Abstract
Background:
The SPRINT-MS trial demonstrated benefit of ibudilast on brain atrophy over 96 weeks in progressive MS. Optical coherence tomography (OCT) was performed in all trial participants.
Objective:
Report the OCT results of the SPRINT-MS trial.
Methods:
OCT was obtained at baseline and every 6 months using spectral domain OCT and analyzed by an OCT reading center. Change in each OCT outcome measure by treatment group was estimated using linear mixed models.
Results:
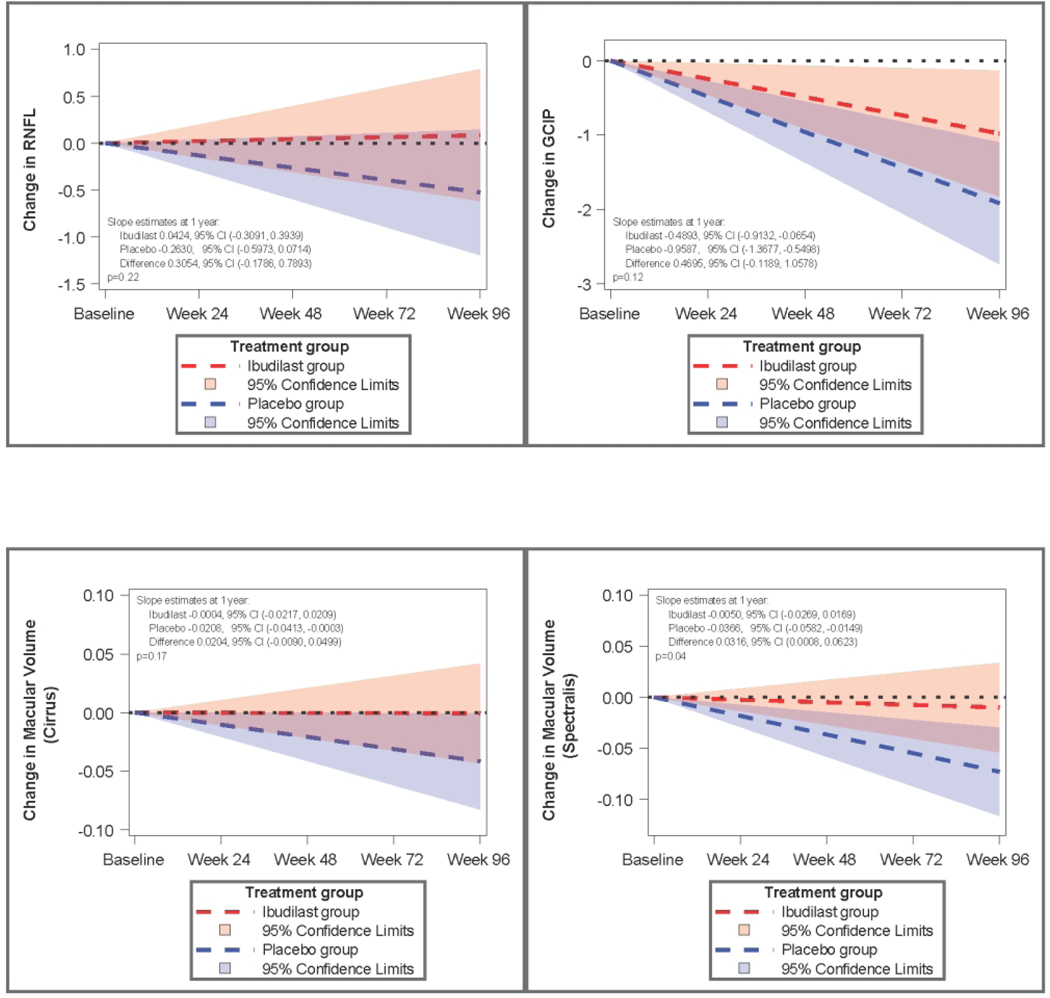
Change in pRNFL thickness was +0.0424uM/year (95% CI: −0.3091 to 0.3939) for ibudilast vs. − 0.2630uM (95%CI −0.5973 to 0.0714) for placebo (n=244, p=0.22). Macular volume change was − 0.00503mm3/year (−0.02693 to 0.01688) with ibudilast vs. −0.03659mm3/year (−0.05824 to −0.01494) for placebo in the Spectralis cohort (n=61, p=0.044). For the Cirrus cohort, macular volume change was −0.00040 mm3/year (−0.02167, 0.020866) with ibudilast compared to −0.02083 mm3/year (−0.04134 to −0.00033) for placebo (n=183, p=0.1734). Ganglion cell-inner plexiform layer thickness change, available from Cirrus, was −0.4893uM/year (−0.9132, −0.0654) with ibudilast vs. −0.9587uM/year (−1.3677, −0.5498) with placebo (n=183, p=0.12)
Conclusion:
Retinal thinning in MS may be attenuated by ibudilast. Sample size estimates suggest OCT can be a viable outcome measure in progressive MS trials if a therapy has a large treatment effect.
NN102/SPRINT-MS ClinicalTrials.gov number, NCT01982942
Keywords: Optical coherence tomography, ibudilast, multiple sclerosis, neuroprotection
Introduction:
Ibudilast is an orally bioavailable molecule used in Asia for the treatment of asthma and post stroke complications, based primarily on its inhibition of several cyclic nucleotide phosphodiesterases1. Subsequent discoveries of effects on macrophage migration inhibitory factor 2 and toll-like receptor 43, potential nociceptive effects, and ability of ibudilast to cross the blood–brain barrier led to further investigation of potential central nervous system effects4. Ibudilast was initially evaluated in relapsing-remitting multiple sclerosis (MS) where it reduced brain volume loss in a dose dependent manner without reducing the occurrence of new focal inflammatory brain lesions, a dichotomy that led to hypotheses about a possible neuroprotective role for ibudilast in MS. There is an enormous unmet need for therapeutics in progressive MS, in which accumulation of neurological disability often proceeds unchecked 5.
SPRINT-MS was a prospective, phase 2 trial in which people with primary or secondary progressive MS were randomized to ibudilast or placebo for 96 weeks, and evaluated with multimodal imaging. There was a 48% reduction of brain volume loss measured by brain parenchymal fraction, the primary outcome measure for the trial6. OCT was included as a key secondary outcome measure.
OCT has frequently been used as an outcome measure in multicenter clinical trials in ophthalmology, including multiple trials in macular degeneration, retinal vascular diseases, and glaucoma. In neurology, longitudinal cohort and pooled observational studies demonstrate that neuronal OCT parameters decline over time in MS, proportional to disease activity disability progression in MS. In particular the peripapillary RNFL (pRNFL), macular volume (MV) and ganglion cell-inner plexiform layer thickness (GCIP) are measures with relevance to MS, to monitor disease progression or evaluate for complication of MS therapies7. OCT has therefore become commonly available as a part of clinical care at tertiary care neurology sites, is relatively low cost, easy to perform, and associated with minimal additional burden during study visits. Challenges and key technical issues with planning and executing a multicenter clinical trial using OCT in neurology include compatibility and prevalence of devices by different manufacturers and need for a specialized OCT reading center8. SPRINT-MS represents the first time that OCT has been systematically incorporated as a secondary outcome measure, acquired within departments of neurology, in a multicenter clinical trial in progressive MS.
Methods:
Patients with either primary progressive MS (PPMS) or secondary progressive MS (SPMS) were enrolled at 28 sites in the United States, with central clinical coordination and data management as part of the Network for Excellence in Neuroscience Clinical Trials (NeuroNEXT) network. The SPRINT-MS study was approved by a central institutional review board and conducted in accordance with the International Council for Harmonisation guidelines for Good Clinical Practice and the Declaration of Helsinki. All patients provided written informed consent prior to screening. Complete trial design and primary outcome are previously published6,9.OCT outcomes were a mandatory part of the study protocol for all sites and all participants. OCT was performed at baseline, week 24, week 48, week 72 and week 96. Neither detailed visual history nor history of optic neuritis were collected as part of the study procedures, though relapse and/or systemic corticosteroid steroid treatment for multiple sclerosis within 3 months of screening was an exclusion criterion for enrollment in the study.
For the OCT component of the study, individual sites were certified by the central reading center as either a Zeiss Cirrus or Heidelberg Spectralis SD-OCT site based on the technology that they had available. Sites were required to become certified, and then use the same SD-OCT device for all study subject visits. All subjects at each site were scanned on the same scanner model, and no site changed scanner model during the trial. All technicians and research coordinators involved in OCT acquisition underwent training for the SPRINT-MS study via live webinar, and each site was supplied with an SD-OCT study procedures handbook and quick reference guide specific to the SPRINT-MS study and the instrument that was used at each site. Individual OCT technologists (in some case research coordinators) were certified to perform the protocol-defined scan set on the SD-OCT for study. Each site was required to certify at least one or more OCT technologists. Certification consisted of each technologist submitting scans from two separate subjects (typically healthy volunteers) they had acquired using the study protocol and correctly uploading the images to the central reading center. Upon review of the certification images by reading center graders, the tech received either approval of certification or feedback on scan quality with instructions to resubmit updated examples. Each individual OCT technologist was registered with the reading center, but devices were not certified separately.
For Cirrus sites, scans of both eyes were acquired using the Macular Cube Scan 512 × 128 and Optic Disc Cube 200 × 200 protocols. Good scan position, iris image clarity, signal strength ≥6 were required by the technician at the time of acquisition. Use of eye tracking and Repeat Scan overlay/registration were utilized if available. For Spectralis sites, scans acquired in both eyes were Glaucoma RNFL 768×496 with an ART of 100, Axonal RNFL-N 1536×496 with an ART of 100, Posterior Pole 61 scan lines spaced 12μm apart, 768A-scans with an ART of 9. Quality was required to be > 25, with entire scan visible in window. The Heidelberg eye tracking software feature was used if possible for all scans, and study visits for each eye were all registered using the baseline visit as the reference scan for follow up scans. For all sites, the scan protocol was performed a minimum of twice on each subject at each study visit, to ensure adequate images of sufficient quality. These quality control standards are similar to, but predate, the published OSCAR-IB criteria10.
Scans were sent from each site to the central reading center via secure web-based interface, and evaluated initially for completeness and scan quality parameters, with sites receiving rapid feedback, to maximize receipt of quality data. Images accepted by the reading center were independently evaluated by a minimum of two (2) certified Graders, who were ophthalmologists employed by the reading center and masked to treatment status. All numeric data were required to match within 7.5% of the mean between Grader 1 and Grader 2. If data was outside this bound, then a third certified Grader was assigned to independently analyze the image and adjudicate the value. All quantitative values are those obtained from either Cirrus or Spectralis software, as defined in the study protocol. Third party software was not utilized for quantification of retinal structures. Values for pRNFL from Cirrus and Spectralis are combined, as supported by Syc et al 11,12. Because they measure different areas of the retina using different methods, the macular volume cannot be combined across devices and are therefore is reported independently by device for each cohort of patients. Ganglion cell/inner plexiform (GCIP) layer thickness was available for and is calculated for Cirrus scans only.
Data from both eyes were incorporated into a statistical model which evaluated for differences between treatment groups while allowing correction for within-subject inter-eye correlation. Analyses were conducted according to actual randomization strata under the modified intent-to-treat principle defined as all the patients who underwent randomization, received at least one dose of a study medication, and had at least one efficacy assessment after baseline. The rate of change in each OCT outcome measure by treatment group was estimated using a linear mixed model with adjustment for randomization strata. A common intercept was assumed to constrain the baseline means to be equal. In addition to patient specific random intercepts and slopes, we induced correlated errors between eyes at each visit to account for the within-subject covariance between measurements taken on both eyes. Akaike information criterion (AIC) based on maximum likelihood estimation suggested the linear model was the optimal fit. Model estimates were obtained by restricted maximum likelihood (REML) using an unstructured covariance matrix. A 3-way interaction model of treatment, time, and MS type was used to explore the potential differential effect of ibudilast in PPMS vs SPMS.
Furthermore, we estimated sample sizes required to achieve various effect sizes for the OCT outcome measures based on trends observed in the SPRINT-MS trial. Due to the complexity of the models for the OCT outcomes, closed form formulas to compute sample size do not exist. Therefore, the required sample sizes were estimated with simulation. The model-estimated fixed and random effects and random error were used to simulate OCT outcomes. The treatment effect estimate was varied to achieve selected effect sizes for the percentage in slope reduction relative to placebo. The power was estimated by the percent of times out of r=1000 replications that the p-value for the test of equal slopes between treatment groups was below the designated alpha level (α=0.10). A grid search method was used to estimate the desired sample size that would achieve approximate 80% power. To explore the impact of the noise seen in the RNFL data on the sample size estimates, while maintaining trial applicability, large outliers were excluded from the model estimates used in the simulations. Specifically, patients with greater than 10uM change in RNFL over any 24-week period were excluded from the model estimates for RNFL along with data values with signal strength below the quality threshold. Statistical analyses were performed in SAS 9.4 Software (SAS Institute, Cary, NC) and R13. Given the exploratory nature of the analysis of this secondary endpoint, multiple comparisons were not accounted for in the analysis.
Results:
Of the 28 sites participating in SPRINT-MS, there were 22 sites that utilized Zeiss Cirrus OCT and 6 sites that utilized Heidelberg Spectralis OCT. This resulted in 183 participants being imaged with Cirrus, and 61 with Spectralis over the course of the trial. Baseline participant characteristics are presented in Table 1. Completeness of data is presented in Supplemental Table 1.
Table 1:
Baseline patient characteristics, modified intent to treat population (n=244)
| ibudilast | placebo | p-value | |
|---|---|---|---|
| N (patients) | 121 | 123 | |
| Age (mean years +/− SD) | 54.7314 ± 7.7063 | 56.8909 ± 6.4984 | 0.02 |
| Disease duration (median, range) | 11 (0 – 41) | 9 (0 – 36) | 0.77 |
| Primary Progressive (n, %) | 63 (52.07%) | 64 (52.03%) | 0.9958 |
| EDSS (median, range) | 6 (2.5–6.5) | 6 (3 – 6.5) | 0.45 |
| 2.5% LCLA (mean number correct +/− SD) | 29.6750 ± 12.2063† | 27.2213 ± 12.6357† | 0.13† |
| pRNFL (mean* +/− SD) | 83.3944 ± 10.5055† | 81.0000 ± 13.1451† | 0.12† |
| Macular Volume (mean +/− SD)(Spectralis sites) | 8.2572 ± 0.4601† | 8.1178 ± 0.4580 | 0.24 |
| Macular Volume (mean +/− SD)(Cirrus sites) | 9.6961 ± 0.5366 | 9.5879 ± 0.5760† | 0.19 |
| GCIP (mean +/− SD) (Cirrus sites only) | 71.6861 ± 8.9790 | 68.8861 ± 9.7125† | 0.05 |
mean of the left and right eye measures. When measures from both eyes were unavailable, the value for the one available eye was used.
denotes unavailable data as follows: 2.5% LCLA, 1 ibudilast subject and 1 placebo subject, pRNFL, 5 ibudilast subjects, 4 placebo subjects; Macular Volume (Spectralis sites), 1 ibudilast subject; Macular Volume (Cirrus sites), 2 placebo subjects; GCIP, 3 placebo subjects.
The primary OCT outcome, difference between treatment groups in pRNFL change over time in all patients, showed an estimated reduction of 0.305uM per year (95% CI −0.1786 to 0.7893, p=0.22) of pRNFL loss with ibudilast compared to placebo. Estimated rate of pRNFL loss per year was −0.2630uM (95% CI −0.5973 to 0.0714) in the placebo group compared to 0.0424uM (−0.3091 to 0.3939) in the ibudilast arm. Macular volume loss was −0.00503mm3 (−0.02693 to 0.01688) per year with ibudilast compared to −0.03659mm3 (−0.05824 to −0.01494) per year in the placebo arm in the Spectralis patients (n=61, p=0.044). In patients followed with the Cirrus OCT, macular volume loss was −0.00040 (−0.02167, 0.020866) with ibudilast compared to −0.02083 mm3 (−0.04134 to 0.00033) for placebo (n=183, p=0.1734). Ganglion cell-inner plexiform (GCIP) layer thickness, segmented using software available only on Cirrus devices, declined by −0.4893uM (−0.9132, −0.0654) per year in the ibudilast arm vs. −0.9587uM (−1.3677, −0.5498) per year for placebo (n=183, p=0.12). A 3-way interaction model evaluating the differential effect of ibudilast in PPMS vs SPMS for RNFL was not significant (p=0.77).
We simulated, based on the performance of the OCT measures as implemented in the SPRINT-MS trial, sample size estimates for 2-year trials using OCT outcomes in progressive MS. Estimates of sample sizes for 2-year trials assuming 80% power to detect therapeutic effects of varying degrees using OCT visits every 6 months are provided in Table 2. Additionally, we calculated sample size requirements for higher power requirements. To achieve 90% power with GCIP as an outcome assuming an 80% effect size would require randomizing n=204 and for GCIP detecting a 70% effect size n=274 would be required. To achieve 90% power with macular volume measured by Spectralis, assuming an 80% effect size, n=152 would need to be randomized, and for 90% power to detect a 70% Effect size n=192 would need to be randomized. Finally for 90% power and macular volume measured by Cirrus, to detect an 80% effect size, n=1096 would need to be randomized. Simulations were also run using only yearly measurements (baseline, 48 wks, and 96 wks) and in that scenario, to achieve 80% power with GCIP as an outcome assuming an 80% effect size would require randomizing n=198 and for GCIP detecting a 70% effect size n=254 would be required. This amounts to a roughly 30% increase in required sample size when moving from OCT every 6 months to every 12 months. Alpha was set to 0.1 for all simulations.
Table 2:
Sample size calculations using OCT outcome measures in progressive MS (total number of patients to randomize assuming 80% power to detect the specified effect size)
| Effect size | |||||
|---|---|---|---|---|---|
| 50% | 60% | 70% | 80% | ||
| Outcome measure | pRNFL | >9500 | 7336 | 5490 | 4050 |
| MV - Cirrus | 1950 | 1370 | 1054 | 852 | |
| MV - Spectralis | 270 | 204 | 172 | 140 | |
| GCIP (every 6 month measurements) | 386 | 264 | 196 | 152 | |
| GCIP (yearly measurements) | 490 | 350 | 254 | 198 | |
Discussion:
In the SPRINT-MS trial we measured OCT every six months for two years. Small differences were seen in OCT measures between ibudilast- and placebo-treated subjects, with point estimates consistently in the direction showing less loss of retinal tissue in ibudilast than placebo, though not all reached statistical significance. Taken together with the trial’s primary outcome measure of benefit on brain atrophy, the overall pattern in OCT outcomes adds support to the potential benefit of ibudilast on slowing neuronal loss in progressive MS.
In SPRINT-MS, the magnitude of the effect size of ibudilast on OCT parameters is similar to the effect size on brain parenchymal fraction (BPF), though confidence intervals for OCT are wide. Estimating with greater precision the magnitude of the ibudilast effect on retinal layer thickness would therefore require confirmation in a second, larger trial. Also, certain parameters, such as GCIP, are only available in a subset of patients, limiting power to detect a treatment effect on those measures. Future work in this dataset will focus on platform-agnostic retinal segmentation analyses, pooled across instruments, which requires third-party, proprietary algorithms. The degree to which advanced analytic techniques can improve the precision of outcomes such as GCIP across platforms within multicenter trials, requires further study. History of previous optic neuritis was not collected as a screening or baseline variable in this trial but could be in future trials, to further define the population of patients who may benefit from neuroprotective therapy and evaluate the effect in subpopulations of patients with vs. without prior involvement of the visual system that exceeded clinical threshold.
As the first multicenter progressive MS trial employing OCT as an outcome, these results also further define the conditions under which OCT can serve as an outcome measure in future progressive MS trials. When designing the SPRINT-MS trial, formal power analyses could not be performed for pRNFL, since its estimated change over time in this patient population was not known. The rate of retinal thinning over time in MS is known to be proportional to inflammatory disease activity, and patients enrolled in SPRINT-MS had a low overall level of disease activity during the study6 The rate of decline in pRNFL in the placebo arm of the SPRINT-MS trial was −0.26uM per year, similar to −0.21 μm/year in one cohort study of a convenience sample of MS and CIS pooled from clinical practice13and −0.31uM per year in a small recent trial in progressive MS15. For GCIP thickness, the rate of decline in the SPRINT-MS placebo arm was −0.96uM/year compared to −0.37 μm/year from a multicenter CIS/RRMS convenience sample13 and −0.29uM per year in a small progressive MS trial14. Sample size calculations based off the actual data from SPRINT-MS suggest that a trial in progressive MS with GCIP as outcome measure would have 80% power to detect a 60% treatment effect with n=264 participants randomized. GCIP, especially if in the future it is able to be combined across more than one brand of spectral domain OCT device, and given it is less susceptible to acute changes in the setting of optic neuritis, may be feasible to serve as an outcome measure in progressive MS trials. Under the conditions employed in this trial, using pRNFL as the sole OCT outcome would require substantially larger sample sizes, reducing feasibility.
Together, these results potentially provide some support of a neuroprotective role for ibudilast in progressive MS, and guide the use of OCT in future trials in progressive MS. Unique aspects of OCT implementation in the SPRINT-MS trial include enrollment and qualification of multiple sites, inclusion of two different spectral domain scanner brands, and quality control and independent analysis facilitated by use of an OCT reading center. Many of these are analogous to how MRI is utilized with a reading center in MRI-driven MS clinical trials. In future studies, preplanning GCIP as primary outcome measure with advanced analytics validated across acquisition platforms would be one potential design improvement. Other methods to reduce noise and increase ability to detect treatment effect between groups could include increasing standards for scan quality, adding assessment points, or changing inclusion criteria to enrich for more active patients with more rapid change over time. More recently described retinal measures such as inner nuclear layer and outer nuclear layer thickness, not analyzed in this study, deserve investigation and may hold promise as more specific markers of retinal atrophy in progressive MS16.
Supplementary Material
Figure 1:
Model-based change in OCT measures over time by treatment group
Acknowledgments
Funding:
This work was supported by grants from the National Institute of Neurological Disorders and Stroke (NINDS) [U01NS082329] and the National Multiple Sclerosis Society [RG 4778-A-6] and by MediciNova through a contract with the National Institutes of Health (NIH). The NeuroNEXT Network is supported by the NINDS (Central Coordinating Center, U01NS077179; Data Coordinating Center, U01NS077352; and individual grants to each trial site).
Footnotes
Disclosures:
RB: has served as a consultant for Biogen, Genzyme, Genentech, and Novartis. He receives research support from Biogen, Genentech, and Novartis, and shares rights to intellectual property underlying the Multiple Sclerosis Performance Test, currently licensed to Qr8 Health and Biogen.
JF: The Author declares that there is no conflict of interest
PK: has received personal compensation from Carl Zeiss Medtec
CN: The Author declares that there is no conflict of interest
JS: The Author declares that there is no conflict of interest
EK: The Author declares that there is no conflict of interest
DW: The Author declares that there is no conflict of interest
JY: The Author declares that there is no conflict of interest
DE: The Author declares that there is no conflict of interest
MC: The Author declares that there is no conflict of interest
RTN: has consulted for Alexion, Alkermes, Bayer AG, Biogen, Celgene, EMD Serono, Genentech, Genzyme, Novartis, TG Therapeutics, Viela Bio.
ECK has received consulting fees from Alexion, Atlas5D, Biogen, Celgene, EMD Serono, Genentech and MedDay and research funding from AbbVie, Atlas5D, Biogen, EMD Serono, Genzyme, and Roche.
ADG: has received personal compensation for consulting from Adamas, EMD-Serono, MedDay, Greenwich Bioscience, Celgene, Teva, Sun Pharma, Novartis, Sanofi Genzyme, Genentech-Roche, Biogen, Atara, Acorda Therapeutics and received research support from Teva, Sun Pharma, Novartis, Sanofi Genzyme, Genentech-Roche, Biogen, Atara, Acorda Therapeutics.
KN has received personal licensing fee from Biogen, consulting fee from NeuroRx, and speaking fee from Sanofi Genzyme and research grant funding from Biogen, Novartis, and Sanofi Genzyme.
CSC The Author declares that there is no conflict of interest
RJF has received personal consulting fees from Actelion, Biogen, Celgene, EMD Serono, Genentech, Immunic, Novartis, Sanofi Gensyme, Teva, and TG therapeutics; served on advisory committees for Actelion, Biogen, Immunic, and Novartis; and received clinical trial contract and research grant funding from Novartis.
References
- 1.Gibson LC, Hastings SF, McPhee I, et al. The inhibitory profile of Ibudilast against the human phosphodiesterase enzyme family. Eur J Pharmacol 2006; 538: 39–42. 2006/05/06. DOI: S0014–2999(06)00201–9 [pii] 10.1016/j.ejphar.2006.02.053. [DOI] [PubMed] [Google Scholar]
- 2.Cho Y, Crichlow GV, Vermeire JJ, et al. Allosteric inhibition of macrophage migration inhibitory factor revealed by ibudilast. Proc Natl Acad Sci U S A 2010; 107: 11313–11318. 2010/06/11. DOI: 10.1073/pnas.1002716107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruiz-Perez D, Benito J, Polo G, et al. The Effects of the Toll-Like Receptor 4 Antagonist, Ibudilast, on Sevoflurane’s Minimum Alveolar Concentration and the Delayed Remifentanil-Induced Increase in the Minimum Alveolar Concentration in Rats. Anesth Analg 2016; 122: 1370–1376. 2016/02/10. DOI: 10.1213/ANE.0000000000001171. [DOI] [PubMed] [Google Scholar]
- 4.Sanftner LM, Gibbons JA, Gross MI, et al. Cross-species comparisons of the pharmacokinetics of ibudilast. Xenobiotica 2009; 39: 964–977. 2009/11/21. DOI: 10.3109/00498250903254340. [DOI] [PubMed] [Google Scholar]
- 5.Ontaneda D, Fox RJ and Chataway J. Clinical trials in progressive multiple sclerosis: lessons learned and future perspectives. Lancet Neurol 2015; 14: 208–223. 2015/03/17. DOI: 10.1016/S1474-4422(14)70264-9S1474-4422(14)70264-9 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox RJ, Coffey CS, Conwit R, et al. Phase 2 Trial of Ibudilast in Progressive Multiple Sclerosis. New Engl J Med 2018; 379: 846–855. DOI: 10.1056/NEJMoa1803583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saidha S, Al-Louzi O, Ratchford JN, et al. Optical coherence tomography reflects brain atrophy in multiple sclerosis: A four-year study. Ann Neurol. 2015;78(5):801–813. DOI: 10.1002/ana.24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keltner JL, Cello KE, Balcer LJ, et al. Stratus OCT Quality Control in Two Multi-Centre Multiple Sclerosis Clinical Trials. Neuroophthalmology 2011; 35: 57–64. 2011/03/20. DOI: 10.3109/01658107.2011.557760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox RJ, Coffey CS, Cudkowicz ME, et al. Design, rationale, and baseline characteristics of the randomized double-blind phase II clinical trial of ibudilast in progressive multiple sclerosis. Contemporary Clinical Trials 2016; 50: 166–177. 10.1016/j.cct.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tewarie P, Balk L, Costello F, et al. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS One 2012; 7: e34823. 2012/04/27. DOI: 10.1371/journal.pone.0034823 PONE-D-11–21092 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warner CV, Syc SB, Stankiewicz AM, et al. The impact of utilizing different optical coherence tomography devices for clinical purposes and in multiple sclerosis trials. PLoS One 2011; 6: e22947. 2011/08/20. DOI: 10.1371/journal.pone.0022947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Syc SB, Warner CV, Hiremath GS, et al. Reproducibility of high-resolution optical coherence tomography in multiple sclerosis. Mult Scler 2010; 16: 829–839. 2010/06/10. DOI: 10.1177/1352458510371640. [DOI] [PubMed] [Google Scholar]
- 13.Team RC. R: A language and environment for statistical computing. 2019. [Google Scholar]
- 14.Ratchford JN, Saidha S, Sotirchos ES, et al. Active MS is associated with accelerated retinal ganglion cell/inner plexiform layer thinning. Neurology 2013; 80: 47–54. 2012/12/26. DOI: 10.1212/WNL.0b013e31827b1a1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winges KM, Murchison CF, Bourdette DN, et al. Longitudinal optical coherence tomography study of optic atrophy in secondary progressive multiple sclerosis: Results from a clinical trial cohort. Mult Scler 2019; 25: 55–62. 2017/11/08. DOI: 10.1177/1352458517739136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sotirchos ES, Gonzalez Caldito N, Filippatou A, et al. Progressive Multiple Sclerosis Is Associated with Faster and Specific Retinal Layer Atrophy. Ann Neurol. 2020;87(6):885–896. DOI: 10.1002/ana.25738 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.