Abstract
Introduction
The incidence of anterior cruciate ligament reconstruction (ACLR) surgeries is increasing and so is the number of revision surgeries for a failed ACLR. The spectrum of ACL failure includes symptoms of recurrent instability, pain, and/or stiffness.
Discussion
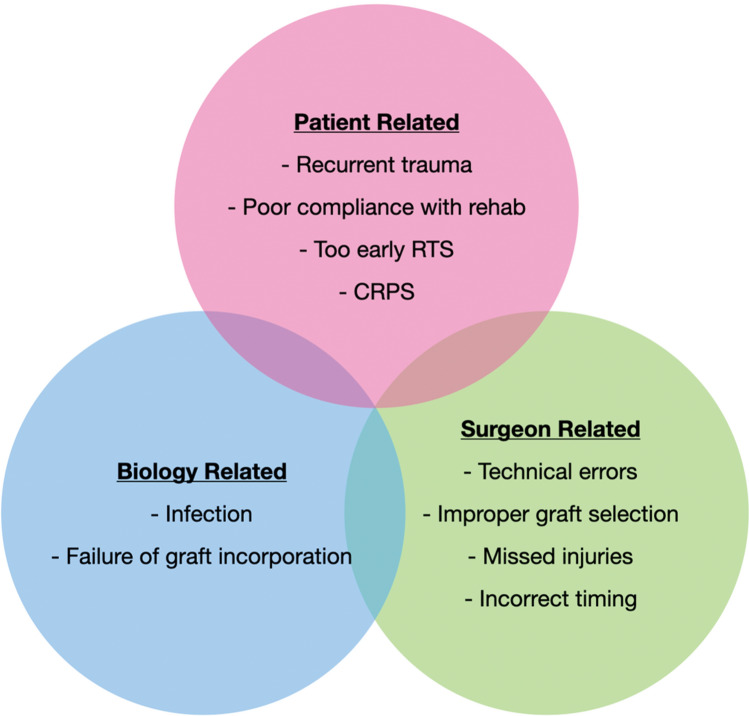
Factors contributing to ACL failure may be classified as patient-related, surgeon-related, and biological factors. Of these, tunnel malposition and recurrent trauma are the most common causes. Detailed patient assessment, imaging, and studying details of the index surgery are critical prior to planning revision surgery. Infection has to be ruled out prior to planning any reconstructive surgical procedure. Osseous malalignment in the coronal or sagittal planes would also need correction along with or prior to revision ACL surgery. Revision ACL reconstruction maybe performed as a one-stage or two-stage procedure. Severe tunnel dilatation, infection, or arthrofibrosis necessitates a two-stage approach. Autografts are preferred for revision ACL due their lesser re-tear rates and better outcomes. Associated meniscus tears and cartilage injuries are more common in revision than in primary surgery and need to be managed appropriately. Extra-articular reconstruction for controlling anterolateral instability is frequently required as well.
Conclusion
Revision ACL reconstruction is a complex undertaking due to limited graft options, compromised anatomy and high frequency of associated injuries. Patient expectations must be tempered because functional outcomes and return to pre-injury sports are inferior to a primary surgery.
Keywords: Anterior cruciate ligament, Anterior cruciate ligament reconstruction, Re-tear, Revision, Tunnel malposition, Tunnel dilatation, One-stage reconstruction, Two-stage reconstruction, Autograft, Posterior tibial slope
Introduction
Anterior cruciate ligament reconstruction (ACLR) is performed to achieve knee stability, prevent secondary injuries, and allow patients to return to pre-injury activity level [1]. With increasing number of primary ACLRs being performed [2], the incidence of revisions for failure of the primary surgery is also increasing. Unfortunately, there is no consensus for the definition of a “failed ACLR”. Noyes and Barber–Westin defined a non-functional ACL graft as one that demonstrates ≥ 6 mm of increased anteroposterior laxity on KT-2000 arthrometer testing or a positive pivot-shift test [3]. However, this definition considers instability as the sole determinant of a failed ACLR. Johnson and Fu proposed a more exhaustive definition of ACLR failure encompassing instability, pain, and stiffness [4]. Each or a combination of these scenarios could be due to multiple etiologies, the end result being a patient with a dysfunctional knee and unable to participate in activities for which the index procedure was performed.
The ACLR revision rate for adults is reported at 4.1% at 5 years in the Danish registry, whilst United States and Norway community registries report 0.9–1.5% revision rates [5, 6]. A meta-analysis of revision ACLRs in children and adolescents reported a revision rate of 4.8% [7]. Differences also exist in the revision rates from various graft types used in primary ACLR. In a recent large meta-analysis, the failure rate of autograft ACLR was found to be around 2.8% [8], and van Eck reported the failure of allograft ACLR to be 13% [9]. This review will analyze the various causes of failure of an ACL reconstruction, and discuss factors and techniques to consider in revision surgery and present the outcomes of revision ACLR.
Why are ACLRs Failing Today?
The reasons for an ACLR failure can be broadly grouped into surgeon, patient, and biological factors (Fig. 1). Surgeon-related or technical factors seem to be the most common reason for ACL failures. In Jaeckers’s series of 167 patients with failed ACLR, technical errors were found in 64.5%, followed by trauma (29.1%) and biological failure (6.4%). Femoral tunnel malposition was the commonest technical cause (83.1%) (Fig. 2) followed by a non-anatomic tibia tunnel (45.1%). They found strong correlations between non-traumatic technical failure and femoral tunnel mal-positioning, transtibial femoral drilling technique, and use of femoral trans-fixation devices [10]. Chen also suggested a non-traumatic etiology to be the most common cause (47%) in patients undergoing re-revisions ACL surgery [11]. Partial anatomic tunnels are reported to be more frequent in the tibia (45%) than the femur (27%) by Achnitch [12]. Malpositioned tunnels lead to inadequate restoration of stability (especially rotational) and graft impingement with the roof of the intercondylar notch, posterior cruciate ligament (PCL) or lateral femoral condyle [13, 14]. While inadequate rotational stability predisposes the graft to mechanical failure, graft impingement can cause attritional rupture or interfere with biological healing.
Fig. 1.
Etiological factors responsible for failure of an anterior cruciate ligament failure (RTS return to sports, CRPS complex regional pain syndrome)
Fig. 2.
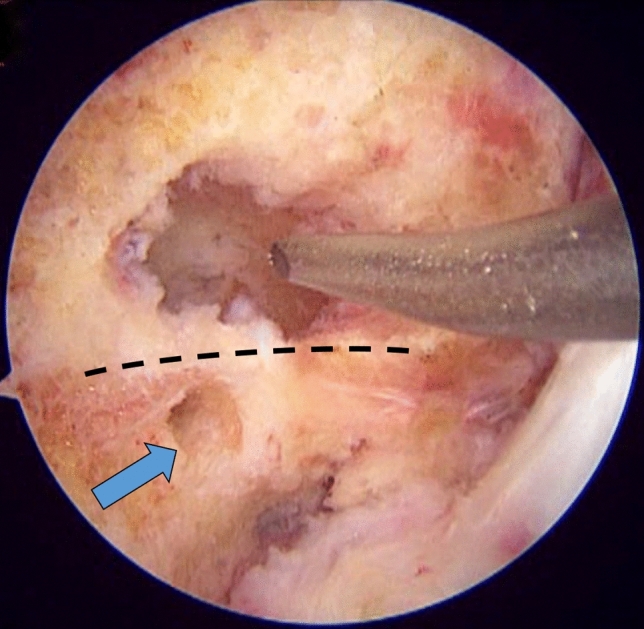
Right knee arthroscopy, viewing from the anterolateral portal, knee in 90º flexion. Malpositioned femoral tunnel (shown with chondral pick) above the intercondylar ridge (dotted line) seen during revision surgery. The anatomic tunnel position shown with blue arrow
Recurrent trauma has been reported to be the second common reason for ACL graft re-rupture. The Multicenter ACL Revision study (MARS) reported a 32% incidence in a cohort of 460 patients [15]. Three years later in 2013, in the same cohort with 1200 patients, the incidence was 54% [11]. Early or premature return to sport and high impact activities was an important predisposing factor.
Biological failure after ACLR with autograft or allograft remains a vexing problem. This can be attributed to disturbances in revascularization, inhibited cellular proliferation, or difficulty in the ligamentization process. Either of these may lead to graft necrosis or failure of incorporation [16]. Infection rate after ACLR is very low, but is an important cause of failure of reconstruction [17]. The risk factors associated with higher incidence of graft rupture are provided in Table 1.
Table 1.
Risk factors for graft rupture after ACL reconstruction
| Risk factor | Description | |
|---|---|---|
| 1 | Allograft ACLR [18–20] | Higher in younger patients and with irradiated/chemically treated grafts |
| 2 | Hamstring autograft [8] | Higher failure than BPTB autograft |
| 3 | Knee hyperextension ≥ 5° [21] | Present in almost 1/3 of revision ACLR cases |
| 4 | Lower BMI [21] | However, those with higher BMI have inferior functional outcomes |
| 5 | Male gender [22] | Based on Registry data from Sweden, Norway, Denmark and Kaiser Permanente. Younger age is the most consistently reported risk factor |
| 6 | Younger age [22] | |
| 7 | Suspensory graft fixation [22] | |
| 8 | Anteromedial portal drilling [22] |
ACLR anterior cruciate ligament reconstruction, BPTB bone–patella tendon–bone, BMI body mass index)
Planning a Revision ACL Reconstruction
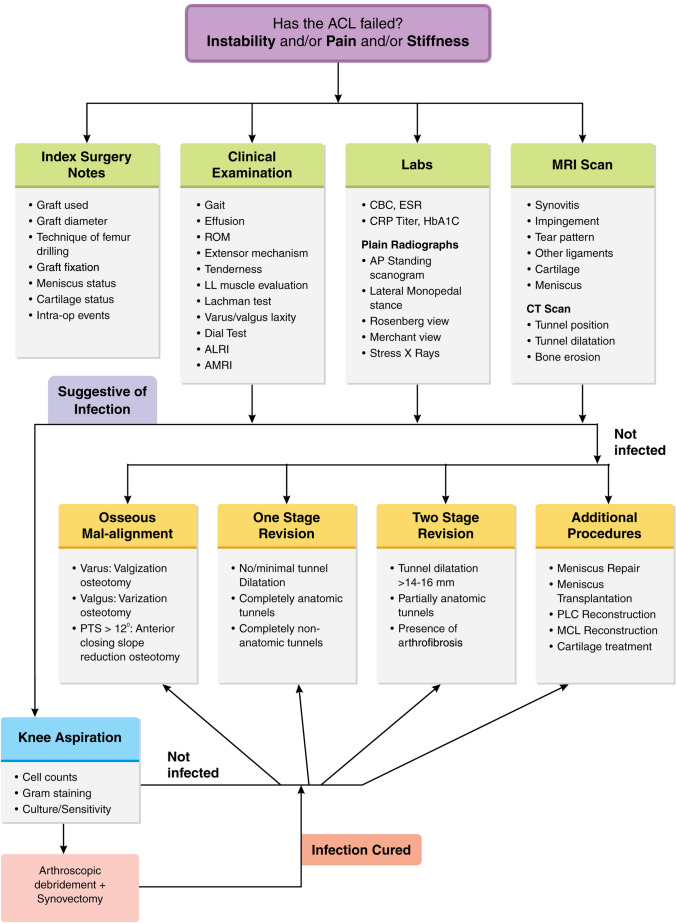
A failed ACLR does not always mandate a revision surgery. Patients with bi/tri-compartmental arthritis or regional pain syndromes, those without recurrent instability, sedentary lifestyle or unwilling to participate in post-operative rehabilitation may not be ideal for revision ACLR [23]. This subset of patients may be managed non-surgically with physical therapy and activity modification. It is prudent to evaluate patients comprehensively before embarking on revision surgery [24]. The authors’ planning and decision-making algorithm is presented in (Fig. 3).
Primary surgery details A prior knowledge of the graft used, drilling technique and fixation methods for the primary ACLR are important. This will help plan the graft choice and the fixation methods for the revision procedure. It is also essential to take cognizance of events during (e.g. tunnel malposition) or after the surgery (e.g. infection) that could have led to failure of the primary surgery.
Clinical examination The patient’s gait (antalgic, stiff knee or varus thrust pattern) can reveal several clues to the diagnosis [25]. The soft tissues of the entire lower limb, including the status of the skin, scars, muscle excursion, muscle strength and neurovascular status need to be assessed carefully. Previous scars may indicate the graft type used as well as sequelae of infection during the healing period. Loss of extension would be suggestive of a cyclops lesion or graft impingement. Flexion loss is more likely to indicate arthrofibrosis [26, 27]. Patients who demonstrate hyperextension should be carefully examined for any soft tissue insufficiency or any bony abnormality. Knee effusion and joint line tenderness may suggest synovial inflammation, meniscal tear or chondral injury. A detailed clinical assessment of the ACL and the collaterals, including any rotatory laxity is essential. Every clinical examination would need to include assessment and comparison with the contralateral knee too.
Imaging Standing anteroposterior, lateral and Rosenberg X-ray views reveal the presence of degenerative changes as well as the location and dilation of the tunnels. They are useful to locate the site and type of hardware that has been used [28]. Stress radiographs may be performed when indicated for assessing collateral stability. An anteroposterior standing scanogram of both lower limbs will quantify the mechanical axis and coronal plane malalignment, if any. The lateral monopedal stance view is useful to assess the proximal posterior tibial slope (PPTS). The femoral and tibial tunnel positions are best assessed on 3D computed tomography (CT) scans. Thin axial sections can be very effectively computed into 3D models for tunnel assessment using compatible software. Magnetic resonance imaging (MRI) scans are useful for identifying graft rupture, synovitis, graft impingement, meniscus tears, cartilage lesions, associated ligament tear(s) and presence of any cysts or intra-articular collection [29]. The Porto KT MRI sequences are useful for dynamic assessment of the anteroposterior and rotational stability [30].
Infection screen The authors recommend ruling out infection prior to planning any revision ACLR surgery. Inflammatory serum markers (ESR and CRP) and joint fluid aspiration are reliable methods with high diagnostic accuracy [31]. Additional assessment with contrast MRI scans may be done if the initial assessment yields positive results for infection [32].
Fig. 3.
Authors’ decision-making algorithm for a patient with a failed ACLR (CBC complete blood count, ESR erythrocyte sedimentation rate, CRP C-reactive protein, ROM range of motion, ALRI anterolateral rotary instability, AMRI anteromedial rotary instability, PLC posterolateral corner, MCL medial collateral ligament)
Graft Choice in Revision ACL
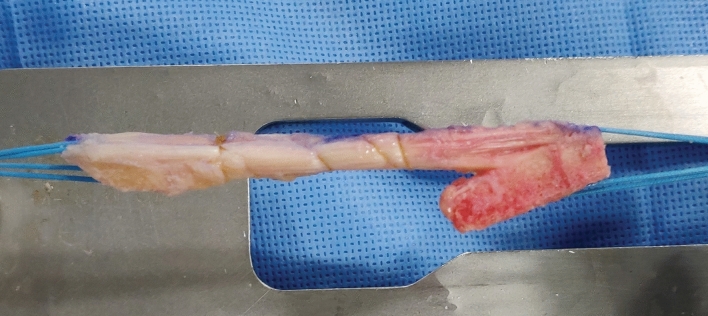
Graft choice plays an important role in the success of the procedure. Allografts are better avoided whenever possible because of higher re-tear rates (2.78 times) and inferior patient-reported outcomes [33–35]. Autografts are preferred, unless they are not available or their choice is compromised by what was used during the primary surgery. Ipsilateral or contralateral hamstrings, BPTB or quadriceps tendon (QT) (preferably with bone block) may be used as graft source. The chosen graft should be of adequate diameter to occupy the tunnel if a one-stage procedure is contemplated. BPTB and QT grafts offer the advantage of providing bone-to-bone healing and are the authors’ preferred graft choice (Fig. 4). The fixation is as important as the graft choice in the revision setting. The fixation should be secure, especially since the bone quality may be compromised. In case of doubt as regards security of graft fixation, it is worthwhile to perform a secondary fixation [36].
Fig. 4.
Bone–patella tendon–bone graft with a tongue of bone on the tibial side which can be used to fill up defect in the tunnel during a one-stage revision ACL reconstruction
One-Stage Revision ACLR
Majority of revision ACL reconstructions can be performed as a one-stage procedure (Table 2). The ideal indication would be completely non-anatomic and minimally dilated previous tunnels. In this situation, the anatomic ACL insertions are identified, and new tunnels are drilled maintaining a sufficient bone bridge between the old and new tunnels. A less ideal indication would be completely anatomic tunnels with no or minimal dilatation. This can be managed by drilling the same tunnel to a larger diameter with fresh bone walls and using a graft of larger diameter. Outside-in drilling of the femur in a changed trajectory may be a useful strategy to achieve healthy bone in tunnel walls whilst maintaining the anatomic insertion site. Tunnel dilatation up to 14–16 mm can be managed in one-stage surgery if the tunnel locations are near anatomic [24, 37]. Strategies may include utilizing bone from the graft to fill the defect (e.g. tendo-achilles allograft with calcaneus bone block) or using a large interference screw to serve both as fixation and a filler. Another option for tackling a dilated femoral tunnel is to perform an over-the-top (OTT) type of reconstruction [38]. For isolated femoral or tibial tunnel dilatation, single-stage bone grafting using allograft dowels and revision ACLR has also been described with favorable outcomes [39, 40]. Schliemann demonstrated that filling up of an incomplete or non-anatomic tibial tunnel with a bioabsorbable screw or press-fit bone graft achieved biomechanical properties similar to a primary ACLR. Back-up cortical fixation must be performed in such a case to prevent failure of the construct [41].
Table 2.
Authors’ indications for staging aseptic revision ACL reconstruction
| Single-stage ACL revision | Two-stage ACL revision surgery |
|---|---|
| Good range of motion | Stiff knee (> 20º flexion deformity, < 80º flexion) |
| Tunnel dilation < 14 mm | Tunnel dilatation > 14 mm |
| Completely incorrect or completely correct tunnel | Partially correct tunnel (2/3 overlap with ideal position) |
| Smaller coronal plane deformity requiring correction (< 10º) | Large coronal plane deformity requiring correction (> 10º) |
| Posterior tibial slope ≤ 10º | Posterior tibial slope > 12º |
Two-Stage Revision ACLR
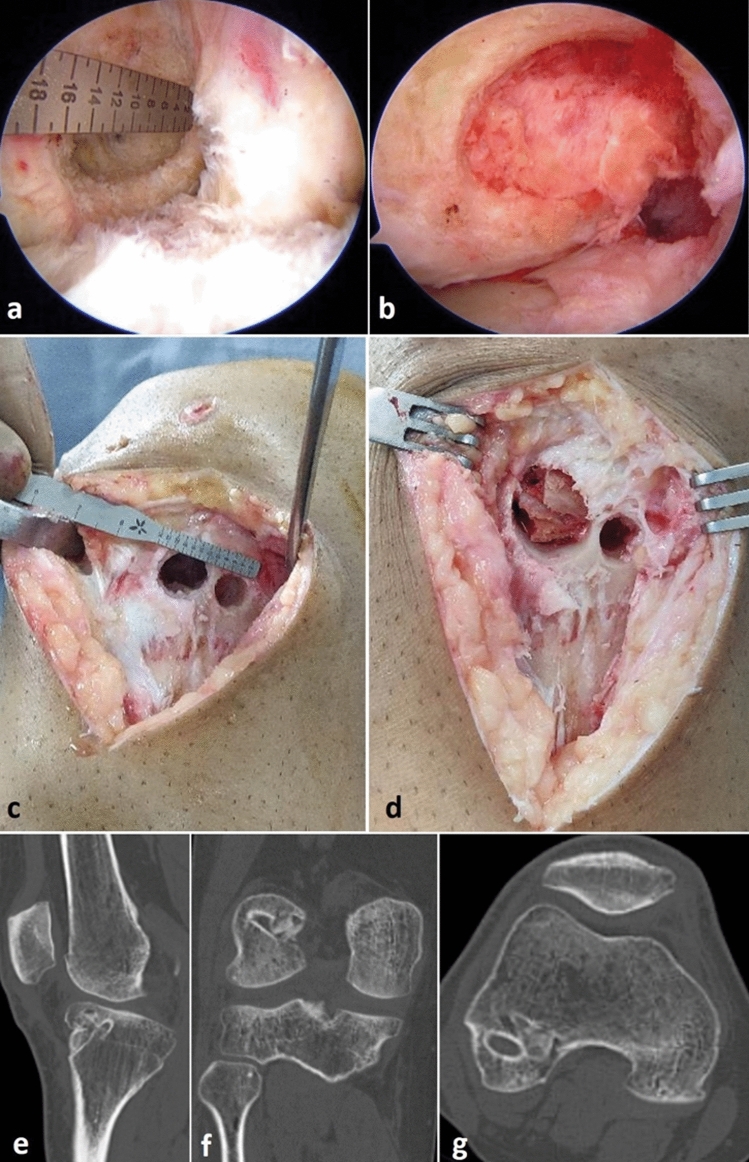
In certain situations, it may be prudent to perform the revision ACLR in two stages to avoid suboptimal graft choice, tunnel position, graft fixation, and intra-articular biology [42]. Tunnel widening is a problem if either tunnel is enlarged by 100% or if the diameter is > 14–16 mm [36, 37] or if they are partially anatomic. In the first stage, removal of any interference screws and bone grafting of the dilated femoral and /or tibial tunnels is performed. In this procedure the tunnels are cleared of any hardware, granulation tissue or graft material, and its sclerotic walls freshened with a curette, burr, or drill to obtain bleeding bone all around. Bone grafting can be performed using either autograft from iliac crest, tibial tuberosity or Gerdy’s tubercle, or allograft in the form of morselized cancellous chips or dowels [43] (Fig. 5). Allograft bone dowels achieve satisfactory osteointegration and avoid all donor site morbidity [44]. Following bone grafting, a CT scan should be repeated at 3–4 months to confirm graft incorporation and filling up of bone within the tunnels [43]. Any collateral ligament reconstruction or osteotomy or meniscus body or root repair may be performed in the first stage itself [43]. The second stage reconstruction is usually performed after 4–6 months [36]. In the presence of extensive arthrofibrosis, the ligament reconstruction should be staged and a synovectomy must be performed initially to achieve full ROM [43].
Fig. 5.
a Arthroscopic image of a dilated femoral tunnel following a double-bundle ACL failure measuring 16 mm. b Bone grafting performed using iliac crest autograft to fill up the defect. c Tibial tunnels were also enlarged but with intact cortical bridge. d Cancellous chips from iliac crest used for grafting the tibial tunnels. CT scan after four months showing bony consolidation in the tunnels seen on sagittal (e), coronal (f) and axial (g) sections
Additional Procedures
Osteotomy Patients undergoing an ACLR revision are more likely to have tibiofemoral varus alignment, advanced radiographic arthritis and medial meniscus tears [45]. The correction of any osseous abnormality, either in the coronal or sagittal plane, will take precedence over ligament reconstruction. A varus of greater than 3° compared to the opposite knee would warrant a valgization osteotomy [25]. A medial open wedge HTO (MOWHTO) is the preferred approach currently owing to its technical ease and the ability to adjust the tibial slope. In spite of its popularity, there is no scientific evidence supporting its superiority over a closing wedge HTO (Fig. 6) [46]. A combined procedure such as a revision ACLR and MOWHTO may pose some technical challenges, such as the use of a shorter ACL graft, avoiding tibial tunnel coalition with screws and decreasing the tibial slope. Patient specific instrumentation for MOWHTO is a safe and accurate alternative to avoid tunnel-screw coalition [47]. Concomitant HTO and ACLR will lead to substantial reduction in knee adduction moment and sustained shift in mediolateral load [48].
Fig. 6.
a Anteroposterior standing full-length scanogram showing the Mikulicz line passing medially at the knee in a patient who had an ACL graft rupture. b High tibial osteotomy performed to correct the varus malalignment
In the sagittal plane, a posterior tibial slope (PTS) of more than 12° is a very strong predictor of ACL re-tear. This effect is more exaggerated in adolescents [49, 50]. The tibial slope has been shown to have a strong linear co-relation with the force experienced by an ACL graft when the knee is axially loaded, in all flexion angles [51]. A de-flexion osteotomy reduces anterior tibial translation on axial loading in both ACL deficient and reconstructed states. It thus protects the ACL graft in a revision scenario [52]. The early functional outcomes of tibial de-flexion (slope reduction) osteotomy and a second revision ACL reconstruction have been reported to be satisfactory [53]. An anterior closing wedge osteotomy can be performed above or at the level of tibial tuberosity. A tibial tuberosity osteotomy is required when the osteotomy is performed at the same level, for improving exposure and preventing changes in the patellar height. In such a situation, it may be prudent to defer the ligament reconstruction to a later stage (Fig. 7).
Extra-articular procedures Anterolateral rotary stability (ALRI) is a much-debated topic in ACL surgery, both for indications and technique (anterolateral ligament reconstruction versus iliotibial band tenodesis). Anterolateral capsular injury, medial or lateral meniscus injury and a high tibial slope all contribute to ALRI [54, 55]. These factors are more frequently encountered in the revision scenario than in a primary ACLR. An extra-articular procedure protects the revised ACL graft and helps to improve rotational stability of the knee. Hence, it is recommended in most revision surgeries and especially so if there is high-grade pivot-shift, generalized joint laxity or if a soft tissue graft is used [56]. This procedure is associated with low re-tear and complication rates and good functional outcomes at mid-term [34, 57, 58].
Additional ligament reconstructions Missed injuries of the medial collateral ligament (MCL) or of the posterolateral corner (PLC) exert increased load on the ACL graft and are contributory to its failure [59, 60]. New injures to these structures at the time of traumatic ACL graft rupture are also possible. These injuries must be diligently sought on clinical examination, stress radiography, and MRI. Reconstruction of MCL or PLC using the surgeon’s preferred technique is crucial at the time of revision ACLR. Due to the added complexity of such concomitant surgery, graft choices, fixation methods and prevention of tunnel coalition requires careful planning.
Cartilage surgery Cartilage lesions in the tibiofemoral and patellofemoral compartments are encountered more commonly in revision ACLR [61]. Excision of the medial or lateral meniscus during primary ACLR is likely to increase the odds of cartilage damage (Outerbridge grade 3 or 4) in the respective compartment [62, 63]. Moreover, increased age is also an independent factor for articular cartilage deterioration in either tibiofemoral compartments, whilst high BMI and an allograft primary ACLR are associated with an increased degeneration in the patellofemoral compartment [63]. Such cartilage defects have to be treated based on their site, size, and depth using any cartilage restoration or regeneration technique as necessary [64].
Meniscus surgery Meniscus root tears are more prevalent in patients undergoing revision ACLR, with lateral tears being four times more common than medial tears [65]. The overall healing rate of the meniscus repairs after the first-stage revision ACLR has been reported as 86%, and as 82.3% with transtibial root repair and 92.4% after an inside-out repair [65]. When a near total meniscectomy was performed during primary ACLR, meniscus allograft transplantation (MAT) is a safe procedure and provides good outcomes in the mid-term (Fig. 8) [66]. A MAT also contributes to the stability of the knee by reducing both anteroposterior and rotational plane laxity [67].
Fig. 7.
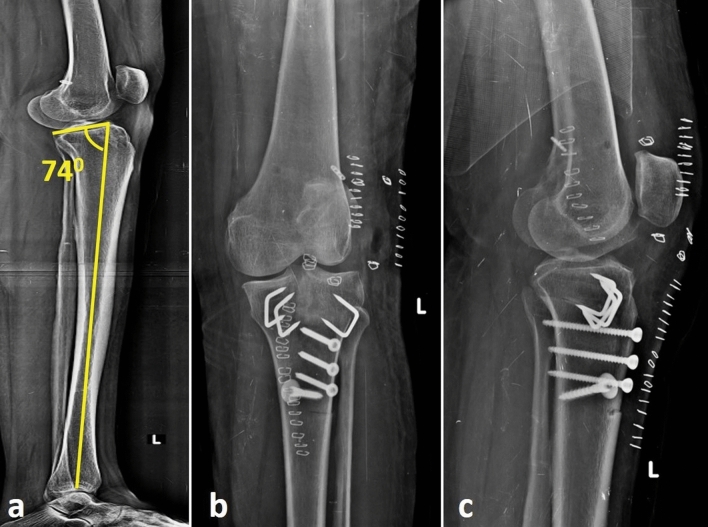
a Full-length lateral radiograph of tibia showing the proximal posterior tibia slope as 74º in a patient whose ACL failed. Anteroposterior (b) and lateral (c) radiographs after a de-flexion osteotomy via a tibial tubercle osteotomy approach to reduce the slope and revision ACL reconstruction with quadriceps tendon graft
Fig. 8.
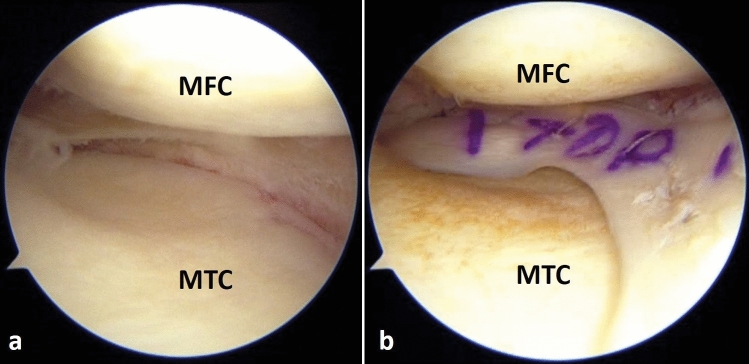
Right knee arthroscopy, viewing from the anterolateral portal, knee is 30º flexion and valgus force applied. a The medial meniscus is absent due to a subtotal meniscectomy performed during primary ACL reconstruction. b Medial meniscus allograft transplantation performed during revision ACL reconstruction (MFC medial femoral condyle, MTC medial tibial condyle)
Results of Revision ACL Reconstruction
The outcomes of revision ACLR markedly differ from those of a primary reconstruction due to several factors. Revision ACLR may restore knee laxity similar to a primary ACLR. However, it is correlated with inferior Lysholm scores, poorer clinician-reported function, and greater radiographic tibiofemoral arthritis [68]. Considering graft re-rupture to be the only sign of failure may actually under-report the actual failure rates of revision ACLR. Grassi et al. defined cumulative failure as presence of knee laxity on arthrometric testing or pivot-shift test and International Knee Documentation Committee (IKDC) score grade C or D in a meta-analysis of 16 case series. They reported such failures as being present in > 5% patients in 15 case series and, > 20% in 5 case series [69] Graft fixation with metal interference screws and avoidance of notchplasty were associated with better outcomes at 2 years. These factors seem to be completely under the control of the surgeon. Factors which adversely affect outcomes include female gender, higher BMI, lower Marx activity score and lesser interval between primary and revision surgery. These certainly are not under the control of the surgeon [70]. Age < 20 years and allograft reconstruction have also been implicated as independent risk factors for revision surgery, especially within 2 years of the index surgery [33].
Data from Swedish national registry suggests inferior outcomes after revision ACLR than primary ACLR at 1 year follow-up. These are further compromised if the patient were to require a PLC reconstruction or have presence of chondral lesions [61]. Graft used for revision surgery also influences the outcome. Allografts have poorer outcomes, as shown in a meta-analysis of 32 studies, with greater laxity, higher rates of re-operations and complications [34]. Autografts were associated with improved sports participation, better patient-reported outcome measures and lower grafter re-rupture rate at 2 years in the MARS cohort [35].
Return to sports (RTS) is considered as the ultimate measure of success of ACLR. Patients returning to a single or multiple sports after revision ACLR demonstrate significantly better patient-reported outcome measures using multiple validated assessment tools [71]. In a meta-analysis of 31 studies Andriolo found that 73% patients had good outcomes on subjective or objective measures but only 43% could return to same level of sporting activity [72]. This is significantly lower compared to RTS after primary ACLR. In the prospective comparative series of Lefevre et al. with 552 patients, there was no difference between overall rates of RTS between primary and revision ACLRs. However, patients with a revision surgery had a significantly lower rate of return to their usual sport [73]. A meta-analysis 13 studies evaluated RTS in athletes after minimum one year. Only 13–69% athletes were able to return to pre-injury level, while rate of RTS at any level ranged from 56 to 100% [74]. In a case series of adolescent athletes undergoing revision ACLR, only 68.4% patients could return to their pre-injury level of sports within 2 years [75]. Thus, patient expectations must be kept realistic with respect to RTS after a revision ACLR.
Conclusion
Revision ACL reconstruction is an increasingly performed procedure today, and is likely to become commoner. It is vital to identify the cause(s) of failure of the previous surgery and rectify them whilst avoiding the same errors again. Revision ACLR maybe a complex undertaking due to limited graft options and compromised anatomy. Associated injuries to meniscus, cartilage, and anterolateral capsule are very common and need to be suitably addressed for optimal results. Patient expectations must be tempered based on the fact that functional outcomes and return to pre-injury sports are decidedly inferior to a primary surgery.
Acknowledgements
Dr. Shantanu Patil, SRM University, Chennai for the help with editing of the manuscript.
Funding
None.
Compliance with Ethical Standards
Conflict of interest
Sachin Tapasvi and Anshu Shekhar declare that they have no conflict of interest.
Ethical standard statement
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Informed consent
For this type of study, informed consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sachin Tapasvi, Email: stapasvi@gmail.com.
Anshu Shekhar, Email: dr.anshushekhar@gmail.com.
References
- 1.Lien-Iversen T, Morgan DB, Jensen C, Risberg MA, Engebretsen L, Viberg B. Does surgery reduce knee osteoarthritis, meniscal injury and subsequent complications compared with non-surgery after ACL rupture with at least 10 years follow-up? A systematic review and meta-analysis. British Journal of Sports Medicine. 2019;54(10):592–598. doi: 10.1136/bjsports-2019-100765. [DOI] [PubMed] [Google Scholar]
- 2.Buller LT, Best MJ, Baraga MG, Kaplan LD. Trends in anterior cruciate ligament reconstruction in the United States. Orthopaedic Journal of Sports Medicine. 2015;3(1):2325967114563664. doi: 10.1177/2325967114563664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noyes FR, Barber-Westin SD. Revision anterior cruciate surgery with use of bone-patellar tendon-bone autogenous grafts. The Journal of Bone and Joint Surgery-American Volume. 2001;83(8):1131–1143. doi: 10.2106/00004623-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Johnson DL, Fu FH. Anterior cruciate ligament reconstruction: Why do failures occur? Instructional Course Lectures. 1995;44:391–406. [PubMed] [Google Scholar]
- 5.Lind M, Menhert F, Pedersen AB. Incidence and outcome after revision anterior cruciate ligament reconstruction. The American Journal of Sports Medicine. 2012;40(7):1551–1557. doi: 10.1177/0363546512446000. [DOI] [PubMed] [Google Scholar]
- 6.Maletis GB, Granan L-P, Inacio MCS, Funahashi TT, Engebretsen L. Comparison of community-based ACL reconstruction registries in the U.S. and Norway. The Journal of Bone and Joint Surgery-American Volume. 2011;93(Suppl 3):31–36. doi: 10.2106/JBJS.K.00905. [DOI] [PubMed] [Google Scholar]
- 7.Frosch K-H, Stengel D, Brodhun T, Stietencron I, Holsten D, Jung C, et al. Outcomes and risks of operative treatment of rupture of the anterior cruciate ligament in children and adolescents. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2010;26(11):1539–1550. doi: 10.1016/j.arthro.2010.04.077. [DOI] [PubMed] [Google Scholar]
- 8.Samuelsen BT, Webster KE, Johnson NR, Hewett TE, Krych AJ. Hamstring autograft versus patellar tendon autograft for ACL reconstruction: Is there a difference in graft failure rate? A meta-analysis of 47,613 patients. Clinical orthopaedics and related research. 2017;475(10):2459–2468. doi: 10.1007/s11999-017-5278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Eck CF, Schkrohowsky JG, Working ZM, Irrgang JJ, Fu FH. Prospective analysis of failure rate and predictors of failure after anatomic anterior cruciate ligament reconstruction with allograft. The American Journal of Sports Medicine. 2012;40(4):800–807. doi: 10.1177/0363546511432545. [DOI] [PubMed] [Google Scholar]
- 10.Jaecker V, Zapf T, Naendrup J-H, Kanakamedala AC, Pfeiffer T, Shafizadeh S. Differences between traumatic and non-traumatic causes of ACL revision surgery. Archives of Orthopaedic and Trauma Surgery. 2018;138(9):1265–1272. doi: 10.1007/s00402-018-2954-5. [DOI] [PubMed] [Google Scholar]
- 11.Chen JL, Allen CR, Stephens TE, Haas AK, Huston LJ, Wright RW, et al. Differences in mechanisms of failure, intraoperative findings, and surgical characteristics between single- and multiple-revision ACL reconstructions: A MARS cohort study. American Journal of Sports Medicine. 2013;41(7):1571–1578. doi: 10.1177/0363546513487980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Achtnich A, Ranuccio F, Willinger L, Pogorzelski J, Imhoff AB, Braun S, et al. High incidence of partially anatomic tunnel placement in primary single-bundle ACL reconstruction. Knee Surgery, Sports Traumatology, Arthroscopy. 2017;26(2):462–467. doi: 10.1007/s00167-017-4555-1. [DOI] [PubMed] [Google Scholar]
- 13.Iriuchishima T, Shirakura K, Fu FH. Graft impingement in anterior cruciate ligament reconstruction. Knee Surgery, Sports Traumatology, Arthroscopy. 2012;21(3):664–670. doi: 10.1007/s00167-012-2014-6. [DOI] [PubMed] [Google Scholar]
- 14.Howell SM, Taylor MA. Failure of reconstruction of the anterior cruciate ligament due to impingement by the intercondylar roof. The Journal of Bone & Joint Surgery. 1993;75(7):1044–1055. doi: 10.2106/00004623-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Wright RW, Huston LJ, Spindler KP, Dunn WR, Haas AK, Allen CR, et al. Descriptive epidemiology of the Multicenter ACL Revision Study (MARS) cohort. American Journal of Sports Medicine. 2010;38(10):1979–1986. doi: 10.1177/0363546510378645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ménétrey J, Duthon VB, Laumonier T, Fritschy D. “Biological failure” of the anterior cruciate ligament graft. Knee Surgery, Sports Traumatology, Arthroscopy. 2008;16(3):224–231. doi: 10.1007/s00167-007-0474-x. [DOI] [PubMed] [Google Scholar]
- 17.Eckenrode BJ, Carey JL, Sennett BJ, Zgonis MH. Prevention and management of post-operative complications following ACL reconstruction. Current Reviews in Musculoskeletal Medicine. 2017;10(3):315–321. doi: 10.1007/s12178-017-9427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westermann R, Lynch T, Spindler K. Hot topics in the multicenterorthopedics outcomes network: Anterior cruciate ligament. The Journal of Knee Surgery. 2016;29(07):539–542. doi: 10.1055/s-0036-1593341. [DOI] [PubMed] [Google Scholar]
- 19.Hulet C, Sonnery-Cottet B, Stevenson C, Samuelsson K, Laver L, Zdanowicz U, et al. The use of allograft tendons in primary ACL reconstruction. Knee Surgery, Sports Traumatology, Arthroscopy. 2019;27(6):1754–1770. doi: 10.1007/s00167-019-05440-3. [DOI] [PubMed] [Google Scholar]
- 20.Maletis GB, Chen J, Inacio MCS, Love RM, Funahashi TT. Increased risk of revision after anterior cruciate ligament reconstruction with soft tissue allografts compared with autografts: Graft processing and time make a difference. American Journal of Sports Medicine. 2017;45(8):1837–1844. doi: 10.1177/0363546517694354. [DOI] [PubMed] [Google Scholar]
- 21.Cooper DE, Dunn WR, Huston LJ, Haas AK, Spindler KP, Allen CR, et al. Physiologic preoperative knee hyperextension is a predictor of failure in an anterior cruciate ligament revision cohort: A report from the MARS group. The American Journal of Sports Medicine. 2018;46(12):2836–2841. doi: 10.1177/0363546518777732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahardja R, Zhu M, Love H, Clatworthy MG, Monk AP, Young SW. Factors associated with revision following anterior cruciate ligament reconstruction: A systematic review of registry data. The Knee. 2020;27(2):287–299. doi: 10.1016/j.knee.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Osti L, Buda M, Osti R, Massari L, Maffulli N. Preoperative planning for ACL revision surgery. Sports Medicine and Arthroscopy Review. 2017;25(1):19–29. doi: 10.1097/JSA.0000000000000140. [DOI] [PubMed] [Google Scholar]
- 24.Burnham JM, Herbst E, Pauyo T, Pfeiffer T, Johnson DL, Fu FH, et al. Technical considerations in revision anterior cruciate ligament (ACL) reconstruction for operative techniques in orthopaedics. Operative Techniques in Orthopaedics. 2017;27(1):63–69. doi: 10.1053/j.oto.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noyes FRSR. The role of high tibial osteotomy in the anterior cruciate ligament-deficient knee with varus alignment. In: DeLee JCDD, editor. Orthopaedic sports medicine principles and practice. Philadelphia: WB Saunders; 1994. pp. 1401–1443. [Google Scholar]
- 26.Noailles T, Chalopin A, Boissard M, Lopes R, Bouguennec N, Hardy A. Incidence and risk factors for cyclops syndrome after anterior cruciate ligament reconstruction: A systematic literature review. Orthopaedics & Traumatology: Surgery & Research. 2019;105(7):1401–1405. doi: 10.1016/j.otsr.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Magit D, Wolff A, Sutton K, Medvecky MJ. Arthrofibrosis of the Knee. Journal of the American Academy of Orthopaedic Surgeons. 2007;15(11):682–694. doi: 10.5435/00124635-200711000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Kosy JD, Mandalia VI. Plain radiographs can be used for routine assessment of ACL reconstruction tunnel position with three-dimensional imaging reserved for research and revision surgery. Knee Surgery, Sports Traumatology, Arthroscopy. 2017;26(2):534–549. doi: 10.1007/s00167-017-4462-5. [DOI] [PubMed] [Google Scholar]
- 29.Ahn JH, Lee YS, Chang MJ, Yim HS. Analysis of revision anterior cruciate ligament reconstruction according to the combined injury, degenerative change, and MRI findings. The Knee. 2011;18(6):382–386. doi: 10.1016/j.knee.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Espregueira-Mendes J, Pereira H, Sevivas N, Passos C, Vasconcelos JC, Monteiro A, et al. Assessment of rotatory laxity in anterior cruciate ligament-deficient knees using magnetic resonance imaging with Porto-knee testing device. Knee Surgery, Sports Traumatology, Arthroscopy. 2012;20(4):671–678. doi: 10.1007/s00167-012-1914-9. [DOI] [PubMed] [Google Scholar]
- 31.Parvizi, J., & Gehrke. T. (2018). Proceedings of the second international consensus on musculoskeletal infection. In J. Parvizi, T. Gehrke (Eds) Brooklandville: Data Trace.
- 32.Gobbi A, Karnatzikos G, Chaurasia S, Abhishek M, Bulgherhoni E, Lane J. postoperative infection after anterior cruciate ligament reconstruction. Sports Health. 2016;8(2):187–189. doi: 10.1177/1941738115618638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding D, Group M. Subsequent surgery after revision anterior cruciate ligament reconstruction. Orthopaedic Journal of Sports Medicine. 2016;4(7_suppl4):2325967116S0013. [Google Scholar]
- 34.Grassi A, Nitri M, Moulton SG, MarcheggianiMuccioli GM, Bondi A, Romagnoli M, et al. Does the type of graft affect the outcome of revision anterior cruciate ligament reconstruction? The Bone & Joint Journal. 2017;99-B(6):714–723. doi: 10.1302/0301-620X.99B6.BJJ-2016-0929.R2. [DOI] [PubMed] [Google Scholar]
- 35.Group M Effect of graft choice on the outcome of revision anterior cruciate ligament reconstruction in the Multicenter ACL Revision Study (MARS) Cohort. American Journal of Sports Medicine. 2014;42(10):2301–2310. doi: 10.1177/0363546514549005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richter DL, Werner BC, Miller MD. Surgical pearls in revision anterior cruciate ligament surgery. Clinics in Sports Medicine. 2017;36(1):173–187. doi: 10.1016/j.csm.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Maak TG, Voos JE, Wickiewicz TL, Warren RF. Tunnel widening in revision anterior cruciate ligament reconstruction. American Academy of Orthopaedic Surgeon. 2010;18(11):695–706. doi: 10.5435/00124635-201011000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Cheatham SA, Johnson DL. Anticipating problems unique to revision ACL surgery. Sports Medicine and Arthroscopy Review. 2013;21(2):129–134. doi: 10.1097/JSA.0b013e3182900739. [DOI] [PubMed] [Google Scholar]
- 39.Werner BC, Gilmore CJ, Hamann JC, Gaskin CM, Carroll JJ, Hart JM, et al. Revision anterior cruciate ligament reconstruction. Journal of the American Academy of Orthopaedic Surgeons. 2016;24(8):581–587. doi: 10.5435/JAAOS-D-15-00572. [DOI] [PubMed] [Google Scholar]
- 40.Dragoo JL, Kalisvaart M, Smith KM, Pappas G, Golish R. Single-stage revision anterior cruciate ligament reconstruction using bone grafting for posterior or widening tibial tunnels restores stability of the knee and improves clinical outcomes. Knee Surgery, Sports Traumatology, Arthroscopy. 2019;27(11):3713–3721. doi: 10.1007/s00167-019-05467-6. [DOI] [PubMed] [Google Scholar]
- 41.Schliemann B, Treder M, Schulze M, Müller V, Vasta S, Zampogna B, et al. Influence of different tibial fixation techniques on initial stability in single-stage anterior cruciate ligament revision with confluent tibial tunnels: A biomechanical laboratory study. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2016;32(1):78–89. doi: 10.1016/j.arthro.2015.06.044. [DOI] [PubMed] [Google Scholar]
- 42.Wilde J, Bedi A, Altchek DW. Revision anterior cruciate ligament reconstruction. Sports Health. 2014;6(6):504–518. doi: 10.1177/1941738113500910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erickson BJ, Cvetanovich G, Waliullah K, Khair M, Smith P, Bach B, et al. Two-stage revision anterior cruciate ligament reconstruction. Orthopedics. 2016;39(3):e456–e464. doi: 10.3928/01477447-20160324-01. [DOI] [PubMed] [Google Scholar]
- 44.Gertel TH, Lew WD, Lewis JL, Stewart NJ, Hunter RE. Effect of anterior cruciate ligament graft tensioning direction, magnitude, and flexion angle on knee biomechanics. The American Journal of Sports Medicine. 1993;21(4):572–581. doi: 10.1177/036354659302100415. [DOI] [PubMed] [Google Scholar]
- 45.Won HH, Chang CB, Je MS, Chang MJ, Kim TK. Coronal limb alignment and indications for high tibial osteotomy in patients undergoing revision ACL reconstruction. Clinical Orthopaedics and Related Research. 2013;471(11):3504–3511. doi: 10.1007/s11999-013-3185-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cantivalli A, Rosso F, Bonasia DE, Rossi R. High tibial osteotomy and anterior cruciate ligament reconstruction/revision. Clinics in Sports Medicine. 2019;38(3):417–433. doi: 10.1016/j.csm.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 47.Chaouche S, Jacquet C, Fabre-Aubrespy M, Sharma A, Argenson J-N, Parratte S, et al. Patient-specific cutting guides for open-wedge high tibial osteotomy: Safety and accuracy analysis of a hundred patients continuous cohort. International Orthopaedics. 2019;43(12):2757–2765. doi: 10.1007/s00264-019-04372-4. [DOI] [PubMed] [Google Scholar]
- 48.Marriott K, Birmingham TB, Kean CO, Hui C, Jenkyn TR, Giffin JR. Five-year changes in gait biomechanics after concomitant high tibial osteotomy and ACL reconstruction in patients with medial knee osteoarthritis. The American Journal of Sports Medicine. 2015;43(9):2277–2285. doi: 10.1177/0363546515591995. [DOI] [PubMed] [Google Scholar]
- 49.Sonnery-Cottet B, Mogos S, Thaunat M, Archbold P, Fayard J-M, Freychet B, et al. Proximal tibial anterior closing wedge osteotomy in repeat revision of anterior cruciate ligament reconstruction. The American Journal of Sports Medicine. 2014;42(8):1873–1880. doi: 10.1177/0363546514534938. [DOI] [PubMed] [Google Scholar]
- 50.Salmon LJ, Heath E, Akrawi H, Roe JP, Linklater J, Pinczewski LA. 20-Year outcomes of anterior cruciate ligament reconstruction with hamstring tendon autograft: The catastrophic effect of age and posterior tibial slope. The American Journal of Sports Medicine. 2017;46(3):531–543. doi: 10.1177/0363546517741497. [DOI] [PubMed] [Google Scholar]
- 51.Bernhardson AS, Aman ZS, Dornan GJ, Kemler BR, Storaci HW, Brady AW, et al. Tibial slope and its effect on force in anterior cruciate ligament grafts: Anterior cruciate ligament force increases linearly as posterior tibial slope increases. The American Journal of Sports Medicine. 2019;47(2):296–302. doi: 10.1177/0363546518820302. [DOI] [PubMed] [Google Scholar]
- 52.Imhoff FB, Mehl J, Comer BJ, Obopilwe E, Cote MP, Feucht MJ, et al. Slope-reducing tibial osteotomy decreases ACL-graft forces and anterior tibial translation under axial load. Knee Surgery, Sports Traumatology, Arthroscopy. 2019;27(10):3381–3389. doi: 10.1007/s00167-019-05360-2. [DOI] [PubMed] [Google Scholar]
- 53.Dejour D, Saffarini M, Demey G, Baverel L. Tibial slope correction combined with second revision ACL produces good knee stability and prevents graft rupture. Knee Surgery, Sports Traumatology, Arthroscopy. 2015;23(10):2846–2852. doi: 10.1007/s00167-015-3758-6. [DOI] [PubMed] [Google Scholar]
- 54.Musahl V, Rahnemai-Azar AA, Costello J, Arner JW, Fu FH, Hoshino Y, et al. The influence of meniscal and anterolateral capsular injury on knee laxity in patients with anterior cruciate ligament injuries. The American Journal of Sports Medicine. 2016;44(12):3126–3131. doi: 10.1177/0363546516659649. [DOI] [PubMed] [Google Scholar]
- 55.Rahnemai-Azar AA, Abebe ES, Johnson P, Labrum J, Fu FH, Irrgang JJ, et al. Increased lateral tibial slope predicts high-grade rotatory knee laxity pre-operatively in ACL reconstruction. Knee Surgery, Sports Traumatology, Arthroscopy. 2016;25(4):1170–1176. doi: 10.1007/s00167-016-4157-3. [DOI] [PubMed] [Google Scholar]
- 56.Miller TK. The role of an extra-articular tenodesis in revision of anterior cruciate ligament reconstruction. Clinics in Sports Medicine. 2018;37(1):101–113. doi: 10.1016/j.csm.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 57.Grassi A, Zicaro JP, Costa-Paz M, Samuelsson K, Wilson A, Zaffagnini S, et al. Good mid-term outcomes and low rates of residual rotatory laxity, complications and failures after revision anterior cruciate ligament reconstruction (ACL) and lateral extra-articular tenodesis (LET) Knee Surgery, Sports Traumatology, Arthroscopy. 2019;28(2):418–431. doi: 10.1007/s00167-019-05625-w. [DOI] [PubMed] [Google Scholar]
- 58.Alessio-Mazzola M, Formica M, Russo A, Sanguineti F, Capello AG, Lovisolo S, et al. Outcome after combined lateral extra-articular tenodesis and anterior cruciate ligament revision in professional soccer players. The Journal of Knee Surgery. 2018;32(09):906–910. doi: 10.1055/s-0038-1672120. [DOI] [PubMed] [Google Scholar]
- 59.Svantesson E, HamrinSenorski E, Alentorn-Geli E, Westin O, Sundemo D, Grassi A, et al. Increased risk of ACL revision with non-surgical treatment of a concomitant medial collateral ligament injury: A study on 19,457 patients from the Swedish National Knee Ligament Registry. Knee Surgery, Sports Traumatology, Arthroscopy: Official Journal of the ESSKA. 2019;27(8):2450–2459. doi: 10.1007/s00167-018-5237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pacheco RJ, Ayre CA, Bollen SR. Posterolateral corner injuries of the knee. The Journal of Bone and Joint Surgery British Volume. 2011;93-B(2):194–197. doi: 10.1302/0301-620X.93B2.25774. [DOI] [PubMed] [Google Scholar]
- 61.Svantesson E, HamrinSenorski E, Kristiansson F, Alentorn-Geli E, Westin O, Samuelsson K. Comparison of concomitant injuries and patient-reported outcome in patients that have undergone both primary and revision ACL reconstruction—a national registry study. Journal of Orthopaedic Surgery and Research. 2020;15(1):9. doi: 10.1186/s13018-019-1532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Borchers JR, Kaeding CC, Pedroza AD, Huston LJ, Spindler KP, Wright RW. Intra-articular findings in primary and revision anterior cruciate ligament reconstruction surgery: A comparison of the MOON and MARS study groups. American Journal of Sports Medicine. 2011;39(9):1889–1893. doi: 10.1177/0363546511406871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Magnussen RA, Borchers JR, Pedroza AD, Huston LJ, Haas AK, Spindler KP, et al. Risk factors and predictors of significant chondral surface change from primary to revision anterior cruciate ligament reconstruction: A MOON and MARS Cohort Study. American Journal of Sports Medicine. 2018;46(3):557–564. doi: 10.1177/0363546517741484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shekhar A, Patil S, Reddy S, Tapasvi S. Management of chondral defects of the knee. In: SM H, editor. Hardikar’s operations: text and Atlas. New Delhi: Jaypee Brothers Medical (P) Ltd.; 2019. [Google Scholar]
- 65.DePhillipo NN, Dekker TJ, Aman ZS, Bernholt D, Grantham WJ, LaPrade RF. Incidence and healing rates of meniscal tears in patients undergoing repair during the first stage of 2-stage revision anterior cruciate ligament reconstruction. The American Journal of Sports Medicine. 2019;47(14):3389–3395. doi: 10.1177/0363546519878421. [DOI] [PubMed] [Google Scholar]
- 66.Zaffagnini S, Grassi A, Romandini I, Marcacci M, Filardo G. Meniscal allograft transplantation combined with anterior cruciate ligament reconstruction provides good mid-term clinical outcome. Knee Surgery, Sports Traumatology, Arthroscopy. 2018;27(6):1914–1923. doi: 10.1007/s00167-018-5078-0. [DOI] [PubMed] [Google Scholar]
- 67.Zaffagnini S, Di Paolo S, Stefanelli F, Dal Fabbro G, Macchiarola L, Lucidi GA, et al. The biomechanical role of meniscal allograft transplantation and preliminary in-vivo kinematic evaluation. Journal of Experimental Orthopaedics. 2019;6(1):27. doi: 10.1186/s40634-019-0196-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grassi A, Ardern CL, MarcheggianiMuccioli GM, Neri MP, Marcacci M, Zaffagnini S. Does revision ACL reconstruction measure up to primary surgery? A meta-analysis comparing patient-reported and clinician-reported outcomes, and radiographic results. British Journal of Sports Medicine. 2016;50(12):716–724. doi: 10.1136/bjsports-2015-094948. [DOI] [PubMed] [Google Scholar]
- 69.Grassi A, Kim C, MarcheggianiMuccioli GM, Zaffagnini S, Amendola A. What is the mid-term failure rate of revision ACL reconstruction? A systematic review. Clinical Orthopaedics and Related Research. 2017;475(10):2484–2499. doi: 10.1007/s11999-017-5379-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Allen CR, Anderson AF, Cooper DE, DeBerardino TM, Dunn WR, Haas AK, et al. Surgical predictors of clinical outcomes after revision anterior cruciate ligament reconstruction. American Journal of Sports Medicine. 2017;45(11):2586–2594. doi: 10.1177/0363546517712952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bigouette JP, Owen EC, Lantz BBA, Hoellrich RG, Huston LJ, Haas AK, et al. Relationship between sports participation after revision anterior cruciate ligament reconstruction and 2-year patient-reported outcome measures. American Journal of Sports Medicine. 2019;47(9):2056–2066. doi: 10.1177/0363546519856348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Andriolo L, Filardo G, Kon E, Ricci M, Della Villa F, Della Villa S, et al. Revision anterior cruciate ligament reconstruction: Clinical outcome and evidence for return to sport. Knee Surgery, Sports Traumatology, Arthroscopy. 2015;23(10):2825–2845. doi: 10.1007/s00167-015-3702-9. [DOI] [PubMed] [Google Scholar]
- 73.Lefevre N, Klouche S, Mirouse G, Herman S, Gerometta A, Bohu Y. Return to sport after primary and revision anterior cruciate ligament reconstruction: A prospective comparative study of 552 patients from the FAST cohort. The American Journal of Sports Medicine. 2016;45(1):34–41. doi: 10.1177/0363546516660075. [DOI] [PubMed] [Google Scholar]
- 74.Glogovac G, Schumaier AP, Grawe BM. Return to sport following revision anterior cruciate ligament reconstruction in athletes: A systematic review. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2019;35(7):2222–2230. doi: 10.1016/j.arthro.2019.01.045. [DOI] [PubMed] [Google Scholar]
- 75.Saper M, Pearce S, Shung J, Zondervan R, Ostrander R, Andrews JR. Outcomes and return to sport after revision anterior cruciate ligament reconstruction in adolescent athletes. Orthopaedic Journal of Sports Medicine. 2018;6(4):2325967118764884. doi: 10.1177/2325967118764884. [DOI] [PMC free article] [PubMed] [Google Scholar]