Abstract
In recent years the role of the intestinal microbiota in health and disease has come to the forefront of medical research. Alterations in the intestinal microbiota and several of its features have been linked to numerous diseases, including type 1 diabetes (T1D). To date, studies in animal models of T1D, as well as studies in human subjects, have linked several intestinal microbiota alterations with T1D pathogenesis. Features that are most often linked with T1D pathogenesis include decreased microbial diversity, the relative abundance of specific strains of individual microbes, and altered metabolite production. Alterations in these features as well as others have provided insight into T1D pathogenesis and shed light on the potential mechanism by which the microbiota plays a role in T1D pathogenesis, yet the underlying factors leading to these alterations remains unknown. One potential mechanism for alteration of the microbiota is through diet and nutrition. Previous studies have shown associations of diet with islet autoimmunity, but a direct contributing factor has yet to be identified. Diet, through introduction of antigens and alteration of the composition and function of the microbiota, may elicit the immune system to produce autoreactive responses that result in the destruction of the beta cells. Here, we review the evidence associating diet induced changes in the intestinal microbiota and their contribution to T1D pathogenesis. We further provide a roadmap for determining the effect of diet and other modifiable factors on the entire microbiota ecosystem, including its impact on both immune and beta cell function, as it relates to T1D. A greater understanding of the complex interactions between the intestinal microbiota and several interacting systems in the body (immune, intestinal integrity and function, metabolism, beta cell function, etc.) may provide scientifically rational approaches to prevent development of T1D and other childhood immune and allergic diseases and biomarkers to evaluate the efficacy of interventions.
Keywords: type 1 diabetes, microbiota, diet, probiotics, disease risk
Introduction
T1D is an autoimmune disease hallmarked by the inability of the body to produce insulin due to the autoimmune destruction of the beta cells in the endocrine pancreas. While the specific underpinnings of the pathogenesis of T1D are largely unknown, there is a large heritability component, and more than 50 genetic loci have been associated with an increase in T1D risk (1). In recent years, the development of more specific genetic risk scores (GRS) have been able to refine T1D risk into a continuous variable. The most novel of these GRS, the GRS2, has shown great discriminatory power in future prediction of T1D (2). However, given the polygenic nature of T1D along with the idea that there are likely other factors or triggers contributing to T1D initiation and progression, these GRS are highly sensitive but not specific, meaning, a large proportion of individuals with a high GRS2 score will never go on to develop T1D.
A major advancement in the field was defining the staging paradigm for T1D, noting that, in individuals with a high risk of developing T1D, i.e., those with a high HLA genetic risk or a family history of T1D, development of two or more islet autoantibodies (stage 1) carried a lifetime risk of developing T1D close to 100% and further refinement of this population based on dysglycemia (stage 2) resulted in an even higher relative risk of T1D (3). However, given the large variability in progression to T1D even in these refined populations, the differentiation between why some individuals progress more rapidly than others is largely unknown and is thought to involve environmental factors. In fact, in recent years, the largest relative increases in T1D in the United States have been in African American and Hispanic youth, further suggesting an increasing importance for environmental factors contributing to T1D, as these populations are considered to carry lower genetic risk (4). Similarly, an earlier report also showed that the largest increase in T1D in Australia was among those with lower HLA risk, suggesting a larger influence of environmental factors (5).
Indeed, in 2004, The Environmental Determinants of Diabetes in the Young (TEDDY) was launched to specifically study the environmental factors contributing to T1D pathogenesis (6). While no single factor has been found, a multitude of factors have been shown to contribute to islet autoimmunity (IA) and/or T1D. Many of these factors have direct or indirect contributions to either the diet or the gut microbiota in children and will be discussed in this review. Additional studies, both in humans and mouse models of T1D, have further implicated the role of both diet and microbiota in T1D pathogenesis.
One potential mechanism that has been implicated in T1D pathogenesis is that of increased intestinal permeability, or a leaky gut. Evidence from both rodent models (7, 8) and humans (9, 10) suggest that intestinal permeability is increased in T1D, suggesting a potential pathogenic mechanism. However, the direct mechanisms by which gut leakiness may contribute to T1D pathogenesis are poorly understood, but may include inflammation, immune responses and a loss of tolerance to β-cell antigens driven by changes in the microbiota.
Herein, we summarize the current studies describing the interplay of genetics, diet, and the microbiota on T1D development and possibilities to alter any one or a combination of these factors to modify T1D progression.
Environmental Factors That Induce Microbiota Changes and Their Potential Role in T1D Pathogenesis
Seminal studies in T1D animal models, such as Non-Obese Diabetic mice (NOD) and Bio Breeding diabetes prone rat (BB-DP), have shown distinct changes in microbiota following the onset of diabetes. In Bio Breeding rats a higher abundance of Bacteroides was found in BB-DP rats, while higher concentrations of Lactobacillus and Bifidobacterium were correlated with diabetes resistant rats (BB-DR). The apparent natural dysbiosis status observed in BB-DR and BB-DP rats correlated with earlier reports of antibiotic-induced dysbiosis. The treatment of BB-DP rats with antibiotics prevented T1D onset (11, 12) while the incidence of T1D in a germ-free environment or following antibiotic treatment accelerated autoimmunity in NOD mice (13–17). Altogether, these data indicate the critical role of the microbiota in the development/onset of T1D.
Due to the high genetic heterogeneity and variable dietary patterns in human populations, the identification of diabetogenic or tolerogenic microbiota in T1D has not been straightforward. An initial study performed in stool samples collected by the Diabetes and Prevention study (DIPP) in Finland identified several bacterial genera that were more abundant in children that have seroconverted when compared to controls (18). Four matched case-control children with high-risk HLA-DQ genotype were selected, and three time points were analyzed, the last time point being seroconversion (presence of at least two islet autoantibodies). It was found that as the cases progressed to seroconversion, a decline in Firmicutes and an increase in Bacteroidetes was observed. At the seroconversion time point, the abundance of five Bacteroidetes species were significantly more abundant in cases than in controls (18). This study also suggested that a higher abundance of bacteria producing the short chain fatty acid (SCFA) butyrate- were present in controls. A similar decrease in microbial diversity was found to be associated with seroconversion in a study performed in 18 subjects from the TRIGR and FINDIA cohorts (19). It was also found that the high abundance of lactate and butyrate producing bacteria was negatively correlated to the number of islet autoantibodies in these cohorts. Kostic et al. (20) evaluated the DIABIMMUNE cohort to identify a possible microbial signature prognostic of T1D onset and potential links of this signature to metabolites in serum and stool. This densely sampled longitudinal study performed in 33 HLA-matched Estonian and Finnish infants (from birth to 3 years of age) compared the alpha diversity of the gut microbiota across time in non-converter (not seroconverted), seroconverted (not diagnosed with T1D), and T1D cases (seroconverted subjects diagnosed with T1D) (20). A significant difference in diversity was found prior to diagnosis of T1D in seroconverters that progressed to clinical disease. The decrease in diversity observed in T1D cases was correlated with high abundance of the Rikenellaceae family, as well as Blautia, Ruminococcus, and Streptococcus genera (20). While these studies were all relatively small, their results provided the initial evidence of a shift in the microbiota composition preceding T1D diagnosis.
Recently, The Environmental Determinants of Diabetes in the Young (TEDDY) study reported their findings on the metagenomes of a much larger multicenter study, which included three clinical locations in Europe (Finland, Germany, Sweden) and three in the United States (Colorado, Washington, Georgia/Florida) (21, 22). The goal of this specific study was to identify fluctuations in the microbiota from children starting at 3 months of age, to elucidate perturbations that may precede IA and T1D diagnosis (21, 22). This study offered the advantage of analyzing metagenomes as well as 16S rRNA gene community profiling. The metagenome data allowed a detailed pathway analyses and a better assignment of specific species that may contribute to the variance observed. The study found that the majority of variability in the metagenomes was explained by inter-subject differences, followed by age, geographical location, and breastfeeding of the subjects. A strong association was found with the ingestion of human milk and the abundance of Bifidobacterium species during the early stages of gut colonization (21, 22). The association is probably driven by the presence of human milk oligosaccharides (HMOs) that are almost predominantly degraded by Bifidobacterium, therefore acting as prebiotics (23). Furthermore, while Bifidobacterium species have been used as probiotics for decades, their role in prevention of T1D onset has not been explored in either animal models of T1D or in clinical trials. The analyses of the TEDDY IA cases and controls indicated a greater presence of Lactobacillus rhamnosus and Bifidobacterium dentium in controls, while IA cases had a higher abundance of Streptococcus species (mitis, oralis, and pneumoniae) (21). Similarly, the analyses of T1D cases and controls indicated controls had higher abundancy of lactic acid producing bacteria (Lactococcus lactis and Streptococcus thermophilus) commonly found in cheese and yogurt, while the T1D cases had a higher abundancy of Bifidobacterium pseudocatenulatum, Roseburia hominis, and Alistipes shahii (21). Detailed metabolic pathway analyses between cases and controls indicated that pathways involved in the synthesis of SCFA (e.g., acetate, butyrate, and propionate) are enriched in control subjects (21). In a separate study of adults with long standing T1D, higher levels of Christensenella associated with T1D and correlated negatively with fecal acetate (24). Together, these findings suggest the protective effect of SCFA, as previously reported in rodent models as well as other human T1D cohorts (21, 24–26).
Altered intestinal permeability has also been implicated in T1D pathogenesis. In an early study of individuals at various stages of disease, intestinal permeability was shown to be increased compared to controls, suggesting that the small intestine was somehow implicated in T1D pathogenesis (27). More recent studies have correlated gut microbiota changes to the changes in intestinal permeability and gut barrier proteins, specifically showing an associated decrease in SCFA-producing bacteria (28, 29). While a direct cause-and-effect has not been shown, there is increasing evidence that augmentation with SCFA-producing bacteria in individuals at-risk of developing T1D holds promise to change the trajectory of disease.
Genetic Factors Contribute to Shaping The Gut Microbiota Linked to T1D
The establishment of a dysbiotic microbiota such as that found in T1D may have multiple causes. External factors such as diet, which provides different substrates for the bacteria to survive can profoundly influence microbiota composition (30). There may be an altered exposure to different communities of bacteria particularly during early life establishment of the microbiota. Furthermore, an altered host environment due to genetic polymorphisms may influence the intestinal environment or immune response to the bacteria. Finally, there can be purely stochastic effects driving variability between individuals (31). In general, it is considered that environmental factors have a stronger influence over the microbiota structure than host genetics (32), with heritability typically having weak effect sizes and few genes reaching genome-wide significance (32–35). Of interest, genetic polymorphism within the lactase gene shows a genome-wide association with the abundance of the Bifidobacterium genus (36), which was recently validated in a meta-analysis of 18,340 individuals (33). Lactase is a brush-border enzyme responsible for digestion of lactose from human and dairy derived milk (37). This suggests a significant diet-genetic-microbiota interaction for colonization of this important health-associated genera which could be relevant to the altered abundance of bifidobacteria seen in some studies of T1D risk (21). When looking at genetic polymorphisms directly linked to T1D, several studies have observed a relationship between T1D susceptibility alleles and an altered gut microbiota. A Swedish study of 403 children found that T1D-risk HLA alleles were significantly associated with gut microbiome composition (P = 0.01), with a range of taxa linked to specific HLA-risk groups (38). In a NOD mouse study, disease protection mediated by the presence of a protective MHC-II allele was found to be dependent on the gut microbiota (39). Similarly, genetic polymorphisms in the interleukin-2 pathway that are strongly linked to T1D risk in both humans and NOD mice, were found to be associated with an altered gut microbiota (40). Notably protective IL2 alleles were linked to increased regulatory T cell frequency and mucin production within the intestine and IL2 therapy could mimic this effect on the gut suggesting that immunotherapy outcomes in T1D may also be linked to the gut microbiota response (40, 41). Together, these studies indicate that host genetics may play a role in shaping the intestinal niche that supports a dysbiotic microbiota in T1D and this could be a barrier to therapies reliant on colonization of new species into gut in individuals with T1D. If host genetics are a barrier, then manipulation of the microbiota may be better achieved early in life, with repeated delivery or combined with immunotherapy, for therapeutic benefit.
Diet Induced Microbiota Changes in T1D
The gut microbiota is established and maintained by the host's genetics and external environmental factors, which include diet. Previous studies have shown that dietary factors are associated with the development of IA or with progression of IA to T1D, but a direct contributing factor has yet to be identified (42). One potential pathway by which diet may increase risk of T1D is by impacting the microbiota. Diet, through introduction of antigens and alteration of the microbiota and microbial metabolites, may influence the immune system to produce or to control autoreactive responses that result in the destruction of the beta cells. Here, we review the dietary factors that have been implicated in IA and T1D and discuss the evidence regarding their ability to induce changes in the intestinal microbiota and how this may contribute to T1D pathogenesis. While several dietary factors have been implicated in T1D, we will focus on those that have been found to be consistently (and with strong study designs) associated with IA and T1D, or progression from IA to T1D.
Breast-Feeding and Infant Diet
Ever since the publication of the ecologic study linking insufficient breast-feeding to T1D incidence over time (43), numerous studies have been conducted on breast-feeding and T1D, and still the role of breastfeeding initiation, and whether an optimal duration of breastfeeding exists, remains unclear. Prospective studies of children at increased risk for T1D have reported inconsistent results regarding the association between breast-feeding and T1D (44–47). A large, population-based study of two birth cohorts in Norway suggests that while initiation of breastfeeding may reduce the risk of T1D, among those who were breastfed there does not appear to be a benefit of prolonging breastfeeding on the risk of T1D (48).
While the findings regarding breastfeeding and T1D may be equivocal, breast milk has a profound impact on the gut microbiota of the infant, as reviewed in Li et al. (49). Breastfeeding could lead to a healthier infant gut microbiota at an early stage of life compared with infants who are never breastfed (50, 51). As mentioned previously, the TEDDY study (21, 22) found that breast milk was the most significant factor associated with the structure of the developing microbiota. Current breast-feeding was associated with higher levels of Bifidobacterium species (B. breve, B. bifidum, and B. longum). B. longum subsp. infantis is a particularly important degrader of human milk oligosaccharide (HMO), and a genetic strain that is specific for the utilization of HMO within a subset of B. longum was present only in samples of breast-fed infants in TEDDY (21). Similarly, in a small Korean study, an increase in Bifidobacterium was observed in newborn infants that were breast-fed, whereas an increase in Klebsiella and Serratia was detected in newborn infants fed infant formula, suggesting a beneficial impact of breast milk (52).
The cessation of breast-feeding results in a functional shift in the gut microbiota (49) and a faster maturation of the gut (22). However, even with this shift, the impact of breastfeeding on the microbiota is still seen in 6-year old children, suggesting a long-term impact of exposure to breastmilk. The relative abundance of genera belonging to Firmicutes and Actinobacteria (and in particular Bifidobacterium, the predominant genus of Actinobacteria), was significantly different at 6 years of age between children that had been exclusively breast-fed for 4 months and those that had not (53). Bifidobacterium are important species in the healthy gut microbiota that can partially breakdown complex starches and oligosaccharides, making them available via “cross-feeding” to support butyrate producing bacterial populations such as Roseburia sp., Eubacterium hallii, and Anaerostipes caccae (54).
Age at introduction of complementary foods in infants has been examined regarding IA and T1D risk. Prospective observational studies suggest that late introduction of cereals (46) or gluten (47) is associated with increased risk of T1D and IA, respectively. However, an intervention in which gluten introduction was delayed to 12 months, vs. 6 months, showed no effect on IA or T1D risk (55, 56). It has been hypothesized that introduction of complementary foods impacts health by altering the microbiota. Introduction of any complementary foods at 3 months of age (vs. later) was associated with increased Bilophila wadsworthia and Roseburia in the infant's gut microbiota after adjustment for breast-feeding (57). However, others suggest that that the maturation of the gut microbiota is driven by the cessation of breast milk rather than the introduction of complementary foods (22). Using samples collected from 44 children from the German BABYDIET study, Endesfelder et al. showed that children with the most complex diet at 6-months of age were dominated by Bacteroides and this Bacteroides dominated community was associated with later development of IA. This suggested that fast-tracking of a more complex and adult-like microbial community may be related to establishment of taxa associated with T1D risk (58).
Early Childhood Intake of Gluten and Fiber
Researchers have long been interested in gluten intake as a risk factor for T1D due to the similarities between and co-occurrence of T1D and celiac disease in individuals and families. The Diabetes Autoimmunity Study in the Young (DAISY) examined early childhood intake of gluten and did not find an association with either development of IA or progression from IA to T1D (59). Related to gluten intake is the intake of dietary fiber. TEDDY reported that intake of soluble fiber was not associated with the development of IA or T1D (60). However, Hakola et al. (61) found that in Finland increased early childhood gluten and dietary fiber intake were both associated with increased risk of IA, though they were not associated with T1D risk. A large nationwide cohort study in Norway confirmed the association between early childhood intake of gluten and a higher risk of T1D (62), but was not able to address the dietary fiber association.
Studies in normal volunteers show that introduction of gluten-free diets changes the composition of the microbiota (63). This gluten-induced dysbiosis may activate an inflammatory pathway that could lead to autoimmune diseases such as T1D (64, 65). There are no data in humans, but NOD mice on a gluten-free diet showed decreased Bifidobacterium, Tanerella, and Barnesiella species and increased Akkermansia species in feces, as well as a reduced diabetes incidence compared with mice not on a gluten-free diet (66).
OMEGA-3 Fatty Acids
Two prospective cohorts of at-risk children (DAISY and DIPP) have shown that increased intake of omega-3 fatty acids (67, 68) or increased biomarker of omega-3 fatty acid status (67, 68) were linked to a reduced risk of IA. Omega-3 fatty acids are associated with alteration of the gut microbiota (69). A trial involving omega-3 fatty supplements for 8 weeks resulted in increases in the Clostridiaceae, Sutterellaceae, and Akkermansiaceae families, which then declined when supplementation was stopped (70). Trials in individuals at risk for metabolic syndrome (71) and with new onset type 2 diabetes (72) showed an impact of fatty acid supplementation on the composition of the microbiota. While it is not clear whether these changes would have an appreciable impact on IA or T1D risk, one can hypothesize that they may lead an increase in the production of anti-inflammatory compounds (69) that may affect the development of IA and T1D.
Vitamin D and 25OHD
Vitamin D is obtained both from the diet and synthesis in the skin, and then metabolized to 25-hydroxyvitamin D (25OHD) in the liver. Higher plasma 25OHD levels were associated with lower risk of IA in both TEDDY (73) and in the TRIGR Ancillary Study (74), but not in other cohort studies of at-risk children (75–77). In children with IA, lower 25OHD levels were not associated with increased risk of progression to T1D (75, 78), suggesting that the protective role of vitamin D occurs early in the disease process. Vitamin D deficiency may contribute to autoimmunity via effects on the composition of the microbiota, although existing evidence is limited to animal studies or small human studies (79). Alternatively, vitamin D may act via direct effects on immune function rather than on the microbiota (80). Studies examining vitamin D intake and the gut microbiota have been inconsistent (81–83). A genome wide association study (GWAS) showed that the vitamin D receptor (VDR) gene was associated with beta diversity of the gut microbiota (84). How vitamin D affects the microbiota in this instance is not clear, although it is likely through an indirect mechanism as bacteria do not express the VDR.
Total Sugar Intake and Dietary Glycemic Index
Increased intake of total sugars, and higher glycemic index of the diet are associated with increased risk of progression to T1D in autoantibody positive individuals (85, 86). A standard deviation (i.e., 49.7 g) increase in total sugar intake per day was associated with a 1.75-fold increased risk of progression to T1D after an average of 10.5 years (86). Sugar intake and glycemic index were not associated with initial development of IA, suggesting that these factors are only related to acceleration of the later stages of T1D. Potential mechanisms by which dietary carbohydrates and sugars may have an effect on T1D include metabolic (87, 88), and immunological pathways (89, 90), as well as pathways involving the microbiota, as reviewed in (91). Studies suggest that the high amount of simple sugars in our diet has lasting and detrimental effects on our microbiota (92–94). It is hypothesized that the replacement of microbiota-accessible carbohydrates (i.e., fiber) with the fats and simple carbohydrates that are characteristic of the Western diet may be negatively shaping the microbiota thereby leading to poor health outcomes.
Uncovering Therapeutic Targets—A Proposed Roadmap Forward
Approaches to Remodeling the Gut Microbiota for T1D Therapy
Several strategies are being considered to attempt to remodel the gut microbiota in T1D with an aim to improving immunological tolerance and beta cell function. Live bacteria (termed probiotics) can be introduced with a goal to either colonize the gut with a species that is absent, to increase the abundance of an existing species, or to provide beneficial metabolites without colonization. Alternatively, the goal may be to increase the overall community diversity by introducing a large bolus of diverse microorganisms with or without depletion of the existing microbiota as is the case with fecal microbiota transplantation (FMT). Another major approach under investigation is the use of “prebiotics” and diet-based therapy where the goal is to provide a food source to encourage the growth of beneficial bacteria. Alternatively, dietary supplements can be designed to directly deliver these bacterial metabolites themselves (95). The advantage of prebiotic approaches is that they do not rely on bacterial colonization, which may be ineffective if the T1D-associated intestinal environment is resistant to the introduction of beneficial bacteria. This has been shown to be the case in NOD mice, which were resistant to passive introduction of new species (96). T1D-associated genetic risk factors are thought to contribute to altering the intestinal immune environment, making it less hospitable to certain bacteria (38, 40, 97). Another approach is to identify the major modifiable lifestyle factors that lead to the establishment of a dysbiotic gut microbiota, but even this may need to be supported by one of the other strategies above, and lifestyle changes may be difficult to implement.
Probiotics to Restore Microbial Dysbiosis in T1D
The notion of modulating gut homeostasis with supplementation of tolerogenic commensal microbes offers the potential for a safe method of intervention in the disease prevention setting. TEDDY reported an association between decreased risk of IA and early supplementation of probiotics (between the age of 0–27 days) in children with the highest risk HLA genotype (DR3/4), whereas the responses were highly variable in other age groups (98). These data suggest that early intervention with probiotics in infancy in T1D-susceptible populations, when the microbiota is being established, may be protective of future disease through probiotic colonization. However, this study did not look at dose or type of probiotic consumed, so more rational approach should be taken to identify disease protective probiotic strains. A number of Lactobacillus and Bifidobacterium species considered “Generally Regarded as Safe” (GRAS) microorganisms are commonly used in dietary supplements as probiotics worldwide and could potentially be trialed in T1D-susceptible newborns.
The use of probiotics for the prevention of T1D was initially tested with heat killed Lactobacillus casei BL21 in the NOD rodent model (99). It was found that the onset of diabetes was significantly higher in the control group than in the group that received L. casei (99). Similar results were obtained by weekly feeding the VSL#3 formulation to NOD rodents (100). VSL#3 is a formulation containing three species of Bifidobacterium (B. longum, B. infantis, and B. breve), four species of Lactobacillus (L. acidophilus, L. paracasei, L. delbrueckii subsp. bulgaricus, and L. plantarum), and Streptococcus thermophilus. The decrease in T1D onset was positively correlated with lower T cell infiltration in the pancreas in the VSL#3 treated group, suggesting a direct mechanistic role of the microbiota in T1D. It was also observed that VSL#3-treated mice was associated with increased IL-10 mRNA expression and with the presence of IL-10-positive infiltrating mononuclear cells (MNCs) in the pancreas. Clinical trials are underway to test the role of the VSL#3 probiotic formulation (Visbiome probiotic, ClinicalTrials.gov identifier NCT04141761, Table 1) on children and adolescents with T1D. In a randomized clinical trial designed to test for the prevention of allergy in a high-risk cohort, VSL#3 provided during the first 6 months of life was not related to the development of T1D (amongst n = 14 cases) or development of islet autoantibodies (amongst n = 25 cases) during the 13-year follow-up (101). However, as this study was not powered to investigate T1D these results should be viewed with caution. Furthermore, a drawback of this formulation is the inability to determine which of the strains within the formulation is the one exerting a beneficial effect.
Table 1.
Clinical trials with completed or recruiting status with probiotic, prebiotics, and FMT interventions for T1D prevention or therapy.
| Clinical Trial identifiera | Status | Study population | Intervention | Sponsor |
|---|---|---|---|---|
| Probiotics | ||||
| NCT03961854 | Recruiting | T1D <1 Year; 8–17 years | Drug: L. johnsonii N6.2 Probiotic Drug: Placebo Capsule |
University of Florida |
| NCT03961347 | Recruiting | T1D <3 Years; 18–45 years | Drug: L. johnsonii N6.2 Probiotic Drug: Placebo Capsule |
University of Florida |
| NCT03423589 | Completedb | Full sibling of someone with T1D; 6–17 years | Dietary Supplement: VSL#3 | Medical College of Wisconsin |
| NCT04141761 | Recruiting | T1D <90 days; 6–17 years | Dietary Supplement: Visbiome (VSL#3) Other: Placebo |
Medical College of Wisconsin |
| NCT03880760 | Recruiting | T1D diagnosis; 6–18 years | Other: L. johnsonii MH-68, B. animalis subsp. lactis CP-9 and L. salivarius AP-32 Other: Placebo |
China Medical University Hospital |
| Prebiotics | ||||
| NCT02442544 | Active, not recruiting | T1D >1 year; 8–17 years | Dietary Supplement: Prebiotic (1:1 oligofructose: inulin) Dietary Supplement: Placebo |
Alberta Children's Hospital |
| NCT04114357 | Not yet recruiting | T1D >4 mos., <24 mos.; 12–16 years | Drug: Acetylated and Butyrylated High Amylose Maize Starch | Indiana University |
| NCT02903615 | Active, not recruiting | T1D diagnosis; 18–70 years | Other: Novel diet (lower carbohydrate, Mediterranean-style, prebiotic fiber focus) Other: Standard diet (Standard diabetes diet recommendations) |
Garvan Institute of Medical Research |
| ACTRN12618001391268 | Completedb | T1D >6 months; 18–45 years | Dietary Supplement: Acetylated and Butyrylated High Amylose Maize Starch | Monash University |
| Fecal transplant | ||||
| NCT04124211 | Recruiting | T1D diagnosis; 18–65 years | Biological: Fecal Microbiota Transplantation (FMT) | The Third Affiliated Hospital of Southern Medical University |
| NL3542 | Completed (41) | T1D <6 weeks; 18–30 years | Biological: Fecal Microbiota Transplantation (FMT) | University of Amsterdam |
Data from 08/09/2020.
Identifiers starting with NCT were collected from clinicaltrials.gov; Identifier starting with ACTRN was collected from the Australia New Zealand Clinical Trials Registry and starting with NL from the Netherlands Trial Register.
No results reported.
Through interventions with different probiotics in different rodent models of disease, it has been observed that the mechanisms of action of probiotics are diverse and strain specific. The effects range from immunomodulatory to immunostimulatory (102). The strain specificity is usually overlooked; however, it is probably the biggest factor to consider in most animal and human studies looking to use probiotics for the prevention or treatment of a disease. In addition, many commercially available probiotic formulations are selected based on their robustness in industrial production and shelf life, instead of their beneficial effects on human subjects. Currently, many clinical trials are underway in order to be able to add health claims into their labels. It is possible that the variability observed on the TEDDY cohort may be due to the use of a diverse array of probiotic products not specifically selected for T1D prevention. Consequently, there is a significant need to identify bacterial strains that are associated with a beneficial health outcome. The availability of large microbiota datasets offers the possibility of (i) testing if GRAS microorganisms are negatively correlated with disease onset and (ii) isolate and test unconventional microbial species that may be used to prevent T1D onset.
L. Johnsonii N6.2 as a Proof-of-Concept Probiotic in T1D
As a proof of concept, Roche et al. performed a culture independent analysis of the bacteria in fecal samples collected from BB-DR and BB-DP rats (103). These experiments showed a significant difference in the intestinal microbiota of DR and DP rats. Valladares et al. (104) used this microbiota guided approach to isolate two strains of Lactobacillus, L. johnsonii N6.2 and L. reuteri TD1, that were abundant in BB-DR rodents, and subsequently administered the strains to BB-DP rats to test their role in T1D onset. It was found that L. johnsonii N6.2, but not L. reuteri, prevented diabetes onset when fed daily to BB-DP rats (104). Diabetes prevention correlated with a Th17 cell bias and elevated IL-23 levels within the mesenteric lymph nodes (105). Further in vitro studies indicated that the modification of dendritic cells (DCs) by oral feeding of L. johnsonii N6.2 contributed to the Th17 bias (105). It was also found that L. johnsonii N6.2 produces a metabolite that strongly inhibits the activity of the host enzyme Indoleamine 2,3-dioxygenase (IDO), in vitro. The substrate for IDO expressed by DCs is free tryptophan, a key regulator of T cell development and immune responses. Mass spectrometry analysis of the IDO catalytic heme-center supported the presence of a molecule in the cell-free culture supernatant of L. johnsonii, that modifies the prosthetic group of this immunoregulatory enzyme, and consequently modulates its activity. An in vivo L. johnsonii feeding assay performed in BB-DP rats showed L. johnsonii N6.2 induced lower levels of intestinal IDO gene transcription, which correlated with a decrease in the concentration of blood plasma kynurenine (106). Altogether, the authors suggest that this probiotic bacterium alters host IDO activity, with putative consequences on T cell development, intestinal physiology and ultimately T1D development.
Translating the use of L. johnsonii N6.2 toward the prevention of T1D in humans first required a pilot study in healthy individuals. Guidelines from the U.S. Food and Drug Administration (FDA) state that new probiotic strains tested in clinical trials with the intent to prevent or treat a disease are considered biologics (http://www.fda.gov/cber/guidelines.htm). As such, the safety, tolerability, and general response to consumption of this microorganism was tested in healthy individuals, where consumption of L. johnsonii N6.2 was found to be safe and well-tolerated (107). Results showed that L. johnsonii N6.2 can survive and may colonize the intestinal tract with minimal effects on the residing microbiota in healthy subjects. Significant changes in the kynurenine pathway metabolites, as well as innate and adaptive immune responses, were observed in serum and peripheral blood in the probiotic treatment group (107). Remarkably, the significant increase in tryptophan concentrations were positively correlated to a progressive increase in the cell counts of Lactobacillus over time in the probiotic group. Additionally, a significant increase in monocyte and NK cell frequencies correlated with a decrease in the percentage of CD4+ T cells. Changes were also observed in most CD8+ T cell subsets, including naïve (Tn), central memory (Tcm), effector memory (Tem) and effector memory expressing CD45RA (Temra). These results indicated that L. johnsonii N6.2 supplementation can evoke strain specific responses that may have the ability to modulate innate and adaptive immunity and potentially T1D progression that have not been previously reported for other probiotics (108–110). Altogether, the data collected thus far indicate that the mechanisms observed in animal models holds true in human subjects. Clinical trials are currently being performed to evaluate the safety of L. johnsonii N6.2 in children and adults with T1D as a stepping-stone for its translation into a preventive therapy for T1D (ClinicalTrials.gov identifiers NCT03961854 and NCT03961347, Table 1).
Identification of Next-Generation Probiotics That May Reduce Progression of ISLET Autoimmunity
Most traditional probiotic bacteria originate from food, typically fermented dairy products or are previously isolated strains now currently used as commercial supplements. For example, Groele et al. plan to trial two common probiotic strains Lactobacillus rhamnosus GG and Bifidobacterium lactis Bb12 in new-onset T1D (111). Probiotic bacteria are not necessarily well-adapted to colonization and modulation of the human gut and other strains, termed “next-generation probiotics,” that are indigenous to the human microbiota may be more successful at inducing long-term benefits in T1D. When looking to identify which species should be introduced, it is important to consider the complex inter-relationships exist between species with different functional roles that support the growth of other community members. For example, one species may produce metabolites or substrates required to support the growth of another species, known as cross-feeding. One such interaction occurs when butyrate producers such as Faecalibacterium prausnitzii stimulate production of mucin by epithelial cells which in turn promotes the growth of mucinophiles such as Akkermansia. Mucin degradation and fermentation by Akkermansia releases oligosaccharides and acetate which in turn further supports the growth of butyrate producers in a feedback loop (112). This has led to the concept that increasing the abundance of a single species such as Akkermansia could then enhance the abundance of other beneficial species resulting in a broader beneficial impact. Indeed, some studies have suggested that a reduction in Akkermansia may be associated with IA or T1D (22, 58). This concept was tested by introduction of Akkermansia muciniphila by oral gavage into NOD mice resulting in an increase in mucin production by goblet cells in the colon, while reduced expression of the inflammatory marker Emr1 and increased expression of the anti-inflammatory marker Ym1 was also observed (113). The combined effect of these changes included increasing immune regulatory factors (increased IL-10 and TGF-beta in the pancreatic LN and Tregs in the islets) and delayed diabetes onset. It is likely that many more species with similar functions may also protect from T1D, at least in animal models. Recent progress in the ability to monoculture many more members of the gut microbiota community means that efforts are now ongoing aiming to systematically screen for taxa that can prevent T1D. These efforts may then identify candidates which can be tested for beneficial effect in human T1D.
Fecal Microbiota Transplantation (FMT)
FMT is the practice of transferring the total bacterial population from a healthy donor stool sample (either by rectal or oral routes into the gastrointestinal tract) with a goal of remodeling the recipient's microbiota for therapeutic benefit. This approach has gained traction due to dramatic benefits found using FMT for treatment of recurrent Clostridium difficile infection (114). In C. difficile, FMT was shown to significantly increase the diversity of the recipient's microbiota 2-weeks after transfer. Using genetic approaches to track donor derived microbial strains, Li et al. showed that donor derived bacteria persisted for 3-months after FMT (115). However, colonization was more successful for species that were already present in the recipient's own microbiota, supporting that host compatibility is a barrier to introduction of new taxa. In a study of individuals with metabolic syndrome, FMT from lean donors induced short-term changes in the recipients gut microbiota composition and improved insulin sensitivity and decreased glycated hemoglobin levels at 6 weeks (116). Taxa that were increased at 6 weeks included acetate producer Bifidobacterium pseudolongum, lactate-producing Lactobacillus salivarius, butyrate-producing Butyrivibrio, Clostridium symbiosum, and Eubacterium species. This suggests that modest benefits to glycemic control can be gained by FMT induced remodeling of the gut microbiota, but that strategies to increase the durability of the effects are needed.
The first study of FMT in T1D was recently published (41). Nieuwdorp and colleagues completed a trial of allogeneic vs. autologous FMT in individuals with new-onset T1D (Netherlands Trial Register identifier NL3542, Table 1). They found that the participants that received back their own autologous stool sample had preserved beta cell function (stimulated C-peptide) compared with those that received FMT from an allogeneic healthy donor (41), and this was correlated with certain microbial metabolites. This may indicate that the microbiota from the subject's own sample were better adapted to re-colonize than species from a non-diabetic donor. Results from this study demonstrate that FMT and microbiota-targeted therapy in general holds promise for promoting immune tolerance and preserving beta cell function in T1D. In the long-term, it is unlikely that FMT in its current form will progress to a wide-spread therapy for T1D due to both safety and palatability considerations. Recently, two cases of drug-resistant E. coli bacteraemia occurred following FMT in two separate clinical trials resulting in the death of one immunocompromised patient (117). It is more likely that if FMT demonstrates therapeutic benefit, knowledge from these studies will lead to the development of defined microbial consortium-transfer based approaches. The challenge will be in how to tailor such products to account for compatibility with core microbiota present in each recipient that together with diet and genetic background strongly influence donor engraftment (115).
Prebiotics and Diet-Based Approaches– Providing the Nutritional Landscape for Beneficial Bacteria
The use of diet-based therapy to target the gut microbiota can circumvent the need for engraftment of donor microbes into the system. These include prebiotics, which are defined as “a substrate that is selectively utilized by host microorganisms conferring a health benefit” (118). These include certain oligosaccharides (including human milk oligosaccharides) and fermentable fibers. Using such an approach, Ho et al. delivered a prebiotic, oligofructose-enriched inulin or placebo, for 12 weeks in children that had T1D for at least 1 year (119). At the completion of the diet (3 months), the prebiotic group (n = 17) had significant preservation of c-peptide compared to the placebo group (n = 21), though this benefit was not sustained at 6 months after the diet was stopped. The prebiotic administration was associated with a significant increase in the Bifidobacterium genera. Other approaches introduce specific modifications to prebiotics to increase the delivery of beneficial metabolites such as SCFAs. For example, high amylose maize starch (HAMS) is a resistant starch that can be modified by acetylation (HAMSA) and/or butyrylation (HAMSB). When HAMSA or HAMSB are fermented in the colon, large quantities of acetate and butyrate are released. In NOD mice, a HAMSAB diet provided before disease onset almost completely prevented progression to autoimmune diabetes (95). Protection was associated with expansion of regulatory T cells and a decreased frequency of islet-specific effector T cells via a mechanism dependent on remodeling of the gut microbiota. HAMSAB is being tested in adults with established T1D in the “TOGeTHER” (Type One Gut Therapy) trial (Australia New Zealand Clinical Trials Registry identifier ACTRN12618001391268, Table 1) for its potential to increase systemic acetate and butyrate delivery and modulate the microbiota, immune tolerance and glycemic control. Similarly, HAMSAB is being tested in new-onset T1D in a randomized cross-over trial (NCT04114357). In a related approach, de Groot et al. gave oral sodium butyrate to adults with established T1D for 1 month (120). Unlike HAMSAB, which is utilized in the colon by the gut microbiota releasing acetate and butyrate, oral sodium butyrate capsules deliver butyrate mainly to the small intestine. In this study, oral butyrate delivery did not lead to increased butyrate availability in the colon, nor did it impact peripheral innate immunity, islet-specific T cell autoimmunity or beta cell function. However, this mode of butyrate delivery, dose, or duration of treatment may not have been appropriate to achieve the disease protective effects seen in animal studies. In studies in healthy subjects, three different resistant starches: maize, potato, and tapioca-based starches with different chemical cross-linking, were compared in a dose-escalation study with a non-resistant (digestible) control starch (121). The authors found that the crystalline maize resistant starch increased butyrate production together with Eubacterium rectale (a major butyrate producer), while the cross-linked tapioca resistant starch increased the SCFA propionate and Parabacteroides distasonis. Parabacteroides distasonis produces succinate, which is then converted to propionate by other bacteria. Neither the potato-based resistant starch nor the control starch increased SCFA concentrations. These data suggest that specialized dietary fibers can be used to target specific SCFA, and those that favor butyrate production could be specifically evaluated in T1D.
New Directions in Microbiota Targeted Therapy for T1D
While much work is focusing on ways to increase SCFA production by the gut bacteria in T1D, many other microbial pathways and bioactive compounds produced by commensal bacteria may be beneficial for immune tolerance and glucose homeostasis. For example, the secondary bile acids isoDCA, isoalloLCA, and lithocholic/3-oxo-lithocholic acids, which are metabolized by bacteria in the colon from primary bile acid waste, have been shown to promote the generation of peripheral Tregs in the colon (122–124). Supplementation of these secondary bile acids was achieved in mice by either delivering them in drinking water, incorporating them into the mouse chow or by transferring a consortium of bacteria engineered with the metabolic capability to produce isoDCA. Thus, translation of such approaches to humans may have potential to reduce intestinal inflammation and potentially autoimmunity. Another example are microbe derived metabolites derived from breakdown of the amino acid tryptophan into indoles. These indoles are ligands for the aryl hydrocarbon receptor (AhR) which then promotes gut barrier integrity, Tregs and IL-22 production. In mouse models of T1D, oral administration of AhR ligands have been shown to expand Tregs, reduce insulitis and diabetes progression (125, 126). The beneficial gut bacteria Faecalibacterium prausnitzii is a high butyrate producer, but also produces an anti-inflammatory protein called MAM which inhibits NFκB activation and alleviates chemically-induced colitis (127). Identification of anti-inflammatory molecules produced by gut bacteria is currently a rich field, and it is likely that many candidates will be identified with promise for human disease modulation including T1D. Such molecules (now termed “postbiotics”) could be delivered as stand-alone drugs or the bacteria that produce these molecules could be targeted for expansion by prebiotics or probiotic based therapies.
While microbiota-based therapies hold much potential, it is quite likely that combination therapy approaches will be needed, especially at disease onset when autoimmunity and beta cell destruction is well-advanced. In cancer, it has recently been shown that the efficacy of check-point inhibitor immunotherapy relies on the gut microbiota (128, 129). At least in mouse models, co-delivery of a probiotic cocktail of Bifidobacterium strains improved the outcome of anti-PDL1 cancer therapy (130) and likewise Bacteroides thetaiotaomicron or Bacteroides fragilis transfer enhanced anti-CTLA-4 cancer therapy (128). There is good rationale that such combination approaches may also be of benefit in T1D. For example, low-dose IL-2 therapy, which protects NOD mice from diabetes (131) and is currently being trialed in human T1D, expanded Treg in the gut mucosa and led to an altered gut microbiota in NOD mice (40). Therefore, there may well be synergy between microbiota targeted and IL-2 based therapy. Similarly, in a rat model of T1D, two immunotherapies which prevent disease: the histone deacetylase inhibitor ITF-2357 and IL-1 receptor antagonist (Anakinra) also modify the gut microbiota. Using this rationale, Chan et al. showed that combining anti-CD3 immunotherapy with a prebiotic oligofructose increased the rate of disease reversal when treatment was started at the onset of hyperglycemia (132). In a more sophisticated approach, Takiishi et al. have used a probiotic strain of Lactococcus lactis modified to secrete the autoantigen proinsulin and IL-10 in combination with anti-CD3 therapy (133, 134). They showed that this combination therapy was more effective than either treatment alone in reversing disease at the onset of hyperglycemia in NOD mice. Hence, combination therapies targeting multiple disease pathways, including via the gut microbiota, could prove beneficial for treatment of T1D.
Highlights and Future Directions
Taken together, these studies provide evidence that the diet and other modifiers of the microbiota can alter T1D autoimmunity. While there remain a number of gaps in determining the exact role for these factors, the evidence suggests an important function. Furthermore, while insulin has been around for 100 years, there are no other treatments for T1D and other approaches are urgently needed. Modifying the microbiota through diet, probiotics, prebiotics or some combination thereof, is an attractive opportunity for disease modification, especially in very young children and/or in primary prevention, where immunotherapies and other therapeutic approaches may have limited accessibility.
Several of the dietary factors that have been associated with either IA or T1D appear to have some influence on the gut microbiota. In order to determine if the mechanism by which diet impacts T1D is through changing the microbiota, one would need to conduct a true causal study. However, thus far, the majority of data to date are all indirect, and the limited clinical trials in this area have yet to show causality. Needless to say, there is sufficient evidence to suggest that clinical studies applying these approaches are both feasible and warranted, given the correlative evidence, the relative safety of such approaches and the need for disease modification in T1D.
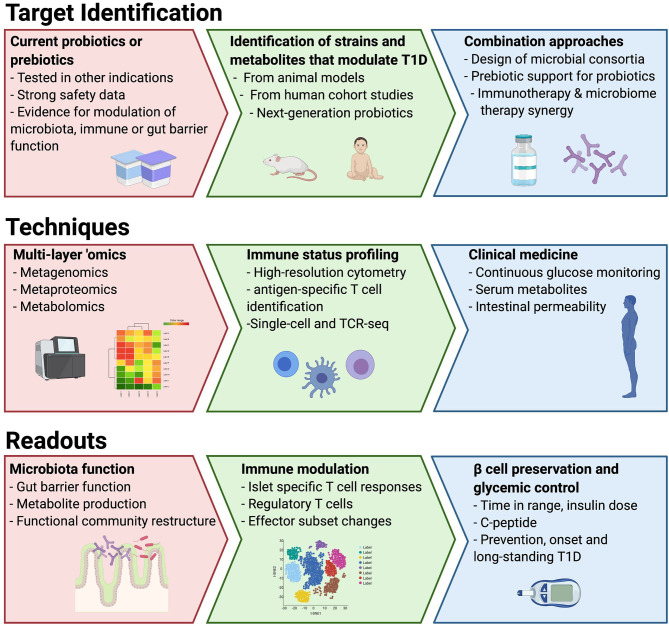
To date, most of the studies of the microbiota in T1D have used stool bacterial composition as a surrogate to understand functional changes in the microbiota, the intestinal environment and immune system. Moreover, few studies have attempted to understand the direct role the microbiota may be playing in destruction of the beta cells, such as those mechanisms that might be in play due to the presence of a leaky gut in T1D linked to microbial dysbiosis (27, 28). To overcome these limitations, the use of well-designed preclinical models as well as quick entry into clinical studies for interventions with good safety profiles should be prioritized (Figure 1). In this way, dietary studies may be the most straightforward interventions, but they are fraught with limitations with respect to adherence. We suggest two parallel approaches to probiotic interventions in T1D: (1) identification and isolation of strains and/or their metabolites important in IA or T1D and (2) testing commercial probiotics with claimed benefits in other immune-based diseases. The use of integrated “omic” technologies can greatly advance our ability to functionally assess the impact of new therapies on the complex gut ecosystem. The benefits of metagenomics have been widely realized to uncover bacterial pathways encoded in the microbiota that may be altered in disease (21). Metaproteomics is an emerging technology with potential to measure the actual functional activity of both the microbes and the host by measuring stool proteins derived from bacteria, the gut and the pancreas (29). Metabolomics of serum has been used to reveal differences in lipid metabolism and nutrient uptake in children prior to the development of autoimmunity (135, 136). As some plasma metabolites can be derived from the gut microbiota, a direct measurement of microbiota metabolite production in stool would help to understand whether circulating metabolites altered in T1D are microbiota derived. Fecal metabolomics has been successfully used to elucidate distinct disease-related microbiota signatures in other diseases (137, 138), but similar studies in T1D are not yet available. Finally, as the evidence suggests in other diseases, microbiota-based approaches may have an added benefit in traditional therapeutics and should be considered as adjunctive therapy.
Figure 1.
Proposed roadmap to utilize different approaches, model systems and techniques to drive to microbiota-targeted therapeutics in T1D.
Author Contributions
JLD, EEH-W, GLL, and JMN conceived the concept and co-wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
JLD is an employee of Janssen Research and Development. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Dr. Ryne J. Debo for early assistance in conceiving the manuscript. Figure created using Biorender.com.
Footnotes
Funding. EEH-W was supported by grants from JDRF (3-SRA-2019-730-S-B) and the Children's Hospital Foundation (2019002539). GLL was partially supported by grants from JDRF (2-SRA-2019-811-M-B) and from the National Institute of Diabetes and Digestive and Kidney Diseases (1RO1DK121130-01). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. JMN was partially supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK104351, R01-DK32493) and from the National Institute of Allergy and Infectious Diseases (R21-AI142483).
References
- 1.Robertson CC, Rich SS. Genetics of type 1 diabetes. Curr Opin Genet Dev. (2018) 50:7–16. 10.1016/j.gde.2018.01.006 [DOI] [PubMed] [Google Scholar]
- 2.Sharp SA, Rich SS, Wood AR, Jones SE, Beaumont RN, Harrison JW, et al. Development and standardization of an improved Type 1 diabetes genetic risk score for use in newborn screening and incident diagnosis. Diabetes Care. (2019) 42:200–7. 10.2337/dc18-1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunne JL, Richardson SJ, Atkinson MA, Craig ME, Dahl-Jorgensen K, Flodstrom-Tullberg M, et al. Rationale for enteroviral vaccination and antiviral therapies in human type 1 diabetes. Diabetologia. (2019) 62:744–53. 10.1007/s00125-019-4811-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Department of Health & Human Services . National Diabetes Statistics Report, 2020. Atlanta, GA: (2020). [Google Scholar]
- 5.Fourlanos S, Varney MD, Tait BD, Morahan G, Honeyman MC, Colman PG, et al. The rising incidence of type 1 diabetes is accounted for by cases with lower-risk human leukocyte antigen genotypes. Diabetes Care. (2008) 31:1546–9. 10.2337/dc08-0239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Group TS. The Environmental Determinants of Diabetes in the Young (TEDDY) study. Ann N Y Acad Sci. (2008) 1150:1–13. 10.1196/annals.1447.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neu J, Reverte CM, Mackey AD, Liboni K, Tuhacek-Tenace LM, Hatch M, et al. Changes in intestinal morphology and permeability in the biobreeding rat before the onset of type 1 diabetes. J Pediatr Gastroenterol Nutr. (2005) 40:589–95. 10.1097/01.MPG.0000159636.19346.C1 [DOI] [PubMed] [Google Scholar]
- 8.Lee AS, Gibson DL, Zhang Y, Sham HP, Vallance BA, Dutz JP. Gut barrier disruption by an enteric bacterial pathogen accelerates insulitis in NOD mice. Diabetologia. (2010) 53:741–8. 10.1007/s00125-009-1626-y [DOI] [PubMed] [Google Scholar]
- 9.Kuitunen M, Saukkonen T, Ilonen J, Akerblom HK, Savilahti E. Intestinal permeability to mannitol and lactulose in children with type 1 diabetes with the HLA-DQB1*02 allele. Autoimmunity. (2002) 35:365–8. 10.1080/0891693021000008526 [DOI] [PubMed] [Google Scholar]
- 10.Secondulfo M, Iafusco D, Carratù R, deMagistris L, Sapone A, Generoso M, et al. Ultrastructural mucosal alterations and increased intestinal permeability in non-celiac, type I diabetic patients. Dig Liver Dis. (2004) 36:35–45. 10.1016/j.dld.2003.09.016 [DOI] [PubMed] [Google Scholar]
- 11.Brugman S, Klatter FA, Visser JT, Wildeboer-Veloo AC, Harmsen HJ, Rozing J, et al. Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes? Diabetologia. (2006) 49:2105–8. 10.1007/s00125-006-0334-0 [DOI] [PubMed] [Google Scholar]
- 12.Schwartz RF, Neu J, Schatz D, Atkinson MA, Wasserfall C. Comment on: Brugman S et al. (2006) Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes? Diabetologia 49:2105-2108. Diabetologia. (2007) 50:220–1. 10.1007/s00125-006-0526-7 [DOI] [PubMed] [Google Scholar]
- 13.Livanos AE, Greiner TU, Vangay P, Pathmasiri W, Stewart D, McRitchie S, et al. Antibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nat Microbiol. (2016) 1:16140. 10.1038/nmicrobiol.2016.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Candon S, Perez-Arroyo A, Marquet C, Valette F, Foray AP, Pelletier B, et al. Antibiotics in early life alter the gut microbiome and increase disease incidence in a spontaneous mouse model of autoimmune insulin-dependent diabetes. PLoS ONE. (2015) 10:e0125448. 10.1371/journal.pone.0125448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown K, Godovannyi A, Ma C, Zhang Y, Ahmadi-Vand Z, Dai C, et al. Prolonged antibiotic treatment induces a diabetogenic intestinal microbiome that accelerates diabetes in NOD mice. ISME J. (2016) 10:321–32. 10.1038/ismej.2015.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. (2008) 455:1109–13. 10.1038/nature07336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alam C, Bittoun E, Bhagwat D, Valkonen S, Saari A, Jaakkola U, et al. Effects of a germ-free environment on gut immune regulation and diabetes progression in non-obese diabetic (NOD) mice. Diabetologia. (2011) 54:1398–406. 10.1007/s00125-011-2097-5 [DOI] [PubMed] [Google Scholar]
- 18.Giongo A, Gano KA, Crabb DB, Mukherjee N, Novelo LL, Casella G, et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. (2011) 5:82–91. 10.1038/ismej.2010.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Goffau MC, Luopajarvi K, Knip M, Ilonen J, Ruohtula T, Harkonen T, et al. Fecal microbiota composition differs between children with beta-cell autoimmunity and those without. Diabetes. (2013) 62:1238–44. 10.2337/db12-0526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kostic AD, Gevers D, Siljander H, Vatanen T, Hyotylainen T, Hamalainen AM, et al. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe. (2015) 17:260–73. 10.1016/j.chom.2015.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vatanen T, Franzosa EA, Schwager R, Tripathi S, Arthur TD, Vehik K, et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature. (2018) 562:589–94. 10.1038/s41586-018-0620-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart CJ, Ajami NJ, O'Brien JL, Hutchinson DS, Smith DP, Wong MC, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. (2018) 562:583–8. 10.1038/s41586-018-0617-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Underwood MA, German JB, Lebrilla CB, Mills DA. Bifidobacterium longum subspecies infantis: champion colonizer of the infant gut. Pediatr Res. (2015) 77:229–35. 10.1038/pr.2014.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Groot PF, Belzer C, Aydin O, Levin E, Levels JH, Aalvink S, et al. Distinct fecal and oral microbiota composition in human type 1 diabetes, an observational study. PLoS ONE. (2017) 12:e0188475. 10.1371/journal.pone.0188475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown CT, Davis-Richardson AG, Giongo A, Gano KA, Crabb DB, Mukherjee N, et al. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS ONE. (2011) 6:e25792. 10.1371/journal.pone.0025792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang J, Pearson JA, Peng J, Hu Y, Sha S, Xing Y, et al. Gut microbial metabolites alter IgA immunity in type 1 diabetes. JCI Insight. (2020) 5:e135718. 10.1172/jci.insight.135718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bosi E, Molteni L, Radaelli MG, Folini L, Fermo I, Bazzigaluppi E, et al. Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia. (2006) 49:2824–7. 10.1007/s00125-006-0465-3 [DOI] [PubMed] [Google Scholar]
- 28.Harbison JE, Roth-Schulze AJ, Giles LC, Tran CD, Ngui KM, Penno MA, et al. Gut microbiome dysbiosis and increased intestinal permeability in children with islet autoimmunity and type 1 diabetes: a prospective cohort study. Pediatr Diabetes. (2019) 20:574–83. 10.1111/pedi.12865 [DOI] [PubMed] [Google Scholar]
- 29.Gavin PG, Mullaney JA, Loo D, Cao KL, Gottlieb PA, Hill MM, et al. Intestinal metaproteomics reveals host-microbiota interactions in subjects at risk for type 1 diabetes. Diabetes Care. (2018) 41:2178–86. 10.2337/dc18-0777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. (2014) 505:559–63. 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walter J, Ley R. The human gut microbiome: ecology and recent evolutionary changes. Annu Rev Microbiol. (2011) 65:411–29. 10.1146/annurev-micro-090110-102830 [DOI] [PubMed] [Google Scholar]
- 32.Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. (2018) 555:210–5. 10.1038/nature25973 [DOI] [PubMed] [Google Scholar]
- 33.Kurilshikov A, Medina-Gomez C, Bacigalupe R, Radjabzadeh D, Wang J, Demirkan A, et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat Genet. (2021) 53:156–65. 10.1038/s41588-020-00763-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonder MJ, Kurilshikov A, Tigchelaar EF, Mujagic Z, Imhann F, Vila AV, et al. The effect of host genetics on the gut microbiome. Nat Genet. (2016) 48:1407–12. 10.1038/ng.3663 [DOI] [PubMed] [Google Scholar]
- 35.Turpin W, Espin-Garcia O, Xu W, Silverberg MS, Kevans D, Smith MI, et al. Association of host genome with intestinal microbial composition in a large healthy cohort. Nat Genet. (2016) 48:1413–7. 10.1038/ng.3693 [DOI] [PubMed] [Google Scholar]
- 36.Goodrich JK, Davenport ER, Beaumont M, Jackson MA, Knight R, Ober C, et al. Genetic determinants of the gut microbiome in UK twins. Cell Host Microbe. (2016) 19:731–43. 10.1016/j.chom.2016.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coluccia E, Iardino P, Pappalardo D, Brigida AL, Formicola V, De Felice B, et al. Congruency of genetic predisposition to lactase persistence and lactose breath test. Nutrients. (2019) 11:1383. 10.3390/nu11061383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russell JT, Roesch LFW, Ordberg M, Ilonen J, Atkinson MA, Schatz DA, et al. Genetic risk for autoimmunity is associated with distinct changes in the human gut microbiome. Nat Commun. (2019) 10:3621. 10.1038/s41467-019-11460-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silverman M, Kua L, Tanca A, Pala M, Palomba A, Tanes C, et al. Protective major histocompatibility complex allele prevents type 1 diabetes by shaping the intestinal microbiota early in ontogeny. Proc Natl Acad Sci USA. (2017) 114:9671–6. 10.1073/pnas.1712280114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mullaney JA, Stephens JE, Costello ME, Fong C, Geeling BE, Gavin PG, et al. Type 1 diabetes susceptibility alleles are associated with distinct alterations in the gut microbiota. Microbiome. (2018) 6:35. 10.1186/s40168-018-0417-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Groot P, Nikolic T, Pellegrini S, Sordi V, Imangaliyev S, Rampanelli E, et al. Faecal microbiota transplantation halts progression of human new-onset type 1 diabetes in a randomised controlled trial. Gut. (2021) 70:92–105. 10.1136/gutjnl-2020-322630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Norris JM, Johnson RK, Stene LC. Type 1 diabetes-early life origins and changing epidemiology. Lancet Diabetes Endocrinol. (2020) 8:226–38. 10.1016/S2213-8587(19)30412-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borch-Johnsen K, Joner G, Mandrup-Poulsen T, Christy M, Zachau-Christiansen B, Kastrup K, et al. Relation between breast-feeding and incidence rates of insulin-dependent diabetes mellitus. a hypothesis. Lancet. (1984) 2:1083–6. 10.1016/S0140-6736(84)91517-4 [DOI] [PubMed] [Google Scholar]
- 44.Lund-Blix NA, Stene LC, Rasmussen T, Torjesen PA, Andersen LF, Ronningen KS. Infant feeding in relation to islet autoimmunity and type 1 diabetes in genetically susceptible children: the MIDIA study. Diabetes Care. (2015) 38:257–63. 10.2337/dc14-1130 [DOI] [PubMed] [Google Scholar]
- 45.Chmiel R, Beyerlein A, Knopff A, Hummel S, Ziegler AG, Winkler C. Early infant feeding and risk of developing islet autoimmunity and type 1 diabetes. Acta Diabetol. (2015) 52:621–4. 10.1007/s00592-014-0628-5 [DOI] [PubMed] [Google Scholar]
- 46.Frederiksen B, Kroehl M, Lamb MM, Seifert J, Barriga K, Eisenbarth GS, et al. Infant exposures and development of type 1 diabetes mellitus: the Diabetes Autoimmunity Study in the Young (DAISY). JAMA Pediatr. (2013) 167:808–15. 10.1001/jamapediatrics.2013.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uusitalo U, Lee HS, Andren Aronsson C, Vehik K, Yang J, Hummel S, et al. Early infant diet and islet autoimmunity in the TEDDY study. Diabetes Care. (2018) 41:522–30. 10.2337/dc17-1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lund-Blix NA, Dydensborg Sander S, Stordal K, Nybo Andersen AM, Ronningen KS, Joner G, et al. Infant feeding and risk of type 1 diabetes in two large scandinavian birth cohorts. Diabetes Care. (2017) 40:920–7. 10.2337/dc17-0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, Faden HS, Zhu L. The response of the gut microbiota to dietary changes in the first two years of life. Front Pharmacol. (2020) 11:334. 10.3389/fphar.2020.00334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Chari RS, et al. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ. (2013) 185:385–94. 10.1503/cmaj.121189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bezirtzoglou E, Tsiotsias A, Welling GW. Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH). Anaerobe. (2011) 17:478–82. 10.1016/j.anaerobe.2011.03.009 [DOI] [PubMed] [Google Scholar]
- 52.Ku HJ, Kim YT, Lee JH. Microbiome study of initial gut microbiota from newborn infants to children reveals that diet determines its compositional development. J Microbiol Biotechnol. (2020) 30:1067–71. 10.4014/jmb.2002.02042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gschwendtner S, Kang H, Thiering E, Kublik S, Fosel B, Schulz H, et al. Early life determinants induce sustainable changes in the gut microbiome of six-year-old children. Sci Rep. (2019) 9:12675. 10.1038/s41598-019-49160-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turroni F, Milani C, Duranti S, Mahony J, van Sinderen D, Ventura M. Glycan utilization and cross-feeding activities by bifidobacteria. Trends Microbiol. (2018) 26:339–50. 10.1016/j.tim.2017.10.001 [DOI] [PubMed] [Google Scholar]
- 55.Hummel S, Pfluger M, Hummel M, Bonifacio E, Ziegler AG. Primary dietary intervention study to reduce the risk of islet autoimmunity in children at increased risk for type 1 diabetes: the BABYDIET study. Diabetes Care. (2011) 34:1301–5. 10.2337/dc10-2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beyerlein A, Chmiel R, Hummel S, Winkler C, Bonifacio E, Ziegler AG. Timing of gluten introduction and islet autoimmunity in young children: updated results from the BABYDIET study. Diabetes Care. (2014) 37:e194–5. 10.2337/dc14-1208 [DOI] [PubMed] [Google Scholar]
- 57.Differding MK, Benjamin-Neelon SE, Hoyo C, Ostbye T, Mueller NT. Timing of complementary feeding is associated with gut microbiota diversity and composition and short chain fatty acid concentrations over the first year of life. BMC Microbiol. (2020) 20:56. 10.1186/s12866-020-01723-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Endesfelder D, Engel M, Davis-Richardson AG, Ardissone AN, Achenbach P, Hummel S, et al. Towards a functional hypothesis relating anti-islet cell autoimmunity to the dietary impact on microbial communities and butyrate production. Microbiome. (2016) 4:17. 10.1186/s40168-016-0163-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lund-Blix NA, Dong F, Marild K, Seifert J, Baron AE, Waugh KC, et al. Gluten intake and risk of islet autoimmunity and progression to type 1 diabetes in children at increased risk of the disease: the Diabetes Autoimmunity Study in the Young (DAISY). Diabetes Care. (2019) 42:789–96. 10.2337/dc18-2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beyerlein A, Liu X, Uusitalo UM, Harsunen M, Norris JM, Foterek K, et al. Dietary intake of soluble fiber and risk of islet autoimmunity by 5 y of age: results from the TEDDY study. Am J Clin Nutr. (2015) 102:345–52. 10.3945/ajcn.115.108159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hakola L, Miettinen ME, Syrjala E, Akerlund M, Takkinen HM, Korhonen TE, et al. Association of cereal, gluten, and dietary fiber intake with islet autoimmunity and type 1 diabetes. JAMA Pediatr. (2019) 173:953–60. 10.1001/jamapediatrics.2019.2564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lund-Blix NA, Tapia G, Marild K, Brantsaeter AL, Njolstad PR, Joner G, et al. Maternal and child gluten intake and association with type 1 diabetes: the Norwegian Mother and Child Cohort study. PLoS Med. (2020) 17:e1003032. 10.1371/journal.pmed.1003032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonder MJ, Tigchelaar EF, Cai X, Trynka G, Cenit MC, Hrdlickova B, et al. The influence of a short-term gluten-free diet on the human gut microbiome. Genome Med. (2016) 8:45. 10.1186/s13073-016-0295-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lerner A, Shoenfeld Y, Matthias T. Adverse effects of gluten ingestion and advantages of gluten withdrawal in nonceliac autoimmune disease. Nutr Rev. (2017) 75:1046–58. 10.1093/nutrit/nux054 [DOI] [PubMed] [Google Scholar]
- 65.Haupt-Jorgensen M, Holm LJ, Josefsen K, Buschard K. Possible prevention of diabetes with a gluten-free diet. Nutrients. (2018) 10:1746. 10.3390/nu10111746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marietta EV, Gomez AM, Yeoman C, Tilahun AY, Clark CR, Luckey DH, et al. Low incidence of spontaneous type 1 diabetes in non-obese diabetic mice raised on gluten-free diets is associated with changes in the intestinal microbiome. PLoS ONE. (2013) 8:e78687. 10.1371/journal.pone.0078687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Norris JM, Yin X, Lamb MM, Barriga K, Seifert J, Hoffman M, et al. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA. (2007) 298:1420–8. 10.1001/jama.298.12.1420 [DOI] [PubMed] [Google Scholar]
- 68.Norris JM, Kroehl M, Fingerlin TE, Frederiksen BN, Seifert J, Wong R, et al. Erythrocyte membrane docosapentaenoic acid levels are associated with islet autoimmunity: the Diabetes Autoimmunity Study in the Young. Diabetologia. (2014) 57:295–304. 10.1007/s00125-013-3106-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Costantini L, Molinari R, Farinon B, Merendino N. Impact of Omega-3 fatty acids on the gut microbiota. Int J Mol Sci. (2017) 18:2645. 10.3390/ijms18122645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Watson H, Mitra S, Croden FC, Taylor M, Wood HM, Perry SL, et al. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut. (2018) 67:1974–83. 10.1136/gutjnl-2017-314968 [DOI] [PubMed] [Google Scholar]
- 71.Pu S, Khazanehei H, Jones PJ, Khafipour E. Interactions between obesity status and dietary intake of monounsaturated and polyunsaturated oils on human gut microbiome profiles in the Canola Oil Multicenter Intervention Trial (COMIT). Front Microbiol. (2016) 7:1612. 10.3389/fmicb.2016.01612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Balfego M, Canivell S, Hanzu FA, Sala-Vila A, Martinez-Medina M, Murillo S, et al. Effects of sardine-enriched diet on metabolic control, inflammation and gut microbiota in drug-naive patients with type 2 diabetes: a pilot randomized trial. Lipids Health Dis. (2016) 15:78. 10.1186/s12944-016-0245-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Norris JM, Lee HS, Frederiksen B, Erlund I, Uusitalo U, Yang J, et al. Plasma 25-hydroxyvitamin D concentration and risk of islet autoimmunity. Diabetes. (2018) 67:146–54. 10.2337/db17-0802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miettinen ME, Niinisto S, Erlund I, Cuthbertson D, Nucci AM, Honkanen J, et al. Serum 25-hydroxyvitamin D concentration in childhood and risk of islet autoimmunity and type 1 diabetes: the TRIGR nested case-control ancillary study. Diabetologia. (2020) 63:780–7. 10.1007/s00125-019-05077-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Simpson M, Brady H, Yin X, Seifert J, Barriga K, Hoffman M, et al. No association of vitamin D intake or 25-hydroxyvitamin D levels in childhood with risk of islet autoimmunity and type 1 diabetes: the Diabetes Autoimmunity Study in the Young (DAISY). Diabetologia. (2011) 54:2779–88. 10.1007/s00125-011-2278-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reinert-Hartwall L, Honkanen J, Harkonen T, Ilonen J, Simell O, Peet A, et al. No association between vitamin D and beta-cell autoimmunity in Finnish and Estonian children. Diabetes Metab Res Rev. (2014) 30:749–60. 10.1002/dmrr.2550 [DOI] [PubMed] [Google Scholar]
- 77.Makinen M, Mykkanen J, Koskinen M, Simell V, Veijola R, Hyoty H, et al. Serum 25-Hydroxyvitamin D concentrations in children progressing to autoimmunity and clinical type 1 diabetes. J Clin Endocrinol Metab. (2016) 101:723–9. 10.1210/jc.2015-3504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Raab J, Giannopoulou EZ, Schneider S, Warncke K, Krasmann M, Winkler C, et al. Prevalence of vitamin D deficiency in pre-type 1 diabetes and its association with disease progression. Diabetologia. (2014) 57:902–8. 10.1007/s00125-014-3181-4 [DOI] [PubMed] [Google Scholar]
- 79.Waterhouse M, Hope B, Krause L, Morrison M, Protani MM, Zakrzewski M, et al. Vitamin D and the gut microbiome: a systematic review of in vivo studies. Eur J Nutr. (2019) 58:2895–910. 10.1007/s00394-018-1842-7 [DOI] [PubMed] [Google Scholar]
- 80.Aranow C. Vitamin D and the immune system. J Investig Med. (2011) 59: 881–6. 10.2310/JIM.0b013e31821b8755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jackson MA, Verdi S, Maxan ME, Shin CM, Zierer J, Bowyer RCE, et al. Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nat Commun. (2018) 9:2655. 10.1038/s41467-018-05184-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luthold RV, Fernandes GR, Franco-de-Moraes AC, Folchetti LG, Ferreira SR. Gut microbiota interactions with the immunomodulatory role of vitamin D in normal individuals. Metabolism. (2017) 69:76–86. 10.1016/j.metabol.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 83.Seura T, Yoshino Y, Fukuwatari T. The relationship between habitual dietary intake and gut microbiota in young Japanese women. J Nutr Sci Vitaminol. (2017) 63:396–404. 10.3177/jnsv.63.396 [DOI] [PubMed] [Google Scholar]
- 84.Wang J, Thingholm LB, Skieceviciene J, Rausch P, Kummen M, Hov JR, et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat Genet. (2016) 48:1396–406. 10.1038/ng.3695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lamb MM, Yin X, Barriga K, Hoffman MR, Baron AE, Eisenbarth GS, et al. Dietary glycemic index, development of islet autoimmunity, and subsequent progression to type 1 diabetes in young children. J Clin Endocrinol Metab. (2008) 93:3936–42. 10.1210/jc.2008-0886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lamb MM, Frederiksen B, Seifert JA, Kroehl M, Rewers M, Norris JM. Sugar intake is associated with progression from islet autoimmunity to type 1 diabetes: the Diabetes Autoimmunity Study in the Young. Diabetologia. (2015) 58:2027–34. 10.1007/s00125-015-3657-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bantle JP. Dietary fructose metabolic syndrome diabetes. J Nutr. (2009) 139:1263S−8S. 10.3945/jn.108.098020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stanhope KL, Schwarz JM, Havel PJ. Adverse metabolic effects of dietary fructose: results from the recent epidemiological, clinical, mechanistic studies. Curr Opin Lipidol. (2013) 24:198–206. 10.1097/MOL.0b013e3283613bca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bjork E, Kampe O, Andersson A, Karlsson FA. Expression of the 64 kDa/glutamic acid decarboxylase rat islet cell autoantigen is influenced by the rate of insulin secretion. Diabetologia. (1992) 35:490–3. 10.1007/BF02342450 [DOI] [PubMed] [Google Scholar]
- 90.Marrack P, Kappler JW. Do MHCII-presented neoantigens drive type 1 diabetes and other autoimmune diseases? Cold Spring Harb Perspect Med. (2012) 2:a007765. 10.1101/cshperspect.a007765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Di Rienzi SC, Britton RA. Adaptation of the gut microbiota to modern dietary sugars and sweeteners. Adv Nutr. (2020) 11:616–29. 10.1093/advances/nmz118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature. (2016) 529:212–5. 10.1038/nature16504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sonnenburg ED, Sonnenburg JL. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. (2014) 20:779–86. 10.1016/j.cmet.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kashyap PC, Marcobal A, Ursell LK, Smits SA, Sonnenburg ED, Costello EK, et al. Genetically dictated change in host mucus carbohydrate landscape exerts a diet-dependent effect on the gut microbiota. Proc Natl Acad Sci USA. (2013) 110:17059–64. 10.1073/pnas.1306070110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marino E, Richards JL, McLeod KH, Stanley D, Yap YA, Knight J, et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat Immunol. (2017) 18:552–62. 10.1038/ni.3713 [DOI] [PubMed] [Google Scholar]
- 96.Mullaney JA, Stephens JE, Geeling BE, Hamilton-Williams EE. Early-life exposure to gut microbiota from disease-protected mice does not impact disease outcome in type 1 diabetes susceptible NOD mice. Immunol Cell Biol. (2019) 97:97–103. 10.1111/imcb.12201 [DOI] [PubMed] [Google Scholar]
- 97.Paun A, Yau C, Meshkibaf S, Daigneault MC, Marandi L, Mortin-Toth S, et al. Association of HLA-dependent islet autoimmunity with systemic antibody responses to intestinal commensal bacteria in children. Sci Immunol. (2019) 4:eaau8125. 10.1126/sciimmunol.aau8125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Uusitalo U, Liu X, Yang J, Aronsson CA, Hummel S, Butterworth M, et al. Association of early exposure of probiotics and islet autoimmunity in the TEDDY study. JAMA Pediatr. (2016) 170:20–8. 10.1001/jamapediatrics.2015.2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Matsuzaki T, Nagata Y, Kado S, Uchida K, Kato I, Hashimoto S, et al. Prevention of onset in an insulin-dependent diabetes mellitus model, NOD mice, by oral feeding of Lactobacillus casei. APMIS. (1997) 105:643–9. 10.1111/j.1699-0463.1997.tb05066.x [DOI] [PubMed] [Google Scholar]
- 100.Calcinaro F, Dionisi S, Marinaro M, Candeloro P, Bonato V, Marzotti S, et al. Oral probiotic administration induces interleukin-10 production and prevents spontaneous autoimmune diabetes in the non-obese diabetic mouse. Diabetologia. (2005) 48:1565–75. 10.1007/s00125-005-1831-2 [DOI] [PubMed] [Google Scholar]
- 101.Savilahti E, Harkonen T, Savilahti EM, Kukkonen K, Kuitunen M, Knip M. Probiotic intervention in infancy is not associated with development of beta cell autoimmunity and type 1 diabetes. Diabetologia. (2018) 61:2668–70. 10.1007/s00125-018-4738-4 [DOI] [PubMed] [Google Scholar]
- 102.Azad MAK, Sarker M, Wan D. Immunomodulatory effects of probiotics on cytokine profiles. Biomed Res Int. (2018) 2018:8063647. 10.1155/2018/8063647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roesch LF, Lorca GL, Casella G, Giongo A, Naranjo A, Pionzio AM, et al. Culture-independent identification of gut bacteria correlated with the onset of diabetes in a rat model. ISME J. (2009) 3:536–48. 10.1038/ismej.2009.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Valladares R, Sankar D, Li N, Williams E, Lai KK, Abdelgeliel AS, et al. Lactobacillus johnsonii N6.2 mitigates the development of type 1 diabetes in BB-DP rats. PLoS ONE. (2010) 5:e10507. 10.1371/journal.pone.0010507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lau K, Benitez P, Ardissone A, Wilson TD, Collins EL, Lorca G, et al. Inhibition of type 1 diabetes correlated to a Lactobacillus johnsonii N6.2-mediated Th17 bias. J Immunol. (2011) 186:3538–46. 10.4049/jimmunol.1001864 [DOI] [PubMed] [Google Scholar]
- 106.Valladares R, Bojilova L, Potts AH, Cameron E, Gardner C, Lorca G, et al. Lactobacillus johnsonii inhibits indoleamine 2,3-dioxygenase and alters tryptophan metabolite levels in BioBreeding rats. FASEB J. (2013) 27:1711–20. 10.1096/fj.12-223339 [DOI] [PubMed] [Google Scholar]
- 107.Marcial GE, Ford AL, Haller MJ, Gezan SA, Harrison NA, Cai D, et al. Lactobacillus johnsonii N6.2 modulates the host immune responses: a double-blind, randomized trial in healthy adults. Front Immunol. (2017) 8:655. 10.3389/fimmu.2017.00655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rask C, Adlerberth I, Berggren A, Ahren IL, Wold AE. Differential effect on cell-mediated immunity in human volunteers after intake of different lactobacilli. Clin Exp Immunol. (2013) 172:321–32. 10.1111/cei.12055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.van Baarlen P, Troost FJ, van Hemert S, van der Meer C, de Vos WM, de Groot PJ, et al. Differential NF-kappaB pathways induction by Lactobacillus plantarum in the duodenum of healthy humans correlating with immune tolerance. Proc Natl Acad Sci USA. (2009) 106:2371–6. 10.1073/pnas.0809919106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sheikhi A, Shakerian M, Giti H, Baghaeifar M, Jafarzadeh A, Ghaed V, et al. Probiotic yogurt culture bifidobacterium animalis subsp. lactis BB-12 and Lactobacillus Acidophilus LA-5 modulate the cytokine secretion by peripheral blood mononuclear cells from patients with ulcerative colitis. Drug Res. (2016) 66:300–5. 10.1055/s-0035-1569414 [DOI] [PubMed] [Google Scholar]
- 111.Groele L, Szajewska H, Szypowska A. Effects of Lactobacillus rhamnosus GG and Bifidobacterium lactis Bb12 on beta-cell function in children with newly diagnosed type 1 diabetes: protocol of a randomised controlled trial. BMJ Open. (2017) 7:e017178. 10.1136/bmjopen-2017-017178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Belzer C, Chia LW, Aalvink S, Chamlagain B, Piironen V, Knol J, et al. Microbial metabolic networks at the mucus layer lead to diet-independent butyrate and Vitamin B12 production by intestinal symbionts. mBio. (2017) 8:e00770-17. 10.1128/mBio.00770-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hanninen A, Toivonen R, Poysti S, Belzer C, Plovier H, Ouwerkerk JP, et al. Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in NOD mice. Gut. (2018) 67:1445–53. 10.1136/gutjnl-2017-314508 [DOI] [PubMed] [Google Scholar]
- 114.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. (2013) 368:407–15. 10.1056/NEJMoa1205037 [DOI] [PubMed] [Google Scholar]
- 115.Li SS, Zhu A, Benes V, Costea PI, Hercog R, Hildebrand F, et al. Durable coexistence of donor and recipient strains after fecal microbiota transplantation. Science. (2016) 352:586–9. 10.1126/science.aad8852 [DOI] [PubMed] [Google Scholar]
- 116.Kootte RS, Levin E, Salojarvi J, Smits LP, Hartstra AV, Udayappan SD, et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. (2017) 26:611–19 e6. 10.1016/j.cmet.2017.09.008 [DOI] [PubMed] [Google Scholar]
- 117.DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med. (2019) 381:2043–50. 10.1056/NEJMoa1910437 [DOI] [PubMed] [Google Scholar]
- 118.Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. (2017) 14:491–502. 10.1038/nrgastro.2017.75 [DOI] [PubMed] [Google Scholar]
- 119.Ho J, Nicolucci AC, Virtanen H, Schick A, Meddings J, Reimer RA, et al. Effect of prebiotic on microbiota, intestinal permeability, and glycemic control in children with type 1 diabetes. J Clin Endocrinol Metab. (2019) 104:4427–40. 10.1210/jc.2019-00481 [DOI] [PubMed] [Google Scholar]
- 120.de Groot PF, Nikolic T, Imangaliyev S, Bekkering S, Duinkerken G, Keij FM, et al. Oral butyrate does not affect innate immunity and islet autoimmunity in individuals with longstanding type 1 diabetes: a randomised controlled trial. Diabetologia. (2020) 63:597–610. 10.1007/s00125-019-05073-8 [DOI] [PubMed] [Google Scholar]
- 121.Deehan EC, Yang C, Perez-Munoz ME, Nguyen NK, Cheng CC, Triador L, et al. Precision microbiome modulation with discrete dietary fiber structures directs short-chain fatty acid production. Cell Host Microbe. (2020) 27:389–404 e6. 10.1016/j.chom.2020.01.006 [DOI] [PubMed] [Google Scholar]
- 122.Campbell C, McKenney PT, Konstantinovsky D, Isaeva OI, Schizas M, Verter J, et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature. (2020) 581:475–9. 10.1038/s41586-020-2193-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Song X, Sun X, Oh SF, Wu M, Zhang Y, Zheng W, et al. Microbial bile acid metabolites modulate gut RORgamma(+) regulatory T cell homeostasis. Nature. (2020) 577:410–5. 10.1038/s41586-019-1865-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hang S, Paik D, Yao L, Kim E, Trinath J, Lu J, et al. Bile acid metabolites control TH17 and Treg cell differentiation. Nature. (2019) 576:143–8. 10.1038/s41586-019-1785-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ehrlich AK, Pennington JM, Wang X, Rohlman D, Punj S, Lohr CV, et al. Activation of the aryl hydrocarbon receptor by 10-Cl-BBQ prevents insulitis and effector t cell development independently of Foxp3+ regulatory T Cells in nonobese diabetic mice. J Immunol. (2016) 196:264–73. 10.4049/jimmunol.1501789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kerkvliet NI, Steppan LB, Vorachek W, Oda S, Farrer D, Wong CP, et al. Activation of aryl hydrocarbon receptor by TCDD prevents diabetes in NOD mice and increases Foxp3+ T cells in pancreatic lymph nodes. Immunotherapy. (2009) 1:539–47. 10.2217/imt.09.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Quevrain E, Maubert MA, Michon C, Chain F, Marquant R, Tailhades J, et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn's disease. Gut. (2016) 65:415–25. 10.1136/gutjnl-2014-307649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. (2015) 350:1079–84. 10.1126/science.aad1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. (2018) 359:91–7. 10.1126/science.aan3706 [DOI] [PubMed] [Google Scholar]
- 130.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. (2015) 350:1084–9. 10.1126/science.aac4255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. (2008) 28:687–97. 10.1016/j.immuni.2008.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chan C, Hyslop CM, Shrivastava V, Ochoa A, Reimer RA, Huang C. Oligofructose as an adjunct in treatment of diabetes in NOD mice. Sci Rep. (2016) 6:37627. 10.1038/srep37627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Takiishi T, Cook DP, Korf H, Sebastiani G, Mancarella F, Cunha JP, et al. Reversal of diabetes in NOD mice by clinical-grade proinsulin and IL-10-Secreting Lactococcus lactis in combination with low-dose Anti-CD3 depends on the induction of Foxp3-Positive T cells. Diabetes. (2017) 66:448–59. 10.2337/db15-1625 [DOI] [PubMed] [Google Scholar]
- 134.Takiishi T, Korf H, Van Belle TL, Robert S, Grieco FA, Caluwaerts S, et al. Reversal of autoimmune diabetes by restoration of antigen-specific tolerance using genetically modified Lactococcus lactis in mice. J Clin Invest. (2012) 122:1717–25. 10.1172/JCI60530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Balzano-Nogueira L, Ramirez R, Zamkovaya T, Dailey J, Ardissone AN, Chamala S, et al. Integrative analyses of TEDDY Omics data reveal lipid metabolism abnormalities, increased intracellular ROS and heightened inflammation prior to autoimmunity for type 1 diabetes. Genome Biol. (2021) 22:39. 10.1186/s13059-021-02262-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Oresic M, Simell S, Sysi-Aho M, Nanto-Salonen K, Seppanen-Laakso T, Parikka V, et al. Dysregulation of lipid and amino acid metabolism precedes islet autoimmunity in children who later progress to type 1 diabetes. J Exp Med. (2008) 205:2975–84. 10.1084/jem.20081800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bao R, Hesser LA, He Z, Zhou X, Nadeau KC, Nagler CR. Fecal microbiome and metabolome differ in healthy and food-allergic twins. J Clin Invest. (2021) 131:e141935. 10.1172/JCI141935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bowerman KL, Rehman SF, Vaughan A, Lachner N, Budden KF, Kim RY, et al. Disease-associated gut microbiome and metabolome changes in patients with chronic obstructive pulmonary disease. Nat Commun. (2020) 11:5886. 10.1038/s41467-020-19701-0 [DOI] [PMC free article] [PubMed] [Google Scholar]