Abstract
Previously, a fungus was isolated from a diseased pigeon group clinically suspected of being infected with Candida. The fungus was subsequently identified as Candida glabrata using morphology, physiology, biochemistry, and molecular biology testing methods. In the present study, to determine the controlling effects of Chinese herbal medicine for C. glabrata, the bacteriostatic effects of the ethanol extracts Acorus gramineus, Sophora flavescens, Polygonum hydropiper, Cassia obtusifolia, Pulsatilla chinensis, Dandelion, and Cortex phellodendri on C. glabrata in vitro were analyzed. The results showed that the minimum inhibitory concentrations (MIC80) of Cortex phellodendri was 0.25 μg/μL. Meanwhile, that of S. flavescens was 32 μg/μL; C. obtusifolia was 56 μg/μL; A. gramineus and Polygonum hydropiper was 64 μg/μL; and P. chinensis was 112 μg/μL. However, MIC80 for Dandelion was undetectable. In addition, improved drug sensitivity tests revealed that colonies had grown after 24 h in the blank group, as well as the Polygonum hydropiper, P. chinensis, Dandelion, and ethanol groups. The colonies first appeared at the 48-hour point in the other drug-sensitive medium of Chinese herbal medicine. However, no colony growth was found in Cortex phellodendri medium, and the formation of the maximum colony diameter in that group was later than the blank group (e.g., 96 h in the blank group and 120 h in the Chinese herbal medicine group). It was observed that only 17 colony-forming units had grown in 125 μg/μL of the S. flavescens medium, which was significantly different from other groups. Also, the final colony diameter was significantly smaller than that of the other experimental groups. Therefore, it was determined that the A. gramineus, S. flavescens, Polygonum hydropiper, Cassia obtusifolia, P. chinensis, and Cortex phellodendri had certain inhibitory effects on the growth of the C. glabrata. Among those, it was observed that the Cortex phellodendri had the strongest inhibitory effects, followed by the S. flavescens. In the future, these Chinese herbal medicines are expected to be used to treat the fungal infections related to C. glabrata in poultry to improve production performance.
Key words: Candida glabrata, isolation and identification, Chinese herbal medicine, bacteriostatic effect
Introduction
At the present time, Candida glabrata has gradually developed into the second largest pathogenic Candidiasis, following closely after Candida albicans (Tscherner et al., 2011; Chen et al., 2017; Grisin et al., 2017), which is usually a type of normal flora found in organisms. When the immunity of a body decreases, the fungi will show pathogenicity to the host and can infect a body through the skin, oral cavities, esophagus, and gastrointestinal tract, as well as other parts. Then, with increased infection, once the strain enters the blood system, it will spread to the entire body, causing systemic candidiasis. Consequently, the heart, brain, nerves, bones, and other parts of the body will be negatively affected (Clemons et al., 2006; Medrano-Diaz et al., 2018; Parin et al., 2018). The current prophylactic measures taken on poultry farms mainly focus on biosecurity, cleaning and disinfection, overall management, acidification of feed and drinking water, and vaccination procedures (Peeters et al., 2019). The use of antibiotics has effectively inhibited bacterial and fungal infections and improved the production performance of animals. However, it has been found that with its large-scale and long-term unrestricted use, multiple antibiotic-resistant bacteria strains have begun to emerge (Roth et al., 2019). Microbial drug resistance is a serious global threat to the health and safety of humans, animals, and environments. Therefore, many countries have gradually prohibited the use of antibiotics as feed additives. In addition, strict standards for the banning and restriction of antibiotic usage in economic animal industries have been established (Maron et al., 2013).
In recent years, with the prohibition and restriction of the use of antibiotics in animals, it has been proven difficult to maintain animal health, and production performances have been greatly affected. Therefore, in order to ensure human health and safety and produce pollution-free and drug-free animal products, researchers have conducted major studies regarding antibiotic substitutes. Plant feed additives that are based on Chinese herbal medicine and its extracts, which are mainly composed of volatile and nonvolatile bioactive substances, have consistently been hot topics in the research field of antibiotic substitutes (Sharma et al., 2020). Chinese herbal medicine includes many types of pure and natural, low-toxic, nonresidual, and pollution-free medicinal plants. Chinese herbal medicine may not only contain common sugars, lipids, and amino acids but also alkaloids, vitamins, flavonoids, and volatile oils. In previous study, the results of Chinese herbal remedies have shown considerable efficacy in enhancing memory and appetite, relieving coughs and asthma systems, and lowering cholesterol levels, as well as displaying antitumor, antioxidation, antivirus, anti-inflammation, and bacteriostasis potential (Cai et al., 2015; Liao and Lin, 2015; Xu et al., 2016; Dai et al., 2018; Fu et al., 2018; Huang et al., 2018; Qian et al., 2018). Studies have shown that Chinese herbal medicines are ideal substitutes for hormones and antibiotics and can be effectively used to develop feed additives. In this study, on the basis of the results of previous related research investigations, 7 Chinese herbs were selected to study their antibacterial effects on clinically isolated avian source C. glabrata through in vitro experiments. These included Acorus gramineus, Sophora flavescens, Polygonum hydropiper, Cassia obtusifolia, Pulsatilla chinensis, Dandelion, and Cortex phellodendri.
Materials and methods
The methods used in this study were approved by the Experimental Animal Ethics Committee of Hebei Agricultural University.
Isolation of the C. glabrata
In the present study, suspected infected pigeons were obtained from Ningxiang Livestock and Poultry Clinic in Baoding (Hebei, China). The pigeons were observed to have white pseudo-membrane or white round protuberant ulcer formations in their mucous membrane, which were similar to candidiasis. The exudate from the crop mucous membrane of the pigeons was obtained using aseptic cotton swabs and placed in 10 mL of aseptic saline. After the dilution process, 100 μL was evenly coated on Sabouraud (SDA) medium and incubated at 37°C for 48 h. The morphological characteristics of the colony samples were then observed, and single colonies were selected. The streaking and culturing processes were continued until a pure culture was obtained. At that point in the experimental process, Gram staining of single colonies was taken, and the shapes of the mycelia and spores were observed under a microscope. The strains were then frozen in glycerol tubes for later use.
Morphological Identification
The preserved bacteria were resuscitated and prepared into 1 × 101 colony-forming units (CFU)/mL bacterial suspension, and 100 μL was evenly smeared on Yeast Extract Peptone Dextrose Medium; TTC-Sabourand Medium; Candida Chromogenic Medium; and 1% Tween80-Corn Meat Agar Medium, respectively, and cultured upside down at 37°C for 48 h. Then, the colony morphology and Gram staining were observed microscopically.
Physiological and Biochemical Identification
The resuscitation strain was prepared into 1 × 106 CFU/mL bacteria suspension, and 100 μL was inoculated in glucose, sucrose, maltose, lactose fermentation tubes, glucose, maltose, sucrose, lactose, galactose, honey disaccharide, raffinose, inositol, xylose, trehalose, and cellubitol assimilation tubes, respectively, at 37°C for 24 h.
Molecular Biological Identifications
The total DNA of the bacterial solution was extracted in accordance with the operation procedures provided in this study's fungal genomic DNA extraction kit (courtesy of OMEGR). The quality of the obtained DNA was detected using a 1% agarose gel electrophoresis method. Fungal universal internal transcribed spacer (ITS) sequence primers (ITS1 5′-TCCGTAGGTGAACCTGCGG-3′) and (ITS2 3′-GCTGCGTTCTTCATCGATGC-5′) were adopted. This study used a PCR reaction system (total system: 25 μL) which included a 12.5-μL KAPA 2G Robust Hot Start Ready Mix; 1 μL forward primer; 1 μL reverse primer; and 5 μL DNA. Finally, 5.5 μL of ddH2O was added to 25 μL. First of all, the samples were predenatured at 95°C for 5 min; then subjected to 35 cycles; denatured at 95°C for 45 s; annealed at 55°C for 50 s; extended at 72°C for 45 s; and finally stored at 4°C for future use. The purified PCR product was sequenced using an Applied Biosystems 3730-xl sequencer. The sequence results were then processed using a web-based blasting program and a Basic Local Alignment Search Tool (BLAST) (http://www.ncbi.nlm.nih.gov/BLAST). The data were compared with those listed in the NCBI/Genebank database, and MEGA7.0 software (Sudhir Kumar, Arizona State University, Phoenix, AZ) was used to build the evolutionary tree.
Animal Infection Tests
For this study's experimental animal tests, 30 healthy four-day-old Roman gray chickens were provided by Dingnong Corporation of Hebei (Baoding, China), and the adopted methods were approved by the Animal Ethics Committee of Hebei Agricultural University. The chickens were raised, managed, and dissected according to the national legislation on animal welfare protection. The experimental animals were randomly divided into 2 groups: group A and group B. Group A was fed 0.5 mL (1 × 107 CFU/mL) of bacterial suspension each day, and group B was fed 0.5 mL of saline each day. The mental states and deaths of the chickens were observed at all times. Then, after 7 d of feeding, the pathogenic symptoms of the groups were observed, and the pathogens were isolated and purified.
Preparation of the Chinese Herbal Medicine
In this research investigation, S. flavescens, A. gramineus, Polygonum hydropiper, Cassia obtusifolia, P. chinensis, Dandelion, and Cortex phellodendri samples were purchased from the Anguo Oriental Medicine City (Hebei, China). First of all, 50-g Chinese herbal medicine samples were soaked in 8 times their volume of 60% ethanol overnight. Then, 60% ethanol solutions were added to further increase the volumes by 10 times (Yi et al., 2006; Tang et al., 2011; Fang et al., 2016; Han et al., 2018; Xiang et al., 2019). Subsequently, after the designed experimental steps, an ultrasonic instrument with a temperature level of 40°C was applied 3 times, with each application being 15 min in length. An ultrasonic extraction method was used to extract the desired liquid, and then vacuum filtration was applied. Any drug residue was discarded, and the samples were subjected to 3,000 r/min centrifugation for 10 min. When the centrifugation process was completed, the precipitation supernatant was placed into a rotary evaporator and steamed at 80°C to obtain a 1.5-g/mL concentration level. After sterilization was performed, the prepared solution was packed into 4-mL centrifuge tubes and stored at a refrigeration temperature of −20°C.
Susceptibility Tests of the Chinese Herbal Medicine In Vitro
In the present study, the MIC of the Chinese herbal medicine was determined according to the M27-E4 standard (Reference method for the broth dilution antifungal susceptibility testing of yeast: Approved standard; Fourth Edition) compiled by the Clinical and Laboratory Standards Institute. In this study, the Chinese herbal medicine (1.5 g/mL) was diluted to 1,024 μg/μL, 512 μg/μL, 256 μg/μL, 128 μg/μL, 64 μg/μL, 32 μg/μL, 16 μg/μL, 8 μg/μL, and 4 μg/μL samples, respectively, by RPMI1640 medium using a double dilution method. Then, 180 μL of the RPMI1640 medium (including the Chinese herbal medicine) was placed into a 96-well plate, and each concentration was set to 3 repetitions. In addition, 100 μL of the RPMI1640 medium was added to both positive control and negative control holes. In the same manner, a 60% ethanol group was set up. The activated colony on the SDA medium was made into 1 × 103–5 × 103 CFU/mL bacterial suspensions using RPMI1640 medium. All the test wells were receiving 100 μL of bacteria suspensions which were expected to be the negative control wells that received 100 μL of RPMI1640 medium, making the final concentrations of each hole of the Chinese herbal medicine 512 μg/μL, 256 μg/μL, 128 μg/μL, 64 μg/μL, 32 μg/μL, 16 μg/μL, 8 μg/μL, and 4 μg/μL, respectively. Then, 96-well plate was placed into a 37°C incubator for 48 h. A microplate reader was used to measure the OD600 values of each well. It was observed that when compared with the negative control, the drug concentration with an OD value of more than 80% was MIC80. The aforementioned experimental process was repeated 3 times.
Drug Sensitivity Tests for the Chinese Herbal Medicine
From the 1.5 g/mL of Chinese herbal medicine, 1 mL was added to 11 mL of SDA medium to prepare a Chinese medicine medium plate with a concentration of 125 μg/μL. Then, a 64-μg/μL Chinese herbal medicine medium plate and 60% ethanol medium plate were prepared using the same method. During the experimental process, 101 CFU/mL bacterial suspension was prepared using normal saline, and 100 μL of the bacterial suspension was evenly spread on the plates of ordinary SDA medium containing different concentrations of Chinese herbal medicine, as well as those without Chinese herbal medicine. The samples were then cultured at 37°C. The colony growth numbers were observed at 24, 48, 72, 96, 120, and 144 h after inoculation, respectively, and the colony diameters were carefully measured.
Statistical Analysis Results
In the present study, all the results were expressed as means ± SEM. Statistical analysis was performed using the SigmaPlot 2000 software (SPSS Inc., Chicago, IL). All the data were analyzed using a one-way analysis of variance method to determine the differences among the groups. In this study, the groups were considered to be significantly different at P < 0.05 and extremely significant at P < 0.01.
Results
Morphology of the Pathogenic Fungi
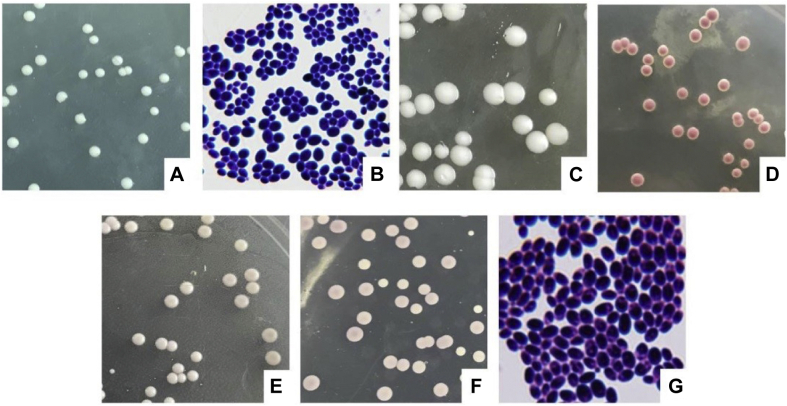
The pathogen isolated from the diseased pigeon group was cultured in SDA medium at 37°C for 48 h. It was observed that the culture grew well and has a yeast-like odor. The colonies had the following characteristics: cream colored; moist; cheese-like; protruding in the middle; neat edges; smooth surfaces; no mycelium growth; colony diameters ranging from 5 to 6 mm (Figure 1A); Gram staining blue-purple; oval spores detected under a microscope; and no hyphae and other invasive structures observable (Figure 1B). The colony morphology in the Yeast Extract Peptone Dextrose medium was found to be similar to that observed in the SDA medium, in which slightly larger, cheese-like, cream-colored, protruding in the middle, neat edges, smooth surfaces, and diameters ranging between 8 and 9 mm had formed (Figure 1C). The TTC-Sabourand medium produced colonies characterized with deep red coloration, moistness, protruding middles, neat edges, smooth surfaces, and no mycelium growth (Figure 1D). In addition, in the Candida chromogenic medium, the colonies were white in color (Figures 1E and 1F). Also, the positive gram staining process was uneven, and the cells were round or oval. There was no mycelium growth found in the 1% Tween 80-corn Agar medium, and gram positive oval sporozoites were identified under a Gram staining microscope. However, posterior wall spores had not been produced (Figure 1G). In addition, there was no germ tube formation after incubation with serum at 37°C for 3 h.
Figure 1.
Colony morphology. (A) Colony morphology of the SDA. (B) Gram staining characteristics (10 μm). (C) Colony morphology of the YEPD. (D) Colony morphology of the TTC-SDA. (E) Positive colony morphology of the Candida chromogenic culture medium. (F) Back of the colony morphology of the Candida chromogenic culture medium. (G) Characteristics of the GRAM staining in the 1% Tween 80-Corn Agar medium (10 μm).
Physiological and Biochemical Characteristics of the Pathogenic Fungi
Physiological and biochemical test tube cultures were examined for 24 h. The results showed that glucose and maltose could produce acid, but not gas. Also, although the lactose and sucrose culture tubes could produce acid yet not gas, it was found that glucose, maltose, trehalose, and cellubitol could not be assimilated. Furthermore, sucrose, lactose, galactose, maltose, maltose, inositol, and xylose also could not be assimilated.
Molecular Identifications
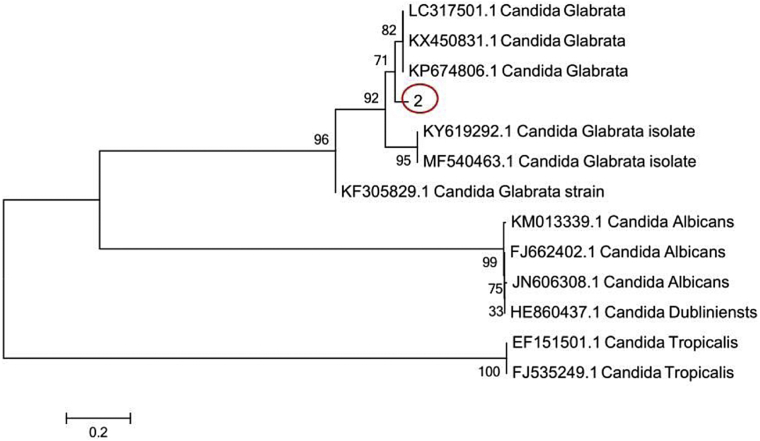
The DNA sequences of the 48-hour cultured samples in the SDA medium were sequenced and compared using the BLAST software of the NCBI website. The homology with sequence numbers MK300697.1, MH973205.1, LC534389.1, LS398122.1, MN699325.1, KX450831.1, LC317501.1, and KP674806.1 had reached 100%. In this study, C. glabrata MF540463.1, KY619292.1, KF305829.1; Candida dubliniensis HE860437.1; Candida albicans JN606308.1 and FJ662402; and Candida tropical EF151501.1 and FJ53249.1 were selected to draw the evolutionary tree using MEGA7.0 software, as shown in Figure 2. The clinical isolates were in the same evolutionary tree branch as the KX450831.1, LC317501.1, and KP674806.1, which belonged to C. glabrata.
Figure 2.
Phylogenetic tree of the Candida glabrata strains. Note: In the figure, “2” indicates the isolated strain obtained from the diseased pigeon group.
Animal Infection Tests
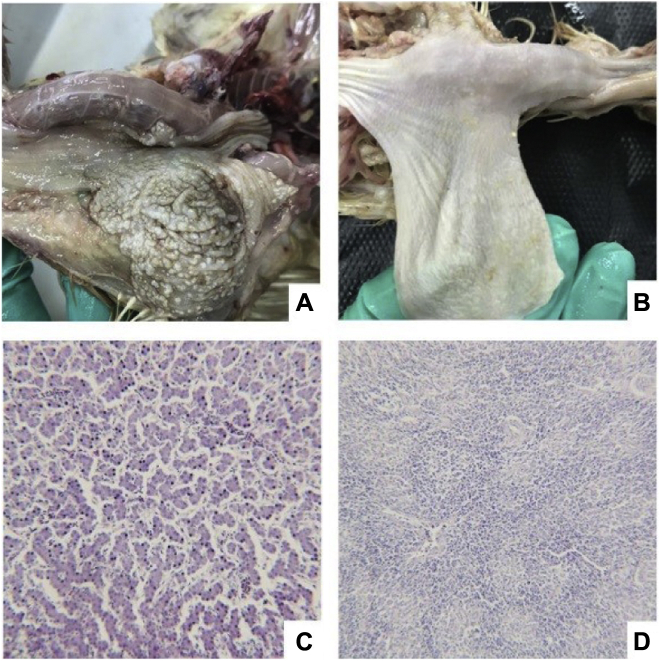
On the seventh day of the infection process, the animal groups displayed hyperplasia and hypertrophy of the stratified flat epithelium; obvious hyperkeratosis of the cuticles; white pseudo-membrane or white round protruding ulcers on the surfaces of mucous membrane; and folds in lesions resembling towels, as detailed in Figure 3A. However, no obvious pathological changes were found in the stomach, liver, lung, spleen, or thymus tissue, and no spores and hyphae were found in the blood smears and liver and lung contact specimens. The exudate was isolated and cultured at 37°C for a period of 48 h. The colonies were observed to be milky white in color, with raised and smooth surfaces and neat edges. The Gram staining results were blue-purple, and oval spores could be seen under microscope, with no hyphae and other invasive structures. The aforementioned white colonies in the Candida chromogenic medium, as well as the physiological, biochemical, and molecular biological characteristics, were determined to be consistent with those of the infected strains.
Figure 3.
Experimental animal groups. (A) Diseased group. (B) Healthy group. (C) Histopathological change of liver (100 μm). (D) Histopathological change of spleen (100 μm).
Susceptibility of the Chinese Herbal Medicines to C. glabrata
The results of this study's MIC80 determinations of the C. glabrata using Chinese herbal medicine extracts are shown in Table 1. It was found that, with the exception of Dandelion having no inhibitory effects on the C. glabrata, as the concentration levels of the Chinese herbal medicine were increased, the growth rates of C. glabrata had decelerated, and the values of the OD600 decreased. The inhibitory effects of Cortex phellodendri on C. glabrata were the strongest among the 7 types of tested Chinese herbal medicine. It was observed that when the concentration of Cortex phellodendri was more than 0.25 μg/μL; the concentration of S. flavescens was greater than 32 μg/μL; the concentration of Cassia obtusifolia was greater than 56 μg/μL; the concentrations of A. gramineus and Polygonum hydropiper were more than 64 μg/μL; and the concentration of P. chinensis was greater than 112 μg/μL, the OD600 values had decreased by more than 80% when compared with the negative control well. In addition, 60% ethanol at 128 μg/μL also was observed to have inhibitory effects on the growth rate of the C. glabrata.
Table 1.
MIC80 of the different groups (n = 3).
| Groups | Control (alcohol) | Acorus gramineus | Sophora flavescens | Polygonum hydropiper | Cassia obtusifolia | Pulsatilla chinensis | Dandelion | Cortex phellodendri |
|---|---|---|---|---|---|---|---|---|
| MIC80 (μg/μl) | 128 | 64 | 32 | 64 | 56 | 112 | 0 | 0.25 |
Note: MIC80 means comparing with the negative control, the lowest drug concentration with an OD value declining more than 80%.
Inhibitory Effects of Chinese Herbal Medicine on the Growth Rates of C. glabrata
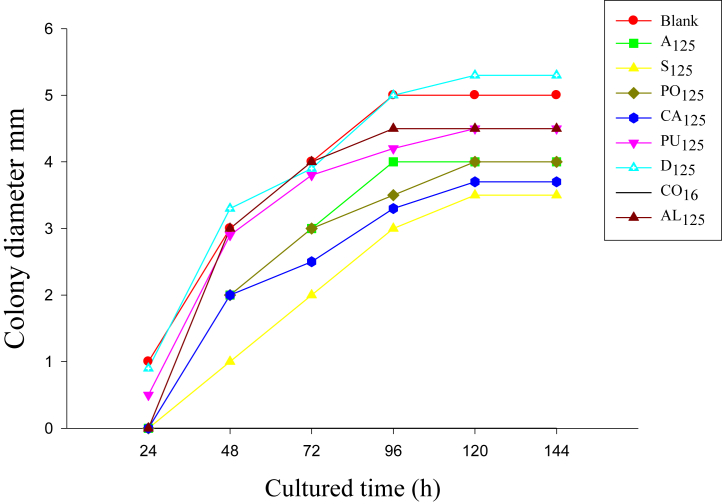
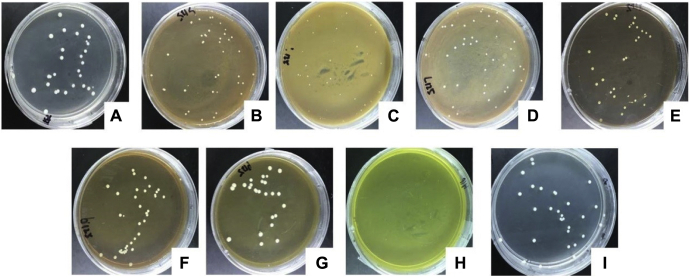
The colony growth rates were observed at 24, 48, 72, 96, 120, and 144 h after infection, during which time different concentrations of Chinese herbal medicine were added to the solid medium. Figure 4 displays the colony morphology in the solid medium after 72 h of culturing. After 24 h of culturing, it was observed that colonies had grown in the blank group; Polygonum hydropiper; Cassia obtusifolia 64 μg/μL; P. chinensis; and Dandelion 64 μg/μL and 125 μg/μL groups. The colony diameters of each Chinese herbal medicine group were smaller than those of the blank group and ethanol control group, with the exception of the Dandelion group. The colonies first appeared at the 48-hour point in the other drug-sensitive medium of the Chinese herbal medicine. When compared with the blank group, the maximum colony diameter had occurred later (96 h in the blank group compared with 120 h in the Chinese herbal medicine group). It was found that when the S. flavescens were at a concentration of 125 μg/μL, only 17 CFU colonies had grown in the culture medium, which was significantly different from the other groups. In addition, there was no colony growth observed in the Cortex phellodendri drug-sensitive medium, as shown in Table 2. The effects of the different concentrations of the Chinese herbal medicine on the growth rates of the C. glabrata are shown in Figure 5. The colony diameters were found to gradually increase over time, but the maximum colony diameter of each Chinese herbal medicine treatment group was found to be lower than that of the blank group and ethanol group. The colony diameter of S. flavescens was only 3.5 mm, which was significantly smaller than that of the other groups (4.5 mm–5 mm). It was found in this study that the addition of Chinese herbal medicine had certain inhibitory effects on the growth rates of the C. glabrata. In this study's comparison of the 7 types of Chinese herbal medicine, it was determined that the Cortex phellodendri had achieved the best antibacterial effects. Furthermore, the inhibitory effects of the S. flavescens were slightly stronger than those of the A. gramineus, Polygonum hydropiper, while Cassia obtusifolia and P. chinensis were observed to achieve the weakest antibacterial effects. Generally speaking, no inhibitory effects on the C. glabrata were observed for the Dandelion.
Figure 4.
Diameters of the colonies on solid medium with different Chinese herbal medicines cultured for various lengths of time.
Table 2.
Effects of the Chinese herbal medicine on the colony growth numbers under different concentration conditions (n = 3).
| Concentration (μg/μL) | Colony no. (24 h) | Colony no. (48 h) | Colony no. (96 h) |
|---|---|---|---|
| Blank | 34.3 ± 0.5 | ||
| A64 | no | 28.3 ± 0.9∗ | |
| A125 | no | 27.5 ± 1.3∗ | |
| S64 | no | 29.0 ± 1.0 | |
| S125 | no | 17.3 ± 1.3∗∗ | |
| PO64 | 27.3 ± 1.8∗ | ||
| PO125 | no | 24.0 ± 1.7∗ | |
| CA64 | 26.3 ± 0.6∗ | ||
| CA125 | no | 25.0 ± 1.2∗ | |
| PU64 | 30.1 ± 0.3 | ||
| PU125 | 29.2 ± 1.1 | ||
| D64 | 30.3 ± 0.8 | ||
| D125 | 31.4 ± 0.2 | ||
| CO16 | no | no | no |
| CO32 | no | no | no |
| CO64 | no | no | no |
| CO125 | no | no | no |
| AL64 | 29.3 ± 1.5 | ||
| AL125 | 30.0 ± 0.8 |
The lower corner is marked with concentration, A single asterisks (∗) indicates statistical significance (P < 0.05), and “∗∗” indicates P < 0.01.
Abbreviations: A, Acorus gramineus; AL, alcohol; CA, Cassia obtusifolia; CO, Cortex phellodendri; D, Dandelion; PO, Polygonum hydropiper; PU, Pulsatilla chinensis; S, Sophora flavescens.
Figure 5.
Colony growth rates on a solid medium with different types of herbal medicine for 72 h. (A) Blank control (solid medium with no herbal medicine). (B) A125 (solid medium with Acorus gramineus at 125 μg/μL). (C) S125 (solid medium with Sophora flavescens at 125 μg/μL). (D) PO125 (solid medium with Polygonum hydropiper at 125 μg/μL). (E) CA125 (solid medium with Cassia obtusifolia at 125 μg/μL). (F) PU125 (solid medium with Pulsatilla chinensis at 125 μg/μL). (G) D125 (solid medium with Dandelion at 125 μg/μL). (H) CO16 (solid medium with Cortex phellodendri at 16 μg/μL). (I) AL125 (solid medium with alcohol at 125 μg/μL).
Discussion
C. albicans and C. glabrata are considered to be the main infections in clinical candidiasis. However, there are known differences between the 2 strains in regard to invasion processes, pathogenicity characteristics, virulence factors, and so on. First of all, in terms of the transformation from yeast to hyphae, 2 forms of C. albicans and hyphae have been found in infected sites, and which form was dominant was determined according to the different infected organs (Lionakis et al., 2011). It was believed that the morphology of the yeast was important for the transmission of the infection, and the morphology of the hyphae was more closely related to attachment, host invasion, and tissue damage (Saville et al., 2003). However, unlike C. albicans, there have been some reports that C. glabrata may form pseudo-hyphae (Csank and Haynes, 2000; Sasani et al., 2016). However, the pathogenicity of C. glabrata appears to be unrelated to morphology, and there may be other ways for C. albicans to invade a body. Second, C. albicans can secrete a large number of tissue destructive factor hydrolase, phospholipase, and other substances. However, the yields of C. glabrata tend to be very low or even zero (Rossoni et al., 2013; Bassyouni et al., 2015; Riceto et al., 2015; Fatahinia et al., 2017; Canela et al., 2018). C. glabrata is known to be famous for its long-lasting survival in macrophages because of its high inherent stress tolerance during pathogenicity. It has been observed that by allowing itself to be absorbed by macrophages, C. glabrata can survive and divide within the macrophages for long periods of time, eventually resulting in cell lysis due to fungal load (Seider et al., 2011; Dementhon et al., 2012). In contrast, C. albicans actively escapes host immunity through mycelial growth and phagocytic cell penetration. During fungal pathogenicity processes, adhesion has been considered to be an important virulence factor. In previous related studies, during the adhesion processes mediated by the Van der Waals research team, the cell surface water absorption of C. glabrata was found to be positively correlated with adhesion. Furthermore, when compared with C. albicans, the relative cell surface water absorption of C. glabrata was observed to be significantly higher than that of C. albicans (Luo and Samaranayake, 2002; Blanco et al., 2010). Differences have been found between C. albicans and C. glabrata from the aspects of structure, cell morphology, enzyme production and secretion, responses to host resistance, and resistance to antifungal drugs. Finally, it was observed that there were very significant differences between the mature biofilm of the C. albicans and C. glabrata. For example, the thicknesses of the biofilm formations in the C. glabrata ranged between 75 and 90 ± 5 μm. These were half the thickness of the normal C. albicans biofilm. In addition, when compared with the C. albicans, it was found that there was much less biomass at the ends of the biofilm formations. The biofilm of the C. albicans was arranged in three-dimensional structures with different shapes and gaps between the cells, in which microchannels had formed. In contrast, the biofilm of the C. glabrata was observed to be thinner. However, the cell density was higher, and the cells were more closely accumulated (Seneviratne et al., 2009; Silva et al., 2009; Kucharkov et al., 2015). The characteristics of the biofilm enabled the fungi to escape disinfection and sterilization, as well as resist the therapeutic effects of antifungal drugs. Therefore, it should be considered in the identifications of fungal infections that it is very important to accurately determine the species of Candida infection, which can provide accurate guidance for antimicrobial therapy in the later stages. In this study, the strains were identified according to the traditional gold standards of pure culture morphology, physiology, biochemistry, molecular biology, and so on (Sun et al., 2014). The accuracy of this identification method was found to be high. Therefore, this study's test results provided important references for the future diagnosis of C. albicans and a basis for the further prevention and treatment of the disease. In recent years, some methods based on multiple PCR and other techniques for the rapid detection of Candida species in blood had been developed. However, there are still some limitations in sensitivity and specificity (Lau et al., 2010; Taira et al., 2014), Therefore, the determination of new and rapid identification methods for strain identification processes may take the lead in the treatment of fungal infections, which will potentially reduce animal mortality and improve production efficiency.
It has been found that adding additives to animal feed to inhibit the growth or germicidal effects of bacteria can effectively alleviate infections related to pathogenic bacteria and protect the health of animals. In recent years, probiotics, amino acids, chitosan, nanoparticles, activated carbon, and plant extracts have been gradually used as feed additives to improve resistance levels to infections caused by pathogenic microorganisms. Good inhibitory effects have been demonstrated on Bacillus, Escherichia coli, Salmonella, Clostridium perfringens, Campylobacter, Coccidia, and other pathogens (Abdel-Hafeez et al., 2017; Hong et al., 2018; Huneau-Salaun et al., 2018; Adegbeye et al., 2019; Burchacka et al., 2019; Mortada et al., 2020). In particular, plant extracts have been widely used in feed additive research studies because of their natural material content and their functions of promoting growth, as well as antibacterial, antioxidation, and anti-inflammation properties. They have also been observed to stimulate digestive and immune system functions, as well as increase animal production performances, by increasing the production of digestive enzymes or improving feed utilization efficiency by enhancing liver function. It has been found that such extracts as garlic, ginseng, primrose, rose, licorice, aloe, and green tea showed effective inhibitory effects on viruses, bacteria, and fungi (Ayyat et al., 2018; Elghandour et al., 2018; Kolling et al., 2018; Liu et al., 2018).
This study was based on the results of previous research investigations regarding the bacteriostatic and anti-inflammatory effects of Chinese herbal medicine. In this study's experimental processes, A. gramineus, S. flavescens, Polygonum hydropiper, Cassia obtusifolia, P. chinensis, Dandelion, and Cortex phellodendri were selected. There effective components were extracted using an ultrasonic alcohol extraction method, which greatly improved the effects of Chinese herbal medicine. It was determined through bacteriostatic tests in vitro that the Chinese herbal medicine which were the most sensitive and effective toward C. glabrata could be successfully screened. The results showed that Cortex phellodendri had displayed the strongest antibacterial activities against C. glabrata in vitro when the MIC80 was 0.25 μg/μL. In addition, it was determined that S. flavescens also showed good antibacterial effects when the MIC80 was 32 μg/μL. The main components in the Cortex phellodendri and S. flavescens were alkaloids and flavonoids. The Cortex phellodendri mainly contained original berberine-type alkaloids, pseudoberberine-type alkaloids, aporphine alkaloids, indole-type alkaloid, and quinoline-type alkaloids (Xiang et al., 2016; Cai et al., 2017). In addition, flavonoids such as citrates and dehydro-citrates isolated from the Cortex phellodendri had displayed strong antioxidant and antibacterial activities. Previous related studies have revealed that citrates have antibacterial effects on streptococcus aureus, E. coli, and so on (Zhu et al., 2009; Li and Xiao, 2018). Forty-one types of alkaloids have been identified, such as sophorine and matrine in S. flavescens, as well as hundreds of flavonoids, including isoflavones, dihydro-flavonoids, and dihydro-flavonols (Wang et al., 2014; Zhu et al., 2018). It has been found that S. flavescens alkaloids have the ability to inhibit the proliferation of gram-negative and gram-positive bacteria to varying degrees. Also, flavonoids have been observed to have certain inhibitory effects on Staphylococcus aureus and E. coli (Cao et al., 2020). In the present study, both Cortex phellodendri and S. flavescens displayed strong inhibitory effects on C. glabrata, which may be the main role of alkaloids and flavonoids. However, which components play major roles have yet to be determined and require further exploration. This study did find that inhibitory effects of Dandelion on C. glabrata could not be found in the determination of MIC80 using microdilution methods. In summary, drug-sensitive medium with Chinese herbal medicine additions showed the clear inhibitory effects on C. glabrata for 7 types of Chinese herbal medicine. It was found that after 24 h of culturing, colonies could be found in the blank group; Polygonum hydropiper 64 μg/μL; P. chinensis; Dandelion; and ethanol groups. However, the colonies were smaller than those of the control group and the ethanol control group. No colony growth was found in the other media. With the exception of the Dandelion, not only were the colony numbers significantly different from those of the blank group but also the colony growth rates and final colony diameters were significantly different. It was observed that the C. glabrata did not grow in the Cortex phellodendri drug-sensitive medium. Also, in the 125-μg/μL drug-sensitive medium of the S. flavescens, only 17 CFU colonies had grown (compared with the blank group's 34 CFU), which indicated obvious inhibitory effects on the C. glabrata. In particular, the medium supplemented with Chinese herbal medicine during the first 72 h of growth indicated strong inhibitory effects on the growth rates of the C. glabrata. Therefore, it was concluded that the Chinese herbal medicine extracts examined in this study, which were demonstrated to sensitive and effective toward C. glabrata, could be considered as poultry feed additives for the prevention of future C. glabrata infections. However, although these Chinese herbal medicines had a certain inhibitory effect on C. glabrata, they could not effectively kill the fungi, when compared with drugs such as fluconazole, amphotericin B, caspofungin, nystatin, and other drugs. This may have been related to the complexity of the components of the Chinese herbal medicine and the low concentrations of the active components. Therefore, it was considered in this study that perhaps the multicomponent active components of the Chinese herbal medicine played roles which were less likely to cause fungal drug resistance. During the course of this study's experiments, only the components of Chinese herbal medicine were extracted. The components were not further separated or purified to better analyze the antifungal properties of the Chinese herbal medicine. Therefore, in future investigations, it will be necessary to further analyze and study the different components of Chinese herbal medicine to develop new antifungal veterinary drugs and feed additives with no pollution or residue, low toxicity, and high effectiveness. The goals of such efforts would be to protect the development of poultry industry, as well as to guard human health and safety.
Conclusions
In this study, it was found that A. gramineus, S. flavescens, Polygonum hydropiper, Cassia obtusifolia, P. chinensis, and Cortex phellodendri had all displayed inhibition effects on the growth of C. glabrata to certain extents. Among those, Cortex phellodendri was confirmed to have displayed the strongest bacteriostatic effects in vitro. This was followed by the S. flavescens, A. gramineus, Polygonum hydropiper, Cassia obtusifolia, and P. chinensis. However, this study was not able to determine if Dandelion had any inhibition effects on the fungi in question. Therefore, based on the findings of this study, the aforementioned herbal extracts are expected to be used as veterinary drugs or feed additives against C. glabrata, with the goal of preventing future C. glabrata infections.
Acknowledgements
This project was supported by the Fund for Scientific Research and Development of the Agricultural University of Hebei, project no. JY2018005.
Disclosures
The authors declare that there were no conflicts of interest regarding the publication of this article.
References
- Abdel-Hafeez H.M., Saleh E.S.E., Tawfeek S.S., Youssef I.M.I., Abdel-Daim A.S.A. Effects of probiotic, prebiotic, and synbiotic with and without feed restriction on performance, hematological indices and carcass characteristics of broiler chickens. Asian-Australas. J. Anim. Sci. 2017;30:672–682. doi: 10.5713/ajas.16.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adegbeye M.J., Elghandour M.M.M.Y., Barbabosa-Pliego A., Monroy J.C., Mellado M., Reddy P.R.K., Salem A.Z.M. Nanoparticles in Equine nutrition: mechanism of action and application as feed additives. J. Equine Vet. Sci. 2019;78:29–37. doi: 10.1016/j.jevs.2019.04.001. [DOI] [PubMed] [Google Scholar]
- Ayyat M.S., Ayyat A.M.N., Al-Sagheer A.A., El-Hais A.E.M. Effect of some safe feed additives on growth performance, blood biochemistry, and bioaccumulation of aflatoxin residues of Nile tilapia fed aflatoxin-B1 contaminated diet. Aquacult. 2018;495:27–34. [Google Scholar]
- Bassyouni R.H., Wegdan A.A., Abdelmoneim A., Said W., AboElnaga F. Phospholipase and aspartyl proteinase activities of candida species causing vulvovaginal candidiasis in patients with type 2 diabetes mellitus. J. Microbiol. Biotechnol. 2015;25:1734–1741. doi: 10.4014/jmb.1504.04009. [DOI] [PubMed] [Google Scholar]
- Blanco M.T., Sacristan B., Lucio L., Blanco J., Perez-Giraldo C., Gomez-Garcia A.C. Cell surface hydrophobicity as an indicator of other virulence factors in Candida albicans. Rev. Iberoam. Micol. 2010;27:195–199. doi: 10.1016/j.riam.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Burchacka E., Łukaszewicz M., Kułażyński M. Determination of mechanisms of action of active carbons as a feed additive. Bioorg. Chem. 2019;93:102804–102810. doi: 10.1016/j.bioorg.2019.02.029. [DOI] [PubMed] [Google Scholar]
- Cai W., Guan Y., Zhou Y., Wang Y., Ji H., Liu Z. Detection and characterization of the metabolites of rutaecarpine in rats based on ultra-high-performance liquid chromatography with linear ion trap-orbitrap mass spectrometer. Pharm. Biol. 2017;55:294–298. doi: 10.1080/13880209.2016.1236392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J.P., Liu L.L., To K.K.W., Lau C.C.Y., Woo P.C.Y., Lau S.K., Guo Y.H., Ngan A.H.Y., Che X.Y., Yuen K.Y. Characterization of the antigenicity of Cpl1, a surface protein of Cryptococcus neoformans var. neoformans. Mycologia. 2015;107:39–45. doi: 10.3852/14-074. [DOI] [PubMed] [Google Scholar]
- Canela H.M.S., Cardoso B., Vitali L.H., Coelho H.C., Martinez R., Ferreira M.E.D.S. Prevalence, virulence factors and antifungal susceptibility of Candida spp. isolated from bloodstream infections in a tertiary care hospital in Brazil. Mycoses. 2018;61:11–21. doi: 10.1111/myc.12695. [DOI] [PubMed] [Google Scholar]
- Cao Y.Q., Wei D.D., Zhang S., Zeng F., Guo S., Guo J.M., Duan J.A. Bacteriostasis of prenylated flavonoids from Sophora Flavescens and Flavonoids from glycyrrhiza uralensis alone and their combination on staphylococcus aureus and experimental mastitis. J. Nanjing Univ. Tradit. Chin. Med. 2020;3:331–338. (in chinese) [Google Scholar]
- Chen S.M., Shen H., Zhang T., Huang X., Liu X.Q., Guo S.Y., Zhao J.J., Wang C.F., Yan L., Xu G.T., Jiang Y.Y., An M.M. Dectin-1 plays an important role in host defense against systemic Candida glabrata infection. Virulence. 2017;8:1643–1656. doi: 10.1080/21505594.2017.1346756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemons K.V., Espiritu M., Parmar R., Stevens D.A. Assessment of the paradoxical effect of caspofungin in therapy of candidiasis. Antimicrob. Agents Chemother. 2006;50:1293–1297. doi: 10.1128/AAC.50.4.1293-1297.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csank C., Haynes K. Candida glabrata displays pseudohyphal growth. FEMS Microbiol. Lett. 2000;189:115–120. doi: 10.1111/j.1574-6968.2000.tb09216.x. [DOI] [PubMed] [Google Scholar]
- Dai Z., Wang L., Wang X., Zhao B., Zhao W., Bhardwaj S.S., Ye J., Yin Z., Zhang J., Zhao S. Oxymatrine induces cell cycle arrest and apoptosis and suppresses the invasion of human glioblastoma cells through the EGFR/PI3K/Akt/mTOR signaling pathway and STAT3. Oncol. Rep. 2018;40:867–876. doi: 10.3892/or.2018.6512. [DOI] [PubMed] [Google Scholar]
- Dementhon K., El-Kirat-Chatel S., Noël T. Development of an in vitro model for the multi-parametric quantification of the cellular interactions between Candida yeasts and phagocytes. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032621. e32621-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elghandour M.M.M.Y., Kanth Reddy P.R., Salem A.Z.M., Ranga Reddy P.P., Hyder I., Barbabosa-Pliego A., Yasaswini D. Plant bioactives and extracts as feed additives in horse nutrition. J. Equine Vet. Sci. 2018;69:66–77. [Google Scholar]
- Fang K., Chen C., Gao R., Wang S., Zhang Y., Zhang Q., Yan B. Ultrasonic extraction of total flavones from sophora flavescentis l. Shandong J. Tradit. Chin. Med. 2016;35:989–991. (in chinese) [Google Scholar]
- Fatahinia M., Halvaeezadeh M., Rezaei-Matehkolaei A. Comparison of enzymatic activities in different Candida species isolated from women with vulvovaginitis. J. Mycol. Med. 2017;27:188–194. doi: 10.1016/j.mycmed.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Fu Y., Wu H.Q., Cui H.L., Li Y.Y., Li C.Z. Gastroprotective and anti-ulcer effects of oxymatrine against several gastric ulcer models in rats: Possible roles of antioxidant, antiinflammatory, and prosurvival mechanisms. Phytother. Res. 2018;32:2047–2058. doi: 10.1002/ptr.6148. [DOI] [PubMed] [Google Scholar]
- Grisin T., Bories C., Loiseau P.M., Bouchemal K. Cyclodextrin-mediated self-associating chitosan micro-platelets act as a drug booster against Candida glabrata mucosal infection in immunocompetent mice. Int. J. Pharm. 2017;19:381–389. doi: 10.1016/j.ijpharm.2017.01.048. [DOI] [PubMed] [Google Scholar]
- Han G., Gao Z., Gao E. Progress in extraction and purification of alkaloids from sophora flavescens, China. Mod. Food Sci. Technol. 2018;5:163–166. (in chinese) [Google Scholar]
- Hong S.W., Kim J.H., Bae H.J., Ham J.S., Yoo J.G., Chung K.S., Oh M.H. Selection and characterization of broad-spectrum antibacterial substance-producing Lactobacillus curvatus PA40 as a potential probiotic for feed additives. Anim. Sci. J. 2018;89:1459–1467. doi: 10.1111/asj.13047. [DOI] [PubMed] [Google Scholar]
- Huang Y., Zhang J., Wang G., Chen X., Zhang R., Liu H., Zhu J. Oxymatrine exhibits anti-tumor activity in gastric cancer through inhibition of IL-21R-mediated JAK2/STAT3 pathway. Int. J. Immunopathol. Pharmacol. 2018;32:1–10. doi: 10.1177/2058738418781634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huneau-Salaun A., Guyard-Nicodeme M., Benzoni G., Gautier X., Quesne S., Poezevara T., Chemaly M. Randomized control trial to test the effect of a feed additive on Campylobacter contamination in commercial broiler flocks up to slaughter. Zoonoses Public Health. 2018;65:404–411. doi: 10.1111/zph.12447. [DOI] [PubMed] [Google Scholar]
- Kolling G.J., Stivanin S.C.B., Gabbi A.M., Machado F.S., Ferreira A.L., Campos M.M., Tomich T.R., Cunha C.S., Dill S.W., Pereira L.G.R., Fischer V. Performance and methane emissions in dairy cows fed oregano and green tea extracts as feed additives. J. Dairy Sci. 2018;101:4221–4234. doi: 10.3168/jds.2017-13841. [DOI] [PubMed] [Google Scholar]
- Kucharkov S., Neirinck B., Sharma N., Vleugels J., Lagrou K., Van Dijck P. In vivo Candida glabrata biofilm development on foreign bodies in a rat subcutaneous model. J. Antimicrob. Chemother. 2015;70:846–856. doi: 10.1093/jac/dku447. [DOI] [PubMed] [Google Scholar]
- Lau A., Halliday C., Chen S.C., Geoffrey Playford E., Stanley K., Sorrell T.C. Comparison of whole blood, serum and plasma for early detection of Candidemia by Multiplex-Tandem PCR. J. Clin. Microbiol. 2010;48:811–816. doi: 10.1128/JCM.01650-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.X., Xiao H.L. Study on the anti-inflammatory and antibacterial effects of Cortex Phellodendron Decoction. Chin. Med. Dis. Edu. 2018;16:90–91. (in chinese) [Google Scholar]
- Liao Y., Lin J. Quercetin intraperitoneal administration ameliorates lipopolysaccharide-induced systemic inflammation in mice. Life Sci. 2015;137:89–97. doi: 10.1016/j.lfs.2015.07.015. [DOI] [PubMed] [Google Scholar]
- Lionakis M.S., Lim J.K., Lee C.R., Murphy P.M. Organ-specific innate immune responses in a mouse model of invasive candidiasis. J. Innate Immun. 2011;13:180–199. doi: 10.1159/000321157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Espinosa C.D., Abelilla J.J., Casas G.A., Lagos L.V., Lee S.A., Kwon W.B., Mathai J.K., Navarro D., Jaworski N.W., Stein H.H. Non-antibiotic feed additives in diets for pigs: a review. Anim. Nutr. 2018;4:113–125. doi: 10.1016/j.aninu.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G., Samaranayake L.P. Candida glabrata, an emerging fungal pathogen, exhibits superior relative cell surface hydrophobicity and adhesion to denture acrylic surfaces compared with Candida albicans. APMIS. 2002;110:601–610. doi: 10.1034/j.1600-0463.2002.1100902.x. [DOI] [PubMed] [Google Scholar]
- Maron D.F., Smith T.J.S., Nachman K.E. Restrictions on antimicrobial use in food animal production: an international regulatory and economic survey. Glob. Health. 2013;9:48–55. doi: 10.1186/1744-8603-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrano-Diaz C.L., Vega-González A., Ruiz-Baca E., Moreno A., Cuéllar-Cruz M. Moonlighting proteins induce protection in a mouse model against Candida species. Microb. Pathog. 2018;124:21–29. doi: 10.1016/j.micpath.2018.08.024. [DOI] [PubMed] [Google Scholar]
- Mortada M., Cosby D.E., Shanmugasundaram R., Selvaraj R.K. In vivo and in vitro assessment of commercial probiotic and organic acid feed additives in broilers challenged with Campylobacter coli. J. Appl. Poult. Res. 2020;29:435–446. [Google Scholar]
- Parin U., Erbas G., Kirkan S., Savasan S., Tugba Yuksel H., Balat G. Detection of Candida species by nested PCR method in one-humped camels (Camelus dromedarius) Trop. Anim. Health Prod. 2018;50:421–425. doi: 10.1007/s11250-017-1452-z. [DOI] [PubMed] [Google Scholar]
- Peeters L., Mostin L., Wattiau P., Boyen F., Dewulf J., Maes D. Efficacy of Clostridium butyricum as probiotic feed additive against experimental Salmonella Typhimurium infection in pigs. Livest. Sci. 2019;221:82–85. [Google Scholar]
- Qian L., Li X., Ye P., Wang G., Dai W., Liu Y., Gao Q., Shen G. Oxymatrine induces apoptosis and inhibits invasion in Gallbladder carcinoma via PTEN/PI3K/AKT pathway. Cytotechnology. 2018;70:83–94. doi: 10.1007/s10616-017-0153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riceto É.B.D.M., Menezes R.D.P., Penatti M.P.A., Pedroso R.D.S. Enzymatic and hemolytic activity in different Candida species. Rev. Iberoam. Micol. 2015;32:79–82. doi: 10.1016/j.riam.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Rossoni R.D., Barbosa J.O., Vilela S.F.G., Santos J.D.D., Jorge A.O.C., Junqueira J.C. Correlation of phospholipase and proteinase production of Candida with in vivo pathogenicity in Galleria mellonella. Braz. J. Oral Sci. 2013;12:199–204. [Google Scholar]
- Roth N., Hofacre C., Zitz U., Mathis G.F., Moder K., Doupovec B., Berghouse R., Domig K.J. Prevalence of antibiotic-resistant E. coli in broilers challenged with a multi-resistant E. coli strain and received ampicillin, an organic acid-based feed additive or a synbiotic preparation. Poult. Sci. 2019;98:2598–2607. doi: 10.3382/ps/pez004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasani E., Khodavaisy S., Afshari S.A.K., Darabian S., Aala F., Rezaie S. Pseudohyphae formation in Candida glabrata due to CO2 exposure. Curr. Med. Mycol. 2016;2:49–52. doi: 10.18869/acadpub.cmm.2.4.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saville S.P., Lazzell A.L., Monteagudo C., Lopez-Ribot J.L. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot. Cell. 2003;2:1053–1060. doi: 10.1128/EC.2.5.1053-1060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seider K., Brunke S., Schild L., Jablonowski N., Wilson D., Majer O., Barz D., Haas A., Kuchler K., Schaller M., Hube B. The facultative intracellular pathogen Candida glabrata subverts macrophage cytokine production and phagolysosome maturation. J. Immunol. 2011;187:3072–3086. doi: 10.4049/jimmunol.1003730. [DOI] [PubMed] [Google Scholar]
- Seneviratne C.J., Silva W.J., Jin L.J., Samaranayake Y.H., Samaranayake L.P. Architectural analysis, viability assessment and growth kinetics of Candida albicans and Candida glabrata biofilms. Arch. Oral Biol. 2009;54:1052–1060. doi: 10.1016/j.archoralbio.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Sharma M.K., Dinh T., Adhikari P.A. Production performance, egg quality, and small intestine histomorphology of the laying hens supplemented with phytogenic feed additive. J. Appl. Poult. Res. 2020;29:362–371. [Google Scholar]
- Silva S., Henriques M., Martins A., Oliveira R., Williams D., Azeredo J. Biofilms of non-Candida albicans Candida species: quantification, structure and matrix composition. Med. Mycol. 2009;47:681–689. doi: 10.3109/13693780802549594. [DOI] [PubMed] [Google Scholar]
- Sun Z., Liu W., Bao Q., Zhang J., Hou Q., Kwok L., Sun T., Zhang H. Investigation of bacterial and fungal diversity in tarag using high-throughput sequencing. J. Dairy Sci. 2014;97:6085–6096. doi: 10.3168/jds.2014-8360. [DOI] [PubMed] [Google Scholar]
- Taira C.L., Okay T.S., Delgado A.F., Ceccon M.E.J.R., Almeida M.T.G.D., Negro G.M.B.D. A multiplex nested PCR for the detection and identification of Candida species in blood samples of critically ill paediatric patients. BMC Infect. Dis. 2014;14:406–412. doi: 10.1186/1471-2334-14-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J., Chen S., Liu R., Xu X., Yu X. Ultrasonic extraction of total flavonoids from polygonum polygonum l. Lishizhen Med. Mater. Med. Res. 2011;22:1387–1388. (in chinese) [Google Scholar]
- Tscherner M., Schwarzmüller T., Kuchler K. Pathogenesis and antifungal drug resistance of the human fungal pathogen Candida glabrata. Pharmaceuticals. 2011;4:169–186. [Google Scholar]
- Wang Z.Y., Wang L.L., Zhang J. Advance on Chemical Constituents in Sophora flavescens. Chin J. Exp. Tradit. Med. Form. 2014;20:205–214. (in chinese) [Google Scholar]
- Xiang Q., Hashi Y., Chen Z. Simultaneous detection of eight active components in Radix Tinosporae by ultra high performance liquid chromatography coupled with electrospray tandem mass spectrometry. J. Sep. Sci. 2016;39:2036–2042. doi: 10.1002/jssc.201600042. [DOI] [PubMed] [Google Scholar]
- Xiang Y., He L., He W., Gao B., Yuan M., Luo S., Xu Z., Hong X. Screening and optimization of extraction technology of total flavonoids from polygonum polygonum l. Chin J. Vet. Drug. 2019;53:40–47. (in chinese) [Google Scholar]
- Xu F., Wu H., Zhang K., Lv P., Zheng L., Zhao J. Pro-neurogenic effect of β-asarone on RSC96 Schwann cells in vitro. In vitro cell. Dev. Biol. Anim. 2016;52:278–286. doi: 10.1007/s11626-015-9980-1. [DOI] [PubMed] [Google Scholar]
- Yi J., Li X., Su J., Tan W. Preparation of volatile oil of acorus gramineus β- cyclodextrin inclusion by ultrasonic method. Lishizhen Med. Mater. Med. Res. 2006;10:381–383. (in chinese) [Google Scholar]
- Zhu C., Dong C., Kong Y., Liu L., Wu Q., Yao Y. Microdilution inhibition test of Chinese herbs to assess their effect against clinical strains of Ureaplasma urealyticum in vitro. J. Nanjing Med. Univ. 2009;23:143–145. (in chinese) [Google Scholar]
- Zhu H., Yang Y., Feng Z., Jiang J., Zhang P. Sophoflavanones A and B, two novel prenylated flavanones from the roots of Sophora flavescens. Bioorg. Chem. 2018;79:122–125. doi: 10.1016/j.bioorg.2018.04.019. [DOI] [PubMed] [Google Scholar]