Abstract
Hepatic sinusoidal obstruction syndrome (HSOS) is a rare hepatic vascular disorder characterized by intrahepatic congestion, liver injury, and post-sinusoidal portal hypertension, and it is frequently associated with hematopoietic stem cell transplantation. In this study, we observed a case of HSOS associated with the ingestion of Gynura segetum, a pyrrolizidine alkaloid (PA)-containing Chinese herb, in a patient with alcoholic cirrhosis. The patient was a 43-year-old man with chief complaints of physical asthenia and a loss of appetite for more than a month. The diagnosis of HSOS combined with alcoholic cirrhosis was confirmed via the histopathological examination of liver tissues. With proper supportive and symptomatic care and anticoagulation therapy using low-molecular-weight heparin, the patient’s condition was stabilized. Because of its nonspecific symptoms in the early stage and a lack of information about PA consumption, PA-induced HSOS (PA-HSOS) has been long neglected, especially in patients with underlying liver diseases. Early identification and intervention are critical for optimizing outcomes. Further efforts are needed to supervise the use of PA-containing herbal medicines and identify accurate biomarkers for PA-HSOS.
Keywords: Hepatic sinusoidal obstruction syndrome, alcoholic cirrhosis, Gynura segetum, pyrrolizidine alkaloids, heparin, herbal medicine, liver
Introduction
Hepatic sinusoidal obstruction syndrome (HSOS) is a potentially life-threatening hepatic vascular disorder associated with intrahepatic congestion, liver injury, and post-sinusoidal portal hypertension, and its manifestations include hyperbilirubinemia, ascites, abdominal distension, and painful hepatomegaly. It has been most commonly reported as a side effect of induction regimens prior to hematopoietic stem cell transplantation (HSCT), and its incidence ranges 10% to 60%.1–3 Other causative agents include high-intensive chemotherapy, immunosuppressive drugs administered after organ transplantation, and radiation.4,5 Pyrrolizidine alkaloid (PA)-containing herbal remedies have emerged as another cause of HSOS. PAs are toxic secondary metabolites generated naturally by more than 6000 types of plants worldwide for defense against herbivores.6 PA intoxication in humans is often caused by self-medication with alkaloid-forming herbs or the unwitting ingestion of contaminated farm and sideline products.7,8 PA-induced HSOS (PA-HSOS) was first reported in 1920 and was described to be related to the ingestion of senecio tea, which is made from a PA-containing herb.9 Gynura segetum, a species of the Asteraceae family, is an alkaloid-forming herb that is also called “Tusanqi” in Chinese, and it shares a similar name as the disparate Chinese herb Panax notoginseng (“sanqi”). Thus, it may be easily misused as a folk medicine in the absence of proper instructions. In addition, although it was reported to promote microcirculation, anti-inflammation, anti-oxidation, and analgesia, people are often unaware of its PA-mediated toxicity because of the lack of medical supervision.10 In addition, PA-induced toxicity is more likely to be overlooked in patients with underlying liver diseases. In this study, we reported a case of HSOS in a patient with underlying alcoholic cirrhosis who had consumed G. segetum for 10 days.
Case description
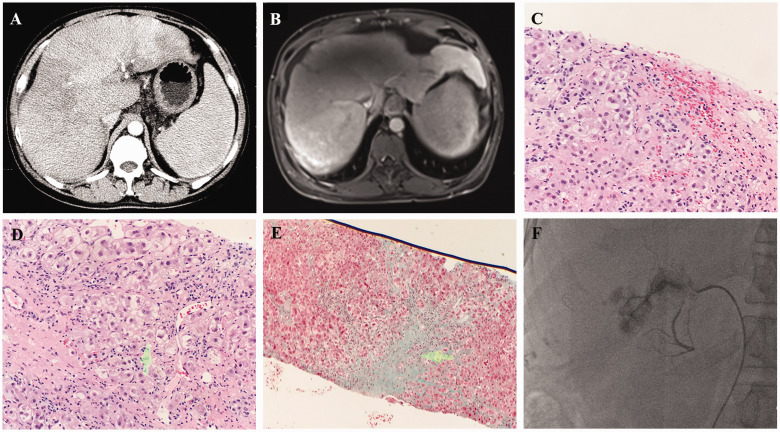
A 43-year-old man was hospitalized in the Affiliated Hangzhou First People’s Hospital of Zhejiang University School of Medicine on 4 December 2017 with physical asthenia and a loss of appetite for more than a month. The patient’s medical history included hypertension for 3 years. He had no history of heart, kidney, hematological, or hereditary metabolic disease. The patient had drunk 450 mL of 30% alcohol daily for 20 years. He consumed 150 g of medicinal liquor containing G. segetum per day for 10 days before developing symptoms. Physical examination revealed severe scleral jaundice, palmar erythema, and hepatosplenomegaly. Laboratory tests disclosed deteriorated liver function as indicated by the following findings: total bilirubin, 217.6 μmol/L; direct bilirubin, 156.6 μmol/L; alanine aminotransferase, 24 U/L; aspartate transaminase, 57 U/L; albumin, 32.5 g/L; alkaline phosphatase, 168 U/L; and γ-glutamate transaminase, 430 U/L. The prothrombin time was 13.7 s (international normalized ratio [INR] = 1.21). Hemogram revealed a normal white blood cell count but a low platelet count (89,000/μL) and low hemoglobin level (11 g/dL). Ascites examination revealed that the fluid was transudative. The total nucleated cell count was 70 × 106/L, the albumin level was 17.1 g/L, and the serum ascites albumin gradient exceeded 11 g/L. Viral hepatitis testing was negative. The patient’s immunoglobulin G level was within normal limits. Serial autoantibodies related to autoimmune liver diseases were not detected. Enhanced abdominal computed tomography (CT) disclosed hepatosplenomegaly, ascites, esophagocardial varices, an uneven density, and diffuse patchy shadows in the whole hepatic lobe (Figure 1a). The hepatic veins were not evident on the images. Abdominal magnetic resonance imaging (MRI) also revealed manifestations of cirrhosis. The liver exhibited heterogeneous enhancement, and the hepatic veins became narrow, making them not clearly visible (Figure 1b). According to the history of G. segetum ingestion; clinical features; blood test results; imaging findings; and the exclusion of viral hepatitis, autoimmune liver diseases, and other types of drug-induced liver injury, the patient was diagnosed with HSOS. For treatment, S-adenosyl-l-methionine injection (1 g/day) and ursodeoxycholic acid capsules (900 mg/day) were applied to promote cholagogic effects. Spironolactone and furosemide tablets were used to correct dehydration. After eliminating the possibility of hemorrhage, low-molecular-weight heparin (100 IU/kg every 12 hours) was preferred for anticoagulant therapy. No adverse events occurred during anticoagulant treatment. After 7 weeks of treatment, the patient’s clinical symptoms were improved. Blooding testing revealed that the total bilirubin level had decreased to 75.5 μmol/L, indicating the amelioration of liver dysfunction. Then, liver biopsy was performed 5 days after the discontinuation of low-molecular-weight heparin. The findings revealed hepatocyte edema, spotty necrosis, and congestive liver, which corresponded to the pathological changes of HSOS (Figure 1c). Histological features of alcoholic cirrhosis, such as pseudolobule formation, hyaline degeneration, and Mallory’s corpuscles (Figure 1d), were also found. Masson’s staining demonstrated fibrinogen thickening in sublobular venules (Figure 1e). After hospital discharge, the patient continued to receive anticoagulant treatment for 3 months. Digital subtraction angiography was performed during a follow-up visit on 23 March 2018. The findings indicated narrowing at the initial part of the left hepatic vein and proximal stenosis of the accessory hepatic vein with collateral circulation formation. There were no sites of narrowing or obstruction in the right and middle hepatic veins. The flow velocity of the three large branches of the hepatic vein was normal (Figure 1f). The laboratory test for liver function tended to be normal. Mild jaundice persisted because of the underlying alcoholic cirrhosis.
Figure 1.
Diagnosis of hepatic sinusoidal obstruction syndrome. (a) Enhanced abdominal computed tomography revealed hepatosplenomegaly, an uneven density, and diffuse patchy shadows in the whole hepatic lobe. (b) Enhanced magnetic resonance imaging revealed heterogeneous hepatic enhancement and significant stenosis in hepatic veins. (c) Hematoxylin–eosin staining of liver tissues revealed congestive liver (×10). (d) An image of Mallory’s corpuscles (arrow, ×10). (e) Masson’s staining revealed fibrinogen thickening of sublobular venules (×4). (f) Digital subtraction angiography disclosed narrowing at the initial part of the left hepatic vein and proximal stenosis of accessory hepatic vein with collateral circulation formation.
Discussion
HSOS is defined as a rare clinical syndrome of post-sinusoidal portal hypertension resulting from injury of the hepatic sinusoidal endothelium and obstruction of the hepatic sinus outflow tract. In recent decades, HSCT-induced HSOS (HSCT-HSOS) has been extensively reported and investigated. However, the ingestion of PA-containing herbals is currently the major cause of HSOS in China. G. segetum, a PA-producing plant, is usually used for bleeding injuries, hypertension, and stasis as a self-medication in rural areas in China.11 However, the severe outcomes of HSOS caused by G. segetum limit its clinical application, and they cannot be ignored.
The mechanisms of PA-HSOS have not been fully clarified. Following PA ingestion, metabolic activation is initiated by catalysis in the liver by cytochrome P450 monooxygenases, and the generated metabolite can rapidly combine with cellular DNA, proteins, or glutathione to form the corresponding adducts.12–14 The formation of protein adducts may impair the biological function of the target protein or activate immune responses, leading to toxicity.15 However, because of the diverse array of potential pyrrole–protein adducts, the specific target proteins and binding sites have not been fully identified. The metabolic activation of PAs also leads to extensive glutathione consumption in sinusoidal endothelial cells (SECs), causing oxidative stress injury in the liver and finally resulting in HSOS.16 The intraportal delivery of exogenous glutathione was demonstrated to protect against PA-induced toxicity based on the observation of clinical and histological improvement in a monocrotaline-induced HSOS rat model.17 This animal model also provided evidence that PA-induced toxicity suppressed the function of endothelial cell progenitors in bone marrow to repair damaged sinusoidal and central vein endothelial cells.18 Moreover, PA-induced cytotoxicity could depolymerize F-actin in SECs, leading to the upregulation of matrix metalloproteinase (MMP)-9 and MMP-2, which contributed to the degradation of the extracellular matrix in the space of Disse. Thus, the dehiscence of SECs and disassembly of the extracellular matrix destroyed the endothelial barrier and allowed blood infiltration into the space of Disse, resulting in the congestion and obstruction of hepatic sinusoids.19 Recently, Zhang et al.20 found that inflammatory signaling pathways and fibrogenic cytokines also played a pivotal role in the development of HSOS.
Currently, a few cases of HSOS caused by G. segetum have been reported in patients with no coexisting illness.21–23 The unique feature of our case is that the patient had alcoholic cirrhosis. He developed HSOS with the nonspecific clinical features of severe jaundice, hepatosplenomegaly, and ascites. Indeed, information about prior PA ingestion is usually difficult to acquire because patients are often unaware of herbal medicinal ingredients or they may ignore the use of herbal remedies, assuming they are safe. It follows that patients could be easily misdiagnosed with severe alcoholic hepatitis or decompensated liver cirrhosis if their medication history is neglected. There is a major change in the differential diagnosis. The patient had no apparent discomfort despite having liver cirrhosis. He did not increase his alcohol consumption, but he developed symptoms after consuming medicinal liquor containing G. segetum for 10 days, which attracted our attention. The combination of PA exposure, clinical manifestation, laboratory data, and CT imaging established the clinical diagnosis of PA-HSOS. The histological characteristics of alcoholic hepatitis are macrovesicular steatosis and lobular infiltration of neutrophils,24 which were not evident in the liver tissues of the patient. Therefore, the histological evidence further confirmed the diagnosis of PA-HSOS and concomitant alcoholic cirrhosis. However, percutaneous liver biopsy is often delayed because of massive ascites, coagulation disorder, or thrombocytopenia, and transjugular liver biopsy can only be performed in a certified hospital. The CT and MRI findings contributed to the diagnosis, including the observation of diffuse patchy liver enhancement, terminal hepatic venous narrowing or occlusion, and ascites.25 Therefore, when we encounter a patient suspicious for HSOS, it is crucial to repeatedly review the medical history and carefully analyze the auxiliary data. Early identification and intervention are critical for treating PA-HSOS. Increased bilirubin content was identified as the major prognostic factor for PA-HSOS.26 Conversely, the patient’s condition was ultimately stabilized, indicating the benefit of early anticoagulation therapy.
To date, there is no international consensus regarding the diagnosis and treatment of PA-HSOS. PA-HSOS differs from HSCT-HSOS in many aspects, including its pathogenesis, underlying diseases, ethnicity, and etiology.27 Therefore, the diagnostic criteria of HSCT-HSOS cannot be directly applicable to patients with PA-HSOS. The latest EBMT criteria28 for diagnosing HSCT-HSOS are based on a less strict version of the Baltimore criteria,29 namely the presence of at least two of the following findings: bilirubin ≥ 34.2 μmol/L, hepatomegaly, ascites, and weight gain > 5%. Cytoreductive therapy prior to HSCT is an essential precondition. The diagnosis of HSCT-HSOS relies on the combination of hemodynamic or/and ultrasound evidence combined with clinical manifestations or histological findings. Because the clinical features of PA-HSOS and HSCT-HSOS are similar, part of the diagnostic criteria of HSCT-HSOS can be applied to PA-HSOS. Recently, the Nanjing criteria30 were proposed by the Chinese Society of Gastroenterology Committee of Hepatobiliary to establish the diagnosis of PA-HSOS as follows: (1) a definite history of PA exposure; (2) the presence of three findings (clinical symptoms, abnormal liver function, and the typical features of enhanced CT or MRI) or histopathological evidence; and (3) the elimination of other known causes of liver injury. Histological diagnosis is the gold standard. Swelling and exfoliation of hepatic sinusoidal endothelial cells are believed to be the initial events in the development of HSOS. The hepatic sinusoids are significantly expansive and congestive. Varying degrees of atrophy and necrosis of hepatocytes can be observed. The venular walls are thickened by fibrin deposition, and narrowing of terminal hepatic venules is present.31 The management of PA-HSOS remains difficult for clinicians. The elimination of PA exposure, supportive care and symptomatic therapy, anticoagulation therapy, and liver transplantation have proven effective for the treatment of PA-HSOS. Symptomatic therapy is a basic and important treatment strategy, and the goals include liver protection, the management of ascites, and the improvement of microcirculation. The beneficial effect of anticoagulation therapy is controversial in patients with HSCT-HSOS.32 In addition, anticoagulation therapy is recommended for treating PA-HSOS, and it should be applied as soon as possible after excluding contraindications.30 Because low-molecular-weight heparin is safe at the recommended dose (100 IU/kg, every 12 hours, subcutaneous injection) and it has a low risk of hemorrhage, it is the preferred anticoagulant, and it can be used simultaneously with or prior to warfarin therapy. When warfarin is orally administered, patients must control INR within the range of 2.0 to 3.0, which may satisfy the needs for high anticoagulation intensity and safety. In addition, clinical manifestations and hepatorenal function are monitored during treatment. Defibrotide has been approved for the prevention and treatment of HSCT-HSOS.33 With its anti-ischemic, anti-inflammatory, thrombolytic, and pro-fibrinolytic effects as well as absence of systemic anti-coagulant properties, defibrotide has exhibited promise in the treatment of PA-HSOS.34 Transjugular intrahepatic portosystemic shunts (TIPS) can be considered to control intractable ascites and portal hypertension when medical therapies are not effective. Whether TIPS can improve long-term outcomes remains to be further investigated. Khoury et al.35 reported the efficacy of high-dose methylprednisolone in 20 patients who developed HSCT-HSOS. The response rate was 60%, and no serious side effects were observed. Evidence of the benefits of corticosteroids in treating PA-HSOS remains limited.
It has been suggested that PA-induced safety issues are widespread globally and inadequately addressed.36,37 Because of the wide distribution of PAs, people are at high risk of exposure, and PA exposure is easily overlooked in patients with underlying liver diseases. Because G. segetum-related HSOS lacks specific symptoms in its early stage, rapid recognition and proper treatment are extremely important. Recent attention has been focused on determining the pyrrole–protein adducts to identify accurate biomarkers for PA-HSOS.38,39 Further efforts are needed to regulate the use of PA-containing herbal medicines under clinical instruction and supervision. Randomized controlled trials are required to determine the optimal treatment options for PA-HSOS.
Acknowledgement
We acknowledge the pathologist for providing the histological images of the patients.
Footnotes
Ethics statement: Written informed consent was obtained from the patient for publication of this case report and related images. Ethics committee approval was not required for the publication of this report.
Declaration of conflicting interest: We declare that there is no conflict of interest.
Funding: This work was supported by Zhejiang Province Natural Science Foundation of China under Grant No. LQ18H070006.
ORCID iD: Jie Jin https://orcid.org/0000-0001-9512-859X
References
- 1.Coppell JA, Richardson PG, Soiffer R, et al. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transplant 2010; 16: 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dai N, Yu Y, Ren T, et al. Gynura root induces hepatic veno-occlusive disease: A case report and review of the literature. World J Gastroenterol 2007; 13: 1628–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helmy A. Review article: updates in the pathogenesis and therapy of hepatic sinusoidal obstruction syndrome. Aliment Pharmacol Ther 2006; 23: 11–25. [DOI] [PubMed] [Google Scholar]
- 4.Fan CQ, Crawford JM. Sinusoidal obstruction syndrome (hepatic veno-occlusive disease). J Clin Exp Hepatol 2014; 4: 332–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wadleigh M, Ho V, Momtaz P, et al. Hepatic veno-occlusive disease: pathogenesis, diagnosis and treatment. Curr Opin Hematol 2003; 10: 451–462. [DOI] [PubMed] [Google Scholar]
- 6.Schramm S, Kohler N, Rozhon W. Pyrrolizidine Alkaloids: Biosynthesis, Biological Activities and Occurrence in Crop Plants. Molecules 2019; 24: 498–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edgar J, Colegate S, Boppré M, et al. Pyrrolizidine alkaloids in food: a spectrum of potential health consequences. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2011; 28: 308–324. [DOI] [PubMed] [Google Scholar]
- 8.Letsyo E, Jerz G, Winterhalter P, et al. Incidence of Pyrrolizidine Alkaloids in Herbal Medicines from German Retail Markets: Risk Assessments and Implications to Consumers. Phytother Res 2017; 31: 1903–1909. [DOI] [PubMed] [Google Scholar]
- 9.Willmot FC, Robertson GW. Senecio disease, or cirrhosis of the liver due to Senecio poisoning. Lancet 1920; 2: 848–849. [Google Scholar]
- 10.Seow LJ, Beh HK, Umar MI, et al. Anti-inflammatory and antioxidant activities of the methanol extract of Gynura segetum leaf. Int Immunopharmacol 2014; 23: 186–191. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Qiao D, Li Y, et al. Risk factors for hepatic veno-occlusive disease caused by Gynura segetum: a retrospective study. BMC Gastroenterol 2018; 18: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He X, Xia Q, Ma L, et al. 7-cysteine-pyrrole conjugate: A new potential DNA reactive metabolite of pyrrolizidine alkaloids. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 2016; 34: 57–76. [DOI] [PubMed] [Google Scholar]
- 13.Xia Q, Ma L, He X, et al. 7-glutathione pyrrole adduct: a potential DNA reactive metabolite of pyrrolizidine alkaloids. Chem Res Toxicol 2015; 28: 615–620. [DOI] [PubMed] [Google Scholar]
- 14.Edgar JA, Molyneux RJ, Colegate SM. Pyrrolizidine Alkaloids: Potential Role in the Etiology of Cancers, Pulmonary Hypertension, Congenital Anomalies, and Liver Disease. Chem Res Toxicol 2015; 28: 4–20. [DOI] [PubMed] [Google Scholar]
- 15.Zhou S, Chan E, Duan W, et al. Drug bioactivation, covalent binding to target proteins and toxicity relevance. Drug Metab Rev 2005; 37: 41–213. [DOI] [PubMed] [Google Scholar]
- 16.Ma J, Xia Q, Fu PP, et al. Pyrrole-protein adducts - A biomarker of pyrrolizidine alkaloid-induced hepatotoxicity. J Food Drug Anal 2018; 26: 965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Kanel GC, DeLeve LD. Support of Sinusoidal Endothelial Cell Glutathione Prevents Hepatic Veno-occlusive Disease in the Rat. Hepatology 2000; 31: 428–434. [DOI] [PubMed] [Google Scholar]
- 18.Harb R, Xie G, Lutzko C, et al. Bone marrow progenitor cells repair rat hepatic sinusoidal endothelial cells after liver injury. Gastroenterology 2009; 137: 704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deleve L, Wang X, Tsai J, et al. Sinusoidal obstruction syndrome (veno-occlusive disease) in the rat is prevented by matrix metalloproteinase inhibition. Gastroenterology 2003; 125: 882–890. [DOI] [PubMed] [Google Scholar]
- 20.Zhang F, Zhou Y, Yang X, et al. Gynura Rhizoma containing pyrrolizidine alkaloids induces the hepatic sinusoidal obstruction syndrome in mice via upregulating fibrosis-related factors. Acta Pharmacol Sin 2019; 40: 781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin G, Wang J, Li N, et al. Hepatic sinusoidal obstruction syndrome associated with consumption of Gynura segetum. J Hepatol 2011; 54: 666–673. [DOI] [PubMed] [Google Scholar]
- 22.Li C, Liang XS, Li CZ. Sinusoidal obstruction syndrome associated with the ingestion of gynura root. Clin Toxicol (Phila) 2010; 48: 962–964. [DOI] [PubMed] [Google Scholar]
- 23.Dai H, Gao Y, Yang M, et al. Hepatic veno-occlusive disease induced by Gymura segetum: report of two cases. Hepatobiliary Pancreat Dis Int 2006; 5: 406–408. [PubMed] [Google Scholar]
- 24.Yang XQ, Ye J, Li X, et al. Pyrrolizidine alkaloids-induced hepatic sinusoidal obstruction syndrome: Pathogenesis, clinical manifestations, diagnosis, treatment, and outcomes. World J Gastroenterol 2019; 25: 3753–3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou H, Wang YX, Lou HY, et al. Hepatic sinusoidal obstruction syndrome caused by herbal medicine: CT and MRI features. Korean J Radiol 2014; 15: 218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Qi X, Guo X. Tusanqi-Related Sinusoidal Obstruction Syndrome in China: A Systematic Review of the Literatures. Medicine (Baltimore) 2015; 94: e942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhuge YZ, Wang Y, Zhang F, et al. Clinical characteristics and treatment of pyrrolizidine alkaloid-related hepatic vein occlusive disease. Liver Int 2018; 38: 1867–1874. [DOI] [PubMed] [Google Scholar]
- 28.Mohty M, Malard F, Abecassis M, et al. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant 2016; 51: 906–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones RJ, Beschorner WE, Vogel VG, et al . Venoocclusive disease of the liver following bone marrow transplantation. Transplantation 1987; 44: 778–783. [DOI] [PubMed] [Google Scholar]
- 30.Zhuge Y, Liu Y, Xie W, et al. Expert consensus on the clinical management of pyrrolizidine alkaloid-induced hepatic sinusoidal obstruction syndrome. J Gastroenterol Hepatol 2019; 34: 634–642. [DOI] [PubMed] [Google Scholar]
- 31.Chen Z, Huo J. Hepatic Veno-Occlusive Disease Associated With Toxicity of Pyrrolizidine Alkaloids in Herbal Preparations. Neth J Med 2010; 68: 252–260. [PubMed] [Google Scholar]
- 32.Imran H, Tleyjeh IM, Zirakzadeh A, et al. Use of prophylactic anticoagulation and the risk of hepatic veno-occlusive disease in patients undergoing hematopoietic stem cell transplantation: a systematic review and meta-analysis. Bone Marrow Transplant 2006; 37: 677–686. [DOI] [PubMed] [Google Scholar]
- 33.Dignan F, Wynn R, Hadzic N, et al. BCSH/BSBMT guideline: diagnosis and management of veno-occlusive disease (sinusoidal obstruction syndrome) following haematopoietic stem cell transplantation. Br J Haematol 2013; 163: 444–457. [DOI] [PubMed] [Google Scholar]
- 34.Morabito F, Gentile M, Gay F, et al. Insights into defibrotide: an updated review. Expert Opin Biol Ther 2009; 9: 763–772. [DOI] [PubMed] [Google Scholar]
- 35.Khoury H, Adkins D, Brown R, et al. Does early treatment with high-dose methylprednisolone alter the course of hepatic regimen-related toxicity? Bone Marrow Transplant 2000; 25: 737–743. [DOI] [PubMed] [Google Scholar]
- 36.Fu P, Chiang H, Xia Q, et al. Quality assurance and safety of herbal dietary supplements. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 2009; 27: 91–119. [DOI] [PubMed] [Google Scholar]
- 37.Li N, Xia Q, Ruan J, et al. Hepatotoxicity and Tumorigenicity Induced by Metabolic Activation of Pyrrolizidine Alkaloids in Herbs. Curr Drug Metab 2011; 12: 823–834. [DOI] [PubMed] [Google Scholar]
- 38.Gao H, Ruan JQ, Chen J, et al. Blood pyrrole-protein adducts as a diagnostic and prognostic index in pyrrolizidine alkaloid-hepatic sinusoidal obstruction syndrome. Drug Des Devel Ther 2015; 9: 4861–4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao H, Li N, Wang JY, et al. Definitive diagnosis of hepatic sinusoidal obstruction syndrome induced by pyrrolizidine alkaloids. J Dig Dis 2012; 13: 33–39. [DOI] [PubMed] [Google Scholar]