Highlights
-
•
Identifying leprosy reactions is important to prevent disability.
-
•
HRUS with CD can be useful compared to NCS to diagnose acute inflammatory activity.
-
•
HRUS with CD helps reactions diagnosis in minimal and complete NCS abnormalities.
Keywords: Leprosy neuropathy, Nerve conduction studies, High-resolution ultrasonography and Color Doppler, Leprosy reactions
Abstract
Objective
To analyze the role of high-resolution ultrasonography with color Doppler (HRUS with CD) to diagnose inflammatory activity (IA) in nerves of leprosy patients under type 1 (RT1) and 2 (RT2) reactions compared to Nerve Conduction Studies (NCS).
Methods
Leprosy patients with signs or symptoms suggestive of neuritis (RT1 and RT2) without corticosteroids use were selected. They were evaluated by NCS and subsequently by HRUS with CD. Subacute segmental demyelination and the presence of blood flow, respectively, were considered signs of IA. The two methods were compared for their ability to diagnose patients with leprosy reactions.
Results
A total of 257 nerves from 35 patients were evaluated. NCS and HRUS with CD diagnosed IA in 68% and 74% of patients, respectively. When both methods were used concomitantly, the diagnosis rate was 91.4%. HRUS with CD was particular helpful when there was minimal neurophysiological compromise in NCS or when motor potentials were not detected.
Conclusion
HRUS with CD was able to detect leprosy reactions, especially when combined with NCS. It was especially useful in two opposite situations: nerves with only minor changes and those without motor response in NCS.
Significance
Our data shows the usefulness of HRUS and CD, similar to NCS, as a tool to diagnose leprosy reactions.
1. Introduction
Leprosy is among the main treatable causes of peripheral neuropathy in developing countries. The treatment challenge and prognosis lie in the adequate management of leprosy reactions (Graham et al., 2020, Schreuder, 1998). The reactions are acute episodes of inflammatory response to a bacillary antigen in the nerve, classified as RT1 or RT2. RT1 is characterized by a predominant cellular immune response, which begins in the Schwann cell, where the bacillus has immunological specificity to bind inside. The RT2 occurs in the clinical forms with more humoral immune response. The RT2 develops a predominant humoral phenomenon due to the inefficient cellular immune response, leading to a greater bacillary quantity in the Schwann cells and in the nerve structures, conjunctive tissue and vessels. The response can provoke acute neutrophilic inflammation with vasculitis and severe intraneural edema. Both reactions can evolve into hypervascularization, an increase in the volume of the nerve trunk and its entrapment in physiological tunnels, with worsening of demyelination and secondary axonal loss (Alves et al., 2014, Tankisi et al., 2005). During the reactions, nerve conduction studies (NCS) reveal signs of demyelination that are related to inflammatory activity (IA). A previous study showed that patients experiencing leprosy nerve reactions presented with characteristic demyelinated features, such as temporal dispersion, conduction block and pronounced reduction in conduction velocities at NCS, which improve after steroid treatment (Garbino et al., 2010). These NCS features of IA were shown in the classical model of inflammatory polyneuropathies, including chronic inflammatory demyelinating polyradiculopathy (CIDP) (Van den Bergh et al., 2010). Such signs of segmental demyelination are also observed in acute or subacute nerve inflammatory injuries and can be used as signs of subacute IA (Garbino et al., 2010, Lau et al., 2020, Tankisi et al., 2005). Recently, studies utilizing high-frequency ultrasound with color Doppler (HFUS with CD) in leprosy neuropathy have described characteristic changes in leprosy neuropathy (Gonzalez and Hobson-Webb, 2019, Martinoli et al., 2000), thus facilitating diagnosis and treatment. Prior descriptions have included: thickening at entrapment sites (Bathala et al., 2017, Jain et al., 2009, Lugão et al., 2015, Martinoli et al., 2000), changes in nerve echotexture differentiating the polar forms of leprosy (tuberculoid and Virchowian) (Bathala et al., 2012, Jain et al., 2009, Martinoli et al., 2000), increased cross-sectional area of the nerve, diffuse or localized (Jain et al., 2009, Lugão et al., 2015) and increased intraneural or perineural vascularization observed with CD (Chaduvula et al., 2018, Jain et al., 2009, Martinoli et al., 2000). The presence of intraneural or perineural vascularization is considered abnormal, given that the presence of blood flow at HFUS with CD can be interpreted as a sign of inflammation and, consequently, a sign of acute and subacute inflammatory reaction in the nerve (Chaduvula et al., 2018, Goedee et al., 2014, Lugão et al., 2015).
1.1. Objective
To compare the reliability of HFUS with CD and NCS methods in detecting leprosy reactions in patients not treated with corticosteroids and evaluate the relevance of HFUS with CD for the diagnosis of IA in the nerves of these patients.
2. Methods
2.1. Subjects’ selection
This is a prospective study. Adult patients diagnosed with leprosy and with signs and/or symptoms of acute inflammatory reaction (RT1 or RT2) were recruited at the Hansenology Outpatient Clinic of Instituto Lauro de Souza Lima, Brazil. The diagnosis of leprosy was established by clinical, histopathological and bacteriological evaluation and classified according to the method of Ridley-Jopling (Ridley and Jopling, 1962). The study was previously approved by the institute's ethics committee, and a free and informed consent form was obtained from each patient.
2.2. Clinical criterial and evaluation
The clinical diagnosis of neuritis was suspected when there were new motor or sensory symptoms in nerve topography, confirmed by physical examination. Each case was classified as RT1 or RT2. Patients reporting other causes for peripheral neuropathy (such as diabetes, hypothyroidism, HIV or nerve injuries secondary to trauma), impossibility of electrophysiological or ultrasound assessment of the limbs, or current use of corticosteroids were excluded.
The patients were evaluated consecutively according to the outpatient referral order. They were evaluated first by NCS and then by HFUS with CD, both examinations on the same day and performed blinded by two different examiners. Neuritis, that is, acute inflammatory nerve involvement, was considered when segmental demyelination in NCS was found (characterized by temporal dispersion, conduction block or severe and/or moderate velocity decrease) and/or signs of nerve hypervascularization at HRUS with CD.
2.3. Neurophysiologic evaluation
NCS were performed by two neurophysiologists experienced with leprosy diagnosis, via a 5-channel Nihon Kohden device with an electromyography system, after proper limb heating at a minimum of 32 degrees Celsius. The abductor pollicis brevis, abductor digiti minimi, extensor digitorum brevis and abductor hallucis brevis muscles were used for registering the compound muscular action potentials (CMAP) of the median, ulnar, deep peroneal and posterior tibial nerves, respectively, and were captured with surface electrodes whose active electrode was on the muscular belly and the reference one on the tendon. The nerve was stimulated by a bipolar stimulator with the cathode oriented distally. This stimulation was performed at two points on the median and posterior tibial nerves and at three points on the ulnar and peroneal nerves – these latter two nerves were studied proximally using stimulus below and above the elbow and behind and above the fibular head, respectively.
Sensory nerve action potentials (SNAP) were measured using the antidromic technique: on the second finger for the median nerve, the fifth finger for the ulnar nerve (with wrist stimuli), and behind the lateral malleolus for the sural nerve (with stimulation on the posterolateral portion of the calf).
The parameters analyzed for neurophysiological classification of myelinic and axonal involvement were distal latency (in milliseconds - msec), segmental conduction velocity (in meters/second - m/s), duration and amplitude (in microvolts – uV for SNAP and millivolts – mV for CMAP); values were evaluated using previously standardized criteria (Preston and Shapiro, 2013). The presence of pathological temporal dispersion (defined as a 30% increase or more in CMAP negative-peak duration between proximal and distal stimulation sites) or conduction block (defined as a 50% or more amplitude reduction of the proximal CMAP in combination with a 30% or less increase in duration) (van den Bergh et al., 2010; Olney, 1999) was considered positive for acute or subacute myelinic involvement (Garbino et al., 2010, Tankisi et al., 2005, van den Bergh et al., 2010).
The abnormality was classified as pronounced and/or moderate myelinic when the conduction velocity (CV) in the upper limbs was lower than 30 m/s and, in the lower limbs, lower than 20 m/s. Minimal changes were diagnosed when any of the following was found: 1) a CV reduction less than 10 m/s compared to normal velocity; 2) a reduction of less than 50% in the amplitudes of the sensory and/or motor potentials compared to normal values or to contralateral studies if they were normal; 3) prolongation of motor distal latency less than 30%; or 4) incipient changes in motor CV through the elbow or fibular head.
Axonal involvement was considered when there was a reduction greater than 50% in the amplitude of the CMAP without a significant increase in its duration (less than 30%). Three groups were considered according to the reduction in the amplitude: 1) mild (between 50% and 80% of normality reference value); 2) moderate/pronounced (reduction greater than 90%) and 3) undetected motor potential.
Needle electromyography was not added to this protocol. It was considered that NCS could be sufficient to assess the activity of leprosy neuropathy, since the focus of this study was on demyelinating nerve injuries. (Garbino et al., 2016, Santos et al., 2017).
2.4. High-resolution ultrasonography and color Doppler
HRUS with CD was performed by an experienced radiologist using the color Doppler ultrasound device Samsung Medison LA3-16AD, model HM70A with a linear transducer with frequency band from 3 to 16 MHz (MHz). The color Doppler settings were chosen to optimize the identification of blood flow in the vessels, with slow speed. The pulse repetition frequency was determined at 1 KHz and the Doppler gain adjusted to the maximum level so as not to produce any artifact. The filter was set to 50 Hz. The median nerves were evaluated in the wrist and in the antecubital fossa, ulnar nerves in the forearm, elbow and distal third of the arm; common peroneal nerves in the popliteal region, head of the fibula and two centimeters distal to it; and tibial nerves in the tarsal tunnel and proximal to the medial malleolus.
HRUS with CD defined the presence or absence of blood flow in the neural vascular plexus (intraneural or perineural), characterizing nerve hypervascularization in inflammatory responses.
2.5. Statistical analysis
Descriptive statistics, namely the average, extreme values, and percentages, were obtained to summarize the data. The Chi-Square Test was performed to verify possible associations between variables, considering a 5% significance level.
3. Results
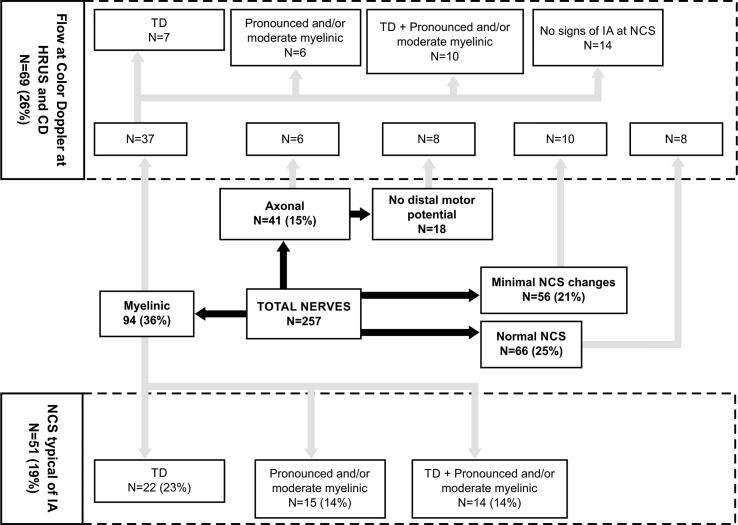
Thirty-five (35) patients were evaluated. The average age was 52 years (minimum of 37 and maximum of 82 years). A total of 257 nerves were analyzed, including the ulnar (n = 68), motor peroneal (n = 67), median (n = 60) and tibial (n = 62). Of the 257 nerves assessed, 94 (36%) showed predominantly myelinic involvement, 41 (15%) predominantly axonal involvement, 56 (21%) only minor changes and 66 (25%) normal nerve conduction.
The results are summarized in Table 1 and Fig. 1: 51 nerves (19%) had NCS with some features suggestive of IA, as temporal dispersion alone, pronounced/moderate isolated myelinic alterations and both changes at the same nerve. No nerve with conduction block was observed.
Table 1.
Neurophysiological and ultrasound features of patients.
| Case No. | Age | Ridley and Jopling Classification | Side | Nerve |
|||
|---|---|---|---|---|---|---|---|
| Median | Ulnar | Fibular | Tibial | ||||
| 1 | 55 | TT | R | N | |||
| 21 | 82 | BB | R | Min | Min | M (TD) | M (TD) |
| L | Min | Min | M (TD) | M (TD) | |||
| 31, 2 | 50 | BL | R | – | ⊕ M (TD) | M (PM) | M (PM) |
| L | – | ⊕ M (TD) | A () | A (∅) | |||
| 42 | 53 | LL | R | ⊕ N | M | N | N |
| L | N | M | N | N | |||
| 51 | 40 | BL | R | – | M (TD) | – | – |
| L | – | – | M (TD) | – | |||
| 61, 2 | 43 | BL | R | – | M (TD; PM) | ⊕ A (TD) | – |
| L | – | M (TD) | ⊕ M | ||||
| 72 | 29 | BL | R | Min | M | N | M |
| L | Min | M | N | N | |||
| 81, 2 | 35 | LL | R | ⊕ Min | N | ||
| L | ⊕ M (TD; PM) | N | |||||
| 91, 2 | 57 | LL | R | ⊕ Min | M (PM) | Min | ⊕ M (PM) |
| L | N | ⊕ M (PM) | M | A (TD) | |||
| 101, 2 | 52 | BL | R | M | ⊕ M | M (TD) | M (TD) |
| L | M | ⊕ Min | N | Min | |||
| 111, 2 | 51 | BL | R | M (PM) | ⊕ M (PM) | N | A |
| L | Min | ⊕ M (PM) | N | ⊕ M (PM) | |||
| 121 | 39 | BT | R | Min | N | A | N |
| L | Min | M (PM) | N | N | |||
| 13 | 41 | BL | R | Min | Min | Min | N |
| L | Min | Min | Min | N | |||
| 142 | 52 | LL | R | Min | Min | Min | N |
| L | Min | Min | A | ⊕ N | |||
| 152 | 41 | TT | R | M | N | N | N |
| L | M | ⊕ Min | ⊕ N | N | |||
| 162 | 54 | BT | R | N | ⊕ M | N | N |
| L | N | ⊕ M | N | N | |||
| 171, 2 | 61 | BL | R | M | ⊕ N | ⊕ A | M (TD) |
| L | M | ⊕ N | Min | ⊕ M (TD; PM) | |||
| 181 | 41 | BT | R | Min | M (PM) | Min | N |
| L | N | M (PM) | Min | N | |||
| 191, 2 | 28 | BT | R | N | N | ⊕ M (TD) | Min |
| L | N | Min | N | N | |||
| 201, 2 | 40 | BB | R | A (TD) | M (TD; PM) | M | ⊕ A (TD) |
| L | ⊕ Min | ⊕ M (TD; PM) | ⊕ A | M (TD) | |||
| 211 | 44 | BL | R | Min | Min | M (TD) | M (TD) |
| L | Min | A | A (∅) | M | |||
| 221, 2 | 73 | BL | R | Min | ⊕ M (TD; PM) | A (∅) | ⊕ A (∅) |
| L | Min | Min | A (∅) | ⊕ A (∅) | |||
| 232 | 21 | BL | R | M | N | Min | N |
| L | Min | ⊕ Min | ⊕ N | N | |||
| 241, 2 | 50 | BL | R | ⊕ M | ⊕ M (TD; PM) | ⊕ M | ⊕ M (TD) |
| L | Min | ⊕ M (TD) | ⊕ A (∅) | A (TD) | |||
| 252 | 75 | BT | R | M | Min | N | N |
| L | ⊕ Min | Min | A | N | |||
| 261, 2 | 71 | BL | R | N | ⊕ M (TD; PM) | A | ⊕ M (TD; PM) |
| L | N | ⊕ M | ⊕ A (∅) | ⊕ A (∅) | |||
| 271, 2 | 56 | BT | R | ⊕ M (TD; PM) | A (TD) | A (∅) | A (∅) |
| L | M (TD; PM) | A (TD) | A (∅) | A (∅) | |||
| 281, 2 | 37 | BB | R | ⊕ M | ⊕ A (∅) | ⊕ A (∅) | M |
| L | M (PM) | ⊕ Min | ⊕ A (∅) | ⊕ A | |||
| 291, 2 | 47 | LL | R | M | Min | Min | ⊕ N |
| L | M | ⊕ M | A (∅) | ⊕ M (TD) | |||
| 301, 2 | 51 | BT | R | M (PM) | ⊕ M | N | A |
| L | M | ⊕ Min | N | A | |||
| 311, 2 | 58 | BL | R | Min | ⊕ M | M (TD) | ⊕ A |
| L | Min | Min | ⊕ M (TD) | ⊕ Min | |||
| 32 | 56 | BT | R | M | N | Min | N |
| L | M | N | N | N | |||
| 332 | 59 | BB | R | ⊕ M | ⊕ N | N | N |
| L | M | ⊕ M | N | Min | |||
| 341, 2 | 53 | BL | R | M | M | ⊕ M (TD; PM) | ⊕ M (PM) |
| L | M | ⊕ M | M (TD; PM) | ⊕ M (TD; PM) | |||
| 351 | 74 | BB | R | M | M (TD) | A (TD) | A |
| L | M | M | A (TD) | A | |||
TT: tuberculoid, BT: borderline tuberculoid, BB: borderline, BL: borderline lepromatous, LL: lepromatous, R: right, L: left, N: normal, M: myelinated changes, A: axonal changes, Min: minimal changes, ⊕: presence of color Doppler flow, DT: temporal dispersion, MP: moderate/pronounced myelin changes, ∅: no distal conduction motor action potential.
Patients with signs of inflammatory activity at nerve conduction studies.
Patients with signs of inflammatory activity at HFUS and CD.
Fig. 1.
Signs of demyelinated nerve injury at Nerve Conduction Studies and presence of flow at High-Resolution Ultrasound with Color Doppler that are suggestive of inflammatory activity (neuritis). Abbreviations: HRUS and CD = High-resolution Ultrasound and Color Doppler; IA = inflammatory activity; NCS = Nerve Conduction Studies; TD = Temporal Dispersion.
The presence of flow detected with color Doppler was observed in 69 nerves (26%); of these, 53% were at nerves that had myelinic involvement at NCS (n = 37), predominantly in nerves with neurophysiological features of IA (n = 23). Fig. 2 displays an example of classical signs of segmental demyelination and inflammatory activity at NCS and HFUS with CD. The correlation between myelinic involvement at NCS and presence of flow at HRUS with CD was statistically significant (p = 0.01). However, in the nerves with no typical neurophysiological signs of IA, such as mild myelinic changes, axonal and minimal changes and at nerves with normal NCS, flow was also observed via color Doppler (n = 46). Blood flow was detected in 14 (34%) of 41 nerves with axonal involvement, of which eight (19%) did not have distal motor potential (p = 0.025). In addition, blood flow was also present at nerves with minimal changes, as well as in those with normal NCS. No statistical correlation was found by analyzing other NCS impairment (mild, moderate and pronounced axonal impairment and mixed changes) and the findings of HRUS with CD.
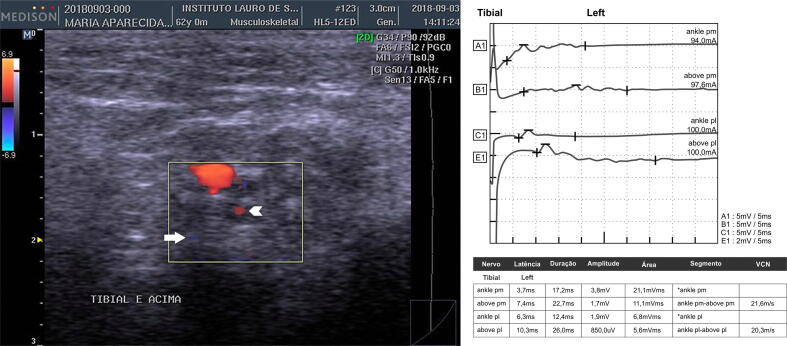
Fig. 2.
An example of neurophysiology and ultrasonography signs of inflammatory activity (patient no. 17). On the left: Left tibial nerve, transverse acquisition, with central (arrowhead) and peripheral blood flow (arrow). On the right, nerve conduction study of the same nerve demonstrating temporal dispersion through the tars.
The clinical, neurophysiology and ultrasonography features of patients are summarized in Table 1. Considering the 35 patients, 68% (n = 24) presented with NCS findings suggestive of IA, whereas blood flow at HRUS with CD was observed in 74% (N = 26). Six (17%) individuals presented findings only in NCS and eight (22%) had findings exclusively in HRUS with CD; in three individuals NCS and HRUS with CD were both normal: two of these patients presented minimal findings in NCS and only one had normal NCS.
Nine (25%) patients had absent motor distal response in at least one of the evaluated nerves. Eight (88%) of these showed both NCS and HRUS with CD features suggestive of neuritis in other nerves studied and only one (2%) patient had only NCS impairment.
Both methods were altered concomitantly in the same patient in 18 of the patients (51%). Considering one or another method for the diagnosis of IA, the positivity rate was 91% (n = 32). Among the seven patients with diagnosis exclusively by HRUS with CD, six (85%) had minimal changes in NCS and only one (14%) showed a normal NCS. Finally, when evaluating all nerves of a single individual, only one patient (2%) tested negative for inflammatory activity in all complementary tests.
4. Discussion
The neurophysiological pattern in leprosy RT1 and RT2 was defined in a previous study (Garbino et al., 2010). The same features of demyelination were also observed in other inflammatory neuropathies, such as Guillain-Barré Syndrome and chronic inflammatory demyelinating polyradiculopathy. (Sander and Latov, 2003, Tankisi et al., 2005, van den Bergh et al., 2010). In both leprosy reactions and other inflammatory neuropathies, the classical neurophysiological signs of segmental demyelination associated or not with temporal dispersion and/or conduction block can be utilized to evaluate the presence of inflammatory activity. Considering that all patients evaluated had clinical characteristics for the diagnosis of leprosy reaction, nerve conduction was able to detect neuritis in 68% (24 of 35) of the patients evaluated, while HRUS with CD successfully diagnosed 74% (26 of 35) of these patients. When the two methods were combined, the detection efficiency reached 91% of the patients. Among the patients in which the diagnosis of the reactions was not obtained by the NCS, the HRUS with CD was able to add positivity in 22% (n = 8) of the cases. Of these cases, six of the patients had minimal electromyographic changes, five of them had RT1 and only one RT2.
This study demonstrates that HRUS with CD can be useful to diagnose leprosy reactions, particularly in two electrophysiological situations Firstly, when the signs of neural compromise are minimal, observed more commonly in patients with RT1, it may be related to an initial phase of the leprosy reaction, with a predominance of cellular immune reaction directed to Schwann cells and secondary hypervascularization, prior to segmental demyelination. Secondly, when no motor potential was detected in at least one nerve, we observed a significant correlation with the presence of flow at CD (p = 0.025). In this subgroup of patients, with distal potentials not obtained in NCS, ultrasound proved to be a useful diagnostic tool to differentiate complete inactive axonal lesions from the presence of ongoing inflammatory activity, therefore assisting in the therapeutic decision.
When considering the typical neurophysiological signs suggestive of inflammatory activity, HRUS with CD demonstrated flow in 24 (39%) of 61 nerves. This finding reinforces the hypothesis that the inflammation begins inside the Schwann cell before an influx of inflammatory cells into the nerve. Therefore, typical neurophysiological criteria for demyelination and inflammatory activity can be accepted as an early NCS sign of reactions. Despite the low agreement of the two methods when assessing nerves individually, considering the global assessment of the patient's nerves, HRUS with CD detected signs of inflammatory activity in 74% of all patients evaluated.
We consider that HRUS with CD can detect inflammatory activity in leprosy reactions, similar to NCS, and can aid in diagnosing these patients. However, we emphasize the benefit of NCS in quantitative analysis of nerve impairment, as well as in comparative time evolution analysis of the patient considering that leprosy neuropathy typically presents multiple episodic reactions.
5. Conclusion
The ultrasound analysis can detect neuritis and is particularly useful in two different neurophysiology situations, at both extremes of the NCS findings: that is, nerves with only minimal changes and those in which no distal motor response was detected. In the first situation, the data aid in the diagnosis of an initial reaction, and in the second, in the differentiation between sequelae of old pronounced neuropathy or the presence of inflammatory activity in progress. By adding the ultrasound assessment in these specific cases, neuritis was diagnosed in a greater proportion (85% total) of the patients. If both diagnostic methods were performed systematically, the detection of patients with neuritis would reach 91% of the individuals.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Alves, E.D., Ferreira, T.L., Ferreira, I.N., 2014. Progress and challenges the leprosy. Chapter 11, pp. 221-222. ISBN 978-85-64593-22-0.
- Bathala L., Kumar K., Pathapati R., Jain S., Visser L.H. Ulnar neuropathy in hansen disease: clinical, high-resolution ultrasound and electrophysiologic correlations. J. Clin. Neurophysiol. 2012;29:190–193. doi: 10.1097/wnp.0b013e31824d969c. [DOI] [PubMed] [Google Scholar]
- Bathala L., Krishnam V.N., Kumar H.K., Neladimmanahally V., Nagaraju U., Kumar H.M., Telleman J.A., Visser L.H., Small P.L.C. Extensive sonographic ulnar nerve enlargement above the medial epicondyle is a characteristic sign in Hansen’s neuropathy. PLoS Negl. Trop. Dis. 2017;11(7):e0005766. doi: 10.1371/journal.pntd.0005766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaduvula M.V., Visser L.H., Suneetha S., Suneetha L., Devaraju B., Ellanti R., Raju R., Jain S. High-resolution sonography as an additional diagnostic and prognostic tool to monitor disease activity in leprosy: A two-year prospective study. Ultraschall. Med. 2018;39:80–89. doi: 10.1055/s-0042-108430. [DOI] [PubMed] [Google Scholar]
- Santos D.F.D., Mendonça M.R., Antunes D.E., Sabino E.F.P., Pereira R.C., Goulart L.R., Goulart I.M.B., Small P.L.C. Revisiting primary neural leprosy: Clinical, serological, molecular, and neurophysiological aspects. PLoS Negl Trop Dis. 2017;11(11):e0006086. doi: 10.1371/journal.pntd.0006086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbino J.A., Heise C.O., Marques W. Assessing nerves in leprosy. Clin. Dermatol. 2016;34(1):51–58. doi: 10.1016/j.clindermatol.2015.10.018. [DOI] [PubMed] [Google Scholar]
- Garbino J.A., Naafs B., Ura S., Salgado M.H., Virmond M. Neurophysiological patterns of ulnar nerve neuropathy in leprosy reactions Leprosy View project Leprous Neuropathy View project. Lepr. Rev. 2010;81(3):206–215. [PubMed] [Google Scholar]
- Goedee H.S., Brekelmans G.J.F., Visser L.H. Multifocal enlargement and increased vascularization of peripheral nerves detected by sonography in CIDP: A pilot study. Clin. Neurophysiol. 2014;125(1):154–159. doi: 10.1016/j.clinph.2013.05.025. [DOI] [PubMed] [Google Scholar]
- Gonzalez N.L., Hobson-Webb L.D. Neuromuscular ultrasound in clinical practice: A review. Clin. Neurophysiol. Pract. 2019;4:148–163. doi: 10.1016/j.cnp.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A., Furlong S., Margoles L.M., Owusu K., Franco-Paredes C. Clinical management of leprosy reactions. Infect Dis. Clin. Pract. 2020;18:235–238. doi: 10.1097/IPC.0b013e3181deba2a. [DOI] [Google Scholar]
- Jain S., Visser L.H., Praveen T.L.N., Rao P.N., Surekha T., Ellanti R., Abhishek T.L.N., Nath I., Phillips R. High-resolution sonography: A new technique to detect nerve damage in leprosy. PLoS Negl. Trop Dis. 2009;3(8):e498. doi: 10.1371/journal.pntd.0000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau Y.H., Mohd Unit H., Lee L.P., Loh W.K., Hiew F.L. Temporal dispersion in demyelination of POEMS syndrome and Castleman disease. Clin. Neurophysiol. Pract. 2020;5:112–117. doi: 10.1016/j.cnp.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugão H.B., Nogueira-Barbosa M.H., Marques Jr. W., Foss N.T., Frade M.A.C., Franco-Paredes C. Asymmetric nerve enlargement: A characteristic of leprosy neuropathy demonstrated by ultrasonography. PLoS Negl. Trop Dis. 2015;9(12):e0004276. doi: 10.1371/journal.pntd.0004276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinoli C., Derchi L.E., Bertolotto M., Gandolfo N., Bianchi S., Fiallo P., Nunzi E. US and MR imaging of peripheral nerves in leprosy. Skeletal Radiol. 2000;29(3):142–150. doi: 10.1007/s002560050584. [DOI] [PubMed] [Google Scholar]
- Preston D.C., Shapiro B.E. W.B. Saunders; 2013. Electromyography and Neuromuscular Disorders – Clinical-Electrophysiologic Correlations; pp. 249–266. [Google Scholar]
- Ridley D.S., Jopling W.H. A classification of leprosy for research purposes. Lepr. Rev. 1962;33:119–128. doi: 10.5935/0305-7518.19620014. [DOI] [PubMed] [Google Scholar]
- Sander H.W., Latov N. Research criteria for defining patients with CIDP. Neurology. 2003;60:S8–S15. doi: 10.1212/wnl.60.8_suppl_3.s8. [DOI] [PubMed] [Google Scholar]
- Schreuder P.M. The occurrence of reactions and impairments in leprosy: experience in the Leprosy Control Program of three provinces in Northeastern Thailand, 1978–1995. I. Overview of the study. Int. J. Lepr. Other Mycobact. Dis. 1998;66:149–158. [PubMed] [Google Scholar]
- Tankisi H., Pugdahl K., Fuglsang-Frederiksen A., Johnsen B., de Carvalho M., Fawcett P.R.W., Labarre-Vila A., Liguori R., Nix W.A., Schofield I.S. Pathophysiology inferred from electrodiagnostic nerve tests and classification of polyneuropathies. Suggested guidelines. Clin. Neurophysiol. 2005;116(7):1571–1580. doi: 10.1016/j.clinph.2005.04.003. [DOI] [PubMed] [Google Scholar]
- van den Bergh P., Hadden R., Bouche P., Cornblath D., Hahn A., Illa I., Koski C., Léger J., Nobile-Orazio E., Pollard J., Sommer C., van Doorn P., van Schaik I. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society-First Revision. J. Peripher. Nerv. Syst. 2010;17:356–363. doi: 10.1111/j.1468-1331.2009.02930.x. [DOI] [PubMed] [Google Scholar]