Abstract
Elevations in peripheral inflammatory markers have been reported in patients with psychosis. Whether this represents an inflammatory process defined by individual or subgroups of markers is unclear. Further, relationships between peripheral inflammatory marker elevations and brain structure, cognition, and clinical features of psychosis remain unclear. We hypothesized that a pattern of plasma inflammatory markers, and an inflammatory subtype established from this pattern, would be elevated across the psychosis spectrum and associated with cognition and brain structural alterations. Clinically stable psychosis probands (Schizophrenia spectrum, n=79; Psychotic Bipolar disorder, n=61) and matched healthy controls (HC, n=60) were assessed for 15 peripheral inflammatory markers, cortical thickness, subcortical volume, cognition, and symptoms. A combination of unsupervised exploratory factor analysis and hierarchical clustering was used to identify inflammation subtypes. Levels of IL6, TNFα, VEGF, and CRP were significantly higher in psychosis probands compared to HCs, and there were marker-specific differences when comparing diagnostic groups. Individual and/or inflammatory marker patterns were associated with neuroimaging, cognition, and symptom measures. A higher inflammation subgroup was defined by elevations in a group of 7 markers in 36% of Probands and 20% of HCs. Probands in the elevated inflammatory marker group performed significantly worse on cognitive measures of visuo-spatial working memory and response inhibition, displayed elevated hippocampal, amygdala, putamen and thalamus volumes, and evidence of gray matter thickening compared to the proband group with low inflammatory marker levels. These findings specify the nature of peripheral inflammatory marker alterations in psychotic disorders and establish clinical, neurocognitive and neuroanatomic associations with increased inflammatory activation in psychosis. The identification of a specific subgroup of patients with inflammatory alteration provides a potential means for targeting treatment with anti-inflammatory medications.
Keywords: Schizophrenia, Bipolar disorder, inflammation, cognition, MRI, factor analysis, clustering
Introduction
Psychotic disorders comprise a clinically, genetically and neurobiologically heterogeneous clinical syndrome (1). Accumulating evidence suggests that cognitive, neurophysiological and neuroanatomic alterations contribute to clinical and functional impairments in psychosis (2,3). Each of these features have been linked to altered inflammatory and immunological pathways as well as disruptions in the blood brain barrier (BBB) and microvascular function (4,5). While this provides an exciting avenue for understanding psychosis pathophysiology and new possibilities for treatment, variation of individual peripheral inflammatory marker may be influenced by a number of factors. The degree to which groups of inflammatory markers co-segregate in relation to neurobiological and clinical features of disease, and whether specific subgroups of patients express these alterations remain to be clarified (6).
While the neuroinflammation hypothesis in psychosis has been challenged (7) in the context of several other biological factors being proposed (including abnormalities in oligodendrocytes (8,9), N-methyl-D-aspartate (NMDA) signaling (10) and dopaminergic transmission (11)), post-mortem studies have identified inflammatory marker differences between cases and controls, with additional evidence suggesting these differences are driven by a subset of people with psychosis (12). A number of studies have established the connectivity between peripheral cytokines and the CNS (13), and further identified alterations of individual peripheral markers (i.e. IL1β, IL1RA, sIL2R, IL4, IL6, IL8, IL10, IL12, CRP, VEGFA, IFNγ, TGFβ, TNFα) linked to inflammation, immune dysregulation, oxidative stress, and microvascular function in psychosis (14–25). It is possible that individuals with elevations in peripheral inflammatory markers may be useful in defining a distinct subgroup of patients with psychotic disorders. Observations that subgroups of patients may respond to inflammation-targeted interventions in clinical trials are consistent with this hypothesis (26–31). Initial approaches to identify patient subtypes using clustering techniques based on different combinations of cortical and peripheral inflammatory marker levels estimated that approximately 40% of people with schizophrenia may be classified into an elevated subtype (12,20,32,33). In postmortem studies, a high inflammatory subtype was associated with cortical gray matter volume reductions (34), gliosis (35), and immune cell transmigration (5) while in vivo studies identified that a high inflammatory subtype was associated with reduced volume in Broca’s area and worse verbal fluency scores (33). Other studies identified inflammatory or microvascular marker associations with both increases and decreases in gray matter thickness and subcortical volumes (24,36–40). This highlights a need to clarify pathophysiological consequences and clinical context to better understand inflammation mechanisms in psychosis (37,38). Separate investigations identified associations between peripheral inflammatory marker elevations and worse positive and negative symptoms (17,19,41), greater past exposure to infectious agents (18), and steeper age-related rates of prefrontal cortical thinning (39).
The degree to which individual or groups of peripheral inflammatory markers are informative for psychosis pathophysiology and the clinical consequences of disease is unclear. Examining these relationships in individuals with a range of phenotypes may provide additional clues about these connections and determine which measures of inflammation may be most informative. To begin to address gaps in our current understanding of inflammatory subgrouping and marker associations with brain dysfunction, we conducted a study in 200 participants from the Bipolar Schizophrenia Network on Intermediate Phenotypes (B-SNIP) to define relationships between inflammation and microvascular markers and psychosis phenotypes using a novel unsupervised exploratory factor analysis approach that also considered important clinical and laboratory variables. We also aimed to establish peripheral inflammatory marker-informed subtypes of psychosis using a hierarchical clustering approach. We hypothesized that combinations of microvascular and inflammatory markers would perform better than individual markers at predicting neuroimaging and related cognitive and clinical outcomes related to psychosis.
Methods and Materials
Study Participants
This study included participants with schizophrenia (SZP) spectrum illness (n=79; schizophrenia n=50, schizoaffective n=29), bipolar 1 disorder with psychosis (BPP n=61) and healthy controls (HC n=60) recruited from the Chicago site of the B-SNIP study who provided blood samples and had clinical and neurobiological phenotypes for study (see supplementary methods and Tamminga et al 2013 (42) for study details).
Cardiometabolic disease (CMD) status was determined based on patient report and/or medical record information indicating a cardiovascular or metabolic disorder including coronary artery disease, hypertension, diabetes, hyperlipidemia or related disorders. CMD was assessed since it has been previously correlated with both peripheral inflammatory markers (43) and body mass index (44), the latter of which was not collected in this study. A medication history interview was performed for both prescription and non-prescription medications. Average daily doses of antipsychotics were converted to chlorpromazine equivalents (CPZ) (45). Genetic ancestry was determined using high density genotype data available on participants and used this information as covariates in our peripheral inflammatory marker analyses (see supplementary methods) (46,47).
Symptom ratings collected included the Positive and Negative Syndrome Scale (PANSS), Young Mania Rating Scale (YMRS), and Montgomery-Asberg Depression Rating Scale (MADRS) (48–53). Cognition was assessed using the Brief Assessment of Cognition in Schizophrenia (BACS) total score, Spatial Span subtest from the Wechsler Memory Scale (forward and backward total scores), dot pattern expectancy test (target detection sensitivity, error rate and reaction time), and antisaccade error rate (54–61). Facial emotion recognition was assessed using the Penn Emotion Recognition-40 (ER40) test (55). Morning blood draws were obtained for inflammatory marker assessments. Exploratory analyses by experimental biotype (BT) categorizations identified by the B-SNIP consortium (experimentally categorized groups based on neurobiological measures that define dimensions of cognitive control and sensorimotor reactivity resulting in three clusters – BT1, BT2, and BT3) was also examined (62) (see supplementary methods).
Peripheral Inflammatory Marker Assays
Peripheral inflammatory markers were selected on the basis of previously described relationships with schizophrenia and bipolar disorder (15,21,23,25,63–65). Serum concentrations of inflammatory, and microvascular markers (IL1α, IL1β, IL2, IL4, IL6, IL8, IL10, IL12/IL23p40, IL12p70, IFNγ, TNFα, TNFβ, CRP, Flt-1, VEGF, VEGFC, VEGFD, and TGFβ1) were determined using customized V-Plex sandwich immunoassays from Meso Scale Discovery (MSD) and the Sector 6000 Microplate ELISA System (Meso Scale Diagnostics, Rockville, MD). Complement 4 (C4a) concentrations were determined using a solid phase sandwich ELISA (Beckton, Dickinson and Company BD Biosciences, San Jose, CA). These assays were performed blind to case-control status. Intra-assay coefficients of variation, lower limits of detection, and concentration units may be found in Supplementary Table 1.
Structural Neuroimaging Processing
Structural T1 MPRAGE (3T GE Signa HDX) scans were available on a subset of participants (101 probands and 53 HC) with acquisition protocol and preprocessing steps described in supplementary methods. We extracted regional gray matter thickness and subcortical volumes including the accumbens, amygdala, caudate, globus pallidus, hippocampus, putamen, thalamus and lateral ventricles. Cortical Gray matter and subcortical regions were studied because the effects of peripheral inflammatory markers have been previously examined using these structures (33,37,66).
Statistical Analyses
All statistical analyses were performed in R version 3.6.1. Demographic variables were compared across groups using chi-squared tests or a one-way analysis of variance (ANOVA). ANOVA was used to test the moderating effect of storage days, hemolysis score, sample set, age, sex, first two genetic ancestry components (predominantly African or European), CMD, CMD medications (CMDM), NSAID, SSRI, lithium, antipsychotic status and CPZ equivalents. Significant predictors (p<0.05) in the initial models were used as covariates in subsequent statistical models. The False Discovery Rate (FDR) (67) method (reported as q values) was used for multiple comparison correction (15 markers) for group comparisons, and separately for correlations by clinical domain (symptoms, cognition and brain measures).
Peripheral Inflammatory Marker Analyses
Among the 19 markers examined, 15 were selected for further analysis based on the following criteria: less than 5% with QNS values, greater than 75% of values reported, and mean coefficient of variation less than 30 (68). IL1α, IL2, IL4, IL12p70 were excluded for failing these criteria. Two markers were significantly impacted by storage days and values exceeding a threshold of >2700 were excluded from analyses (VEGFC n=55; TGFβ1 n=55). All markers were visually assessed for normality using histograms and data were natural log transformed for IL1β, IL6, IL8, IL10, IL12/IL23p40, IFNγ, TNFβ, VEGFD and CRP. All remaining 15 markers were winsorized to 3 SD. Inter-marker correlations within HC and probands was performed using partial Pearson’s correlation and clustered suing the hierarchical method.
Group Contrasts and Correlations
Probands had a significantly greater number of storage days compared to controls but were matched on hemolysis scores (Table 1). Storage days, sample set and hemolysis scores were significantly associated with several peripheral inflammatory marker measurements (Supplementary Table 2). Therefore, peripheral marker data was pre-adjusted using the intercept adjustment method for these variables. There were few effects of CMD or medications (NSAID, CPZ, Lithium, SSRI or CMDM) on marker concentrations and therefore these variables were not included in subsequent models. A small number of markers were differentially associated with age, sex or ancestry. For consistency, these variables were used as covariates in all of our analyses. Intracranial volume was used as an additional covariate in subcortical volume analyses. General linear models were used controlling for confounding variables in group contrasts. Partial Spearman correlations were used to quantify associations between markers and symptoms, global and social functioning, cognition, gray matter thicknesses, and subcortical volumes.
Table 1:
Demographic and Clinical Measures in Probands and Controls
| HC (n=60) | Probands (n=140) | |
|---|---|---|
| Age (mean, SD) | 37.6 (13.5) | 34.3 (13.3) |
| Sex (Male/Female) | 26/34 | 60/80 |
| Ancestry (EU/AF) | 46/14 | 97/43 |
| Diagnosis (SZP, BPP) | - | 79/61 |
| Antipsychotic (yes/no) | ||
| 1st Generation Antipsychotic | - | 14/124 |
| 2nd Generation Antipsychotic | - | 96/42 |
| Daily CPZ equivalent (mean, SD) | - | 504 (441) |
| Lithium (yes/no) | - | 21/117 |
| SSRI (yes/no) | - | 43/95 |
| Age of onset (mean, SD) | - | 15.8 (6.8) |
| Storage Days (mean, SD)* | 2398 (293.4) | 2523 (279.4) |
| Hemolysis score (mean, SD) | 1.9 (1.0) | 1.8 (0.9) |
| NSAID (yes/no) | 17/43 | 25/113 |
| CMDM (yes/no)* | 2/58 | 29/110 |
| CMD (yes/no)* | 4/84 | 45/86 |
| GAF (mean, SD)* | 84.5 (5.4) | 50.4 (12.9) |
| SFS total (mean, SD)* | 163.5 (17.6) | 130.8 (21.9) |
| PANSS Positive total (mean, SD) | - | 16.5 (5.8) |
| PANSS Negative total (mean, SD) | - | 16.7 (6.6) |
| YMRS total (mean, SD) | - | 6.8 (6.3) |
| MADRS total (mean, SD) | - | 11.1 (9.2) |
Note: SD = standard deviation; EU = European; AF = African; HC = healthy control; CPZ = chlorpromazine; SSRI = Selective Serotonin Reuptake Inhibitor; NSAID = non-steroidal anti-inflammatory agent; CMDM = cardiovascular medicine; CMD = cardiometabolic disorder; GAF = global assessment of function; SFS = Birchwood social functioning scale; PANSS = Positive and Negative Syndrome Scale; YMRS = Young Mania Rating Scale; MADRS = Montgomery–Åsberg Depression Rating Scale.
Proband group vs HC comparisons, p < 0.05
Exploratory Factor Analysis and Cluster Analysis of Peripheral Inflammatory Markers
An unsupervised exploratory analysis was conducted to identify a shared-factor model that uniquely explained the variance of the peripheral inflammatory markers while controlling for covariates (storage days, hemolysis score, sample set, age, sex and ancestry) using markers without missing data (see supplementary methods). The markers included in the factor analysis were CRP, Flt1, IFNγ, IL1β, IL6, IL8, IL10, IL12/IL23p40, TNFα, TNFβ, VEGF, VEGFD, C4a. Covariates were controlled for by fitting a multivariate multiple linear regression model with peripheral inflammatory markers as dependent variables and covariates as independent variables. Principle component analysis (PCA) was then performed on the residual from the fitted multivariate multiple linear regression. The Kaiser rule and elbow method were used to select the optimal number of principal components for the peripheral inflammatory data, which resulted in a 5-factor solution. To assess whether factor loading scores differed between subgroups of patients, unsupervised hierarchical clustering was applied. A dissimilarity matrix was calculated to quantify the Euclidean distance between every pair of subjects in this 5-dimensional feature space. The agglomerative coefficient was then calculated to determine the optimal number of factors to include in the dimensional feature space, as well as the best agglomerative method to use (average, single, complete or Wards method) to identify the optimal clustering solution. The optimal number of clusters for confirmed using the Silhouette method. Partial least squares regression was also performed in the whole sample for the five factor loadings and brain imaging measures to examine whether findings were consistent between different analytical approaches.
Code availability
Cleaning and analysis code if available upon request to the corresponding author.
Results
Demographic and Clinical Characteristics
Psychosis probands and healthy control participants were matched on age, sex, ancestry, and NSAID status. Probands were more likely to have cardiometabolic disorders and therefore more likely to be taking cardiovascular medications than healthy controls (Table 1). In general, the proband group exhibited mild symptoms of psychosis and depression and remitted mania symptoms (Table 1). Comparisons across diagnostic or BSNIP biotype groups and controls are described in Supplementary Table 3.
Peripheral Inflammatory Marker Comparisons
Figure 1A depicts the study design and analysis pipeline. Compared to HCs, probands had elevated TNFα, CRP, VEGF, and IL6 levels (p<0.05, Figure 1B). SZP also had higher CRP (q<0.05), IL6 (q<0.05), and VEGF (p<0.05) levels compared to HCs (Figure 1C). SZP also had higher CRP and IL6 levels compared to BPP (p<0.05). BPP showed higher TNFα levels compared to HCs (Figure 1C). Mean, standard deviation and effect size values by diagnostic group are summarized in Supplementary Table 4. Secondary analyses of BSNIP Biotypes are included in Supplemental Figure 1. Inter-marker correlations are presented in Supplemental Figure 2.
Figure 1: Study Design and Group Comparisons.
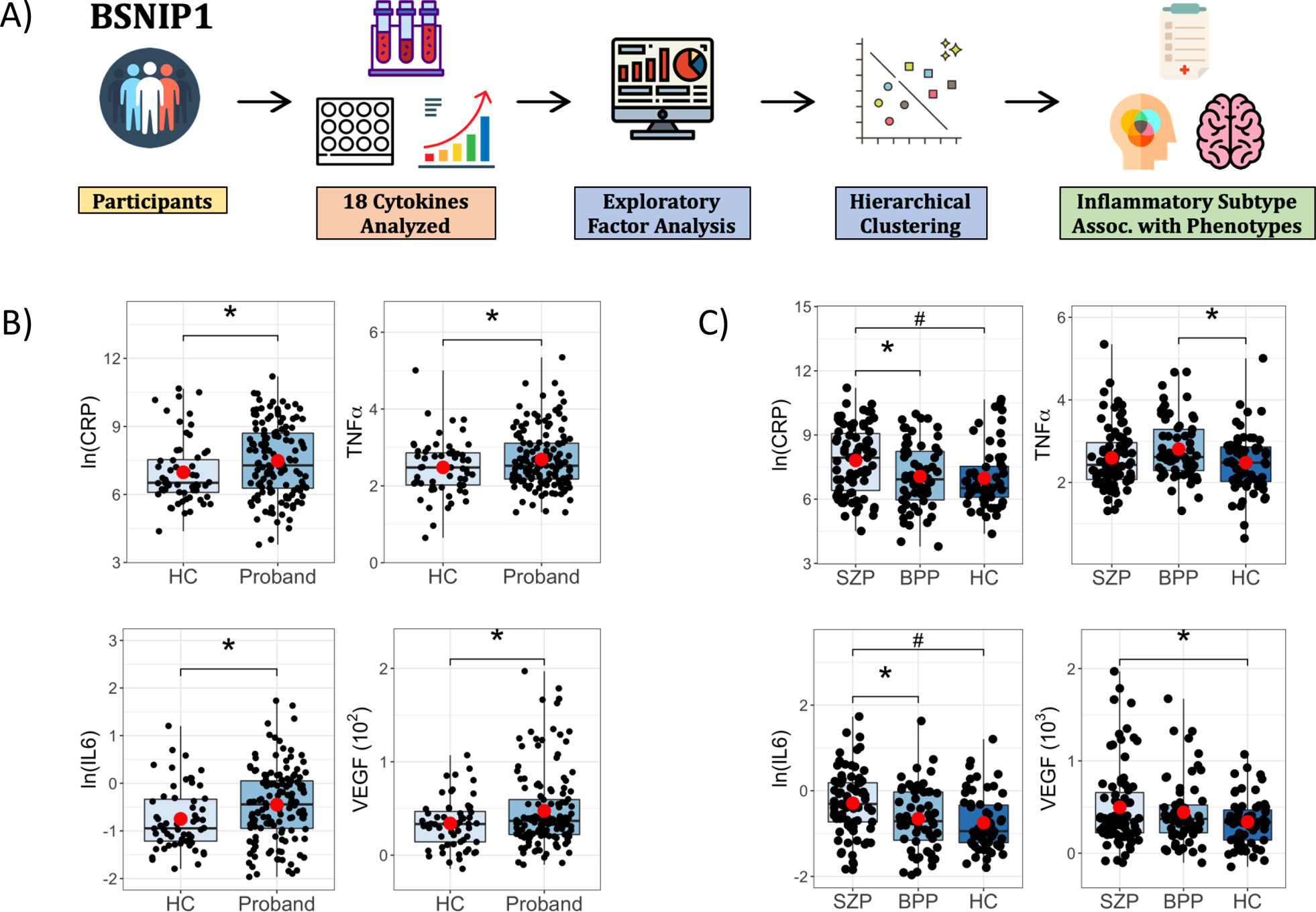
A) Participants from the B-SNIP1 study underwent cytokine level measurements and 18 cytokines were selected for group comparisons. This was followed by unsupervised exploratory factor analysis and unsupervised hierarchical clustering to identify inflammatory subtypes that were associated with psychosis phenotypes. B) Boxplots showing pairwise contrasts of CRP, IL6, TNFa and VEGF between probands and healthy control (HC) subjects and C) between schizophrenia (SZP), psychotic bipolar group (BPP) and HC subjects. ln = natural log; *p<0.05, #q<0.05, (•) mean.
Peripheral Inflammatory Marker Relationships with Structural MRI, Cognition, and Symptoms
The four peripheral inflammatory markers that differentiated probands from controls were used for correlational analyses with imaging measures in the full patient proband group (CRP, IL6, TNFα and VEGF). In probands, higher TNFα levels were associated with higher right medial orbital frontal thickness, right middle temporal thickness, and left thalamic volumes (q’s<0.05). In probands, higher CRP levels were associated with thinner left post central, left supramarginal, left transverse temporal, right cuneus and precuneus thickness, and higher volume in left putamen (q’s<0.05). In HCs, higher IL6 levels were associated with thinner left pars opercularis and right bank superior temporal sulcus (q’s<0.05) (Table 2).
Table 2:
Correlations between Cytokines and Cognitive or Cortical/Subcortical Measures
| Cytokine | Group | Measure | r-value | p-value | q-value | |
|---|---|---|---|---|---|---|
| Cognition | ||||||
| IL6 | Proband | BACS composite | −0.296 | <0.001 | 0.002 | |
| CRP | Proband | BACS composite | −0.216 | 0.011 | 0.022 | |
| CRP | Proband | Antisaccade ER | 0.192 | 0.024 | 0.048 | |
| VEGF | Proband | Antisaccade ER | 0.263 | 0.002 | 0.008 | |
| TNFa | Proband | DPX RT | 0.229 | 0.009 | 0.037 | |
| Thickness/Volume Measures | ||||||
| IL6 | HC | L Pars Orbitalis | −0.413 | 0.002 | 0.009 | |
| IL6 | HC | R Superior Temporal Sulcus | −0.382 | 0.004 | 0.019 | |
| TNFa | Proband | R Medial Orbital Frontal | 0.282 | 0.004 | 0.017 | |
| TNFa | Proband | R Middle Temporal | 0.251 | 0.011 | 0.046 | |
| TNFa | Proband | L Thalamus volume | 0.293 | 0.003 | 0.028 | |
| CRP | Proband | L Post Central | −0.251 | 0.011 | 0.045 | |
| CRP | Proband | L Supramarginal | −0.281 | 0.004 | 0.018 | |
| CRP | Proband | L Transverse Temporal | −0.258 | 0.009 | 0.037 | |
| CRP | Proband | L Putamen volume | 0.324 | 0.001 | 0.018 | |
| CRP | Proband | R Cuneus | −0.256 | 0.009 | 0.037 | |
| CRP | Proband | R Precuneus | −0.256 | 0.009 | 0.039 | |
Note: Partial Spearman correlations were performed between cytokines and clinical, cognitive or cortical thickness measures. Cytokines were pre-adjusted for storage days, sample set and hemolysis score. Correlations were performed using age, sex and ancestry as covariates. Volume measures were additionally covaried for intracranial volume. Multiple comparison correction was performed separately in each domain tested. No correlations between peripheral inflammation markers and clinical symptoms survived multiple comparison correction. BACS = Brief Assessment of Cognition in Schizophrenia; DPX RT = dot pattern expectancy task reaction time; Antisaccade ER = error rate. Only those cognitive and brain measures showing significant effects are reported in the table.
Higher IL6 and CRP levels were associated with poorer cognition (BACS composite) in probands (q’s<0.05) (Table 2, see supplementary results text for BACS subscale data). Higher levels of CRP and VEGF were associated with worse antisaccade error rate in probands (q’s<0.05). There were no correlations between peripheral inflammatory markers and cognition in the HCs. There were no significant relationships surviving FDR correction between peripheral inflammatory markers and clinical symptom measures in probands.
Exploratory Factor Analysis using Peripheral Inflammatory Marker Measures
Unsupervised exploratory factor analysis using 13 markers (excluding VEGFC and TGFβ1) resulted in a five-factor model (Figure 2A, Supplementary Figure 3). This figure depicts how each inflammatory marker contributes to each factor and significant factor-marker loading pairs were identified. For example, factor 1 had greater loading for CRP, IFNγ, IL1β, IL8, IL10, TNFα, and VEGF, with smaller loading magnitudes for the remaining markers (Figure 2A). Thus, factor 1 is primarily defined by CRP, IFNγ, IL1β, IL8, IL10, TNFα, and VEGF. Factor 1 distinguished probands (p=0.005) from HC, while none of the other factors distinguished cases from HCs (Figure 2B). Comparisons of factor loading across diagnostic and biotype groups are presented in Supplementary Table 5 and supplementary results. Correlations between factors 1 through 5 and phenotypes are included in Supplementary Table 6 and supplementary results. Partial least squares regression was also performed in the whole sample for the five factor loadings and brain imaging measures and analysis resulted in a similar solution as the partial Spearman correlations with 8 out of 10 findings being identified by approaches).
Figure 2: Exploratory Factor Analysis and Group Comparisons.
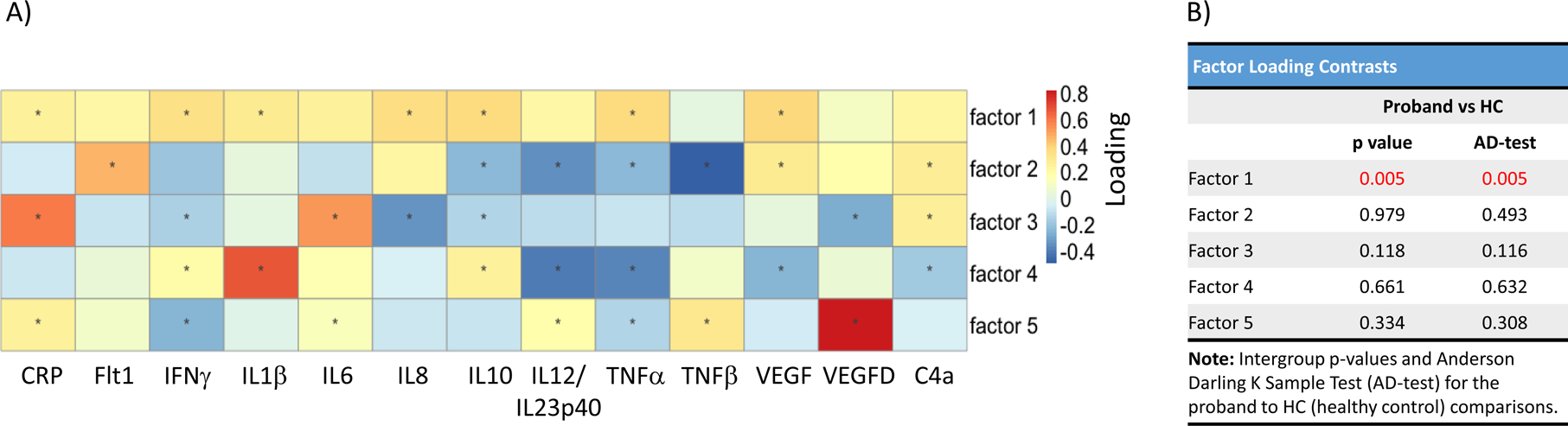
A) 13 cytokines with <10% of missing data were used in the exploratory factor analysis across the whole sample. Five factors made up 70% of the explained variance. Factors with lower explained variance have smaller magnitude loadings. This is illustrated in the heatmap. For example, factor 1 is made up of CRP, IFNg, IL1b, IL8, IL10, TNFa, and VEGF. B) Factor loadings for each individual were used for group comparisons and the intergroup p-values and the Anderson-Darling k-sample test (AD-test) are listed in the table. *, p<0.05; Red indicates significant values.
Subtyping Based on Peripheral Inflammatory Marker Data
Unsupervised hierarchical clustering using factors 1 through 5 did not result in a better agglomerative coefficient when compared to a model using only the factor 1 loading scores. Thus, the combination of factor 1 loadings and Ward’s method provided the best agglomerative coefficient (0.999). While there were additional potential clustering solutions evident within the nested groups, a two-cluster solution was optimal for defining relatively homogenous subgroups and for ensuring individual cluster sample sizes that provided sufficient statistical power to detect biologically meaningful differences between the subgroups (Figure 3A). This clustering solution provided a reasonable clustering score (Silhouette score = 0.59). Based on factor 1 loadings (notably CRP, IFNγ, IL1β, IL8, IL10, TNFα, and VEGF) we identified significant subgroups of individuals (χ2=200, df=3, p<0.001) with relatively higher peripheral inflammatory marker levels (total n=63; 51 Proband-High [36%] and 12 HC-High [20%]) and relatively lower marker levels (total n=137; 89 Proband-Low [64%] and 48 HC-Low [80%]) (Figure 3B). It is important to note that factor 1 loading was not significantly correlated with CMD (r=0.086, p=0.157). In general, the Proband-High and HC-High groups had higher marker levels compared to the low inflammatory groups (see Figure 3C for effect size values per contrasted groups). There was no diagnostic (χ2=1.18, df=2, p=0.554) or biotype (χ2=3.43, df=2, p=0.178) group enrichment in the higher marker group (Supplementary Table 7). Furthermore, inflammatory subtypes were matched on age, sex, ancestry, hemolysis score, and NSAID status (Supplementary Table 7). Within probands, the inflammatory subtypes were additionally matched on antipsychotic, lithium, NSAID, and SSRI status, as well as antipsychotic dosage and age of onset. The Proband-High and Proband-Low groups had greater rates of cardiometabolic disorders and cardiometabolic medications compared to the HC-High or -Low group, but not between each other.
Figure 3: Hierarchical clustering defines two cytokine-based groups (Inflammatory Subtypes).
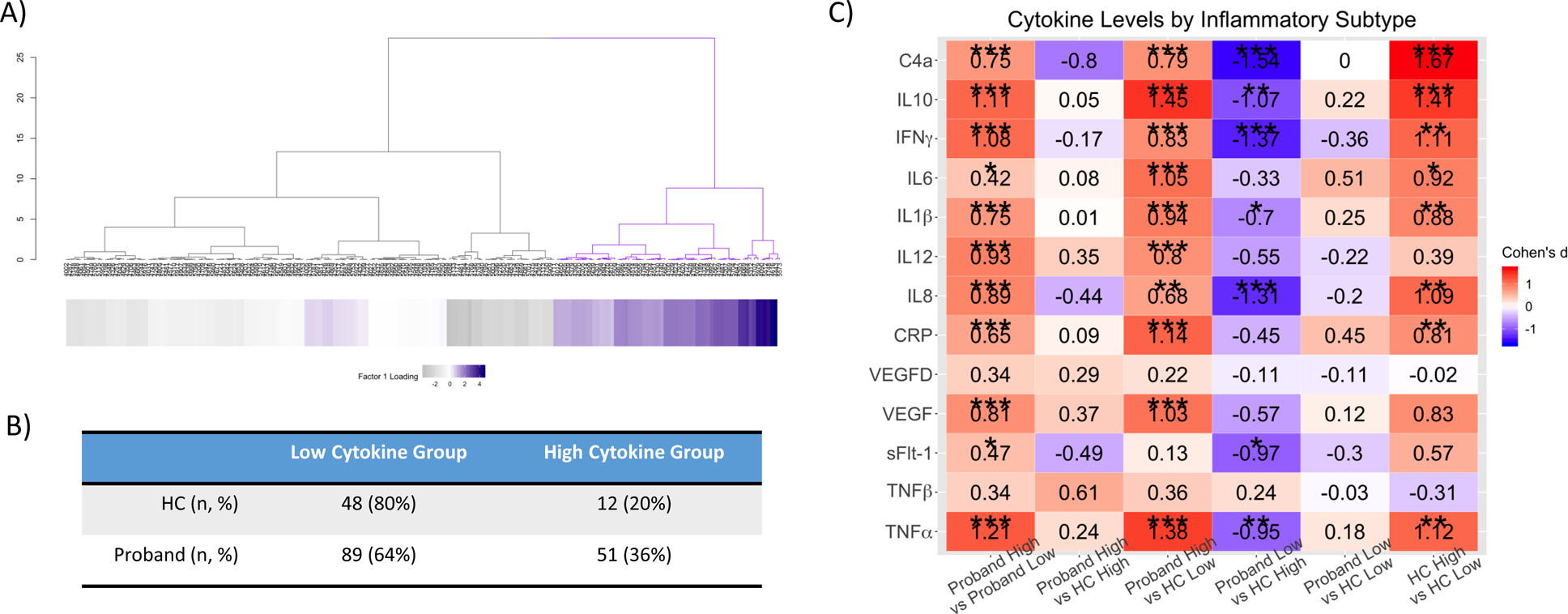
A) Hierarchical clustering analysis with the height of each linkage in the dendrogram representing the distance between the clusters joined by that link. The heatmap consists of factor loadings with gray indicating lower scores and purple indicating higher scores. B) Table showing that one subgroup contains elevated cytokine levels while the other subgroup has lower cytokine levels. The high cytokine subgroups comprise 36% of the psychosis group and 20% of the control group. C) Pairwise contrasts of cytokine levels between Proband High, Proband Low, healthy control (HC) High and HC Low subjects. Cohen’s d estimates were adjusted for age, sex, and ancestry. *, p < 0.05; **, p <0.01; ***, p < 0.001.
Spatial span forward (visual-spatial working memory) and antisaccade error rate (response inhibition) differed between the Proband-High and Proband-Low subtypes (Figure 4). The Proband-High and Proband-Low subtypes both demonstrated poorer overall cognition (BACS composite) and antisaccade error rate compared to the HC-low group. No differences existed between the HC-High or HC-Low group for any of the cognitive variables. Additionally, there were no significant differences between the Proband-High and Proband-Low subtypes in terms of psychotic symptoms, mania, depression, impulsivity or global functioning.
Figure 4: Cognition Differences Between Inflammatory Subtypes.
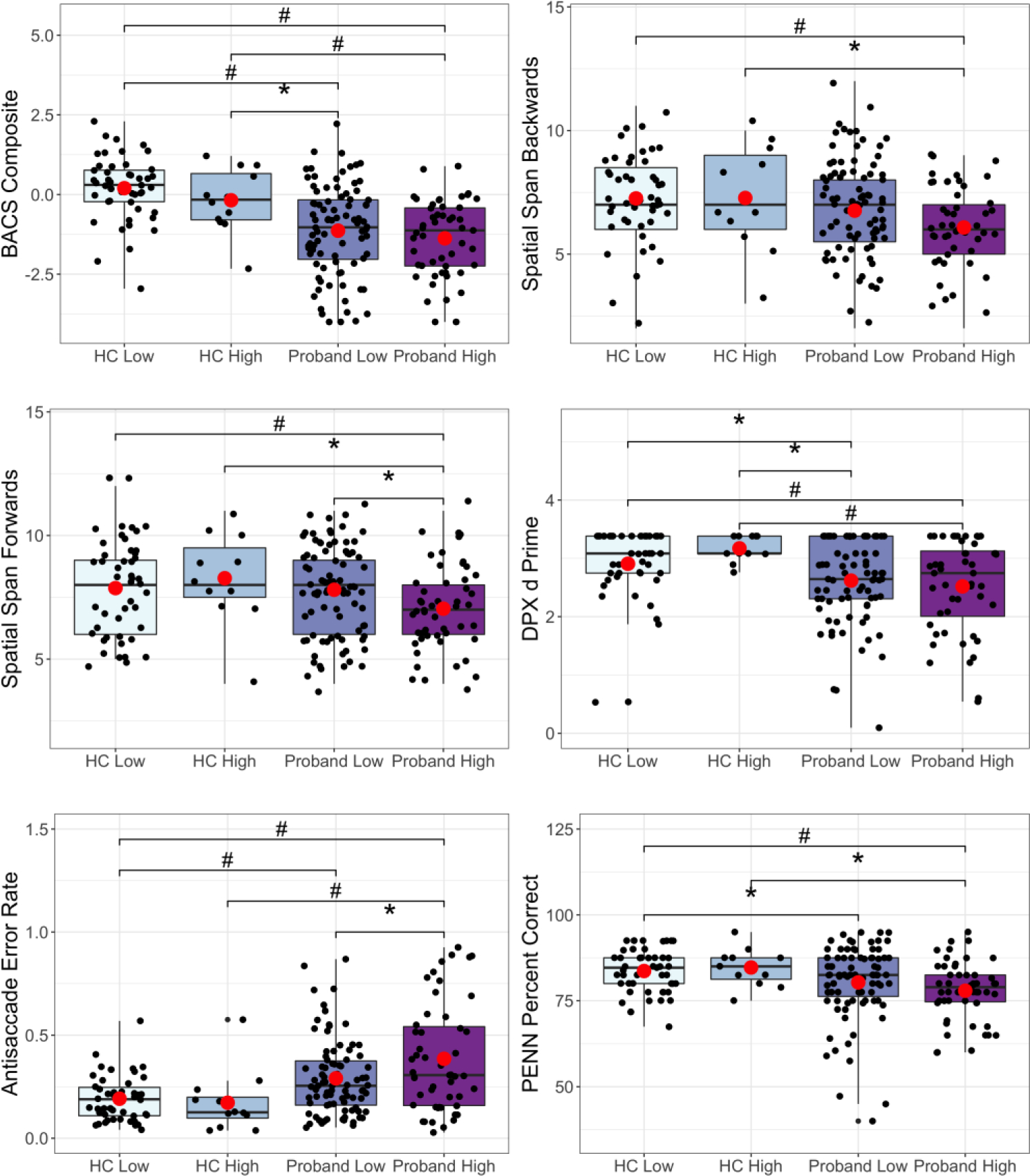
Pairwise contrasts of cognition variables between Proband High, Proband Low, healthy control (HC) high, and HC low subjects. BACS = Brief Assessment of Cognition in Schizophrenia; DPX d Prime = dot pattern expectancy d Prime; PENN = Penn Emotion Recognition Task). *q<0.1, #q<0.05, (•) mean.
Proband-High compared to the Proband-Low had significantly (q<0.05) greater volumes in the bilateral hippocampus, amygdala, and putamen, as well as the left thalamus (Figure 5A) and increased thickness in several gray matter regions (Figure 5B). The Proband-Low group had significantly (q<0.05) reduced cortical thickness (frontal, temporal, and occipital regions) and significantly (q<0.05) smaller subcortical volumes (hippocampus, amygdala, and right thalamus) compared to the HC-Low group (Figure 5A and 5B). Similar to some of the thinning observed in the Proband-Low group as compared to HC-Low, the Proband-High subtype was found to have lower right rostral middle frontal (d=−0.76, q=0.06), bilateral medial orbital frontal (right, d=−0.65, q=0.06; left, d=−0.67, q=0.08) and left inferior temporal (d=−0.75, q=0.06) thickness compared to the HC-Low group (data not shown). There were no regions where the Proband-High group had greater thickness or volume compared to the HC-Low group. Additionally, there were no other cortical or subcortical differences between the inflammatory subtypes within or between the HC or Proband groups that survived multiple comparisons corrections.
Figure 5: Gray Matter Thickness and Subcortical Volume Differences Between Inflammatory Subtypes.
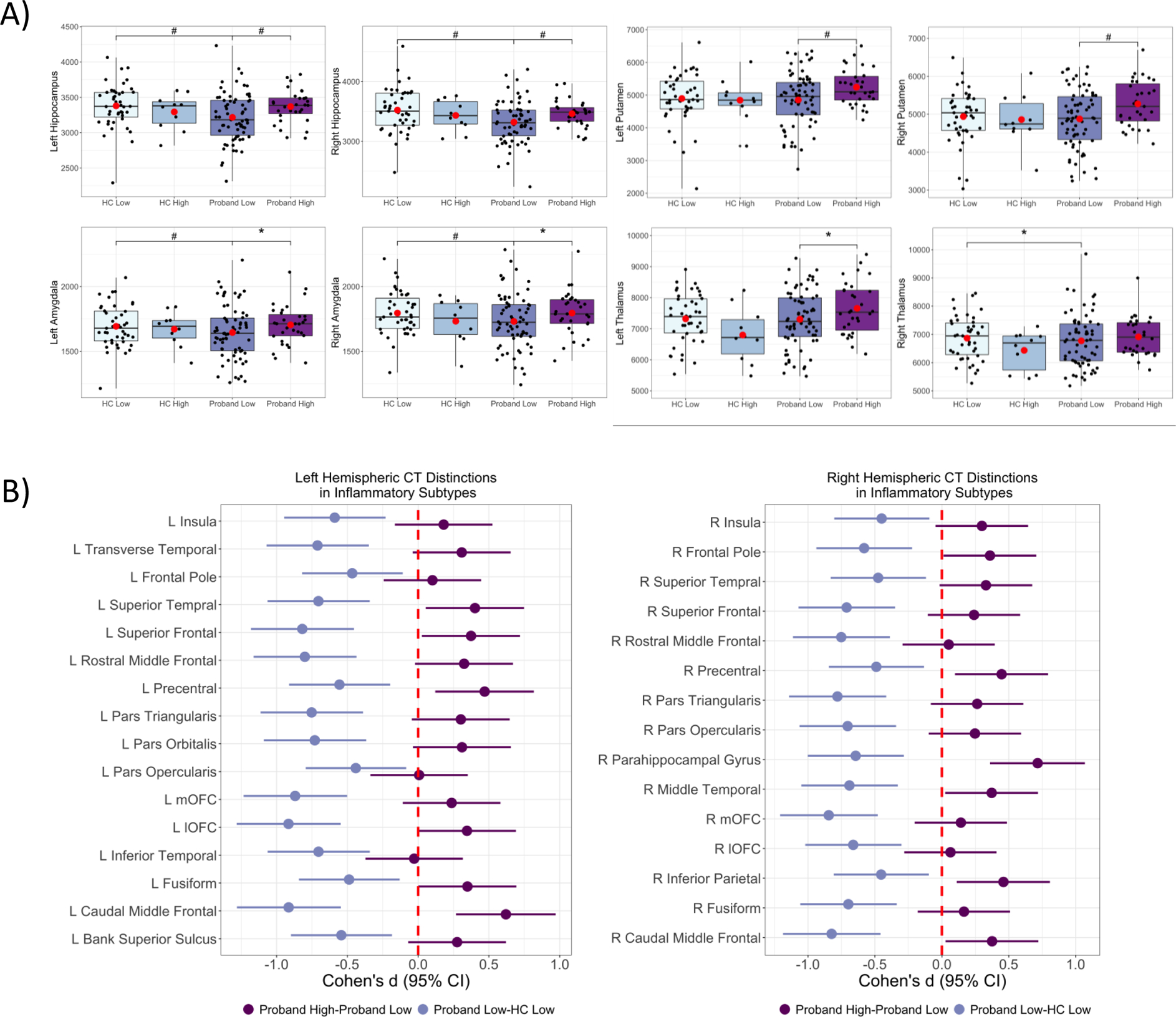
A) Pairwise contrasts of subcortical volume measures between Proband High, Proband Low, healthy control (HC) High and HC Low subjects. B) Pairwise contrasts of gray matter cortical thickness (CT) variables between Proband High, Proband Low, and healthy control (HC) Low subjects. Only the regions in the Proband Low versus HC low contrast survived multiple comparison correction (q<0.05). There are several regions that are significant (p<0.05 and confidence intervals exclude zero) in the Proband High versus Proband Low comparison. Solid circles indicate Cohen’s d, and solid lines indicate confidence intervals. Cohen’s d estimates were adjusted for age, sex, and ancestry. R = Right; L = Left; mOFC = medial orbitofrontal cortex; IOFC = lateral OFC. *, q<0.1; #q<0.05, (•) mean.
Discussion
In the largest transdiagnostic study of deeply phenotyped psychosis subjects to date, we used a panel of 15 peripheral inflammatory markers and a novel factor analysis/clustering approach to identify an inflammatory subtype. The finding that 36% of psychosis probands had a pattern of elevated peripheral inflammatory marker levels is consistent with previous reports using postmortem tissue (12), peripheral mRNA (33), and blood levels (20). The present findings highlight individual and composite inflammatory marker associations with brain abnormalities, but that using a combination of markers may be more clinically and pathophysiologically informative than single measures. While cognitive and neuroanatomical data suggest a clinical relevance for peripheral inflammatory marker abnormalities, determining whether the marker-defined higher inflammation subgroup represents a patient subgroup that may preferentially benefit from anti-inflammatory treatment will require future examination.
Individual Peripheral Inflammatory Markers Relationships
Of the 15 peripheral inflammatory markers measured, only TNFα, IL6, VEGF and CRP were elevated in psychosis probands. Inter-marker analyses showed that IL6, VEGF and CRP were positively correlated in probands, but not TNFα, and that each one of these clustered with a different set of markers (Supplementary Figure 2). These markers have been previously investigated in separate studies of SZP and bipolar disorder. Our assessment of inflammatory markers in deeply phenotyped patients representing the psychosis spectrum with a collection of clinical and neurobiological measures used herein adds to the existing knowledge about potential mechanistic relationships. These markers are known to be increased in both SZP and bipolar disorder during either acute (TNFα, IL6, CRP) or chronic stages of illness (IL6, VEGF, CRP) suggesting the possibility of a common underlying mechanism of immune dysfunction in these disorders (15,21,25,64). TNFα, IL6, and VEGF are pleiotropic factors that are activated during conditions of injury or stress and result in the maintenance and perpetuation of immunological reactions signaled through the expression of the acute phase protein, CRP, and together can lead to BBB disruption (4,21,69–71). Findings described herein raise the plausible possibility that patients with psychosis are abnormally sensitive to immune activation and that the effects on cognition are potentially mediated more robustly by TNFα, IL6, VEGF and CRP than other marker measures. To our knowledge, we are the first group to demonstrate that elevations in VEGF and CRP are associated with worse inhibitory behavioral control (i.e., elevated antisaccade error rate). See supplementary discussion section for a description regarding peripheral inflammatory marker correlations with brain structure.
Subgrouping Based on Multiple Peripheral Inflammatory Markers
Diagnostic heterogeneity continues to be a major obstacle in the understanding and treatment of psychosis patients. A lack of rigorously defined physiological targets has limited advances in precision medicine for determining optimal treatments or the development of new interventions. Previous investigations have used composite scores (39), unsupervised k-means clustering (33) or regularized regression (19) to identify patients with patterns of peripheral inflammatory marker alterations. The combined exploratory factor analysis and hierarchical clustering approach employed herein, extends this prior work by defining the underlying structure of a set of peripheral inflammatory marker variables related to cognition and brain structure.
We empirically defined two psychosis subtypes associated with different patterns of inflammatory marker levels, which were not enriched in any diagnostic or biotype group and supports the utility of transdiagnostic studies when examining biomarkers in psychosis. The clinical relevance of these findings is underscored by associations between cognitive and neuroimaging measures. These in turn informed the pathophysiologic effects of elevated peripheral information on neuroanatomy and function, which would not have been possible when examining psychosis probands by categorically defined diagnostic groups. This approach also clarified that in HCs, inflammatory subtypes do not appear to identify any adverse cognitive effects or brain structural differences. Findings in probands support the hypothesis that persons with psychosis may have a particular vulnerability that might be explained by microvascular and/or microglia dysfunction. The absence of observed relationships between inflammatory subtypes and clinical symptoms in the present study suggests that anatomic and cognitive features may be more sensitive to alterations in a generalized inflammatory response and that neuroinflammatory alterations may represent one potential path to psychosis. The lack of association may also be a function of the relatively clinically stable participants. While these analyses only considered structural MRI, additional complementary imaging approaches that examine cerebral perfusion (72), extracellular free-water (73,74) or fMRI connectivity networks (e.g. default mode (75), central executive (76) and visual networks (77)) linked to these regions may improve our understand the pathophysiological effects of peripheral inflammation on microvascular dysfunction, neuroinflammation and functional connectivity.
In this cohort, there were 1) three regions of shared vulnerability (right rostral middle frontal, bilateral medial orbitofrontal, and the left inferior temporal cortex) within the Proband-High and -Low groups as compared to HC-Low, 2) greater thickness and volume was observed for the Proband-High group compared to the Proband-Low group in various brain regions, and 3) profound thinning was observed in the Proband-Low compared to the HC-Low group. A potential explanation for the first observation is that proinflammatory markers elicit the conversion of tryptophan to kynurenine in the periphery, which increases kynurenic acid, a known factor leading to rostral middle frontal volume loss (78). A possible interpretation for the second observation (significantly greater volume in the hippocampus, amygdala, putamen, and left thalamus in the Proband-High group compared to the Proband-Low group, as well as thickening in the Proband-High group) is that peripheral inflammation is mediating brain parenchymal expansion. Why these subcortical structures are particularly sensitive and why we did not observe cortical thinning in the Proband High group remains to be determined. It is plausible that peripheral inflammation leads to BBB disruption and subsequent gray matter thickening (i.e. pseudothickening) due to inflammation-associated increases in vessel dilation, and increased fluid impacting volume and thickness measures (4). This interpretation would also apply to the profound thinning observed in the Proband-Low group, which suggests that low levels of peripheral inflammation may not affect the brain’s microvasculature and therefore greater brain matter loss is observed. Therefore, it is important to consider the stage of psychotic illness, illness acuity, potential confounders, and inflammatory status when determining whether brain volume loss (resolved inflammation) or brain volume expansion (active low-grade inflammation) is to be expected. Future studies are necessary to examine the stability of this relationship and the potential for perfusion-, neuroinflammation-, and/or BBB permeability-based imaging in determining the pathophysiologic consequences of peripheral inflammation in the brain.
Implications for Novel strategies to Treat Psychosis
The present findings highlight relationships between inflammation status defined by multiple peripheral inflammatory markers and may help overcome challenges observed in initial studies exploring the use of targeted anti-inflammatory drugs, such as Infliximab (26) or Tocilizumab (29) as augmentation strategies to treat depression or psychosis. These studies have primarily been negative, but have not stratified using baseline peripheral inflammatory marker levels. Post hoc analysis by Raison and colleagues demonstrated that participants with a CRP levels ≥5mg/L were more likely to respond to treatment with Infliximab (26), however, McIntyre and colleagues performed a study whereby patients with bipolar depression were stratified based on their CRP levels and they did not identify a reduction in depressive symptoms (27). On the other hand, Tocilizumab remains promising as there may be the potential for this medication to improve cognition (28,29) and that this effect may be predicted by baseline peripheral inflammatory marker levels (29). Lastly, research focusing on repurposing drugs with anti-inflammatory properties such as celecoxib, minocycline, N-acetylcysteine, statins, and aspirin in the treatment of psychosis have been promising (79), but many studies have not used a biomarker-based approach to predict treatment response. Taken together, individual markers may not be sufficient to predict treatment outcome using anti-inflammatory drugs, and further work is needed to determine whether a combination of inflammatory markers or markers from diverse pathways are required.
Limitations
The present study had a number of limitations that require consideration when interpreting the results and generalizability of the findings. Relationships described here in were observed in a somewhat small cross-sectional study design of clinically stable patients with psychosis and a number of confounding factors (quantification of lifetime antipsychotic exposure, smoking, body mass index, medical comorbidities, trauma, depression, anxiety, and sleep related issues) that could have an impact on the observed associations were not measured (80,81). Autoimmune antibody levels or past exposure to infectious agents were not measured (82), and could potentially contribute to and/or explain some aspects of the peripheral inflammatory marker elevations observed. Therefore, in future larger studies we suggest incorporating these potential confounding factors along with those factors that we identified to be important in our study (sample storage days, hemolysis score, sample set, age, sex, and ancestry), as well as assessing autoimmune and infectious exposure status.
Conclusion
Results described herein specify individual and group peripheral inflammatory marker alterations in psychotic disorders and establish clinical, neurocognitive and neuroanatomic associations with increased inflammation. A subgroup of patients with greater inflammation defined by a group of inflammatory markers provides a potential means for identifying individuals who may benefit from treatment with anti-inflammatory medications.
Supplementary Material
Acknowledgments
Supported in part by NIMH grants MH-077851 (to Dr. Tamminga), MH-078113 (to Dr. Keshavan), MH-077945 (to Dr. Pearlson), MH-077852 (to Dr. Thaker), and MH-077862 (to Dr. Sweeney); MH-083888 (to Dr. Bishop); the Commonwealth Research Center (grant SCDMH82101008006 to Dr. Keshavan); the NIH’s National Center for Advancing Translational Sciences (pilot grant to Dr. Bishop supported by UL1TR000114); and a Dupont Warren and Livingston Award from Harvard Medical School (to Dr. Lizano). Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Poster Presentation at the American College of Neuropsychopharmacology, Orlando, FL, December 8–12, 2019
Disclosures
CAT has served on the advisory board for drug development for Intra-Cellular Therapies, Inc., as an ad hoc consultant for Eli Lilly, Sunovion, Astellas, Pfizer, and Merck, has been a council member and unpaid volunteer for the National Alliance on Mental Illness, and served as deputy editor for the American Psychiatric Association. MSK has received research support from Sunovion and GlaxoSmithKline. Remaining authors report no competing interests.
References
- 1.Haller C, Padmanabhan J, Lizano P, Torous J, Keshavan M. Recent advances in understanding schizophrenia. F1000Prime Rep [Internet]. 2014. July 8 [cited 2019 Dec 29];6. Available from: http://www.f1000.com/reports/m/6/57/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keshavan MS, Collin G, Guimond S, Kelly S, Prasad KM, Lizano P. Neuroimaging in Schizophrenia. Neuroimaging Clin N Am. 2020. February;30(1):73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marder SR, Cannon TD. Schizophrenia. Ropper AH, editor. N Engl J Med. 2019. October 31;381(18):1753–61. [DOI] [PubMed] [Google Scholar]
- 4.Kamintsky L, Cairns KA, Veksler R, Bowen C, Beyea SD, Friedman A, et al. Blood-brain barrier imaging as a potential biomarker for bipolar disorder progression. NeuroImage Clin. 2019. October;102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai HQ, Catts VS, Webster MJ, Galletly C, Liu D, O’Donnell M, et al. Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol Psychiatry [Internet]. 2018. September 13 [cited 2019 Dec 29]; Available from: http://www.nature.com/articles/s41380-018-0235-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berk M, Walker AJ, Nierenberg AA. Biomarker-Guided Anti-inflammatory Therapies: From Promise to Reality Check. JAMA Psychiatry. 2019. August 1;76(8):779. [DOI] [PubMed] [Google Scholar]
- 7.Trépanier MO, Hopperton KE, Mizrahi R, Mechawar N, Bazinet RP. Postmortem evidence of cerebral inflammation in schizophrenia: a systematic review. Mol Psychiatry. 2016. August;21(8):1009–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roussos P, Haroutunian V. Schizophrenia: susceptibility genes and oligodendroglial and myelin related abnormalities. Front Cell Neurosci [Internet]. 2014. [cited 2020 Aug 4];8. Available from: http://journal.frontiersin.org/article/10.3389/fncel.2014.00005/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karoutzou G, Emrich HM, Dietrich DE. The myelin-pathogenesis puzzle in schizophrenia: a literature review. Mol Psychiatry. 2008. March;13(3):245–60. [DOI] [PubMed] [Google Scholar]
- 10.Stephan KE, Friston KJ, Frith CD. Dysconnection in Schizophrenia: From Abnormal Synaptic Plasticity to Failures of Self-monitoring. Schizophr Bull. 2009. March 30;35(3):509–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abi-Dargham A Schizophrenia: Overview and Dopamine Dysfunction. J Clin Psychiatry. 2014. November 24;75(11):e31–e31. [DOI] [PubMed] [Google Scholar]
- 12.Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013. February;18(2):206–14. [DOI] [PubMed] [Google Scholar]
- 13.Banks WA. The blood-brain barrier in neuroimmunology: Tales of separation and assimilation. Brain Behav Immun. 2015. February;44:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller BJ, Gassama B, Sebastian D, Buckley P, Mellor A. Meta-Analysis of Lymphocytes in Schizophrenia: Clinical Status and Antipsychotic Effects. Biol Psychiatry. 2013. May;73(10):993–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016. December;21(12):1696–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraguas D, Díaz-Caneja CM, Ayora M, Hernández-Álvarez F, Rodríguez-Quiroga A, Recio S, et al. Oxidative Stress and Inflammation in First-Episode Psychosis: A Systematic Review and Meta-analysis. Schizophr Bull. 2019. June 18;45(4):742–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perkins DO, Jeffries CD, Addington J, Bearden CE, Cadenhead KS, Cannon TD, et al. Towards a Psychosis Risk Blood Diagnostic for Persons Experiencing High-Risk Symptoms: Preliminary Results From the NAPLS Project. Schizophr Bull. 2015. March;41(2):419–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes LN, Severance EG, Leek JT, Gressitt KL, Rohleder C, Coughlin JM, et al. Inflammatory Molecular Signature Associated With Infectious Agents in Psychosis. Schizophr Bull. 2014. September;40(5):963–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.the OPTiMiSE Study Group, Martinuzzi E, Barbosa S, Daoudlarian D, Bel Haj Ali W, Gilet C, et al. Stratification and prediction of remission in first-episode psychosis patients: the OPTiMiSE cohort study. Transl Psychiatry. 2019. December;9(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boerrigter D, Weickert TW, Lenroot R, O’Donnell M, Galletly C, Liu D, et al. Using blood cytokine measures to define high inflammatory biotype of schizophrenia and schizoaffective disorder. J Neuroinflammation. 2017. December;14(1):188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandes BS, Steiner J, Bernstein H-G, Dodd S, Pasco JA, Dean OM, et al. C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: meta-analysis and implications. Mol Psychiatry. 2016. April;21(4):554–64. [DOI] [PubMed] [Google Scholar]
- 22.Katsel P, Roussos P, Pletnikov M, Haroutunian V. Microvascular anomaly conditions in psychiatric disease. Schizophrenia – angiogenesis connection. Neurosci Biobehav Rev. 2017. June;77:327–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lizano PL, Keshavan MS, Tandon N, Mathew IT, Mothi SS, Montrose DM, et al. Angiogenic and immune signatures in plasma of young relatives at familial high-risk for psychosis and first-episode patients: A preliminary study. Schizophr Res. 2016. January;170(1):115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lizano P, Lutz O, Ling G, Padmanabhan J, Tandon N, Sweeney J, et al. VEGFA GENE variation influences hallucinations and frontotemporal morphology in psychotic disorders: a B-SNIP study. Transl Psychiatry. 2018. 11;8(1):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Misiak B, Stramecki F, Stańczykiewicz B, Frydecka D, Lubeiro A. Vascular endothelial growth factor in patients with schizophrenia: A systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2018. August;86:24–9. [DOI] [PubMed] [Google Scholar]
- 26.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A Randomized Controlled Trial of the Tumor Necrosis Factor Antagonist Infliximab for Treatment-Resistant Depression: The Role of Baseline Inflammatory Biomarkers. JAMA Psychiatry. 2013. January 1;70(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McIntyre RS, Subramaniapillai M, Lee Y, Pan Z, Carmona NE, Shekotikhina M, et al. Efficacy of Adjunctive Infliximab vs Placebo in the Treatment of Adults With Bipolar I/II Depression: A Randomized Clinical Trial. JAMA Psychiatry. 2019. August 1;76(8):783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller BJ, Dias JK, Lemos HP, Buckley PF. An Open-Label, Pilot Trial of Adjunctive Tocilizumab in Schizophrenia. J Clin Psychiatry. 2016. February 24;77(02):275–6. [DOI] [PubMed] [Google Scholar]
- 29.Girgis RR, Ciarleglio A, Choo T, Haynes G, Bathon JM, Cremers S, et al. A Randomized, Double-Blind, Placebo-Controlled Clinical Trial of Tocilizumab, An Interleukin-6 Receptor Antibody, For Residual Symptoms in Schizophrenia. Neuropsychopharmacology. 2018. May;43(6):1317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng W, Cai D-B, Yang X-H, Ungvari GS, Ng CH, Müller N, et al. Adjunctive celecoxib for schizophrenia: A meta-analysis of randomized, double-blind, placebo-controlled trials. J Psychiatr Res. 2017. September;92:139–46. [DOI] [PubMed] [Google Scholar]
- 31.Xiang Y-Q, Zheng W, Wang S-B, Yang X-H, Cai D-B, Ng CH, et al. Adjunctive minocycline for schizophrenia: A meta-analysis of randomized controlled trials. Eur Neuropsychopharmacol. 2017. January;27(1):8–18. [DOI] [PubMed] [Google Scholar]
- 32.Fillman SG, Sinclair D, Fung SJ, Webster MJ, Shannon Weickert C. Markers of inflammation and stress distinguish subsets of individuals with schizophrenia and bipolar disorder. Transl Psychiatry. 2014. February;4(2):e365–e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fillman SG, Weickert TW, Lenroot RK, Catts SV, Bruggemann JM, Catts VS, et al. Elevated peripheral cytokines characterize a subgroup of people with schizophrenia displaying poor verbal fluency and reduced Broca’s area volume. Mol Psychiatry. 2016. August;21(8):1090–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Catts VS, Sheedy D, McCrossin T, Kril JJ, Shannon Weickert C. Cortical grey matter volume reduction in people with schizophrenia is associated with neuro-inflammation. Transl Psychiatry. 2016. December;6(12):e982–e982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Catts VS, Wong J, Fillman SG, Fung SJ, Shannon Weickert C. Increased expression of astrocyte markers in schizophrenia: Association with neuroinflammation. Aust N Z J Psychiatry. 2014. August;48(8):722–34. [DOI] [PubMed] [Google Scholar]
- 36.Lizano PL, Yao JK, Tandon N, Mothi SS, Montrose DM, Keshavan MS. Association of sFlt-1 and worsening psychopathology in relatives at high risk for psychosis: A longitudinal study. Schizophr Res. 2017. May;183:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pillai A, Howell KR, Ahmed AO, Weinberg D, Allen KM, Bruggemann J, et al. Association of serum VEGF levels with prefrontal cortex volume in schizophrenia. Mol Psychiatry. 2016. May;21(5):686–92. [DOI] [PubMed] [Google Scholar]
- 38.Jacomb I, Stanton C, Vasudevan R, Powell H, O’Donnell M, Lenroot R, et al. C-Reactive Protein: Higher During Acute Psychotic Episodes and Related to Cortical Thickness in Schizophrenia and Healthy Controls. Front Immunol. 2018. October 10;9:2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cannon TD, Chung Y, He G, Sun D, Jacobson A, van Erp TGM, et al. Progressive Reduction in Cortical Thickness as Psychosis Develops: A Multisite Longitudinal Neuroimaging Study of Youth at Elevated Clinical Risk. Biol Psychiatry. 2015. January;77(2):147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu D, Lv P, Li F, Zhang W, Fu G, Dai J, et al. Association of peripheral cytokine levels with cerebral structural abnormalities in schizophrenia. Brain Res. 2019. 01;1724:146463. [DOI] [PubMed] [Google Scholar]
- 41.Dickerson F, Stallings C, Origoni A, Boronow J, Yolken R. C-reactive protein is associated with the severity of cognitive impairment but not of psychiatric symptoms in individuals with schizophrenia. Schizophr Res. 2007. July;93(1–3):261–5. [DOI] [PubMed] [Google Scholar]
- 42.Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, et al. Clinical Phenotypes of Psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry. 2013. November;170(11):1263–74. [DOI] [PubMed] [Google Scholar]
- 43.Lopez-Candales A, Hernández Burgos PM, Hernandez-Suarez DF, Harris D. Linking Chronic Inflammation with Cardiovascular Disease: From Normal Aging to the Metabolic Syndrome. J Nat Sci. 2017. April;3(4). [PMC free article] [PubMed] [Google Scholar]
- 44.Lyall DM, Celis-Morales C, Ward J, Iliodromiti S, Anderson JJ, Gill JMR, et al. Association of Body Mass Index With Cardiometabolic Disease in the UK Biobank: A Mendelian Randomization Study. JAMA Cardiol. 2017. August 1;2(8):882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho B-C. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010. February 1;67(3):255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lencer R, Mills LJ, Alliey-Rodriguez N, Shafee R, Lee AM, Reilly JL, et al. Genome-wide association studies of smooth pursuit and antisaccade eye movements in psychotic disorders: findings from the B-SNIP study. Transl Psychiatry. 2017. 24;7(10):e1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alliey-Rodriguez N, Grey TA, Shafee R, Asif H, Lutz O, Bolo NR, et al. NRXN1 is associated with enlargement of the temporal horns of the lateral ventricles in psychosis. Transl Psychiatry. 2019. 17;9(1):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr Bull. 1987. January 1;13(2):261–76. [DOI] [PubMed] [Google Scholar]
- 49.Young RC, Biggs JT, Ziegler VE, Meyer DA. A Rating Scale for Mania: Reliability, Validity and Sensitivity. Br J Psychiatry. 1978. November;133(5):429–35. [DOI] [PubMed] [Google Scholar]
- 50.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995. November;51(6):768–74. [DOI] [PubMed] [Google Scholar]
- 51.Montgomery SA, Åsberg M. A New Depression Scale Designed to be Sensitive to Change. Br J Psychiatry. 1979. April;134(4):382–9. [DOI] [PubMed] [Google Scholar]
- 52.Jones SH, Thornicroft G, Coffey M, Dunn G. A Brief Mental Health Outcome Scale: Reliability and Validity of the Global Assessment of Functioning (GAF). Br J Psychiatry. 1995. May;166(5):654–9. [DOI] [PubMed] [Google Scholar]
- 53.Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S. The Social Functioning Scale the Development and Validation of a New Scale of Social Adjustment for use in Family Intervention Programmes with Schizophrenic Patients. Br J Psychiatry. 1990. December;157(6):853–9. [DOI] [PubMed] [Google Scholar]
- 54.Keefe R The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004. June 1;68(2–3):283–97. [DOI] [PubMed] [Google Scholar]
- 55.Ruocco AC, Reilly JL, Rubin LH, Daros AR, Gershon ES, Tamminga CA, et al. Emotion recognition deficits in schizophrenia-spectrum disorders and psychotic bipolar disorder: Findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Schizophr Res. 2014. September;158(1–3):105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reid N Wide Range Achievement Test: 1984 Revised Edition. J Couns Dev. 1986. April;64(8):538–9. [Google Scholar]
- 57.Jones JAH, Sponheim SR, MacDonald AW. The dot pattern expectancy task: Reliability and replication of deficits in schizophrenia. Psychol Assess. 2010;22(1):131–41. [DOI] [PubMed] [Google Scholar]
- 58.Harris MSH, Reilly JL, Thase ME, Keshavan MS, Sweeney JA. Response suppression deficits in treatment-naïve first-episode patients with schizophrenia, psychotic bipolar disorder and psychotic major depression. Psychiatry Res. 2009. December 30;170(2–3):150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hill SK, Reilly JL, Keefe RSE, Gold JM, Bishop JR, Gershon ES, et al. Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Am J Psychiatry. 2013. November;170(11):1275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reilly JL, Hill SK, Gold JM, Keefe RSE, Clementz BA, Gershon E, et al. Impaired Context Processing is Attributable to Global Neuropsychological Impairment in Schizophrenia and Psychotic Bipolar Disorder. Schizophr Bull. 2017. 01;43(2):397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kristian Hill S, Buchholz A, Amsbaugh H, Reilly JL, Rubin LH, Gold JM, et al. Working memory impairment in probands with schizoaffective disorder and first degree relatives of schizophrenia probands extend beyond deficits predicted by generalized neuropsychological impairment. Schizophr Res. 2015. August;166(1–3):310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, et al. Identification of Distinct Psychosis Biotypes Using Brain-Based Biomarkers. Am J Psychiatry. 2016. April;173(4):373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buckley PF, Mahadik S, Pillai A, Terry A. Neurotrophins and schizophrenia. Schizophr Res. 2007. August;94(1–3):1–11. [DOI] [PubMed] [Google Scholar]
- 64.Fernandes BS, Steiner J, Molendijk ML, Dodd S, Nardin P, Gonçalves C-A, et al. C-reactive protein concentrations across the mood spectrum in bipolar disorder: a systematic review and meta-analysis. Lancet Psychiatry. 2016. December;3(12):1147–56. [DOI] [PubMed] [Google Scholar]
- 65.Schizophrenia Working Group of the Psychiatric Genomics Consortium, Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016. February;530(7589):177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goff DC, Zeng B, Ardekani BA, Diminich ED, Tang Y, Fan X, et al. Association of Hippocampal Atrophy With Duration of Untreated Psychosis and Molecular Biomarkers During Initial Antipsychotic Treatment of First-Episode Psychosis. JAMA Psychiatry. 2018. April 1;75(4):370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B Methodol. 1995. January;57(1):289–300. [Google Scholar]
- 68.Cui ZC. Allowable limit of error in clinical chemistry quality control. Clin Chem. 1989. April 1;35(4):630–1. [PubMed] [Google Scholar]
- 69.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015. May;16(5):448–57. [DOI] [PubMed] [Google Scholar]
- 70.Pollak TA, Drndarski S, Stone JM, David AS, McGuire P, Abbott NJ. The blood-brain barrier in psychosis. Lancet Psychiatry. 2018;5(1):79–92. [DOI] [PubMed] [Google Scholar]
- 71.Hsuchou H, Kastin AJ, Mishra PK, Pan W. C-reactive protein increases BBB permeability: implications for obesity and neuroinflammation. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2012;30(5):1109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Najjar S, Pahlajani S, De Sanctis V, Stern JNH, Najjar A, Chong D. Neurovascular Unit Dysfunction and Blood–Brain Barrier Hyperpermeability Contribute to Schizophrenia Neurobiology: A Theoretical Integration of Clinical and Experimental Evidence. Front Psychiatry. 2017. May 23;8:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lyall AE, Pasternak O, Robinson DG, Newell D, Trampush JW, Gallego JA, et al. Greater extracellular free-water in first-episode psychosis predicts better neurocognitive functioning. Mol Psychiatry. 2018. March;23(3):701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Di Biase MA, Katabi G, Piontkewitz Y, Cetin-Karayumak S, Weiner I, Pasternak O. Increased extracellular free-water in adult male rats following in utero exposure to maternal immune activation. Brain Behav Immun. 2020. January;83:283–7. [DOI] [PubMed] [Google Scholar]
- 75.Yin L, Xu X, Chen G, Mehta ND, Haroon E, Miller AH, et al. Inflammation and decreased functional connectivity in a widely-distributed network in depression: Centralized effects in the ventral medial prefrontal cortex. Brain Behav Immun. 2019. August;80:657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nusslock R, Brody GH, Armstrong CC, Carroll AL, Sweet LH, Yu T, et al. Higher Peripheral Inflammatory Signaling Associated With Lower Resting-State Functional Brain Connectivity in Emotion Regulation and Central Executive Networks. Biol Psychiatry. 2019. July;86(2):153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Balter LJT, Bosch JA, Aldred S, Drayson MT, Veldhuijzen van Zanten JJCS, Higgs S, et al. Selective effects of acute low-grade inflammation on human visual attention. NeuroImage. 2019. November;202:116098. [DOI] [PubMed] [Google Scholar]
- 78.Kindler J, Lim CK, Weickert CS, Boerrigter D, Galletly C, Liu D, et al. Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Mol Psychiatry [Internet]. 2019. April 3 [cited 2019 Dec 29]; Available from: http://www.nature.com/articles/s41380-019-0401-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Çakici N, van Beveren NJM, Judge-Hundal G, Koola MM, Sommer IEC. An update on the efficacy of anti-inflammatory agents for patients with schizophrenia: a meta-analysis. Psychol Med. 2019. October;49(14):2307–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Jager W, Bourcier K, Rijkers GT, Prakken BJ, Seyfert-Margolis V. Prerequisites for cytokine measurements in clinical trials with multiplex immunoassays. BMC Immunol. 2009;10(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leng SX, McElhaney JE, Walston JD, Xie D, Fedarko NS, Kuchel GA. ELISA and Multiplex Technologies for Cytokine Measurement in Inflammation and Aging Research. J Gerontol A Biol Sci Med Sci. 2008. August 1;63(8):879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Upthegrove R, Barnes NM. The immune system and schizophrenia: an update for clinicians. Adv Psychiatr Treat. 2014. March;20(2):83–91. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


