Abstract
Background
In recent decades there has been growing interest in the use of volatile organic compounds (VOCs) in exhaled breath as biomarkers for the diagnosis of multiple variants of cancer. This review aimed to evaluate the diagnostic accuracy and current status of VOC analysis in exhaled breath for the detection of cancer in the digestive tract.
Methods
PubMed and the Cochrane Library database were searched for VOC analysis studies, in which exhaled air was used to detect gastro-oesophageal, liver, pancreatic, and intestinal cancer in humans, Quality assessment was performed using the QUADAS-2 criteria. Data on diagnostic performance, VOCs with discriminative power, and methodological information were extracted from the included articles.
Results
Twenty-three articles were included (gastro-oesophageal cancer n = 14, liver cancer n = 1, pancreatic cancer n = 2, colorectal cancer n = 6). Methodological issues included different modalities of patient preparation and sampling and platform used. The sensitivity and specificity of VOC analysis ranged from 66.7 to 100 per cent and from 48.1 to 97.9 per cent respectively. Owing to heterogeneity of the studies, no pooling of the results could be performed. Of the VOCs found, 32 were identified in more than one study. Nineteen were reported as cancer type-specific, whereas 13 were found in different cancer types. Overall, decanal, nonanal, and acetone were the most frequently identified.
Conclusion
The literature on VOC analysis has documented a lack of standardization in study designs. Heterogeneity between the studies and insufficient validation of the results make interpretation of the outcomes challenging. To reach clinical applicability, future studies on breath analysis should provide an accurate description of the methodology and validate their findings.
This review provides an overview of the diagnostic performance and current status of volatile organic compound (VOC) analysis in exhaled breath for the detection of cancer in the digestive tract, including gastro-oesophageal, liver, pancreatic, and colorectal cancers. Some VOCs were cancer type-specific, whereas others were found in different cancer types. Their use in cancer screening is discussed.
Breath analysis and gastrointestinal cancer screening
Introduction
Cancer is one of the leading causes of premature death. With an increasing worldwide life expectancy, the prevalence of cancer and its burden on society is growing1. Early-stage cancers are often asymptomatic and therefore difficult to detect. Treatment options for cancer, and ultimately their success rates, are greatly dependent on the disease stage at the time of diagnosis. The 5-year survival rate of stage I colorectal cancer is approximately 97.7 per cent, but it drops to 43.9 per cent for stage IV. Similar reductions in 5-year survival rate are seen in other cancer types, including gastro-oesophageal, liver, and pancreatic cancers2. These survival rates indicate the importance of screening programmes in detecting cancers in an early stage. Current screening and diagnostic techniques are often invasive and not patient friendly. This review focuses on the detection of digestive tract malignancies, including colorectal, gastro-oesophageal, liver, and pancreatic cancers, by analysis of exhaled air.
Colorectal cancer is one of the largest causes of cancer-related deaths. Screening for colorectal cancer with known tumour markers, such as carcinoembryonic antigen or cancer antigen 19.9, are not ideal owing to low sensitivity and specificity3,4. Faecal blood tests, such as the guaiac faecal occult blood test and the more recent faecal immunohistochemical test (FIT), can be used as screening tools for colorectal cancer5. In 2014, a national screening programme was introduced in the Netherlands using the FIT, leading to earlier diagnosis of colorectal cancer. In the event of a positive test, which indicates an increased risk of colorectal cancer, colonoscopy is recommended6. Although the FIT is non-invasive and has a sensitivity of over 80 per cent, a malignancy is found during colonoscopy after only about 8 per cent of positive tests7.
Next to colorectal cancer, gastric carcinoma is a common digestive tract malignancy with reported late diagnosis and high mortality rates8. In high-incidence countries, including Japan, screening programmes using gastroscopy have shown a decrease in mortality9. However, a major drawback of this screening programme is the invasive character of the endoscopic procedures used and the risk of complications.
Liver cancer is far less common. Screening using regular ultrasound imaging is performed in patients with underlying risk factors, such as chronic viral hepatitis or alcohol intake10. Although it is non-invasive, its sensitivity is relatively low and is operator-dependent11.
The same holds for pancreatic cancer. Pancreatic tumours in less than 20 per cent of patients are operable at the time of diagnosis12, and screening (using endoscopic ultrasonography or MRI) is currently recommended only for patients with a genetic predisposition13. However, the prognosis is poor, symptoms are associated with disease progression, and deaths from the disease are increasing globally1,14,15.
There is a general need for improvement in screening techniques for digestive tract malignancies. The sensitivity and specificity of most screening tools are not high enough to reach clinically valuable post-test probabilities in a screening setting. Thus, it remains a challenge within global healthcare to develop more suitable diagnostic tools for tumour detection16,17.
In recent years, detection of cancer by analysis of volatile organic compounds (VOCs) in body materials has shown promising results. VOC analysis has a long-standing history in medical research. By 1971, the Nobel Prize winner Linus Pauling18 had detected 250 different compounds in breath using gas chromatography. Today, the value of VOC analysis in exhaled breath has been examined as monitoring tool in many diseases19, including the heart transplant rejection breath test20.
VOCs are carbon-based organic molecules, and their presence in exhaled breath can be divided into exogenously or endogenously derived compounds, according to their origin. Exogenous VOCs originate from environmental factors, such as food and beverage consumption, smoking, or other environmental exposures. Endogenous VOCs are produced as by- or end-products of human or microbial metabolism. Apart from breath, VOCs can be detected in sweat, blood, tissue samples, urine, and faeces21,22. At present, more than 800 different breath VOCs have been registered in the Chemical Abstracts Service system22. The composition of VOCs in exhaled breath can be altered owing to pathological processes such as the presence of cancer. Tumour-associated inflammation leading to enhanced oxidative stress, altered glucose metabolism, and redox regulation in cancer cells can lead to different VOC signatures in patients with cancer23–25. Breath analysis methods might be able to identify ‘breath signatures’ specific to those with cancer. This could be of value in clinical practice.
Analysis of VOC profiles can be performed using a variety of analytical platforms26. Currently, the most common systems in use are gas chromatography mass spectrometry (GC-MS), proton transfer reaction mass spectrometry (PTR-MS), and selected ion flow tube mass spectrometry (SIFT-MS). In addition, pattern recognition sensor systems are emerging that detect total VOC-binding patterns instead of individual VOCs. The latter systems are commonly referred to as an electronic nose or E-nose26,27. All systems have their strengths and limitations. Systems that allow selective quantification of VOCs are usually more laborious, require trained personnel, and are expensive in comparison to systems that register unselective VOC binding patterns, such as portable E-nose systems17.
The non-invasive nature of breath analysis makes it interesting for clinical use. Despite a long history of breath research, there are currently only a few applications in the clinic. This review provides an overview of the current literature on the identification of digestive tract cancer by means of VOC analysis in exhaled breath. The aim was to examine the diagnostic performance of VOC analysis and also to identify potential pitfalls in order to improve future research in this field.
Methods
Search strategy
An electronic search of PubMed and the Cochrane Library was performed in May 2019. Neoplasm, cancer, tumour, electronic nose, volatile organic compounds, VOC, exhaled breath, predictive value of tests, sensitivity, and specificity were used as search terms, and were combined using AND–OR combinations.
Studies of cancer diagnosis that met the following criteria were included: at least two different groups of patients were included in the study, with regard to the presence of cancer; the index test was analysis of endogenous VOCs in exhaled breath; and the disease type was cancer of the digestive tract (oesophagus, stomach, liver, pancreas, and bowel). Studies were excluded if they were published before 2000, were not performed in adult humans, did not analyse malignant diseases, or analysed biofluids (such as breath condensate, urine, blood, and faeces).
The selection of potentially eligible articles was performed according to the PRISMA guidelines28. Discrepancies between the selections were solved in a consensus meeting between the reviewers. The following information was gathered independently and tabulated from the articles by type of cancer: author(s), year of publication, index test, reference test, method of data analysis, comparison groups, sensitivity, specificity, accuracy, and area under the curve (AUC). All VOCs identified in the studies were tabulated.
Quality assessment
The methodological quality of the articles was assessed by means of the Quality Assessment of Diagnostic Studies 2 tool (QUADAS-2)29; a modified version was used30 (Table S1). The assessment was performed by two independent researchers and discrepancies were resolved by consensus.
Results
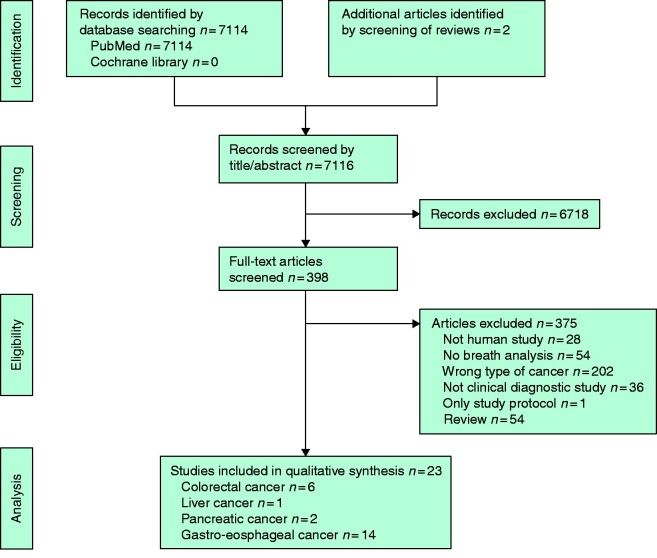
A total of 7114 studies were identified by the search in PubMed. After applying the eligibility criteria 21 articles were identified. Two articles were retrieved by manual search, and finally 23 articles31–53 were included in the review (Fig. 1).
Fig. 1.
PRISMA diagram showing selection of articles for review
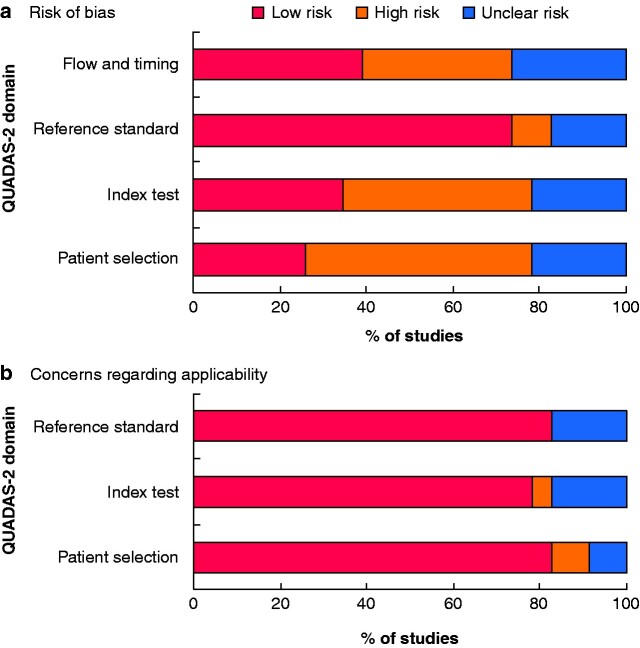
Quality assessment of the studies
An overview of the results of quality assessment is provided in Table 1 and Fig. 2. The risk of bias was highest for patient selection. The most common reasons for unclear or high risk of bias were unclear specification, or issues regarding the eligibility criteria. For the index test criterion, the most common reason for a high-risk assessment was not having performed a blinded validation of the diagnostic model.
Table 1.
Quality assessment for each article
| Reference | Risk of bias |
Concerns regarding applicability |
|||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| Gastro-oesophageal cancer | |||||||
| Abela et al.40 | + | – | – | – | + | – | + |
| Amal et al.32 | + | + | + | + | + | + | + |
| Amal et al.31 | + | – | + | ? | + | + | + |
| Chen et al.33 | + | – | + | ? | + | + | + |
| Daniel and Thangavel34 | + | ? | + | + | + | ? | + |
| Duran-Acevedo et al.35 | ? | – | + | ? | + | + | + |
| Kumar et al.42 | – | – | + | + | + | + | + |
| Kumar et al.41 | + | + | + | + | + | + | + |
| Markar et al.43 | – | + | + | + | + | + | + |
| Schuermans et al.36 | + | + | + | – | + | + | + |
| Shehada et al.37 | ? | + | ? | ? | + | + | ? |
| Tong et al.38 | ? | ? | ? | ? | + | – | ? |
| Xu et al.39 | – | + | + | ? | + | + | + |
| Zou et al.44 | ? | – | ? | ? | + | + | ? |
| Colorectal cancer | |||||||
| Altomare et al.49 | + | + | + | ? | + | + | + |
| Altomare et al.48 | – | – | + | – | – | + | ? |
| Amal et al.50 | + | + | + | + | + | + | + |
| Peng et al.51 | – | – | ? | – | + | – | + |
| van de goor et al.52 | + | + | + | ? | – | + | + |
| Wang et al.53 | – | – | + | + | + | + | + |
| Liver cancer | |||||||
| Qin et al.45 | ? | – | + | – | ? | + | + |
| Pancreatic cancer | |||||||
| Markar et al.46 | + | + | + | + | + | + | + |
| Princivalle et al.47 | ? | – | ? | – | + | + | + |
+, Low risk; –, high risk; ?, unclear risk.
Fig. 2.
Summary of risk of bias and concerns regarding applicability for included studies
a Risk of bias and b concerns regarding applicability.
For flow and timing, the most common reason for high risk of bias was not having attempted to limit exogenous and endogenous influences on VOC composition. Regarding the applicability of the studies to the study question, the overall applicability concern was scored tolerantly and assessed as relatively low.
Study characteristics
Fourteen articles describing studies in gastro-oesophageal cancer were included. The cancer population size ranged from 14 to 162 patients. Most studies (9) looked only at patients with gastric cancer31–39, whereas four studies40–43 included mixed oesophagogastric cancer. One study44 included only patients with oesophageal cancer. In most studies, the diagnosis was proven histologically; however, in four studies37,38,40,44, oesophagogastroduodenoscopy to rule out malignancy was not performed in controls.
Only one study45 included patients with liver cancer (30 patients). The patients had histologically proven stage I–V cancer and were compared with a group of healthy volunteers, and with a group of patients with hepatitis B-induced liver cirrhosis. The two control groups did not receive the same reference test as the cancer group. Patients with hepatitis B and cirrhosis were untreated and the disease confirmed histologically or cytologically. The healthy volunteers, however, who were the patient’s relatives and hospital staff with no history of cancer or other chronic disease, did not undergo any reference test.
Two studies46,47 included patients with histologically proven pancreatic cancer (25 and 65 patients respectively). The control groups consisted of perceived healthy controls in one study47, and patients suspected to have pancreatic disease who were scheduled for pancreatic imaging and found to be negative for malignancy in the other46.
Six studies48–53 analysed breath samples from patients with colorectal cancer. The study population ranged from 20 to 65 patients with cancer. Patients with stage I–IV disease were included in all but one study48–52; the other study53 included only patients with stage I–III tumours. The control group consisted of healthy controls in four studies49–51,53. One study52 compared VOCs from patients with colorectal cancer with those from patients with head and neck cancer (squamous cell carcinoma) or breast cancer. The remaining study48 was a follow-up analysis in which patients with colorectal cancer were compared with those with colorectal cancer from the original study49, who meanwhile had been treated and declared tumour-free. In addition, the follow-up patients were compared with healthy controls. All studies used histologically proven colorectal cancer as reference.
In general, many factors were heterogeneous across the studies. The eligibility criteria were sometimes not described clearly. Some studies included benign disease, whereas this was an exclusion criterion in other studies. There was also no consensus regarding how to deal with co-morbidities, and the timing of the index test compared with the reference test was not always at the same stage of the diagnostic process.
Patient preparation and sample collection
Measures to reduce influences of ambient air were taken in 21 of 23 studies (Table S2). Performing a lung washout was done in 10 of 23 studies, and sampling ambient air as a reference value was performed in 9 of 23. Nineteen of 23 studies described having taken measures to limit the influence of food and/or beverages. The timing of fasting before breath collection ranged from 2 h to more than 24 h. Withholding from alcohol consumption and/or smoking before measurement was mentioned explicitly in 10 of 23 studies, and was at least recorded in 17 of 23 studies. Other preparatory measures described were withholding from physical exercise, being in an emotional balance, gurgling with water before breath collection, and restraining from the use of toothpaste.
The timing of breath collection in the diagnostic process differed between the studies. Sample collection was performed using the following systems: Mylar® bags, Tedlar®bags, syringes, inert steel bags or chambers, nalophan sampling bags, BioVOCtm breath sampler, directly into PTR-MS instrument, and directly into e-nose. Research groups then stored and analysed the samples themselves or transported them to a laboratory that had access to the required analytical platform.
Analytical platforms and data analysis
A variety of methods were used to analyse VOCs from exhaled breath (Table 2). GC-MS and sensor array systems were most often used to analyse exhaled breath (8 studies)32,33,35,39,43,46,50,51, followed GC-MS alone (6 studies) 31,38,45,48,49,53. Other systems used were: SIFT-MS (2 studies)41,42, home-made PTR-MS (1)44, ultrasensitive tuneable diode laser spectrometer (1 study)40, trichloro(phenethyl)silane field effect transistor (1 study)37, and IMR-MS (1 study)47. Only three studies34,36,52 used sensor systems for analysis: AEONOSE (eNose company) (2 studies) and breath analyser (Figaro, USA) (1 study).
Table 2.
Overview of included articles by cancer type
| Reference | Analytical platform and data analysis | Reference test | Cancer type and stage, and group size | Sensitivity (%) | Specificity (%) | Accuracy (%) | AUC |
|---|---|---|---|---|---|---|---|
| Gastro-oesophageal cancer (n = 14) | |||||||
| Abela et al.40 | Ultrasensitive TDLS; Mann–Whitney U test and Kruskal–Wallis test |
TG: histology
CG: selected from University of Glasgow database for ethane levels in healthy adults, without history of GI tumours |
OC/GaC stage II–IV (n = 20) and HC (n = 10) | n.a. | n.a. | n.a. | n.a. |
| Amal et al.32 |
GC-MS; t test
Cross-reactive nanoarrays (GNPs and SWCNTs covered with different ligands); DFA |
TG: morphologically confirmed adenocarcinomas
CG: upper endoscopy |
Training phase
1) GaC stage I–IV (n = 69) and OLGIM 0–IV (n = 230) 2) GaC stage I–IV (n = 69) and OLGIM 0 (n = 109) 3) GaC stage I–IV (n = 69) and OLGIM 0–II (n = 204) 4) GaC stage I–IV (n = 69) and OLGIM III–IV (n = 24) 5) GaC stage I–IV (n = 69) and OLGIM I–IV (n = 120) 6) GaC stage I–IV (n = 69) and PUD (n = 38) Validation phase 1) GaC stage I–IV (n = 30) and OLGIM 0–IV (n = 95) 2) GaC stage I–IV (n = 30) and OLGIM 0 (n = 46) 3) GaC stage I–IV (n = 30) and OLGIM 0–II (n = 87) 4) GaC stage I–IV (n = 30) and OLGIM III–IV (n = 10) 5) GaC stage I–IV (n = 30) and OLGIM I–IV (n = 50) 6) GaC stage I–IV (n = 30) and PUD (n = 30) |
Training phase
1) 84 2) 93 3) 94 4) 96 5) 94 6) 93 Validation phase 1) 73 2) 90 3) 97 4) 93 5) 93 6) 87 |
Training phase
1) 86 2) 84 3) 91 4) 83 5) 95 6) 89 Validation phase 1) 98 2) 80 3) 84 4) 80 5) 80 6) 87 |
Training phase
1) 85 2) 88 3) 92 4) 92 5) 95 6) 92 Validation phase 1) 92 2) 84 3) 87 4) 90 5) 85 6) 87 |
n.a. |
| Amal et al.31 | TD-GC-MS; Wilcoxon/Kruskal–Wallis test |
TG: upper endoscopy + histology
CG: upper endoscopy |
China
1) GaC stage I–IV (n = 37) and control (combined) (n = 61) Latvia 2) GaC stage I–IV (n = 37) and control (combined) (n = 61) |
n.a. | n.a. | n.a. | n.a. |
| Chen et al.33 |
SPME-GC-MS; t test
SERS sensor; ANOVA (Diagnostic tool developed based on simulated breath samples and validated on real patients) |
TG: gastroscopy + histology
CG: endoscopy + histology |
1) EGC (n = 55) and control (n = 56) + AGC (n = 89)
2) AGC (n = 89) and control (n = 56) + EGC (n = 55) |
1) 87.3
2) 89.9 |
1) 94.1
2) 92.0 |
n.a. | n.a. |
| Daniel and Thangavel34 |
3 metal oxide semiconductor gas sensor arrays (TGS813, TGS822, TGS2620) (Figaro); ANN—CFBP and FFBP
(Diagnostic model trained with 90 per cent of data and 10 per cent used for validation) |
TG: gastroscopy and biopsy when abnormalities discovered
CG: gastroscopy |
GaC (n = 49) versus controls (mix) (n = 112) | 94.4 | 89.9 | 93 | n.a. |
| Duran-Acevedo et al.35 |
SPME-GC-MS (GC/Q-TOF); PCA
And/or chemical gas sensor with AGD; PCA |
TG: gastroscopy + histology
CG: gastroscopy (+ histology) |
GC-MS analysis
1) GaC (n = 14) and controls (mix) (n = 16) Chemical sensor analysis 2) CG (n = 11) and controls (mix) (n = 16) |
GC-MS analysis
1) 93 Chemical sensor analysis 2) 100 |
GC-MS analysis
1) 87 Chemical sensor analysis 2) 93 |
GC-MS analysis
1) 90 Chemical sensor analysis 2) 97 |
n.a. |
| Kumar et al.42 | SIFT-MS + MIM; Mann–Whitney U test and LLR |
TG: histology
CG: OGD |
Diagnostic model based on 4 VOCs
OC/GaC (n = 18) and control (benign) (n = 18) |
n.a. | n.a. | n.a. | 0.91 |
| Kumar et al.41 |
SIFT-MS; Mann Whitney U test
(Diagnostic model based on binary LLR; 2/3 for model development, 1/3 for validation with accuracy based on mean of 10× monte Carlo simulations) |
TG: OGD + histology
CG: OGD |
VOC data analysis
1) GaC stage I–III (n = 33) and HC (n = 62) 2) GaC stage I–III (n = 33) and control (mix) (n = 129) 3) OC stage I–III (n = 48) and HC (n = 62) 4) OC stage I–III (n = 48) and control (mix) (n = 129) Diagnostic prediction model: training phase 5) GaC/OC stage I–III (n = 53) and control (mix) (n = 85) Diagnostic prediction model: validation phase 6) GaC/OC stage I–III (n = 28) and control (mix) (n = 44) |
VOC data analysis
1) 100 2) 87.9 3) 98 4) 87.5 Diagnostic prediction model: training phase 5) 89.3 Diagnostic prediction model: validation phase 6) 86.7 |
VOC data analysis
1) 92.2 2) 88.5 3) 91.7 4) 82.9 Diagnostic prediction model: training phase 5) 83.7 Diagnostic prediction model: validation phase 6) 81.7 |
n.a. |
VOC data analysis
1) 0.98 2) 0.92 3) 0.97 4) 0.90 Diagnostic prediction model; training phase 5) 0.92 Diagnostic prediction model: validation phase 6) 0.87 |
| Markar et al.43 |
SIFT-MS (+ cross validation with GC-MS)
(5-VOC-predictive model based on multivariable LLR) |
TG: histologically proven OG cancer (non-metastatic)
CG: gastroscopy |
OC/GaC stage I–IV (n = 162) and controls (benign mix) (n = 163) | 80 | 81 | n.a. | 0.85 |
| Schuermans et al.36 | AEONOSE; ANN |
TG: after confirmed tumour diagnosis
CG: family members screened by endoscopy and negative for gastric malignancies |
GaC (n = 16) and HC (n = 28) | 81 | 71 | 75 | 0.83 |
| Shehada et al.37 |
TPS-SiNW FET (individually modified); DFA
(Diagnostic model based on DFA with 75 per cent of samples used as training set and 25 per cent for blinded validation) |
n.a. |
Training phase
1) GaC stage I–IV (n = 22) and control (mix) (n = 58) Validation phase 2) GaC stage I–IV (n = 8) and control (mix) (n = 19) |
Training phase
1) 87 Validation phase 2) 71 |
Training phase
1) 81 Validation phase 2) 89 |
Training phase
1) 83 Validation phase 2) 85 |
n.a. |
| Tong et al.38 | SPME-GC-MS; PCA, PLSDA (with VIP) and two-sided Welch 2-sample t test | n.a. |
1) GC (n = 24) and HC (n = 32)
2) GC (n = 24) and PUD (n = 24) 3) CG (n = 24) and gastritis (n = 48) |
n.a. | n.a. | n.a. | n.a. |
| Xu et al.39 |
GC-MS; Wilcoxon Kruskal–Wallis test
Nanomaterial-based sensor array; DFA (training on 100 per cent of samples, validation on blinded 25 per cent of samples) |
TG: endoscopy and histology
CG: endoscopy (+biopsy) |
1) GaC stage I–IV (n = 37) and control (benign mix) (n = 93)
2) GaC stage I–IV (n = 37) and GU (n = 32) and control (benign) (n = 61) 3) GaC stage I–IV (n = 6) and control (benign mix) (n = 26) |
Training phase
1) 89 Validation phase 3) 83 |
Training phase
1) 90 Validation phase 3) 96 |
Training phase
1) 90 2) 77 Validation phase 3) 94 |
n.a. |
| Zou et al.44 | Home-made PTR-MS (Ion Sniffer 2020Q); Mann–Whitney U test and SDA |
TG: diagnosed with OC, not further specified
CG: not specified |
SDA analysis based on 20 VOCS
1) OC stage I–IV (n = 29) and HC (n = 57) 2) OC stage I (n = 1) and HC (n = 57) 3) OC stage II (n = 7) and OC (n = 57) 4) OC stage III (n = 7) and HC (n = 57) 5) OC stage IV (n = 14) and HC (n = 57) ROC analysis using 7 VOCs 6) OC stage I–IV (n = 29) and HC (n = 57) |
SDA analysis based on 20 VOCS
1) 86.2 |
SDA analysis based on 20 VOCS
1) 89.5 |
SDA analysis based on 20 VOCS
2) 100 3) 71 4) 86 5) 93 |
ROC analysis
6) 0.943% |
| Liver cancer (n = 1) | |||||||
| Qin et al.45 |
SPME-GC-MS; Mann–Whitney U test
(Diagnostic model based on Fisher linear discriminant functions, cross-validation and leave-1-out procedure) |
TG: cytology or histology
CG (cirrhosis): clinically diagnosed with hepatocirrhosis induced by hepB virus CG: relatives and hospital staff |
Per VOC analysis in different groups
HCC stage I–IV + hepB (n = 30) and HC (n = 36) 1) 3-Hydroxy-2-butanone 2) Styrene 3) Decane HCC stage I–IV +HepB (n = 30) and cirrhosis +HepB (n = 27) 4) 3-Hydroxy-2-butanone 5) Styrene 6) Decane Diagnostic model 7) HCC stage I–IV + HepB (n = 30) and HC (n = 36) |
Per VOC analysis
HCC and HC 1) 83.3 2) 66.7 3) 86.7 HCC and cirrhosis 4) 70.0 5) 66.7 6) 76.7 Diagnostic model 7) 86.7 |
Per VOC analysis
HCC and HC 1) 91.7 2) 94.4 3) 58,3 HCC and cirrhosis 4) 70.4 5) 70.4 6) 48.1 Diagnostic model 7) 91.7 |
n.a. |
Per VOC analysis
HCC and HC 1) 0.926 2) 0.812 3) 0.798 HCC and cirrhosis 4) 0.745 5) 0.686 6) 0.637 |
| Pancreatic cancer (n = 2) | |||||||
| Markar et al.46 | TD-GC-MS; Mann– Whitney U test and LLR |
TG: CT abdomen/endoscopic ultrasonography + histologically by FNA
CG: recruited with a pancreatic condition or other patients scheduled for pancreatic ultrasonography or abdominal CT, included when negative on imaging |
Training phase
1) PC (mix) (n = 25) and no cancer mix (n = 43) 2) AC (n = 17) and no cancer mix (n = 43) 3) AC, local (n = 7) and no cancer (n = 43) Validation phase 4) PC (mix) (n = 32) and no cancer mix (n = 32) 5) AC (n = 28) and no cancer mix (n = 32) 6) AC, local (n = 14) and no cancer (n = 32) |
Training phase
1) 80 2) 94 3) 100 Validation phase 4) 81 5) 70 6) 79 |
Training phase
1) 95 2) 91 3) 100 Validation phase 4) 51 5) 74 6) 81 |
n.a. |
Training phase
1) 0.901 (0.819-0.982) 2) 0.99 (0.973-1.00) 3) 0.10 Validation phase 4) 0.736 (0.614-0.858) 5) 0.744 (0.615-0.873) 6) 0.855 (0.732-0.914) |
| Princivalle et al.47 |
IMR-MS (AirSense analyser; V&F); LASSO and LLR
(Diagnostic model based on age + 10 VOCs) |
TG: cytohistology
CG: perceived healthy controls |
PDA (n = 65) and HC (n = 102) |
Diagnostic model
100 |
Diagnostic model
84.3 |
n.a. |
Diagnostic model
0.987 |
| Colorectal cancer (n = 6) | |||||||
| Altomare et al.49 | GC-MS; PNN |
TG: histology
CG: colonoscopy Validation phase: not specified |
Trial phase
1) CRC stage I–IV (n = 37) and HC (n = 41) Validation phase 2) CRC stage I–IV (n = 15) and HC (n = 10) |
Trial phase
Validation phase 2) n.a. |
Trial phase
1) 83 (68, 93)* Validation phase 2) n.a. |
Trial phase
1) 85 Validation phase 2) 76 |
Trial phase
1) 0.85.2 |
| Altomare et al.48 | TD-GC-MS; PNN and Mann– Whitney U test |
TG: histology
CG: colonoscopy |
31-VOC model
1) CRC stage I–IV (n = 48) and CRC-FU (n = 32) 2) CRC-FU (n = 32) and HC (n = 55) 11-VOC model (overlapping VOCs with previous study) 3) CRC stage I–IV (n = 48) and CRC-FU (n = 32) 4) CRC-FU (n = 32) and HC (n = 55) |
31-VOC model
1) 100 (92.6, 100)* 2) 100 11-VOC model 3) 100 4) 100 |
31-VOC model
1) 95.8 (83.8, 99.9)* 2) 96.4 11-VOC model 3) 97.9 4) 90.9 |
31-VOC model
1) 97.5 2) 97.7 11-VOC model 3) 98.8 4) 94.3 |
31-VOC model
1) 0.993 2) 0.992 11-VOC model 3) 0.10 4) 0.959 |
| Amal et al.50 |
GC-MS; Student’s t test
Cross reactive nanoarrays; DFA |
TG: histology
CG: negative medical history and colonoscopy |
Training phase
1) CRC stage I–IV (n = 45) and HC (n = 86) 2) CRC stage I–IV (n = 45) and adenoma (n = 22) 3) HC (n = 86) and adenoma (n = 22) 4) NAA (n = 10) and AA (n = 12) Validation phase 5) CRC stage I–IV (n = 20) and HC (n = 36) 6) CRC stage I–IV (n = 16) and adenoma (n = 16) 7) HC (n = 16) and adenoma (n = 16) 8) NAA (n = 8) and AA (n = 8) |
Training phase
1) 93 (82, 98)* 2) 95 3) 94 4) 100 Validation phase 5) 94 (62, 97)* 6) 88 7) 94 8) 88 |
Training phase
1) 88 (89, 99)* 2) 90 3) 94 4) 89 Validation phase 5) 91 (81, 99)* 6) 91 7) 94 8) 94 |
Training phase
1) 90 2) 92 3) 94 4) 95 Validation phase 5) 91 6) 91 7) 94 8) 94 |
n.a. |
| Peng et al.51 |
GNP sensor array; PCA
SPME-GC-MS, ADMIS |
TG: imaging and histology
CG: n.a. Exclusions: n.a. |
CRC stage I–IV (n = 26) and HC (n = 22) | n.a. | n.a. | n.a. | n.a. |
| van de Goor et al.52 | AEONOSE; ANN |
TG: histology
CG: histology |
1) CRC stage I–IV (n = 28) and HNSCC stage 0–IV (n = 100)
2) CRC stage I–IV (n = 28) and BC stage 0–IV (n = 40) |
1) 79
2) 88 |
1) 81
2) 79 |
1) 83
2) 84 |
1) 0.83 (0.74-0.92) 2) 0.90 (0.81-0.98) |
| Wang et al.53 | SPME-GC-MS; PCA and PLSDA |
TG: histology
CG: negative medical history and colonoscopy |
CRC stage I–III (n = 20) and HC (n = 20) | n.a. | n.a. | n.a. | n.a. |
Values in parentheses are 95 per cent confidence interval. AUC, area under the curve; TDLS, tuneable diode laser spectrometer; TG, test group; CG, control group; GI, gastrointestinal; HC, healthy controls; OC, oesophageal cancer; GaC, gastric cancer; n.a., not available; GC-MS, gas chromatography–mass spectrometry; GNP, gold nanoparticles; SWCNT, single-wall carbon nanotube; DFA, discriminant function analysis; OLGIM, operative link on gastric intestinal metaplasia; PUD, peptic ulcer disease; TD-GC-MS, thermal desorption GC-MS; SPME-GC-MS, solid-phase micro extraction GC-MS; SERS, surface enhanced Raman scattering; EGC, early gastric cancer; AGC, advanced gastric cancer; ANN, artificial neural network; CFBP, cascade forward back propagation; FFBP, feed forward back propagation; GC/Q-TOF, quadrupole time-of-flight gas chromatography mass spectrometry; PCA, principal component analysis; AGD, advanced gas deposition system; SIFT-MS, selected ion flow tube mass spectrometry; MIM, multiple ion monitoring; LLR, logistic regression; VOC, volatile organic compound; OGD, oesophagogastric duodenoscopy; TPS-SiNW FET, trichloro(phenethyl)silane field effect transistor; PLSDA, partial least squared discriminant analysis; VIP, variable importance in the projection; GU, gastric ulcer; PTR-MS, proton transfer reaction mass spectrometry; SDA, stepwise discriminant analysis; ROC, receiver operating characteristic; hepB, hepatitis B; HCC, hepatocellular carcinoma; FNA, fine-needle aspiration; PC, pancreatic cancer; AC, adenocarcinoma; IMR-MS, ion molecule reaction mass spectrometry; LASSO, least absolute shrinkage and selection operator; PDA, pancreatic ductal adenoma; PNN, probabilistic neural network; CRC, colorectal cancer; CRC-FU, follow-up 1 year after CRC surgery (+ chemotherapy); AA, advanced adenoma; NAA, non-advanced adenoma; ADMIS, automated mass spectral deconvolution and identification system; HNSCC, head and neck squamous cell carcinoma; BC, breast cancer.
Data analysis was performed by a variety of techniques, involving the following methods: principal component analysis (PCA), probabilistic neural networks, partial least squared discriminant analysis (PLSDA), discriminant function analysis, artificial neural networks, Fisher least discriminant function analysis, least shrinkage and selection operator logistic regression (LLR), Mann–Whitney U test with LLR, predictive probability models, Mann–Whitney U test with binary logistic regression model, PCA with PLSDA with variable importance in the projection model, t test with ANOVA, and PCA with stepwise discriminant analysis. A detailed explanation of these methods is beyond the scope of this review.
Diagnostic test performance and validation
A summary of the diagnostic performance of the individual studies is provided in Table 2. The results were divided into four groups based on the cancer type studied. Data on sensitivity, specificity, accuracy, and AUC were retrieved from the articles. Where a study compared the index group with multiple reference groups, the results of these comparisons are also included. Five authors did not report diagnostic performance. The sensitivity ranged from 66.7 to 100 per cent, whereas specificity ranged from 48.1 to 97.9 per cent. In most studies, the sensitivity and specificity were lower in the validation phase than the training phase. Owing to heterogeneity of the studies, no meta-analysis could be performed. Internal, external or cross-validation was performed in one third of the studies (Table 2).
Both the largest (484 patients)32 and the smallest (30)40 studies, including patients and controls, analysed VOCs from patients with gastro-oesophageal cancer.
Reported volatile organic compounds
In total, 106 different VOCs were identified. For most VOCs, there was a statistically significant difference in presence between the groups. Some of the identified VOCs were only significant within a subgroup. Of the VOCs recorded, 32 were identified by more than one study (Table S3). These VOCs were either found to be cancer type-specific in multiple studies (19 VOCs), or were found in different cancer types (13 VOCs) and were therefore more general cancer VOCs. The VOCs that were identified in the most studies (4 studies each) were decanal, nonanal, and acetone.
Seventeen studies reported on VOCs that were present at significantly different levels in the exhaled breath of patients with cancer and control groups. All 106 VOCs identified in the studies are summarized in Table S4 (VOCs identified in multiple studies are highlighted in different colours). In total, 32 of the 106 VOCs were present differently in multiple studies. Ranked from high to low based on number of studies they were mentioned in, these were: decanal (4), nonanal (4), acetone (4), 1,3-dimethylbenzene (3), 2-methylpentane (3), 3-methylpentane (3), 2-propenenitrile (3), furfural (3), 4-methyloctane (3), isoprene (3), 1,2-pentadiene (2), 1,4-dimethylbenzene (2), 1,2,3-trimethylbenzene (2), undecande (2), dodecane (2), 4-methyl-2-pentanone (2), hexane (2), cyclohexane (2), methylcyclopentane (2), methylcyclohexane (2), ammonia (2), pentane (2), tetradecane (2), butanal (2), butyric acid (2), hexaoic acid (2), pentanoic acid (2), 2-butoxy-ethanol (2), 6-methyl-5-hepten-2-one (2), methanol (2), ethyl phenol (2), hexadecane (2).
Thirteen compounds identified in multiple studies were described for different cancer types. Acetone was found to be significantly different in the oesophageal cancer/gastric cancer, pancreatic cancer, and colorectal cancer groups. 2-Methylpentane, 3-methypentane, 4-methyloctane, dodecane, decanal, and nonanal were found in the oesophageal cancer/gastric cancer and colorectal cancer groups. Pentane,undecane,tetradecane, hexane, ammonia and 1,2,3-trimethylbenzene were found in the pancreatic cancer and colorectal cancer groups. The remaining 19 VOCs were found only in studies of the same cancer.
Discussion
The diagnostic performance of breath analysis for diagnosing cancer has shown promising results, with good sensitivity and specificity. The potential use of breath analysis as a non-invasive test that can be applied clinically may differ for each specific type of digestive tract malignancy as it depends on the cancer prevalence and existing diagnostic alternatives. Breath analysis could be considered as an additional screening tool to supplement faecal blood testing in colorectal cancer, or a screening tool for gastric cancer in countries with a high incidence, such as Asian countries, including Japan. Another option could be monitoring of patients with Barrett’s oesophagus to detect a potential conversion to malignancy. Breath analysis might be of special interest for pancreatic cancer, as its incidence is rising and the prognosis is poor, partly because it is often missed in the early stages14. A non-invasive test with the ability to distinguish between benign and malignant masses would be welcome. Despite the amount of research already done, there is currently no breath test being used for the detection of gastrointestinal tract malignancies, and the majority of clinical investigations are proof-of-concept studies. Most of these studies have been performed in small populations using different analytical techniques with poor standardization. VOCs are a product of metabolic processes and so their presence in exhaled breath greatly depends on the metabolic state of the patient. Alterations in breath profiles could not only be induced by cancer but also by other potential endogenous and exogenous influences, such as fasting status, microbiome, smoking, medication, co-morbidities, and exposure to varying ambient air pollutants; all these issues should be taken into consideration when designing a diagnostic study on breath analysis21.
Several initiatives are under way to develop protocols for standardization of sampling and analytical measurements in the International Association of Breath Research54–56 and the European Respiratory Society57. In a recent review30, a proposed framework for conducting and reporting future studies investigating the role of VOCs in cancer diagnosis was formulated. Applying standardization would contribute to improved quality of individual studies and enhance comparison between studies, leading to faster implementation of this promising diagnostic tool in clinical practice.
Although there is an abundance of possibilities for performing VOC analysis, a disadvantage in most of the currently available studies is possible overestimation of the predictive value and lack of external validation. Prediction models generally perform better on data on which the model was developed than on new data. Owing to relatively small sample sizes in most of the studies, there is a lack of external validation leading to a possible reduction in reproducibility58. According to the TRIPOD statement59, it is highly recommended for studies of prediction models to at least perform internal validation of the findings. Truly reliable results will only be generated by also validating the results externally.
There are many different analytical methods being used in studies of VOCs, and a distinction can be made between the so called real-time and offline analysis techniques22. The majority of the included studies used an offline combination of GC-MS systems with a sensor array system. An advantage of this approach is that specific discriminative VOCs can be identified and used to develop sensor systems applicable to clinical settings. However, certain conditions must be fulfilled for development of a breath test for use in the clinic. For clinical use, it is most important that the device is easy to carry, gives quick results, is non-invasive, should not be susceptible to environmental influences, and has both a high sensitivity and specificity.
VOCs that appeared in multiple studies might have the most discriminative value for discriminating cancer from non-cancer conditions. Some VOCs, such as acetone, 2-methylpentane, 3-methylpetane, decanal, nonanal, pentane, and tetradecane, were identified in studies of different cancer types. This suggests that VOCs can be cancer type-specific, but also general markers for cancer. The vast majority of the VOCs, however, were only identified in single studies. Of the single VOCs that were identified in multiple studies, including decanal, nonanal and acetone, not all can be attributed directly to certain (patho)physiological processes. However, it is known that cancers often show metabolic abnormalities, such as dysregulation of glucose, fatty acid, and amino acid metabolism60. One should keep in mind that not only cancers but also other metabolic abnormalities might cause alterations in breath profiles. For example, an increase in acetone can be a result of diabetic ketoacidosis. However, acetone is a ketone strongly related to fatty acid oxidation. Fatty acids consist of a carboxyl group and a hydrocarbon chain that can be saturated or unsaturated, and are required for synthesis of membranes and signalling molecules in cellular proliferation, as seen in cancers60–62.
Headspace analysis of healthy intestinal epithelial cells and colonic cancer cells has already shown differences in release of VOCs. This indicates that metabolic abnormalities of cancer cells might contribute to the differences in exhaled breath profiles63. As the pathophysiological mechanisms that lead to the altered VOC production in patients with cancer have not yet been elaborated sufficiently, it remains difficult to determine the origin of the distinctive VOCs.
More recent studies using sensor systems, such as the Aenose, have shown promising results of exhaled breath analysis for diagnosing malignancies. However, these studies were unable to identify individual compounds as they used sensor measurements that were analysed using pattern recognition techniques64. Additionally, they can be criticized for showing poor linear reproducibility of the results and they also seem to be particularly sensitive to exogenous influences, such as humidity17.
As for use in clinical practice, it would be of interest to determine whether a breath test could be applied not only to distinguish between healthy patients and those with cancer, but also between similar diseases such as cancer and benign conditions of the same organ22. Therefore, one should consider also including patients with benign diseases in breath analysis studies. During the review process, an additional study65 was published that met the search criteria for the present analysis. Breath analysis was performed using the Aenose for diagnosing colorectal cancer. The final model for distinguishing colorectal cancer from healthy controls showed a sensitivity of 95 per cent and specificity of 64 per cent, with an AUC of 0.84. Benign conditions such as advanced adenoma, non-advanced adenomas or hyperplastic polyps were also taken into account. Although the Aenose was able to distinguish patients with colorectal cancer from healthy controls, it was not able to differentiate colorectal cancer from advanced adenomas, or advanced adenomas from non-advanced adenomas, suggesting that the VOC profiles are too similar65. A different study66 using the Aenose for a known precursor of oesophageal carcinoma, Barrett’s oesophagus, had shown promising results, with a sensitivity of 91 per cent and specificity of 74 per cent for differentiating patients with Barrett’s oesophagus from healthy controls. These findings demonstrate that exhaled breath analysis may be of use in the early detection of precancerous conditions, enabling better surveillance or earlier treatment. However, as discussed above, a number of steps still need to be taken to develop clinically applicable breath tests.
Currently, multiple systems are used for VOC detection, which have similar diagnostic performance. However, comparison and pooling of the studies proved to be difficult in the present analysis owing to wide heterogeneity between the studies. A consensus on how studies that analyse VOCs in exhaled breath should be performed will greatly advance progress in this field.
The appearance of some of VOCs in multiple studies of the same cancer type, but also different cancer types, suggests that there could be tumour-specific and also general cancer-associated VOCs. Further studies are needed to determine whether such VOCs could be used to improve cancer diagnostics.
Funding
This study was supported by the Dutch Digestive Foundation (MLDS career development grant CDG16-12 to T.L.)
Conflict of interest.
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Supplementary Material
Contributor Information
K F H Hintzen, Department of Surgery, Maastricht University Medical Centre, Maastricht, the Netherlands; Department of Pharmacology and Toxicology, Maastricht University, Maastricht, the Netherlands.
J Grote, Department of Surgery, Maastricht University Medical Centre, Maastricht, the Netherlands.
A G W E Wintjens, Department of Surgery, Maastricht University Medical Centre, Maastricht, the Netherlands.
T Lubbers, Department of Surgery, Maastricht University Medical Centre, Maastricht, the Netherlands.
M M M Eussen, Department of Surgery, Maastricht University Medical Centre, Maastricht, the Netherlands.
F J van Schooten, Department of Pharmacology and Toxicology, Maastricht University, Maastricht, the Netherlands.
N D Bouvy, Department of Surgery, Maastricht University Medical Centre, Maastricht, the Netherlands.
A Peeters, Department of Clinical Epidemiology and Medical Technology Assessment, Maastricht University Medical Centre, Maastricht, the Netherlands.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424 [DOI] [PubMed] [Google Scholar]
- 2. Hawkes N. Cancer survival data emphasise importance of early diagnosis. BMJ 2019;364:l408. [DOI] [PubMed] [Google Scholar]
- 3. Nikolaou S, Qiu S, Fiorentino F, Rasheed S, Tekkis P, Kontovounisios C. Systematic review of blood diagnostic markers in colorectal cancer. Tech Coloproctol 2018;22:481–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nicholson BD, Shinkins B, Pathiraja I, Roberts NW, James TJ, Mallett S et al. Blood CEA levels for detecting recurrent colorectal cancer. Cochrane Database Syst Rev 2015;2015(12)CD011134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jodal HC, Helsingen LM, Anderson JC, Lytvyn L, Vandvik PO, Emilsson L. Colorectal cancer screening with faecal testing, sigmoidoscopy or colonoscopy: a systematic review and network meta-analysis. BMJ Open 2019;9:e032773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Elferink MAG, Toes-Zoutendijk E, Vink GR, Lansdorp-Vogelaar I, Meijer GA, Dekker E et al. Landelijk bevolkingsonderzoek naar colorectaal carcinoom. Ned Tijdschr Geneeskd. 2018;162:1–6 [PubMed] [Google Scholar]
- 7. Toes-Zoutendijk E, Kooyker AI, Dekker E, Spaander MCW, Opstal-van Winden AWJ, Ramakers C et al. Incidence of interval colorectal cancer after negative results from first-round fecal immunochemical screening tests, by cutoff value and participant sex and age. Clin Gastroenterol Hepatol 2020;18:1493–1500. [DOI] [PubMed] [Google Scholar]
- 8. Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh KG, Goh KL et al. ; Asia Pacific Working Group on Gastric Cancer. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol 2008;9:279–287 [DOI] [PubMed] [Google Scholar]
- 9. Zhang X, Li M, Chen S, Hu J, Guo Q, Liu R et al. Endoscopic screening in Asian countries is associated with reduced gastric cancer mortality: a meta-analysis and systematic review. Gastroenterology 2018;155:347e.9–354.e9 [DOI] [PubMed] [Google Scholar]
- 10. European Association for the Study of the Liver; European Organisation for Research and Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908–943 [DOI] [PubMed] [Google Scholar]
- 11. Colli A, Fraquelli M, Casazza G, Massironi S, Colucci A, Conte D et al. Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol 2006;101:513–523 [DOI] [PubMed] [Google Scholar]
- 12. Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV et al. Pancreatic cancer. Nat Rev Dis Primers 2016;2:16022. [DOI] [PubMed] [Google Scholar]
- 13. Canto MI, Harinck F, Hruban RH, Offerhaus GJ, Poley JW, Kamel I et al. ; International Cancer of Pancreas Screening (CAPS) Consortium. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 2013;62:339–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol 2019;10:10–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. GBD2017 Pancreatic Cancer Collaborators. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2019;4:934–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Duffy MJ. Clinical uses of tumor markers: a critical review. Crit Rev Clin Lab Sci 2001;38:225–262 [DOI] [PubMed] [Google Scholar]
- 17. Schmidt K, Podmore I. Current challenges in volatile organic compounds analysis as potential biomarkers of cancer. J Biomark 2015;2015:981458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pauling L, Robinson AB, Teranishi R, Cary P. Quantitative analysis of urine vapor and breath by gas–liquid partition chromatography. Proc Natl Acad Sci U S A 1971;68:2374–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boots AW, Bos LD, van der Schee MP, van Schooten FJ, Sterk PJ. Exhaled molecular fingerprinting in diagnosis and monitoring: validating volatile promises. Trends Mol Med 2015;21:633–644 [DOI] [PubMed] [Google Scholar]
- 20. Phillips M, Boehmer JP, Cataneo RN, Cheema T, Eisen HJ, Fallon JT et al. Heart allograft rejection: detection with breath alkanes in low levels (the HARDBALL study). J Heart Lung Transplant 2004;23:701–708 [DOI] [PubMed] [Google Scholar]
- 21. Blanchet L, Smolinska A, Baranska A, Tigchelaar E, Swertz M, Zhernakova A et al. Factors that influence the volatile organic compound content in human breath. J Breath Res 2017;11:016013. [DOI] [PubMed] [Google Scholar]
- 22. Amann A, Costello B, Miekisch W, Schubert J, Buszewski B, Pleil J et al. The human volatilome: volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J Breath Res 2014;8:034001. [DOI] [PubMed] [Google Scholar]
- 23. McFadyen MC, Melvin WT, Murray GI. Cytochrome P450 enzymes: novel options for cancer therapeutics. Mol Cancer Ther 2004;3:363–371 [PubMed] [Google Scholar]
- 24. Elfaki I, Mir R, Almutairi FM, Duhier FMA. Cytochrome P450: polymorphisms and roles in cancer, diabetes and atherosclerosis. Asian Pac J Cancer Prev 2018;19:2057–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Antoniou SX, Gaude E, Ruparel M, van der Schee MP, Janes SM, Rintoul RC; LuCID Group. The potential of breath analysis to improve outcome for patients with lung cancer. J Breath Res 2019;13:034002. [DOI] [PubMed] [Google Scholar]
- 26. Ahmed WM, Lawal O, Nijsen TM, Goodacre R, Fowler SJ. Exhaled volatile organic compounds of infection: a systematic review. ACS Infect Dis 2017;3:695–710 [DOI] [PubMed] [Google Scholar]
- 27. de Boer NK, de Meij TG, Oort FA, Ben Larbi I, Mulder CJ, van Bodegraven AA et al. The scent of colorectal cancer: detection by volatile organic compound analysis. Clin Gastroenterol Hepatol 2014;12:1085–1089 [DOI] [PubMed] [Google Scholar]
- 28. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–341 [DOI] [PubMed] [Google Scholar]
- 29. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB et al. ; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–536 [DOI] [PubMed] [Google Scholar]
- 30. Hanna GB, Boshier PR, Markar SR, Romano A. Accuracy and methodologic challenges of volatile organic compound-based exhaled breath tests for cancer diagnosis: a systematic review and meta-analysis. JAMA Oncol 2019;5:e182815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Amal H, Leja M, Broza YY, Tisch U, Funka K, Liepniece-Karele I et al. Geographical variation in the exhaled volatile organic compounds. J Breath Res 2013;7:047102. [DOI] [PubMed] [Google Scholar]
- 32. Amal H, Leja M, Funka K, Skapars R, Sivins A, Ancans G et al. Detection of precancerous gastric lesions and gastric cancer through exhaled breath. Gut 2016;65:400–407 [DOI] [PubMed] [Google Scholar]
- 33. Chen Y, Zhang Y, Pan F, Liu J, Wang K, Zhang C et al. Breath analysis based on surface-enhanced Raman scattering sensors distinguishes early and advanced gastric cancer patients from healthy persons. ACS Nano 2016;10:8169–8179 [DOI] [PubMed] [Google Scholar]
- 34. Daniel DA, Thangavel K. Breathomics for gastric cancer classification using back-propagation neural network. J Med Signals Sens 2016;6:172–182 [PMC free article] [PubMed] [Google Scholar]
- 35. Duran-Acevedo CM, Jaimes-Mogollon AL, Gualdron-Guerrero OE, Welearegay TG, Martinez-Marin JD, Caceres-Tarazona JM et al. Exhaled breath analysis for gastric cancer diagnosis in Colombian patients. Oncotarget 2018;9:28 805–28 817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schuermans VNE, Li Z, Jongen A, Wu Z, Shi J, Ji J et al. Pilot study: detection of gastric cancer from exhaled air analyzed with an electronic nose in Chinese patients. Surg Innov 2018;25:429–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shehada N, Bronstrup G, Funka K, Christiansen S, Leja M, Haick H. Ultrasensitive silicon nanowire for real-world gas sensing: noninvasive diagnosis of cancer from breath volatolome. Nano Lett 2015;15:1288–1295 [DOI] [PubMed] [Google Scholar]
- 38. Tong H, Wang Y, Li Y, Liu S, Chi C, Liu D et al. Volatile organic metabolites identify patients with gastric carcinoma, gastric ulcer, or gastritis and control patients. Cancer Cell Int 2017;17:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xu ZQ, Broza YY, Ionsecu R, Tisch U, Ding L, Liu H et al. A nanomaterial-based breath test for distinguishing gastric cancer from benign gastric conditions. Br J Cancer 2013;108:941–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Abela JE, Skeldon KD, Stuart RC, Padgett MJ. Exhaled ethane concentration in patients with cancer of the upper gastrointestinal tract—a proof of concept study. Biosci Trends 2009;3:110–114 [PubMed] [Google Scholar]
- 41. Kumar S, Huang J, Abbassi-Ghadi N, Mackenzie HA, Veselkov KA, Hoare JM et al. Mass spectrometric analysis of exhaled breath for the identification of volatile organic compound biomarkers in esophageal and gastric adenocarcinoma. Ann Surg 2015;262:981–990 [DOI] [PubMed] [Google Scholar]
- 42. Kumar S, Huang J, Abbassi-Ghadi N, Španěl P, Smith D, Hanna GB. Selected ion flow tube mass spectrometry analysis of exhaled breath for volatile organic compound profiling of esophago-gastric cancer. Anal Chem 2013;85:6121–6128 [DOI] [PubMed] [Google Scholar]
- 43. Markar SR, Wiggins T, Antonowicz S, Chin ST, Romano A, Nikolic K et al. Assessment of a noninvasive exhaled breath test for the diagnosis of oesophagogastric cancer. JAMA Oncol 2018;4:970–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zou X, Zhou W, Lu Y, Shen C, Hu Z, Wang H et al. Exhaled gases online measurements for esophageal cancer patients and healthy people by proton transfer reaction mass spectrometry. J Gastroenterol Hepatol 2016;31:1837–1843 [DOI] [PubMed] [Google Scholar]
- 45. Qin T, Liu H, Song Q, Song G, Wang HZ, Pan YY et al. The screening of volatile markers for hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev 2010;19:2247–2253 [DOI] [PubMed] [Google Scholar]
- 46. Markar SR, Brodie B, Chin ST, Romano A, Spalding D, Hanna GB. Profile of exhaled-breath volatile organic compounds to diagnose pancreatic cancer. Br J Surg 2018;105:1493–1500 [DOI] [PubMed] [Google Scholar]
- 47. Princivalle A, Monasta L, Butturini G, Bassi C, Perbellini L. Pancreatic ductal adenocarcinoma can be detected by analysis of volatile organic compounds (VOCs) in alveolar air. BMC Cancer 2018;18:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Altomare DF, Di LM, Porcelli F, Travaglio E, Longobardi F, Tutino M et al. Effects of curative colorectal cancer surgery on exhaled volatile organic compounds and potential implications in clinical follow-up. Ann Surg 2015;262:862–867 [DOI] [PubMed] [Google Scholar]
- 49. Altomare DF, Di Lena M, Porcelli F, Trizio L, Travaglio E, Tutino M et al. Exhaled volatile organic compounds identify patients with colorectal cancer. Br J Surg 2013;100:144–150 [DOI] [PubMed] [Google Scholar]
- 50. Amal H, Leja M, Funka K, Lasina I, Skapars R, Sivins A et al. Breath testing as potential colorectal cancer screening tool. Int J Cancer 2016;138:229–236 [DOI] [PubMed] [Google Scholar]
- 51. Peng G, Hakim M, Broza YY, Billan S, Abdah-Bortnyak R, Kuten A et al. Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Br J Cancer 2010;103:542–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. van de Goor RM, Leunis N, van Hooren MR, Francisca E, Masclee A, Kremer B et al. Feasibility of electronic nose technology for discriminating between head and neck, bladder, and colon carcinomas. Eur Arch Otorhinolaryngol 2017;274:1053–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang C, Ke C, Wang X, Chi C, Guo L, Luo S et al. Noninvasive detection of colorectal cancer by analysis of exhaled breath. Anal Bioanal Chem 2014;406:4757–4763 [DOI] [PubMed] [Google Scholar]
- 54. Gaude E, Nakhleh MK, Patassini S, Boschmans J, Allsworth M, Boyle B et al. Targeted breath analysis: exogenous volatile organic compounds (EVOC) as metabolic pathway-specific probes. J Breath Res 2019;13:032001. [DOI] [PubMed] [Google Scholar]
- 55. Herbig J, Beauchamp J. Towards standardization in the analysis of breath gas volatiles. J Breath Res 2014;8:037101. [DOI] [PubMed] [Google Scholar]
- 56. Malaskova M, Henderson B, Chellayah PD, Ruzsanyi V, Mochalski P, Cristescu SM et al. Proton transfer reaction time-of-flight mass spectrometric measurements of volatile compounds contained in peppermint oil capsules of relevance to real-time pharmacokinetic breath studies. J Breath Res 2019;13:046009. [DOI] [PubMed] [Google Scholar]
- 57. Horvath I, Barnes PJ, Loukides S, Sterk PJ, Hogman M, Olin AC et al. A European Respiratory Society technical standard: exhaled biomarkers in lung disease. Eur Respir J 2017;49:1600965. [DOI] [PubMed] [Google Scholar]
- 58. Fijten RRR, Smolinska A, Drent M, Dallinga JW, Mostard R, Pachen DM et al. The necessity of external validation in exhaled breath research: a case study of sarcoidosis. J Breath Res 2017;12:016004. [DOI] [PubMed] [Google Scholar]
- 59. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ 2015;350:g7594. [DOI] [PubMed] [Google Scholar]
- 60. Li Z, Zhang H. Reprogramming of glucose, fatty acid and amino acid metabolism for cancer progression. Cell Mol Life Sci 2016;73:377–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Currie E, Schulze A, Zechner R, Walther TC, Farese RV Jr. Cellular fatty acid metabolism and cancer. Cell Metab 2013;18:153–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Koundouros N, Poulogiannis G. Reprogramming of fatty acid metabolism in cancer. Br J Cancer 2020;122:4–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu M, Li Y, Wang G, Guo N, Liu D, Li D et al. Release of volatile organic compounds (VOCs) from colorectal cancer cell line LS174T. Anal Biochem 2019;581:113340. [DOI] [PubMed] [Google Scholar]
- 64. Waltman CG, Marcelissen TAT, van Roermund JGH. Exhaled-breath testing for prostate cancer based on volatile organic compound profiling using an electronic nose device (Aeonose™): a preliminary report. Eur Urol Focus 2018 [DOI] [PubMed] [Google Scholar]
- 65. van Keulen KE, Jansen ME, Schrauwen RWM, Kolkman JJ, Siersema PD. Volatile organic compounds in breath can serve as a non-invasive diagnostic biomarker for the detection of advanced adenomas and colorectal cancer. Aliment Pharmacol Ther 2020;51:334–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Peters Y, Schrauwen RWM, Tan AC, Bogers SK, de Jong B, Siersema PD. Detection of Barrett's oesophagus through exhaled breath using an electronic nose device. Gut 2020;69:1169–1172 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.