Abstract
Olive leaves are rich in bioactive substances which exert anti-inflammatory, antioxidant, insulin-sensitizing and antihypertensive effects. The aim of this study was to analyze the possible beneficial effects of an olive leaf extract (OLE) rich in secoiridoids and phenolic compounds on the aging-induced metabolic and vascular alterations. Three experimental groups of rats were used: 3-month-old rats, 24-month-old rats and 24-month-old rats supplemented 21 days with OLE (100 mg/kg). Administration of OLE to aged rats decreased the weight of adrenal glands and prevented the aging-induced loss of body weight and muscle mass. In the serum, OLE reduced the circulating levels of LDL-cholesterol and IL-6 and increased the concentrations of leptin and adiponectin. In the liver OLE attenuated the decreased gene expression of SOD-1, GSR, GCK and GSK-3β and reduced the aging-induced overexpression of NOX-4, Alox-5, iNOS and TNF-α. In aorta segments, OLE prevented endothelial dysfunction and vascular insulin resistance and improved vasoconstriction in response to KCl and NA. Improvement in vascular function was associated with the attenuation of the alterations in the gene expression of COX-2, IL-6, GPx, NOX-1 and IL-10. In conclusion, OLE exerts anti-inflammatory and antioxidant effects in aged rats and attenuates the alterations in vascular function associated with aging.
Subject terms: Plant sciences, Cardiology, Diseases, Endocrinology
Introduction
Aging is related with structural and functional alterations of vital organs. This is associated with the loss of physiological integrity, resulting in a progressive decline of homeostasis and a reduced capacity to respond to environmental stimuli, which contributes to the apparition of age-related co-morbidities1. Aging-associated co-morbidities include neurological, respiratory, oncologic, immune and/or metabolic disorders2. Among the latter, metabolic syndrome, a condition that is characterized by the co-existence of abdominal obesity, hypertriglyceridemia, fasting hyperglycemia, decreased HDL serum concentrations and hypertension, is one of the most prevalent among aged individuals3. Moreover, aging is considered the major risk factor for the development of cardiovascular diseases, which represent the leading cause of death in developed countries4. Aging-related cardiovascular alterations are the result, at least in part, of increased vasoconstriction and reduced endothelium-induced vasodilation which derives in enhanced incidence of arterial hypertension and atherosclerosis5.
In the last decades, the progressive increase in the number of elderly persons has represented a major sanitary and economic problem worldwide, due to the increment of age-related diseases. Furthermore, the proportion of the world’s population over 65 years is expected to double in the next decades, with this fact producing an increased incidence of age-related diseases and therefore a huge burden on healthcare systems6. For this reason, there is a strong interest in the development of new procedures and strategies that may slow the apparition of these disturbances.
Mediterranean diet, which includes the consumption of olive-derived products, is reported to exert several beneficial effects in old patients7,8 and for this reason, is recommended in the dietary guidelines for a healthy lifestyle9. In addition to olive oil, leaves from olive tree have been also used in traditional herbal medicine, and recent studies demonstrate a positive effect of olive leaf extracts (OLE) improving the alterations associated with the metabolic syndrome, such as dyslipidemia and insulin resistance10,11. These beneficial effects are attributed to the phenolic compounds present in the olive leaves that have potent antioxidant effects12. Indeed, OLE have been shown to exert antioxidant effects on culture cells13, invertebrates14 and mammals15,16.
In addition, since OLE reduces inflammation in several tissues and organs including vascular endothelial cells17, it may also be effective for the treatment/and or prevention of the age-related cardiovascular impairment. In this regard, there are some studies reporting the beneficial effects of OLE improving arterial hypertension both in rodents18,19 and in humans20,21, although the exact mechanisms of this antihypertensive effects are not well known.
Even though there is ample evidence about the beneficial effects of OLE on dyslipidemia and insulin resistance in metabolic syndrome10,22, its effects on insulin sensitivity and vascular function in the context of aging has not been studied yet. Thus, the aim of this study was to analyze the possible beneficial effects of a new OLE on the cardiometabolic alterations associated with aging in rats, and the possible antioxidant and/or anti-inflammatory mechanisms that may underlie this protection.
Results
Chemical characterization of OLE
According to the method described by Talhaoui et al.23 total flavonoid concentration was over 1.0% (1.15 ± 0.06%, dry basis).
The used RP-HPLC-PAD-MS analytical methodology allowed the identification of 32 constituents of the studied olive-tree leaf extract (Table 1). Eleven of them, protocatechuic acid, hydroxytyrosol, tyrosol, oleuropein, verbascoside, oleacein, quercetin-3-O-rutinoside, quercetin-3-O-glucoside, apigenin-7-O-rutinoside, luteolin and apigenin were identified unambiguously by co-elution and comparison with the retention time and the mass and UV spectra of commercial reference substances. The rest of the peaks were identified taking into account their chromatographic (retention time and order of elution), spectroscopic (ultraviolet–visible (UV–VIS) spectra), and spectrometric (molecular and typical fragment ions) characteristics.
Table 1.
Identification of phenolic compounds and flavonoids by HPLC–MS in the OLE. For each compound, the ultraviolet–visible absorption spectrum (UV–VIS), [M-H]− and fragment ions are shown.
| TR (min) | Molecule | UV–VIS (nm) | [M-H]− (m/z) | Product ions (m/z) |
|---|---|---|---|---|
| 14.1 | 3,4-DHBA (protocatechuic acid) | 225/260/294 | 153.0 | |
| 14.8 | Loganic accid | 230 | 375.0 | 213.0 |
| 15.6 | 3,4-DHPE (hydroxytyrosol) | 227/280 | 153.1 (305.2)* | 123.2 |
| 18.0 | 3,4-DHPE-glucoside | 228/278 | 315.0 | 153.1 |
| 20.6 | DM-EA glucoside (isomer 1) | 233 |
389.1 (779.2)* |
227.0, 183.0 |
| 34.0 | DM-EA glucoside (isomer 2) | 236 | 389.2 | 345.1 |
| 37.9 | EA glucoside (isomer 1) | 240 | 403.0 | 241.1, 139.1 |
| 40.6 | EA glucoside (isomer 2) | 240 | 403.0 (807.3)* | 100.9 |
| 50.4 | EA glucoside (isomer 3) | 238 |
403.1 (807.1)* |
|
| 56.6 | EA glucoside (isomer 4) + hydroxyloganin | 246/247/323 |
403.1 405.1 (811.0)* |
240.9 |
| 62.7 | EA (isomer 1) | 239 | 241.0 | 165.1, 139.1, 127.1, 121.1, 111.2, 101.0 |
| 64.5 | quercetin-3-O-rutinoside | 258/304sh/358 | 609.0 | 301.1 |
| 66.0 | quercetin-3-O-glucoside | 242/299sh/333 | 436.0 | 301.0 |
| 66.7 | EA (isomer 2) | 240 | 241.0 | |
| 70.2 | Verbascoside + luteolin-O-hexoside |
239/288sh/334 228/267sh/341 |
623.1 447.1 (895.2)* |
477.0 |
| 72.6 | 3,4-DHPE-DHEA-glucoside + 3,4-DHPE-DA-EDA (oleacein) | 230/277 |
543.0 (1087.4)* 319.0 |
513.0 275.1, 241.1, 139.2 |
| 78.1 | Apigenin-7-O-rutinoside | 257/333 | 577.0 | |
| 85.2 | Diosmin + luteolin-O-hexoside | 270/332 |
607.0 447.1 |
461.1 285.0 |
| 86.7 | 3,4-DHPE-EA-glucoside (oleuropein) | 280 | 539.1 | 377.0 |
| 91.8 | 3,4-DHPE-EA-glucoside (oleuropein,isomer 2) | 282 | 539.2 | 403.1, 377.0 |
| 95.9 | 3,4-DHPE-EA-glucoside (oleuropein,isomer 3) | 281 | 539.9 | 403.1, 377.1, 345.0, 307.0, 275.0 |
| 97.0 | Luteolin | 227/267/350 | 285.0 | |
| 98.9 | 4-HPE-EA-glucoside (ligustroside) | 281 | 523.2 | 509.0, 361.0 |
| 112.9 | Apigenin | 232/287sh/314 | 269.0 | |
| 115.6 | 3,4-DHPE-EA (isomer 1) | 281 | 377.1 | 307.1, 241.0 |
| 118.1 | 3,4-DHPE-EA (isomer 2) | 282 | 377.0 | 345.1, 307.0, 275.0, 149.1, 139.1 |
| 119.9 | 3,4-DHPE-EA (isomer 3) | 282 | 377.1 | 275.1, 240.9, 139.1 |
| 122.6 | 3,4-DHPE-EA (isomer 4) | 281 | 377.1 | 307.0, 275.0, 241.2, 138.9 |
3,4-DHBA = 3,4-dihydroxybenzoic acid; 3,4-DHPE = 3,4-dihydroxyphenylethanol (hydroxytyrosol); 4-HPE = 4-hydroxyphenylethanol (tyrosol); DM = demethyl; EA = elenolic acid; DHEA = dihydro elenolic acid; DA-EDA = deacetoxy elenolic acid dialdehyde form; * double ion.
The results in Table 1 showed the presence of a wide diversity of secondary olive leaf metabolites that include secoiridoids, simple and flavonoid phenolic compounds and iridoids. Among secoiridoids, four elenolic acid (EA) and two demethyl (DM) elenolic acid glucosides were identified, together with two isomers of elenolic acid. Regarding simple phenolic compounds, the most diverse was the group of phenylethanoids, mainly represented by hydroxytyrosol and its four isomer esters with elenolic acid, one ester with deacetoxy elenolic acid dialdehyde form (oleacein), three isomer esters with elenolic acid glucoside (oleuropein), one ester with dihydro elenolic acid glucoside, one glucoside and one ester of tyrosol with elenolic acid glucoside (ligustroside). This group includes also the hydroxytyrosol derivative verbascoside and the dihydroxybenzoic acid, protocatechuic acid. Flavonoids were composed by the two flavonols quercetin-3-O-rutinoside and quercetin-3-O-glucoside, and six flavones, luteolin, two luteolin hexoside isomers, diosmin, apigenin and apigenin-7-O-rutinoside. Two iridoids were detected also, loganic acid and hydroxyloganin.
Body weight and food and water intake changes induced by aging and OLE treatment
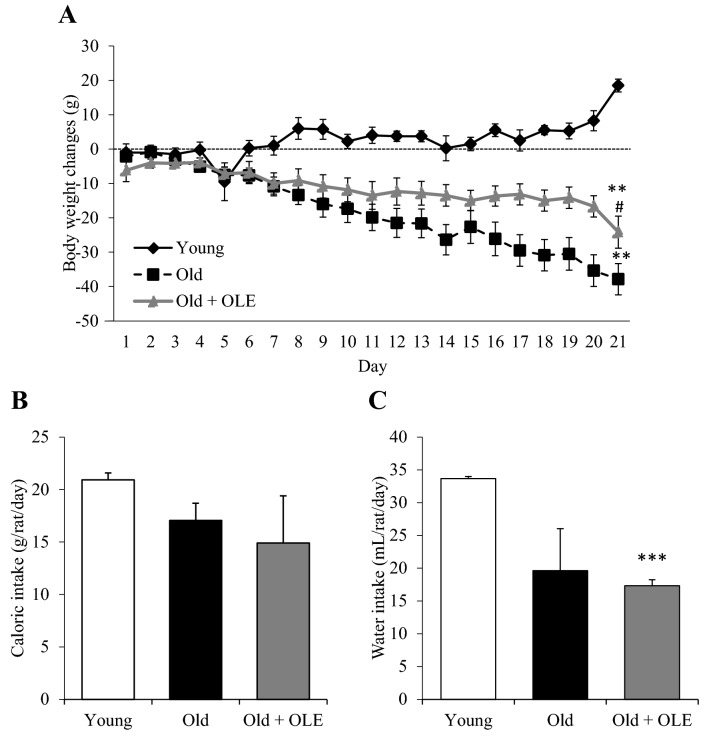
Body weight gain is shown in Fig. 1A. The two-way ANOVA revealed significant differences among experimental groups for both factors (Time: p < 0.001 and experimental group: p < 0.001). The post-hoc analysis showed that whereas the young rats gained weight over the three-week treatment, the old rats significantly lost weight (p < 0.01). This body weight loss was attenuated in aged rats treated with OLE (p < 0.01). Daily water intake was significantly decreased in old rats treated with OLE compared to young animals (p < 0.001) (Fig. 1C). However, caloric intake was unchanged between groups (Fig. 1B).
Figure 1.
Effects of aging and a 21-day treatment with the OLE on rat body weight gain (A), daily food (B) and water (C) intake. Values are represented as mean ± SEM. **p < 0.01 vs. Young; #p < 0.05 vs. Old.
Effects of aging and OLE on organ weights
Absolute and relative organ weights from young and old rats treated with vehicle or with OLE are shown in Table 2. Compared to young animals, old rats showed increased absolute weights of heart (p < 0.01, liver (p < 0.05), epidydimal visceral (p < 0.001), white lumbar subcutaneous (p < 0.01), interscapular brown (p < 0.05) and periaortic (p < 0.05) adipose tissues. Treatment with OLE prevented the aging-induced decrease in the absolute weight of gastrocnemius muscle (p < 0.05). Relative organ weights followed the same pattern than absolute weights except for the heart, and the interscapular and periaortic adipose tissue depots that were not significantly increased in old untreated rats compared to young rats. OLE treatment increased the relative weight of the interscapular brown adipose tissue compared to untreated old rats (p < 0.05).
Table 2.
Organ weights of young rats, old rats and old rats treated with olive leaves extract (OLE).
| Young | Old | Old + OLE | |
|---|---|---|---|
| Heart (mg) | 1,503.8 ± 78.8 | 1,891.3 ± 55.6** | 1,845.8 ± 78.9** |
| Heart (mg/100 g body weight) | 314.6 ± 10.8 | 291.0 ± 14.4 | 264.6 ± 13.7* |
| Epidydimal visceral adipose tissue (mg) | 9,843.1 ± 1075.0 | 20,475.4 ± 1451.5*** | 21,545.9 ± 1908.0*** |
| Epidydimal visceral adipose tissue (mg/100 g body weight) | 2,107.1 ± 183.7 | 3,201.8 ± 139.6*** | 2,970.7 ± 265.1** |
| Lumbar subcutaneous adipose tissue (mg) | 4,992.4 ± 787.5 | 27,905.7 ± 6192.6*** | 28,619.6 ± 2906.6*** |
| Lumbar subcutaneous adipose tissue (mg/100 g body weight) | 1,063.1 ± 139.6 | 3,974.2 ± 661.4*** | 4,249.9 ± 396.9*** |
| Interscapular brown adipose tissue (mg) | 496.3 ± 50.0 | 810.8 ± 119.4* | 1011.8 ± 154.6*** |
| Interscapular brown adipose tissue (mg/100 g body weight) | 105.6 ± 9.8 | 111.1 ± 12.9 | 140.4 ± 14.4*# |
| Periaortic adipose tissue (mg) | 180.0 ± 26.0 | 307.9 ± 47.7** | 397.5 ± 57.8*** |
| Periaortic adipose tissue (mg/100 g body weight) | 39.3 ± 5.9 | 47.8 ± 7.5 | 55.2 ± 5.9* |
| Liver (mg) | 13,465.0 ± 425.6 | 14,886.9 ± 566.9* | 15,733.9 ± 588.5** |
| Liver (mg/100 g body weight) | 2,932.8 ± 103.6 | 2,204.2 ± 138.6*** | 2,343.0 ± 92.6** |
| Soleus (mg) | 180.8 ± 12.6 | 163.4 ± 10.8 | 173.1 ± 11.6 |
| Soleus (mg/100 g body weight) | 39.2 ± 2.1 | 26.0 ± 1.6*** | 24.8 ± 2.0*** |
| Gastrocnemius (mg) | 2,473.4 ± 50.7 | 2,239.1 ± 101.9* | 2,560.9 ± 123.0# |
| Gastrocnemius (mg/100 g body weight) | 534.4 ± 12.1 | 329.4 ± 15.7*** | 375.5 ± 11.1***# |
Data are represented as mean value ± SEM; n = 6–11 samples/group.
*p < 0.05 vs. Young; **p < 0.01 vs. Young; ***p < 0.001 vs. Young; #p < 0.05 vs. Old.
Effects of aging and OLE on lipid profile, serum levels of metabolic hormones and HOMA-IR index
Aging was associated with increased circulating levels of total lipids (p < 0.05), triglycerides (p < 0.05), total cholesterol (p < 0.05), LDL-cholesterol (p < 0.01), insulin (p < 0.01), leptin (p < 0.01), adiponectin (p < 0.001) and HOMA-Index (p < 0.05). Treatment with OLE prevented the aging-induced increase in LDL-cholesterol and further increased the serum levels of leptin and adiponectin (p < 0.05 for both) (Table 3).
Table 3.
Lipid profile, hormone concentrations and inflammatory parameters in the serum of young rats, old rats and old rats treated with olive leaves extract (OLE).
| Young | Old | Old + OLE | |
|---|---|---|---|
| Glycemia (mg/dL) | 90.7 ± 3.8 | 72.4 ± 11.1 | 62.7 ± 10.7* |
| Total Lipids (mg/dL) | 853 ± 63 | 1051 ± 37* | 1173 ± 87** |
| Triglycerides (mg/dL) | 97.6 ± 13.5 | 158.5 ± 36.4* | 169.5 ± 11.7** |
| Total Cholesterol (mg/dL) | 135.1 ± 15.3 | 199.3 ± 13.9* | 194.0 ± 26.3 |
| LDL-cholesterol (mg/dL) | 28.8 ± 2.7 | 47.8 ± 2.6** | 33.6 ± 1.4### |
| HDL-cholesterol (mg/dL) | 15.7 ± 0.6 | 15.7 ± 2.7 | 15.8 ± 0.6 |
| Insulin (ng/mL) | 17.7 ± 5.2 | 81.3 ± 32.2** | 30.0 ± 3.4 |
| HOMA-Index | 1.75 ± 0.3 | 13.1 ± 5.4* | 5.5 ± 2.3* |
| Leptin (ng/mL) | 11.82 ± 1.4 | 30.2 ± 5.9** | 53.0 ± 7.7***# |
| Adiponectin (mg/dL) | 67.1 ± 6.7 | 108.9 ± 4.3*** | 123.5 ± 5.5***# |
| Interleukin-6 (pg/mL) | 135.6 ± 7.1 | 188.7 ± 24.1* | 146.7 ± 7.9# |
| TNFα (pg/mL) | 0.1 ± 0.1 | 1.8 ± 0.9* | 0.82 ± 0.6 |
Data are represented as mean value ± SEM; n = 6–11 samples/group.
*p < 0.05 vs. Young; **p < 0.01 vs. Young; ***p < 0.001 vs. Young; #p < 0.05 vs. Old; ###p < 0.001 vs. Old.
Effects of aging and OLE on serum inflammatory parameters
Old rats showed higher serum levels of the inflammatory markers IL-6 and TNFα compared to young rats (p < 0.05 for both) and treatment with the OLE prevented the aging-induced increase in IL-6 (p < 0.05) (Table 3).
Effects of aging and OLE on gene expression of insulin sensitivity-related enzymes in the liver
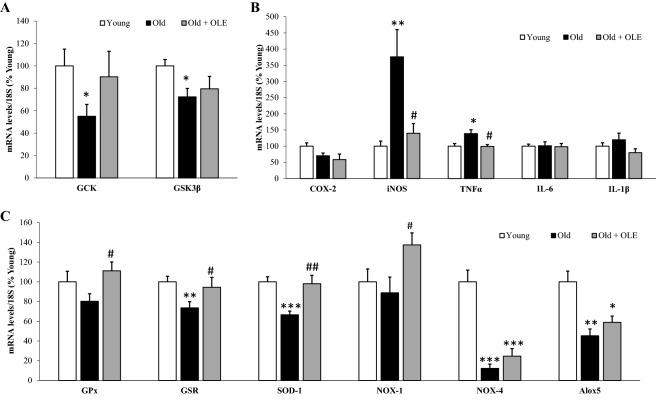
The mRNA levels of the insulin sensitivity-related enzymes GCK and GSK3β were significantly downregulated in untreated old rats compared to young animals (Fig. 2A; p < 0.05 for both) but not in aged rats treated with OLE.
Figure 2.
Effects of aging and a 21-day treatment with the OLE on mRNA concentrations of Glucokinase and Glycogen synthase kinase 3β (A), ciclooxigenase-2, inducible Nitric Oxide Synthase, Tumor Necrosis Factor α, Interleukin 6 and Interleukin 1β (B) and Glutathione Peroxidase, Glutathione Reductase, Super Oxide Dismutase 1, NADPH oxidase 1 and 4, and Lipoxygenase (C) in rat livers. Values are represented as mean ± SEM. *p < 0.05 vs. Young; **p < 0.01 vs. Young; ***p < 0.001 vs. Young; #p < 0.05 vs. Old; ##p < 0.01 vs. Old.
Effects of aging and OLE on gene expression of inflammatory and oxidative stress markers in the liver
The hepatic overexpression of the pro-inflammatory markers iNOS (p < 0.01) and TNFα (p < 0.05) associated with aging was prevented by the treatment with OLE (p < 0.05 for both) (Fig. 2B).
Old rats also showed reduced gene expression in the liver of the antioxidant enzymes GSR and SOD-1 (p < 0.05 and p < 0.001, respectively), as well as NOX-4 (p < 0.001) and Alox5 (p < 0.01). The treatment of old rats with OLE did not affect the gene expression of NOX-4 and Alox5, but it prevented both the aging-induced reduction of GSR (p < 0.05) and SOD-1 (p < 0.01) and significantly upregulated the mRNA levels of GPx (p < 0.05) and NOX-1 (p < 0.05) (Fig. 2).
Effects of aging and OLE on gene expression of inflammatory and oxidative stress markers in cardiac tissue
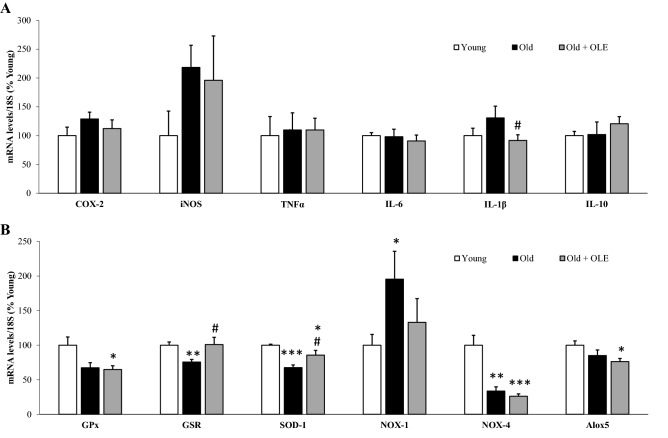
There were no significant changes in the mRNA levels of inflammatory markers in the cardiac tissue of old rats compared to young ones. However, supplementation with OLE to old rats significantly downregulated the gene expression of IL-1β (p < 0.05) compared to untreated ones (Fig. 3A).
Figure 3.
Effects of aging and a 21-day treatment with the OLE on mRNA concentrations of ciclooxigenase-2, inducible Nitric Oxide Synthase, Tumor Necrosis Factor α, Interleukin 6, Interleukin 1β and Interleukin 10 (A) and Glutathione Peroxidase, Glutathione Reductase, Super Oxide Dismutase 1, NADPH oxidase 1 and 4, and Lipoxygenase (B) in rat hearts. Values are represented as mean ± SEM.* p < 0.05 vs. Young; **p < 0.01 vs. Young; ***p < 0.001 vs. Young; #p < 0.05 vs. Old.
The gene expression of the antioxidant enzymes GSR and SOD-1 was reduced in old compared to young rats (p < 0.01 and p < 0.001, respectively) whereas the mRNA levels of the pro-oxidant enzyme NOX-1 were significantly increased (p < 0.05). Treatment with OLE prevented the aging-induced reduction in GSR and SOD-1 mRNA levels (p < 0.05 for both). The gene expression of NOX-4 was reduced in old rats regardless of whether they had been treated (p < 0.001) or not (p < 0.01) with OLE (Fig. 3B).
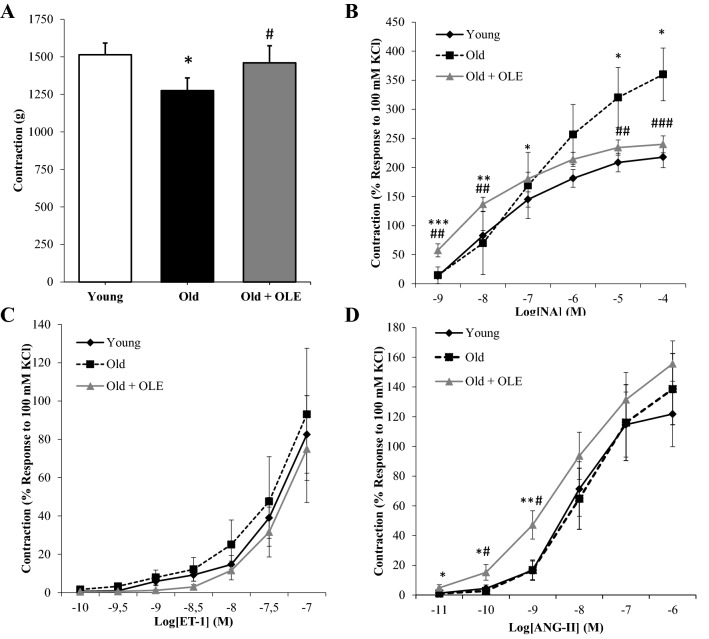
Aortic vasoconstriction changes induced by aging and OLE treatment
Arterial vasoconstriction of abdominal aorta segments to KCl (A), Noradrenaline (B), Endothelin-1 (C) and Angiotensin II (D) is shown in Fig. 4. Aging did not modify arterial vasoconstriction in response to either ET-1 (Fig. 4C) or Ang-II (Fig. 4D). However, aorta segments from old untreated rats showed a decreased vasoconstrictor response to KCl (p < 0.05; Fig. 4A), and an increased vascular contraction in response to accumulative concentrations of NA (p < 0.01; Fig. 5B).
Figure 4.
Effects of aging and a 21-day treatment with the OLE on the contraction response of abdominal aortic segments to potassium chloride 100 mM (A), norepinephrine (B), endothelin-1 (C) and to angiotensin-II (D). Values are represented as mean ± SEM. *p < 0.05 vs. Young; **p < 0.01 vs. Young; ***p < 0.001 vs. Young; #p < 0.05 vs. Old; ##p < 0.01 vs. Old; ###p < 0.001 vs. Old.
Figure 5.
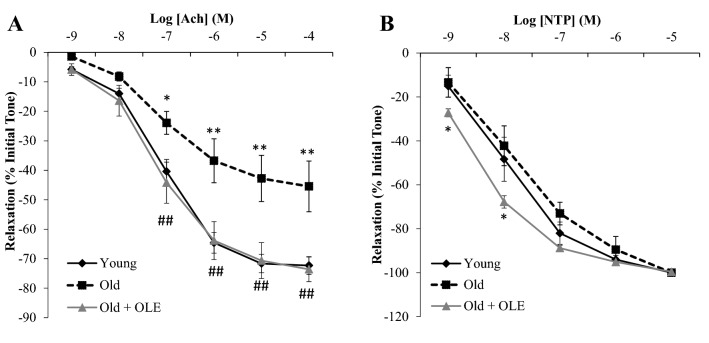
Effects of aging and a 21-day treatment with the OLE on the relaxation response of thoracic aortic segments to acetylcholine (A) and to sodium nitroprusside (B). Values are represented as mean ± SEM. *p < 0.05 vs. Young; **p < 0.01 vs. Young; ##p < 0.01 vs. Old.
Treatment with OLE prevented the aging-induced decrease in arterial vasoconstriction in response to KCl (p < 0.05) and the aging-induced increased vasoconstriction in response to NA (p < 0.01). Nonetheless, the contraction to AngII was significantly increased at low doses by treatment with the OLE (p < 0.05; Fig. 4D).
Changes on endothelium-dependent and independent relaxation in aorta segments induced by aging and OLE treatment
The endothelium-dependent relaxation in response to acetylcholine was significantly reduced in aorta segments from old rats (p < 0.01; Fig. 5A) and this effect was totally reverted by OLE treatment (p < 0.01; Fig. 5A). On the other hand, the endothelium-independent relaxation in response to sodium nitroprusside (NTP) was not modified in untreated old rats, whereas OLE treatment significantly increased endothelium-independent relaxation at low concentrations of NTP (p < 0.05; Fig. 5B).
Aortic insulin vascular relaxation and activation of PI3K/Akt pathway
Despite insulin induced dose‐dependent relaxation of aorta segments in all experimental groups (Fig. 6A), aging was related to a reduced relaxation response compared to young rats (p < 0.05) and significantly improved when supplemented with OLE (p < 0.01).
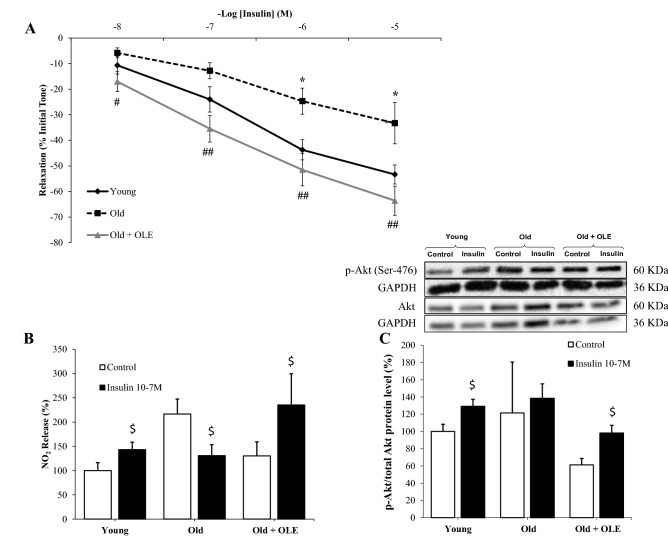
Figure 6.
Effects of aging and a 21-day treatment with the OLE on the relaxation response of thoracic aortic segments to insulin (A), on the nitrites release from aorta segments to culture medium in presence/absence of insulin (10−7 M) (B), and on the ratio between protein levels of p-Akt and Akt in aorta (c). Values are represented as mean ± SEM. *p < 0.05 vs. Young; #p < 0.05 vs. Old; ##p < 0.01 vs. Old; $p < 0.05 vs. Control.
Incubation of aorta segments with insulin increased the release of nitrites to the culture medium and the ratio p-Akt/Akt in arterial tissue from young (p < 0.05) and old rats treated with the OLE (p < 0.05), but not in untreated old rats (Fig. 6B and C).
Effects of aging and OLE on gene expression of inflammatory and oxidative stress markers in the aorta
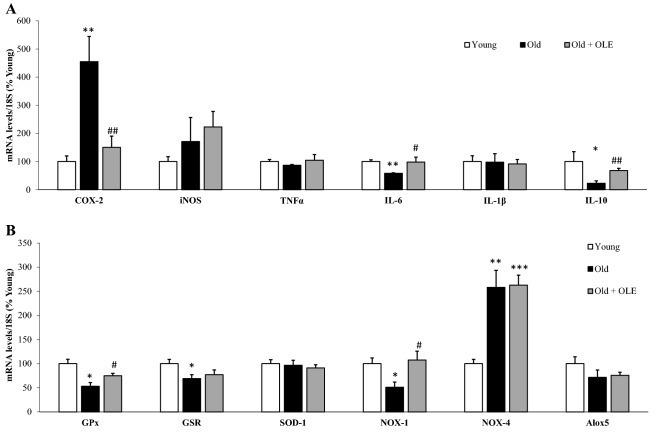
Untreated old rats showed a significant increase in the aortic gene expression of COX-2 (p < 0.01) and NOX-4 (p < 0.001) and a significant reduction in the mRNA levels of IL-6 (p < 0.01), IL-10 (p < 0.05), GPx (p < 0.05), GSR (p < 0.05) and NOX-1 (p < 0.05). The treatment with OLE attenuated the aging-induced changes in COX-2 (p < 0.01), IL-6 (p < 0.05), GPx (p < 0.05), NOX-1 (p < 0.05) and IL-10 (p < 0.01) mRNA levels, but not in NOX-4 (Fig. 7).
Figure 7.
Effects of aging and a 21-day treatment with the OLE on mRNA concentrations of ciclooxigenase-2, inducible Nitric Oxide Synthase, Tumor Necrosis Factor α, Interleukin 6, Interleukin 1β and Interleukin 10 (A) and Glutathione Peroxidase, Glutathione Reductase, Super Oxide Dismutase 1, NADPH oxidase 1 and 4, and Lipoxygenase (B) in rat aortas. Values are represented as mean ± SEM. *p < 0.05 vs. Young; **p < 0.01 vs. Young; ***p < 0.001 vs. Young; #p < 0.05 vs. Old; ##p < 0.05 vs. Old.
Discussion
This study provides evidence about the effects of a new OLE rich in flavonoids and secoiridoids alleviating the cardiometabolic disorders associated with aging. There are previous studies reporting the positive effects of treatments with olive leaf extracts decreasing oxidative stress in major organs of aged rats15 and reducing aging-induced neurodegeneration in humans24 and in rodents25. However, to our knowledge, this is the first study that demonstrates the beneficial metabolic and cardiovascular effects of an olive leaf extract in aged animals. Importantly, our results showed that the OLE used in this study exerts its cardiometabolic protective effects at a considerably lower dose than the extract used by other authors (100 mg/kg vs 500 and 1000 mg/kg)15 and in a shorter period of time (three weeks vs 6 months)25. The dose used in this study was selected based in previous studies found in the literature in which the same dose of OLE had significant effects in rats preventing fluoxetine-induced hepatotoxicity through attenuation of oxidative stress, inflammation, and apoptosis26. Likewise, a very recent study shows how the same dose of OLE prevents type-2 diabetes in rats and decreases the expression of liver superoxide dismutase as well as the total antioxidant capacity in the plasma27.
Aged rats showed increased adiposity, increased plasma levels of leptin, total and LDL-cholesterol and triglycerides and decreased muscle mass. It is reported that, in addition to adiposity and dyslipidemia28, this loss of muscle mass, so called sarcopenia, is a common characteristic among individuals suffering from metabolic syndrome29. In addition, several parameters related to insulin sensitivity were also impaired with aging, like elevated insulin plasma levels and HOMA index, and decreased gene expression of GCK and GSK3β in the liver. These alterations were in agreement with some of the characteristics of metabolic syndrome in elderly patients3.
OLE supplementation in the drinking water for three weeks to old rats partially prevented age-induced weight loss over the three-week treatment. Since there were no significant changes in cumulative intake among the three experimental groups, this effect was not attributable to modifications in caloric intake. The effect of OLE attenuating body weight loss in old rats may be explained, at least in part, by the significant increase in muscle mass in old treated rats since skeletal muscle accounts for approximately 40% of total body mass. In agreement with this, it is reported that supplementation with secoiridoids from olive oil attenuates sarcopenia in overweight and obese older adults30.
It is also reported that aging is associated with a chronic state of low-grade inflammation, with this fact being involved in the development of aging-associated comorbidities31, and particularly in the mechanisms of the metabolic impairment in this condition32. In agreement with this, untreated old rats exhibited elevated circulating levels of IL-6 and TNFα and these concentrations were reduced by treatment with the OLE in the case of IL-6. Likewise, other studies have demonstrated the systemic anti-inflammatory capacity of different extracts of olive leaves both in humans20,33,34 and in rodents35,36. This effect is most likely due to the presence of phenolic compounds in the extract since it is reported in several in vivo and in vitro models that the flavonoids and phenolic secoiridoids identified in the OLE used in this study, such as luteolin and its derivatives, verbascoside, oleacein and most of the identified hydroxytyrosol elenolates, exert powerful anti-inflammatory effects, decreasing both the systemic and tissue levels of IL-637–39. The OLE-induced decrease in the circulating levels of IL-6 may be related, at least in part, with its beneficial effects attenuating muscle loss, since a positive relationship between serum IL-6 and sarcopenia has been reported40. Moreover, OLE treatment also prevented the aging-induced upregulation of IL-6 mRNA levels in gastrocnemius muscle (Young: 100 ± 8; Old: 152 ± 12**; Old + OLE: 119 ± 11#), pointing to a local anti-inflammatory effect at the muscular level.
OLE treatment to old rats not only decreased the circulating levels of IL-6 but also improved dyslipidemia reducing the plasma levels of LDL-cholesterol. Consistent with our results, treatment with OLE is reported to improve the lipid profile both in rodents subjected to high fat diets35,41–44 and in a cohort of pre-hypertensive men20. However, OLE treatment increased circulating leptin and adiponectin levels in aged rats, which may be related with the slightly higher adiposity of these rats compared to the untreated ones. Although the increase in leptin levels in individuals with increased adiposity, both humans and experiments animals, is clearly demonstrated45 , the association of circulating adiponectin levels with obesity and metabolic syndrome is controversial. In this regard, it is extensively reported that adiponectin levels are decreased in obese individuals46,47 and in experimental models of genetic obesity48. However, it is not that clear in experimental models of diet-induced obesity in which both decreased48 and increased49 circulating levels of this adipokine have been described. Indeed, increased adiponectin serum levels have been found increased overtime in a study that examined prospectively the course of circulating adiponectin levels during development of metabolic alterations in a diet-induced rat model of metabolic syndrome50. Likewise, in experimental models of aging both unchanged51 and increased adiponectin serum concentrations have been found52. Thus, it is possible that the commonly decreased adiponectin serum levels observed in humans in a context of obesity and/or metabolic syndrome are not necessarily reproduced in experimental models of metabolic syndrome induced by either aging or diet.
Glycemia and hepatic pAkt/Akt ratio (Supplementary data; Fig. 3A) were unchanged between young and old rats fed ad libitum but they were significantly decreased in aged rats treated with OLE. These results are in agreement with other studies in which OLE is reported to exert antidiabetic effects both in rats53,54 and in humans54. Although the differences in HOMA index or insulin plasma levels between treated and untreated old rats were not statistically significant, our results showed evidence of preserved insulin sensitivity in the liver of treated animals. Likewise, other authors have reported increased insulin sensitivity in the liver of diabetic rats treated with OLE55. However, although in basal conditions the mRNA levels of GSK3β were significantly decreased in old untreated rats and not in old rats treated with OLE, the ratio pGSK3β/GSK3β was unchanged with aging and significantly increased in OLE treated animals (Supplementary data; Fig. 3B). Thus, further studies are required to dilucidate the differences between hepatic GSK3β gene and protein expression, not only in basal conditions but also in response to insulin stimulation.
Based on previous studies, the insulin sensitizing effects of OLE may be attributed to the presence of phenolic secoiridoids such as oleuropein and its products of hydrolysis, but also to other compounds such as hydroxytyrosol, tyrosol and ligustroside10. Since the development of insulin resistance and type 2 diabetes is related to increased inflammation and oxidative stress56, the increased insulin sensitivity in the liver may be related, at least in part, with the effect of OLE reducing the mRNA levels of the pro-inflammatory markers iNOS and TNF-α and increasing the gene expression of the antioxidant enzymes GPx, GSR and SOD-1. These results are in agreement with those described in other experimental models of liver damage, in which treatment with an OLE attenuates oxidative stress26,57,58, decreases the hepatic gene expression of proinflammatory cytokines26,35,55,58,59 and increases hepatic insulin sensitivity55. However, in heart and aorta tissues the gene expression of TNF-α was not affected neither by aging nor by OLE treatment. The OLE-induced reduction of TNF-α mRNA levels in the liver and not in other tissues could be explained, at least in part, because the liver, due to its key role in metabolic and detoxifying pathways, is probably more exposed to inflammatory stress and OLE may be effective only when there is inflammatory activation above basal levels. This may be related with the non-significant reduction of TNF-α circulating levels in OLE treated rats, since TNF-α plasma levels represent an average production in all organs and, for this reason, they show a higher variability.
Moreover, decreased hepatic insulin resistance in old rats treated with OLE may also be related to the increased circulating levels of the insulin sensitizing hormone adiponectin, as previously reported in adipose tissue of ovariectomized rats60 and obese mice treated with OLE59. Adiponectin has insulin-sensitizing and endothelium-protecting effects61, so these elevated levels may be related to the improved metabolic and vascular function in OLE treated rats. However, adiponectin levels are also correlated with incident falls62, which may be the result of a bone weakening effect of this hormone63,64 due to the stimulation of osteoclastogenesis65. Thus, further studies are required to determine OLE effects on bone density.
Our results show that aged rats not only suffered metabolic but also vascular alterations. The endothelium-dependent relaxation to acetylcholine was reduced in the aortas of untreated old rats indicating, as previously described66,67, that the ability of the endothelium to regulate the vascular tone in an aging context is altered. Likewise, the contraction to KCl was reduced indicating that smooth muscle function is also impaired67. However, the arterial contraction to norepinephrine was increased, suggesting that there is hypersensitivity of adrenergic receptors, as previously reported in an aging context both in humans and in rodents66. These vascular alterations were reversed by treatment with OLE, which normalized both the relaxation to acetylcholine and the contraction to KCl and norepinephrine. OLE also increased the endothelium-independent relaxation in response to NTP and the contraction to Ang II which were not altered by aging, suggesting that the action of OLE on the vascular system may be more extensive beyond counteracting the effects of aging. Other authors have reported beneficial effects of OLE in cardiovascular function reducing both cardiac output and total peripheral vascular resistance in spontaneously hypertensive rats68 and enhancing endothelium-dependent relaxation and eNOS activation through the reduction of oxidative stress19. In the present study OLE also improved the aging-induce endothelial dysfunction and reduced the adrenergic hypersensitivity in the aorta of old rats, which may also contribute to the antihypertensive effect. Paradoxically, a significant increase in the contraction of aorta segments to angiotensin II was also found. This finding may be explained by the effect of OLE reducing the angiotensin converting enzyme, as it has been described previously69, which may decrease the circulating levels of angiotensin II, thus resulting in compensatory hypersensitivity of angiotensin receptors.
As previously described70, our results also showed that aging is not only associated with insulin resistance in metabolic tissues, but also in the cardiovascular system where it decreases the vasodilation of aorta segments in response to insulin through an impaired activation of the PI3K/Akt pathway and decreased nitric oxide release. In a previous study, we reported that this impairment can be attenuated by a moderate protocol of caloric restriction70. Similarly, in this study we demonstrated that the treatment with OLE improved vasodilation of aorta segments in response to insulin and increased both the release of nitric oxide and the activation of the PI3K/Akt pathway in arterial tissue. To our knowledge this is the first study that reports a positive effect of OLE attenuating aging-induced insulin vascular resistance.
The protective effects of OLE on cardiovascular function may also be related to its anti-inflammatory and antioxidant effects, as treatment with OLE to old rats significantly reduced the gene expression of IL-1β in the heart and the mRNA levels of the inflammatory marker COX-2 in the aorta and increased the gene expression of the antioxidant enzyme GPx in the aorta and the expression of GSR and SOD-1 in the heart. Moreover, OLE treatment prevented the aging-induced downregulation of anti-inflammatory cytokine IL-10 in arterial tissue. Paradoxically an overexpression of the inflammatory marker IL-6 and the prooxidative enzyme NOX-1 in the aorta was also found. However, it has been proposed that in some cases nutritional phytochemicals may induce hormetic mechanisms which are based on activation of a moderate oxidative and/or inflammatory stress that activates compensatory mechanisms71,72.
Our results of gene expression of inflammatory and oxidative stress markers in cardiac and arterial tissue agree with previous studies that report the effects of both OLE and its components attenuating inflammation and DNA damage in human arterial endothelial cells17, preventing cardiac stiffness and fibrosis44 and reducing myocardial oxidative damage and atherosclerosis73.
In general, this study demonstrates that an OLE rich in flavonoids and secoiridoids alleviates the cardiometabolic disorders associated with aging. Further studies are required to assess if this treatment is also useful to prevent cardiometabolic alterations in aged humans as well as in experimental models of metabolic syndrome. Likewise, further studies are required to identify which of the specific compounds present in this extract are responsible for the protective cardiometabolic effects, although the functional effects of the OLE shown in this manuscript are probably not due to the contribution of individual molecules but to the overall composition of this natural extract.
Conclusion
In conclusion, the results of the present study indicate that OLE is effective attenuating the metabolic and vascular alterations associated with aging possibly through the reduction of inflammation and oxidative stress. Therefore, it may constitute a useful strategy to improve cardiometabolic alterations in aged patients.
Material and methods
All methods were carried out in accordance with relevant guidelines and regulations. Plant manipulations comply with national and international guidelines and legislation.
Materials
Water soluble samples of olive leaf extract (OLE) from Olea europaea L. standardized in 30% of ortho-diphenols by UV/Vis were provided by Pharmactive Biotech Products S.L. (Madrid, Spain). All of them were in powder form and were stored in darkness until their addition into the feeding bottles.
For High Performance Liquid Chromatography (HPLC) analysis, milli-Q grade water was produced in house by a Milli-Q purification system (Millipore Corp., Bedford, MA, USA). Acetonitrile and methanol (HPLC solvents) were purchased from Merck (Dramstadt, Germany), and acetic acid from Labbox Labware SL (Madrid, Spain). For peak identification, the following reference substances were used: the HPLC grade 3,4-DHBA (protocatechuic acid) (> 97%), 3,4-DHPE (hydroxytyrosol, > 98%), 4-HPE (tyrosol, 98%), 3,4-DHPE-EA-glucoside (oleuropein, > 98%), and verbascoside (> 99%) were from Merck (Dramstadt, Germany). The 3,4-DHPE-DA-EDA (oleacein), quercetin-3-O-rutinoside (rutin), quercetin-3-O-glucoside, apigenin-7-O-rutinoside, luteolin and apigenin were from Extrasynthèse (Genay, France).
Chemical characterization of OLE
Analysis of total flavonoid content by HPLC–DAD
Determination of total flavonoids as luteolin equivalents was carried out according to Talhaoui et al.23.
Identification of phenolic compounds by RP-HPLC-PAD-MS(ESI)
Solutions of 10 mg/mL of OLE were prepared in water (in duplicate) and were analysed quantitatively by reversed phase high performance liquid chromatography coupled to photo-diode array detector and mass spectrometry detector with electrospray ionization source (RP-HPLC-PAD-MS(ESI)) as described in Silván et al.74. Briefly, the chromatographic system was an Agilent 1100 series (binary pump, autosampler, photodiode array detector (PAD) (Palo Alto, CA, USA)), coupled to an ESI source and quadrupole mass analyser. Stationary phase was an ACE-C18-AR (200 mm × 4.6 mm, 3 μm particle size) column from Advanced Chromatography Technologies (Aberdeen, UK). The mobile phase was a linear gradient of eluent A (2% (v/v) acetic acid in water), and eluent B (2% (v/v) acetic acid in acetonitrile) and was pumped at a flow rate of 0.6 mL/min as follows: from 0 to 80 min, 0% to 20% of B; from 80 to 115 min, 20% to 29% of B; from 115 to 120 min, 29% to 100% of B; during 10 min, 100% of B; and from 130 to 140 min, 100% to 0% of B. The PAD was set at 240, 280 and 330 nm and the injection volume was 10 µL. ESI operation parameters were fixed at: nebulizing pressure of 40 psi and a capillary tension of 4000 V. Drying gas was N2, at a flow of 10 L/min at 340 °C. Mass spectra were registered by scanning negative ions from m/z less than 200 Da, at 100 V, m/z 200–1000 Da, at 200 V, and m/z 1000–2500 Da, at 250 V74.
In vivo study
Animals
Three (Young; n = 11) and 24-months-old (Old; n = 14) male Wistar rats were fed ad libitum with a standard chow (322.6 kcal/100 g) and housed in climate-controlled quarters with a 12 h light cycle and under controlled conditions of humidity (50–60%) and temperature (22–24 °C). All the experiments and handling of animals were conducted with the approval of the Animal Care and Use Committee of the Community of Madrid (Spain) (PROEX 048-18) according to the European Union Legislation and in compliance with the ARRIVE guidelines.
Treatment
Three weeks before sacrifice half of the old rats (Old + OLE) were treated daily with 100 mg/kg of the OLE by dissolved in the drinking water. The other half of the old rats and the young rats received just tap water. Rats were allowed to drink ad libitum. To ensure the correct OLE dose (100 mg/kg/day), the amount of OLE that was added to the drinking water was adjusted every three days depending on both water intake and body weight.
As described at González-Hedström et al.67, a daily control of body weight was performed over the three-week treatment. Each day, the change in body weight was calculated by subtracting the daily body weight of each animal from the their body weight at the beginning of treatment. Food intake was assessed weekly by placing a specific amount of chow in each cage and measuring the remaining amount one week later. The day of sacrifice, all animals were injected an overdose of sodium pentobarbital (100 mg/kg) and killed by decapitation after overnight fasting. Before sacrifice, glycemia was measured by venous tail puncture using Glucocard G (Arkray Factory, Inc., Koji Konan-cho, Koka, Shiga, Japan).
To obtain the serum after sacrifice, trunk blood was collected and centrifuged at 3000 rpm for 20 min. After that, visceral (epididymal), subcutaneous (lumbar), brown (interscapular) and perivascular (aortic) adipose tissue depots as well as kidneys, adrenal glands, spleen, liver and heart were immediately removed, weighed and stored at -80 °C for further analysis.
Serum measurements
Lipid profile and serum levels of metabolic hormones and inflammatory markers were measured as previously described in González-Hedström et al.67.
Metabolic hormones
The serum concentrations of leptin, insulin and adiponectin were measured by ELISA kits (Merck Millipore, Dramstadt, Germany) following the manufacturer’s instructions. The sensitivity of the method for leptin, insulin and adiponectin was 0.04, 0.2, and 0.16 ng/mL respectively. The intraassay variation was between 1.9–2.5% for leptin, 0.9–8.4% for insulin, and 0.43–1.96% for adiponectin.
Lipid profile
Triglycerides, total lipids, total cholesterol, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) were measured in the serum using commercial kits from Spin React S.A.U (Sant Esteve de Bas, Gerona, Spain) following the manufacturer’s instructions.
Pro-inflammatory mediators
Interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) plasma levels were measured by an ELISA kit (Cusabio, Wuhan, China) following the manufacturer’s instructions. The sensitivity of the method was 0.078 pg/mL for IL-6 and 1.56 pg/mL for TNF-α. The intraassay and interassay variations were < 8% and < 10% respectively for both.
Incubation of aorta segments in presence/absence of insulin (10−7 M)
As previously described67, 2 mm-long segments from the thoracic aorta were placed in 6 well culture plates and incubated with 1 mL of Dulbecco’s Modified Eagle’s Medium and Ham’s F-12 medium (DMEM/F-12) with glutamine from Gibco (1:1 mix; Invitrogen, Carlsbad, CA, USA), supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin (Invitrogen, Carlsbad, CA, USA) in the presence/absence of insulin (10−7 M) (Sigma-Aldrich, St. Louis, MO, USA) at 37 °C in a 95% O2 and 5% CO2 incubator. The segments and the culture media were collected and stored at − 80 °C for further analysis after 30 min of incubation.
Nitrite and nitrate concentrations in the culture medium
As in González-Hedström et al.67, using a modified method of the Griess assay75, nitrite and nitrate concentrations were measured in the culture medium from aorta segment incubations. Briefly, to 100 μL of culture medium on a 96-well plate, 100 μL of vanadium chloride (Sigma‐Aldrich, St. Louis, MO, USA) were added. Immediately after, 100 μL of the Griess reagent (1:1 mixture of 1% sulfanilamide (Merck Millipore, Darmstadt, Germany) and 0.1% naphthylethylenediamine dihydrochloride (Merck Millipore, Darmstadt, Germany)) were added to each well and incubated at 37 °C for 30 min. Absorbance was measured at 540 nm after incubation. A NaNO2 standard curve was used to calculate nitrite and nitrate concentrations. Results were expressed in μM.
Protein quantification by Western Blot
An amount of 100 mg of arterial tissue was homogenized using RIPA buffer. After centrifugation (12.500 rpm; 4○C, 20 min), the supernatant was collected, and total protein content was analyzed by the Bradford method76. As previously described67, for each assay, resolving gels with SDS acrylamide (10%) (Bio-Rad, Hercules, CA, USA) were used and 100 μg of protein were loaded in each well. After electrophoresis, proteins were transferred to polyvinylidine difluoride (PVDF) membranes (Bio-Rad, Hercules, CA, USA) and transfer efficiency was determined by Ponceau red dyeing (Sigma-Aldrich, St. Louis, MO, USA). Membranes were then blocked with Tris-buffered saline (TBS) containing 5% (w/v) non-fat dried milk for non-phosphorylated protein or with 5% (w/v) bovine serum albumin (BSA) for phosphorylated protein and incubated with the appropriate primary antibody for Akt (1:1000; Merck Millipore; Dramstadt, Germany) and p-Akt (Ser473) (1:500; Cell Signaling Technology; Danvers, MA, USA). Membranes were subsequently washed and incubated with the corresponding secondary antibody conjugated with peroxidase (1:2000; Pierce, Rockford, IL, USA). Peroxidase activity was visualized by chemiluminescence and quantified by densitometry using BioRad Molecular Imager ChemiDoc XRS System (Hércules, CA, USA). All data are referred to % of control values (samples from young rats) on each gel.
Experiments of vascular reactivity
After sacrifice, the aorta was dissected and cut into 2 mm long segments for performing experiments of vascular reactivity as previously described67. Each segment was prepared for isometric tension recording in a 4-mL organ bath containing modified Krebs–Henseleit solution at 37 °C (mM): NaCl, 115; KCl, 4.6; KH2PO4, 1.2; MgSO4, 1.2; CaCl2, 2.5; NaHCO3, 25; glucose, 11. The solution was equilibrated with 95% O2 and 5% CO2 to a pH of 7.3–7.4. Briefly, two fine steel wires (100 µm) were passed through the lumen of the vascular segment. One wire was fixed to the organ bath wall while the other was connected to a strain gauge for isometric tension recording (Universal Transducing Cell UC3 and Statham Microscale Accessory UL5, Statham Instruments, Inc.). This arrangement allows to apply passive tension in a perpendicular plane to the long axis of the vascular cylinder. The changes in isometric force were recorded using a PowerLab data acquisition system (AD Instruments, Colorado Springs, CO, USA). After the setting process, an optimal passive tension of 1 g was applied to the vascular segments. After 60–90 min of equilibration, the vascular segments were stimulated with potassium chloride (100 mM) to determine the contractility of smooth muscle. Segments that failed to contract at least 0.5 g to KCl were discarded.
Using abdominal aortic segments, the vasoconstrictor response to accumulative doses of noradrenaline (10−9–10−4 M), endothelin-1 (ET-1) (10−9–10−7 M) or angiotensin-II (AngII) (10−11–10−6 M) was recorded. Results were expressed as percentage of the contraction to 100 mM KCl.
Thoracic aortic segments were used to study the vasodilator response. They were precontracted with phenylephrine 10−7.5 M to subsequently perform a cumulative dose–response curve in response to acetylcholine (10−9–10−4 M), sodium-nitroprusside (10−9–10−5 M) or insulin (10−8–10−5 M). Relaxation was expressed as percentage of the initial tone.
All drugs were obtained from Sigma-Aldrich, (St. Louis, MO, USA).
RNA extraction and purification
As in González-Hedström et al.67, total RNA was extracted from myocardial, liver and arterial tissue according to the Tri-Reagent protocol77 and quantified with Nanodrop 2000 (Thermo Fisher Scientific, Hampton, NH, USA). From 1 µg of total RNA, cDNA was synthesized by a high capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA)67, total RNA was extracted from myocardial, liver and arterial tissue according to the Tri-Reagent protocol77 and quantified with Nanodrop 2000 (Thermo Fisher Scientific, Hampton, NH, USA). From 1 µg of total RNA, cDNA was synthesized by a high capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA).
Quantitative real-time PCR
The gene expression of interleukin 1β (IL-1β, Rn00580432_m1), interleukin 6 (IL-6, Rn01489669_m1), interleukin 10 (IL-10, Rn01483988_g1), tumoral necrosis factor alpha (TNF-α, Rn01525859_g1), cyclooxigenase-2 (COX-2, Rn01483828_m1), inducible Nitric Oxide Synthase (iNOS, Rn00561646_m1), NADPH oxidase 1 (NOX1, n00586652_m1), NADPH oxidase 4 (NOX4, Rn00585380_m1), glutathione reductase (GSR, Rn01482159_m1), glutathione peroxidase (GPx, Rn00574703_m1), glucokinase (GCK, Rn00561265_m1), glycogen synthase kinase 3β (GSK3β, Rn01444108_m1), superoxide dismutase-1 (SOD1, Rn00566938_m1) and lipoxygenase (Alox5, Rn00563172_m1) were assessed in myocardial, liver and arterial tissues by quantitative real-time polymerase chain reaction (qPCR) using assay-on-demand kits (Applied Biosystems, Foster City, CA, USA) as previously described67. TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA, USA) was used for amplification according to the manufacturer’s protocol in a Step One machine (Applied Biosystems, Foster City, CA, USA). Values were normalized to the housekeeping gene 18S (Rn01428915_g1). According to manufacturer’s guidelines, the ∆∆CT method was used to determine relative expression levels78.
Statistical analysis
Values are expressed as means ± standard error of the mean (SEM) and analyzed by one-way ANOVA followed by Bonferroni post-hoc test using GraphPad Prism 5.0. (San Diego, California, USA). Body weight gain over time was analyzed by two-way ANOVA. A p value of < 0.05 was considered significant.
Supplementary Information
Acknowledgements
This project was funded by the call “Doctorados Industriales 2017” (IND2017/BIO7701), a grant from Community of Madrid (Spain). This program aims to promote the effective collaboration between Universities and Companies and provides funding for both, the development of the research project in the University and the Company, and to hire a PhD student (Daniel González-Hedström) over a three-year period by the Company (Pharmactive Biotech Products S.L.). Community of Madrid also funded the contract of María de la Fuente-Fernández through the Youth Employment Program (PEJ-2018-AI/SAL-11315).
Author contributions
M.G. participated in the conception and design of the study. M.G, D.G.H., A.L.G.V., S.A., M.F.F., P.A., M.P., T.P., A.I.M. and A.M.I.G. participated in the acquisition, analysis and interpretation of data. M.G., D.G.H. and A.L.G.V. wrote the manuscript. M.G. revised the manuscript. M.G. and A.M.I.G. managed the funding acquisition.
Data availability
All data generated or analyzed during this study are included in this published article.
Competing interests
As this work was carried out in collaboration with the pharmaceutical company Pharmactive Biotech Products S.L., authors González-Hedström D, Almodóvar P and Inarejos-García AM may have conflict of interest. However, the RP-HPLC-PAD-MS(ESI) analysis of the extract and the in vivo study has been performed by the academic researchers from Universidad Autónoma de Madrid and Universidad Complutense de Madrid, so, authors García Villalón AL, Amor S, de la Fuente-Fernández M, Prodanov M, Priego T, Martín AI and Granado M declare no potential conflict of interest. The sponsors had no role in the design, execution, interpretation and writing of the manuscript.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-87628-7.
References
- 1.Buckley BM. Healthy ageing: ageing safely. Eur Heart J. 2001;3(Suppl N):N6–10. doi: 10.1016/s1520-765x(01)90131-2. [DOI] [PubMed] [Google Scholar]
- 2.Tuchweber B, Salas M. Experimental pathology of aging. Methods Achiev Exp Pathol. 1975;7:167–226. [PubMed] [Google Scholar]
- 3.Dominguez LJ, Barbagallo M. The biology of the metabolic syndrome and aging. Curr Opin Clin Nutr Metab Care. 2016;19:5–11. doi: 10.1097/MCO.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 4.Tuomilehto J. Impact of age on cardiovascular risk: implications for cardiovascular disease management. Atheroscler Suppl. 2004;5:9–17. doi: 10.1016/j.atherosclerosissup.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Fleg JL, Strait J. Age-associated changes in cardiovascular structure and function: a fertile milieu for future disease. Heart Fail Rev. 2012;17:545–554. doi: 10.1007/s10741-011-9270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacNee W, Rabinovich RA, Choudhury G. Ageing and the border between health and disease. Eur Respir J. 2014;44:1332–1352. doi: 10.1183/09031936.00134014. [DOI] [PubMed] [Google Scholar]
- 7.Perez-Lopez FR, Chedraui P, Haya J, Cuadros JL. Effects of the Mediterranean diet on longevity and age-related morbid conditions. Maturitas. 2009;64:67–79. doi: 10.1016/j.maturitas.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Virruso C, et al. Nutraceutical properties of extra-virgin olive oil: a natural remedy for age-related disease? Rejuvenation Res. 2014;17:217–220. doi: 10.1089/rej.2013.1532. [DOI] [PubMed] [Google Scholar]
- 9.Barzi F, et al. Mediterranean diet and all-causes mortality after myocardial infarction: results from the GISSI-Prevenzione trial. Eur J Clin Nutr. 2003;57:604–611. doi: 10.1038/sj.ejcn.1601575. [DOI] [PubMed] [Google Scholar]
- 10.Potential Effects on Glycemia and Lipidemia Acar-Tek, N. & Agagunduz, D. Olive Leaf (Olea europaea L. folium) Ann Nutr Metab. 2020;76:10–15. doi: 10.1159/000505508. [DOI] [PubMed] [Google Scholar]
- 11.El SN, Karakaya S. Olive tree (Olea europaea) leaves: potential beneficial effects on human health. Nutr Rev. 2009;67:632–638. doi: 10.1111/j.1753-4887.2009.00248.x. [DOI] [PubMed] [Google Scholar]
- 12.Zuntar, I. et al. Phenolic and antioxidant analysis of olive leaves extracts (Olea europaea L.) obtained by high voltage electrical discharges (HVED). Foods8, doi:10.3390/foods8070248 (2019). [DOI] [PMC free article] [PubMed]
- 13.De la Ossa, J. G. et al. Waste Autochthonous Tuscan Olive Leaves (Olea europaea var. Olivastra seggianese) as Antioxidant Source for Biomedicine. Int J Mol Sci20, doi:10.3390/ijms20235918 (2019). [DOI] [PMC free article] [PubMed]
- 14.Luo, S. et al. In Vivo and In Vitro Antioxidant Activities of Methanol Extracts from Olive Leaves on Caenorhabditis elegans. Molecules24, doi:10.3390/molecules24040704 (2019). [DOI] [PMC free article] [PubMed]
- 15.Coban J, et al. Olive leaf extract decreases age-induced oxidative stress in major organs of aged rats. Geriatr Gerontol Int. 2014;14:996–1002. doi: 10.1111/ggi.12192. [DOI] [PubMed] [Google Scholar]
- 16.Mattioli, S. et al. Effect of feed supplemented with selenium-enriched olive leaves on plasma oxidative status, mineral profile, and leukocyte DNA damage in growing rabbits. Animals (Basel)10, doi:10.3390/ani10020274 (2020). [DOI] [PMC free article] [PubMed]
- 17.Burja B, et al. Olive leaf extract attenuates inflammatory activation and DNA damage in human arterial endothelial cells. Front Cardiovasc Med. 2019;6:56. doi: 10.3389/fcvm.2019.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khayyal MT, et al. Blood pressure lowering effect of an olive leaf extract (Olea europaea) in L-NAME induced hypertension in rats. Arzneimittelforschung. 2002;52:797–802. doi: 10.1055/s-0031-1299970. [DOI] [PubMed] [Google Scholar]
- 19.Romero M, et al. Antihypertensive effects of oleuropein-enriched olive leaf extract in spontaneously hypertensive rats. Food Funct. 2016;7:584–593. doi: 10.1039/c5fo01101a. [DOI] [PubMed] [Google Scholar]
- 20.Lockyer S, Rowland I, Spencer JPE, Yaqoob P, Stonehouse W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: a randomised controlled trial. Eur J Nutr. 2017;56:1421–1432. doi: 10.1007/s00394-016-1188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Susalit E, et al. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: comparison with Captopril. Phytomedicine. 2011;18:251–258. doi: 10.1016/j.phymed.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Saibandith, B., Spencer, J. P. E., Rowland, I. R. & Commane, D. M. Olive Polyphenols and the Metabolic Syndrome. Molecules22, doi:10.3390/molecules22071082 (2017). [DOI] [PMC free article] [PubMed]
- 23.Talhaoui, N., Gómez-Caravaca, A. M., León, L., De la Rosa, R., Segura-Carretero, A., Fernández-Gutiérrez, A. Determination of phenolic compounds of “Sikitita” olive leaves by HPLC-DAD-TOF-MS. Comparison with its parents “Arbequina” and “Picual” olive leaves. LWT- Food Sci. Technol.58, 28–34 (2014).
- 24.Casamenti F, Stefani M. Olive polyphenols: new promising agents to combat aging-associated neurodegeneration. Expert Rev Neurother. 2017;17:345–358. doi: 10.1080/14737175.2017.1245617. [DOI] [PubMed] [Google Scholar]
- 25.Mehraein F, Sarbishegi M, Golipoor Z. Different effects of olive leaf extract on antioxidant enzyme activities in midbrain and dopaminergic neurons of Substantia Nigra in young and old rats. Histol Histopathol. 2016;31:425–431. doi: 10.14670/HH-11-687. [DOI] [PubMed] [Google Scholar]
- 26.Elgebaly HA, et al. Olive oil and leaf extract prevent fluoxetine-induced hepatotoxicity by attenuating oxidative stress, inflammation and apoptosis. Biomed Pharmacother. 2018;98:446–453. doi: 10.1016/j.biopha.2017.12.101. [DOI] [PubMed] [Google Scholar]
- 27.Motlagh S, V. M., Arabsolghar R. The protective effects of Olive leaf extract on Type 2 diabetes, the expression of liver superoxide dismutase and total antioxidant capacity of plasma in rats. Trends in Pharmaceutical Sci.March, 43–52, doi:10.30476/TIPS.2020.82251.1038 (2020).
- 28.Wang HH, Lee DK, Liu M, Portincasa P, Wang DQ. Novel insights into the pathogenesis and management of the metabolic syndrome. Pediatr Gastroenterol Hepatol Nutr. 2020;23:189–230. doi: 10.5223/pghn.2020.23.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richter-Stretton GL, Fenning AS, Vella RK. Skeletal muscle: a bystander or influencer of metabolic syndrome? Diabetes Metab Syndr. 2020;14:867–875. doi: 10.1016/j.dsx.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Villani A, Wright H, Slater G, Buckley J. A randomised controlled intervention study investigating the efficacy of carotenoid-rich fruits and vegetables and extra-virgin olive oil on attenuating sarcopenic symptomology in overweight and obese older adults during energy intake restriction: protocol paper. BMC Geriatr. 2018;18:2. doi: 10.1186/s12877-017-0700-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov. 2009;3:73–80. doi: 10.2174/187221309787158371. [DOI] [PubMed] [Google Scholar]
- 32.Park MH, et al. Age-related inflammation and insulin resistance: a review of their intricate interdependency. Arch Pharm Res. 2014;37:1507–1514. doi: 10.1007/s12272-014-0474-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Javadi, H., Yaghoobzadeh, H., Esfahani, Z., Reza Memarzadeh, M. & Mehdi Mirhashemi, S. Effects of Olive Leaf Extract on Metabolic Response, Liver and Kidney Functions and Inflammatory Biomarkers in Hypertensive Patients. Pak. J. Biol. Sci.22, 342–348, doi:10.3923/pjbs.2019.342.348 (2019). [DOI] [PubMed]
- 34.Lockyer S, Corona G, Yaqoob P, Spencer JP, Rowland I. Secoiridoids delivered as olive leaf extract induce acute improvements in human vascular function and reduction of an inflammatory cytokine: a randomised, double-blind, placebo-controlled, cross-over trial. Br J Nutr. 2015;114:75–83. doi: 10.1017/S0007114515001269. [DOI] [PubMed] [Google Scholar]
- 35.Fki I, et al. Comparative study on beneficial effects of hydroxytyrosol- and oleuropein-rich olive leaf extracts on high-fat diet-induced lipid metabolism disturbance and liver injury in rats. Biomed Res Int. 2020;2020:1315202. doi: 10.1155/2020/1315202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong D, et al. Mechanisms of olive leaf extract-ameliorated rat arthritis caused by kaolin and carrageenan. Phytother Res. 2012;26:397–402. doi: 10.1002/ptr.3567. [DOI] [PubMed] [Google Scholar]
- 37.Aziz N, Kim MY, Cho JY. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J Ethnopharmacol. 2018;225:342–358. doi: 10.1016/j.jep.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Yu H, Jin Y, Li M, Qu C. Verbascoside alleviates atopic dermatitis-like symptoms in mice via its potent anti-inflammatory effect. Int Arch Allergy Immunol. 2018;175:220–230. doi: 10.1159/000486958. [DOI] [PubMed] [Google Scholar]
- 39.Ma H, Qin S, Zhao S. Osteoarthritis is prevented in rats by verbascoside via nuclear factor kappa B (NF-kappaB) pathway downregulation. Med Sci Monit. 2020;26:e921276. doi: 10.12659/MSM.921276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson D, Jackson T, Sapey E, Lord JM. Frailty and sarcopenia: the potential role of an aged immune system. Ageing Res Rev. 2017;36:1–10. doi: 10.1016/j.arr.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Cheurfa, M. et al. Hypocholesterolaemic and antioxidant properties of Olea europaea L. leaves from Chlef province, Algeria using in vitro, in vivo and in silico approaches. Food Chem. Toxicol.123, 98–105, doi:10.1016/j.fct.2018.10.002 (2019). [DOI] [PubMed]
- 42.Mahmoudi A, et al. Oleuropein and hydroxytyrosol rich extracts from olive leaves attenuate liver injury and lipid metabolism disturbance in bisphenol A-treated rats. Food Funct. 2018;9:3220–3234. doi: 10.1039/c8fo00248g. [DOI] [PubMed] [Google Scholar]
- 43.Olmez E, et al. Olive leaf extract improves the atherogenic lipid profile in rats fed a high cholesterol diet. Phytother Res. 2015;29:1652–1657. doi: 10.1002/ptr.5445. [DOI] [PubMed] [Google Scholar]
- 44.Poudyal H, Campbell F, Brown L. Olive leaf extract attenuates cardiac, hepatic, and metabolic changes in high carbohydrate-, high fat-fed rats. J Nutr. 2010;140:946–953. doi: 10.3945/jn.109.117812. [DOI] [PubMed] [Google Scholar]
- 45.Shah NR, Braverman ER. Measuring adiposity in patients: the utility of body mass index (BMI), percent body fat, and leptin. PLoS ONE. 2012;7:e33308. doi: 10.1371/journal.pone.0033308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arita Y, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 47.Ghadge AA, Diwan AG, Harsulkar AM, Kuvalekar AA. Gender dependent effects of fasting blood glucose levels and disease duration on biochemical markers in type 2 diabetics: A pilot study. Diabetes Metab Syndr. 2017;11(Suppl 1):S481–S489. doi: 10.1016/j.dsx.2017.03.041. [DOI] [PubMed] [Google Scholar]
- 48.Yamauchi T, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 49.Griffin TM, Huebner JL, Kraus VB, Yan Z, Guilak F. Induction of osteoarthritis and metabolic inflammation by a very high-fat diet in mice: effects of short-term exercise. Arthritis Rheum. 2012;64:443–453. doi: 10.1002/art.33332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aslam M, Madhu SV. Development of metabolic syndrome in high-sucrose diet fed rats is not associated with decrease in adiponectin levels. Endocrine. 2017;58:59–65. doi: 10.1007/s12020-017-1403-5. [DOI] [PubMed] [Google Scholar]
- 51.Kmiec Z, et al. Effects of fasting and refeeding on serum leptin, adiponectin and free fatty acid concentrations in young and old male rats. Gerontology. 2005;51:357–362. doi: 10.1159/000088698. [DOI] [PubMed] [Google Scholar]
- 52.Li FH, et al. Beneficial alterations in body composition, physical performance, oxidative stress, inflammatory markers, and adipocytokines induced by long-term high-intensity interval training in an aged rat model. Exp Gerontol. 2018;113:150–162. doi: 10.1016/j.exger.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 53.Abunab H, Dator WL, Hawamdeh S. Effect of olive leaf extract on glucose levels in diabetes-induced rats: a systematic review and meta-analysis. J Diabetes. 2017;9:947–957. doi: 10.1111/1753-0407.12508. [DOI] [PubMed] [Google Scholar]
- 54.Wainstein J, et al. Olive leaf extract as a hypoglycemic agent in both human diabetic subjects and in rats. J Med Food. 2012;15:605–610. doi: 10.1089/jmf.2011.0243. [DOI] [PubMed] [Google Scholar]
- 55.Liu YN, Jung JH, Park H, Kim H. Olive leaf extract suppresses messenger RNA expression of proinflammatory cytokines and enhances insulin receptor substrate 1 expression in the rats with streptozotocin and high-fat diet-induced diabetes. Nutr Res. 2014;34:450–457. doi: 10.1016/j.nutres.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 56.Keane, K. N., Cruzat, V. F., Carlessi, R., de Bittencourt, P. I., Jr. & Newsholme, P. Molecular events linking oxidative stress and inflammation to insulin resistance and beta-cell dysfunction. Oxid Med Cell Longev2015, 181643, doi:10.1155/2015/181643 (2015). [DOI] [PMC free article] [PubMed]
- 57.Jemai H, et al. Hepatoprotective effect of oleuropein-rich extract from olive leaves against cadmium-induced toxicity in mice. Biomed Res Int. 2020;2020:4398924. doi: 10.1155/2020/4398924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park JH, Jung JH, Yang JY, Kim HS. Olive leaf down-regulates the oxidative stress and immune dysregulation in streptozotocin-induced diabetic mice. Nutr Res. 2013;33:942–951. doi: 10.1016/j.nutres.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 59.Vezza, T. et al. The metabolic and vascular protective effects of olive (Olea europaea L.) leaf extract in diet-induced obesity in mice are related to the amelioration of gut microbiota dysbiosis and to its immunomodulatory properties. Pharmacol Res150, 104487, doi:10.1016/j.phrs.2019.104487 (2019). [DOI] [PubMed]
- 60.Yoon L, Liu YN, Park H, Kim HS. Olive leaf extract elevates hepatic PPAR alpha mRNA expression and improves serum lipid profiles in ovariectomized rats. J Med Food. 2015;18:738–744. doi: 10.1089/jmf.2014.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Achari, A. E. & Jain, S. K. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci18, doi:10.3390/ijms18061321 (2017). [DOI] [PMC free article] [PubMed]
- 62.Huang C, et al. High serum adiponectin levels predict incident falls among middle-aged and older adults: a prospective cohort study. Age Ageing. 2016;45:366–371. doi: 10.1093/ageing/afw043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johansson H, et al. High serum adiponectin predicts incident fractures in elderly men: osteoporotic fractures in men (MrOS) Sweden. J Bone Miner Res. 2012;27:1390–1396. doi: 10.1002/jbmr.1591. [DOI] [PubMed] [Google Scholar]
- 64.Komorita Y, et al. Serum adiponectin predicts fracture risk in individuals with type 2 diabetes: the Fukuoka Diabetes Registry. Diabetologia. 2017;60:1922–1930. doi: 10.1007/s00125-017-4369-1. [DOI] [PubMed] [Google Scholar]
- 65.Luo XH, et al. Adiponectin stimulates RANKL and inhibits OPG expression in human osteoblasts through the MAPK signaling pathway. J Bone Miner Res. 2006;21:1648–1656. doi: 10.1359/jbmr.060707. [DOI] [PubMed] [Google Scholar]
- 66.Gocmez SS, et al. Age Impaired endothelium-dependent vasodilation is improved by resveratrol in rat mesenteric arteries. J Exerc Nutrition Biochem. 2016;20:41–48. doi: 10.20463/jenb.2016.03.20.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gonzalez-Hedstrom, D. et al. A Mixture of Algae and Extra Virgin Olive Oils Attenuates the Cardiometabolic Alterations Associated with Aging in Male Wistar Rats. Antioxidants (Basel)9, doi:10.3390/antiox9060483 (2020). [DOI] [PMC free article] [PubMed]
- 68.The role of oleuropein Ivanov, M. et al. Highly potent antioxidant Olea europaea L. leaf extract affects carotid and renal haemodynamics in experimental hypertension. EXCLI J. 2018;17:29–44. doi: 10.17179/excli2017-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hansen K, et al. Isolation of an angiotensin converting enzyme (ACE) inhibitor from Olea europaea and Olea lancea. Phytomedicine. 1996;2:319–325. doi: 10.1016/S0944-7113(96)80076-6. [DOI] [PubMed] [Google Scholar]
- 70.Amor S, et al. Study of insulin vascular sensitivity in aortic rings and endothelial cells from aged rats subjected to caloric restriction: Role of perivascular adipose tissue. Exp Gerontol. 2018;109:126–136. doi: 10.1016/j.exger.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 71.Martel J, et al. Hormetic effects of phytochemicals on health and longevity. Trends Endocrinol Metab. 2019;30:335–346. doi: 10.1016/j.tem.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 72.Speciale A, Chirafisi J, Saija A, Cimino F. Nutritional antioxidants and adaptive cell responses: an update. Curr Mol Med. 2011;11:770–789. doi: 10.2174/156652411798062395. [DOI] [PubMed] [Google Scholar]
- 73.Efentakis P, et al. Effects of the olive tree leaf constituents on myocardial oxidative damage and atherosclerosis. Planta Med. 2015;81:648–654. doi: 10.1055/s-0035-1546017. [DOI] [PubMed] [Google Scholar]
- 74.Silván, J. M., Pinto-Bustillos, M. A., Vásquez-Ponce, P., Prodanov, M., Martínez-Rodríguez, A. Olive mill wastewater as a potential source of antibacterial and anti-inflammatory compounds against the food-borne pathogen Campylobacter.51, 177–185 (2018).
- 75.Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 76.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 77.Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques. 1993;15(532–534):536–537. [PubMed] [Google Scholar]
- 78.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.