Abstract
Objective
Burn injuries are among the most common accidental health problems worldwide, frequently leading to health and socio-economic challenges. Despite this, no standard protocol for managing burn injuries can overcome the adverse effects of currently used drugs. The present study sets out to develop and evaluate the efficacy of new herbal ointments in providing synergistic anti-inflammatory, antimicrobial, antioxidant, and cell-proliferating activities. It also investigates the high-performance liquid chromatography (HPLC) characterisation of these new herbal ointments.
Method
Three different concentrations of the new herbal ointment, which incorporates extracts of Matricaria aurea flower heads, arial parts of Calendula tripterocarpa, Rosmarinus officinalis leaves, Alkanna tinctoria roots, and myrrh were developed and evaluated. Ointments designed to promote burn-wound healing were prepared and compared with β-sitosterol ointment and silver sulfadiazine cream, as a commercial standards.
Results
According to statistical and histopathological analyses and visual inspections, the new herbal formulas showed faster wound healing, more tolerability, and less toxicity than the commercial standards.
Conclusion
The new herbal ointments, developed in our study, have shown promising results. The formula offers mechanical protection without any release of non-biodegradable particles. It maintains the optimum moisture and pH of the skin, while minimising scar-tissue formation. These advantages, in addition to availability, low costs, and easy handling, may support the use of this new herbal formula as an effective and safe alternative treatment, designed to promote the healing of burn injuries.
Keywords: Alkanna tinctoria, Burn, Calendula tripterocarpa, Matricaria aurea, Myrrh
الملخص
أهداف البحث
الإصابة بالحروق هي واحدة من أكثر المشاكل الصحية شيوعا، التي تحدث بشكل عرضي في جميع أنحاء العالم وتؤدي إلى أضرار صحية واجتماعية واقتصادية كبيرة. وبرغم ذلك فإنه لا يوجد بروتوكول مثالي و معتمد لعلاج الحروق قادر على تجنب الآثار السلبية للعلاجات الحالية. صُمم البحث الحالي لتطوير وتقييم فاعلية وصفة عشبية جديدة على شكل مرهم محب للماء لتوفير أنشطة تآزرية مضادة للالتهابات والميكروبات والأكسدة وتحفز نموالخلايا بالإضافة إلى التحليل الوصفي بجهازكروماتوجرافيا السائل عالي الآداء لهذا المرهم للمساعدة في اختبارات قياس الجودة لهذه الوصفة.
طرق البحث
تم تطوير ثلاثة تراكيز مختلفة من المرهم العشبي الجديد الذي يحتوي على مستخلصات كل من أزهار البابونج الذهبي، والأجزاء العلوية من الحنوة، وأوراق اكليل الجبل، وجذور الخواجوا بالإضافة إلى المرّ. وتم تقييمها كمحفز لشفاء الجروح مقارنة مع مرهم بيتاسيتوستيرول وكريم سلفاديازين الفضة كأدوية تجارية متداولة لعلاج الحروق.
النتائج
وفقا للتحليلات الإحصائية والنسيجية المرضية والفحص البصري، أظهرت الصيغ العشبية الجديدة تأثيرا سريعا في إعادة التئام الجروح، ومزيدا من تحمل الجسم له دون أضرار وأقل سمية مقارنة بتلك الموجودة في الأدوية التجارية.
الاستنتاجات
أظهرت هذه المراهم العشبية نتائج واعدة، حيث أنها قدمت حماية ميكانيكية، ولم تطلق جزيئات غير قابلة للتحلل. كما حافظت على درجات الترطيب والحموضة المثلى للجلد، وقللت من تكوين الندب النسيجية. هذه المزايا بالإضافة إلى وفرة المكونات، والتكلفة المنخفضة، وسهولة التحضير تمثل مجموعة من الخصائص الجيدة التي تدعم استخدام هذه التركيبات العشبية الجديدة كعلاج بديل، فعال وآمن لعلاج الحروق وتحفيز بناء الجلد السليم.
الكلمات الأساسية: الخواجوا, علاج الحروق, الحنوة, البابونج الذهبي, المرّ
Introduction
A burn is defined as skin damage caused by excessive heat sources or chemicals that account for more than 7.1 million fire-related burns per year, according to WHO estimates.1 A burn injury is an inflammatory condition that occurs beneath the stratum corneum of the skin; it can progress to spontaneous healing or deteriorate into further complications, including long-term disability and disfigurement, prolonged hospitalisation, loss of body extremities, and even death. Burns also impose significant socio-economic damages on affected individuals.2, 3, 4, 5, 6 Treatment strategies rely chiefly on the topical application of medicaments designed to enhance wound healing, minimise the body's inflammatory response, and – most importantly – prevent opportunistic infections that are commonly associated with severe wound injuries.7, 8, 9 Although many topical therapies are already available for the treatment of burn injuries, including 1% silver sulfadiazine (SSD), β-sitosterol in moist exposed burn ointment (MEBO), and mafenide acetate 10% cream etc. However, over time, problems associated with these treatments have emerged. For example, there is disturbing evidence that SSD can stimulate inflammatory reactions, provoke allergic reactions, and cause delayed or improper healing and serious cytotoxic activity in host cells.10,11 It has been shown that MEBO treatment is not always dependable, with some wounds becoming worse during the healing process.4 The present study was designed to develop an efficient herbal formula, in the form of a hydrophilic ointment, to achieve all of the criteria of healthy, re-formed skin after a burn accident. Based on a literature review and local folk medicine, a combination of five herbal extracts was chosen to fulfil this objective. These herbal extracts act synergistically to promote healing because they contain anti-inflammatory, antimicrobial, antioxidant, and antiulcerative properties, as well as stimulants for cell regeneration. The herbal extracts were obtained from Matricaria aurea flower heads,12, 13, 14 the arial parts of Calendula tripterocarpa,15, 16, 17 Rosmarinus officinalis leaves,18, 19, 20, 21, 22, 23 Alkanna tinctoria roots,24, 25, 26 and myrrh tears – a dried oleo-gum-resin exudate obtained from the indigenous tree, Commiphora myrrha.27,28 The gum content of myrrh acts as an adsorbent and moisturising agent.29,30 Gum also helps to distribute the phenolic and other hydrophilic content of herbal extracts, improving skin penetration and absorption (hydrophilic or diadermic ointment). It has also been used successfully to treat wounds and ulcers.31, 32, 33 Natural gums swell in the presence of water and form three-dimensional polymer networks, known as gels, which impart hydrophilic properties to the vehicle.34 The hydrophilic ointment base was proposed as a vehicle for this herbal formula to improve exudation humidity, resist ointment erosion, and control the release of active compounds.35 The investigation parameters for evaluating the therapeutic efficacy of this new herbal formulation were chosen to enable a pre- and post-healing assessment of healthy (non-complicated) healing. In addition to evaluating the organoleptic properties of the herbal formulas, the present study has monitored animal behaviour after the application of topical preparations, watched the healing progress, calculated the daily wound contraction, carried out a histopathological analysis of the scald area at the end of the experiment, and conducted an in-vitro antimicrobial susceptibility test of all test and control samples. HPLC analyses of the individual extracts and the herbal ointment extract were also conducted to ensure quality control in studying the herbal formulas.
Materials and Methods
Plant material
The flower heads of M. aurea (Loefl.) Sch.Bip., the arial parts of C. tripterocarpa Rupr., and the leaves of R. officinalis L., were collected in April 2019 from a wild area in Rafha City, the Northern Borders Region, KSA, while A. tinctoria (L.) tausch roots and myrrh were purchased from a local market in Rafha City. The plant samples were identified and voucher specimens were deposited in the department of Phytochemistry and Natural Products, Faculty of Pharmacy, Northern Border University, Rafha, KSA. The fresh plants were air-dried in the shade, finely ground, and then extracted with methanol. They were concentrated and dried under reduced pressure using a rotatory evaporator. The amount (and percentage) of matter extracted from M. aurea, C. tripterocarpa, R. officinalis, and A. tinctoria were 14.08 g, (16.56%); 18.34 g, (19.73%); 36.22 g (22.64%); and 6.56 g (4.54%), respectively. The extracts were kept in the refrigerator until use. No extraction procedure was needed for myrrh because it contains no plant tissues.
Chemicals and apparatus
The following chemicals were purchased from a pharmacy: Ketamine (Tekam® 10 mg/ml, 10 ml vials) produced by Hikma Pharmaceutical Co. in Amman, Jordan; Xylazine (Seton® 2%, 50 ml vials) produced by Laboratorios Calier S.A. Barcelones, 26 (Poligono Industrial El Ramassá) 08520 Les Franqueses del Valles (Barcelona) España/Spain; β-sitosterol 0.25% w/w ointment 30 g (Avomeb®), manufactured by Middle East Pharmaceutical Industries Co., Ltd. (Avalon Pharma), Riyadh-KSA; and silver sulfadiazine 1% w/w cream 50 g (Flamazine®) manufactured by Riyadh Pharma Medical and Cosmetic Products Co. Ltd., Riyadh, KSA. The HPLC apparatus was a Waters® 2545 Quaternary Gradient Module pump, equipped with a Waters® 2998 diode array detector, using Empower™ 3 Software. The detailed procedure used to conduct the HPLC analysis of the ointment preparation and individual plant extracts is discussed in a supplementary file (Suppl. 1).
Experimental animals
The experimental protocol was approved by the Institutional Animal Ethics Committee, under the supervision of the Scientific Research Review Committee, Faculty of Pharmacy, Northern Border University, Certificate No. (PHARM-NBU-2019-1). It was performed in accordance with the guidelines of the National Institutes of Health Principles of Laboratory Animal Care (NIH publication no. 85–23, revised 1985). Male, Wister albino rats weighing 220 ± 10 g were used in this study. The animals were kept and maintained under laboratory conditions in the animal house at the Faculty of Pharmacy, Northern Border University, KSA. Animals were fed with standard laboratory feed and water ad libitum. They were exposed to natural cycles of light and dark.
Preparation of the ointment samples
Three concentrations of the new herbal recipe (300 g each) were prepared in ointment bases, according to the following formulas.
The weighed amount of myrrh tears was finely ground in a porcelain mortar, triturated with the specified amount of Dist H2O to obtain soft yellow emulsion, and then mixed thoroughly with other plant extracts. Liquid paraffin was added and the mixture was triturated thoroughly. White soft paraffin was added gradually and mixed continuously until a homogenous ointment preparation was obtained.
Organoleptic evaluation of herbal ointments
All herbal ointments were visually inspected for appearance, homogeneity, and colour. The ointment preparation was tested for characteristic odour, texture,36 pH value,37 loss on drying,36 and topical sensitivity (i.e. any signs of an allergic reaction).38
Preparation of animal models and the induction of full thickness burns
The animals were divided into 6 groups (6 animals each) as follows: Group I: Control group; these scald burn animals were left without treatment. Group II: This group was treated with a standard drug (silver sulphadiazine cream). Group III: This group was treated with a standard drug (β-sitosterol ointment). Group IV: This test group of animals treated with a new herbal formulation (2.5% ointment) Group V: This test group of animals treated with a new herbal formulation (5% ointment). Group VI: This test group of animals treated with a new herbal formulation (10% ointment). All the animals were anaesthetised using an intraperitoneal injection mixture of Ketamine 50 mg/kg and Xylazine 5 mg/kg. The rats then received 2 ml of saline subcutaneously beneath the dorsum skin to protect their spinal cords. Following this, the hair on their dorsa was shaved off to ensure even burn wounding, and a full-thickness burn (∼10 cm2) was induced in all of the experimental animals, using the hot-water model.6 This model has gained acceptance and is in widespread use. According to some experts, it is the standard method used in animal burn models. Indeed, hot liquids are the most frequent cause of burns in children and elderly people.39 All herbal ointments and commercial standards were applied twice daily.
Gross condition and monitoring wound contraction
The gross condition of the burn wounds was visually inspected daily to determine the time required for scabs to fall and to observe the healing progress of the skin lesion. The re-epithelialisation period was calculated by the number of days required for the scab to fall off the burn-wound surface without leaving a raw wound underneath. This happens because epidermal cells form and migrate beneath the scab, separating it from the wound surface.40 Progressive changes in the wound area were monitored planimetrically. The size of the wound was traced on a transparent piece of paper every 72 h throughout the experiment period. The tracing was then transferred to a 1 mm2 graph sheet, making it possible to measure the wound-surface area. The measured area was then used to calculate the percentage of wound contraction, taking the initial size of the wound as 100% and using the following equation: % of wound contraction = [(WSi-WS)/WSi] x 100, where WSi is the initial wound size and WS is the wound size on a specific day.
Histopathology of the skin tissue
On the 21st day, the animals were euthanised after being anesthetised; skin-tissue samples were collected for a histopathological examination. These tissue samples were fixed on thick hard paper, preserved in a 10% neutral buffered formalin solution, and passed on to the histopathology lab at the Prince Naif Bin Abdul-Aziz Health Research Center, King Saud University. There, the tissue sections were stained with haematoxylin-eosin stain and examined using light microscopy. Digital photomicrographs were captured at representative locations using a digital camera attached to a microscope. Tissue samples were evaluated for the extent of dermal bleedings, epidermal exfoliation and scabbing, inflammatory cell responses, proliferation of fibrous tissues, and regeneration of hairs and sweat glands.
In-vitro antimicrobial activity
The cup plate agar-diffusion method41 was used to compare the antimicrobial activity of the prepared ointments with those of standard preparations for Candida albicans RCMB 005003 (1) ATCC 10231, Methicillin-Resistant Staphylococcus aureus (MRSA), S. aureus ATCC 25923, Bacillus subtilis RCMB 015 (1) NRRL B-543, Pseudomonas aeruginosa ATCC 27853, and Escherichia coli ATCC 25922. Inhibition zones that developed as a consequence of active antimicrobial metabolites were measured after 24 h of bacteria incubation and after 48 h of fungi incubation. Antibiotic gentamycin and ketoconazole were used as the antibacterial and antifungal positive controls, respectively.
Statistical analysis
A statistical analysis was carried out using SPSS Program Version 24. The groups' mean and standard errors were calculated for each dataset. The qualitative data, including the dermatological effect and physical properties of the samples, were reported as descriptive information. A repeated measure analysis was conducted to verify the effect of time within each group, using Wilks's Lambda test. An ANOVA test was carried out to compare the groups within each time point. The P value was considered statistically significant if P < 0.05.
Results
Organoleptic evaluation and quality control study of herbal ointments
The results of the organoleptic and quality-control tests of the herbal ointments are summarised in Table 1, Table 2.
Table 1.
Prescriptions for the pharmaceutical formulation of the new herbal ointments.
| Ingredient | 2.5% ointment (Amount in g) | 5% ointment (Amount in g) | 10% ointment (Amount in g) |
|---|---|---|---|
| M. aurea ext. | 1.5 | 3 | 6 |
| C. tripterocarpa ext. | 1.5 | 3 | 6 |
| R. officinalis ext. | 1.5 | 3 | 6 |
| A. tinctoria ext. | 0.75 | 1.5 | 3 |
| Myrrh (solid tears) | 2.25 | 4.5 | 9 |
| Dist H2O | 10 | 20 | 30 |
| White soft paraffin | 247.5 | 225 | 190 |
| Liquid paraffin | 30 | 30 | 30 |
Table 2.
Results of the organoleptic and quality-control tests of the herbal ointments.
| Evaluation parameters | 2.5% ointment | 5% ointment | 10% ointment |
|---|---|---|---|
| Colour | Light green | Green | Green |
| Odour | Characteristic Rosemary odour | Characteristic Rosemary odour | Characteristic Rosemary odour |
| Consistency | Soft semisolid | Soft semisolid | Soft semisolid |
| Phase separation | Nil | Nil | Nil |
| Texture | Smooth | Smooth | Smooth |
| pH | 6.15 | 6.14 | 6.09 |
| Homogeneity | Homogenous | Homogenous | Homogenous |
| Loss on drying | 3.83% | 7.51% | 11.35% |
| Topical sensitivity test | No allergic reactions | No allergic reactions | No allergic reactions |
Gross examination and Re-epithelialisation
The scald wounds in Group I (untreated group) showed thick, hard, dry, and yellowish-brown-to-dark-brown scabs that were intact from the 2nd day to the 21st day. The scald wounds in the SSD group (Group II) were thick and dry but thinner than those of Group I, with yellowish-brown-to-brown scabs that were intact from the 2nd day to the 13th day. After that, the scabs started to narrow down while the skin was still rough and erythematous. The scald wounds in the β-sitosterol group (Group III) showed thick, dry, and yellowish-brown scabs that partly fell down or dissolved as a result of liquefaction, due to the ointment application leaving an oozing surface. The scabs fell completely after 13 days, while the skin was still rough and erythematous. However, Groups IV, V, and VI had moderately thick scabs after 2–3 days and fell after 13, 10, and 12 days, respectively. The skin colour was generally normal, like non-burned skin. Out of all of the samples, Group V had the best results, with scabs falling rapidly, normal skin colouration, and healing with regenerated hairs, all perfectly attained. The epithelialisation periods (mean ± SE) of Groups I, II, III, IV, V, and VI were >21, 18.5 ± 0.6, 11.9 ± 1.2, 13.4 ± 0.9, 10.6 ± 0.7, and 11.2 ± 1.5 days, respectively.
Scald wound contraction
Table 3 presents the wound-area measurements over a period of 21 days, showing the average percentage of wound contraction among the different groups.
Table 3.
Average wound areas in mm2/rat ± SE and wound-contraction percentages.
| Group | I | II | III | IV | V | VI |
|---|---|---|---|---|---|---|
| Average wound area in mm2/rat ± SE (% contraction) | ||||||
| Day 0 | 1104 ± 8.9 (0.0) | 1053 ± 12.02 (0.0) | 1086 ± 12.73 (0.0) | 1098 ± 18.77 (0.0) | 1072 ± 19.29 (0.0) | 1084 ± 12.99 (0.0) |
| Day 3 | 1097 ± 23.36 (0.63) | 1047 ± 27.11 (0.57) | 1057 ± 29.04 (2.67) | 1045 ± 25.57 (4.83) | 998 ± 32.21 (6.90) | 1025 ± 26.59 (5.44) |
| Day 6 | 992 ± 22.49 (10.15) | 970 ± 22.68 (7.88) | 988 ± 26.43 (9.02) | 887 ± 26.8 (19.22) | 753 ± 17.92 (29.76) | 845 ± 25.88 (22.05) |
| Day 9 | 882 ± 27.84 (20.11) | 841 ± 21 (20.13) | 905 ± 14.56 (16.67) | 832 ± 11.82 (24.23) | 676 ± 14.38 (36.94) | 766 ± 12.15 (29.34) |
| Day 12 | 821 ± 31.24 (25.63) | 743 ± 19.81 (29.44) | 772 ± 14.06 (28.91) | 431 ± 12.15 (60.75) | 361 ± 12.96 (66.32) | 427 ± 12.51 (60.61) |
| Day 15 | 769 ± 41.26 (30.34) | 665 ± 13.81 (36.85) | 623 ± 16.16 (42.63) | 204 ± 9.78 (81.42) | 131 ± 9.34 (87.78) | 218 ± 13.56 (79.89) |
| Day 18 | 749 ± 33.04 (32.16) | 418 ± 16.11 (60.30) | 302 ± 16.44 (72.19) | 99 ± 14.25 (90.98) | 52 ± 12.55 (95.15) | 96 ± 14.99 (91.14) |
| Day 21 | 685 ± 40.43 (37.95) | 215 ± 24.83 (79.58) | 176 ± 22.65 (83.79) | 45 ± 12.02 (95.90) | 18 ± 6 (98.32) | 31 ± 9.7 (97.14) |
In terms of the effect of time within each group, there was a statistically significant difference between each pair of time points with p < 0.001. Comparing the groups in an endpoint observation resulted in a statistically significant difference between all pairs, apart from pairs II & III, IV & V, IV & VI, and V & VI, where the difference was not significant.
Histopathological examination
Group I showed severe exfoliation and scabbing, accompanied by an oozing surface and a large ulcerative area involving mild inflammation and dermal bleeding. These characteristic features indicated incomplete healing of the scald area (Figure 1). Group II showed the granulation stage and incomplete layers of epidermal tissue hypertrophy; however, the stratum corneum, stratum lucidum, and stratum granulosum were partly reformed. Fewer inflammatory cells were observed in Group II than in Group I. The healing process in Group III was better than that in both Groups I and II. An almost continuous layer of hypertrophic epidermal tissues was developed, and the dermal layer showed numerous sweat glands and hair follicles. Groups IV, V, and VI showed better healing properties than the other groups; the epidermal layers were almost continuously and completely regenerated, with all the detailed cellular structures of normal tissue. Numerous sweat glands and hair follicles were scattered throughout the barely inflamed dermal tissue. The best results were observed in Group V, where the normal thickness of the epidermis, dermis, and hypodermis layers, with their special detailed structures, were clearly marked. These results coincided well with the gross examination.
Figure 1.
The histopathology of the skin at the 21st day of treatment, stained with haematoxylin-eosin stain. The skin of the non-treated group (Group I) shows ulceration and oedema, an abundance of mononuclear inflammatory cells, and early epithelialisation and granulated tissue. The skin of Group II shows an incomplete epidermal layer, the presence of oedema, and an abundance of mononuclear inflammatory cells with granulated tissue. The skin of Group III shows and almost complete hypertrophic epidermal layer and the presence of oedema and inflammation. The skin of Groups IV, V, and VI showed healed skin structures and complete epithelialisation; the layers of epidermis and dermis are as normal as unburned skin. Numerous hair follicles and sweat glands can be seen, along with adnexa restoration and extensive fibrosis and collagen tissue within the dermis.
In-vitro antimicrobial activity
The results of the antimicrobial activity test are presented in Table 4. The antimicrobial activity results of the herbal preparations were comparable to those of the silver sulfadiazine cream, especially against the MRSA strain. As expected, the β-sitosterol-based ointment showed no activity because the preparation was free from antimicrobial constituents. However, the antimicrobial-activity results of the control and test samples were lower than those of the standard antimicrobial agents (ketoconazole and gentamycin). These results may be due to the sample formulation, where the release of active compounds from the ointment base (lipophilic) into the agar media (hydrophilic) is very low.
Table 4.
Antimicrobial activities (expressed in mms of inhibition zone) against a range of pathogenic microorganisms.
| Test microorganisms | II | III | IV | V | VI | Control |
|---|---|---|---|---|---|---|
| Candida albicans RCMB 005003 (1) ATCC 10231 | NA | NA | NA | NA | NA | 20 |
| Methicillin-Resistant Staphylococcus aureus (MRSA) | 8 | NA | 8 | 8 | 11 | 15 |
| Staphylococcus aureus ATCC 25923 | 8 | NA | NA | NA | 8 | 24 |
| Bacillus subtilis RCMB 015 (1) NRRL B-543 | 10 | NA | NA | NA | NA | 26 |
| Pseudomonas aeruginosa ATCC 27853 | 13 | NA | NA | NA | 9 | 27 |
| Escherichia coli ATCC 25922 | 11 | NA | NA | NA | NA | 30 |
RCMB: Regional Center for Mycology and Biotechnology.
NA = No significant activity.
HPLC analysis
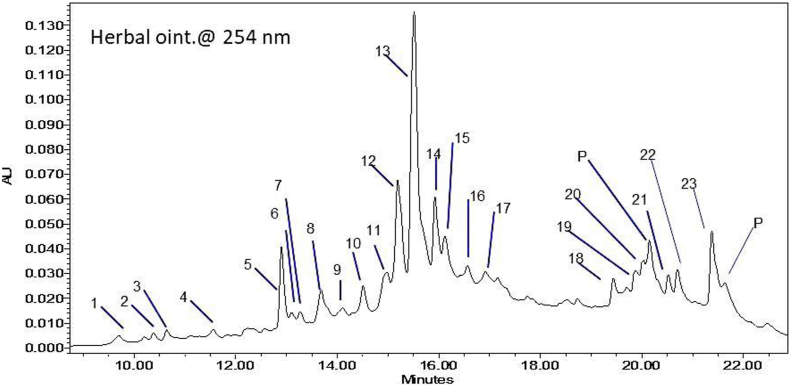
The HPLC analysis of herbal ointment (5% formulation), as well as various plant extracts at 254 nm, shows the characteristic peak-retention time, corresponding to each phytoconstituent in the herbal formula and original extract (see Suppl. 1). However, a magnified part of the HPLC chromatogram of the herbal ointment (Figure 2) shows the peak numbering of the detected phytoconstituents. Table 5 records the peak retention times, corresponding to the present phenolic compounds in the herbal ointment (5% formulation) and their identification in the original plant samples, based on a comparison of retention times and characteristic UV spectra.
Figure 2.
Magnified section of the HPLC chromatogram of the herbal ointment at 254 nm, showing characteristic peaks that correspond to different phytoconstituents.
Table 5.
HPLC characterisation of different phytoconstituents in ointment preparation (5%) and their occurrence in each plant extract.
| Peak no. | R. time | % Area | M. aurea | C. tryp. | Rosemary | Alkanna | Myrrh |
|---|---|---|---|---|---|---|---|
| 1 | 9.696 | 1.3 | ∗ | ∗ | ∗ | ||
| 2 | 10.382 | 0.49 | ∗ | ||||
| 3 | 10.640 | 0.48 | ∗ | ∗ | |||
| 4 | 11.555 | 0.51 | ∗ | ∗ | |||
| 5 | 12.900 | 6.45 | ∗ | ∗ | ∗ | ∗ | |
| 6 | 13.104 | 0.37 | ∗ | ||||
| 7 | 13.270 | 0.72 | ∗ | ||||
| 8 | 13.661 | 3.73 | ∗ | ∗ | ∗ | ||
| 9 | 14.105 | 0.86 | ∗ | ∗ | |||
| 10 | 14.508 | 2.52 | ∗ | ∗ | |||
| 11 | 14.923 | 2.63 | ∗ | ∗ | |||
| 12 | 15.189 | 11.21 | ∗ | ∗ | ∗ | ||
| 13 | 15.515 | 35.49 | ∗ | ∗ | ∗ | ||
| 14 | 15.922 | 4.90 | ∗ | ∗ | ∗ | ||
| 15 | 16.123 | 1.70 | ∗ | ∗ | |||
| 16 | 16.569 | 1.05 | ∗ | ∗ | |||
| 17 | 16.914 | 0.72 | ∗ | ∗ | |||
| 18 | 19.433 | 1.81 | ∗ | ∗ | ∗ | ||
| 19 | 19.876 | 1.91 | ∗ | ∗ | ∗ | ||
| 20 | 20.033 | 2.17 | ∗ | ||||
| P# | 20.144 | 4.50 | ∗ | ∗ | ∗ | ∗ | ∗ |
| 21 | 20.522 | 1.55 | ∗ | ||||
| 22 | 20.701 | 2.49 | ∗ | ∗ | |||
| 23 | 21.376 | 8.52 | ∗ | ||||
| P# | 21.634 | 2.19 | ∗ | ∗ | ∗ | ∗ | ∗ |
∗ = presence of the phytoconstituent in the sample.
P# = phthalates (plasticisers), i.e. solvent impurities.
Discussion
The results reveal that the mean period of epithelialisation decreased significantly in the herbal-ointment-treated groups, in comparison to Groups I, II, and III. The histopathological changes in the skin lesions were significantly less evident in the herbal-ointment groups than in the negative and positive control groups, which exhibited incomplete epithelial-layer formation, neutrophilic infiltration, and perivascular inflammatory changes. By contrast, reduced inflammation and enhanced wound contraction, re-epithelialisation, the regeneration of granulation tissue, angiogenesis, and collagen deposition were detected in the herbal-treated wounds (Figure 1). Based on visual inspections (Figure 3) and statistical and histopathological analyses, Group I showed the slowest improvement among all the groups. The inflammatory reactions and wound contamination were very clear in this group. All the animals in Group II (SSD group) suffered from skin itching and allergic dermatitis immediately after each application of the control ointment (see Suppl. 2), in addition to skin erythema throughout the whole experimental period. In Group III (the β-sitosterol group), the applied ointment had edible characteristics (Suppl. 3) and some rats were observed licking the ointment from the dorsa of neighbouring animals. Hyper-liquefaction of the wound after the ointment was applied was also observed, particularly during the first 12 days. This was caused by the hypertonic effect of the ointment preparation (the induction of fluid secretion from the skin lesion), which, in turn, delayed re-epithelialisation and wound healing in this group. Interestingly, Groups IV, V and VI showed no skin erythema, no allergic reactions, and no hyper-liquefaction throughout the experimental period.
Figure 3.
Wound contraction and the gross appearance of scald wound in all groups.
This new herbal formula was developed to promote scald-wound healing and evaluated using various investigative parameters. The results were promising when the formula was compared to commercial burn treatments. The wound-contraction percentages in the herbal ointment samples (Groups IV, V, and VI) were significantly higher than those in either the non-treated group or the standard groups (Groups II & III, see Figure 4).
Figure 4.
Burn-wound area in relation to days of treatment.
Conclusion
The search for an effective and safer topical burn therapy that can kill microbes and promote tissue regeneration, while limiting tissue damage, remains a major challenge. Given concerns about the toxicity of purified chemicals, health authorities and health-care professionals will continue to switch to traditional and alternative herbal medicines. Their low toxicity, low cost, easy of handling, and availability are factors that may encourage practitioners to consider using this new herbal formula as an alternative healing ointment for burn injuries. To the best of our knowledge, this is the first test of C. tripterocarpa and M. aurea as burn-injury healing promoters. This is also the first time this new herbal formula has been tested in three different concentrations to assess its ability to promote healing in full-thickness scald burns. Given these promising results and the study's ability to implement quality-control testing through an HPLC analysis, this herbal formula could be approved as an effective and safe alternative ointment to treat burn wounds and promote healing.
Source of funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
The experimental protocol was approved by the Institutional Animal Ethics Committee, under the supervision of the Scientific Research Review Committee, Faculty of Pharmacy, Northern Border University, Certificate No. (PHARM-NBU-2019-1), dated 30 October, 2019. It was carried out in accordance with the guidelines of the National Institutes of Health Principles of Laboratory Animal Care (NIH publication no. 85–23, revised 1985).
Authors’ contributions
MA: Study design, experimental work, scientific writing, and manuscript revision; AF: Study design, experimental work, and manuscript revision; FA: experimental work. BA: Study design, experimental work and manuscript revision; AY: Prepared the study animals and induced burns. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Acknowledgments
The authors are thankful to Dr Mohamed Attia at the histopathology lab at the Prince Naif Bin AbdulAziz Health Research Center, King Saud University for his valuable help in our histopathological examination of the tissue samples. The authors also appreciate the efforts of Prof Dr Mostafa Alhoseini, Public Health Dept., Faculty of Medicine, Ain Shams University for Statistical Analysis.
Footnotes
Peer review under responsibility of Taibah University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtumed.2020.10.023.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Othman N., Kendrick D. Epidemiology of burn injuries in the East Mediterranean Region: a systematic review. BMC Publ Health. 2010;10:83. doi: 10.1186/1471-2458-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGill V., Kowal-Vern A., Fisher S.G., Kahn S., Gamelli R.L. The impact of substance use on mortality and morbidity from thermal injury. J Trauma. 1995;38(6):931–934. doi: 10.1097/00005373-199506000-00019. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia N., Singh A., Sharma R., Singh A., Soni V., Singh G. Evaluation of burn wound healing potential of aqueous extract of Morus alba based cream in rats. J Phytopharmacol. 2014;3(6):378–383. [Google Scholar]
- 4.Li H.L., Deng Y.T., Zhang Z.R., Fu Q.R., Zheng Y.H., Cao X.M. Evaluation of effectiveness in a novel wound healing ointment-crocodile oil burn ointment. Afr J Tradit Complement Altern Med. 2017;14(1):62–72. doi: 10.21010/ajtcam.v14i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krug E. WHO Press; 20 Avenue Appia, 1211, Geneva 27, Switzerland: 2011. Burn prevention: success stories and lessons learned. World Health Organization. [Google Scholar]
- 6.Abdullahi A., Amini-Nik S., Jeschke M.G. Animal models in burn research. Cell Mol Life Sci. 2014;71(17):3241–3255. doi: 10.1007/s00018-014-1612-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brul S., Coote P. Preservative agents in foods: mode of action and microbial resistance mechanisms. Int J Food Microbiol. 1999;50(1–2):1–17. doi: 10.1016/s0168-1605(99)00072-0. [DOI] [PubMed] [Google Scholar]
- 8.Trøstrup H., Thomsen K., Calum H., Høiby N., Moser C. Animal models of chronic wound care: the application of biofilms in clinical research. Chron Wound Care Manag Res. 2016;3:123–132. [Google Scholar]
- 9.Li J., Chen J., Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25(1):9–18. doi: 10.1016/j.clindermatol.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Fuller F.W. The side effects of silver sulfadiazine. J Burn Care Res. 2009;30(3):464–470. doi: 10.1097/BCR.0b013e3181a28c9b. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X.G., Li X.M., Zhou X.X., Wang Y., Lai W.Y., Liu Y. The wound healing effect of Callicarpa nudiflora in scalded rats. Evid Based Complement Alternat Med. 2019:1860680. doi: 10.1155/2019/1860680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qnais E. The analgesic effect of the ethanolic extract of Matricaria aurea. Turk J Biol. 2011;35(3):347–352. [Google Scholar]
- 13.Abdelhalim A., Aburjai T., Hanrahan J., Abdel-Halim H. Medicinal plants used by traditional healers in Jordan, the Tafila region. Phcog Mag. 2017;13(49, suppl.4):95–101. doi: 10.4103/0973-1296.203975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed A.A., Abou-Elela M.A. Highly oxygenated bisabolenes and an acetylene from Matricaria aurea. Phytochemistry. 1999;51(4):551–554. doi: 10.1016/s0031-9422(99)00020-5. [DOI] [PubMed] [Google Scholar]
- 15.Al-Saleem M.S., Awaad A.S., Alothman M.R., Alqasoumi S.I. Phytochemical standardization and biological activities of certain desert plants growing in Saudi Arabia. Saudi Pharm J. 2018;26(2):198–204. doi: 10.1016/j.jsps.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Rifai A. Identification and evaluation of in-vitro antioxidant phenolic compounds from the Calendula tripterocarpa Rupr. South Afr J Bot. 2018;116:238–244. [Google Scholar]
- 17.Nasef M., Kadry H.A., Ashour M.A., Nassar S.L., Selim N.M., El-Raey M.A. Biological investigations of a new natural recipe expected to promote healing of superficial burns. Int J Pharm Clinical Res. 2016;8(8):1230–1239. [Google Scholar]
- 18.Abdolghaffari A.H., Mahdaviani P., Fallah-Bonekohal S., Ghasemi-Niri S.-F., Hagiaghaei R., Banan-Khojaste S.-M. Wound healing effect of Rosemary and Chamomile combination in rat. Pharmacol Online. 2010;3:139–145. [Google Scholar]
- 19.de Oliveira J.R., Camargo S.E.A., de Oliveira L.D. Rosmarinus officinalis L. (rosemary) as therapeutic and prophylactic agent. J Biomed Sci. 2019;26:5. doi: 10.1186/s12929-019-0499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medicherla K., Ketkar A., Sahu B.D., Sudhakar G., Sistla R. Rosmarinus officinalis L. extract ameliorates intestinal inflammation through MAPKs/NF-kB signaling in a murine model of acute experimental colitis. Food Funct. 2016;7(7):3233–3243. doi: 10.1039/c6fo00244g. [DOI] [PubMed] [Google Scholar]
- 21.Andrade J.M., Faustino C., Garcia C., Ladeiras D., Reis C.P., Rijo P. Rosmarinus officinalis L.: an update review of its phytochemistry and biological activity. Future Sci OA. 2018;4(4):FSO283. doi: 10.4155/fsoa-2017-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amaral G.P., de Carvalho N.R., Barcelos R.P., Dobrachinski F., Portella RdeL., da Silva M.H. Protective action of ethanolic extract of Rosmarinus officinalis L. in gastric ulcer prevention induced by ethanol in rats. Food Chem Toxicol. 2013;55:48–55. doi: 10.1016/j.fct.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 23.Karthik D., Viswanathan P., Anuradha C.V. Administration of rosmarinic acid reduces cardiopathology and blood pressure through inhibition of p22phox NADPH oxidase in fructose-fed hypertensive rats. J Cardiovasc Pharmacol. 2011;58:514–521. doi: 10.1097/FJC.0b013e31822c265d. [DOI] [PubMed] [Google Scholar]
- 24.Azab A., Nassar A., Azab A.N. Anti-inflammatory activity of natural products. Molecules. 2016;21(10):1321. doi: 10.3390/molecules21101321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kourounakis A.P., Assimopoulou A.N., Papageorgiou V.P., Gavalas A., Kourounakis P.N. Alkannin and Shikonin: effect on free radical processes and on inflammation - a preliminary pharmacochemical investigation. Archiv Der Pharmazie. 2002;335(6):262–266. doi: 10.1002/1521-4184(200208)335:6<262::AID-ARDP262>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 26.Sahakyan N., Petrosyan M., Trchounian A. The activity of Alkanna species in vitro culture and intact plant extracts against antibiotic resistant bacteria. Curr Pharmaceut Des. 2019;25(16):1861–1865. doi: 10.2174/1381612825666190716112510. [DOI] [PubMed] [Google Scholar]
- 27.Elzayat E.M., Auda S.H., Alanazi F.K., Al-Agamy M.H. Evaluation of wound healing activity of henna, pomegranate and myrrh herbal ointment blend. Saudi Pharm J. 2018;26(5):733–738. doi: 10.1016/j.jsps.2018.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Germano A., Occhipinti A., Barbero F., Maffei M.E. A pilot study on bioactive constituents and analgesic effects of MyrLiq® a Commiphora myrrha extract with high furanodiene content. BioMed Res Int. 2017:3804356. doi: 10.1155/2017/3804356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmad S., Ahmad M., Manzoor K., Purwar R., Ikram S. A review on latest innovations in natural gums based hydrogels: preparations & applications. Int J Biol Macromol. 2019;136:870–890. doi: 10.1016/j.ijbiomac.2019.06.113. [DOI] [PubMed] [Google Scholar]
- 30.Gim S., Zhu Y., Seeberger P.H., Delbianco M. Carbohydrate-based nanomaterials for biomedical applications. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019;11(5):e1558. doi: 10.1002/wnan.1558. [DOI] [PubMed] [Google Scholar]
- 31.Tirant M., Lotti T., Gianfaldoni S., Tchernev G., Wollina U., Bayer P. Integrative Dermatology – the use of herbals and nutritional supplements to treat dermatological conditions. Maced J Med Sci. 2018;6(1):185–202. doi: 10.3889/oamjms.2018.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soliman A.M., Teoh S.L., Ghafar N.A., Das S. Molecular concept of diabetic wound healing: effective role of herbal remedies. Mini Rev Med Chem. 2019;19(5):381–394. doi: 10.2174/1389557518666181025155204. [DOI] [PubMed] [Google Scholar]
- 33.Sarbaz Z., Yazdanpanahi Z., Hosseinkhani A., Nazari F., Akbarzadeh M. The effect of sitz bath of hydro-alcoholic extract of myrrh gum on episiotomy wound healing in nulliparous women. J Family Reprod Health. 2019;13(2):89–97. [PMC free article] [PubMed] [Google Scholar]
- 34.Ribeiro D.M.L., Júnior A.R.C., Vale de Macedo G.H.R., Chagas V.L., Silva LdS., Cutrim BdS. Polysaccharide-based formulations for healing of skin-related wound infections: lessons from animal models and clinical trials. Biomolecules. 2020;10(1):63. doi: 10.3390/biom10010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shigeyama M., Ohgaya T., Takeuchi H., hino T., Kawashima Y. Formulation design of ointment base suitable for healing of lesions in treatment of bedsores. Chem Pharm Bull. 2001;49(2):129–133. doi: 10.1248/cpb.49.129. [DOI] [PubMed] [Google Scholar]
- 36.Addotey J.N.A., Nyansah M.M.S. Quality assessment of some topical polyherbal preparations on the ghanaian market. World J Pharm Pharmaceut Sci. 2016;5(4):461–472. [Google Scholar]
- 37.Rajasree P.H., Vishwanad V., Cherian M., Eldhose J., Singh R. Formulation and evaluation of antiseptic polyherbal ointment. Int J Pharm Life Sci. 2012;3(10):2021–2031. [Google Scholar]
- 38.Majumder P., Majumder S. Preparation and characterization of some herbal ointment formulations with evaluation of antimicrobial property. Indian J Res Pharm Biotechnol. 2013;1(3):385–390. [Google Scholar]
- 39.Hiyama Y., Marshall A.H., Kraft R., Qa’aty N., Arno A., Herndon D.N. Effects of metformin on burn-induced hepatic endoplasmic reticulum stress in male rats. Mol Med. 2013;19(1):1–6. doi: 10.2119/molmed.2012.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hosgood G. Wound repair and specific tissue response to injury. In: Slatter D., editor. Text book of small animal surgery. Saunders; Philadelphia: 2002. pp. 66–86. [Google Scholar]
- 41.Ashour M.A., Abdelwahab M.F. Antimicrobial and cytotoxic activities of plant-derived endophytic metabolites from northern border region, KSA. Indo Am J Pharm Sci. 2018;5:1122–1132. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.