Abstract
The implementation of nanotechnology to develop efficient antimicrobial systems has a significant impact on the prospects of the biomedical field. Nanogels are soft polymeric particles with an internally cross-linked structure, which behave as hydrogels and can be reversibly hydrated/dehydrated (swollen/shrunken) by the dispersing solvent and external stimuli. Their excellent properties, such as biocompatibility, colloidal stability, high water content, desirable mechanical properties, tunable chemical functionalities, and interior gel-like network for the incorporation of biomolecules, make them fascinating in the field of biological/biomedical applications. In this review, various approaches will be discussed and compared to the newly developed nanogel technology in terms of efficiency and applicability for determining their potential role in combating infections in the biomedical area including implant-associated infections.
Keywords: Nanogels, Coatings, Antibacterial activity, Antifouling, Biocompatibility
Graphical abstract
Highlights
-
•
Nanogels are novel soft nanoparticles with high potential impact with the biomedical field.
-
•
Nanogels are applied both as bioactive materials in suspension and at interfaces.
-
•
Hydrogel nanoparticles have tremendous impact on infection related issues.
-
•
Nanogels offer antifouling possibilities and provided controlled release possibilities.
-
•
Nanogels can be tuned in their physicochemical properties to match the biomedical application.
1. Introduction
Bacterial infections are the major causes of death worldwide. Reducing the mortality rate caused by bacterial infections is considered one of the most critical challenges. The infections are mostly occurring due to the bacterial biofilms formed by initial bacterial attachment and subsequent proliferation and colonization of bacterial cells [[1], [2], [3], [4]]. Bacterial biofilm and infection complications are not affecting only the biomedical field; it creates problems also in the textile and food packaging industries, marine equipment, and water purification systems. When microorganisms colonize on textile materials, this leads to undesired hygienic problems [5]. For food packaging materials, biofilm formation creates a health risk for the consumer and also reduces the production efficacy of the industry itself [6]. For the marine industry, biofilm can be the cause of corrosion and biofouling of marine equipment, resulting in its degradation and high economic costs [7]. Bacteria accumulation in water purification systems decreases the quality of home drinking water creating health threats [8]. Medical implants and devices, such as hip and knee joints, pacemakers, intraocular lenses, and vascular catheters, are crucial in health care. Infections can develop in or around these devices, which impairs the function of the device and can even lead to device failure. Besides, these complications place the health of the patient at high risk, sometimes even leading to death [[9], [10], [11]]. Over the past decades, the situation is becoming even worse due to increasing antibiotic resistance [12]. Antibiotic resistance occurs when bacteria develop the capability to become unresponsive towards the drug, which then results in infections that are complicated to treat [13,14]. Therefore, novel antibacterials, delivery systems and surface coatings are urgently needed to fight infections arising within the biomedical context in which the implant-associated infections take a prominent place but certainly is not the only urgent type of infection.
Recently, the development of hydrogels has become an attractive strategy and the design of tissue engineering scaffolds, antifouling coatings on implant surfaces, drug delivery, and wound dressing systems have been widely studied [15,16]. Hydrogels are soft materials with cross-linked networks of hydrophilic polymers with high water content, which contribute to their biocompatibility and make them highly useful for biomedical applications; hence the development of hydrogels are one of the most studied areas at the interface of engineering and medicine [[17], [18], [19]]. The translation and usage of hydrogels into the clinical applications can be seen in many examples such as contact lenses, dermal fillers in soft tissue augmentation for aesthetic products, encapsulation and release systems for cancer products, mechanical support and scaffolding materials for spinal fusion applications [20]. While many applications involve hydrogels, these are often of macroscopic nature and for many interesting applications, particularly when interacting with tissue cells and bacterial cells, nano- and microstructures are of high interest because these interact with cells on a different level and even have the capability of being internalized. Hence, many efforts have been directed towards the development of nano- and microgels.
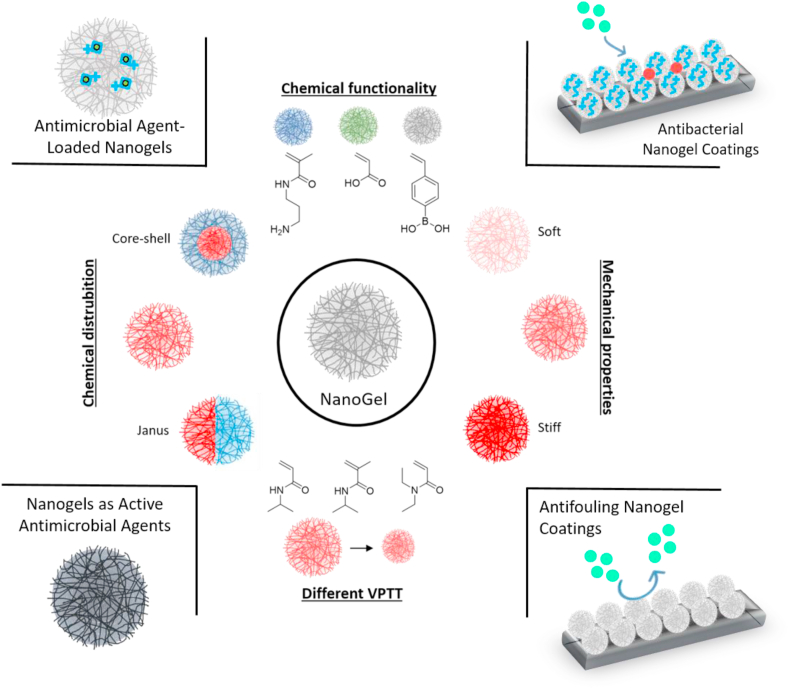
Nanogels are newly emerging nano-sized hydrogel-like polymeric materials and have overlapping properties of both nanoparticles and hydrogels [21]. Nanoparticles, both inorganic (gold, silver, etc.) and organic (liposomes), are already present in the clinic as well as many natural and synthetic hydrogels [22]. To this end, nanogels provide a cross-over for combinations of hydrogels and nanomaterials. They have advantages over macro-sized networks because their size enables them to interact with cells in a more specific fashion and even be internalized and due to their soft nature behave differently from solid and self-assembled polymer-based drug delivery systems, including polymer-based nanoparticles, micelles, and liposomes [[23], [24], [25]]. Nanogels consist of physically or chemically cross-linked three-dimensional polymer networks that can also be functionalized and integrated easily with pharmaceutical agents. Up to now, various nanogels have been widely used in the delivery of pharmaceuticals, mainly antitumor agents and proteins [26]. More recently, increasing attention has been paid to enhance their biocompatibility as well as using them as antimicrobial and antifouling surface coatings. In this context, different approaches have recently been followed in the biomedical field; for example, the antifouling performance to inhibit protein, macrophage, and bacteria adhesion to the surface, the enzyme uptake capability for biosensors, the capacity to control the cell proliferation and cell adhesion of nanogel coatings have been investigated [[27], [28], [29], [30], [31], [32]]. Their features such as size, charge, porosity, amphiphilicity, and softness can be tuned by changing the chemical composition during the synthesis or post-modification [33]. Moreover, the properties of nanogels can be modified by adding stimuli-responsive functional groups, which respond to external stimuli such as temperature and pH by swelling or collapsing [34]. The responsiveness can be ideal for a combination with drug release, in order to create a combined antimicrobial and antifouling coating [35]. Different physicochemical properties of nanogels and their applications are represented in Scheme 1.
Scheme 1.
Schematic representation of the different strategies of nanogel design and their applications in biomedical field.
Despite the promising progress made in the past years, much development is still needed. This review will describe the recent advances of highly biocompatible nanogel particles with particular emphasis as antimicrobial delivery carriers and surface coatings, including antifouling coatings, for biological and biomedical applications.
2. Synthesis, properties and biocompatibility of nanogels
2.1. Synthesis
Nanogels can be fabricated by numerous techniques that have been summarized recently in various excellent reviews [[36], [37], [38], [39], [40], [41], [42], [43]]. Generally, the synthesis methods can be divided into three major categories: 1) polymerization of monomers, 2) physical or chemical cross-linking of polymer precursors or natural polymers, 3) template-assisted nanofabrication. A brief overview of the commonly used techniques for the preparation of nanogels that have been studied in the context of antimicrobial applications will be provided.
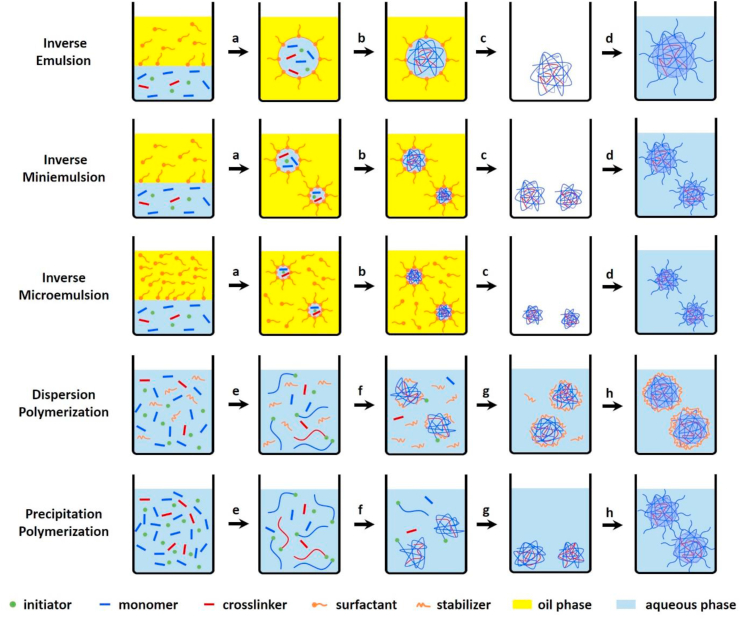
Heterogeneous polymerization is the most extensively employed method used to synthesize stable nanogels by free radical polymerization of monomers with the aid of cross-linkers and functional co-monomers. The polymerization is generally performed in aqueous matrix since most of the monomers and cross-linkers are hydrophilic. Approaches for such heterogeneous polymerization reactions include inverse emulsion, inverse miniemulsion and inverse microemulsion polymerization [[44], [45], [46], [47]], dispersion polymerization [[48], [49], [50]], and precipitation polymerization [42,51,52], as shown in Fig. 1.
Fig. 1.
Schematic description of nanogel formation by heterogeneous polymerization. The inverse emulsion, inverse miniemulsion and inverse microemulsion polymerization are considered to proceed as follows: (a) emulsification and homogenization, (b) polymerization, (c) removal of excess surfactant, and (d) transfer to good solvent. The dispersion and precipitation polymerization are considered to proceed as follows: (e) initiation and chain growth, (f) precipitation and nucleation by polymer chains collapse when the temperature is far above the lower critical solution temperature or by colloidal stabilizer on the surface of the unstable particles, (g) particle growth and (h) transfer to good solvent or decrease of temperature below the volume phase transition temperature.
The most appropriate polymerization processes depend on the desired properties of nanogels and the nature of the monomers. The inverse emulsion, inverse miniemulsion, and inverse microemulsion polymerization methods proceed via the polymerization of hydrophilic (ionic) monomers and cross-linkers within “(nano/micro) reactors” (the aqueous droplets), where the polymerization occurs upon the addition of the polymerization initiator [53]. One main difference between inverse emulsion and inverse miniemulsion processes is the initial size of the dispersed aqueous phase. In the case of inverse emulsion polymerizations, monomer droplets are formed by mechanical stirring and the size range from 1 to 20 μm [54]. In contrast, inverse miniemulsion polymerization has been broadly used to synthesize poly (N-isopropylacrylamide ((p (NIPAM)) nanogels and the nanogel size is around 150–300 nm [[55], [56], [57]]. The aqueous droplets are generated by applying high shear stress, e.g., by ultrasonication or high-pressure homogenizer, and stabilized with surfactants in a continuous organic phase. The used surfactant concentration is below or near its critical micellar concentration (CMC), so the miniemulsions are only kinetically stable where the stability greatly depends on the formation conditions. Thermodynamic stability is obtained by a further addition of surfactant above the CMC leading to inverse microemulsions. The inverse microemulsion is used to produce p (NIPAM), polyacrylamide ((P (AAM)), and poly (vinylpyrrolidone) (PVP) nanogels with a diameter usually less than 100 nm [45,[58], [59], [60], [61], [62], [63]]. In contrast to the heterogeneous inverse emulsion polymerizations, dispersion and precipitation polymerizations are initiated by a homogeneous nucleation mechanism that contains all ingredients, including the monomers, cross-linker, initiator and/or surfactants that are initially dissolved in the solvent. Following the polymerization, the polymer chains start growing until an insoluble critical chain length is reached and ultimately leads to the formation of stable distributed polymeric nanogels. Dispersion polymerization is mainly used to synthesize nanospheres of hydrophobic polymers such as polystyrene (PS) [64]. Recently, the efforts have been made towards the synthesis of nanogels in aqueous systems (water or water/ethanol mixture) using reversible addition-fragmentation chain transfer (RAFT) dispersion polymerization [49,50,[65], [66], [67]]. Dispersion polymerization generally yields smaller nanogels because of the presence of a colloidal stabilizer [68]. Differently, precipitation polymerization is frequently used to synthesize thermosensitive p (NIPAM) and poly (N-vinylcaprolactam) (PVCL) nanogels utilizing the phase separation behavior from swollen to a collapsed state since the reaction temperature (50–80 °C) is far above the volume phase transition temperature (VPTT). The detailed polymerization mechanism of precipitation polymerization has been addressed previously [42,69,70]. Briefly, initiators produce free radicals at the polymerization temperature, which attack water-soluble monomers followed by radical propagation and chain-growth to form oligoradicals. The growing polymer chains subsequently collapse when they reach a critical length and form precursor particles because the temperature is far above the lower critical solution temperature (LCST) of the formed polymers. The formed precursor particles can grow by aggregation with other precursor particles to form larger colloidal particles, by deposition onto the surface of existing particles, or by further addition of monomers. Once the nanogel particles reach the critical size, they are stabilized by the use of surfactants or electrostatic stabilization originated from charges of the initiator. When the reaction stops and cools down to room temperature (below VPTT), the nanogels swell to a “fuzzy” morphology and are stabilized by steric mechanisms due to the formation of hydrogen bonds between polymer segments and water molecules. There are some advantages using precipitation polymerization for the preparation of aqueous nanogels: 1) controllable nanogel size by the use of surfactants or co-monomers; 2) narrow nanogel size distribution; 3) desired properties and complex architectures can be obtained by integration of different co-monomers or even encapsulation of nanoparticles during the polymerization process.
Apart from the heterogeneous polymerization of monomers, nanogels prepared by cross-linking of synthetic or natural polymer precursors provides opportunities for producing more biocompatible nanogels [41,71]. Synthetic polymers with the desired function can be first synthesized by controlled polymerization to finely tune the composition, molecular weight, functional groups, and architecture. Functionalized natural polymers, such as polypeptides, alginate, pullulan, chitosan, dextran, and hyaluronic acid contribute to the improvement of biocompatibility and biodegradability of nanogels [[72], [73], [74], [75], [76], [77]]. There is a variety of cross-linking approaches, including “click” chemistry, thiol-disulfide exchange, Schiff-base reactions, photo-induced cross-linking have been developed for the synthesis of nanogels from the polymer precursors [21,75,[78], [79], [80], [81], [82], [83]]. Complementary to chemical cross-linking, physical self-assembly offers a reversible property since they are stabilized by relatively weak interactions between polymer chains such as hydrogen bonding, hydrophobic interactions, van der Waals interactions, and electrostatic interactions. Usually, the physical self-assembly is conducted under mild conditions in an aqueous medium. The size of nanogels is controlled by the polymer concentration, amphiphilic character, functional groups, pH, ionic strength, and temperature [84].
Different from the other previously mentioned polymerization approaches where templates were used such as emulsion droplets, another template-assisted nanofabrication, such as photolithography [85,86], is a new top-down particle lithographic technique called PRINT (Particle Replication In Non-wetting Templates), which provides the opportunity to fabricate a stable nanogel system with precise control over size, shape, deformability, and surface functionality [84,87]. Step and Flash Imprint Lithography (S-FIL) is a method that was recently developed as a nanofabrication method that is able to directly harvest nanogels from a silicon wafer substrate into aqueous buffers using a simple and biocompatible process in a high-throughput manner [88]. With this technique, the shape and surface chemistry of nanogels can be finely controlled, which is difficult using other bottom‐up methods.
2.2. Properties
Polymer nanogels, also known as microgels -since the first usage of the term “Microgel” in 1949 by W. O. Baker, have a sub-micron confined network resulting from chemical cross-linking between polymer strands and have attracted considerable attention in theoretical studies regarding soft matter [[89], [90], [91]]. At low concentrations, a nanogel solution acts like a dilute colloidal system, and the size of these well-dispersed nanogel particles and the local concentration of the cross-linked polymer chains in each particle can be adjusted by changing the cross-link density and surfactant amount during the polymerization [92,93]. By the time, the term microgel gave its place to the term “nanogels” since smaller microgels were started to be synthesized. Thanks to the unique behaviors of nanogels, such as their three-dimensional, macromolecular polymer network of colloidal size swollen by the dispersing solvent, and their versatile features like biocompatibility, degradability, high colloidal stability, and high loading capacity make them important candidates for biomedical applications for drug delivery, cell imaging, and tissue engineering [[94], [95], [96]]. Moreover, the ability to rapidly respond to environmental changes such as temperature, light, pH, ionic strength offers them distinct advantages over other types of nanomaterials in the biomedical field [21,97]. Through the advanced chemical design and synthesis methods mentioned above, the important parameters as size, shape, swelling degree, and chemical, as well as the topological composition, can be tuned to achieve the unique properties of nanogels [94]. Various reviews have summarized the fundamental research of nanogel structure, properties and applications in materials science and the biomedical field [21,38,40,41,43,83,94,95,98]. Within this review, the focus lies on the selective properties of nanogels specific for antimicrobial applications.
First of all, aqueous colloidal stability under physiological conditions is the most important requirement for any type of nanomaterials to be applied in antimicrobial applications, which will ultimately decide the biological efficiency, in vivo distribution, and toxicity of nanoparticles. Nanogels show better stability compared to macromolecules, micelles, inorganic nanoparticles, and other nanoparticles, which is derived from intra-particle cross-linking of the polymer chains and their hydrophilic nature [39,94,95].
To apply nanogels as a delivery system to combat bacterial infections, the versatility of the architecture and the tunable pore size of nanogels allows for the incorporation of numerous guest molecules ranging from inorganic nanoparticles to biomacromolecules such as proteins and DNA or small therapeutic molecules without compromising their hydrogel-like behavior and colloidal stability [53,[99], [100], [101], [102], [103]]. To achieve long circulation half-life of their cargo and improve the retention at the target site in vivo, meanwhile preventing the degradation of enzymes or genetic material, the size, softness, charge, and surface properties of nanogels play crucial roles. As compared to the size of biomolecules, the nanoscale size of nanogels helps to avoid rapid renal segregation, enhances bloodstream transport, easily enables tissue permeation, and increases passive targeting by the enhanced permeation and retention (EPR) effect [21]. Another unique characteristic that helps nanogels prevent fast renal clearance is their softness and deformability, which help them easily pass through the splenic filtration bed leading to longer circulation half-life and lower splenic accumulation [[104], [105], [106], [107]]. Further, nanogels can be designed to be stimuli-responsive and react to external stimuli to help achieve a controlled, triggered response at the infection region. The external stimuli can be the physiological environmental change within the body such as temperature, pH, enzyme presence, and redox conditions, or the stimuli can be applied externally such as light, electrochemical signal, pressure, and magnetic field. These stimuli can cause conformational or structural changes and then alter the hydrophilicity and/or hydrophobicity of the nanogels, subsequently resulting in swelling or collapse of the nanogel network [108,109]. The extent of swelling depends on the chemical composition, hydrophilicity of cross-linkers, ionization of functional groups, and the degree of cross-linking of the nanogel network, which controls the polymer mobility and the interaction of the polymer chains with water [[110], [111], [112]]. Depending on the desired stimulus and the utilized antimicrobial agents and target ligands, the cargo can be either chemically conjugated to nanogels or physically loaded into the network to achieve an on-demand release nanogel delivery system.
The hydrophilic nature of the hydrogel facilitates uses in vivo due to the presence of numerous polar groups such as –OH, –COOH, –CONH2 and –SO3H distributed along the polymer chain [41,113]. Besides, to facilitate the renal clearance of the final degraded products, many labile cross-links have been introduced in the chemical design of nanogels such as hydrazone, disulfide, carbonate ester, and siloxane to develop degradable nanogels [82,99,114,115]. Besides, nanogels composed of natural biodegradable polymers such as chitosan, pullulan, dextran, hyaluronic acid, alginate, and gelatin can be degraded by biological microenvironments [99].
2.3. Biocompatibility of nanogels
Nanogels coatings and particles have great potential in the development of smart biomaterials and are gaining interest in the biomedical field [116]. Because nanogels may interact with tissues and cells, they must be non-cytotoxic, non-apoptotic, and non-necrotic. A biomaterial is said to be biocompatible when it optimally performs its desired function without eliciting any local or systemic harmful effects [117]. Introduction of a biomaterial into a host organism triggers the foreign body response (FBR), which is preceded by the formation of a provisional matrix as a result of blood-biomaterial interactions, acute and chronic inflammation and coupled with the formation of foreign body giant cells [118]. The aim of the FBR is to enclose the biomaterial in a fibrotic capsule, isolating it from the surrounding tissue [119]. This inevitable enclosure in a fibrotic capsule has led to a revision of the definition of biocompatibility [117] where a distinction has been made between biotolerability and biocompatibility. The former is the ability of a biomaterial to reside in the host for a long period of time while eliciting a low degree of inflammation and the latter is defined as the ability of a material to locally trigger and guide non-fibrotic wound healing, reconstruction, and tissue integration [120]. Biocompatibility, and specifically cytotoxicity is often tested in vitro. The present in vitro assays are insufficient to allow in vitro assessment of biotolerability. Therefore, information on biotolerability is obtained from in vivo studies.
Nanogels are often found to be non-cytotoxic, although sometimes it is a dose and time dependent phenomenon. Temperature sensitive poly (N-vinylprolactam)-based nanogels were found to be non-cytotoxic to both 9-day and 16-day neurons from the cerebral cortex of rat embryos for up to 24 h [131] based on the outcome of both MTT and LDH assays. Rosmarinic nanogel particles, cross-linked products of Rosmarinic acid, showed improved cytotolerance of COS-1 cells lines at concentrations below 100 μg/mL when compared to rosmarinic acid, a non-biocompatible antioxidant and bioflavanoid. Also, Rosmarinic acid nanogels were blood compatible only at concentrations below 50 μg/mL as it showed a dose dependent stimulation of blood clotting and hemolysis at 100 μg/mL [127]. Similarly, hybrid core shell nanogels based on poly (NIPAM-co-3-acrylamidophenylboronic acid-co-dextran-maleic acid) coated nanoparticles with a great potential for temperature-controlled insulin release, showed higher cell viability of A549 cells from 2 to 4 days of exposure at 400 μg/mL with respect to 3-(Methacryloxy) propyltrimethoxysilane coated silica nanoparticles according to the results of MTT assay. Even increasing the concentration of the nanogels was accompanied with a slight increase in cytotolerance after 2 and 4 days [132].
Conjugation of nanogels to biomaterials are often applied in order to modulate their biocompatibility. Khan et al. [133] developed gold nanorod-nanogel (Au NRs-nanogels) composite particles by connecting gold nanorods to pNIPAM based nanogels via electrostatic interactions. The Au NRs-nanogels showed reduced cytotoxicity to MCF-7 cells based on MTT and LDH. In addition, the nanogel almost eliminated the hemolytic activity of the gold nanorods on blood agar [133]. Ketoprofen, an anti-inflammatory drug, inhibits cyclooxygenase and shows high gastrointestinal toxicity. Encapsulation by nanogels based on cellulose acetate phthalate and hydroxyethyl methacrylate (CAP-co-poly (HEMA)), reduced the cytotoxicity of ketoprofen against Vero cells after 24 h incubation at a concentration range of 1–20 μg/mL [130]. 2-hydroxy-1-(4-(2-hydroxyethoxy)phenyl)-2-methyl-1-propanone (Irgacure 2959) is an often used photoinitiator for water borne photo-curing of biomaterials because of its excellent water solubility and low toxicity [134]. However, Irgacure 2959 is characterized by a high migration of the photolysis fragments into the liquid environment due to its low molecular weight, which is potentially harmful for tissue cells. Incorporation of Irgacure 2959 into nanogels based on oligo (ethylene glycol) monomethyl ether methacrylate (OEOMA) with a molecular weight of 950 g/mol and polyethylene glycol dimethacrylate (PEGDMA) enhanced its thermostability and also reduced the migration of the photolysis fragments of the photo-cured film when compared with Irgacure 2959. Furthermore, MTT assay was used to demonstrate excellent cell viability of HeLa Cells after 24 h exposure to the nanogels in comparison to Irgacure 2959 [134].
Besides the reduction of cytotoxicity of compounds used in biomedical applications through encapsulation in nanogels, the biocompatibility of nanogels themselves can be affected by further functionalization [130]. Modification of the zwitterionic betaine of polyethyleneimine (PEI) via treatment with 1,3-propane sultone to improve the antifouling properties of PEI, reduced its proapoptotic and pronecrotic properties as well as its cytotoxicity to Vero79 cells in comparison with bare PEI after 48 h incubation. However, as a result, the antimicrobial ability of bare PEI was lost after modification [135]. Gum Arabic is widely employed as a thickening and emulsifying agent in the food industry. Nanogels of gum arabic crosslinked with gelatin is cytotolerant at concentration 0.125–1 mg/mL and hemocompatible at concentrations 0.2–1 mg/mL under ISO/TR 7406 [136]. Biocompatibility of Gum Arabic gel particles conjugated with diethylenediamine (GA-DETA) and taurine (GA-TA) in an attempt to tune the properties of the nanogels was studied. On one hand, DETA introduced positive charges which improved protein adsorption and in turn contributed to its improved cytotolerance on fibroblast L929 cells in comparison with unmodified Gum Arabic nanogels and on the other hand, taurine resulted in increased cytotoxicity on fibroblast L929 cells at 0–200 μg/mL. While both GA-DETA and GA-TA nanogels were found to induce blood clotting, GA-TA showed a higher capacity to induce apoptosis by reducing intracellular calcium concentrations [129].
On one hand, hydrophilic nanogel drug carriers resist opsonization and protein adhesion in a host but are poor transporters of hydrophobic drugs. On the other hand, the blood circulation time and therapeutic potential of hydrophobic nanogels is low because of their removal by the reticuloendothelial system. Amphiphilic nanogels are a new family of nanogels that combine resistance to opsonization and removal with increased uptake of hydrophobic drugs due to the presence of both hydrophilic and hydrophobic groups respectively [137]. The cytotolerance of amphiphilic nanogels is important since they can induce toxicity through interaction with cellular lipids. Combining 80 mol% of hydrophilic 2-hydroxypropylamin (HPA) groups with 20 mol% of either benzyl (BENZA-20), hexyl (HEXA-20), cholesteryl (CHOlA-20) or dodecyl (DODA-20) showed similar cell viability (circa 100%) of monocytic-like THP-1 cells at 20 -100 μg/mL after 2 h incubation in comparison to the biocompatible pentafluorophenyl methacrylate (PFPMA) as determined by WST-1 assay. Additionally, opsonization and protein adhesion was dependent on the hydrophobicity and structure of the nanogels while the former determined the corona composition of the nanogels, which will in turn determine their circulation time [137]. Magnetic nanogels can be guided to areas of interest to release contrast agents in magnetic resonance imaging [138]. Microscopy was employed to evaluate the biocompatibility of magnetic nanogels, which consisted of PDEAEMA-based nanogels encapsulated with Fe3O4 nanoparticles on red blood cells and platelets. In autologous plasma, the presence of biomolecules such as proteins, carbohydrates and lipids adhered to the surface of these magnetic nanogels, which prevented interaction with red blood cells and preserved their morphology while red blood cells were lysed after 15 min contact with these magnetic nanogels in physiological saline solution. In both situations, no interaction with platelets was observed [139]. Table 1 provides a short overview of common in vitro biocompatibility tests taken for various nanogel-based systems.
Table 1.
Summary of in vitro biocompatibility tests.
| Nano/microgel description | Intended application | Test model | Biocompatibility test | Ref |
|---|---|---|---|---|
| Silica nanoparticles coated with N-propyl acrylamidophenyl boronic acid-co-dextran-maleic acid nanogels | Insulin delivery | A549 cells | MTT assay | [55] |
| Rosmarinic acid crosslinked with trimethylolpropane triglycidyl ether | Drug conjugation and delivery | Cos-1 cells | Hemolysis and blood clotting test Toluidine blue staining |
[127] |
| Au-NR and N-isopropylacrylamide-co-acrylic acid nanogel composite | Drug delivery Photothermal therapy |
MCF-7 cells | MTT assay LDH assay Cell hemolysis test on blood agar |
[133] |
| PEI microgels functionalized with 1,3-propane sultone | Drug delivery Gene delivery Tissue engineering |
Vero 79 cells | WST-1 assay | [135] |
| Gum Arabic-gelatin nanogels | Drug delivery Gene delivery |
MCF-7 cells | Hemolysis test MTT |
[136] |
| 80 mol% 2-hydroxypropylamin groups with BENZA-20 or HEXA-20 or CHOlA-20 or DODA-20) | Drug delivery | THP-1 cells | WST-1 assay | [137] |
| Poly (N-(2-mercaptoethyl) acrylamide) | Antioxidant Antibacterial |
L929 fibroblasts DLD-1 cells |
WST-1 assay Hemolysis test Blood clotting index Hoechst and Propidium iodide staining |
[130] |
| Gum Arabic nanogels modified with diethylenediamine and taurine | Antibacterial | L929 fibroblasts | WST-1 assay Hemolysis test Blood clotting index Hoechst and Propidium iodide staining |
[129] |
| Oligo (ethylene) mono methyl ether methacrylate | Photo initiator in water borne photocuring | HeLa cells | MTT | [134] |
| PDEAMA-based nanogels labelled with Fe3O4 magnetic nanoparticles | Clinical diagnostics and therapy | Red blood cells | Microscopic assessment | [139] |
Biocompatibility can be influenced by physico-chemical properties of the nanogels. For example, the temperature at which MTT and LDH assays are performed for some temperature sensitive nanogels can influence the outcome of these tests, which in particular is relevant in cases of temperature triggered drug release. The cell viability of both Human breast cancer cell line (MCF-7) and Jurkat cells were approximately 100% when incubated with poly N,N′-diethylacrylamide (DEEam) at concentration range of 0.1–2.5 mg/mL and poly (N-isopropylacrylamide) (NipAAm) at concentrations up to 1 mg/mL for 24 h when MTT and LDH assays were performed at 37 °C. However at 22 °C, which is below the critical temperature, the permeability of the cell membranes of both MCF-7 and Jurkat cells for LDH increased at concentration up to and above 1 mg/mL for polyDEEAm and polyNiPAAm respectively due to their improved solubility and interaction with the cell membrane [140].
2.4. In vitro biocompatibility tests
Biocompatibility testing is part of the main conformity assessment to demonstrate the safety of the product for use in medical devices. In this respect the ISO-standard used in the biological evaluation of medical device, ISO 10993, is guiding the conformity assessment, e.g. with respect to cytotoxicity testing as in ISO 10993:5. According to this standard, cytoxicity tests can be assessed by morphological means or measurements of cell damage, cell growth, and specific aspects of cellular metabolism. Rapid and inexpensive calorimetric assays such as the (3-(4,5-dimethylthiazol-2-yl)-2–5-diphenyltetrazolium bromide (MTT), 2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfo-phenyl)-2H-tetrazolium, monosodium (WST-1) and lactate dehydrogenase (LDH) assays are routinely performed to evaluate the cyto-tolerance of newly synthesized nanogels based on cellular metabolism [121]. The MTT assay measures the viability of cells by determining the activity of mitochondrial NAD(P)H-dependent cellular oxidoreductase enzyme which is capable of reducing MMT to an insoluble purple formazan. Similarly, the WST-1 assay predicts relative cell proliferation through the reduction of the tetrazolium WST-1 salt to formazan by mitochondrial dehydrogenases while the LDH assay quantitatively measures the leakage of LDH from the cytoplasm as a result of cell membrane damage [121].
Apart from these standard methods, several methods have been employed to evaluate the apoptotic, necrotic, and genotoxic effects of nano-sized gels. A double staining with Propidium iodide and Hoechst applied to a cellular suspension in the presence of various concentrations of the gel has been utilized to assess apoptotic and necrotic effects. These are sometimes paired with methods like the comet assay [[122], [123], [124]] and the annexin-v staining [125] to detect single strand breaks in DNA and the translocation of phosphatidylserine on the outer leaflet of the cell membrane respectively. In addition to this, the generation of reactive oxygen species is measured with the 20,70-dichlorofluorescein diacetate assay [124] and microscopic [126] assessment are utilized to estimate the cytotoxicity of nanogels [127,128].
In order to prevent the inhibition of oxygen transport and encourage proper blood clotting, nanogels, which may directly or indirectly come into contact with blood, should not cause the lysis or coagulation of red blood cells and platelets respectively. Hemocompability is usually evaluated with hemolysis and blood clotting tests because a biomaterial may influence the mechanisms of coagulation and thrombosis [129,130].
2.5. Biotolerability
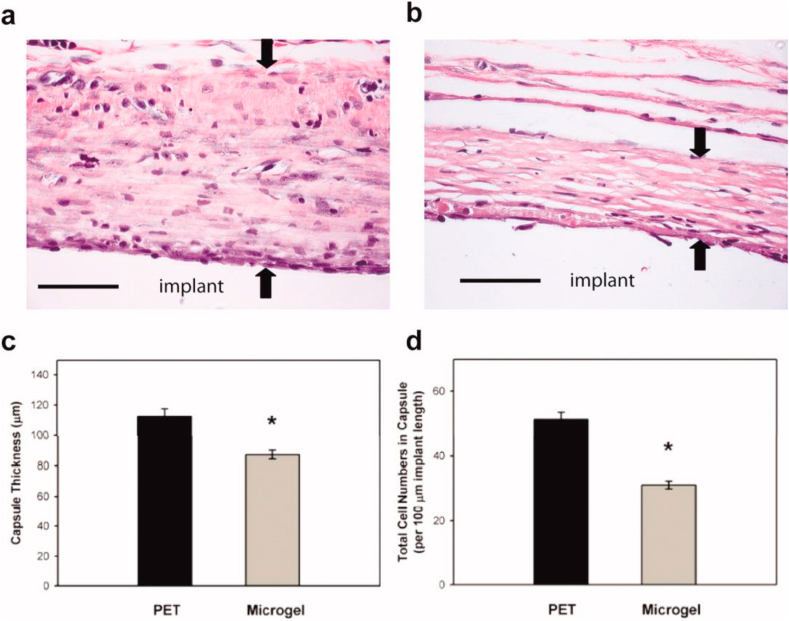
As far as we know, no standard procedures are available to test the biotolerability of nanogels, nor for the FBR against biomaterials in general. In vivo studies, however, often gives a clear picture of the wide repertoire of interactions that occur once nanogels are introduced into a living host [141,142]. The provisional matrix, which is formed by the non-specific adhesion of serum proteins, attracts fibroblasts and other cells involved in acute inflammation. Therefore preventing the adhesion of proteins to the surface of the biomaterial may limit the extent of the FBR [143]. Coating poly (ethylene terephthalate) (PET) with films of pNIPAm cross-linked with the non-fouling poly (ethylene glycol), reduced cellular adhesion both in vitro and in vivo in comparison with uncoated PET substrates. Histological assessment of the implants after intraperitoneal implantation in mice at 48 h showed a thinner and denser collagen capsule on coated PET in comparison to uncoated PET as shown in Fig. 2, indicating a shorter chronic inflammation and a faster resolution of the immune response on the coating [144].
Fig. 2.
Microgel coatings reduce chronic inflammation associated with materials implanted subcutaneously in the rat dorsum for 4 wk. Explants were evaluated for fibrous encapsulation by staining collagen (pink), elastin (black), and nuclei (black). Representative images for unmodified PET (a) and microgel-coated PET (b) disks, and the original implant location is designated. Black arrows indicate capsule measurements. Microgel coatings reduced fibrous capsule thickness by 22% compared to unmodified PET controls as quantified in (c), *p < 0.04. The density of capsule-associated cells was also significantly reduced in microgel-coated samples (*p < 5.6 × 10−3) compared to unmodified PET controls as quantified in (d). Data is represented as the average value ± standard error of the mean using n = 4–7 samples per treatment group. Scale bar is 50 μm. (Reproduced with permission from ref 144. (Copyright 2010 Wiley).
Since nanogels are hydrogels at nanometer scales, zwitterionic nanogels may promote a non-fibrotic incorporation of biomaterials in the host tissue. The zwitterionic polycarboxybetaine methacrylate (pCBMA) is the only hydrogel known to resist the formation of a fibrotic capsule after 3 months of subcutaneous implantation in mice [145]. However, a disadvantage of zwitterionic hydrogels is their low mechanical strength. To improve the mechanical strength of zwitterionic hydrogels while mitigating the foreign body response, triazole groups were introduced in pCB to form triazole zwitterionic hydrogels (TR-ZW) [146]. This resulted in a 250% percent tensile strain, which is higher than the 65% tensile strain of pCB. In vitro assessment showed similar cell adhesion to the TR-ZW when compared to pCB and the biocompatible poly (2-hydroxyethyl methacrylate) (PHEMA). The expression of interleukin 10 was also increased, which is a characteristic of the pro healing macrophage phenotype. Histological evaluation after subcutaneous implantation of the TR-ZW in mice showed that the introduction of hydrophobic triazole groups increased the adsorption of plasma (3–10 ng/cm2) on the TR-ZW hydrogels in comparison to pCB (0.3 ng/cm2). However, this did not interfere with the attenuation of a fibrotic capsule and increased blood vessel formation when compared to PHEMA [146]. Temperature sensitive PNIPAM nanogels elicited mild inflammation, which kept declining in severity from one week up to 3 months after injection into the right kidney of rabbits at an embolization rate of 0.10 mL/s. After 3 months, no signs of neovascularization, foreign body granuloma, extravasation, mural hemorrhage and vascular spasms were found. However, fibrous tissues were found in embolized vessels together with the PNIPAM nanogels, which contributed to the persistence of the embolic effect 1 and 2 months after the operation [147].
Biocompatibility and biotolerability are dynamic and multifactorial properties that depend on the concentration and physico-chemical properties of the biomaterial, location, the types of interaction between material and host tissue. The intended functionality is essential since the given definition of biotolerability is not applicable to nanogels used as embolic agents. The research presented above proves the complexity involved in defining the biocompatibility or biotolerability of nanogels. Changes in the biomaterial or in the host environment can potentially cause a once biocompatible biomaterial to become toxic or non-compatible [142]. This makes a single definition of biocompatibility unrealistic. Rather, biocompatibility and biotolerability could be redefined for classes of nanogels that have similar biomedical applications. In addition to this, the biocompatibility of nanogels should not be based only on MTT and LDH assays as sometimes found in the literature. The MTT assay can be influenced by factors such as pH and the presence of superoxide, polyphenols, and pyruvate analogs. Superoxide is capable of reducing MTT to Formazan, resulting in an inaccurate estimation of cell viability [148]. Moreover, MTT formazan formed needle-like crystals at nanomolar concentrations that could induce apoptosis of SH-SY5Y cells which resulted in increased plasma permeability and leakage of cell content [149]. Therefore, these assays should always be combined with tests that evaluate apoptotic, necrotic and hemolytic effects of newly synthesized nanogels.
In contrast to drugs, the preclinical and clinical testing of biomaterials is not well defined [150]. Therefore, standard tests can help avoid inaccurate claims of biocompatibility. In addition, choosing the appropriate standard toxicity tests provide some understanding about the physiological, chemical and biochemical processes that become operative in cells or tissues upon contact with nanogels and this is key in defining their biocompatibility and biotolerability [151]. This knowledge may in turn facilitate the development of nanogels with defined properties that may result in successful clinical application [152].
3. Nanogel-based antimicrobial systems
Over the past few decades, multidrug-resistant bacteria have become a serious health concern due to the abuse and misuse of antibiotics [153,154]. A high therapeutic dosage of antibiotics is required to treat the resistant bacterial infection, therewith further contributing to the increased emergence of drug resistance. Therefore, it is imperative to develop novel antimicrobial agents and new treatment strategies [155,156]. The recent advancements in the development of antimicrobial nanogels offered a promising approach to tackle multidrug-resistant bacteria [[157], [158], [159], [160]]. This section will first provide the applications of nanogels used as delivery tools for conventional biocidal agents; then, the focus will be on the recent development of nanogels with intrinsic antimicrobial actions.
3.1. Nanogels as antimicrobial delivery vehicles
To reduce the applied dosage of biocidal agents and enhance the bioavailability at the same time, the conventional biocidal agents can be conjugated or encapsulated into nanogels and enriched in the infected zone, and released to kill bacteria. The widely used biocidal agents in literature include but are not limited to antibiotics [[161], [162], [163], [164], [165], [166], [167]], bioactive antimicrobial agents [[168], [169], [170], [171], [172]], silver and gold nanoparticles [[173], [174], [175], [176], [177], [178], [179], [180], [181], [182]], and antimicrobial peptides [[183], [184], [185], [186], [187]].
3.1.1. Antimicrobial agent-loaded nanogels
In addition to developing new antibiotics, reduction of the administered dose is also important to cope with the rise of drug-resistant bacteria. Small molecular active antibiotics are easily cleared and quickly inactivated, while the remaining active antibiotics have difficulty penetrating the tissue barrier and killing bacteria in the infected area. To enhance the bioavailability of antibiotics, conventional antibiotics can be encapsulated into nanogels. Encapsulation protects the drugs from degradation and may improve the penetration by tuning specific (bio)chemical interactions with the cell wall, and subsequently (triggered) release of the antibiotics to the infected zone to improve the therapeutic concentrations [162,163].
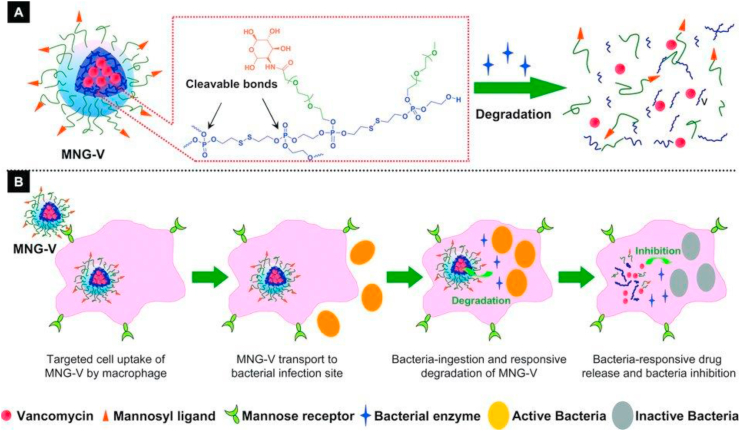
Among all the commercially available antibiotics, vancomycin is considered a last resort medicine for the treatment of sepsis and lower respiratory tract infections caused by Gram-positive bacteria. Vancomycin is a glycopeptide antimicrobial as alternative penicillin to treat penicillinase-producing strains of Staphylococcus aureus, and it is also widely used for the treatment of serious infections involving methicillin-resistant S. aureus (MRSA) [188,189]. However, the incidence of infections with vancomycin-resistant bacteria strains have greatly increased [190,191]. Recently, several studies have used nanogels to deliver vancomycin to the bacterial infection site [161,166,192,193]. However, the hydrophilic nature of vancomycin may cause undesired leakage or non-specific release when loaded into nanogels. To overcome the problem, a bacterial-lipase sensitive polymeric triple-layered nanogel (TLN) was synthesized by a convenient arm-first procedure using an amphiphilic diblock copolymer mPEG-PCL (monomethoxy poly (ethylene glycol)-b-poly (ε-caprolactone)) to initiate the ring-opening polymerization of the difunctional monomer DEGDP (3-oxapentane-1,5-diyl bis(ethylene phosphate)) [161]. In this approach, the bacterial lipase-sensitive hydrophobic PCL interlayer formed a hydrophobic and compact molecular fence surrounding the cross-linked polyphosphoester core to prevent vancomycin release from the drug reservoir before reaching the infection sites. The approach eliminates potential adverse side effects due to non-specific drug leakage. After reaching the bacterial infection sites, the TLN sensed the lipase-secreting bacteria and the PCL fence degraded to release the loaded vancomycin. To further achieve the selective eradication of intracellular bacteria, a macrophage targeted antibiotic delivery nanogel (MNG-V) was developed by the same group using a mannosylated PEG-arms shell and a vancomycin-loaded polyphosphoester cross-linked core, as shown in Fig. 3 [192]. The conjugated mannose moieties could target the mannose receptors expressed on macrophages and facilitated the uptake of nanogels to accumulate the drug at the bacterial infection site in vivo through macrophage transport [194]. Subsequently, after bacteria are phagocytosed by macrophages, the rapid triggered vancomycin release was achieved in the presence of the bacterial enzymes (e.g., phosphatase or phospholipase) by degrading the polyphosphoester core of the nanogel. The released vancomycin further killed the bacteria, when the absolute concentration of vancomycin was increased to 10 μg/mL, the counting the colony forming units (CFU) of surviving intracellular bacteria, treated by MNG-V, was about 50 times lower than in cells treated with free vancomycin, which also significantly improves the therapeutic efficacy of vancomycin in the infected zebrafish embryo model. However, the increased macrophage uptake of nanogels may also speed up the immune clearance to reduce the therapeutic efficacy [195].
Fig. 3.
(A) Schematic illustration of a vancomycin‐loaded mannosylated nanogels (MNG‐V) and the bacteria‐responsive drug release; (B) Schematic illustration of targeted uptake of MNG‐V, transport, degradation, drug release and bacteria inhibition. (Reproduced with permission from ref 192. (Copyright 2012 Wiley)).
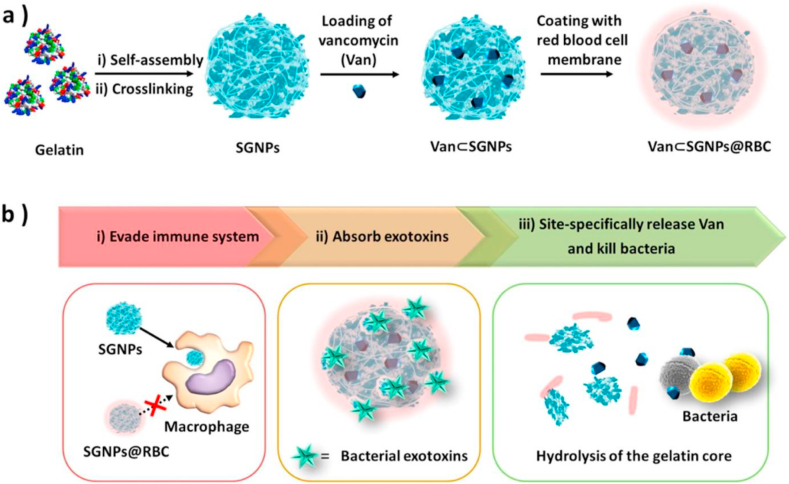
To avoid the immune clearance by macrophages, Li et al. reported red blood cell (RBC) membrane coated vancomycin-loaded supramolecular gelatin nanoparticles (SGNPs) for adaptive and “on-demand” antibiotic delivery [193]. As shown in Fig. 4, the RBC membranes also act as detoxifiers to absorb the exotoxins produced by bacteria to relieve bacteria-induced inflammation. Meanwhile, the overexpressed bacterial gelatinases in the infection microenvironment could effectively hydrolyze SGNPs into small fragments, and triggered the release of loaded vancomycin to kill pathogenic bacteria locally. In this way, the bacterial infection was treated by the biomimetic antibiotic delivery system with a minimum dose of antibiotics. Another group combined the two unique strategies, toxin neutralization property of RBC membranes and the ‘on-demand’ drug release nanogel, to achieve the specific intracellular release of vancomycin to treat the MRSA infection [166]. Different from the prior wrapping of RBC membranes onto pre-formed nanogels is that they first prepared the cell membrane-derived vesicles and then polymerize the hydrogel cores inside the vesicles by a redox-responsive cross-linker containing a disulfide bond. Therefore, in an extracellular environment, the RBC-nanogels effectively neutralized MRSA-associated toxins, which in turn promoted further bacterial uptake by macrophages. The intracellular reducing environment facilitated an accelerated drug release profile, which resulted in more effective bacterial inhibition.
Fig. 4.
(a) Preparation of vancomycin encapsulated supramolecular gelatin nanoparticles with RBC membrane coating layer (Van⊂SGNPs@RBC). (b) Schematic representation of adaptive and multifunctional Van⊂SGNPs@RBC in the treatment of a bacterial infection. (Reproduced with permission from ref 193. (Copyright 2014 American Chemical Society)).
Alternatives to synthetic antibiotics, a growing number of antibacterial studies using natural antimicrobial compounds, have emerged to solve the severe issues mainly raised from the overuse of antibiotics [[196], [197], [198]]. However, the intrinsic problems of natural compounds, such as low activity, low availability, and instability, hinder their applications. Recent advances have been made by introducing nanogels to transport and protect these natural compounds for enhanced effectiveness [[199], [200], [201]]. For instance, essential oils consist of multiple phenylpropanoids, which are natural antifungal agents but with intrinsic hydrophobicity and rapid evaporation. These oils have been encapsulated into chitosan nanogels to improve antimicrobial activity and stability and are applied in the food industry [[202], [203], [204]]. Curcumin, a natural yellow phenolic compound present in turmeric, has numerous pharmacological properties such as anticancer, anti-inflammatory, antimicrobial, anti-malarial, and antioxidant, as studied in both preclinical and clinical studies [[205], [206], [207]]. However, the rapid degradation of curcumin at physiological pH and poor aqueous solubility limits their application. To tackle the problem, Li and coworkers fabricated a strawberry-like novel temperature-sensitive nanogel using (p (NIPAM), temperature-sensitive polymer to encapsulate poly (3,4-ethylene dioxythiophene) nanoparticles (PEDOT, photothermal agents) and curcumin through reformative precipitation polymerization [201]. The release of curcumin was achieved by the shrinkage of p (NIPAM) networks triggered by a light-induced temperature increase of PEDOT. This temperature-sensitive nanogel system showed excellent antioxidant and antibacterial performance.
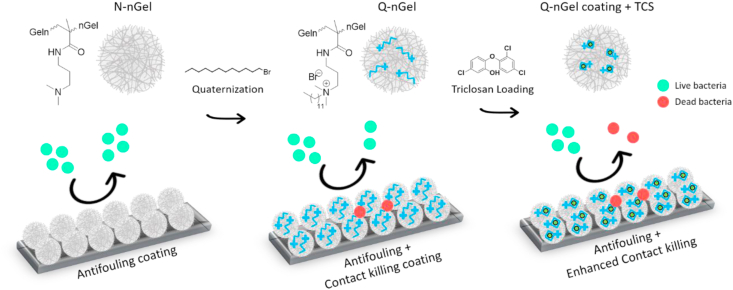
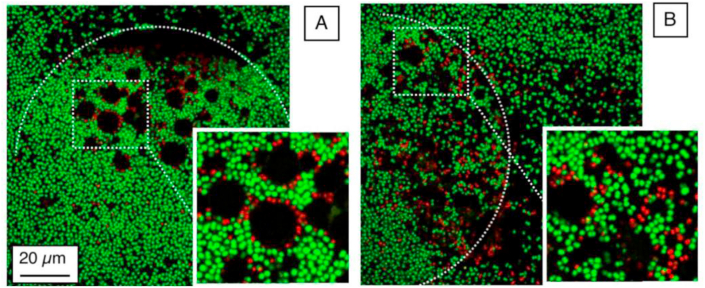
Other than the conventional antibiotics and natural antimicrobial compounds, some chemical antiseptic and disinfection moieties, such as chlorhexidine and Triclosan, have received great attention in oral and skin health applications, for instance, periodontal therapy and wound healing [208,209]. The mechanisms of action of chlorhexidine are bactericidal as well as bacteriostatic, causing membrane disruption [210]. Triclosan combats bacteria by non-specific interaction with the cell membrane but also blocks the lipid synthesis explicitly to inhibit the bacterial growth [211]. However, both of them are small molecules that have poor bioavailability; especially, Triclosan has poor solubility in aqueous solutions, and is under scrutiny because of its environmental and potential hazardous effects [212], which makes it important to provide systems that greatly diminish the used concentration without losing the antimicrobial effects. Therefore, controlled delivery of them to the target sites can help to reduce the toxicity and enhance the biological effectiveness. Some investigations have used nanogels as a carrier to be applied in both aqueous dispersion and surface coatings, which will be addressed in the coating section [[213], [214], [215], [216]]. Recently, in our group, a p (NIPAM-co-DMAPMA) based nanogel quaternized with 1-bromododecane was designed to induce intraparticle micellization and thereby create a hydrophobic domain inside the nanogel to host hydrophobic drug, e.g., Triclosan [217]. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) results showed that the synergy of physical destruction of the microbial cell membrane by quaternary ammonium compounds and active nano-injection of Triclosan dramatically enhanced the antimicrobial efficiency 1018 fold as compared to free Triclosan against S. aureus.
Notably, bacteria generally have negatively charged cell membranes consisting of lipid layers and peptidoglycan [218]. Therefore, nanogel carriers with a positive surface charge could enhance the interaction between the nanogel and the surface of the bacteria. Paunov and coworkers have conducted a series of experiments on the enhancement of the antimicrobial effect of antibiotics or cationic antimicrobial agents using nanogel carriers with cationic surface functionality [[219], [220], [221], [222]]. In one of these studies, the antibacterial agent berberine was loaded into polyacrylic acid-based nanogels, the surface of which was subsequently coated with a layer of cationic polyelectrolyte, poly (diallyl dimethylammonium chloride) (PDAC) [221]. The PDAC-coated nanogels encapsulated with berberine exhibited enhanced antimicrobial efficacy against both Escherichia coli (E. coli) and Chlamydomonas reinhardtii, compared to the free berberine. In another work, the biocompatible cationic polyelectrolyte polyethyleneimine (PEI) was used to functionalize the polyacrylic acid-based nanogel. The nanogel was loaded with two small cationic antimicrobial molecules, tetracycline hydrochloride and lincomycin hydrochloride, which can potentially overcome antibiotic resistance [220]. The functionalized antibiotic-loaded cationic nanogel carriers enhanced the antimicrobial activity by specific electrostatic adhesion to the microbial cell wall, and due to accumulation, a higher local antibiotic concentration was achieved.
3.1.2. Silver-loaded nanogels
Metal nanoparticles exhibit unique chemical, physical, and biological properties that are significantly different from bulk metals or isolated molecules. For example, silver nanoparticles (AgNPs) display antimicrobial and anti-inflammatory properties, thus have been exploited as antimicrobial agents [223,224]. The proposed mechanism of action of AgNPs vary: the destruction of the bacterial cell wall by their favorable surface properties; generation of free radicals that can alter the membrane permeability; inactivation of indispensable enzymes by thiol group modification due to the release of silver ions [225,226]. To achieve the controlled or sustained release of AgNPs locally while limiting the cellular uptake and possible cytotoxicity of AgNPs, encapsulation of AgNPs inside nanogels, so-called hybrid nanogels or nanocomposites have been developed [[227], [228], [229], [230], [231]]. However, most of the hybrid nanogels were prepared with prefabricated AgNPs, followed by the addition of AgNPs into the polymer network [232,233]. Alternatively, efforts are heading towards studying greener and easier methods for AgNPs containing antimicrobial hybrid nanogels [177,[234], [235], [236], [237]]. Ferrer and coworkers developed the hybrid nanogels (~160 nm) consisting of a lysozyme rich core and a dextran rich shell containing AgNPs (~5 nm), which were synthesized “in situ” in the nanogel solution without requiring additional reducing agents [238]. Lysozyme was found to enhance nucleation and stabilization of AgNPs while limiting their growth. Furthermore, with varying lysozyme content, they were able to tune the size of both AgNPs and nanogels and the loading capacity; larger nanogels with greater loading of smaller AgNPs were obtained following increased lysozyme concentration [236]. Similarly, Khan et al. also reported an environmentally greener reducing agent, glucose, to form the AgNPs nanogel “in situ”, but with a microwave-assisted heating technique [237]. Also, AgNPs nanogel have been synthesized and stabilized simultaneously “in situ” with the help of gamma irradiation, the mean size of AgNPs ranged from 10 to 50 nm [177]. Analogously, Choi et al. developed a one-step process to synthesize nanogels containing AgNPs involving electron beam irradiation of silver nitrate and poly (acrylic acid). The size of the prepared nanogels decreased with increasing irradiation doses, while the antibacterial effects increased due to a larger surface area for bacterial interaction of smaller AgNPs [234].
3.1.3. Antimicrobial peptide-loaded nanogels
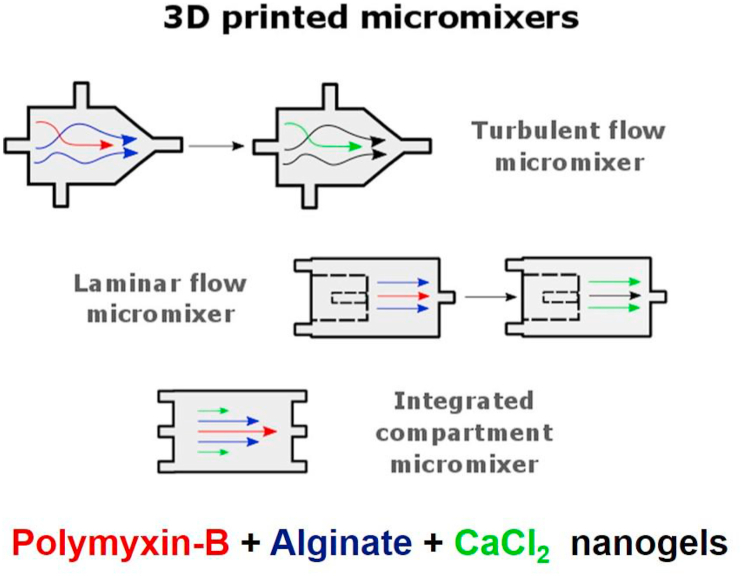
As an attractive alternative to commercial antibiotics, antimicrobial peptides (AMPs) have gained large interest over the last decades and some found their way into clinical practice [239]. AMPs are natural compounds driven from innate immune systems and can kill bacteria by non-specific membrane disruption, which is less likely to induce bacterial resistance [240]. With their both hydrophobic and hydrophilic moieties, AMPs affect bacteria in several ways: AMPs destabilize the membrane by pore formation, causing the leakage of intracellular components, and the carpet mechanisms where the coverage of AMPs on the outer and inner membrane is uneven, which induces the imbalanced surface tension resulting in cell membrane collapse [241,242]. However, some undesirable characteristics, such as high toxicity, easy degradation, and low bioavailability by binding to serum proteins, mucins, and other anionic components, have impaired the clinical translation of AMPs [240,241]. Therefore, recently increased attention has been placed on nanogels as carriers for the delivery of AMPs to improve the antimicrobial performance [[243], [244], [245], [246], [247], [248], [249], [250]]. The Malmsten group investigated cationic AMPs incorporated into anionic poly (ethyl acrylate-co-methacrylic acid) (MAA) nanogels in different ways that influenced the encapsulation efficiency and release of AMPs, and the membrane interactions and antimicrobial effects of those nanogels. To investigate the mechanisms of membrane interactions of free and nanogel-loaded AMPs, the dimyristoylphosphatidylcholine/dimyristoylphosphatidylglycerol (DMPC/DMPG) (75/25) was chosen as model cellular membrane to obtain solid-supported bilayers with an anionic charge to mimic the bacterial membranes. They found that the peptide incorporation increased with increasing nanogel charge density and peptide amphiphilicity. The nanogel displayed a negative zeta potential even at high peptide loading and therefore did not bind to the bacteria-mimicking membranes. Instead, membrane disruption and antimicrobial effects against E. coli relied largely on peptide release, nanogel charge density, primarily influencing the peptide loading and release rate [245]. They also investigated how poly (ethylene glycol) conjugation (PEGylation) of AMPs affected their loading and release behavior from MAA nanogels. They found that the PEG content influenced the nanogel loading and release rather than the PEG conjugation site. PEGylation appeared to shift peptide localization towards the corona instead of in the core of the nanogel [249]. Building on the studies of AMPs-loaded nanogels synthesized by emulsion polymerization, recently the same group prepared AMPs-loaded nanogels using continuous microfluidic particle generation based on 3D-printed micromixers by complexation of polymyxin B with Ca2+ cross-linked alginate, as shown in Fig. 5 [248]. As such, Polymyxin B was localized in the particle core and caused particle growth with increasing peptide loading up to > 80%, and the peptide release was strongly accelerated at physiological ionic strength. To offer better stability during the production of AMPs-loaded nanogels, Parilti and coworkers used supercritical carbon dioxide as a green alternative for the conventional solvents to synthesize AMP-loaded poly (2-hydroxyethyl methacrylate) (poly (HEMA)) nanogel via one-pot free-radical dispersion polymerization as a powder [243]. The antibacterial tests revealed the sustained release of the AMP from this poly (HEMA) nanogel that was swollen in water.
Fig. 5.
Preparation of Ca2+-cross-linked alginate nanogels loaded with the AMP polymyxin B in a continuous process using 3D-printed micromixers with three different geometric designs: turbulent flow micromixers, laminar flow micromixers, or integrated compartment micromixers. (Reproduced with permission from ref 248. (Copyright 2019 Elsevier)).
3.2. Nanogels as active antimicrobial agents
Although antimicrobials-containing nanogels significantly show antibacterial efficacy and potentially slow down the emergence of multidrug-resistant bacteria, the development of new materials with intrinsic antimicrobial activities is still in urgent demand. Metal-based nanoparticles can be directly used but, as mentioned before, usually with high toxicity. Nanogels provide a versatile platform to generate novel intrinsically antimicrobial structures due to their large surface area to mass ratio allowing multivalent interactions with bacteria, their chemical reactivity, and highly compatible surface for facile functionalization, and their high colloidal stability and excellent biocompatibility.
Whereas in the previous section nanogels were applied as carriers for antimicrobials and the susceptibility of microorganisms to these antimicrobials are in most cases expected to be unchanged (or in some cases enhanced), the susceptibility of microorganisms towards nanogels with intrinsic antimicrobial action need to be assessed separately. Antimicrobial susceptibility tests have been designed to evaluate the time or concentration dependent antimicrobial potential of newly synthesized antimicrobial agent by determining the minimum inhibitory concentration (MIC) against microorganisms, indicating the antimicrobial concentration that inhibits visible growth of a planktonic bacterial suspension after incubation [251,252]. Because the MIC can be influenced by factors such as the inoculum preparation method and concentration, type of growth medium and incubation time, these tests have been standardized by the Clinical and Laboratory Standards Institute, International Standard Organization (ISO) and European committee of antimicrobial susceptibility testing (EUCAST), making clinical evaluation possible, however these guidelines do not guarantee clinical relevance [251].
Recently, the developments of nanogels with intrinsic antimicrobial moieties, such as quaternary ammonium compounds (QACs) [[253], [254], [255], [256]], guanidine [257], AMPs and their synthetic mimics [258], have been subject of investigation but is still underdeveloped as compared to the previously mentioned systems. Positively charged moieties cause physical damage to the bacterial cell membrane and are one of the major mechanisms of antimicrobial activity of functional nanogels. This strong charge interaction leads to the generation of pores and alteration in membrane permeability and, eventually, bacterial cell death [259]. Among the different cationic compounds that have been used in nanogel functionalization, QACs are the most explored ones. Echeverría and coworkers synthesized a tertiary amine group-containing nanogel by copolymerization of NIPAM and dimethylaminoethyl methacrylate (DMAEMA), and further quaternized with methyl iodide and butyl iodide [256]. The MIC results showed that compared with pNIPAM nanogel (MIC > 10 mg/mL) and unquaternized nanogels (MIC = 5 mg/mL), the methyl quaternized nanogels with highest incorporation of DMAEMA up to 25 wt% provide some antimicrobial ability, although the MIC was still high (MIC = 2.5 mg/mL).
In addition to quaternized nanogels, nanogels with cationic guanidine groups on the surface inhibits bacterial growth by attacking them through electrostatic attraction on the bacteria cell surface [260]. Han et al. prepared a guanidine nanogel by copolymerization of styrene, polycaprolactone-hydroxyethyl methacrylate, and polyhexamethylene guanidine hydrochloride methacrylate. The resultant nanogels exhibit a strong ability to kill S. aureus and E. coli by destroying the cell membrane and causing the cell lysis. Besides, the nanogels had a positive effect on preventing wound infection as a result of the antibacterial activity [257].
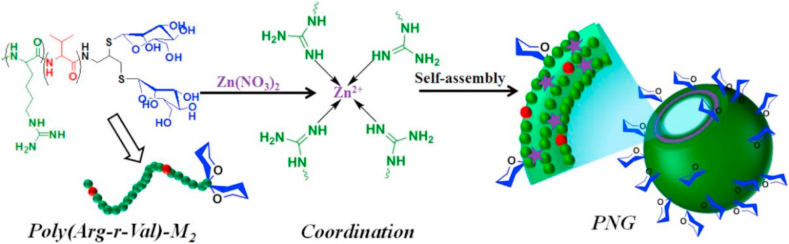
As addressed above, AMPs show broad and effective antimicrobial activity against bacteria, viruses, and fungi, but their high manufacturing cost, susceptibility to proteolysis, and poorly understood pharmacokinetics due to the complexity of their native structures hindered their applicability as drugs. Therefore, there has been increasing research interest towards preparing synthetic AMPs with the amino acids, lysine, arginine, and histidine as cationic components and leucine, valine, and phenylalanine as their hydrophobic analogs [261]. Except being delivered by nanogels, a series of polypeptide nanogels (PNGs) have been fabricated by coordination-assisted self-assembly of a mannose-conjugated AMP, poly (arginine-r-valine)-mannose, as a ligand and Zn2+ ion as a metal ion source to minimize the toxicity of the pristine polypeptide without compromising the antimicrobial activity, as shown in Fig. 6 [258]. The PNGs showed the potential bactericidal effect as revealed from their MIC results (2–16 μg/mL) against S. aureus and E. coli, which were significantly lower than the traditionally used antibiotic, vancomycin (>128 μg/mL; E. coli). Notably, the PNGs exhibited higher cell viability (above 80%) against mammalian cells with a negligible hemolytic effect compared to the pristine polypeptide (viability below 35%). Determination of the MIC in the above studies is necessary to evaluate the susceptibility of microbes to these nanogels with antimicrobial functionality.
Fig. 6.
Schematic presentation of the fabrication of PNG by coordination-assisted self-assembly of a mannose-conjugated antimicrobial polypeptide, poly (arginine-r-valine)-mannose with Zn2+ ions. (Reproduced with permission from ref 258. (Copyright 2019 American Chemical Society)).
4. Antimicrobial nanogel surface coatings for biomedical applications
Bacterial attachment and subsequent biofilm formation on implant surfaces is a severe problem from both an economical and healthcare perspective, since bacterial biofilms show a high resistance towards antibiotics and biomaterial associated infections are notoriously persistent and difficult to eradicate [262,263]. In recent decades, considerable focus has been placed on the creation of surface coatings to prevent initial bacterial adhesion and biofilm formation as an alternative platform since antibiotic resistance is becoming a serious threat. These antimicrobial surface coatings are mainly based on three strategies: an antifouling strategy that can significantly reduce the amount of initial bacterial attachment, an antibacterial (bactericidal) strategy, based on killing bacteria before or after coming in contact with the surface, and a third strategy based on triggered bacterial detachment [264]. Some methods combine strategies to achieve multi-functional surfaces [265].
Based on their physical adsorption capabilities, colloidal particles are known to have excellent ability to form well-organized films regardless of the substrate material [[266], [267], [268], [269]]. Many attempts have been made to perform surface coatings with hard colloidal particles such as polystyrene nanoparticles. However, these coatings were found to be unstable under physiological conditions since the contact point between the particle and surface is limited [270]. From this point of view, nanogels, as being soft deformable colloidal particles, can stick to the surface firmly and create remarkably homogenous coatings [271,272]. Nanogel-based surface coatings can be easily prepared by physical adsorption without requiring any harsh chemical procedures such as grafting or covalent binding [268]. Besides, the characteristics of the nanogel-coated surface can be simply modified by just modulating the chemical functionalities of the adsorbed nanogel particles.
Nanogels have great potential as antifouling and/or antimicrobial coatings on the surface of medical implants. Their advantages, such as high water content, desirable chemical and physical structures, good mechanical properties, and excellent biocompatibility, make them useful candidates as antimicrobial surface coatings [273,274].
4.1. Nanogel-based antifouling coatings
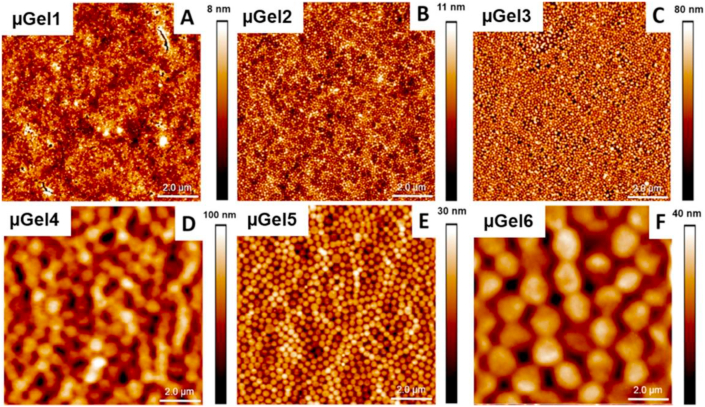
Antifouling surfaces are designed to reduce the initial bacteria attachment by repelling the bacteria and consequently preventing biofilm formation [275]. Apart from zwitterion-based surfaces and polymer brush coatings, these surface coatings are usually created using hydrogels, which can form a hydration layer in an aqueous environment. Hydrogels have been used in the field of biomaterials due to their affinity to water and the formation of a hydration layer, which works as a physical barrier resisting protein adsorption and bacterial adhesion [145,276]. Antifouling hydrogel surfaces can possess strong hydration properties also under physiological conditions such as in serum or salt solutions [[277], [278], [279]]. As mentioned before, nanogels are possessing similar behaviors as hydrogels with a nano-sized feature. Many applications of nanogels inspired by hydrogels, such as antifouling surfaces, have been developed in the biomedical field, based on the excellent tunable-chemical properties and film-forming capabilities of nanogels. Poly (N-isopropylmetacrylamide) (p (NIPMAM)) nanogel coatings have been previously studied to prevent adhesion of cells and proteins on the surface [27,268,280]. More recently, our group focused on these nanogel surfaces as an antifouling coating system that repels bacteria and subsequently prevents biofilm formation and a possible infection [29]. In this work, internal cross-linking density and the size of the nanogels and thickness of the coating was investigated to find the highest reduction of initial bacterial adhesion. As shown in Fig. 7, all nanogel coatings provided a homogeneous monolayer, with a surface coverage of over 90%. The results presented that the thickest and softest (lowest cross-linking density) nanogel coating exhibited more than 98% reduction in the number of initial bacterial adhesion. These findings clearly suggested that a promising antifouling surface coating was successfully developed.
Fig. 7.
Atomic force microscopy images of the p (NIPMAM) nanogel coated glass surfaces with different internal stiffness/cross-linking density and hydrodynamic radii Rh at 23 °C in the dry state. (A) nGel1, Rh = 114 nm, (B) nGel2, Rh = 109 nm, (C) nGel3, Rh = 101 nm, (D) nGel4, Rh = 787 nm, (E) nGel5, Rh = 301 nm, (F) nGel6, Rh = 650 nm at 30 °C. (Reproduced with permission from ref 29. (Copyright 2019 American Chemical Society)).
Similarly, Mergel et al. investigated the effect of electrochemical switching of poly (N-isopropylacrylamide-co-vinylferrocene) p (NIPAM-co-VFc) nanogel coatings prepared with nanogels that have a ferrocene (Fc)-enriched (collapsed/hard) core and a NIPAM-rich shell on the antifouling behavior [281]. The stiffness on the surface was altered by switching the oxidation states of the nanogels with electrochemical stimuli. Quantitative analysis proved that upon oxidation, nanogels coated on the glass surface turn into a significantly softer, highly swollen layer. Furthermore, the bacterial adhesion was examined under flow conditions on coated surfaces on the oxidized and non-oxidized state of the nanogels and also on bare glass. Although the difference in stiffness did not affect the fouling behavior the nanogel coated surfaces showed a remarkable antifouling performance compared to bare glass. These results indicated that the alteration in stiffness of the nanogels is not sufficient to provide a difference in bacteria attachment.
Very recently, addressing the potential use of surface-bound nanogels as coatings for improving the non-fouling properties of the biomaterials, Huang et al. introduced zwitterionic nanogel coated surfaces [282]. Poly (sulfobetaine methacrylate) (PSBMA)/poly (ether sulfone) (PES) nanogels were blended within the PES polymer matrix and further cast on glass surfaces. The biofilm formation of E. coli and S. aureus on the surfaces both in static and flow conditions was investigated with confocal laser scanning microscopy (CLSM). The CLSM results highlighted that the reduced biomass and biofilm thickness were proving the decreased bacterial attachment on the surface for both Gram-negative E. coli and Gram-positive S. aureus bacteria due to the presence of the hydration layers provided by nanogels. On the other hand, the developed zwitterionic system displayed strong anti-abrasion properties and chemical durability, even in harsh chemical environments.
Addressing the temperature-responsive characteristics of nanogels, Saha and co-workers investigated a synthetic strategy to obtain zwitterionic nanogels that exhibit tunable dual-VPTT [283]. These nanogels were covalently bound on activated SiO2 quartz sensors, and the anti-fouling activity was tested by quartz crystal microbalance-dissipation (QCM-D) experiments under a flow of protein solution. The nanogel-coated surface showed excellent antifouling characteristics resulting in remarkably reduced protein adsorption. Thus, these dual-stimuli responsive nanogel coated systems can be used as a coating material to prevent protein adsorption, which may suggest reduced bacterial adhesion as well.
Similarly, addressing the potential use of nanogels as coatings for biomaterials, Brosel-Oliu et al. profited from the nanogel antifouling properties for biosensors to measure the bacterial response to antibiotics, avoiding time consuming culturing procedures [284]. To this end, a p (NIPMAM) nanogel coating was employed to prevent the attachment of E. coli on top of insulating barriers of the biosensor, in order to optimize the sensors sensitivity towards bacterial adhesion on the electrodes of the device. Successful nanogel deposition and immobilization of bacteria was found by confocal microscopy analysis. This approach exhibited the bacterial response to ampicillin when measured by electrochemical impedance spectroscopy, which promotes novel applications in biosensing related to toxicity validations.
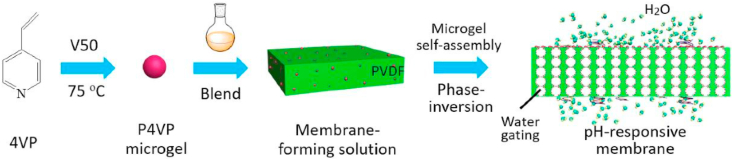
In an attempt to further explore the performance of the antifouling behavior of surface-bound nanogels, Liu et al. fabricated pH-responsive membranes with poly (4-vinyl pyridine) (P4VP) nanogels [285]. P4VP nanogels were self-assembled on the membrane surface and in the inner channels, through the phase-inversion procedure of membrane casting solution, as shown in Fig. 8. The nanogels on the surface of the membrane promoted the antifouling properties and self-cleaning performance, which were examined by water flux experiments. Herein the fouling experiments firstly performed by measuring pure water flux before and after the pollution by pure milk filtration. In the inner channels of the membrane, nanogels can respond to pH changes. Upon this environmental trigger, the channels alter their diameter due to the protonation/deprotonation ability of pyridine groups, providing an excellent pH-responsive water gating capability to membranes. Therefore, such nanogels can be potentially used in smart separation applications, in addition to their antifouling properties.
Fig. 8.
Fabrication diagram of the pH-responsive membrane prepared with nanogels. (Reproduced with permission from ref 285. (Copyright 2019 Elsevier)).
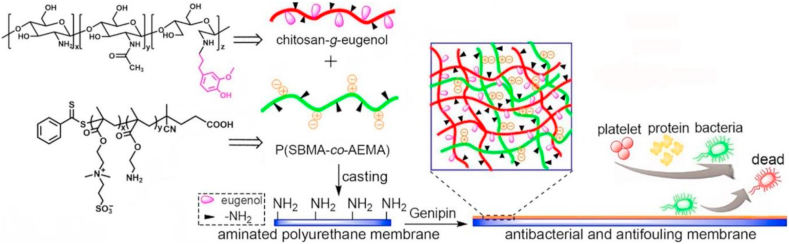
Furthermore, Ji et al. explored the use of heparin-mimicking nanogels to prevent bacteria and protein accumulation on membrane surfaces and also to improve the hemocompatibility of these membranes [286]. Polyethersulfone (PES) membranes were fabricated by a simple physical blending of heparin-mimicking nanogels, namely poly (acrylic acid-co-N-vinyl-2-pyrrolidone) (P (AA-VP)) and poly (2-acrylamido-2-methylpropanesulfonic acid-co-acrylamide) (P (AMPS-AM)), obtained through conventional free-radical copolymerization. Results revealed that nanogel modified membranes inhibited not only the bacterial adhesion but also the protein adsorption on the surface due to the hydrophilic characteristics provided by the nanogels. In addition to the antifouling behavior of the heparin mimicking nanogel an increase of the adhesion of the L929 cells and an enhanced cytocompatibility was shown on the surface of nanogel-modified membranes, as was also found earlier [287]. Thus, nanogel-blended PES membranes have great potential use for various biomedical applications such as blood purification membranes. Another antifouling and antibacterial membrane approach was investigated by, Li et al. (Fig. 9) [288]. Briefly, electrospun polycarbonate urethane substrate membranes were coated with a nanogel layer by cross-linking of eugenol-modified chitosan and the zwitterionic copolymer poly (sulfobetaine methacrylate-co-2-aminoethyl methacrylate) (PSA). The prepared membranes not only presented a significant bacteria-repelling performance towards E. coli and S. aureus but also a lower non-specific protein adsorption on the surface. Furthermore, antibacterial results showed that the nanogel-coated membranes could inhibit not only initial bacteria attachment but also kill the adhered bacteria. Moreover, prepared membranes showed no serious cytotoxicity against L929 fibroblasts, with more than 80% of relative cell viability which; thus, it is considered to have a potential use for biomedical implant applications. According to ISO 10993-5 procedure, percentages of cell viability above 80% are considered as non-cytotoxic; within 80%–60% weak; 60%–40% moderate and below 40% strong cytotoxic, respectively [289].
Fig. 9.
Integrated antibacterial and antifouling membranes via cross-linking eugenol-modified chitosan and a zwitterionic copolymer on the electrospun polyurethane surface. (Reproduced with permission from ref 288. (Copyright 2018 Elsevier)).
Investigating similar designs of both antifouling and antimicrobial surface coatings, Nyström et al. studied the peptide-loaded poly (ethyl acrylate-co-methacrylic acid) nanogels [290]. Therefore, nanogel films were prepared by covalent immobilization of nanogel on silica substrates with or without the incorporation of peptides. To assess the antifouling properties, nanogel-coated surfaces were immersed in an E. coli bacterial suspension under static conditions. The analysis showed that all coatings displayed a strong anti-adhesive effect on E. coli bacteria, as shown in Fig. 10. Moreover, peptide-loaded nanogel coatings exhibited efficient bacteria-killing properties at the same time.
Fig. 10.
(A) Bacterial adhesion of E. coli to surface-modified glass slides after 4 h of incubation in Tris buffer with or without additional 150 mM NaCl (top) and viability of the adhered E. coli quantified using BacLight LIVE/DEAD staining (bottom). Data were normalized against E. coli killed in 70% isopropanol. (B) Representative CLSM images of adhered E. coli on surface-modified glass slides in 10 mM Tris buffer. Images are presented as z-projections of stacks of six images or more, with increased brightness and contrast added after data analyses for improved visualization. (Reproduced with permission from ref 290. (Copyright 2018 American Chemical Society)).
Although the primary emphasis has been placed on biomaterials or biomedical devices, nanogel-coated surfaces offer opportunities also in other industries, e.g., for marine equipment to prevent biofouling and its costly consequences. Chen and co-workers developed a self-healing underwater–oil-repellent and biofouling-resistant coating prepared with nanogel spheres modified with hydrophilic polymeric chains, that were then prepared by self-assembly on a glass substrate [291]. The results show that this coating is highly stable under harsh acidic conditions and prevents biofouling meanwhile also displaying a self-healing ability when mechanically damaged. Albeit these coatings are created to avoid biofouling in marine equipment, it can also be used as a new platform within the biomedical field. Even though, nowadays, most of the coatings for marine equipment are made by environmentally friendly techniques and materials, the biocompatibility and cytotoxicity regulations must be carefully fulfilled when antifouling coatings are used in the body [292].
4.2. Nanogel-based antibacterial coatings
Antibacterial surfaces are identified as surfaces that can kill bacteria. Antibacterial coatings can be categorized into two groups: Contact-killing antibacterial coatings and release-based antibacterial coatings. Contact killing occurs when the bacteria touch the surface and are killed by molecules attached to the surface possessing antimicrobial activity or by the surface pattern itself [[293], [294], [295]]. Release-based antimicrobial coatings are pre-loaded with drugs that are released to kill the bacteria in the near vicinity [296].
As already discussed above, the formation of nanogel-based coatings for antifouling applications has attracted some interest. Apart from these strategies to control the biomaterials associated infections, the research on antibacterial coatings facilitated by nanogels has developed in recent years, as will be described in this section briefly. Nanogels can be combined with conventional antibacterial agents to enhance the killing-mechanism based on a release approach as well as can be functionalized with long tethering molecules to activate the contact killing mechanism.
In our very recent study, we prepared antibacterial surfaces with poly (N-isopropylacrylamide-co-N-[3 (dimethylamino)propyl]methacrylamide) (P(NIPAM-co-DMAPMA)-based nanogels [297]. Through the tertiary amine in the DMAPMA comonomer, nanogels are quaternized which introduced the antibacterial activity due to the bacterial membrane binding and the intercalating ability of the aliphatic QAC. Subsequently, the quaternized nanogels enabled the formation of intraparticle hydrophobic domains allowing for Triclosan incorporation as a very common antimicrobial agent (Fig. 11). The coating with Triclosan-loaded nanogels provided excellent bacteria-killing properties with a killing efficacy of up to 99.99% of adhering bacteria on the surface while still possessing an antifouling activity. This study effectively demonstrated that the first time, the possibility to achieve Triclosan encapsulation into the nanogel for a powerful antibacterial effect.
Fig. 11.
Schematic representation of antifouling and antimicrobial nanogel coatings (Reproduced with permission from ref 297. (Copyright 2019 Elsevier)).
In one of the recent studies on antibacterial nanogel coated surfaces, Zhao et al. reported the bactericidal activity of coatings formed by QAC-based p (NIPAM) nanogels synthesized with 1-vinyl imidazole and 1,6-dibromohexane [298]. Monolayer nanogel films were prepared on different substrates such as silicon, gold, polystyrene and polydimethylsiloxane. Next, the antibacterial efficiency, due to the QAC moieties in the nanogel coating was examined using E. Coli MG1655 bacteria; the bacteria viability was investigated by fluorescent staining and also by colony counting method on agar plates. The nanogel-coated surfaces exhibited almost 100% efficacy in bacteria-killing. This bactericidal effect of this QAC-based p (NIPAM) nanogel coating was established by showing that after inoculating 104 E. Coli MG1655 bacteria in 1 mL of buffer and exposure of the suspension to the coated surface resulted in only one viable colony on the utilized agar plate. Moreover, they proved that these robust coatings could be performed on many different substrates with a desired cytocompatibility.
In a similar line, another research is introduced by Xue et al. also using QAC-based p (NIPAM) nanogels [299]. Herein, firstly the thin films (quaternized p (NIPAM) nanogel, QPM film) were prepared with quaternized nanogels on silicon wafers by drip coating. Then a glycopolymer (P(SS-co-MAG)), which is containing both sulfonate groups and sugar units, was attached via a chemical modification to the QPM films. E. coli bacteria were subsequently used as a model microbe to examine the bactericidal properties of nanogel films. Both nanogel films QPM and QPM modified with P(SS-co-MAG) showed excellent antibacterial activity compared to the bare silicon wafer surface. The bactericidal efficacy of the nanogel coating was solely based on a Live/Dead staining assay after a 2 h incubation in 107/mL E. coli culture without a corresponding viable cell count to show whether the level of bacterial killing answers the 99% killing required by ISO20743 and JIS Z 2801. However, no significant differences were observed between P(SS-co-MAG) modified and QPM surface; thus, it can be concluded that the killing efficiency was not affected by modification with P(SS-co-MAG). Notably, after the P(SS-co-MAG) modification, the films showed an improved cytocompatibility.
According to ISO20743 and the Japanese industry standard JIS Z 2801, dealing with coated materials, the efficacy of antimicrobial agents is determined by the difference in the logarithmic value of viable cell counts after incubating an inoculum with the antimicrobial compound and its corresponding inert control. This difference is only microbiologically relevant when the reduction in viable cell count is more than two log values or more than 99% when linearly expressed [300]. Furthermore, the initial inoculum concentration must correspond to the microbial challenge associated with the intended application of the antimicrobial agent since microbial density can influence the MIC [300,301].
Furthermore, Chattopadhyay et al. explored the use of physically cross-linked nanogels (PCNGs) to form protective coatings on mica and highly oriented pyrolytic graphite surfaces [302]. The nanogels used in this study were synthesized in water as a solvent with C-10 alkyl chains modified poly (ethylene imine) (PEI) and azetidinium groups, thus yielding amphiphilic characteristics. PCNGs were possessing a high colloidal stability due to the hydrophobic interactions of long alkyl chains and the ionic repulsion of the azetidinium groups, thus spontaneously self-assembled. Consequently, prepared PCNGs demonstrated excellent antimicrobial activity against a varied range of bacteria. As shown in this work, the nanogels are stable in water up to 120 days. Considering the stability results of these nanogels, their coatings can be a potential candidate for water-based biomedical applications. To this end, an optimized and successful coating method needs to be performed and the stability tests of the coatings needs to be completed.
Sproul et al. prepared thin nanogel films by using platelet-like particles (PLP), which were synthesized with NIPAM-co-acrylic acid with antimicrobial gold through non-covalent and covalent methods to develop nanogold composites (NGCs) [303]. Antimicrobial assay results suggested that all fabricated thin films by NGC PLPs possessed an effective activity for inhibiting bacterial attachment and growth. Here, viable cell counts were performed at time 0, 3, 6, 12, and 24 h using a testing solution which was made by a 1:10,000 dilution of 105/mL CFU* 0.2mL/sample. Meanwhile, the antimicrobial effects of these particles on inhibiting bacterial growth in suspension were not significant due to the available gold particle surface area which facilitates the bacteria killing; therefore, in the future, applications of NCG PLP's should be applied as a coating on the surface to prevent infections.
Addressing mechanisms confirming antimicrobial effects of nanogel-bound antimicrobial peptides (AMPs), Nyström et al. examined the incorporation of AMPs into a nanogel by electrostatic interactions [290]. Bactericidal properties by contact-killing and release of incorporated peptides of the peptide-loaded nanogels were investigated. The antimicrobial efficiency was dependent on the release rate of the bactericidal peptides from the nanogel network, and it was speculated that the ionic strength in the solution controlled their release. At low ionic strength, peptides remained attached to the nanogels by electrostatic interactions; therefore, the killing mechanism is mostly due to contact killing and partly by the release of the peptides. At high ionic strength, however, the antimicrobial effect was mainly based on peptide release. Such nanogel coatings containing peptides exhibited enhanced antimicrobial and anti-inflammatory properties to control the inflammatory response to biomaterials.
In a similar line of research Nyström et al. investigated the formation of peptide-loaded poly (ethyl acrylate-co-methacrylic acid) (MAA) nanogel multilayers [304]. These nanogels were synthesized with biotin, and the nanogel multilayers were formed by alternating biotin containing nanogels and avidin with layer-by-layer deposition method since avidin can strongly bind to biotin. Next, the loading and release of the antimicrobial peptide into the formed nanogel multilayers were examined at physiological conditions with pH 7.4 and ionic strength 150 mM. At this high ionic strength, the peptide release was observed to be slow because the peptide diffusion through the multilayer from the outside was kinetically hindered. As a result the antimicrobial effects on E. coli bacteria of peptide-loaded multilayers were decreased when compared to a peptide-loaded nanogel monolayer. Furthermore, it was proven that the increased loading of the antimicrobial peptide resulted in an improved antimicrobial activity on the surface, both for planktonic and adhered bacteria. In conclusion, cross-linked nanogel multilayers show great potential as a useful antimicrobial surface coating. However, more attention needs to be given to peptide release phenomena.
Additionally, Liang et al. examined the coated surfaces prepared by poly (acrylic acid)-based anionic nanogels loaded with the antimicrobial (lipo)peptides [305]. They showed that the contact with macrophages and osteoblasts was not able to trigger antimicrobial release; it was only possible with bacteria. Very interestingly, the bacterial contact was found to trigger the release of these AMPs, consequently resulting in local bacterial killing as shown in Fig. 12. Here, a microprobe experiments is performed in order to understand the contact transfer between bacteria and nanogels loaded with antimicrobial peptides, as L5 and Sub5. The bacteria in contact with the peptide loaded nanogels were killed as can be seen in Fig. 12. The loaded antimicrobials continued being stably complexed within the nanogels for extended periods, even for weeks. Thus, this coating can be assessed as a promising antimicrobial triggered bacteria-release system.
Fig. 12.
Contact transfer kills S. epidermidis on AMP loaded microgel-modified surfaces. Confocal images showing live (green) and dead (red) S. epidermidis after 30 min of contact with microgels loaded with: (A) Antimicrobial peptide L5 (in 0.07 M ionic strength buffer); and (B) Antimicrobial peptide Sub5 (in 0.28 M ionic strength buffer). The dashed lines outline the outer diameter of the probe tip. (Reproduced with permission from ref 305. (Copyright 2019 Elsevier)).
Some examples of nanogels and their applications as coatings can be found in Table 2.
Table 2.
Nanogels and their coating applications.
| Coating Applications | Nanogels | Ref. |
|---|---|---|
| Reduced protein adhesion | p (NIPAM) nanogels cross-linked with PEG diacrylate | [27] |
| Prevented bacteria attachment on the surface for biomaterials | p (NIPMAM) nanogel coatings | [29] |
| Allowed cell adhesion and spreading | p (NIPAM) nanogels | [268] |
| Antifouling surface towards bacteria on the surface | p (NIPAM-co-VFc) nanogels | [281] |
| Decreased bacterial attachment and biofilm thickness for biomaterials | PSBMA/PES nanogels | [282] |
| Reduced protein adsorption SiO2 quartz sensors | (PVCL-co-PGMA-g-PSB) zwitterionic nanogels | [283] |
| Antifouling properties for biosensors | p (NIPMAM) nanogels | [284] |
| Antifouling properties and self-cleaning performance for the membrane | (P4VP) nanogels | [285] |
| Inhibited the bacterial adhesion and the protein adsorption on the membrane | P (AA-VP) and (P (AMPS-AM)) nanogels | [286,287] |
| Antifouling and antibacterial membrane surface | poly (sulfobetaine methacrylate-co-2-aminoethyl methacrylate) nanogels by cross-linking of eugenol-modified chitosan | [288] |
| Antifouling and antimicrobial surface coatings | Peptide-loaded poly (ethyl acrylate-co-methacrylic acid) nanogels | [290] |
| Prevent biofouling for marine equipment | NIPAM, methacrylic acid (MAA), and poly (ethylene glycol) diacrylate (PEGDA) based nanogel spheres | [291] |
| Antibacterial and antifouling coating | Quaternized and Triclosan incorporated p (NIPAM-co-DMAPMA)-based nanogels | [297] |
| Antibacterial surface on silicon, gold, polystyrene and PDMS | QAC-based p (NIPAM) nanogels | [298] |
| Antibacterial surface on silicon wafers | p (NIPAM) nanogels modified with P(SS-co-MAG) | [299] |
| Antibacterial surface on mica and graphite surface | Nanogels with C-10 alkyl chains, modified PEI and azetidinium groups | [302] |
| Antibacterial thin films | p (NIPAM-co-acrylic acid) nanogels modified with gold | [303] |
| Antimicrobial and anti-inflammatory properties | Nanogel-bound antimicrobial peptides (AMPs) | [290] |
| Antimicrobial multi and monolayers | Antimicrobial peptide-loaded poly (ethyl acrylate-co-methacrylic acid) (MAA) nanogels | [304] |
| Antimicrobial triggered bacteria-release system | Poly (acrylic acid)-based anionic nanogels loaded with the antimicrobial (lipo)peptides | [305] |
5. Conclusions and outlook
Biomedical applications of nanogels have made quick progress in the last years. These developments have allowed an expansion in the field of drug delivery and antimicrobial approaches, by controlling characteristics of nanogels such as size, stability, surface functionality for biodegradability. A current challenge is the development of strategies for the nanogel surface coatings to have both antifouling and antimicrobial features, both in vitro and in vivo. To address this matter, combining different approaches as exemplified by e.g. the Triclosan loading of the quaternized nanogels earlier presented in this article, is an important example of possible concepts that can give rise to advances in creating effective nanoparticles to prevent infections, both in suspension and on biomedical implant surfaces.
Taken together, nanogels have demonstrated to be not only versatile drug delivery systems but also, when bound onto the substrate, an effective antimicrobial coating system to combat infections on the biomaterial surface is created. One future goal should be the usage and development of these nanogels as multifunctional particles with several additional attributes incorporated to be used for a broad spectrum of biomedical applications with multiple functions, robust biocompatible features, and programmed responses that can be controlled by stimuli and triggers under physiological conditions. Such integrated nanogel-based systems will provide superior material characteristics, especially in the field of biomedical research, to combat biomaterial-associated infections as well as general pathogenic intrusions within the body. Extensive biocompatibility and biotolerability testing of nanogels is a prerequisite for clinical trials and application. To help reach this goal, ISO-standard toxicity tests are designed to guide researchers in acquiring knowledge that will facilitate a better understanding of the interactions between living tissues and nanogels.
The clinical impact that these nanogel-based systems could have reaches far beyond the antimicrobial applications as depicted in this review. General drug delivery approaches would certainly benefit from these nanogels and would be an excellent addition to the liposomal and polymersomal approaches. Therefore, it is expected that nanogels will also impact diseases such as cancer but also due to the diverse range of possible modifications including metallic structures, metal-ions, small molecular conjugates, peptides, antibodies and other biomacromolecules will provide possibilities for applications in imaging, theranostics, biosensing, and gene-delivery. Although hydrogels themselves are already known and used in clinical applications for long times, nanogels require special attentions with respect to their size as they belong to the class of nanotechnology, which is under particular scrutiny in all fields as it is regarded as difficult to predict long-term outcomes.
Declaration of competing interest
The authors declare the following conflict of interests: P. van Rijn also is co-founder, scientific advisor, and shareholder of BiomACS BV, a biomedical-oriented screening company.
Acknowledgments
The manuscript was written through the contributions of all authors. All authors have approved the final version of the manuscript. The authors acknowledge the financial support of the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No 713482 (ALERT Cofound) and the China Scholarship Council (CSC; G. Z no.201706890012).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Jelmer Sjollema, Email: j.sjollema@umcg.nl.
Patrick van Rijn, Email: p.van.rijn@umcg.nl.
References
- 1.Algburi A., Comito N., Kashtanov D., Dicks L.M.T., Chikindas M.L. Control of biofilm formation: antibiotics and beyond, appl. Environ. Microbiol. 2017;83:e02508–e02516. doi: 10.1128/AEM.02508-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rostad C.A., Wehrheim K., Kirklin J.K., Naftel D., Pruitt E., Hoffman T.M., L'Ecuyer T., Berkowitz K., Mahle W.T., Scheel J.N. Bacterial infections after pediatric heart transplantation: epidemiology, risk factors and outcomes. J. Heart Lung Transplant. 2017;36:996–1003. doi: 10.1016/j.healun.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Shepshelovich D., Tau N., Green H., Rozen-Zvi B., Issaschar A., Falcone M., Coussement J., Zusman O., Manuel O., Mor E., Torre-Cisneros J., Yahav D. Immunosuppression reduction in liver and kidney transplant recipients with suspected bacterial infection: a multinational survey. Transpl. Infect. Dis. 2019;21:e13134. doi: 10.1111/tid.13134. [DOI] [PubMed] [Google Scholar]
- 4.Mücke M.M., Rumyantseva T., Mücke V.T., Schwarzkopf K., Joshi S., Kempf V.A.J., Welsch C., Zeuzem S., Lange C.M. Bacterial infection-triggered acute-on-chronic liver failure is associated with increased mortality. Liver Int. 2018;38:645–653. doi: 10.1111/liv.13568. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt-Emrich S., Stiefel P., Rupper P., Katzenmeier H., Amberg C., Maniura-Weber K., Ren Q. Rapid Assay to Assess Bacterial Adhesion on Textiles, Mater. (Basel, Switzerland) 2016;9:249. doi: 10.3390/ma9040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galié S., García-Gutiérrez C., Miguélez E.M., Villar C.J., Lombó F. Biofilms in the food industry: health aspects and control methods. Front. Microbiol. 2018;9:898. doi: 10.3389/fmicb.2018.00898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Passerini D., Fécamp F., Marchand L., Kolypczuk L., Bonnetot S., Sinquin C., Verrez-Bagnis V., Hervio-Heath D., Colliec-Jouault S., Delbarre-Ladrat C. Characterization of biofilm extracts from two marine bacteria. Appl. Sci. 2019;9 doi: 10.3390/app9224971. [DOI] [Google Scholar]
- 8.Budeli P., Moropeng R.C., Mpenyana-Monyatsi L., Momba M.N.B. Inhibition of biofilm formation on the surface of water storage containers using biosand zeolite silver-impregnated clay granular and silver impregnated porous pot filtration systems. PloS One. 2018;13 doi: 10.1371/journal.pone.0194715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arciola C.R., Campoccia D., Montanaro L. Implant infections: adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018;16:1–13. doi: 10.1038/s41579-018-0019-y. [DOI] [PubMed] [Google Scholar]
- 10.Schierholz J.M., Beuth J. Implant infections: a haven for opportunistic bacteria. J. Hosp. Infect. 2001;49:87–93. doi: 10.1053/jhin.2001.1052. [DOI] [PubMed] [Google Scholar]
- 11.Zimmerli W. Clinical presentation and treatment of orthopaedic implant-associated infection. J. Intern. Med. 2014;276:111–119. doi: 10.1111/joim.12233. [DOI] [PubMed] [Google Scholar]
- 12.Cassini A., Högberg L.D., Plachouras D., Quattrocchi A., Hoxha A., Simonsen G.S., Colomb-Cotinat M., Kretzschmar M.E., Devleesschauwer B., Cecchini M., Ouakrim D.A., Oliveira T.C., Struelens M.J., Suetens C., Monnet D.L., Strauss R., Mertens K., Struyf T., Catry B., Latour K., Ivanov I.N., Dobreva E.G., Tambic Andraševic A., Soprek S., Budimir A., Paphitou N., Žemlicková H., Schytte Olsen S., Wolff Sönksen U., Märtin P., Ivanova M., Lyytikäinen O., Jalava J., Coignard B., Eckmanns T., Abu Sin M., Haller S., Daikos G.L., Gikas A., Tsiodras S., Kontopidou F., Tóth Á., Hajdu Á., Guólaugsson Ó., Kristinsson K.G., Murchan S., Burns K., Pezzotti P., Gagliotti C., Dumpis U., Liuimiene A., Perrin M., Borg M.A., de Greeff S.C., Monen J.C.M., Koek M.B.G., Elstrøm P., Zabicka D., Deptula A., Hryniewicz W., Caniça M., Nogueira P.J., Fernandes P.A., Manageiro V., Popescu G.A., Serban R.I., Schréterová E., Litvová S., Štefkovicová M., Kolman J., Klavs, Korošec A., Aracil B., Asensio A., Pérez-Vázquez M., Billström H., Larsson S., Reilly J.S., Johnson A., Hopkins S. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect. Dis. 2019;19:56–66. doi: 10.1016/S1473-3099(18)30605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartell J.A., Sommer L.M., Haagensen J.A.J., Loch A., Espinosa R., Molin S., Johansen H.K. Evolutionary highways to persistent bacterial infection. Nat. Commun. 2019;10:629. doi: 10.1038/s41467-019-08504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munita J.M., Arias C.A. Mechanisms of antibiotic resistance. Virulence Mech. Bact. Pathog. 2016:481–511. doi: 10.1128/9781555819286.ch17. [DOI] [Google Scholar]
- 15.Mao A.S., Mooney D.J. Regenerative medicine: current therapies and future directions. Proc. Natl. Acad. Sci. Unit. States Am. 2015;112 doi: 10.1073/pnas.1508520112. 14452 LP – 14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spicer C.D. Hydrogel scaffolds for tissue engineering: the importance of polymer choice. Polym. Chem. 2020;11:184–219. doi: 10.1039/C9PY01021A. [DOI] [Google Scholar]
- 17.Li X., Sun Q., Li Q., Kawazoe N., Chen G. Functional hydrogels with tunable structures and properties for tissue engineering applications. Front. Chem. 2018;6:499. doi: 10.3389/fchem.2018.00499. https://www.frontiersin.org/article/10.3389/fchem.2018.00499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keskin D., Mokabbar T., Pei Y., van Rijn P. The relationship between bulk silicone and benzophenone-initiated hydrogel coating properties. Polymers. 2018;10:534. doi: 10.3390/polym10050534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clegg J.R., Wagner A.M., Shin S.R., Hassan S., Khademhosseini A., Peppas N.A. Modular fabrication of intelligent material-tissue interfaces for bioinspired and biomimetic devices. Prog. Mater. Sci. 2019;106:100589. doi: 10.1016/j.pmatsci.2019.100589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mandal A., Clegg J.R., Anselmo A.C., Mitragotri S. Hydrogels in the clinic. Bioeng. Transl. Med. 2020;5 doi: 10.1002/btm2.10158. e10158–e10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soni K.S., Desale S.S., Bronich T.K. Nanogels: an overview of properties, biomedical applications and obstacles to clinical translation. J. Contr. Release. 2016;240:109–126. doi: 10.1016/j.jconrel.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anselmo A.C., Mitragotri S. Nanoparticles in the clinic: an update. Bioeng. Transl. Med. 2019;4 doi: 10.1002/btm2.10143. e10143–e10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danhier F., Ansorena E., Silva J.M., Coco R., Le Breton A., Préat V. PLGA-based nanoparticles: an overview of biomedical applications. J. Contr. Release. 2012;161:505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 24.Gong J., Chen M., Zheng Y., Wang S., Wang Y. Polymeric micelles drug delivery system in oncology. J. Contr. Release. 2012;159:312–323. doi: 10.1016/j.jconrel.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Allen T.M., Cullis P.R. Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Deliv. Rev. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 26.Song Q., Yin Y., Shang L., Wu T., Zhang D., Kong M., Zhao Y., He Y., Tan S., Guo Y., Zhang Z. Tumor microenvironment responsive nanogel for the combinatorial antitumor effect of chemotherapy and immunotherapy. Nano Lett. 2017;17:6366–6375. doi: 10.1021/acs.nanolett.7b03186. [DOI] [PubMed] [Google Scholar]
- 27.Nolan C.M., Reyes C.D., Debord J.D., García A.J., Lyon L.A. Phase transition behavior, protein adsorption, and cell adhesion resistance of poly(ethylene glycol) cross-linked microgel particles. Biomacromolecules. 2005;6:2032–2039. doi: 10.1021/bm0500087. [DOI] [PubMed] [Google Scholar]
- 28.South A.B., Whitmire R.E., García A.J., Lyon L.A. Centrifugal deposition of microgels for the rapid assembly of nonfouling thin films. ACS Appl. Mater. Interfaces. 2009;1:2747–2754. doi: 10.1021/am9005435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keskin D., Mergel O., van der Mei H.C., Busscher H.J., van Rijn P. Inhibiting bacterial adhesion by mechanically modulated microgel coatings. Biomacromolecules. 2019;20:243–253. doi: 10.1021/acs.biomac.8b01378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.V Sigolaeva L., V Pergushov D., Oelmann M., Schwarz S., Brugnoni M., Kurochkin I.N., Plamper F.A., Fery A., Richtering W. Surface functionalization by stimuli-sensitive microgels for effective enzyme uptake and rational design of biosensor setups. Polymers. 2018;10:791. doi: 10.3390/polym10070791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lynch I., Miller I., Gallagher W.M., Dawson K.A. Novel method to prepare morphologically rich polymeric surfaces for biomedical applications via phase separation and arrest of microgel particles. J. Phys. Chem. B. 2006;110:14581–14589. doi: 10.1021/jp061166a. [DOI] [PubMed] [Google Scholar]
- 32.Xia Y., Tang D., Wu H., Wang X., Cao M., He H., Wang S. Cell attachment/detachment behavior on poly(N-isopropylacrylamide)-based microgel films: the effect of microgel structure and swelling ratio. J. Mater. Sci. 2018;53:8795–8806. doi: 10.1007/s10853-018-2217-4. [DOI] [Google Scholar]
- 33.Özlem Nazli K., Pester C.W., Konradi A., Böker A., van Rijn P. Cross-linking density and temperature effects on the self-assembly of SiO2—PNIPAAm core–shell particles at interfaces. Chem. Eur J. 2013;19:5586–5594. doi: 10.1002/chem.201203900. [DOI] [PubMed] [Google Scholar]
- 34.Pester C.W., Konradi A., Varnholt B., van Rijn P., Böker A. Responsive macroscopic materials from self-assembled cross-linked SiO2-PNIPAAm core/shell structures. Adv. Funct. Mater. 2012;22:1724–1731. doi: 10.1002/adfm.201102802. [DOI] [Google Scholar]
- 35.Kargupta R., Bok S., Darr C.M., Crist B.D., Gangopadhyay K., Gangopadhyay S., Sengupta S. Coatings and surface modifications imparting antimicrobial activity to orthopedic implants. Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology. 2014;6:475–495. doi: 10.1002/wnan.1273. [DOI] [PubMed] [Google Scholar]
- 36.Bin Hamzah Y., Hashim S., Rahman W.A.W.A. Synthesis of polymeric nano/microgels: a review. J. Polym. Res. 2017;24 doi: 10.1007/s10965-017-1281-9. [DOI] [Google Scholar]
- 37.Zhang H., Zhai Y., Wang J., Zhai G. New progress and prospects: the application of nanogel in drug delivery. Mater. Sci. Eng. C. 2016;60:560–568. doi: 10.1016/j.msec.2015.11.041. [DOI] [PubMed] [Google Scholar]
- 38.Liu G., An Z. Frontiers in the design and synthesis of advanced nanogels for nanomedicine. Polym. Chem. 2014;5:1559–1565. doi: 10.1039/c3py01502e. [DOI] [Google Scholar]
- 39.Kousalová J., Etrych T. Polymeric nanogels as drug delivery systems. Physiol. Res. 2018;67:s305–s317. doi: 10.33549/physiolres.933979. [DOI] [PubMed] [Google Scholar]
- 40.Sahu P., Das D., Kashaw V., Iyer A.K., Kashaw S.K. 2017. Nanogels: A New Dawn in Antimicrobial Chemotherapy. [DOI] [Google Scholar]
- 41.Hajebi S., Rabiee N., Bagherzadeh M., Ahmadi S., Rabiee M., Roghani-Mamaqani H., Tahriri M., Tayebi L., Hamblin M.R. Stimulus-responsive polymeric nanogels as smart drug delivery systems. Acta Biomater. 2019;92:1–18. doi: 10.1016/j.actbio.2019.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pich A., Richtering W. 2010. Microgels by Precipitation Polymerization: Synthesis, Characterization, and Functionalization; pp. 1–37. [DOI] [Google Scholar]
- 43.Oh J.K., Drumright R., Siegwart D.J., Matyjaszewski K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2008 doi: 10.1016/j.progpolymsci.2008.01.002. [DOI] [Google Scholar]
- 44.Sarika P.R., Anil Kumar P.R., Raj D.K., James N.R. Nanogels based on alginic aldehyde and gelatin by inverse miniemulsion technique: synthesis and characterization. Carbohydr. Polym. 2015;119:118–125. doi: 10.1016/j.carbpol.2014.11.037. [DOI] [PubMed] [Google Scholar]
- 45.McAllister K., Sazani P., Adam M., Cho M.J., Rubinstein M., Samulski R.J., DeSimone J.M. Polymeric nanogels produced via inverse microemulsion polymerization as potential gene and antisense delivery agents. J. Am. Chem. Soc. 2002;124:15198–15207. doi: 10.1021/ja027759q. [DOI] [PubMed] [Google Scholar]
- 46.Wutzel H., Richter F.H., Li Y., Sheiko S.S., Klok H.A. Poly[N-(2-hydroxypropyl)methacrylamide] nanogels by RAFT polymerization in inverse emulsion. Polym. Chem. 2014;5:1711–1719. doi: 10.1039/c3py01280h. [DOI] [Google Scholar]
- 47.Averick S.E., Paredes E., Irastorza A., Shrivats A.R., Srinivasan A., Siegwart D.J., Magenau A.J., Cho H.Y., Hsu E., Averick A.A., Kim J., Liu S., Hollinger J.O., Das S.R., Matyjaszewski K. Preparation of cationic nanogels for nucleic acid delivery. Biomacromolecules. 2012;13:3445–3449. doi: 10.1021/bm301166s. [DOI] [PubMed] [Google Scholar]
- 48.Warren N.J., Armes S.P. Polymerization-induced self-assembly of block copolymer nano-objects via RAFT aqueous dispersion polymerization. J. Am. Chem. Soc. 2014;136:10174–10185. doi: 10.1021/ja502843f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen W., Chang Y., Liu G., Wang H., Cao A., An Z. Biocompatible, antifouling, and thermosensitive core-shell nanogels synthesized by RAFT aqueous dispersion polymerization. Macromolecules. 2011;44:2524–2530. doi: 10.1021/ma200074n. [DOI] [PubMed] [Google Scholar]
- 50.Rieger J., Grazon C., Charleux B., Alaimo D., Jérôme C. Pegylated thermally responsive block copolymer micelles and nanogels via in situ RAFT aqueous dispersion polymerization. J. Polym. Sci. Part A Polym. Chem. 2009;47:2373–2390. doi: 10.1002/pola.23329. [DOI] [Google Scholar]
- 51.Kawaguchi H. Thermoresponsive microhydrogels: preparation, properties and applications. Polym. Int. 2014;63:925–932. doi: 10.1002/pi.4675. [DOI] [Google Scholar]
- 52.Ramos J., Imaz A., Forcada J. Temperature-sensitive nanogels: poly(N-vinylcaprolactam) versus poly(N-isopropylacrylamide) Polym. Chem. 2012;3:852–856. doi: 10.1039/c2py00485b. [DOI] [Google Scholar]
- 53.Kabanov A.V., Vinogradov S.V. Nanogels as pharmaceutical carriers: finite networks of infinite capabilities. Angew. Chem. Int. Ed. 2009;48:5418–5429. doi: 10.1002/anie.200900441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanson N., Rieger J. Synthesis of nanogels/microgels by conventional and controlled radical crosslinking copolymerization. Polym. Chem. 2010;1:965–977. doi: 10.1039/c0py00010h. [DOI] [Google Scholar]
- 55.Sun Q., Deng Y. In situ synthesis of temperature-sensitive hollow microspheres via interfacial polymerization. J. Am. Chem. Soc. 2005;127:8274–8275. doi: 10.1021/ja051487k. [DOI] [PubMed] [Google Scholar]
- 56.Jung K.O., Siegwart D.J., Il Lee H., Sherwood G., Peteanu L., Hollinger J.O., Kataoka K., Matyjaszewski K. Biodegradable nanogels prepared by atom transfer radical polymerization as potential drug delivery carriers: synthesis, biodegradation, in vitro release, and bioconjugation. J. Am. Chem. Soc. 2007;129:5939–5945. doi: 10.1021/ja069150l. [DOI] [PubMed] [Google Scholar]
- 57.Oh J.K., Bencherif S.A., Matyjaszewski K. Atom transfer radical polymerization in inverse miniemulsion: a versatile route toward preparation and functionalization of microgels/nanogels for targeted drug delivery applications. Polymer. 2009;50:4407–4423. doi: 10.1016/j.polymer.2009.06.045. [DOI] [Google Scholar]
- 58.Braun O., Selb J., Candau F. Synthesis in microemulsion and characterization of stimuli-responsive polyelectrolytes and polyampholytes based on N-isopropylacrylamide. Polymer. 2001;42:8499–8510. doi: 10.1016/S0032-3861(01)00445-1. [DOI] [Google Scholar]
- 59.Fernandez V.V.A., Tepale N., Sánchez-Díaz J.C., Mendizábal E., Puig J.E., Soltero J.F.A. Thermoresponsive nanostructured poly (N-isopropylacrylamide) hydrogels made via inverse microemulsion polymerization. Colloid Polym. Sci. 2006;284:387–395. doi: 10.1007/s00396-005-1395-1. [DOI] [Google Scholar]
- 60.Bhardwaj P., Singh V., Aggarwal S., Mandal U.K. Poly(acrylamide-co-2-acrylamido-2-methyl-1-propanesulfonic acid) nanogels made by inverse microemulsion polymerization. J. Macromol. Sci. Part A. 2009;46:1083–1094. doi: 10.1080/10601320903256497. [DOI] [Google Scholar]
- 61.Bartoň J. Inverse microemulsion polymerization of oil‐soluble monomers in the presence of hydrophilic polyacrylamide nanoparticles. Macromol. Symp. 2002;179:189–208. doi: 10.1002/1521-3900(200203)179:1<189::AID-MASY189>3.0.CO;2-X. [DOI] [Google Scholar]
- 62.Kaneda I., Sogabe A., Nakajima H. Water-swellable polyelectrolyte microgels polymerized in an inverse microemulsion using a nonionic surfactant. J. Colloid Interface Sci. 2004;275:450–457. doi: 10.1016/j.jcis.2004.02.086. [DOI] [PubMed] [Google Scholar]
- 63.Bharali D.J., Sahoo S.K., Mozumdar S., Maitra A. Cross-linked polyvinylpyrrolidone nanoparticles: a potential carrier for hydrophilic drugs. J. Colloid Interface Sci. 2003;258:415–423. doi: 10.1016/S0021-9797(02)00099-1. [DOI] [PubMed] [Google Scholar]
- 64.Lee H., Lee J.M., Shim S.E., Lee B.H., Choe S. Synthesis of carboxylic acid functionalized nanoparticles by reversible addition-fragmentation chain transfer (RAFT) miniemulsion polymerization of styrene. Polymer. 2005;46:3661–3668. doi: 10.1016/j.polymer.2005.03.034. [DOI] [Google Scholar]
- 65.Xu Y., Li Y., Cao X., Chen Q., An Z. Versatile RAFT dispersion polymerization in cononsolvents for the synthesis of thermoresponsive nanogels with controlled composition, functionality and architecture. Polym. Chem. 2014;5:6244–6255. doi: 10.1039/c4py00867g. [DOI] [Google Scholar]
- 66.Ma K., Xu Y. Z. An, templateless synthesis of polyacrylamide-based nanogels via RAFT dispersion polymerization. Macromol. Rapid Commun. 2015;36:566–570. doi: 10.1002/marc.201400730. [DOI] [PubMed] [Google Scholar]
- 67.Liu G., Qiu Q., An Z. Development of thermosensitive copolymers of poly(2-methoxyethyl acrylate-co-poly(ethylene glycol) methyl ether acrylate) and their nanogels synthesized by RAFT dispersion polymerization in water. Polym. Chem. 2012;3:504–513. doi: 10.1039/c2py00533f. [DOI] [Google Scholar]
- 68.Sanson N., Rieger J. Synthesis of nanogels/microgels by conventional and controlled radical crosslinking copolymerization. Polym. Chem. 2010;1:965–977. doi: 10.1039/c0py00010h. [DOI] [Google Scholar]
- 69.Zetterlund P.B., Kagawa Y., Okubo M. Controlled/living radical polymerization in dispersed systems. Chem. Rev. 2008;108:3747–3794. doi: 10.1021/cr800242x. [DOI] [PubMed] [Google Scholar]
- 70.Qiu J., Charleux B., Matyjaszewski K. Controlled/living radical polymerization in aqueous media: homogeneous and heterogeneous systems. Prog. Polym. Sci. 2001;26:2083–2134. doi: 10.1016/S0079-6700(01)00033-8. [DOI] [Google Scholar]
- 71.Raghupathi K., Eron S.J., Anson F., Hardy J.A., Thayumanavan S. Utilizing inverse emulsion polymerization to generate responsive nanogels for cytosolic protein delivery. Mol. Pharm. 2017;14:4515–4524. doi: 10.1021/acs.molpharmaceut.7b00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim K., Bae B., Kang Y.J., Nam J.M., Kang S., Ryu J.H. Natural polypeptide-based supramolecular nanogels for stable noncovalent encapsulation. Biomacromolecules. 2013;14:3515–3522. doi: 10.1021/bm400846h. [DOI] [PubMed] [Google Scholar]
- 73.Maciel D., Figueira P., Xiao S., Hu D., Shi X., Rodrigues J., Tomás H., Li Y. Redox-responsive alginate nanogels with enhanced anticancer cytotoxicity. Biomacromolecules. 2013;14:3140–3146. doi: 10.1021/bm400768m. [DOI] [PubMed] [Google Scholar]
- 74.Noh Y.-W., Kong S.-H., Choi D.-Y., Park H.S., Yang H.-K., Lee H.-J., Kim H.C., Kang K.W., Sung M.-H., Lim Y.T. 2012. Near-Infrared Emitting Polymer Nanogels for Efficient Sentinel Lymph Node Mapping. [DOI] [PubMed] [Google Scholar]
- 75.Chen J., Zou Y., Deng C., Meng F., Zhang J., Zhong Z. Multifunctional click hyaluronic acid nanogels for targeted protein delivery and effective cancer treatment in vivo. Chem. Mater. 2016;28:8792–8799. doi: 10.1021/acs.chemmater.6b04404. [DOI] [Google Scholar]
- 76.Sun M., Wang X., Cheng X., He L., Yan G., Tang R. TPGS-functionalized and ortho ester-crosslinked dextran nanogels for enhanced cytotoxicity on multidrug resistant tumor cells. Carbohydr. Polym. 2018;198:142–154. doi: 10.1016/j.carbpol.2018.06.079. [DOI] [PubMed] [Google Scholar]
- 77.Arteche Pujana M., Pérez-Álvarez L., Cesteros Iturbe L.C., Katime I. Biodegradable chitosan nanogels crosslinked with genipin. Carbohydr. Polym. 2013;94:836–842. doi: 10.1016/j.carbpol.2013.01.082. [DOI] [PubMed] [Google Scholar]
- 78.Li X.M., Wu Z.Z., Zhang B., Pan Y., Meng R., Chen H.Q. Fabrication of chitosan hydrochloride and carboxymethyl starch complex nanogels as potential delivery vehicles for curcumin. Food Chem. 2019;293:197–203. doi: 10.1016/j.foodchem.2019.04.096. [DOI] [PubMed] [Google Scholar]
- 79.Wei X., Xiong H., He S., Wang Y., Zhou D., Jing X., Huang Y. A facile way to prepare functionalized dextran nanogels for conjugation of hemoglobin. Colloids Surf. B Biointerfaces. 2017;155:440–448. doi: 10.1016/j.colsurfb.2017.04.047. [DOI] [PubMed] [Google Scholar]
- 80.Ji X., Liu J., Liu L., Zhao H. Enzyme-polymer hybrid nanogels fabricated by thiol-disulfide exchange reaction. Colloids Surf. B Biointerfaces. 2016;148:41–48. doi: 10.1016/j.colsurfb.2016.08.043. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Y., Ding J., Li M., Chen X., Xiao C., Zhuang X., Huang Y., Chen X. One-step “click chemistry”-synthesized cross-linked prodrug nanogel for highly selective intracellular drug delivery and upregulated antitumor efficacy. ACS Appl. Mater. Interfaces. 2016;8:10673–10682. doi: 10.1021/acsami.6b00426. [DOI] [PubMed] [Google Scholar]
- 82.Zhang X., Malhotra S., Molina M., Haag R. Micro- and nanogels with labile crosslinks-from synthesis to biomedical applications. Chem. Soc. Rev. 2015;44:1948–1973. doi: 10.1039/c4cs00341a. [DOI] [PubMed] [Google Scholar]
- 83.Jiang Y., Chen J., Deng C., Suuronen E.J., Zhong Z. Click hydrogels, microgels and nanogels: emerging platforms for drug delivery and tissue engineering. Biomaterials. 2014;35:4969–4985. doi: 10.1016/j.biomaterials.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 84.Sasaki Y., Akiyoshi K. Nanogel engineering for new nanobiomaterials: from chaperoning engineering to biomedical applications. Chem. Rec. 2010;10 doi: 10.1002/tcr.201000008. [DOI] [PubMed] [Google Scholar]
- 85.Perry J.L., Herlihy K.P., Napier M.E., Desimone J.M. PRINT: a novel platform toward shape and size specific nanoparticle theranostics. Acc. Chem. Res. 2011;44:990–998. doi: 10.1021/ar2000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jaiswal M.K., Banerjee R., Pradhan P., Bahadur D. Thermal behavior of magnetically modalized poly(N-isopropylacrylamide)-chitosan based nanohydrogel. Colloids Surf. B Biointerfaces. 2010;81:185–194. doi: 10.1016/j.colsurfb.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 87.Gratton S.E.A., Ropp P.A., Pohlhaus P.D., Luft J.C., Madden V.J., Napier M.E., DeSimone J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. U.S.A. 2008;105:11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Glangchai L.C., Caldorera-Moore M., Shi L., Roy K. Nanoimprint lithography based fabrication of shape-specific, enzymatically-triggered smart nanoparticles. J. Contr. Release. 2008;125:263–272. doi: 10.1016/j.jconrel.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 89.Baker W.O. Microgel, A new macromolecule. Ind. Eng. Chem. 1949;41:511–520. doi: 10.1021/ie50471a016. [DOI] [Google Scholar]
- 90.Saunders B.R., Vincent B. Microgel particles as model colloids: theory, properties and applications. Adv. Colloid Interface Sci. 1999;80:1–25. doi: 10.1016/S0001-8686(98)00071-2. [DOI] [Google Scholar]
- 91.Senff H., Richtering W. Temperature sensitive microgel suspensions: colloidal phase behavior and rheology of soft spheres. J. Chem. Phys. 1999;111:1705–1711. doi: 10.1063/1.479430. [DOI] [Google Scholar]
- 92.Pelton R.H., Chibante P. Preparation of aqueous latices with N-isopropylacrylamide. Colloid. Surface. 1986;20:247–256. doi: 10.1016/0166-6622(86)80274-8. [DOI] [Google Scholar]
- 93.Wu X., Pelton R.H., Hamielec A.E., Woods D.R., McPhee W. The kinetics of poly(N-isopropylacrylamide) microgel latex formation. Colloid Polym. Sci. 1994;272:467–477. doi: 10.1007/BF00659460. [DOI] [Google Scholar]
- 94.Plamper F.A., Richtering W. Functional microgels and microgel systems. Acc. Chem. Res. 2017;50:131–140. doi: 10.1021/acs.accounts.6b00544. [DOI] [PubMed] [Google Scholar]
- 95.Karg M., Pich A., Hellweg T., Hoare T., Lyon L.A., Crassous J.J., Suzuki D., Gumerov R.A., Schneider S., Potemkin I.I., Richtering W. Nanogels and microgels: from model colloids to applications, recent developments, and future trends. Langmuir. 2019;35:6231–6255. doi: 10.1021/acs.langmuir.8b04304. [DOI] [PubMed] [Google Scholar]
- 96.Agrawal G., Agrawal R. Functional microgels: recent advances in their biomedical applications. Small. 2018;14:1801724. doi: 10.1002/smll.201801724. [DOI] [PubMed] [Google Scholar]
- 97.Li D., van Nostrum C.F., Mastrobattista E., Vermonden T., Hennink W.E. Nanogels for intracellular delivery of biotherapeutics. J. Contr. Release. 2017;259:16–28. doi: 10.1016/j.jconrel.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 98.Ramos J., Forcada J., Hidalgo-Alvarez R. 2013. Cationic Polymer Nanoparticles and Nanogels: from Synthesis to Biotechnological Applications. [DOI] [PubMed] [Google Scholar]
- 99.Li Y., Maciel D., Rodrigues J., Shi X., Tomás H. Biodegradable polymer nanogels for drug/nucleic acid delivery. Chem. Rev. 2015;115:8564–8608. doi: 10.1021/cr500131f. [DOI] [PubMed] [Google Scholar]
- 100.Li D., van Nostrum C.F., Mastrobattista E., Vermonden T., Hennink W.E. Nanogels for intracellular delivery of biotherapeutics. J. Contr. Release. 2017;259:16–28. doi: 10.1016/j.jconrel.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 101.Eckmann D.M., Composto R.J., Tsourkas A., Muzykantov V.R. Nanogel carrier design for targeted drug delivery. J. Mater. Chem. B. 2014;2:8085–8097. doi: 10.1039/c4tb01141d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sharma A., Garg T., Aman A., Panchal K., Sharma R., Kumar S., Markandeywar T. Nanogel—an advanced drug delivery tool: current and future, Artif. Cells. Nanomedicine, Biotechnol. 2016;44:165–177. doi: 10.3109/21691401.2014.930745. [DOI] [PubMed] [Google Scholar]
- 103.Chacko R.T., Ventura J., Zhuang J., Thayumanavan S. Polymer nanogels: a versatile nanoscopic drug delivery platform. Adv. Drug Deliv. Rev. 2012;64:836–851. doi: 10.1016/j.addr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang L., Cao Z., Li Y., Ella-Menye J.R., Bai T., Jiang S. Softer zwitterionic nanogels for longer circulation and lower splenic accumulation. ACS Nano. 2012;6:6681–6686. doi: 10.1021/nn301159a. [DOI] [PubMed] [Google Scholar]
- 105.Merkel T.J., Jones S.W., Herlihy K.P., Kersey F.R., Shields A.R., Napier M., Luft J.C., Wu H., Zamboni W.C., Wang A.Z., Bear J.E., DeSimone J.M. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc. Natl. Acad. Sci. U.S.A. 2011;108:586–591. doi: 10.1073/pnas.1010013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Peracchia M.T., Fattal E., Desmaële D., Besnard M., Noël J.P., Gomis J.M., Appel M., D'Angelo J., Couvreur P. Stealth(®) PEGylated polycyanoacrylate nanoparticles for intravenous administration and splenic targeting. J. Contr. Release. 1999;60:121–128. doi: 10.1016/S0168-3659(99)00063-2. [DOI] [PubMed] [Google Scholar]
- 107.Hendrickson G.R., Lyon L.A. Microgel translocation through pores under confinement. Angew. Chem. Int. Ed. 2010;49:2193–2197. doi: 10.1002/anie.200906606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lai H., Wu P. A infrared spectroscopic study on the mechanism of temperature-induced phase transition of concentrated aqueous solutions of poly(N-isopropylacrylamide) and N-isopropylpropionamide. Polymer. 2010;51:1404–1412. doi: 10.1016/j.polymer.2010.01.036. [DOI] [Google Scholar]
- 109.Jochum F.D., Theato P. Temperature- and light-responsive smart polymer materials. Chem. Soc. Rev. 2013;42:7468–7483. doi: 10.1039/c2cs35191a. [DOI] [PubMed] [Google Scholar]
- 110.Pikabea A., Aguirre G., Miranda J.I., Ramos J., Forcada J. Understanding of nanogels swelling behavior through a deep insight into their morphology. J. Polym. Sci. Part A Polym. Chem. 2015;53:2017–2025. doi: 10.1002/pola.27653. [DOI] [Google Scholar]
- 111.Motornov M., Roiter Y., Tokarev I., Minko S. Stimuli-responsive nanoparticles, nanogels and capsules for integrated multifunctional intelligent systems. Prog. Polym. Sci. 2010;35:174–211. doi: 10.1016/j.progpolymsci.2009.10.004. [DOI] [Google Scholar]
- 112.Tamura G., Shinohara Y., Tamura A., Sanada Y., Oishi M., Akiba I., Nagasaki Y., Sakurai K., Amemiya Y. Dependence of the swelling behavior of a pH-responsive PEG-modified nanogel on the cross-link density. Polym. J. 2012;44:240–244. doi: 10.1038/pj.2011.123. [DOI] [Google Scholar]
- 113.Hamidi M., Azadi A., Rafiei P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008;60:1638–1649. doi: 10.1016/j.addr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 114.Zu G., Mergel O., Ribovski L., Bron R., Zuhorn I., van Rijn P. Nanogels with selective intracellular reactivity for intracellular tracking and delivery. Chem. Eur J. 2020 doi: 10.1002/chem.202001802. chem.202001802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Agrawal G., Agrawal R., Pich A. Dual responsive poly( N -vinylcaprolactam) based degradable microgels for drug delivery. Part. Part. Syst. Char. 2017;34:1700132. doi: 10.1002/ppsc.201700132. [DOI] [Google Scholar]
- 116.Medeiros S.F., Filizzola J.O.C., Oliveira P.F.M., Silva T.M., Lara B.R., Lopes M.V., Rossi-Bergmann B., Elaissari A., Santos A.M. Fabrication of biocompatible and stimuli-responsive hybrid microgels with magnetic properties via aqueous precipitation polymerization. Mater. Lett. 2016;175:296–299. doi: 10.1016/j.matlet.2016.04.004. [DOI] [Google Scholar]
- 117.Kulkarni A.A., Rao P.S. Nanomater. Tissue Eng. Fabr. Appl. Elsevier Ltd; 2013. Synthesis of polymeric nanomaterials for biomedical applications; pp. 27–63. [DOI] [Google Scholar]
- 118.Klopfleisch R., Jung F. The pathology of the foreign body reaction against biomaterials. J. Biomed. Mater. Res. 2017;105:927–940. doi: 10.1002/jbm.a.35958. [DOI] [PubMed] [Google Scholar]
- 119.J.M. Anderson, A. Rodriguez, D.T. Chang, FOREIGN BODY REACTION TO BIOMATERIALS, (n.d). [DOI] [PMC free article] [PubMed]
- 120.B.D. Ratner, The Biocompatibility Manifesto: Biocompatibility for the Twenty-First Century, (n.d.). 10.1007/s12265-011-9287-x. [DOI] [PubMed]
- 121.Aslantürk Ö.S. Genotoxicity - A Predict. Risk to Our Actual World. InTech; 2018. In vitro cytotoxicity and cell viability assays: principles, advantages, and disadvantages. [DOI] [Google Scholar]
- 122.Langie S.A.S., Azqueta A., Collins A.R. The comet assay: past, present, and future. Front. Genet. 2015;6:266. doi: 10.3389/fgene.2015.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gerecke C., Edlich A., Giulbudagian M., Schumacher F., Zhang N., Said A., Yealland G., Lohan S.B., Neumann F., Meinke M.C., Ma N., Calderón M., Hedtrich S., Schäfer-Korting M., Kleuser B. Biocompatibility and characterization of polyglycerol-based thermoresponsive nanogels designed as novel drug-delivery systems and their intracellular localization in keratinocytes. Nanotoxicology. 2017;11:267–277. doi: 10.1080/17435390.2017.1292371. [DOI] [PubMed] [Google Scholar]
- 124.Gheran C., Rigaux G., Callewaert M., Berquand A., Molinari M., Chuburu F., Voicu S., Dinischiotu A. Biocompatibility of Gd-loaded chitosan-hyaluronic acid nanogels as contrast agents for magnetic resonance cancer imaging. Nanomaterials. 2018;8:201. doi: 10.3390/nano8040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Weldrick P.J., Hardman M.J., Paunov V.N. 2019. Enhanced Clearing of Wound-Related Pathogenic Bacterial Biofilms Using Protease-Functionalized Antibiotic Nanocarriers. [DOI] [PubMed] [Google Scholar]
- 126.Haag A.F., Bagnoli F. 2015. The Role of Two-Component Signal Transduction Systems in Staphylococcus aureus Virulence Regulation; pp. 145–198. [DOI] [PubMed] [Google Scholar]
- 127.Sahiner M., Blake D.A., Fullerton M.L., Suner S.S., Sunol A.K., Sahiner N. Enhancement of biocompatibility and carbohydrate absorption control potential of rosmarinic acid through crosslinking into microparticles. Int. J. Biol. Macromol. 2019;137:836–843. doi: 10.1016/j.ijbiomac.2019.07.032. [DOI] [PubMed] [Google Scholar]
- 128.Haag S.L., Bernards M.T. Enhanced biocompatibility of polyampholyte hydrogels. Langmuir. 2020;36:3292–3299. doi: 10.1021/acs.langmuir.0c00114. [DOI] [PubMed] [Google Scholar]
- 129.Farooq M., Sagbas S., Sahiner M., Siddiq M., Turk M., Aktas N., Sahiner N. Synthesis, characterization and modification of Gum Arabic microgels for hemocompatibility and antimicrobial studies. Carbohydr. Polym. 2017;156:380–389. doi: 10.1016/j.carbpol.2016.09.052. [DOI] [PubMed] [Google Scholar]
- 130.Sengel S.B., Sahiner N. Synthesis and characterization of poly(N‐(2‐mercaptoethyl) acrylamide) microgel for biomedical applications. Polym. Adv. Technol. 2019;30:2109–2121. doi: 10.1002/pat.4644. [DOI] [Google Scholar]
- 131.Imaz A., Forcada J. N-vinylcaprolactam-based microgels for biomedical applications. J. Polym. Sci. Part A Polym. Chem. 2010;48:1173–1181. doi: 10.1002/pola.23876. [DOI] [Google Scholar]
- 132.L. Sun, X. Zhang, C. Zheng, Z. Wu, X. Xia, C. Li, Glucose-and Temperature-Responsive Core-Shell Microgels for Controlled Insulin Release, (n.d.). 10.1039/c2ra21408c. [DOI]
- 133.Khan A., Khan T.H., Ahamed M., El-Toni A.M., Aldalbahi A., Alam J., Ahamad T. Temperature-responsive polymer microgel-gold nanorods composite particles: physicochemical characterization and cytocompatibility. Polymers. 2018;10 doi: 10.3390/polym10010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wei M., Gao Y., Jiang S., Nie J., Sun F. Design of photoinitiator-functionalized hydrophilic nanogels with uniform size and excellent biocompatibility. Polym. Chem. 2019;10:2812–2821. doi: 10.1039/c9py00054b. [DOI] [Google Scholar]
- 135.Sahiner N., Demirci S. Can PEI microgels become biocompatible upon betainization? Mater. Sci. Eng. C. 2017;77:642–648. doi: 10.1016/j.msec.2017.03.285. [DOI] [PubMed] [Google Scholar]
- 136.Sarika P.R., James N.R. Preparation and characterisation of gelatin-gum Arabic aldehyde nanogels via inverse miniemulsion technique. Int. J. Biol. Macromol. 2015;76:181–187. doi: 10.1016/j.ijbiomac.2015.02.038. [DOI] [PubMed] [Google Scholar]
- 137.Bewersdorff T., Gruber A., Eravci M., Dumbani M., Klinger D., Haase A. p>Amphiphilic nanogels: influence of surface hydrophobicity on protein corona, biocompatibility and cellular uptake</p>. Int. J. Nanomed. 2019;14:7861–7878. doi: 10.2147/IJN.S215935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Curcio A., Marotta R., Palumberi D., Falqui A., Pellegrino T. Magnetic pH-responsive nanogels as multifunctional delivery tools for small interfering RNA (siRNA) molecules and iron oxide nanoparticles (IONPs) Chem. Commun. 2012;48:2400–2402. doi: 10.1039/c2cc17223b. [DOI] [PubMed] [Google Scholar]
- 139.Pikabea A., Ramos J., Papachristos N., Stamopoulos D., Forcada J. Synthesis and characterization of PDEAEMA-based magneto-nanogels: preliminary results on the biocompatibility with cells of human peripheral blood. J. Polym. Sci. Part A Polym. Chem. 2016;54:1479–1494. doi: 10.1002/pola.27996. [DOI] [Google Scholar]
- 140.Panayiotou M., Pöhner C., Vandevyver C., Wandrey C., Hilbrig F., Freitag R. Synthesis and characterisation of thermo-responsive poly(N,N′-diethylacrylamide) microgels. React. Funct. Polym. 2007;67:807–819. doi: 10.1016/j.reactfunctpolym.2006.12.008. [DOI] [Google Scholar]
- 141.Fan Z., Deng J., Li P.Y., Chery D.R., Su Y., Zhu P., Kambayashi T., Blankenhorn E.P., Han L., Cheng H. A new class of biological materials: cell membrane-derived hydrogel scaffolds. Biomaterials. 2019;197:244–254. doi: 10.1016/j.biomaterials.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Siswomihardjo W. Adv. Struct. Mater. Springer Verlag; 2016. Biocompatibility issues of biomaterials; pp. 41–65. [DOI] [Google Scholar]
- 143.Major M.R., Wong V.W., Nelson E.R., Longaker M.T., Gurtner G.C. The foreign body response: at the interface of surgery and bioengineering. Plast. Reconstr. Surg. 2015;135:1489–1498. doi: 10.1097/PRS.0000000000001193. [DOI] [PubMed] [Google Scholar]
- 144.Bridges A.W., Whitmire R.E., Singh N., Templeman K.L., Babensee J.E., Lyon L.A., García A.J. Chronic inflammatory responses to microgel-based implant coatings. J. Biomed. Mater. Res. 2010;94A:252–258. doi: 10.1002/jbm.a.32669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang L., Cao Z., Bai T., Carr L., Ella-Menye J.-R., Irvin C., Ratner B.D., Jiang S. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat. Biotechnol. 2013;31:553. doi: 10.1038/nbt.2580. [DOI] [PubMed] [Google Scholar]
- 146.Liu Q., Chiu A., Wang L., An D., Li W., Chen E.Y., Zhang Y., Pardo Y., McDonough S.P., Liu L., Liu W.F., Chen J., Ma M. Developing mechanically robust, triazole-zwitterionic hydrogels to mitigate foreign body response (FBR) for islet encapsulation. Biomaterials. 2020;230:119640. doi: 10.1016/j.biomaterials.2019.119640. [DOI] [PubMed] [Google Scholar]
- 147.H. Zhao, C. Zheng, G. Feng, Y. Zhao, H. Liang, H. Wu, G. Zhou, B. Liang, Y. Wang, X. Xia, Temperature-Sensitive poly(N-Isopropylacrylamide-Co-Butyl Methylacrylate) Nanogel as an Embolic Agent: Distribution, Durability of Vascular Occlusion, and Inflammatory Reactions in the Renal Artery of Rabbits, (n.d.). 10.3174/ajnr.A3177. [DOI] [PMC free article] [PubMed]
- 148.Wang S., Yu H., Wickliffe J.K. Limitation of the MTT and XTT assays for measuring cell viability due to superoxide formation induced by nano-scale TiO 2. Toxicol. Vitro. 2011;25:2147–2151. doi: 10.1016/j.tiv.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 149.Lü L., Zhang L., Wai M.S.M., Yew D.T.W., Xu J. Exocytosis of MTT formazan could exacerbate cell injury. Toxicol. Vitro. 2012;26:636–644. doi: 10.1016/j.tiv.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 150.Williams D.F. Regulatory biocompatibility requirements for biomaterials used in regenerative medicine. J. Mater. Sci. Mater. Med. 2015;26 doi: 10.1007/s10856-015-5421-7. [DOI] [PubMed] [Google Scholar]
- 151.Williams D.F. On the mechanisms of biocompatibility. Biomaterials. 2008;29:2941–2953. doi: 10.1016/j.biomaterials.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 152.Hernández-Adame L., Angulo C., García-Silva I., Palestino G., Rosales-Mendoza S. An overview of nanogel-based vaccines. Expert Rev. Vaccines. 2019;18:951–968. doi: 10.1080/14760584.2019.1647783. [DOI] [PubMed] [Google Scholar]
- 153.Roca I., Akova M., Baquero F., Carlet J., Cavaleri M., Coenen S., Cohen J., Findlay D., Gyssens I., Heure O.E., Kahlmeter G., Kruse H., Laxminarayan R., Liébana E., López-Cerero L., MacGowan A., Martins M., Rodríguez-Baño J., Rolain J.M., Segovia C., Sigauque B., Taconelli E., Wellington E., Vila J. The global threat of antimicrobial resistance: science for intervention. New Microbes New Infect. 2015;6:22–29. doi: 10.1016/j.nmni.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Alanis A.J. Resistance to antibiotics: are we in the post-antibiotic era? Arch. Med. Res. 2005;36:697–705. doi: 10.1016/j.arcmed.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 155.Prestinaci F., Pezzotti P., Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog. Glob. Health. 2015;109:309–318. doi: 10.1179/2047773215Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Neu H.C. The crisis in antibiotic resistance. Science. 1992;257(80):1064–1073. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- 157.Anjum S., Gupta B. Bioengineering of functional nanosilver nanogels for smart healthcare systems. Glob. Challenges. 2018;2:1800044. doi: 10.1002/gch2.201800044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Plamper F.A., Richtering W. Functional microgels and microgel systems. Acc. Chem. Res. 2017;50:131–140. doi: 10.1021/acs.accounts.6b00544. [DOI] [PubMed] [Google Scholar]
- 159.Molina M., Asadian-Birjand M., Balach J., Bergueiro J., Miceli E., Calderón M. Stimuli-responsive nanogel composites and their application in nanomedicine. Chem. Soc. Rev. 2015;44:6161–6186. doi: 10.1039/c5cs00199d. [DOI] [PubMed] [Google Scholar]
- 160.Mura S., Nicolas J., Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 161.Xiong M.H., Bao Y., Yang X.Z., Wang Y.C., Sun B., Wang J. Lipase-sensitive polymeric triple-layered nanogel for “on-demand” drug delivery. J. Am. Chem. Soc. 2012;134:4355–4362. doi: 10.1021/ja211279u. [DOI] [PubMed] [Google Scholar]
- 162.Zhou K., Wang X., Chen D., Yuan Y., Wang S., Li C., Yan Y., Liu Q., Shao L., Huang L., Yuan Z., Xie S. Enhanced treatment effects of tilmicosin against staphylococcus aureus cow mastitis by self-assembly sodium alginate-chitosan nanogel. Pharmaceutics. 2019;11 doi: 10.3390/pharmaceutics11100524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.S.N. Kłodzińska, F. Wan, H. Jumaa, C. Sternberg, T. Rades, H.M. Nielsen, Utilizing nanoparticles for improving anti-biofilm effects of azithromycin: a head-to-head comparison of modified hyaluronic acid nanogels and coated poly (lactic-<em>co</em>-glycolic acid) nanoparticles, J. Colloid Interface Sci.. 10.1016/j.jcis.2019.08.006. [DOI] [PubMed]
- 164.T. Chen, L. Chen, H. Li, Y. Chen, H. Guo, Y. Shu, Z. Chen, C. Cai, L. Guo, X. Zhang, L. Zhou, Q. Zhong, Design and in vitro evaluation of a novel poly(methacrylic acid)/metronidazole antibacterial nanogel as an oral dosage form, Colloids Surf. B Biointerfaces. 118 (2014) 65–71. 10.1016/J.COLSURFB.2014.02.011. [DOI] [PubMed]
- 165.Wu T., Liao W., Wang W., Zhou J., Tan W., Xiang W., Zhang J., Guo L., Chen T., Ma D., Yu W., Cai X. Genipin-crosslinked carboxymethyl chitosan nanogel for lung-targeted delivery of isoniazid and rifampin. Carbohydr. Polym. 2018;197:403–413. doi: 10.1016/j.carbpol.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 166.Zhang Y., Zhang J., Chen W., Angsantikul P., Spiekermann K.A., Fang R.H., Gao W., Zhang L. Erythrocyte membrane-coated nanogel for combinatorial antivirulence and responsive antimicrobial delivery against Staphylococcus aureus infection. J. Contr. Release. 2017;263:185–191. doi: 10.1016/J.JCONREL.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chen T., Li Q., Guo L., Yu L., Li Z., Guo H., Li H., Zhao M., Chen L., Chen X., Zhong Q., Zhou L., Wu T. Lower cytotoxicity, high stability, and long-term antibacterial activity of a poly(methacrylic acid)/isoniazid/rifampin nanogel against multidrug-resistant intestinal Mycobacterium tuberculosis. Mater. Sci. Eng. C. 2016;58:659–665. doi: 10.1016/j.msec.2015.08.055. [DOI] [PubMed] [Google Scholar]
- 168.Aminu N., Chan S.-Y., Yam M.-F., Toh S.-M. A dual-action chitosan-based nanogel system of triclosan and flurbiprofen for localised treatment of periodontitis. Int. J. Pharm. 2019;570:118659. doi: 10.1016/j.ijpharm.2019.118659. [DOI] [PubMed] [Google Scholar]
- 169.Kettel M.J., Heine E., Schaefer K., Moeller M. Chlorhexidine loaded cyclodextrin containing PMMA nanogels as antimicrobial coating and delivery systems. Macromol. Biosci. 2017;17:1600230. doi: 10.1002/mabi.201600230. [DOI] [PubMed] [Google Scholar]
- 170.Chen Z., Lv X., Zhao M., Zhang P., Ren X., Mei X. Encapsulation of green tea polyphenol by pH responsive, antibacterial, alginate microgels used for minimally invasive treatment of bone infection. Colloids Surf. B Biointerfaces. 2018;170:648–655. doi: 10.1016/J.COLSURFB.2018.06.065. [DOI] [PubMed] [Google Scholar]
- 171.Zhao Y., Sun W., Saldaña M.D.A. Nanogels of poly-N-isopropylacrylamide, poly-N,N-diethylacrylamide and acrylic acid for controlled release of thymol. J. Polym. Res. 2018;25:253. doi: 10.1007/s10965-018-1644-x. [DOI] [Google Scholar]
- 172.Sahiner N. Amino acid‐derived Poly(L‐Lysine) (p (LL)) microgel as a versatile biomaterial: hydrolytically degradable, drug carrying, chemically modifiable and antimicrobial material, Polym. Adv. Met. Technol. 2020 doi: 10.1002/pat.4936. pat.4936. [DOI] [Google Scholar]
- 173.El-Feky G.S., El-Banna S.T., El-Bahy G.S., Abdelrazek E.M., Kamal M. Alginate coated chitosan nanogel for the controlled topical delivery of Silver sulfadiazine. Carbohydr. Polym. 2017;177:194–202. doi: 10.1016/J.CARBPOL.2017.08.104. [DOI] [PubMed] [Google Scholar]
- 174.S. Du, X. Chen, X. Chen, S. Li, G. Yuan, T. Zhou, J. Li, Y. Jia, D. Xiong, H. Tan, Covalent chitosan‐cellulose hydrogels via schiff‐base reaction containing macromolecular microgels for pH‐sensitive drug delivery and wound dressing, Macromol. Chem. Phys. (n.d.). 10.1002/macp.201900399. [DOI]
- 175.Qasim M., Udomluck N., Chang J., Park H., Kim K. Antimicrobial activity of silver nanoparticles encapsulated in poly-N-isopropylacrylamide-based polymeric nanoparticles. Int. J. Nanomed. 2018;13:235–249. doi: 10.2147/IJN.S153485. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 176.Ballesteros C.A.S., Bernardi J.C., Correa D.S., Zucolotto V. 2019. Controlled Release of Silver Nanoparticles Contained in Photoresponsive Nanogels. [DOI] [PubMed] [Google Scholar]
- 177.Gupta B., Gautam D., Anjum S., Saxena S., Kapil A. Radiation synthesis of nanosilver nanohydrogels of poly(methacrylic acid) Radiat. Phys. Chem. 2013;92:54–60. doi: 10.1016/j.radphyschem.2013.07.020. [DOI] [Google Scholar]
- 178.Khan A., Khan T.H., El-Toni A.M., Aldalbahi A., Alam J., Ahamad T. In situ formation and immobilization of silver nanoparticles using thermo-responsive microgel particles and their cytotoxicity evaluation. Mater. Lett. 2019;235:197–201. doi: 10.1016/j.matlet.2018.10.041. [DOI] [Google Scholar]
- 179.Ballesteros C.A.S., Correa D.S., Zucolotto V. Polycaprolactone nanofiber mats decorated with photoresponsive nanogels and silver nanoparticles: slow release for antibacterial control. Mater. Sci. Eng. C. 2020;107 doi: 10.1016/j.msec.2019.110334. [DOI] [PubMed] [Google Scholar]
- 180.Rama Subba Reddy P., Krishna Rao K.S.V., Madhusudana Rao K., Sivagangi Reddy N., Eswaramma S. PH sensitive poly(methyl methacrylate-co-acryloyl phenylalanine) nanogels and their silver nanocomposites for biomedical applications. J. Drug Deliv. Sci. Technol. 2015;29:181–188. doi: 10.1016/j.jddst.2015.07.002. [DOI] [Google Scholar]
- 181.Ulker D., Tuncer C., Sezgin S.B., Toptas Y., Cabuk A., Bütün V. An antibacterial composite system based on multi-responsive microgels hosting monodisperse gold nanoparticles. J. Polym. Res. 2017;24:169. doi: 10.1007/s10965-017-1336-y. [DOI] [Google Scholar]
- 182.E.P. Sproul, S. Nandi, E. Chee, S. Sivadanam, B.J. Igo, L. Schreck, A.C. Brown, Development of Biomimetic Antimicrobial Platelet-like Particles Comprised of Microgel Nanogold Composites, (n.d.). 10.1007/s40883-019-00121-6. [DOI] [PMC free article] [PubMed]
- 183.Parilti R., Caprasse J., Riva R., Alexandre M., Vandegaart H., Bebrone C., Dupont-Gillain C., Howdle S.M., Jérôme C. Antimicrobial peptide encapsulation and sustained release from polymer network particles prepared in supercritical carbon dioxide. J. Colloid Interface Sci. 2018;532:112–117. doi: 10.1016/J.JCIS.2018.07.125. [DOI] [PubMed] [Google Scholar]
- 184.Singh S., Datta A., Borro B.C., Davoudi M., Schmidtchen A., Bhunia A., Malmsten M. Conformational aspects of high content packing of antimicrobial peptides in polymer microgels. ACS Appl. Mater. Interfaces. 2017;9:40094–40106. doi: 10.1021/acsami.7b13714. [DOI] [PubMed] [Google Scholar]
- 185.Nordström R., Browning K.L., Parra-Ortiz E., Damgaard L.S.E., Häffner S.M., Maestro A., Campbell R.A., Cooper J.F.K., Malmsten M. Membrane interactions of antimicrobial peptide-loaded microgels. J. Colloid Interface Sci. 2020;562:322–332. doi: 10.1016/j.jcis.2019.12.022. [DOI] [PubMed] [Google Scholar]
- 186.Kłodzińska S.N., Molchanova N., Franzyk H., Hansen P.R., Damborg P., Nielsen H.M. Biopolymer nanogels improve antibacterial activity and safety profile of a novel lysine-based α-peptide/β-peptoid peptidomimetic. Eur. J. Pharm. Biopharm. 2018;128:1–9. doi: 10.1016/J.EJPB.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 187.Chen Y.-F., Chen G.-Y., Chang C.-H., Su Y.-C., Chen Y.-C., Jiang Y., Jan J.-S. TRAIL encapsulated to polypeptide-crosslinked nanogel exhibits increased anti-inflammatory activities in Klebsiella pneumoniae-induced sepsis treatment. Mater. Sci. Eng. C. 2019;102:85–95. doi: 10.1016/J.MSEC.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 188.Marsot A., Boulamery A., Bruguerolle B., Simon N. Vancomycin: a review of population pharmacokinetic analyses. Clin. Pharmacokinet. 2012;51:1–13. doi: 10.2165/11596390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 189.Levine D.P. Vancomycin: a history. Clin. Infect. Dis. 2006;42 doi: 10.1086/491709. S5–S12. [DOI] [PubMed] [Google Scholar]
- 190.Courvalin P. Vancomycin resistance in gram-positive cocci. Clin. Infect. Dis. 2006;42 doi: 10.1086/491711. S25–S34. [DOI] [PubMed] [Google Scholar]
- 191.Appelbaum P.C., Bozdogan B. Vancomycin resistance in Staphylococcus aureus. Clin. Lab. Med. 2004;24:381–402. doi: 10.1016/j.cll.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 192.Xiong M.-H., Li Y.-J., Bao Y., Yang X.-Z., Hu B., Wang J. Bacteria-responsive multifunctional nanogel for targeted antibiotic delivery. Adv. Mater. 2012;24:6175–6180. doi: 10.1002/adma.201202847. [DOI] [PubMed] [Google Scholar]
- 193.Li L.L., Xu J.H., Bin Qi G., Zhao X., Yu F., Wang H. Core-shell supramolecular gelatin nanoparticles for adaptive and “on-demand” antibiotic delivery. ACS Nano. 2014;8:4975–4983. doi: 10.1021/nn501040h. [DOI] [PubMed] [Google Scholar]
- 194.Wijagkanalan W., Kawakami S., Higuchi Y., Yamashita F., Hashida M. Intratracheally instilled mannosylated cationic liposome/NFκB decoy complexes for effective prevention of LPS-induced lung inflammation. J. Contr. Release. 2011;149:42–50. doi: 10.1016/j.jconrel.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 195.Hubbell J.A., Chilkoti A. Nanomaterials for drug delivery. Science. 2012;337(80):303–305. doi: 10.1126/science.1219657. [DOI] [PubMed] [Google Scholar]
- 196.Baker S.J., Payne D.J., Rappuoli R., De Gregorio E. Technologies to address antimicrobial resistance. Proc. Natl. Acad. Sci. U.S.A. 2018;115:12887–12895. doi: 10.1073/pnas.1717160115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bassolé I.H.N., Juliani H.R. Essential oils in combination and their antimicrobial properties. Molecules. 2012;17:3989–4006. doi: 10.3390/molecules17043989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Movert E., Wu Y., Lambeau G., Kahn F., Touqui L., Areschoug T. Using patient pathways to accelerate the drive to ending tuberculosis. J. Infect. Dis. 2013;208:2025–2035. doi: 10.1093/INFDIS. [DOI] [PubMed] [Google Scholar]
- 199.Chen Z., Lv X., Zhao M., Zhang P., Ren X., Mei X. Encapsulation of green tea polyphenol by pH responsive, antibacterial, alginate microgels used for minimally invasive treatment of bone infection. Colloids Surf. B Biointerfaces. 2018;170:648–655. doi: 10.1016/J.COLSURFB.2018.06.065. [DOI] [PubMed] [Google Scholar]
- 200.Zhao Y., Sun W., Saldaña M.D.A. Nanogels of poly-N-isopropylacrylamide, poly-N,N-diethylacrylamide and acrylic acid for controlled release of thymol. J. Polym. Res. 2018;25:253. doi: 10.1007/s10965-018-1644-x. [DOI] [Google Scholar]
- 201.L. Li, L. Fu, X. Ai, J. Zhang, J. Zhou, Design and fabrication of temperature-sensitive nanogels with controlled drug release properties for enhanced photothermal sterilization, Chem. Eur J. (n.d.). 10.1002/chem.201704202. [DOI] [PubMed]
- 202.Zhaveh S., Mohsenifar A., Beiki M., Khalili S.T., Abdollahi A., Rahmani-Cherati T., Tabatabaei M. Encapsulation of Cuminum cyminum essential oils in chitosan-caffeic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. Ind. Crop. Prod. 2015;69:251–256. doi: 10.1016/j.indcrop.2015.02.028. [DOI] [Google Scholar]
- 203.Beyki M., Zhaveh S., Khalili S.T., Rahmani-Cherati T., Abollahi A., Bayat M., Tabatabaei M., Mohsenifar A. Encapsulation of Mentha piperita essential oils in chitosan-cinnamic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. Ind. Crop. Prod. 2014;54:310–319. doi: 10.1016/j.indcrop.2014.01.033. [DOI] [Google Scholar]
- 204.Khalili S.T., Mohsenifar A., Beyki M., Zhaveh S., Rahmani-Cherati T., Abdollahi A., Bayat M., Tabatabaei M. Encapsulation of Thyme essential oils in chitosan-benzoic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2015;60:502–508. doi: 10.1016/j.lwt.2014.07.054. [DOI] [Google Scholar]
- 205.Naksuriya O., Okonogi S., Schiffelers R.M., Hennink W.E. Curcumin nanoformulations: a review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials. 2014;35:3365–3383. doi: 10.1016/j.biomaterials.2013.12.090. [DOI] [PubMed] [Google Scholar]
- 206.Zaman M.S., Chauhan N., Yallapu M.M., Gara R.K., Maher D.M., Kumari S., Sikander M., Khan S., Zafar N., Jaggi M., Chauhan S.C. Curcumin nanoformulation for cervical cancer treatment. Sci. Rep. 2016;6 doi: 10.1038/srep20051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mirzaei H., Shakeri A., Rashidi B., Jalili A., Banikazemi Z., Sahebkar A. Phytosomal curcumin: a review of pharmacokinetic, experimental and clinical studies, Biomed. Pharma. 2017;85:102–112. doi: 10.1016/j.biopha.2016.11.098. [DOI] [PubMed] [Google Scholar]
- 208.Jones R.D., Jampani H.B., Newman J.L., Lee A.S. Triclosan: a review of effectiveness and safety in health care settings. Am. J. Infect. Contr. 2000;28:184–196. doi: 10.1067/mic.2000.102378. [DOI] [PubMed] [Google Scholar]
- 209.Jones C.G. Chlorhexidine: is it still the gold standard? Periodontol. 2000;15:55–62. doi: 10.1111/j.1600-0757.1997.tb00105.x. 1997. [DOI] [PubMed] [Google Scholar]
- 210.Moshrefi A. Chlorhexidine., J. West. Soc. Periodontol. Periodontal. Abstr. 2002;50:5–9. [PubMed] [Google Scholar]
- 211.McMurry L.M., Oethinger M., Levy S.B. Triclosan targets lipid synthesis [4] Nature. 1998;394:531–532. doi: 10.1038/28970. [DOI] [PubMed] [Google Scholar]
- 212.Weatherly L.M., Gosse J.A. Triclosan exposure, transformation, and human health effects. J. Toxicol. Environ. Health B Crit. Rev. 2017;20:447–469. doi: 10.1080/10937404.2017.1399306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Aminu N., Chan S.-Y., Yam M.-F., Toh S.-M. A dual-action chitosan-based nanogel system of triclosan and flurbiprofen for localised treatment of periodontitis. Int. J. Pharm. 2019;570:118659. doi: 10.1016/j.ijpharm.2019.118659. [DOI] [PubMed] [Google Scholar]
- 214.Kettel M.J., Heine E., Schaefer K., Moeller M. Chlorhexidine loaded cyclodextrin containing PMMA nanogels as antimicrobial coating and delivery systems. Macromol. Biosci. 2017;17:1600230. doi: 10.1002/mabi.201600230. [DOI] [PubMed] [Google Scholar]
- 215.Wu D.-Q., Cui H.-C., Zhu J., Qin X.-H., Xie T. Novel amino acid based nanogel conjugated suture for antibacterial application. J. Mater. Chem. B. 2016;4:2606–2613. doi: 10.1039/C6TB00186F. [DOI] [PubMed] [Google Scholar]
- 216.Zhu J., Li F., Wang X., Yu J., Wu D. Hyaluronic acid and polyethylene glycol hybrid hydrogel encapsulating nanogel with hemostasis and sustainable antibacterial property for wound healing. ACS Appl. Mater. Interfaces. 2018;10:13304–13316. doi: 10.1021/acsami.7b18927. [DOI] [PubMed] [Google Scholar]
- 217.Zu G., Steinmuller M., Keskin D., van der Mei H.C., Mergel O., van Rijn P. Antimicrobial nanogels with nano-injection capabilities for delivery of hydrophobic antibacterial agent triclosan. Appl. Polym. Mater. 2020;2(12):5779–5789. doi: 10.1021/acsapm.0c01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Silhavy T.J., Kahne D., Walker S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010;2:a000414. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Al-Awady M.J., Weldrick P.J., Hardman M.J., Greenway G.M., Paunov V.N. Amplified antimicrobial action of chlorhexidine encapsulated in PDAC-functionalized acrylate copolymer nanogel carriers. Mater. Chem. Front. 2018;2:2032–2044. doi: 10.1039/c8qm00343b. [DOI] [Google Scholar]
- 220.Weldrick P.J., Iveson S., Hardman M.J., Paunov V.N. Breathing new life into old antibiotics: overcoming antibacterial resistance by antibiotic-loaded nanogel carriers with cationic surface functionality. Nanoscale. 2019;11:10472–10485. doi: 10.1039/C8NR10022E. [DOI] [PubMed] [Google Scholar]
- 221.Al-Awady M.J., Fauchet A., Greenway G.M., Paunov V.N. Enhanced antimicrobial effect of berberine in nanogel carriers with cationic surface functionality. J. Mater. Chem. B. 2017;5:7885–7897. doi: 10.1039/C7TB02262J. [DOI] [PubMed] [Google Scholar]
- 222.Weldrick P.J., Hardman M.J., Paunov V.N. Enhanced clearing of wound-related pathogenic bacterial biofilms using protease-functionalized antibiotic nanocarriers. ACS Appl. Mater. Interfaces. 2019 doi: 10.1021/acsami.9b16119. acsami.9b16119. [DOI] [PubMed] [Google Scholar]
- 223.Zhang X.-F., Liu Z.-G., Shen W., Gurunathan S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016;17:1534. doi: 10.3390/ijms17091534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Shanmuganathan R., Karuppusamy I., Saravanan M., Muthukumar H., Ponnuchamy K., Ramkumar V.S., Pugazhendhi A. Synthesis of silver nanoparticles and their biomedical applications - a comprehensive review. Curr. Pharmaceut. Des. 2019;25:2650–2660. doi: 10.2174/1381612825666190708185506. [DOI] [PubMed] [Google Scholar]
- 225.Kim J.S., Kuk E., Yu K.N., Kim J.H., Park S.J., Lee H.J., Kim S.H., Park Y.K., Park Y.H., Hwang C.Y., Kim Y.K., Lee Y.S., Jeong D.H., Cho M.H. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 226.Eckhardt S., Brunetto P.S., Gagnon J., Priebe M., Giese B., Fromm K.M. Nanobio silver: its interactions with peptides and bacteria, and its uses in medicine. Chem. Rev. 2013;113:4708–4754. doi: 10.1021/cr300288v. [DOI] [PubMed] [Google Scholar]
- 227.Qasim M., Udomluck N., Chang J., Park H., Kim K. Antimicrobial activity of silver nanoparticles encapsulated in poly-N-isopropylacrylamide-based polymeric nanoparticles. Int. J. Nanomed. 2018;13:235–249. doi: 10.2147/IJN.S153485. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 228.Rama Subba Reddy P., Krishna Rao K.S.V., Madhusudana Rao K., Sivagangi Reddy N., Eswaramma S. PH sensitive poly(methyl methacrylate-co-acryloyl phenylalanine) nanogels and their silver nanocomposites for biomedical applications. J. Drug Deliv. Sci. Technol. 2015;29:181–188. doi: 10.1016/j.jddst.2015.07.002. [DOI] [Google Scholar]
- 229.Ballesteros C.A.S., Correa D.S., Zucolotto V. Polycaprolactone nanofiber mats decorated with photoresponsive nanogels and silver nanoparticles: slow release for antibacterial control. Mater. Sci. Eng. C. 2020;107 doi: 10.1016/j.msec.2019.110334. [DOI] [PubMed] [Google Scholar]
- 230.Ravindra S., Varaprasad K., Rajinikanth V., Mulaba-Bafubiandi A.F., Venkata Surya Ramam K., Mulaba-bafubiandi A.F. Studies on curcumin loaded poly(N-isopropylacrylamide) silver nanocomposite hydrogels for antibacterial and drug releasing applications. J. Macromol. Sci. Part A. 2013;50:1230–1240. doi: 10.1080/10601325.2013.843406. [DOI] [Google Scholar]
- 231.Ozay O., Akcali A., Otkun M.T., Silan C., Aktas N., Sahiner N. P(4-VP) based nanoparticles and composites with dual action as antimicrobial materials. Colloids Surf. B Biointerfaces. 2010;79:460–466. doi: 10.1016/j.colsurfb.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 232.El-Sherif H., El-Masry M., Kansoh A. Hydrogels as template nanoreactors for silver nanoparticles formation and their antimicrobial activities. Macromol. Res. 2011;19:1157–1165. doi: 10.1007/s13233-011-1109-0. [DOI] [Google Scholar]
- 233.Murali Mohan Y., Lee K., Premkumar T., Geckeler K.E. Hydrogel networks as nanoreactors: a novel approach to silver nanoparticles for antibacterial applications. Polymer. 2007;48:158–164. doi: 10.1016/j.polymer.2006.10.045. [DOI] [Google Scholar]
- 234.Choi J.-B., Park J.-S., Khil M.-S., Gwon H.-J., Lim Y.-M., Jeong S.-I., Shin Y.-M., Nho Y.-C., Choi J.-B., Park J.-S., Khil M.-S., Gwon H.-J., Lim Y.-M., Jeong S.-I., Shin Y.-M., Nho Y.-C. Characterization and antimicrobial property of poly(acrylic acid) nanogel containing silver particle prepared by electron beam. Int. J. Mol. Sci. 2013;14:11011–11023. doi: 10.3390/ijms140611011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ballesteros C.A.S., Bernardi J.C., Correa D.S., Zucolotto V. 2019. Controlled Release of Silver Nanoparticles Contained in Photoresponsive Nanogels. [DOI] [PubMed] [Google Scholar]
- 236.Coll Ferrer M.C., Dastgheyb S., Hickok N.J., Eckmann D.M., Composto R.J. Designing nanogel carriers for antibacterial applications. Acta Biomater. 2014;10:2105–2111. doi: 10.1016/J.ACTBIO.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Khan A., Khan T.H., El-Toni A.M., Aldalbahi A., Alam J., Ahamad T. In situ formation and immobilization of silver nanoparticles using thermo-responsive microgel particles and their cytotoxicity evaluation. Mater. Lett. 2019;235:197–201. doi: 10.1016/j.matlet.2018.10.041. [DOI] [Google Scholar]
- 238.Coll Ferrer M.C., Ferrier R.C., Eckmann D.M., Composto R.J. A facile route to synthesize nanogels doped with silver nanoparticles. J. Nanoparticle Res. 2013;15:1–7. doi: 10.1007/s11051-012-1323-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wang G., Li X., Wang Z. APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016;44:D1087–D1093. doi: 10.1093/nar/gkv1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Izadpanah A., Gallo R.L. Antimicrobial peptides. J. Am. Acad. Dermatol. 2005;52:381–390. doi: 10.1016/j.jaad.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 241.T.-H. Lee, K. N. Hall, M.-I. Aguilar, Antimicrobial Peptide Structure and Mechanism of Action: A Focus on the Role of Membrane Structure, (n.d.). [DOI] [PubMed]
- 242.Shen W., He P., Xiao C., Chen X. From antimicrobial peptides to antimicrobial poly(α-amino acid)s. Adv. Healthc. Mater. 2018;7:1800354. doi: 10.1002/adhm.201800354. [DOI] [PubMed] [Google Scholar]
- 243.Parilti R., Caprasse J., Riva R., Alexandre M., Vandegaart H., Bebrone C., Dupont-Gillain C., Howdle S.M., Jérôme C. Antimicrobial peptide encapsulation and sustained release from polymer network particles prepared in supercritical carbon dioxide. J. Colloid Interface Sci. 2018;532:112–117. doi: 10.1016/J.JCIS.2018.07.125. [DOI] [PubMed] [Google Scholar]
- 244.Kłodzińska S.N., Molchanova N., Franzyk H., Hansen P.R., Damborg P., Nielsen H.M. Biopolymer nanogels improve antibacterial activity and safety profile of a novel lysine-based α-peptide/β-peptoid peptidomimetic. Eur. J. Pharm. Biopharm. 2018;128:1–9. doi: 10.1016/J.EJPB.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 245.Singh S., Datta A., Borro B.C., Davoudi M., Schmidtchen A., Bhunia A., Malmsten M. Conformational aspects of high content packing of antimicrobial peptides in polymer microgels. ACS Appl. Mater. Interfaces. 2017;9:40094–40106. doi: 10.1021/acsami.7b13714. [DOI] [PubMed] [Google Scholar]
- 246.Nordström R., Browning K.L., Parra-Ortiz E., Damgaard L.S.E., Häffner S.M., Maestro A., Campbell R.A., Cooper J.F.K., Malmsten M. Membrane interactions of antimicrobial peptide-loaded microgels. J. Colloid Interface Sci. 2020;562:322–332. doi: 10.1016/j.jcis.2019.12.022. [DOI] [PubMed] [Google Scholar]
- 247.Nordström R., Nyström L., Andrén O.C.J., Malkoch M., Umerska A., Davoudi M., Schmidtchen A., Malmsten M. Membrane interactions of microgels as carriers of antimicrobial peptides. J. Colloid Interface Sci. 2018;513:141–150. doi: 10.1016/J.JCIS.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 248.Borro B.C., Bohr A., Bucciarelli S., Boetker J.P., Foged C., Rantanen J., Malmsten M. Microfluidics-based self-assembly of peptide-loaded microgels: effect of three dimensional (3D) printed micromixer design. J. Colloid Interface Sci. 2019;538:559–568. doi: 10.1016/j.jcis.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 249.Nordström R., Nyström L., Ilyas H., Atreya H.S., Borro B.C., Bhunia A., Malmsten M. Microgels as carriers of antimicrobial peptides – effects of peptide PEGylation. Colloids Surfaces A Physicochem. Eng. Asp. 2019;565:8–15. doi: 10.1016/J.COLSURFA.2018.12.049. [DOI] [Google Scholar]
- 250.Chen Y.-F., Chen G.-Y., Chang C.-H., Su Y.-C., Chen Y.-C., Jiang Y., Jan J.-S. TRAIL encapsulated to polypeptide-crosslinked nanogel exhibits increased anti-inflammatory activities in Klebsiella pneumoniae-induced sepsis treatment. Mater. Sci. Eng. C. 2019;102:85–95. doi: 10.1016/J.MSEC.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 251.Balouiri M., Sadiki M., Ibnsouda S.K. Methods for in vitro evaluating antimicrobial activity: a review. J. Pharm. Anal. 2016;6:71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Schumacher A., Vranken T., Malhotra A., Arts J.J.C., Habibovic P. In vitro antimicrobial susceptibility testing methods: agar dilution to 3D tissue-engineered models. Eur. J. Clin. Microbiol. Infect. Dis. 2018;37:187–208. doi: 10.1007/s10096-017-3089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Sagbas S., Aktas N., Sahiner N. Modified biofunctional p(tannic acid) microgels and their antimicrobial activity. Appl. Surf. Sci. 2015;354:306–313. doi: 10.1016/J.APSUSC.2015.06.163. [DOI] [Google Scholar]
- 254.Silan C., Akcali A., Otkun M.T., Ozbey N., Butun S., Ozay O., Sahiner N. Novel hydrogel particles and their IPN films as drug delivery systems with antibacterial properties. Colloids Surf. B Biointerfaces. 2012;89:248–253. doi: 10.1016/J.COLSURFB.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 255.Richardson K.E., Xue Z., Huang Y., Seo Y., Lapitsky Y. Physicochemical and antibacterial properties of surfactant mixtures with quaternized chitosan microgels. Carbohydr. Polym. 2013;93:709–717. doi: 10.1016/J.CARBPOL.2012.12.054. [DOI] [PubMed] [Google Scholar]
- 256.Echeverría C., Aragón-Gutiérrez A., Fernández-García M., Muñoz-Bonilla A., López D., Echeverría C., Aragón-Gutiérrez A., Fernández-García M., Muñoz-Bonilla A., López D. Thermoresponsive poly(N-Isopropylacrylamide-co-Dimethylaminoethyl methacrylate) microgel aqueous dispersions with potential antimicrobial properties. Polymers. 2019;11:606. doi: 10.3390/polym11040606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Han H., Zhu J., Wu D.Q., Li F.X., Wang X.L., Yu J.Y., Qin X.H. Inherent guanidine nanogels with durable antibacterial and bacterially antiadhesive properties. Adv. Funct. Mater. 2019 doi: 10.1002/adfm.201806594. [DOI] [Google Scholar]
- 258.Panja S., Bharti R., Dey G., Lynd N.A., Chattopadhyay S. Coordination-assisted self-assembled polypeptide nanogels to selectively combat bacterial infection. ACS Appl. Mater. Interfaces. 2019;11:33599–33611. doi: 10.1021/acsami.9b10153. [DOI] [PubMed] [Google Scholar]
- 259.Wang Y., Yang Y., Shi Y., Song H., Yu C. Antibiotic‐free antibacterial strategies enabled by nanomaterials: progress and perspectives. Adv. Mater. 2020;32:1904106. doi: 10.1002/adma.201904106. [DOI] [PubMed] [Google Scholar]
- 260.Qian L., Xiao H., Zhao G., He B. Synthesis of modified guanidine-based polymers and their antimicrobial activities revealed by AFM and CLSM. ACS Appl. Mater. Interfaces. 2011;3:1895–1901. doi: 10.1021/am200094u. [DOI] [PubMed] [Google Scholar]
- 261.Muñoz-Bonilla A., Fernández-García M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012;37:281–339. doi: 10.1016/j.progpolymsci.2011.08.005. [DOI] [Google Scholar]
- 262.Otto M. Staphylococcal biofilms. Gram‐Positive Pathog. 2019:699–711. doi: 10.1128/9781683670131.ch43. [DOI] [Google Scholar]
- 263.Talsma S.S. 2007. Biofilms on Medical Devices, Home Healthc. Now. 25.https://journals.lww.com/homehealthcarenurseonline/Fulltext/2007/10000/Biofilms_on_Medical_Devices.7.aspx [DOI] [PubMed] [Google Scholar]
- 264.Tan R., Yoo J., Jang Y. In: Engineering Approaches to Create Antibacterial Surfaces on Biomedical Implants and Devices BT - Racing for the Surface: Pathogenesis of Implant Infection and Advanced Antimicrobial Strategies. Li B., Moriarty T.F., Webster T., Xing M., editors. Springer International Publishing; Cham: 2020. pp. 313–340. [DOI] [Google Scholar]
- 265.Yu Q., Wu Z., Chen H. Dual-function antibacterial surfaces for biomedical applications. Acta Biomater. 2015;16:1–13. doi: 10.1016/j.actbio.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 266.Lyon L.A., Debord J.D., Debord S.B., Jones C.D., McGrath J.G., Serpe M.J. Microgel colloidal crystals. J. Phys. Chem. B. 2004;108:19099–19108. doi: 10.1021/jp048486j. [DOI] [Google Scholar]
- 267.Hellweg T., Dewhurst C.D., Brückner E., Kratz K., Eimer W. Colloidal crystals made of poly(N-isopropylacrylamide) microgel particles. Colloid Polym. Sci. 2000;278:972–978. doi: 10.1007/s003960000350. [DOI] [Google Scholar]
- 268.Schmidt S., Zeiser M., Hellweg T., Duschl C., Fery A., Möhwald H. Adhesion and mechanical properties of PNIPAM microgel films and their potential use as switchable cell culture substrates. Adv. Funct. Mater. 2010;20:3235–3243. doi: 10.1002/adfm.201000730. [DOI] [Google Scholar]
- 269.Schmidt S., Hellweg T., von Klitzing R. Packing density control in P(NIPAM-co-AAc) microgel monolayers: effect of surface charge, pH, and preparation technique. Langmuir. 2008;24:12595–12602. doi: 10.1021/la801770n. [DOI] [PubMed] [Google Scholar]
- 270.Schubert J., Chanana M. Coating matters: review on colloidal stability of nanoparticles with biocompatible coatings in biological media, living cells and organisms. Curr. Med. Chem. 2018;25:4553–4586. doi: 10.2174/0929867325666180601101859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Saxena S., Spears M.W., Jr., Yoshida H., Gaulding J.C., García A.J., Lyon L.A. Microgel film dynamics modulate cell adhesion behavior. Soft Matter. 2014;10:1356–1364. doi: 10.1039/C3SM52518J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Yang Y., Maldonado-Valderrama J., Martín-Molina A. Temperature and electrostatics effects on charged poly(N-isopropylacrylamide) microgels at the interface. J. Mol. Liq. 2020;303:112678. doi: 10.1016/j.molliq.2020.112678. [DOI] [Google Scholar]
- 273.Wang Y., Guo L., Dong S., Cui J., Hao J. Microgels in biomaterials and nanomedicines. Adv. Colloid Interface Sci. 2019;266:1–20. doi: 10.1016/j.cis.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 274.Saunders B.R., Laajam N., Daly E., Teow S., Hu X., Stepto R. Microgels: from responsive polymer colloids to biomaterials. Adv. Colloid Interface Sci. 2009;147–148:251–262. doi: 10.1016/j.cis.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 275.Babi A. Antifouling surfaces: peeling back the solid layers. Nat. Rev. Mater. 2017;2:17035. doi: 10.1038/natrevmats.2017.35. [DOI] [Google Scholar]
- 276.Hu B., Owh C., Chee P.L., Leow W.R., Liu X., Wu Y.-L., Guo P., Loh X.J., Chen X. Supramolecular hydrogels for antimicrobial therapy. Chem. Soc. Rev. 2018;47:6917–6929. doi: 10.1039/C8CS00128F. [DOI] [PubMed] [Google Scholar]
- 277.Lee S.Y., Lee Y., Le Thi P., Oh D.H., Park K.D. Sulfobetaine methacrylate hydrogel-coated anti-fouling surfaces for implantable biomedical devices. Biomater. Res. 2018;22:3. doi: 10.1186/s40824-017-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Banerjee S.L., Bhattacharya K., Samanta S., Singha N.K. Self-healable antifouling zwitterionic hydrogel based on synergistic phototriggered dynamic disulfide metathesis reaction and ionic interaction. ACS Appl. Mater. Interfaces. 2018;10:27391–27406. doi: 10.1021/acsami.8b10446. [DOI] [PubMed] [Google Scholar]
- 279.Wu C., Zhou Y., Wang H., Hu J., Wang X. Formation of antifouling functional coating from deposition of a zwitterionic-co-nonionic polymer via “grafting to” approach. J. Saudi Chem. Soc. 2019;23:1080–1089. doi: 10.1016/j.jscs.2019.05.011. [DOI] [Google Scholar]
- 280.Wang S., Zan F., Ke Y., Wu G. Cells may feel a hard substrate even on a grafted layer of soft hydrogel. J. Mater. Chem. B. 2018;6:1734–1743. doi: 10.1039/C7TB02967E. [DOI] [PubMed] [Google Scholar]
- 281.Mergel O., Schneider S., Tiwari R., Kühn P.T., Keskin D., Stuart M.C.A., Schöttner S., de Kanter M., Noyong M., Caumanns T., Mayer J., Janzen C., Simon U., Gallei M., Wöll D., van Rijn P., Plamper F.A. Cargo shuttling by electrochemical switching of core–shell microgels obtained by a facile one-shot polymerization. Chem. Sci. 2019;10:1844–1856. doi: 10.1039/C8SC04369H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Huang Z., Nazifi S., Jafari P., Karim A., Ghasemi H. Networked zwitterionic durable antibacterial surfaces. ACS Appl. Bio Mater. 2020;3:911–919. doi: 10.1021/acsabm.9b00982. [DOI] [PubMed] [Google Scholar]
- 283.Saha P., Santi M., Frenken M., Palanisamy A.R., Ganguly R., Singha N.K., Pich A. Dual-temperature-responsive microgels from a zwitterionic functional graft copolymer with superior protein repelling property. ACS Macro Lett. 2020;9:895–901. doi: 10.1021/acsmacrolett.0c00304. [DOI] [PubMed] [Google Scholar]
- 284.Brosel-Oliu S., Mergel O., Uria N., Abramova N., van Rijn P., Bratov A. 3D impedimetric sensors as a tool for monitoring bacterial response to antibiotics. Lab Chip. 2019;19:1436–1447. doi: 10.1039/C8LC01220B. [DOI] [PubMed] [Google Scholar]
- 285.Liu H., Yang S., Liu Y., Miao M., Zhao Y., Sotto A., Gao C., Shen J. Fabricating a pH-responsive membrane through interfacial in-situ assembly of microgels for water gating and self-cleaning. J. Membr. Sci. 2019;579:230–239. doi: 10.1016/j.memsci.2019.03.010. [DOI] [Google Scholar]
- 286.Ji H., Xiong L., Shi Z., He M., Zhao W., Zhao C. Engineering of hemocompatible and antifouling polyethersulfone membranes by blending with heparin-mimicking microgels. Biomater. Sci. 2017;5:1112–1121. doi: 10.1039/C7BM00196G. [DOI] [PubMed] [Google Scholar]
- 287.Qazi T.H., Mooney D.J., Pumberger M., Geißler S., Duda G.N. Biomaterials based strategies for skeletal muscle tissue engineering: existing technologies and future trends. Biomaterials. 2015;53:502–521. doi: 10.1016/j.biomaterials.2015.02.110. [DOI] [PubMed] [Google Scholar]
- 288.Li Z., Hu W., Zhao Y., Ren L., Yuan X. Integrated antibacterial and antifouling surfaces via cross-linking chitosan-g-eugenol/zwitterionic copolymer on electrospun membranes. Colloids Surf. B Biointerfaces. 2018;169:151–159. doi: 10.1016/j.colsurfb.2018.04.056. [DOI] [PubMed] [Google Scholar]
- 289.Iso 10993-5:2009 . International Organization for Standardization; Geneva, Switzerland: 2009. Biological Evaluation of Medical Devices. Part 5: Tests for in Vitro Cytotoxicity. [Google Scholar]
- 290.Nyström L., Strömstedt A.A., Schmidtchen A., Malmsten M. Peptide-loaded microgels as antimicrobial and anti-inflammatory surface coatings. Biomacromolecules. 2018;19:3456–3466. doi: 10.1021/acs.biomac.8b00776. [DOI] [PubMed] [Google Scholar]
- 291.Chen K., Zhou S., Wu L. Self-healing underwater superoleophobic and antibiofouling coatings based on the assembly of hierarchical microgel spheres. ACS Nano. 2016;10:1386–1394. doi: 10.1021/acsnano.5b06816. [DOI] [PubMed] [Google Scholar]
- 292.Liu X., Rodeheaver D.P., White J.C., Wright A.M., Walker L.M., Zhang F., Shannon S. A comparison of in vitro cytotoxicity assays in medical device regulatory studies. Regul. Toxicol. Pharmacol. 2018;97:24–32. doi: 10.1016/j.yrtph.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 293.Francolini I., Vuotto C., Piozzi A., Donelli G. Antifouling and antimicrobial biomaterials: an overview. Apmis. 2017;125:392–417. doi: 10.1111/apm.12675. [DOI] [PubMed] [Google Scholar]
- 294.Kaur R., Liu S. Antibacterial surface design – contact kill. Prog. Surf. Sci. 2016;91:136–153. doi: 10.1016/j.progsurf.2016.09.001. [DOI] [Google Scholar]
- 295.Hasan J., Webb H.K., Truong V.K., Pogodin S., Baulin V.A., Watson G.S., Watson J.A., Crawford R.J., Ivanova E.P. Selective bactericidal activity of nanopatterned superhydrophobic cicada Psaltoda claripennis wing surfaces. Appl. Microbiol. Biotechnol. 2013;97:9257–9262. doi: 10.1007/s00253-012-4628-5. [DOI] [PubMed] [Google Scholar]
- 296.Siedenbiedel F., Tiller J.C. Antimicrobial polymers in solution and on surfaces: overview and functional principles. Polymers. 2012;4:46–71. doi: 10.3390/polym4010046. [DOI] [Google Scholar]
- 297.Keskin D., Tromp L., Mergel O., Zu G., Warszawik E., van der Mei H.C., van Rijn P. Highly efficient antimicrobial and antifouling surface coatings with triclosan-loaded nanogels. ACS Appl. Mater. Interfaces. 2020;12:57721–57731. doi: 10.1021/acsami.0c18172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Zhao Z., Ma X., Chen R., Xue H., Lei J., Du H., Zhang Z., Chen H. Universal antibacterial surfaces fabricated from quaternary ammonium salt-based PNIPAM microgels. ACS Appl. Mater. Interfaces. 2020;12:19268–19276. doi: 10.1021/acsami.0c00791. [DOI] [PubMed] [Google Scholar]
- 299.Xue H., Zhao Z., Chen S., Du H., Chen R., Brash J.L., Chen H. Antibacterial coatings based on microgels containing quaternary ammonium ions: modification with polymeric sugars for improved cytocompatibility. Colloid Interface Sci. Commun. 2020;37:100268. doi: 10.1016/j.colcom.2020.100268. [DOI] [Google Scholar]
- 300.Sjollema J., Zaat S.A.J., Fontaine V., Ramstedt M., Luginbuehl R., Thevissen K., Li J., van der Mei H.C., Busscher H.J. In vitro methods for the evaluation of antimicrobial surface designs. Acta Biomater. 2018;70:12–24. doi: 10.1016/j.actbio.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 301.Udekwu K.I., Parrish N., Ankomah P., Baquero F., Levin B.R. Functional relationship between bacterial cell density and the efficacy of antibiotics. J. Antimicrob. Chemother. 2009;63:745–757. doi: 10.1093/jac/dkn554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Chattopadhyay S., Heine E., Mourran A., Richtering W., Keul H., Möller M. Waterborne physically crosslinked antimicrobial nanogels. Polym. Chem. 2016;7:364–369. doi: 10.1039/C5PY01566A. [DOI] [Google Scholar]
- 303.Sproul E.P., Nandi S., Chee E., Sivadanam S., Igo B.J., Schreck L., Brown A.C. Development of biomimetic antimicrobial platelet-like particles comprised of microgel nanogold composites. Regen. Eng. Transl. Med. 2019 doi: 10.1007/s40883-019-00121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Nyström L., Al-Rammahi N., Malekkhaiat Häffner S., Strömstedt A.A., Browning K.L., Malmsten M. Avidin–biotin cross-linked microgel multilayers as carriers for antimicrobial peptides. Biomacromolecules. 2018;19:4691–4702. doi: 10.1021/acs.biomac.8b01484. [DOI] [PubMed] [Google Scholar]
- 305.Liang J., Wang H., Libera M. Biomaterial surfaces self-defensive against bacteria by contact transfer of antimicrobials. Biomaterials. 2019;204:25–35. doi: 10.1016/j.biomaterials.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]