Abstract
Background: Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2, has become a global pandemic. Based on symptoms, COVID-19 cases can be classified as symptomatic or asymptomatic. However, there is limited information about the differences between COVID-19 patients with and without pneumonia. Our study aimed to further discuss the spectrum and clinical characteristics of symptomatic and asymptomatic COVID-19 patients with and without pneumonia.
Methods: In China, all COVID-19 cases are hospitalized in designated hospitals until two continuous negative oropharyngeal swabs obtained, which allows the professional monitoring of symptoms and clinical characteristics. We stratified all COVID-19 cases in our database and evaluated clinical characteristics in different COVID-19 subgroups (symptomatic with pneumonia, symptomatic without pneumonia, asymptomatic with pneumonia, and asymptomatic without pneumonia).
Results: According to symptoms and laboratory and radiologic findings, COVID-19 cases were defined as symptomatic with pneumonia, symptomatic without pneumonia, asymptomatic with pneumonia, or asymptomatic without pneumonia. There were differences in the clinical characteristics and prognosis among the four groups. Both non-invasive mechanical ventilation (18, 4.2%) and invasive mechanical ventilation (11, 2.6%) were applied in only the symptomatic with pneumonia group. Likewise, extracorporeal membrane oxygenation and continuous renal replacement therapy were applied in only the symptomatic with pneumonia group. There were no differences in viral load, the durations of viral shedding, and hospitalization among the four groups.
Conclusion: We have defined a comprehensive spectrum of COVID-19 with and without pneumonia. The symptomatic with pneumonia group consumed more medical resources than the other groups, and extra caution and monitoring should be applied in this group. The asymptomatic COVID-19 group had a similar viral load and viral shedding duration as the symptomatic COVID-19 group.
Keywords: spectrum, characteristics, asymptomatic COVID-19, symptomatic, pneumonia
Introduction
Coronavirus disease 2019 (COVID-19) emerged since December 2019 in Wuhan, Hubei province, central-south China, and is an ongoing global pandemic (1). As of November 2, 2020, there were more than 45 million COVID-19 patients worldwide, and more than 1 million patients lost their lives (2). The causative pathogen has been identified as a novel enveloped RNA beta coronavirus with phylogenetic similarity to severe acute respiratory syndrome coronavirus (SARS-CoV) (3) and has been named SARS-CoV-2 by the World Health Organization. Antiviral drugs were effective for SARS-CoV-2 only in vitro, and a recent clinical trial of lopinavir–ritonavir, an antiviral therapy, was not associated with any benefits in COVID-19 patients (4). Because of pandemic transmission and ineffective therapeutics, early diagnosis and quarantine seem to be crucial to combat COVID-19. The spectrum of COVID-19 ranges from asymptomatic or mild, self-limiting respiratory tract illness to severe progressive pneumonia and acute respiratory distress syndrome (ARDS) (1, 5). Approximately 80% of COVID-19 patients have non-severe illness, but the asymptomatic ratio varies widely in the literatures, which could be explained by different definitions of asymptomatic COVID-19 cases and the late onset of symptoms (5–7) during the disease course. Despite inconsistencies in the asymptomatic case proportions, it is well-accepted that asymptomatic COVID-19 patients can serve as transmission sources (7–9).
SARS-CoV-2 relies on the angiotensin-converting enzyme 2 (ACE2) receptor for cellular entry, and the expression of ACE2 has been confirmed in nasal goblet cells and alveolar epithelial cells, indicating that SARS-CoV-2 can infect both the upper and lower airways (10). However, there is limited information about differences between upper airway and lower airway (pneumonia) infections in COVID-19 patients. To better understand and identify the spectrum of COVID-19, we conducted this retrospective study to discuss the clinical characteristics in different COVID-19 groups stratified by the presence or absence of symptoms and pneumonia.
Methods
Study Design
This study was approved and supervised by the Medical Research Ethics Committee of the Second Xiangya Hospital, Central South University. This retrospective study was performed at the Public Health Treatment Center of Changsha, People's Hospital of Junshan District, Loudi Central Hospital, People's Hospital of Lucheng District, Xiangtan Central Hospital, and People's Hospital of Yunyang District, which were designated COVID-19 hospitals in Hunan and Hubei provinces. COVID-19 patients were admitted to a designated hospital, once they were diagnosed with COVID-19. A total of 228 patients were hospitalized in Changsha, the closest neighboring capital city of Wuhan. COVID-19 was confirmed by real-time reverse transcription–polymerase chain reaction (RT-PCR) detecting SARS-CoV-2 as described previously (3). Open reading frame 1ab (ORF1ab) and nucleocapsid protein (N) were the two targeted genes simultaneously amplified and tested. Clinical samples were quantified and expressed as a cycle threshold value (Ct value), which detected ORF1ab and N as the two targeted genes. The viral load of patients' nasopharyngeal swab samples was estimated by Ct values of N gene, when RT-PCR results were considered positive at the first time. To analyze the characteristics and outcomes of symptomatic and asymptomatic COVID-19 patients with or without pneumonia, we reviewed medical records and enrolled all COVID-19 patients (n = 498) from the above designated hospitals who had been discharged or died before March 30.
The following medical information was obtained: demographics, symptoms during the whole course of COVID-19 (fever, cough, expectoration, dyspnea, temperature, and respiratory rate), laboratory findings (arterial blood pH, arterial blood Pao2, white blood cell count, neutrophils, lymphocyte count, and serum lactate dehydrogenase), chest computed tomography (CT) findings, comorbidities and concomitant diseases, duration of hospitalization, use of antivirus treatments, and concomitant treatments during admission (corticosteroids and antibiotics).
Variables and Definitions
Temperature was examined at least three times a day, and fever was defined as an axillary temperature > 37.3°C (include 37.3°C). Chest CT was conducted every 3–5 days during hospitalization, and the classification of abnormal CT findings associated with COVID-19–related pneumonia followed those of previous research (11). Clinical improvement was defined as no fever for >3 days, the resolution of symptoms, and radiologic improvement (1). Patients were discharged after clinical improvement and the receipt of two negative continued SARS-CoV-2 RT-PCR tests with an interval of more than 24 h.
Severe COVID-19 was defined as a respiratory rate ≥30 breaths/min, blood oxygen saturation (Sao2) ≤93%, a Pao2/fraction of inspired oxygen (Fio2) ratio <300 mm Hg, and/or lung infiltrates in >50% of the lung field within 24–48 h (1). Patients with respiratory failure, septic shock, and/or multiple organ dysfunction/failure were defined as critical COVID-19 patients (1). All the critical patients were admitted into intensive care unit (ICU).
The duration of viral shedding for mild or moderate COVID-19 patients was defined as the time from the date of symptom onset to the date of the last negative result from two consecutive throat swab samples with an interval of more than 24 h, without positive result in subsequent test. The duration of viral shedding for asymptomatic COVID-19 patients was defined as the time from the first-time positive SARS-CoV-2 RT-PCR test to the date of the second consecutive negative RT-PCR results, with 24-h interval and without a positive subsequent test (12, 13).
According to the presence symptoms (fever, cough, expectoration, dyspnea, fatigue, muscle soreness, and headache), COVID-19 patients were divided into asymptomatic and symptomatic COVID-19 groups. Following the pneumonia guidelines from the American Thoracic Society (14), asymptomatic patients with normal chest CT findings during hospitalization were classified as the asymptomatic without pneumonia group. In contrast, asymptomatic patients with abnormal laboratory and radiologic findings during hospitalization were classified as the asymptomatic with pneumonia group. Likewise, symptomatic COVID-19 patients were classified into symptomatic with pneumonia groups and symptomatic without pneumonia groups. Symptoms, laboratory examination results, chest CT findings, and outcomes were confirmed by two independent pulmonologists.
Statistical Analysis
Continuous variables are presented as medians [interquartile ranges (IQRs)], and categorical variables are presented as n (%). We used the Kruskal–Wallis test, Mann–Whitney U-test, χ2 test, or Fisher exact test to compare differences between groups. A two-sided α of < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS software (version 23.0; SPSS Inc., Chicago, IL).
Results
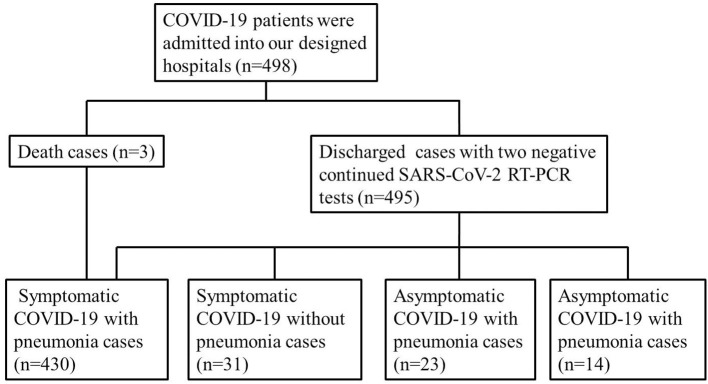
In our study, 498 patients with confirmed COVID-19 were admitted to designated hospitals, and all of them were discharged or died before March 30. A total of 461 patients (92.6%) had symptoms and were included in the symptomatic group, whereas the other 37 patients (7.4%) were included in the asymptomatic group. Of the 461 symptomatic patients, 430 (93.3%) had abnormal laboratory and chest CT findings and were included in the symptomatic with pneumonia group, whereas the other 31 (6.2%) were included in the symptomatic without pneumonia group. Of the 37 asymptomatic COVID-19 patients, 23 (62.2%) had abnormal laboratory and chest CT findings and were classified in the asymptomatic with pneumonia group, whereas the other 14 (37.8%) were classified in the asymptomatic without pneumonia group (Figure 1). The greater frequency of pneumonia in the symptomatic group than in the asymptomatic group (p < 0.001 by χ2 test) supports the theory that symptomatic COVID-19 might be more likely to be associated with lower airway infection than with upper airway infection.
Figure 1.
Flowchart of eligible patients.
Demographic Characteristics and Reported Symptoms
The median age of the 430 symptomatic COVID-19 with pneumonia patients was 45.0 years (IQR, 34.0–57.0 years), and 171 (39.8%) of the 430 patients were male (Table 1). The median ages of the 31 symptomatic COVID-19 without pneumonia patients, 23 asymptomatic COVID-19 with pneumonia patients, and 14 asymptomatic COVID-19 without pneumonia patients were 35.0 years (17.0–50.0 years), 48.0 years (38.0–59.0 years), and 25.0 years (11.0–51.5 years), respectively (Table 1). Statistical analysis showed that COVID-19 patients without pneumonia were younger than COVID-19 patients with pneumonia (p = 0.001, Table 1). Of the 23 asymptomatic pneumonia patients, only 6 (26.1%) were male, presenting a significantly lower proportion of males than females (Table 1). There were no differences in comorbidities, except for a higher frequency of hypertension (39.1%) in the asymptomatic with pneumonia group, compared with the others (Table 1).
Table 1.
Epidemiological and clinical characteristics in symptomatic and asymptomatic COVID-19 cases.
| Symptomatic COVID-19 | Asymptomatic COVID-19 | ||||
|---|---|---|---|---|---|
| Items | With pneumonia (n = 430) | Without pneumonia (n = 31) | With pneumonia (n = 23) | Without pneumonia (n = 14) | p-value |
| Age, years | 45.0 (34.0–57.0)* | 35.0 (17.0–50.0)* | 48.0 (38.0–59.0) | 25.0 (11.0–51.5)* | 0.001 |
| Sex, male | 206 (47.9) | 17 (54.8) | 6 (26.1)* | 7 (50.0) | 0.175 |
| Exposure to Wuhan | 171 (39.8) | 12 (38.7) | 6 (26.1) | 1 (7.1)* | 0.055 |
| Family clusters# | 92 (47.9)* | 13 (72.2) | 8 (88.9) | 5 (55.6) | 0.018 |
| Comorbidity | 86 (20.0) | 4 (12.9) | 7 (30.4) | 2 (14.3) | 0.424 |
| Influenza A or B# | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA |
| Cardiovascular disease | 17 (4.0) | 2 (6.5) | 1 (4.3) | 0 (0) | 0.790 |
| Diabetes mellitus | 37 (8.6) | 0 (0) | 4 (17.4) | 0 (0) | 0.020 |
| Hypertension | 63 (14.7) | 4 (12.9) | 9 (39.1)* | 1 (7.1) | 0.032 |
| COPD | 12 (2.8) | 1 (3.2) | 0 (0) | 0 (0) | 0.374 |
| Chronic liver disease | 14 (3.3) | 0 (0) | 0 (0) | 0 (0) | 0.173 |
| Chronic kidney disease | 2 (0.5) | 0 (0) | 0 (0) | 0 (0) | 0.611 |
| Malignancy | 4 (0.9) | 0 (0) | 1 (4.3) | 1 (7.1) | 0.028 |
| Cerebrovascular disease | 9 (2.1) | 0 (0) | 0 (0) | 0 (0) | 0.278 |
| Rheumatic disease | 3 (0.7) | 0 (0) | 0 (0) | 0 (0) | 0.533 |
| Signs and symptoms at admission | |||||
| Temperature at admission, °C | 36.8 (36.5–37.3)* | 36.7 (36.3–37.0) | 36.6 (36.5–37.0) | 36.6 (36.4–36.9) | 0.021 |
| Respiratory rate | 20.0 (20.0–20.0)* | 20.0 (20.0–20.0) | 20.0 (20.0–20.0) | 20.0 (18.0–20.0) | 0.042 |
| Fever | 312 (72.6) | 18 (58.1) | 0 (0) | 0 (0) | <0.001 |
| Cough | 297 (69.1)** | 14 (45.2) | 0 (0) | 0 (0) | <0.001 |
| Expectoration | 178 (41.4)** | 7 (22.6) | 0 (0) | 0 (0) | <0.001 |
| Dyspnea | 36 (8.4) | 0 (0) | 0 (0) | 0 (0) | 0.012 |
| Fatigue | 156 (36.3) | 8 (25.8) | 0 (0) | 0 (0) | <0.001 |
| Muscle soreness | 56 (13.0) | 5 (16.1) | 0 (0) | 0 (0) | 0.016 |
| Headache | 34 (7.9) | 1 (3.2) | 0 (0) | 0 (0) | 0.053 |
Values are presented as median (IQR) or number (percentage).
p < 0.05 compared with other groups combined,
p < 0.05 compared with symptomatic COVID-19 with pneumonia cases.
Data were only analyzed using cases from Changsha (n = 228); p-values were compared by Kruskal–Wallis test, χ2 test, Fisher exact test, or one-way analysis of variance.
COPD, chronic obstructive pulmonary disease; NA, not available.
Moreover, we found that the proportion of patients reporting an exposure history to Wuhan was significantly lower in the asymptomatic without pneumonia group (7.1%) than in the other groups (Table 1), implicating that asymptomatic patients without pneumonia were more likely to be secondary cases than index cases. We have only collected family cluster history in 228 COVID-19 patients from the Public Health Treatment Center of Changsha. The results showed that symptomatic COVID-19 patients with pneumonia were less likely to be family cluster cases (47.9%, Table 1) than their counterparts.
As expected, the symptomatic with pneumonia group had a higher rate of symptoms, including a higher body temperature at admission (°C) (36.8 [IQR, 36.5–37.3]) and respiratory rate at admission (rate per min) (20 [IQR, 20–20]), cough (69.1%), and expectoration (41.4%) than the other groups (Table 1).
Laboratory and Radiographic Findings
Routine blood tests showed a higher white blood cell count (× 109 per L) (6.5 [IQR, 4.7–7.5]) and lymphocyte count (× 109 per L) (2.4 [IQR, 1.7–3.0]) in the asymptomatic without pneumonia group than in the other groups (Table 2). In contrast, the symptomatic with pneumonia group had a higher frequency of lymphocytopenia (37.7%) and lower platelet count (× 109 per L) (187.5 [IQR, 147.0–246.0]) than the other groups (Table 2). However, there were no differences in hemoglobin levels among groups.
Table 2.
Laboratory findings in symptomatic and asymptomatic COVID-19 cases.
| Symptomatic COVID-19 | Asymptomatic COVID-19 | ||||
|---|---|---|---|---|---|
| Items | With pneumonia (n = 430) | Without pneumonia (n = 31) | With pneumonia (n = 23) | Without pneumonia (n = 14) | p-value |
| White blood cell count, × 109/L | 4.6 (3.6–5.8)* | 5.7 (4.0–6.8) | 5.7 (4.8–7.8)* | 6.5 (4.7–7.5)* | 0.001 |
| <4 × 109/L | 148 (34.4)* | 9 (29.0) | 3 (13.0)* | 3 (21.4) | 0.095 |
| >10 × 109/L | 10 (2.3) | 1 (3.2) | 1 (4.3) | 2 (14.3) | 0.021 |
| Neutrophil count, × 109/L | 2.9 (2.2–3.8) | 3.3 (2.2–3.8) | 3.6 (3.0–4.5)* | 3.4 (2.1–4.6) | 0.167 |
| Lymphocyte count, × 109/L | 1.2 (0.8–1.6)* | 1.8 (1.3–2.7)* | 1.5 (1.3–2.0)* | 2.4 (1.7–3.0)* | <0.001 |
| Lymphocytopenia | 162 (37.7)* | 4 (12.9)* | 3 (13.0)* | 3 (21.4) | 0.001 |
| Hemoglobin, g/L# | 130.0 (119.0–140.0) | 129.0 (119.8–147.8) | 133.0 (127.0–151.0) | 125.0 (119.0–139.5) | 0.431 |
| Platelet, × 109/L | 187.5 (147.0–246.0)* | 221.0 (163.5–246.0) | 238.0 (217.0–305.0)* | 232.5 (187.8–261.0) | 0.002 |
| Alanine aminotransferase, U/L | 21.3 (15.0–30.4)* | 17.7 (11.7–24.7)* | 20.5 (16.3–23.4) | 16.7 (15.1–45.1) | 0.139 |
| Aspartate aminotransferase, U/L | 23.4 (18.8–31.0) | 23.3 (18.7–28.0) | 20.8 (17.8–24.9)* | 24.1 (18.3–33.4) | 0.249 |
| Total bilirubin, mmol/L | 11.0 (8.1–17.0) | 9.6 (6.6–12.6) | 10.7 (9.0–19.5) | 11.2 (8.3–13.5) | 0.296 |
| Lactose dehydrogenase, U/L | 177.1 (145.6–221.0)* | 155.7 (138.5–173.5)* | 146.2 (133.0–185.0)* | 146.5 (128.0–198.3) | 0.002 |
| Creatinine, μmol/L | 62.0 (49.0–76.0) | 57.0 (38.8–75.6) | 59.8 (49.9–73.0) | 51.2 (29.1–66.1) | 0.280 |
| D-dimer, mg/L | 0.31 (0.18–0.52)* | 0.24 (0.12–0.37) | 0.26 (0.17–0.37) | 0.14 (0.09–0.24)* | 0.009 |
| Prothrombin time, s | 12.0 (11.1–12.7)* | 11.8 (11.1–12.8) | 11.1 (10.3–12.4)* | 11.8 (10.8–12.2) | 0.066 |
| Activated partial thromboplastin time, s | 32.4 (29.2–35.6) | 31.3 (30.3–33.7) | 31.8 (29.6–36.8) | 34.1 (32.0–36.1) | 0.622 |
| Creatine kinase, U/L | 66.9 (43.0–104.9) | 78.0 (58.6–94.7) | 67.1 (57.0–109.0) | 76.2 (50.3–104.9) | 0.736 |
| Erythrocyte sedimentation rate, mm/h# | 44.0 (22.0–67.3)* | 18.0 (11.0–28.5)* | 33.0 (9.5–65.5) | 19.0 (7.0–22.8)* | 0.001 |
| CT value of nucleic SARS-CoV-2 acid test | 33.0 (27.6–35.8) | 33.0 (28.8–36.3) | 32.3 (29.2–36.3) | 32.3 (24.6–35.9) | 0.933 |
Values are presented as median (IQR) or number (percentage).
p < 0.05 compared with other groups combined.
Data were only analyzed using cases from Changsha (n = 228); p values were compared by Kruskal–Wallis test, χ2 test, Fisher exact test, or one-way analysis of variance.
Elevated alanine aminotransferase levels (U per L) and lactose dehydrogenase (U per L) were observed in the symptomatic with pneumonia group compared with the symptomatic without pneumonia group (21.3 [IQR, 15.0–30.4] vs. 17.7 [IQR, 11.7–24.7] and 177.1 [IQR, 145.6–221.0] vs. 155.7 [IQR, 138.5–173.5], respectively) (Table 2). Compared with those in the other groups, the symptomatic with pneumonia group had a significantly higher level of d-dimer (mg per L) (0.31 [IQR, 0.18–0.52]) (Table 2). In contrast, a significantly lower level of d-dimer was observed in the asymptomatic without pneumonia group (mg per L) (0.14 [IQR, 0.09–0.24]) than in the other groups (Table 2).
We also found a higher erythrocyte sedimentation rate (mm per hour) (44.0 [IQR, 22.0–67.3]) and C-reactive protein (CRP) (U per L) (8.6 [IQR, 2.8–24.0]) in symptomatic with pneumonia group than in the other groups, whereas the asymptomatic without pneumonia group had a lower level of CRP (U per L) (1.7 [IQR, 0.3–3.0]) than the other groups (Table 2). Accordingly, among the 228 COVID-19 patients from the Public Health Treatment Center of Changsha, symptomatic patients with COVID-19 with pneumonia were more likely to have procalcitonin levels > 0.05 ng per mL (54, 28.1%) than the others (Table 2).
Antigens of influenza A and B were detected in designed hospitals, but antibodies were not detected. Interestingly, the results showed that there was no patient suffering with influenza A or B during hospitalization.
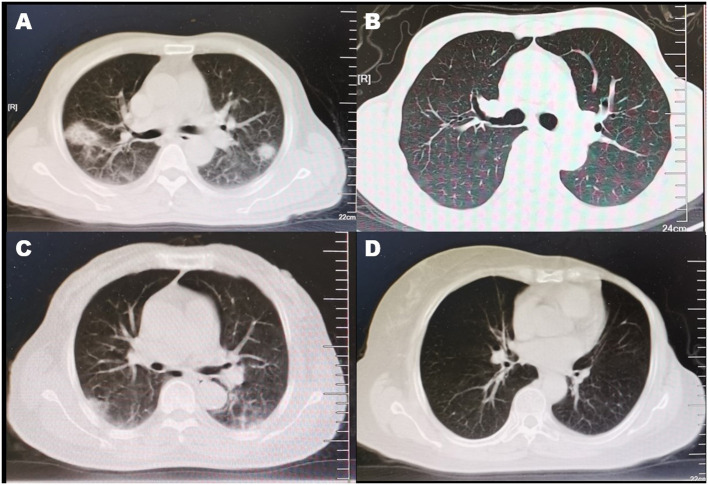
CT scan was conducted repeatedly during the hospitalization. As previously described (11), the CT findings in pneumonia patients were varied, including ground-glass opacity, consolidation, subpleural brand, and so on (Figure 2).
Figure 2.
CT findings of COVID-19 patients. (A) Enlargement of bronchi and vascular and mixed consolidation and GGO were found in this symptomatic COVID-19 with pneumonia patient. (B) There was absence of abnormality in CT scan of this symptomatic COVID-19 without pneumonia patient. (C) Although this asymptomatic pneumonia patient had absence of symptoms, the CT scan found enlargement of bronchi and vascular and subpleural GGO and band. (D) This asymptomatic COVID-19 without pneumonia patient was observed with normal radiographic presentation in CT scan.
Treatments and Outcomes
Excluding 10 patients who were only treated with traditional Chinese medicine in the symptomatic with pneumonia group and 1 patient who was treated with traditional Chinese medicine in the asymptomatic without pneumonia group, 487 patients received antiviral treatment, including arbidol, lopinavir/ritonavir, interferon, ribavirin, and chloroquine phosphate. In total, 254 patients (51.0%) received antibiotics, indicating the presence of secondary or concomitant bacterial infection. The symptomatic with pneumonia group had higher frequencies of antibiotic use (54.2%) and corticosteroid use (28.8) than the other groups (Table 3).
Table 3.
Treatments and outcomes in symptomatic and asymptomatic COVID-19 cases.
| Symptomatic COVID-19 | Asymptomatic COVID-19 | ||||
|---|---|---|---|---|---|
| Items | With pneumonia | Without pneumonia | With pneumonia | Without pneumonia | p-value |
| (n = 430) | (n = 31) | (n = 23) | (n = 14) | ||
| Treatments | |||||
| Antiviral therapy | 420 (97.7) | 31 (100) | 23 (100) | 13 (92.9) | 0.865 |
| Abidol | 200 (46.5) | 16 (51.6) | 14 (60.9) | 6 (42.9) | 0.543 |
| Lopinavir/ritonavir | 310 (72.1) | 24 (77.4) | 10 (43.5)* | 8 (57.1) | 0.022 |
| Interferon | 259 (60.2) | 24 (77.4) | 14 (60.9) | 8 (57.1) | 0.295 |
| Ribavirin | 44 (10.2) | 2 (6.5) | 2 (8.7) | 1 (7.1) | 0.876 |
| Chloroquine phosphate | 53 (12.3) | 2 (6.5) | 4 (17.4) | 2 (14.3) | 0.635 |
| Antibiotic therapy | 233 (54.2)* | 11 (35.5) | 7 (30.4)* | 3 (21.4)* | 0.004 |
| Administration of corticosteroids | 124 (28.8)* | 2 (6.5)* | 0 (0)* | 0 (0)* | <0.001 |
| Non-invasive mechanical ventilation | 18 (4.2) | 0 (0) | 0 (0) | 0 (0) | 0.121 |
| Invasive mechanical ventilation | 11 (2.6) | 0 (0) | 0 (0) | 0 (0) | 0.229 |
| ECMO | 7 (1.6) | 0 (0) | 0 (0) | 0 (0) | 0.339 |
| CRRT | 9 (2.1) | 0 (0) | 0 (0) | 0 (0) | 0.278 |
| Clinical outcomes | |||||
| Severe cases | 61 (14.2)* | 3 (9.7) | 0 (0) | 0 (0) | 0.011 |
| Critical cases (admission to ICU) | 46 (10.7)* | 1 (3.2) | 0 (0) | 0 (0) | 0.019 |
| ARDS | 18 (4.2) | 0 (0) | 0 (0) | 0 (0) | 0.121 |
| Death | 3 (0.7) | 0 | 0 | 0 | 0.533 |
| Duration of viral shedding, days | 14.0 (9.0–21.0) | 15.0 (8.8–23.8) | 13.0 (7.0–19.0) | 13.0 (8.3–17.8) | 0.524 |
| Duration of hospitalization, days | 16.0 (11.5–24.0) | 16.0 (10.0–23.0) | 16.0 (11.0–22.0) | 12.0 (8.8–17.0)* | 0.234 |
Values are presented as median (IQR) or number (percentage).
p < 0.05 compared with other groups combined; p-values were compared by Kruskal–Wallis test, χ2 test, Fisher exact test, or one-way analysis of variance.
ECMO, extracorporeal membrane oxygenation; CRRT, continuous renal replacement therapy; ARDS, acute respiratory distress syndrome; ICU, intensive care unit.
Both non-invasive mechanical ventilation (18, 4.2%) and invasive mechanical ventilation (11, 2.6%) were used in only the symptomatic with pneumonia group (Table 3). Likewise, extracorporeal membrane oxygenation (ECMO) and continuous renal replacement therapy were employed in only the symptomatic with pneumonia group (Table 3). The above results indicated that patients in the symptomatic with pneumonia group suffered from more severe conditions and consumed more medical resources than those in the other groups.
All 64 severe cases occurred in symptomatic COVID-19 patients; 47 of them were critical cases and in ICU, of whom 18 developed ARDS and 3 died. Of the 430 symptomatic with pneumonia patients, 61 (14.2%) had severe COVID-19, and 46 (10.7%) were admitted to the ICU, implying that patients in the symptomatic with pneumonia group were more likely to develop severe COVID-19 and be admitted to the ICU than those in the other groups (Table 3). Interestingly, we did not find differences in the viral load, duration of viral shedding, and hospitalization among the groups (Tables 2, 3).
The follow-up of patients discharged from the Public Health Treatment Center of Changsha is ongoing. Only four of them were re-positive during 2 weeks after discharge, and all of them were from symptomatic COVID-19 with pneumonia. Moreover, continued SARS-CoV-2 RT-PCR tests showed that the contacts of the re-positive patients were not COVID-19 cases. Because the data were limited, the statistical analysis was not conducted in this study.
Discussion
As in Middle East respiratory syndrome patients (15, 16), mounting evidence supports that presymptomatic (17, 18) or asymptomatic COVID-19 patients are infectious (6, 8, 19). In contrast with the amount of research about asymptomatic and symptomatic COVID-19 patients, there is limited research on the differences between COVID-19 patients with and without pneumonia. It is well-acknowledged that pneumonia should be confirmed by radiography findings (14). Therefore, this retrospective study describes the spectrum of COVID-19, including symptomatic COVID-19 with pneumonia, symptomatic COVID-19 without pneumonia, asymptomatic COVID-19 with pneumonia, and asymptomatic COVID-19 without pneumonia, mainly according to symptoms and CT findings. This study observed and identified characteristics and differences among the four groups, suggesting that not only symptoms, but also the presence or absence of pneumonia should be considered when evaluating the spectrum of COVID-19. Unlike some other presymptomatic or incubational cohorts (6, 17, 18), the asymptomatic COVID-19 patients in our study were discharged and monitored or evaluated according to symptoms and laboratory findings by professional healthcare workers during hospitalization, to ensure the absence of symptoms and laboratory and radiologic abnormalities during the whole disease process.
Because senior patients are vulnerable to pneumonia (14), the higher prevalence of pneumonia in the symptomatic group could be explained by the older age of the symptomatic patients. Lower levels of lymphocyte and white blood cells were observed in the symptomatic groups and were associated with better outcomes than those in the asymptomatic groups, implying that lymphocyte and white blood cells might play a protective role in COVID-19. The prevalence of hypertension was higher in the asymptomatic groups than in the symptomatic groups, suggesting that antihypertension treatment, especially ACE inhibitors might be effective for COVID-19.
A number of studies have demonstrated SARS-CoV-2 viral loads in the upper airway (19, 20) and that SARS-CoV-2 entry factors are highly expressed in upper airway cells (10). Moreover, Roman et al. (7) isolated live SARS-CoV-2 from upper airway specimens and found separate genotypes in the upper and lower airways samples. All of the above findings demonstrate that SARS-CoV-2 can infect the upper airway. Therefore, we speculate that COVID-19 without pneumonia might be only the SARS-CoV-2 upper airway infection. As expected, lower prevalence rates of severe cases and pneumonia were found in asymptomatic COVID-19 patients, and asymptomatic patients without pneumonia presented fewer abnormal laboratory findings and a lower risk of developing severe COVID-19 than symptomatic patients. The results suggested that SARS-CoV-2 infection in only the upper airway might be self-limiting, leading to a lack of symptoms and a favorable prognosis. Alba et al. (21) found targeted T-cell responses to SARS-CoV-2 in individuals not exposed to SARS-CoV-2, but possibly exposed to other coronaviruses. Therefore, we speculate that the asymptomatic COVID-19 patients without pneumonia might have been recently infected by other coronaviruses, resulting in the production of T cells targeting SARS-CoV-2, limiting the infected region and attenuating the severity of COVID-19. To save and make effective use of medicine sources, asymptomatic COVID-19 patients without pneumonia should be quarantined in primary hospitals or even Fangcang shelter hospitals (22), rather than tertiary hospitals if possible.
COVID-19 originated in Wuhan in November 2019, and exposure to Wuhan was considered important in the medical history of patients in previous studies (5, 23). The lower frequency of exposure to Wuhan in the asymptomatic without pneumonia group indicated a lower percentage of index cases in this group than in the other groups. Coronaviruses have the potential to mutate, and SARS-CoV-2 has exhibited patient-derived mutations and varying pathogenicity according to subtype (24, 25). Therefore, it is possible that SARS-CoV-2 in secondary patients has mutated, leading to decreased pathogenicity and asymptomatic COVID-19. Because of airborne transmission, family clusters play a role in SARS-CoV-2 spread (26, 27). Awareness of social distancing and personal protection caused by obvious symptoms in family members could explain the lower percentage of family clusters in the symptomatic with pneumonia group than in the other groups. On the other hand, the absence of influenza A or B in these COVID-19 patients also could be explained by the awareness of social distancing and personal protection.
Both the symptoms and laboratory findings in results support that symptomatic COVID-19 patients suffer from more severe disease than asymptomatic COVID-19 patients. A higher rate of ECMO and a higher frequency of ICU admission in the symptomatic with pneumonia group indicated not only the greater consumption of medical resources but also the need of additional monitoring in this group. In contrast, we did not find differences in viral load, viral shedding, and hospitalization durations between the symptomatic and asymptomatic groups as in previous studies (19, 28). The results suggested that asymptomatic COVID-19 might present similar pathogenicity and process of viral shedding as symptomatic COVID-19.
Asymptomatic COVID-19 with pneumonia patients also presented significant characteristics. Compared with the other patients, they are more likely to be male with hypertension and more frequently presented with lymphocytopenia and dysregulation of aminotransferase and prothrombin time. Although the asymptomatic with pneumonia subgroup showed favorable outcomes without ventilation and severe cases, statistical analysis could not find any difference between the only two pneumonia groups (symptomatic vs. asymptomatic). The limited sample size of asymptomatic with pneumonia might be a reason, and further study with larger asymptomatic with pneumonia cases is needed.
Because of a lack of significant clinical symptoms, using symptoms to screen for asymptomatic COVID-19 is difficult (17, 29, 30). In China, the Center for Disease Control and Prevention conducted SARS-CoV-2 PCR testing for all close contacts of COVID-19 patients (1). Because of the strict and comprehensive screening strategy, the prevalence of asymptomatic cases seems to be higher in our study than in other studies (17). As asymptomatic COVID-19 patients can be transmission sources (7–9), and our results showed that 7.4% of COVID-19 patients were asymptomatic, a more comprehensive screening strategy for COVID-19 is urgently needed. On the other hand, follow-up showed that there were four re-positive patients, and they were all from the symptomatic with pneumonia group. Because the data were limited, there is a need to collect more re-positive cases to discuss characteristics.
There are some limitations to this study. Because almost all the patients accepted antiviral treatment, it was possible that some presymptomatic COVID-19 patients did not develop symptomatic disease and were thus classified in the asymptomatic group. However, effective antiviral drugs targeting COVID-19 have not been identified thus far (31). Therefore, we considered the confusion of presymptomatic and asymptomatic COVID-19 patients under professional monitor during hospitalization might be rare. Because of the limited sample size and a lack of reliable evidences about Chinese traditional treatment against COVID-19, the authors did not exclude or analyze the Chinese traditional medicine–treated samples separately. Moreover, to assess the mutations and the infectivity of SARS-CoV-2, viral sequencing should be conducted in future studies.
Conclusion
This spectrum of COVID-19 includes symptomatic COVID-19 with pneumonia, symptomatic COVID-19 without pneumonia, asymptomatic COVID-19 with pneumonia, and asymptomatic COVID-19 without pneumonia. The symptomatic with pneumonia group consumed more medical resources than the other groups, and extra caution of monitoring should be applied in this group. Asymptomatic COVID-19 case presented a similar viral load and viral shedding duration as symptomatic COVID-19 case.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the institutional ethics committee of The Second Xiangya Hospital (2020-010). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
Every author contributed to reviewing the paper. HZ performed, designed the work, and drafted the manuscript. YM performed the statistical analyses. ZZ, WL, PH, MJ, and QL treated and monitored COVID-19 cases. HL and PC inspired the research questions and helped to draft the manuscript. YC was the principal investigator of the study and supervised the study and preparation of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was supported by the National Key Clinical Specialist Construction Programs of China; the National Natural Science Foundation of China 81400032; the Natural Science Foundation of Hunan Province 2019JJ50877; and Emergency Project of Prevention and Control for COVID-19 of Central South University 160260009.
References
- 1.Organization WH . Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). Geneva: WHO; (2020). [Google Scholar]
- 2.Organization WH . Coronavirus Disease 2019 (COVID-19): Situation Report, 76. Geneva. (2020). [Google Scholar]
- 3.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. (2020) 382:1787–99. 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 32:1702–20. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishiura H, Kobayashi T, Miyama T, Suzuki A, Jung S, Hayashi K, et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19). medRxiv. (2020). 10.1101/2020.02.03.20020248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehmann KZ, Drosten C, Wendtner C, Zange M, Vollmar P, Rosina Ehmann D, et al. Virological assessment of hospitalized cases of coronavirus disease 2019. Nature. (2020) 581:465–9. 10.1038/s41586-020-2196-x [DOI] [PubMed] [Google Scholar]
- 7.Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. (2020) 382:970–1. 10.1056/NEJMc2001468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai Y, Yao L, Wei T, Tian F, Jin D-Y, Chen L, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. (2020) 323:1406–7. 10.1001/jama.2020.2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. (2020) 26:1–7. 10.1038/s41591-020-0868-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. (2020) 382:727–33. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. (2020) 296:200343. 10.1148/radiol.2020200343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avanzato VA, Matson MJ, Seifert SN, Pryce R, Williamson BN, Anzick SL, et al. Case study: prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell. (2020) 183:1901–12. e9. 10.1016/j.cell.2020.10.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou R, Li F, Chen F, Liu H, Zheng J, Lei C, et al. Viral dynamics in asymptomatic patients with COVID-19. Int J Infect Dis. (2020) 96:288–90. 10.1016/j.ijid.2020.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American thoracic society and infectious diseases society of America. Am J Respirat Crit Care Med. (2019) 200:e45–67. 10.1164/rccm.201908-1581ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hui DS, Azhar EI, Kim Y-J, Memish ZA, Oh M-d, Zumla A. Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect Dis. (2018) 18:e217–27. 10.1016/S1473-3099(18)30127-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Ahmadi K, Alahmadi M, Al-Zahrani A. Spatial association between primary Middle East respiratory syndrome coronavirus infection and exposure to dromedary camels in Saudi Arabia. Zoo Public Health. (2020) 67:382–90. 10.1111/zph.12697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arons MM, Hatfield KM, Reddy SC, Kimball A, James A, Jacobs JR, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. (2020) 382:2081–90. 10.1056/NEJMoa2008457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu P, Zhu J, Zhang Z, Han Y. A familial cluster of infection associated with the 2019 novel coronavirus indicating possible person-to-person transmission during the incubation period. J Infect Dis. (2020) 221:1757–61. 10.1093/infdis/jiaa077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. (2020) 382:1177–9. 10.1056/NEJMc2001737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng PK, Wong DA, Tong LK, Ip S-M, Lo AC, Lau C-S, et al. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet. (2004) 363:1699–700. 10.1016/S0140-6736(04)16255-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. (2020) 181:1489–501. 10.1016/j.cell.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen S, Zhang Z, Yang J, Wang J, Zhai X, Bärnighausen T, et al. Fangcang shelter hospitals: a novel concept for responding to public health emergencies. Lancet. (2020) 395:1305–14. 10.1016/S0140-6736(20)30744-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. (2020) 382:1199–207. 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao H-P, Lu X, Chen Q, Xu K, Chen Y, Cheng L, et al. Patient-derived mutations impact pathogenicity of SARS-CoV-2. medRxiv. (2020). 10.2139/ssrn.3578153 [DOI] [Google Scholar]
- 25.Tang X, Wu C, Li X, Song Y, Yao X, Wu X, et al. On the origin and continuing evolution of SARS-CoV-2. Natl Sci Rev. (2020) nwaa036. 10.1093/nsr/nwaa036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou X, Li Y, Li T, Zhang W. Follow-up of asymptomatic patients with SARS-CoV-2 infection. Clin Microbiol Infect. (2020) 26:957–9. 10.1016/j.cmi.2020.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan JF-W, Yuan S, Kok K-H, To KK-W, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. (2020) 395:514–23. 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S, Kim T, Lee E, Lee C, Kim H, Rhee H, et al. Clinical course and molecular viral shedding among asymptomatic and symptomatic patients with SARS-CoV-2 infection in a community treatment center in the Republic of Korea. JAMA Inter Med. (2020) 180:1–6. 10.1001/jamainternmed.2020.3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai C-C, Liu YH, Wang C-Y, Wang Y-H, Hsueh S-C, Yen M-Y, et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARSCoV-2): facts and myths. J Microbiol Immunol Infect. (2020) 53:404–12. 10.1016/j.jmii.2020.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. (2020) 323:1824–36. 10.1001/jama.2020.6019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.