Abstract
The conciliation between career and family is a relevant issue for working women, in particular during the first years of motherhood. Data about the state of the cardiac autonomic regulation in working women with preschoolers are lacking. Aim of this study was to compare the cardiac autonomic profile of female healthcare professionals with and without preschoolers via the analysis of the variability of the time distance between two consecutive R-wave peaks (RR) from standard 24-h Holter electrocardiogram (ECG). Fifty healthy active female healthcare professionals were enrolled: 25 with at least one preschooler (W_KID) and 25 without (W_NOKID). A standard Holter ECG was obtained during a regular working day. Segments of 5000 consecutive RRs were selected during daytime (DAY) and nighttime (NIGHT). Heart rate variability analysis was performed and the following parameters were considered for comparison between the two groups: mean (μRR), variance (σ2RR), and the absolute power in high frequency component (HF) of RR (HFRR) series. HFRR was considered as a marker of vagal cardiac modulation. Only µRR significantly increased from DAY to NIGHT in both groups (699 ± 88 vs 887 ± 140 ms in W_KID and 728 ± 90 vs 942 ± 166 ms in W_NOKID). Instead, σ2RR and HFRR increased from DAY to NIGHT only in W_NOKID (from 3334 ± 2153 to 4816 ± 4063 ms2 and from 356 ± 334 to 1397 ± 1629 ms2, respectively). W_KID showed lower σ2RR and HFRR during NIGHT, compared to W_NOKID (2336 ± 3170 vs 4816 ± 4063 ms2 and 556 ± 950 vs 1397 ± 1629 ms2, respectively). The perceived stress according to the visual analogue scale was similar in the two groups (4.7 ± 2.1 in W_KID, 5.7 ± 2.1 in W_NOKID). The presence of preschoolers lowered nocturnal cardiac vagal modulation in female healthcare professionals. This might represent an adaptation with a finalistic purpose, scilicet the facilitation of a prompt reaction in case of a child’s need.
Subject terms: Physiology, Cardiology, Health occupations, Risk factors
Introduction
The conciliation between career and family is a relevant issue. Women are often sandwiched between more and more competitive and demanding working activities and traditional family duties. Debate exists about the impact of the multiple-role engagement, i.e. family and work roles, on psychological stress and quality of life. The “Depletion Hypothesis” considers the multiple-role engagement as a factor contributing to overload and strain, while the “Enrichment Hypothesis” as a factor enhancing individual’s resources, social connections, power, prestige and emotional gratification1,2. In any case, there might be a potentially stressful condition that, in turn, might influence women’s health status.
Independently from the family engagement, the stress induced by the working load has been associated to an increased risk for cardiovascular diseases development, together with other risk factors, such as physical inactivity, smoking, poor diet habits, overweight, hypertension, dyslipidemia, and diabetes3–6. This increased risk could be related to an altered cardiac neural regulation due to a reduced vagal and/or an augmented sympathetic modulation to the heart. The sympathovagal imbalance would entail a diminished adaptive capability of the cardiac control to respond to external perturbations5.
The cardiac autonomic profile (CAP) can be studied by means of the heart rate variability (HRV), i.e. beat-to-beat variation of the cardiac cycle defined as the time interval between two successive R-wave peaks (RR) from the electrocardiogram (ECG). HRV analysis allows to infer vagal modulation directed to the sinus node. Indications about the state of sympathetic modulation can be deduced only under the hypothesis that sympatho-vagal balance is working7,8. These indices vary physiologically in response to a number of daily life challenges, such as orthostatism and physical activity, denoting the transitional adjustment of the system with a finalistic purpose9–13.
The same indices assume prognostic significance when modified as the result of pathological conditions. For instance, decreased overall HRV, increased sympathetic and/or decreased vagal activity directed to the heart after a myocardial infarction, in hypertension and heart failure are related to increased mortality and morbidity14–17. On the other hand, therapeutic interventions that improve the sympathovagal balance may positively affect prognosis18,19.
In addition, HRV studies have demonstrated that also pregnancy and menopause influence the CAP20–24. During pregnancy, a high sympathetic modulation directed to heart and vessels has been found in women at the late stage of pregnancy compared to the early stage25. After menopause, an overall decreased HRV and a cardiac neural sympathetic predominance have been described23. In these conditions it is still unclear whether the modified CAP is expression of a physiological adaptation or of an increased risk of unfavorable events.
Maternity also represents a delicate and intense period of life, in terms of both physical and mental engagement, in particular in the first years. In fact, it is known that anxiety and depression easily develop26 with possible repercussions on both mothers, offspring and their future health status26,27. In this perspective, the study of the CAP in this setting might add important information in understanding physiological and pathophysiological mechanisms. We hypothesize that the CAP of working women might be influenced by the strain determined by the double burden, i.e. workload and child care.
To test this hypothesis, we compared the CAP of two groups of female healthcare professionals28, one group with at least one preschooler, and a second group without. HRV from a standard 24-h ECG recording provided the indices of the daily and nocturnal CAP. As it is recognized that stress could influence the state of the CAP29, we also evaluated the degree of the perceived stress in the study population.
Methods
Population
The study population included 50 healthy women: 25 with at least one preschooler (W_KID) and 25 women without (W_NOKID). The sample was of convenience. They all were full-time active healthcare professionals working at IRCCS Istituti Clinici Scientifici Maugeri in Milan, Italy. Sample size calculation was performed over preliminary data collected on a subgroup of W_KID (9) and W_NOKID (8) on the RR variance parameter assuming a power of 0.90 and a level of significance of 0.05. Inclusion criteria were: (1) fertile age between 25 and 45 years; (2) absence of ECG abnormalities. Exclusion criteria were: (1) part-time workers or on maternity leave; (2) any history of cardiovascular, respiratory or metabolic disease; (3) any current pharmacological therapy known to influence the CAP; (4) any ongoing acute disease; (5) alcohol consumption > 24 g/day (> 250 ml of wine, > 660 ml of beer, > 80 ml of spirits)30; (6) moderate to heavy smoking (> 8 cigarettes daily)31. Criteria were verified at a pre-enrolment screening visit. The standard 12-lead ECG was acquired using a standard ECG equipment (Eli 250, Mortara Instrument, USA).
The protocol adhered to the principles of the Declaration of Helsinki and was approved by the local ethics committee “CE ICS Maugeri-IRCCS, Pavia” (2131CE). Each enrolled subject signed a written informed consent before participating in the experimental protocol.
Experimental protocol
At enrollment, each subject filled out a detailed questionnaire to collect information on demographics (date and place of birth, professional role, working experience), clinical history (smoking and alcoholic habits, menstrual cycle information, use of medications, previous or current medical problems), working schedules, social (type and hours of social activities per week), exercise (type and hours of physical exercise per week), and sleeping (hours of sleep per night, number and length of nocturnal awakenings) habits. A complete physical examination of all districts, checks of height, weight and arterial blood pressure via standard manual sphygmomanometer were performed.
At the beginning of a regular working day, avoiding the 3 days after a night shift and the 7 days after the first day of the menstrual cycle, a 3-lead 24-h ECG (360° eMotion FAROS, Mega Electronics, Finland; Sylco srl, Monza, Italy) was positioned. We asked the participants to avoid alcoholic and caffeinated beverages, as well as heavy physical activity, in the 24 hours preceding and during the ECG recording. The electrodes were adequately positioned to minimize noise. The sampling rate was 500 Hz. Participants were asked to go to bed by midnight and take diary of all activities. Before starting the recording, each subject rated the perceived stress on a Visual Analogue Scale (VAS), that consisted of an unmarked ruler with endpoints labelled as “no perceived stress” (0) and “very high perceived stress” (10)32,33. The scale therefore yielded a subjective score on perceived degree of stress between 0 and 10.
Time series extraction
The modified lead II was chosen to favor R wave identification. From the ECG signal, RR interval time series were derived by an automatic algorithm. RR interval was defined as the temporal distance between two consecutive R-wave peaks. Automatic detection of R-wave peaks was manually checked to avoid missing beats or errors. In presence of premature beats or artifacts, correction by means of cubic spline interpolation was implemented. The fraction of corrections never exceeded 5% of the total considered samples. Segments of 5000 consecutive RR intervals were selected during daytime (DAY, from 1 to 5 p.m.) and during nighttime (NIGHT, from 1 to 4 a.m.) for further analyses. On these selections, an iterated analysis on windows of 250 consecutive RR intervals, with superposition of 200, was performed. All the time and spectral indices were calculated over each time window and the median of the whole distribution was taken as representative34. After linear detrending, mean (μRR) and variance (σ2RR) of RR series were calculated, and expressed in ms and ms2, respectively.
Power spectral analysis
Parametric power spectral analysis was performed on RR series described by an autoregressive model. The model order was chosen according to Akaike information criterion and power spectral density was decomposed into spectral components characterized by a central frequency. Each frequency component was labeled as high frequency (HF) component if its central frequency dropped in the HF (0.15–0.4 Hz) band7. The absolute power in HF band of the RR series, HFRR, defined as the sum of power spectral components classified as HF and expressed in ms2, was considered as an index of the vagal modulation directed to the heart8,35.
Statistical analysis
Categorical data were reported as absolute number (percentage) and continuous data as mean ± standard deviation. The differences between W_KID and W_NOKID in continuous variables were tested by two-tailed Student t test in case of normal distribution (tested by means of Kolmogorov–Smirnov test) or Mann–Whitney rank test in case of non-normal distribution, while for categorical variables χ2 test was applied. Two-way repeated measures analysis of variance (one factor repetition, Holm-Sidak test for multiple comparisons) was performed to check the differences between the two groups (i.e. W_KID and W_NOKID) within the same experimental conditions (i.e. DAY and NIGHT) and the differences between experimental conditions within the same group. Pearson and Spearman correlation analyses were carried out to assess the correlation between CAP indices and the perceived degree of stress and the children’s age. Correlation analysis was carried out separately during DAY and NIGHT over the single groups (i.e. W_NOKID and W_KID) and pooling the data together regardless of groups and experimental condition. A p < 0.05 was always considered significant. Statistical analyses were carried out using Sigmaplot, Systat Software, Inc., Chicago, IL, version 11.0.
Results
All subjects completed the experimental protocol and referred a regular night of sleep during the Holter ECG recording. Awakenings were recorded on the personal diary. Eight W_NOKID and 10 W_KID reported 1 to 2 nocturnal awakenings. These periods were not considered for the analysis. An adequate period for HRV analysis was available in all subjects. The demographic and clinical features of the enrolled population are shown in Table 1. Ten women were on birth control pills (6 in W_NOKID and 4 in W_KID). No other regular medications were declared. As regards W_KID, an only child was present in 48% of cases, 2 children in 52% of cases. The mean age of children was 33.6 ± 18.1 months. The number of holidays and shifts was similar for W_KID and for W_NOKID. The percentage of subjects performing regular exercise (at least 1 day/week for at least 4 weeks) was also homogenous in the two groups, as well as the amount of sleeping hours per night.
Table 1.
Demographic and clinical features of the enrolled population.
| W_NOKID (n = 25) | W_KID (n = 25) | p | Cohen’s d | |
|---|---|---|---|---|
| Age, years | 35.4 ± 7.2 | 37.7 ± 5.6 | 0.220 | 0.357 |
| BMI (kg/m2) | 22.7 ± 3.7 | 23.4 ± 3.1 | 0.496 | 0.205 |
| SAP (mmHg) | 108 ± 11 | 109 ± 13 | 0.893 | 0.083 |
| DAP (mmHg) | 67 ± 10 | 70 ± 10 | 0.459 | 0.300 |
| Sleep per night (h) | 6.6 ± 0.9 | 6.4 ± 1.3 | 0.472 | 0.179 |
| Smoking, n (%) | 7 (28) | 5 (20) | 0.741 | 0.094 |
| Regular physical exercise, n (%) | 12 (48) | 8 (32) | 0.386 | 0.247 |
| Physical exercise (h/week) | 3.0 ± 1.6 | 2.7 ± 1.9 | 0.604 | 0.171 |
| Regular social activities, n (%) | 23 (92) | 17 (68) | 0.077 | 0.516 |
| Social activities (h/week) | 7.4 ± 8.0 | 3.3 ± 2.3 | 0.060 | 0.696 |
| Employment | 0.212 | 0.629 | ||
| Nurse, n (%) | 7 (28) | 10 (40) | ||
| Physician, n (%) | 4 (16) | 6 (24) | ||
| Physiotherapist, n (%) | 13 (52) | 6 (24) | ||
| Nursing assistant, n (%) | 1 (4) | 3 (12) | ||
| Working hours (h/day) | 7.9 ± 1.0 | 7.7 ± 1.4 | 0.174 | 0.164 |
| Working experience (years) | 10.9 ± 5.7 | 12.6 ± 4.5 | 0.280 | 0.332 |
W_NOKID, women without preschoolers; W_KID, women with at least one preschooler; BMI, body mass index; SAP, systolic arterial pressure; DAP, diastolic arterial pressure; p, type I error probability; Cohen’s d, Cohen’s standardized mean difference between groups. Data are presented as mean ± standard deviation or number (percentage).
The perceived stress according to the VAS was surprisingly similar in the two groups (W_KID had 4.7 ± 2.1 and W_NOKID 5.7 ± 2.1, p = 0.087), as shown in Fig. 1.
Figure 1.
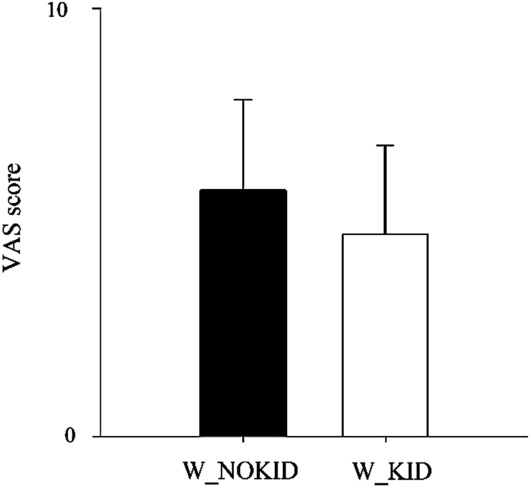
Results of perceived stress evaluation according to the Visual Analogue Scale (VAS) in W_NOKID (dark bar) and W_KID (white bar). Results are presented as mean ± standard deviation.
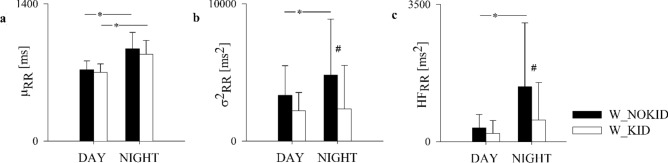
The results of the HRV analysis did differ as shown in Fig. 2. Only µRR significantly increased from DAY to NIGHT in both W_KID and W_NOKID (Fig. 2a), that is, as expected, healthy subjects are more bradycardic during the night. Instead, the expected increase of σ2RR and HFRR from DAY to NIGHT was observed only in W_NOKID. Thus, compared to W_NOKID, W_KID showed lower σ2RR and HFRR during NIGHT (Fig. 2b,c). In the W_KID group, the presence of one or two children did not differentiate the two small subgroups (12 vs 13 women).
Figure 2.
Results of power spectral analysis of heart rate variability during DAY and NIGHT in W_NOKID group (dark bars) and W_KID group (white bars). The indices μRR (a), σ2RR (b), and HFRR (c) are shown. Results are presented as mean ± standard deviation. The symbol * indicates p < 0.05 DAY vs NIGHT. The symbol # indicates p < 0.05 W_KID vs W_NOKID.
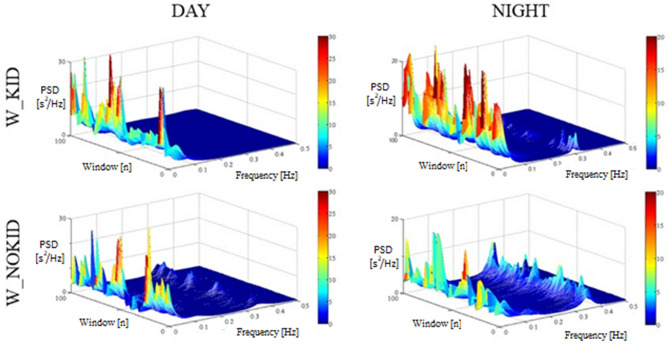
Figure 3 shows two representative examples of the power spectrum of the RR series during daytime (left side) and during nighttime (right side) in a 30-year-old female physician with a 2-year-old child (top panel) and in a 28-year-old nulliparous physician (bottom panel). Each 3D graph represents the power spectral density of the RR series (z-axis) for the considered frequency (x-axis), associated to each window of 250 consecutive RR intervals (y-axis). Of notice, during the night, the relative absence of peaks in the HF band in the woman with a child compared to the higher activity in the HF band of the nulliparous woman emphasizes the paradoxical modulation directed to the heart in presence of offspring.
Figure 3.
Illustrative examples of power spectral density of RR variability during DAY (left panels) and during NIGHT (right panels) computed in one subject of the W_KID group (top panels) and in one subject of W_NOKID group (bottom panels). Each 3D graph shows the power spectral density (PSD, z-axis) of the RR series as a function of the progressive frame of analysis (window, y-axis) and frequency (x-axis).
Table 2 summarizes the results of the correlation analysis between HRV indices and the level of perceived stress assessed by the VAS. No significant correlations were found nor considering W_KID and W_NOKID separately, nor pooling the data together regardless of groups and experimental conditions. Thus, CAP modifications seemed to be unrelated to the degree of perceived stress.
Table 2.
Results of the correlation between heart rate variability indices and VAS scale of perceived stress.
| W_NOKID | W_KID | |||||||
|---|---|---|---|---|---|---|---|---|
| r | p | ρ | p | r | p | ρ | p | |
| μRR during DAY (ms) | − 0.336 | 0.101 | 0.220 | 0.287 | − 0.009 | 0.965 | 0.033 | 0.873 |
| σ2RR during DAY (ms2) | − 0.089 | 0.670 | 0.075 | 0.717 | 0.001 | 0.999 | − 0.063 | 0.761 |
| HFRR during DAY (ms2) | − 0.028 | 0.894 | 0.066 | 0.750 | − 0.062 | 0.768 | 0.032 | 0.876 |
| μRR during NIGHT (ms) | − 0.139 | 0.508 | − 0.197 | 0.341 | 0.171 | 0.413 | 0.220 | 0.287 |
| σ2RR during NIGHT (ms2) | − 0.053 | 0.801 | − 0.028 | 0.890 | − 0.154 | 0.464 | 0.075 | 0.717 |
| HFRR during NIGHT (ms2) | − 0.014 | 0.946 | − 0.096 | 0.644 | − 0.166 | 0.428 | 0.067 | 0.750 |
W_NO KID, women without preschoolers; W_KID, women with preschoolers; RR, RR interval; μRR, mean of RR; σ2RR, RR variance; HFRR, absolute power in high frequency band of the RR; DAY, daytime; NIGHT, nighttime; r, Pearson product moment correlation coefficient; ρ, Spearman rank correlation coefficient; p, type I error probability. Data are presented as mean ± standard deviation.
Also the correlation analysis between HRV indices and children’s age showed no statistically significant results (Table 3).
Table 3.
Results of the correlation between heart rate variability indices and children’s age.
| r | p | ρ | p | |
|---|---|---|---|---|
| μRR during DAY (ms) | 0.098 | 0.642 | 0.104 | 0.615 |
| σ2RR during DAY (ms2) | − 0.197 | 0.345 | − 0.200 | 0.334 |
| HFRR during DAY (ms2) | − 0.072 | 0.732 | − 0.259 | 0.334 |
| μRR during NIGHT (ms) | − 0.065 | 0.759 | − 0.048 | 0.815 |
| σ2RR during NIGHT (ms2) | − 0.280 | 0.175 | − 0.277 | 0.178 |
| HFRR during NIGHT (ms2) | − 0.219 | 0.293 | − 0.228 | 0.270 |
DAY, daytime; NIGHT, nighttime; μRR, mean of RR; σ2RR, RR variance; HFRR, absolute power in high frequency band of the RR series; r, Pearson product moment correlation coefficient; ρ, Spearman rank correlation coefficient; p, type I error probability.
Discussion
In the present study, we demonstrated that the cardiac autonomic profile of a group of female healthcare professionals with preschoolers is different compared to that of a matched group of working women without children. During nighttime a decreased vagal modulation was demonstrated in the former group. However, such autonomic nervous system changes seemed to be unrelated to stress.
The enrolled subjects in the two groups were similar in respect of demographic and clinical parameters, life and working habits, and they were all healthy. The perceived stress was also similar in the two groups, as witnessed by the VAS scores. This finding was somehow surprising, as based on common experience and literature, women with double burdens (child care and workload) would be expected to define themselves as more stressed36,37. This seems to support the enrichment hypothesis1,2, according to which women who are involved in multiple social roles experience satisfaction rather than stress.
Thus, the presence of a commitment to childcare, rather than stress per se, would influence the CAP of the working women of this study.
In healthy subjects, the sympathovagal balance shifts towards an active mode38, instead the sleep is used as a period of cardiovascular relaxation and autonomic quiescence32.
In this study, heart rate physiologically decreased during nighttime in both groups, as mirrored by the µRR increase. Instead, the expected physiological increase of the vagal cardiac activity during the night, mirrored by higher σ2RR and higher HFRR, compared to daytime, was observed only in W_NOKID. Indeed, an altered variation of the vagal activity from daytime to nighttime was clearly identified in W_KID30.
While during daily hours the two groups of women were characterized by similar CAP, during the nighttime, W_NOKIDS turned to an autonomic “sleep mode”, while W_KID remained in an “active mode”, i.e. they diverted the expected physiological modification of CAP pattern during nighttime7,35,39. Of notice, this result seemed to be unrelated with the preschoolers’ age and the number of siblings.
This raises the question whether such modifications may jeopardize these women’s health, as it is well known that a decreased vagal modulation in resting conditions is an independent risk factor for all-cause mortality in several pathological situations, including myocardial infarction, hypertension, heart failure and diabetes14–17. On the other hand, it has to be underlined that W_KID showed a normal sympathovagal modulation during daytime with a paradoxical behavior only during the night. This CAP modification could be the results of an adaption modality to maintain a high level of alert aimed at maximizing the survival of both herself and the offspring throughout the 24 hours. Based on the fight or flight theory40, the sympathetic branch of the autonomic nervous system is more active, causing a concomitant de-activation of the vagal one, in presence of external challenges of the system or emergency situations, to cope with the acute stress13,41. As an example, in reaction to the passive orthostatic challenge applied during tilt test, the reduction of the vagal activity aims at maintaining the homeostasis11,13. In the case of a woman with the baby sleeping next door, a low level of vagal activity during the nocturnal hours would facilitate a prompt reaction in case of a child’s cry of hunger or a threat.
This is in keeping with the theory that cardiac vagal control may regulate behavioral, cognitive, and emotional responses by inhibiting the central autonomic network42.
Nevertheless, some important questions remain open. A prolonged or continuous decreased level of vagal activity could represent for the young mothers an additional risk factor for the development of future cardiovascular events13,41. Indeed, a reduced vagal activity directed to the heart during daytime was recently observed in mothers up to 2 years postpartum with symptoms of anxiety and depression43.
Moreover, stress and lack of sleep are recognized causes of altered heart rate variability29,44–46. Therefore, further longitudinal studies are advocated to verify if the observed vagal hypotonia is only transient, to deepen the relation between the cardiac controls and the mothers’ psychological status, and to eventually include a male population.
The main limitations of the present study are the lack of information about hormonal levels and detailed sleep assessment, which should be evaluated in future studies. However, in the present study, gross sleep parameters, i.e. bedtime, sleeping hours, number of awakenings were similar in both groups. A further limitation is the lack of an accurate evaluation of the perceived stress of the participants. The results of VAS score, although a weak tool given its subjectivity, suggest that this would be an interesting issue to explore. More sophisticated and objective measures of stress should be employed in future studies to add important insights.
Although this is a cross-sectional study of a single 24-h ECG recording, there is strong suggestion that the group of female healthcare professionals engaged in the care of young children seem to have a different cardiac autonomic profile from their peers without such commitment, that is, a reduced nocturnal vagal activity that can favor a prompt response and greater reactivity in case of any need of the offspring.
Supplementary Information
Acknowledgements
We thank all the participants who adhered to the experimental protocol. We thank Valeria Mansi, Chiara Andreotti and Lorena Grano de Oro for their precious help in organizing the experimental protocol during the first months.
Author contributions
L.A.D.V. devised the research protocol; V.D.G., L.A.D.V. and F.P. enrolled the population; L.A.D.V. and B.D.M. performed the experiments; L.A.D.V., B.D.M. and L.C. analyzed the data; B.D.M. prepared figures and tables; B.D.M. and L.A.D.V. drafted the manuscript; B.D.M., G.C., L.C., V.D.G., F.P., A.P. and L.A.D.V. edited and revised the manuscript; All authors approved the final version of the manuscript.
Data availability
Anonymized data are available upon reasonable request to the corresponding author. Potential data exchange will be conducted in accordance with the European legislation about data protection.
Competing interests
The authors declare no conflicts of interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-87596-y.
References
- 1.Sumra MK, Schillaci MA. Stress and the multiple-role woman: Taking a closer look at the "superwoman". PLoS ONE. 2015;10:e0120952. doi: 10.1371/journal.pone.0120952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chrouser CJ, Ryff CD. Multiple roles and well-being: Sociodemographic and psychological moderators. Sex Roles. 2006;50:230–238. [Google Scholar]
- 3.Rozanski A, Blumenthal JA, Davidson KW, Saab PG, Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: The emerging field of behavioral cardiology. J. Am. Coll. Cardiol. 2005;45:637–651. doi: 10.1016/j.jacc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Lucini D, Riva S, Pizzinelli P, Pagani M. Stress management at the worksite: Reversal of symptoms profile and cardiovascular dysregulation. Hypertension. 2007;49:291–297. doi: 10.1161/01.HYP.0000255034.42285.58. [DOI] [PubMed] [Google Scholar]
- 5.Jarczok MN, et al. Autonomic nervous system activity and workplace stressors—A systematic review. Neurosci. Biobehav. Rev. 2013;37:1810–1823. doi: 10.1016/j.neubiorev.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Hirokawa K, et al. Occupational status and job stress in relation to cardiovascular stress reactivity in Japanese workers. Prev. Med. Rep. 2016;4:61–67. doi: 10.1016/j.pmedr.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malik M, Bigger JT, Camm AJ, Kleiger RE, Malliani A, Moss AJ, Schwartz PJ. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 1996;17:354–381. doi: 10.1093/oxfordjournals.eurheartj.a014868. [DOI] [PubMed] [Google Scholar]
- 8.Pagani M, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ. Res. 1986;59:178–193. doi: 10.1161/01.RES.59.2.178. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi AC, et al. Aging reduces complexity of heart rate variability assessed by conditional entropy and symbolic analysis. Intern. Emerg. Med. 2012;7:229–235. doi: 10.1007/s11739-011-0512-z. [DOI] [PubMed] [Google Scholar]
- 10.De Maria B, et al. Cardiac baroreflex hysteresis is one of the determinants of the heart period variability asymmetry. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019;317:R539–R551. doi: 10.1152/ajpregu.00112.2019. [DOI] [PubMed] [Google Scholar]
- 11.Dalla Vecchia LA, et al. Can strenuous exercise harm the heart? Insights from a study of cardiovascular neural regulation in amateur triathletes. PLoS ONE. 2019;14:e0216567. doi: 10.1371/journal.pone.0216567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalla Vecchia L, Traversi E, Porta A, Lucini D, Pagani M. On site assessment of cardiac function and neural regulation in amateur half marathon runners. Open Heart. 2014;1:e000005. doi: 10.1136/openhrt-2013-000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malliani A. Principles of Cardiovascular Neural Regulation in Health and Disease. Kluwer Academic Publishers; 2000. [Google Scholar]
- 14.La Rovere MT, Bigger JT, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–484. doi: 10.1016/S0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 15.Pinna GD, et al. Different estimation methods of spontaneous baroreflex sensitivity have different predictive value in heart failure patients. J. Hypertens. 2017;35:1666–1675. doi: 10.1097/HJH.0000000000001377. [DOI] [PubMed] [Google Scholar]
- 16.Saxena T, Ali AO, Saxena M. Pathophysiology of essential hypertension: An update. Expert Rev. Cardiovasc. Ther. 2018;16:879–887. doi: 10.1080/14779072.2018.1540301. [DOI] [PubMed] [Google Scholar]
- 17.Sessa F, et al. Heart rate variability as predictive factor for sudden cardiac death. Aging (Albany NY) 2018;10:166–177. doi: 10.18632/aging.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalla Vecchia L, Mangini A, Di Biasi P, Santoli C, Malliani A. Improvement of left ventricular function and cardiovascular neural control after endoventriculoplasty and myocardial revascularization. Cardiovasc. Res. 1998;37:101–107. doi: 10.1016/S0008-6363(97)00236-8. [DOI] [PubMed] [Google Scholar]
- 19.Dalla Vecchia L, et al. Favorable effects of carotid endarterectomy on baroreflex sensitivity and cardiovascular neural modulation: A 4-month follow-up. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;304:R1114–R1120. doi: 10.1152/ajpregu.00078.2013. [DOI] [PubMed] [Google Scholar]
- 20.Giampaoli S, et al. Cardiovascular health in Italy. Ten-year surveillance of cardiovascular diseases and risk factors: Osservatorio Epidemiologico Cardiovascolare/Health Examination Survey 1998–2012. Eur. J. Prev. Cardiol. 2015;22:9–37. doi: 10.1177/2047487315589011. [DOI] [PubMed] [Google Scholar]
- 21.Koenig J, Thayer JF. Sex differences in healthy human heart rate variability: A meta-analysis. Neurosci. Biobehav. Rev. 2016;64:288–310. doi: 10.1016/j.neubiorev.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Kappus RM, et al. Sex differences in autonomic function following maximal exercise. Biol. Sex. Differ. 2015;6:28–015. doi: 10.1186/s13293-015-0046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Holzen JJ, Capaldo G, Wilhelm M, Stute P. Impact of endo- and exogenous estrogens on heart rate variability in women: A review. Climacteric. 2016;19:222–228. doi: 10.3109/13697137.2016.1145206. [DOI] [PubMed] [Google Scholar]
- 24.Harvey PJ, et al. After-exercise heart rate variability is attenuated in postmenopausal women and unaffected by estrogen therapy. Menopause. 2016;23:390–395. doi: 10.1097/GME.0000000000000568. [DOI] [PubMed] [Google Scholar]
- 25.Lucini D, Strappazzon P, Dalla Vecchia L, Maggioni C, Pagani M. Cardiac autonomic adjustments to normal human pregnancy: Insight from spectral analysis of R–R interval and systolic arterial pressure variability. J. Hypertens. 1999;17:1899–1904. doi: 10.1097/00004872-199917121-00019. [DOI] [PubMed] [Google Scholar]
- 26.Pawluski JL, Lonstein JS, Fleming AS. The neurobiology of postpartum anxiety and depression. Trends Neurosci. 2017;40:106–120. doi: 10.1016/j.tins.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Schultz LF, et al. Association of maternal depression and anxiety symptoms with sleep duration in children at preschool age. Matern. Child Health J. 2020;24:62–72. doi: 10.1007/s10995-019-02843-z. [DOI] [PubMed] [Google Scholar]
- 28.Bridgeman PJ, Bridgeman MB, Barone J. Burnout syndrome among healthcare professionals. Am. J. Health. Syst. Pharm. 2018;75:147–152. doi: 10.2146/ajhp170460. [DOI] [PubMed] [Google Scholar]
- 29.Carnevali L, Sgoifo A. Vagal modulation of resting heart rate in rats: The role of stress, psychosocial factors, and physical exercise. Front. Physiol. 2014;5:118. doi: 10.3389/fphys.2014.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stockwell T, et al. Do "moderate" drinkers have reduced mortality risk? A systematic review and meta-analysis of alcohol consumption and all-cause mortality. J. Stud. Alcohol. Drugs. 2016;77:185–198. doi: 10.15288/jsad.2016.77.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baumert J, et al. Determinants of heavy cigarette smoking: Are there differences in men and women? Results from the population-based MONICA/KORA Augsburg surveys. Nicotine Tob. Res. 2010;12:1220–1227. doi: 10.1093/ntr/ntq172. [DOI] [PubMed] [Google Scholar]
- 32.Lesage FX, Berjot S, Deschamps F. Clinical stress assessment using a visual analogue scale. Occup. Med. (Lond) 2012;62:600–605. doi: 10.1093/occmed/kqs140. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell AM, Crane PA, Kim Y. Perceived stress in survivors of suicide: Psychometric properties of the Perceived Stress Scale. Res. Nurs. Health. 2008;31:576–585. doi: 10.1002/nur.20284. [DOI] [PubMed] [Google Scholar]
- 34.Porta A, et al. An integrated approach based on uniform quantization for the evaluation of complexity of short-term heart period variability: Application to 24 h Holter recordings in healthy and heart failure humans. Chaos. 2007;17:015117. doi: 10.1063/1.2404630. [DOI] [PubMed] [Google Scholar]
- 35.Akselrod S, et al. Power spectrum analysis of heart rate fluctuation: A quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 36.Robinson GE. Stresses on women physicians: Consequences and coping techniques. Depress. Anxiety. 2003;17:180–189. doi: 10.1002/da.10069. [DOI] [PubMed] [Google Scholar]
- 37.Ross LE, McLean LM. Anxiety disorders during pregnancy and the postpartum period: A systematic review. J. Clin. Psychiatry. 2006;67:1285–1298. doi: 10.4088/JCP.v67n0818. [DOI] [PubMed] [Google Scholar]
- 38.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N. Engl. J. Med. 1993;328:303–307. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 39.Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84:482–492. doi: 10.1161/01.CIR.84.2.482. [DOI] [PubMed] [Google Scholar]
- 40.Cannon WB. Bodily Changes in Pain, Hunger, Fear and Rage. Appleton-Century-Crofts; 1915. [Google Scholar]
- 41.Curtis BM, O'Keefe JH. Autonomic tone as a cardiovascular risk factor: The dangers of chronic fight or flight. Mayo Clin. Proc. 2002;77:45–54. doi: 10.4065/77.1.45. [DOI] [PubMed] [Google Scholar]
- 42.Balzarotti S, Biassoni F, Colombo B, Ciceri MR. Cardiac vagal control as a marker of emotion regulation in healthy adults: A review. Biol. Psychol. 2017;130:54–66. doi: 10.1016/j.biopsycho.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 43.Izumi M, Manabe E, Uematsu S, Watanabe A, Moritani T. Autonomic nervous system activity and anxiety and depressive symptoms in mothers up to 2 years postpartum. J. Psychosom. Obstet. Gynaecol. 2016;37:51–56. doi: 10.3109/0167482X.2016.1142970. [DOI] [PubMed] [Google Scholar]
- 44.Virtanen I, Kalleinen N, Urrila AS, Leppanen C, Polo-Kantola P. Cardiac autonomic changes after 40 hours of total sleep deprivation in women. Sleep Med. 2015;16:250–257. doi: 10.1016/j.sleep.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Hintsanen M, et al. Effort-reward imbalance, heart rate, and heart rate variability: the Cardiovascular Risk in Young Finns Study. Int. J. Behav. Med. 2007;14:202–212. doi: 10.1007/BF03002994. [DOI] [PubMed] [Google Scholar]
- 46.Riese H, Van Doornen LJ, Houtman IL, De Geus EJ. Job strain in relation to ambulatory blood pressure, heart rate, and heart rate variability among female nurses. Scand. J. Work Environ. Health. 2004;30:477–485. doi: 10.5271/sjweh.837. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data are available upon reasonable request to the corresponding author. Potential data exchange will be conducted in accordance with the European legislation about data protection.