply to the myocardium. In patients with comorbidities such as CVD or hypertension, the mismatch between oxygen supply and demand is amplified even further increasing the chances of CI [[50], [51], [52], [53]]. SARS-CoV-2 may also directly attack the cardiomyocytes by interacting with ACE2 expressed in the cardiac tissue, triggering a cascade of events that may lead to a hyperinflammatory state. Consequently, patients with underlying CVD may be more affected by the direct viral attack [50,54]. Therefore, it is evident that comorbid CVD may increase the risk of developing CI during the course of infection. However, it is important to learn about the chances of developing CI in COVID-19 patients who never had underlying CVD. Although six out of eight cohort studies reported a higher proportion of COVID-19 patients with comorbid CVD, both Barman et al. and Mahmoud-Elsayed et al. found no underlying comorbid CVD in the CI group indicating that the progression of COVID-19 to develop CI is not always primarily caused by chronic CVD [12,16]. Moreover, it shows that not all types of cardiac injury are associated with underlying CVD. When CI was defined as troponin elevation, those with CI had a significantly higher chance of having underlying CVD. This was not the case when CI was defined as cardiac imaging abnormalities. Given this heterogeneity in the clinical characteristics of patients with CI, it is important to understand what other risk factors may lead to CI and whether hyperinflammation mediates CI or not. Case reports/series gave a better insight into the associated factors with CI in each individual. In this review, 11 case studies showed that 78% of COVID-19 patients with CI, were children/adolescents had none of them had any underlying CVD [20,21,[23], [24], [25], [26], [27],30,[32], [33], [34]]. Furthermore, 50% of the adult COVID-19 patients who developed CI did not have any comorbid CVD. This suggests that neither older age nor comorbidities are always the main triggers of CI in COVID-19 patients. Further research is urgently required to investigate other possible risk factors that may increase the chances of developing CI in order to develop optimal prevention and intervention strategies.
4.4. Types of CI in adults
Out of the 443 patients who developed CI as reported by the eight included cohort studies, 341 had myocardial injury which was identified by elevated troponin T or I [12–5,[17], [18], [19]]. RV dilation and dysfunction was detected in 20 patients and was identified by TTE [16]. Furthermore, six patients developed myocarditis out of which four have died. The patients who died developed further complications such as ST elevation, myocardial infraction, arrhythmias [28], suspected myopericarditis with ventricular dysfunction [29], severe necrotizing myocarditis and cardiogenic shock [35], as well as fulminant myocarditis with MODS [40]. However, the two other patients who developed cardiomyopathy, cardiogenic shock [23], and pericardial effusion [31] secondary to myocarditis have recovered. One patient had Takostubo [36] syndrome and three developed other types of CI such as intramural hemorrhage [37], decompensated heart failure with reduced ejection fraction (HfrEF), atrial dysrhythmias [38] and decreased ejection fraction with left arterial enlargement [39], all of which have recovered.
4.5. Types of CI in children/adolescents
Myocarditis was detected in 29 patients below the age of 16 five of which developed Kawasaki like symptoms. Furthermore, one patient developed Kawasaki like symptoms only and five had other types of CI. All children/adolescents with CI recovered except for one patient who developed multiple organ dysfunction syndrome (MODS). Blondiaux et al. and Greene et al. reported cases of multisystem inflammatory syndrome in children (MIS-C) which is characterized by COVID-19 associated Kawasaki disease-like symptoms together with toxic shock syndrome and cardiac inflammation [20,26]. Kawasaki disease affects children below five years of age and is characterized by systemic inflammation which mainly affects the medium-sized arteries during the acute febrile phase in addition to the appearance of coronary artery dilatation or aneurysm [55]. Blondiaux et al. observed similar clinical features of Kawasaki disease in some of the COVID-19 patients except for the coronary artery dilatation or aneurysm [20]. Their findings also agreed with other Kawasaki disease histopathologic cardiac analysis which indicated little evidence of myocardial cell degeneration or necrosis indicating that viral myocarditis is not the driving factor of the cardiac injury. However, cell infiltration of macrophages and neutrophils in myocardial interstitium was detected which supports systemic inflammation as the potential mechanism for myocardial manifestations of Kawasaki disease and the COVID-19 associated MIS-C [20,56].
4.6. Which triggers COVID-19-associated CI, systemic inflammation or direct viral attack?
Previous reports including comparing the effect of cardiotropic and non-cardiotropic viruses showed that the cardiotropic viruses may cause arrhythmia, atrioventricular block, myocardial infarction, cardiogenic shock, congestive heart failure while the non-cardiopathic viruses may cause arrhythmia, myocardial infarction, cardiogenic shock and congestive heart failure [57]. It is consequently hard to distinguish between both types of viruses by solely relying on clinical presentation. However, differences were observed in the histology where lymphocytic myocarditis was mainly caused by the non-cardiotropic viruses while lymphocytic, eosinophilic and giant-cell myocarditis and cardiac sclerosis were caused by the cardiotropic viruses [57]. Gatta and Dolan reported that the autopsy-proven myocardial localization of the virus has rarely been reported [58]. Lindner et al. on the other hand reported the possibility of detecting the virus within the myocardium of the deceased COVID-19 patients [59]. Furthermore, Tavazzi et al. described the first case of acute cardiac injury directly linked to myocardial localization of SARS-CoV-2 [35]. Endomyocardial biopsy from a 69-year-old COVID-19 patient with cardiogenic shock demonstrated low-grade myocardial inflammation and viral particles in the myocardium. However, this observation could be attributed to either viremic or infected macrophage migration from the lung. Therefore, it is not yet clear whether this injury is caused by the direct viral attack of the cardiomyocytes or due to the systemic hyperinflammation triggered during COVID-19. In this review, 16 out of 21 of case studies without control included patients who developed CI due to COVID-19 without having any underlying CVD or HTN [20,21,[23], [24], [25], [26], [27], [28],30,31,[32], [33], [34], [35],37,40]. This could be explained by the other hypothesized mechanisms for the development of CI other than the direct viral attack. CI could be caused by systemic inflammation and the dysregulated immune response during the progression of COVID-19. This systematic hyperinflammatory response may lead to multiorgan dysfunction including cardiac injury [50,[60], [61], [62], [63]]. Li et al.a reported that 14 out of 39 COVID-19 patients with CI who had signs of immune dysregulation developed CI nine days after admission [15]. This delay may indicate that the progression of systemic inflammation is most likely to be the cause of cardiac injury rather than the direct viral attack. Inflammatory cytokines may lead to apoptosis or necrosis of the myocardial cells. Furthermore, systemic hyperinflammation may reduce coronary blood flow, destabilize other coronary plaque, and lead to micro-thrombogenesis, particularly in patients with comorbid CVD [13].
4.7. RAAS as additional evidence supporting the involvement of hyperinflammation rather than direct viral attack in developing CI in COVID-19 patients
RAAS inhibitors are widely used to treat hypertension and heart failure. Several studies reported that RAAS inhibitors upregulate ACE2 expression [64,65]. This led to some arguments regarding the possible role of RAAS inhibitors in increasing the risk and/or the severity of COVID-19 and some recommended to discontinue the medications during the pandemic [66,67]. Interestingly, Sattar et al. reported that they stopped the initial regimen of the RAAS inhibitor drug in their patient in response to these recommendations [35]. The patient who had comorbid hypertension and diabetes died due to respiratory failure. Several studies reported that RAAS inhibitors did not affect the outcome of COVID-19 while some described their protective role by reducing serum inflammatory and cardiac markers and reducing mortality [68,69,70]. SARS CoV-2 was found to downregulate ACE2 receptors in the cardiopulmonary system, leading to the accumulation of ATII which is known to have proinflammatory properties [71]. This may also play a role in the hyperinflammatory state triggered by the virus. Therefore, RAAS inhibitors may play two contradictory roles during the course of SARS-CoV-2 infection by upregulating ACE2 which may increase the risk of infection but meanwhile reduce inflammation. As RAAS inhibitors have not been reported to increase the severity of COVID-19, this may support the hypothesis of systemic inflammation being the main causative factor behind CI. If CI is caused by the direct viral attack of the cardiomyocytes, RAAS inhibitors should have been associated with worse prognosis in COVID-19 patients who used the drugs. It is worth noting that the users of RAAS inhibitors have comorbidities that may even increase the severity and mortality due to COVID-19.
4.8. Why do some, but not all, COVID-19 patients develop severe hyperinflammation?
While multiple lines of evidence support the involvement of hyperinflammation in the development of cardiac injury, it is still unclear why some but not all COVID-19 patients develop a severe hyperinflammatory response. In general, it was reported that SARS-CoV-2 may inhibit different antiviral defense mechanisms including the IFN-I signaling pathway through various mechanisms. IFN-I is known to create an antiviral state and to inhibit viral replication. Therefore, the impairment of the IFN-I signaling pathways may lead to uncontrolled viral replication which may cause the dysregulated inflammatory response [72,73]. Accordingly, several clinical trials are being conducted to test the efficiency of treating COVID-19 patients with IFN-I drugs [73]. On the other hand, Bastard et al. conducted a study that included 987 patients and reported that at least 10.2% of the severe cases of COVID-19 had neutralizing IgG autoantibodies against IFN-I [74]. These findings reveal a new risk factor that may partially explain the inconsistent outcomes of COVID-19 in patients who do not have any of the previously reported risk factors including age and comorbidities. This must be also considered when the IFN-I drugs are developed to avoid drug neutralization by the autoantibodies for those individuals who naturally produce the anti-IFN-I antibodies. IFN-alpha 1b was used to treat COVID-19 patients in two of the included studies [21,40].
4.9. Targeting hyperinflammation to prevent/treat CI in COVID-19 patients
Due to the accumulating evidence suggesting that hyperinflammation mediates many of the complications of COVID-19, the use of potent anti-inflammatory drugs as part of the treatment protocols has been an important aspect to consider. Based on previous experience with SARS-CoV, different treatments and interventions have been implemented as an attempt to treat COVID-19. Among those treatments are the potent anti-inflammatory glucocorticoids which are used to suppress hyperinflammation. Initially, observational studies showed that glucocorticoid benefit was dependent on serum CRP level of COVID-19 patients [75]. The RECOVERY trial group however showed that treatment with 6 mg/day of dexamethasone for 10 days reduced mortality in hospitalised COVID-19 patients who required supplemental oxygen or mechanical ventilation [76]. Some studies reported other types of anti-inflammatory drugs such as IL-1 receptor antagonist [27]. Furthermore, tocilizumab, which blocks the IL-6 receptor has been reported by several studies as one of the drugs being used to treat COVID-19 [22,26,27,32,36,38].
5. Conclusions
While it is evident that underlying CVD may increase the risk of cardiac injury, many COVID-19 patients included in this study developed different types of cardiac injury without previously having any of the reported risk factors including age and comorbidities. Overall, multiple evidences suggest that cardiac injury is triggered by the systemic hyperinflammation induced in response to the viral infection. These evidences include the consistently reported elevated inflammatory markers in the severe cases of COVID-19, the detected infiltration of the inflammatory immune cells with scarce damage in the cardiac biopsies, the scarcity of viral detection in the cardiac autopsies and the lack of evidence that RAAS inhibitors increase the severity of COVID-19 by upregulating the expression of ACE2. Based on this, the implication of potent anti-inflammatory drugs and anticoagulants as part of the treatment protocols could be beneficial. However, every care must be taken to adapt the treatment protocols based on each patients' condition including comorbidities and the initial level of inflammatory markers. While little evidence is available about the possible localization of SARS-CoV-2 to the myocardium and the possible direct viral attack as the driving factor of cardiac injury, further investigations are required to understand the underlying mechanisms of pathogenicity as an urgent requirement to develop the appropriate treatment and prevention strategies.
Acknowledgments
Acknowledgements
We would like to thank Mr. Sa'ad Laws for his help during the early phases of this project including developing the search strategy and importing papers. We thank Qatar National Library for funding the publication of this article. We would like also to thank Weill Cornell Medicine-Qatar for the continuous support.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The publication of this article was funded by the Qatar National Library.
Declaration of competing interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.carrev.2021.04.007.
Appendix A. Supplementary data
Cohort study demographics, presentation and outcomes of COVID-19-associated cardiovascular injury.
Cohort study comorbidities, laboratory investigations and treatments of COVID-19 patients with cardiovascular injury.
Case reports, case series and studies without control group demographics, presentation comorbidities, diagnostics, laboratory investigations and outcomes of COVID-19 patients with cardiovascular injury.
Reference ranges and units for cardiac and inflammatory markers.
References
- 1.Dhakal B.P., Sweitzer N.K., Indik J.H., Acharya D., William P. SARS-CoV-2 infection and cardiovascular disease: COVID-19 heart. Heart Lung Circ. 2020;29:973–987. doi: 10.1016/j.hlc.2020.05.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Coronavirus Disease (COVID-19) Dashboard | WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/
- 3.Momtazmanesh S., Shobeiri P., Hanaei S., Mahmoud-Elsayed H., Dalvi B., Malakan Rad E. Cardiovascular disease in COVID-19: a systematic review and meta-analysis of 10,898 patients and proposal of a triage risk stratification tool. Egypt Heart J. 2020;72 doi: 10.1186/s43044-020-00075-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boukhris M., Hillani A., Moroni F., Annabi M.S., Addad F., Ribeiro M.H., et al. Cardiovascular implications of the COVID-19 pandemic: a global perspective. Can J Cardiol. 2020;36:1068–1080. doi: 10.1016/j.cjca.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H., Yang P., Liu K., Guo F., Zhang Y., Zhang G., et al. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18:290–301. doi: 10.1038/cr.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel S., Rauf A., Khan H., Abu-Izneid T. Renin-angiotensin-aldosterone (RAAS): the ubiquitous system for homeostasis and pathologies. Biomed Pharmacother. 2017;94:317–325. doi: 10.1016/j.biopha.2017.07.091. [DOI] [PubMed] [Google Scholar]
- 8.Benigni A., Cassis P., Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2:247–257. doi: 10.1002/emmm.201000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrario C.M. Angiotension-(1-7) and antihypertensive mechanisms. J Nephrol. 1998;11:278–283. [PubMed] [Google Scholar]
- 10.Crowley S.D., Rudemiller N.P. Immunologic effects of the renin-angiotensin system. J Am Soc Nephrol. 2017 doi: 10.1681/ASN.2016101066. (Published online February 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X., Guan B., Su T., Liu W., Chen M., Waleed K.B., et al. Impact of cardiovascular disease and cardiac injury on in-hospital mortality in patients with COVID-19: a systematic review and meta-analysis. Heart. 2020;106:1142–1147. doi: 10.1136/heartjnl-2020-317062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barman H.A., Atici A., Sahin I., Alici G., Tekin E.A., Baycan ÖF, et al. Prognostic significance of cardiac injury in COVID-19 patients with and without coronary artery disease. Coron Artery Dis. 2020 doi: 10.1097/MCA.0000000000000914. (Published online July 15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lingzhi Li, Shudi Zhang, Bing He, Xiaobei Chen, Qingyan Zhao. Retrospective study of risk factors for myocardial damage in patients with critical coronavirus disease 2019 in Wuhan. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dongze Li, Y Chen, Yu Jia, Tong J, Tong J, Wang W, et al. SARS-CoV-2–induced immune dysregulation and myocardial injury risk in China. Circ Res. 2020;127:397–399. doi: 10.1161/CIRCRESAHA.120.317070. [DOI] [PubMed] [Google Scholar]
- 16.Mahmoud-Elsayed H.M., Moody W.E., Bradlow W.M., Khan-Kheil A.M., Senior J., Hudsmith L.E., et al. Echocardiographic findings in patients with COVID-19 pneumonia. Can J Cardiol. 2020;36:1203–1207. doi: 10.1016/j.cjca.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi S., Qin M., Cai Y., Liu T., Shen B., Yang F., et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41:2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei J.-F., Huang F.-Y., Xiong T.-Y., Liu Q., Chen H., Wang H., et al. Acute myocardial injury is common in patients with COVID-19 and impairs their prognosis. Heart. 2020;106:1154–1159. doi: 10.1136/heartjnl-2020-317007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blondiaux E., Parisot P., Redheuil A., Tzaroukian L., Levy Y., Sileo C., et al. Cardiac MRI in children with multisystem inflammatory syndrome associated with COVID-19. Radiology. 2020;297:E283–E288. doi: 10.1148/radiol.2020202288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai X., Ma Y., Li S., Chen Y., Rong Z., Li W. Clinical characteristics of 5 COVID-19 cases with non-respiratory symptoms as the first manifestation in children. Front Pediatr. 2020;8 doi: 10.3389/fped.2020.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coyle J., Igbinomwanhia E., Sanchez-Nadales A., Danciu S., Chu C., Shah N. A recovered case of COVID-19 myocarditis and ARDS treated with corticosteroids, tocilizumab, and experimental AT-001. JACC Case Rep. 2020;2:1331–1336. doi: 10.1016/j.jaccas.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barba P.D., Canarutto D., Sala E., Frontino G., Guarneri M.P., Camesasca C., et al. COVID-19 cardiac involvement in a 38-day old infant. Pediatr Pulmonol. 2020;55:1879–1881. doi: 10.1002/ppul.24895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giacomet V., Manfredini V.A., Meraviglia G., Peri C.F., Sala A., Longoni E., et al. Acute inflammation and elevated cardiac markers in a two-month-old infant with severe acute respiratory syndrome coronavirus 2 infection presenting with cardiac symptoms. Pediatr Infect Dis J. 2020;39:e149–e151. doi: 10.1097/INF.0000000000002750. [DOI] [PubMed] [Google Scholar]
- 25.Gnecchi M., Moretti F., Bassi E.M., Leonardi S., Totaro R, Perotti L., et al. Myocarditis in a 16-year-old boy positive for SARS-CoV-2. The Lancet. 2020;395 doi: 10.1016/S0140-6736(20)31307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greene A.G., Saleh M., Roseman E., Sinert R. Toxic shock-like syndrome and COVID-19: a case report of multisystem inflammatory syndrome in children (MIS-C) Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.05.117. (Published online June 6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grimaud M., Starck J., Levy M., Marais C., Chareyre J., Khraiche D., et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care. 2020;10:69. doi: 10.1186/s13613-020-00690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kariyanna P.T., Hossain N., Jayarangaiah A., Hossain N.A., Francois J.C., Marmur J.D., et al. Pharmaco-invasive therapy for STEMI in a patient with COVID-19: a case report. Am J Med Case Rep. 2020;8:192–196. [Google Scholar]
- 29.Kazi D.S., Martin L.M., Litmanovich D., Pinto D.S., Clerkin K.J., Zimetbaum P.J., et al. Case 18-2020: a 73-year-old man with hypoxemic respiratory failure and cardiac dysfunction. N Engl J Med. 2020;382:2354–2364. doi: 10.1056/NEJMcpc2002417. doi: 10.1056/NEJMcpc2002417. (Published online June 10) [DOI] [PubMed] [Google Scholar]
- 30.Latimer G., Corriveau C., DeBiasi R.L., Jantausch B., Delaney M., Jacquot C., et al. Cardiac dysfunction and thrombocytopenia-associated multiple organ failure inflammation phenotype in a severe paediatric case of COVID-19. Lancet Child Adolesc Health. 2020;4:552–554. doi: 10.1016/S2352-4642(20)30163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luetkens J.A., Isaak A., Zimmer S, Nattermann J., Sprinkart A.M., Boesecke C, et al. Diffuse myocardial inflammation in COVID-19 associated myocarditis detected by multiparametric cardiac magnetic resonance imaging. Circ Cardiovasc Imaging. 2020;13 doi: 10.1161/CIRCIMAGING.120.010897. [DOI] [PubMed] [Google Scholar]
- 32.Oberweis M.-L., Codreanu A., Boehm W., Olivier D., Pierron C., Tsobo C., et al. Pediatric life-threatening coronavirus disease 2019 with myocarditis. Pediatr Infect Dis J. 2020;39:e147–e149. doi: 10.1097/INF.0000000000002744. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Gonzalez M., Rodríguez-Campoy P., Sánchez-Códez M., Gutiérrez-Rosa I., Castellano-Martinez A., Rodríguez-Benítez A. New onset severe right ventricular failure associated with COVID-19 in a young infant without previous heart disease. Cardiol Young. 2020;30:1346–1349. doi: 10.1017/S1047951120001857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rauf A., Vijayan A., John S.T., Krishnan R., Latheef A. Multisystem inflammatory syndrome with features of atypical Kawasaki disease during COVID-19 pandemic. Indian J Pediatr. 2020;87:745–747. doi: 10.1007/s12098-020-03357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tavazzi G., Pellegrini C., Maurelli M., Belliato M., Sciutti F., Bottazzi A., et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taza F., Zulty M., Kanwal A., Grove D. Takotsubo cardiomyopathy triggered by SARS-CoV-2 infection in a critically ill patient. BMJ Case Rep CP. 2020;13 doi: 10.1136/bcr-2020-236561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terzi F., Cefarelli M., Fattori R., Eusanio M.D. Intramural hematoma as unexpected complication of COVID-19 infection. AORTA. 2020;08:074–075. doi: 10.1055/s-0040-1713107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villanueva D.-D.H., Lusby H.P., Islam S.P., Gupte A.A., Beatty N.L. Heart failure exacerbation as only presenting sign of COVID-19. IDCases. 2020;21 doi: 10.1016/j.idcr.2020.e00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warchoł I., Dębska-Kozłowska A., Karcz-Socha I., Książczyk M., Szymańska K., Lubiński A. Terra incognita: clinically suspected myocarditis in a patient with severe acute respiratory syndrome coronavirus 2 infection. Pol Arch Intern Med. 2020;130:446–448. doi: 10.20452/pamw.15309. [DOI] [PubMed] [Google Scholar]
- 40.Zeng J.-H., Liu Y.-X., Yuan J., Wang F.-X., Wu W.-B., Li J.-X., et al. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020;48:773–777. doi: 10.1007/s15010-020-01424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garg M., Sharma A.L., Singh S. Advancement in biosensors for inflammatory biomarkers of SARS-CoV-2 during 2019–2020. Biosens Bioelectron. 2021;171:112703. doi: 10.1016/j.bios.2020.112703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng F., Huang Y., Guo Y., Yin M., Chen X., Xiao L., et al. Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Int J Infect Dis. 2020;96:467–474. doi: 10.1016/j.ijid.2020.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang P., Shi L., Xu J., Wang Y., Yang H. Elevated interleukin-6 and adverse outcomes in COVID-19 patients: a meta-analysis based on adjusted effect estimates. Immunogenetics. 2020:1–7. doi: 10.1007/s00251-020-01179-1. (Published online October 17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henry B.M., MHS de Oliveira, Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med CCLM. 2020;58:1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 45.Chen L.Y.C., Hoiland R.L., Stukas S., Wellington C.L., Sekhon M.S. Confronting the controversy: interleukin-6 and the COVID-19 cytokine storm syndrome. Eur Respir J. 2020;562003006 doi: 10.1183/13993003.03006-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reddy K., Rogers A.J., McAuley D.F. Delving beneath the surface of hyperinflammation in COVID-19. Lancet Rheumatol. 2020;2:e578–e579. doi: 10.1016/S2665-9913(20)30304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manson J.J., Crooks C., Naja M., Ledlie A., Goulden B., Liddle T., et al. COVID-19-associated hyperinflammation and escalation of patient care: a retrospective longitudinal cohort study. Lancet Rheumatol. 2020;2:e594–e602. doi: 10.1016/S2665-9913(20)30275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lowenstein Charles J., Solomon Scott D. Severe COVID-19 is a microvascular disease. Circulation. 2020;142:1609–1611. doi: 10.1161/CIRCULATIONAHA.120.050354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu Huayan, Hou Keke, Xu Rong, Li Z, Fu H, Wen L, et al. Clinical characteristics and risk factors of cardiac involvement in COVID-19. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tajbakhsh A., Hayat S.M.G., Taghizadeh H., et al. COVID-19 and cardiac injury: clinical manifestations, biomarkers, mechanisms, diagnosis, treatment, and follow up. Expert Rev Anti Infect Ther. 2020;0:1–13. doi: 10.1080/14787210.2020.1822737. [DOI] [PubMed] [Google Scholar]
- 51.Khan I.H., Zahra S.A., Zaim S., Harky A. At the heart of COVID-19. J Card Surg. 2020;35:1287–1294. doi: 10.1111/jocs.14596. [DOI] [PubMed] [Google Scholar]
- 52.Wu C.-H., Mohammadmoradi S., Chen J.Z., Sawada H., Daugherty A., Lu H.S. Renin-angiotensin system and cardiovascular functions. Arterioscler Thromb Vasc Biol. 2018;38:e108–e116. doi: 10.1161/ATVBAHA.118.311282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alhogbani T. Acute myocarditis associated with novel middle east respiratory syndrome coronavirus. Ann Saudi Med. 2016;36:78–80. doi: 10.5144/0256-4947.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020 doi: 10.1093/cvr/cvaa078. (Published online March 30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCrindle B.W., Rowley A.H., Newburger J.W., Burns J.C., Bolger A.F., Gewitz M., et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 56.Rowley A.H., Shulman S.T. The epidemiology and pathogenesis of Kawasaki disease. Front Pediatr. 2018;6 doi: 10.3389/fped.2018.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen C., Li H., Hang W., Wang D.W. Cardiac injuries in coronavirus disease 2019 (COVID-19) J Mol Cell Cardiol. 2020;145:25–29. doi: 10.1016/j.yjmcc.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gatta F., Dolan C. Pathophysiology and cardiac autopsy in COVID-19 related myocarditis. Cardiol Cardiovasc Med. 2020;04 doi: 10.26502/fccm.92920134. [DOI] [Google Scholar]
- 59.Lindner D., Fitzek A., Bräuninger H., Aleshcheva G., Edler C., Meissner K., et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5:1281. doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng Y.-Y., Ma Y.-T., Zhang J.-Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020:1–2. doi: 10.1038/s41569-020-0360-5. (Published online March 5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond Engl. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wong C.K., Lam C.W.K., Wu A.K.L., Ip W.K., Lee N.L.S., Chan I.H.S., et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zaim S., Chong J.H., Sankaranarayanan V., Harky A. COVID-19 and multiorgan response. Curr Probl Cardiol. 2020;45:100618. doi: 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A., et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 65.Soler M.J., Ye M., Wysocki J., William J., Lloveras J., Batlle D. Localization of ACE2 in the renal vasculature: amplification by angiotensin II type 1 receptor blockade using telmisartan. Am J Physiol-Ren Physiol. 2009;296:F398–F405. doi: 10.1152/ajprenal.90488.2008. [DOI] [PubMed] [Google Scholar]
- 66.Milne S., Yang C.X., Timens W., Bossé Y., Sin D.D. SARS-CoV-2 receptor ACE2 gene expression and RAAS inhibitors. Lancet Respir Med. 2020;8:e50–e51. doi: 10.1016/S2213-2600(20)30224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8 doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Naveed H., Elshafeey A., Al-Ali D., Janjua E., Nauman A., Kawas H., et al. The interplay between the immune system, the renin angiotensin aldosterone system (RAAS) and RAAS inhibitors may modulate the outcome of COVID-19: a systematic review. J Clin Pharmacol. 2021 doi: 10.1002/jcph.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lam K.W., Chow K.W., Vo J., Hou W., Li H., Richman P.S., et al. Continued in-hospital angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker use in hypertensive COVID-19 patients is associated with positive clinical outcome. J Infect Dis. 2020;222:1256–1264. doi: 10.1093/infdis/jiaa447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang Z., Cao J., Yao Y., Jin X., Luo Z., Xue Y., et al. The effect of RAS blockers on the clinical characteristics of COVID-19 patients with hypertension. Ann Transl Med. 2020;8:430. doi: 10.21037/atm.2020.03.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ni W., Yang X., Yang D., Bao J., Li R., Xiao Y., et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020;24:422. doi: 10.1186/s13054-020-03120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lei X., Dong X., Ma R., Wang W., Xiao X., Tian Z., et al. Activation and evasion of type I interferon responses by SARS-CoV-2. Nat Commun. 2020;11:3810. doi: 10.1038/s41467-020-17665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schreiber G. The role of type I interferons in the pathogenesis and treatment of COVID-19. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.595739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.-H, Zhang Y., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Keller Maria J. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;15 doi: 10.12788/jhm.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., et al. RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. (Published online July 17) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cohort study demographics, presentation and outcomes of COVID-19-associated cardiovascular injury.
Cohort study comorbidities, laboratory investigations and treatments of COVID-19 patients with cardiovascular injury.
Case reports, case series and studies without control group demographics, presentation comorbidities, diagnostics, laboratory investigations and outcomes of COVID-19 patients with cardiovascular injury.
Reference ranges and units for cardiac and inflammatory markers.