Abstract
As one of the most common metastatic sites, bone has a unique microenvironment for the growth and prosperity of metastatic tumor cells. Bone metastasis is a common complication for tumor patients and accounts for 15–20% of systemic metastasis, which is only secondary to lung and liver metastasis. Cancers prone to bone metastasis include lung, breast, and prostate cancer. Extracellular vesicles (EVs) are lipid membrane vesicles released from different cell types. It is clear that EVs are associated with multiple biological phenomena and are crucial for intracellular communication by transporting intracellular substances. Recent studies have implicated EVs in the development of cancer. However, the potential roles of EVs in the pathological exchange of bone cells between tumors and the bone microenvironment remain an emerging area. This review is focused on the role of tumor-derived EVs in bone metastasis and possible regulatory mechanisms.
Keywords: extracellular vesicles, osteoblast, osteoclast, bone metastasis, tumor
Introduction
Malignant tumors are the second leading cause of death worldwide. Distant metastasis of tumor cells is the most common cause of cancer-related death (Krzeszinski and Wan, 2015; Hiraga, 2019; Karayazi Atici et al., 2020). Bone is a major target for cancer metastasis, only secondary to the lung and liver (Yin et al., 2005; Su et al., 2015). Bone has a unique anatomical and physiopathological state that enhances the metastasis of different cancer types, including prostate, breast, lung, and gastric cancer and melanoma (Liu et al., 2020; Ma et al., 2020; Wang et al., 2020). Tumor cells may migrate from their original location to distant skeletal tissues, where they may exhibit accelerated growth and infiltrate surrounding tissues to form distant metastasis, characteristics associated with the heterogeneity of tumors (Zhang et al., 2019; Scimeca et al., 2020). Various physiopathological processes will be induced by the occurrence of affected bones and metastatic tumor cells, such as the adhesion molecules secreted by tumor cells bound to trabecular bone and stromal cells. Additionally, tumor cells can induce angiogenesis and bone absorption factors (Zhang et al., 2019). Once patients develop bone metastasis, there are no effective therapeutic strategies, and the 5-year survival rate of these patients is significantly lower. Moreover, bone metastasis may be accompanied by many complications (e.g., serious bone pain, pathological fracture, hypercalcemia, and spinal cord compression) that affect a patient’s life expectancy and quality of life (Krzeszinski and Wan, 2015; Vicent et al., 2015; Yao et al., 2020).
In recent years, the mechanisms underlying bone metastasis have been a hot research topic, and more and more data support the “seed and soil” theory, which says that tumor cells can only grow in a microenvironment suitable for their growth (Marazzi et al., 2020; Tamura et al., 2020; Yao et al., 2020). Thus, they form metastatic lesions in specific tissues and organs. Primary tumor cells proliferate, invade blood vessels, and form tumor blood vessels. Tumor cells reach each system in the body through the vasculature, proliferate in the microenvironment of specific organs and tissues suitable for their growth, destroy normal tissue structures, and form metastatic lesions. Bone metastasis involves two processes: (1) cancer cells reach bone by a certain pathway; (2) cancer cell survival and growth in the bone. Under normal conditions, the activities of osteoblasts and osteoclasts are balanced (Laranga et al., 2020; Wang et al., 2020). When bone metastasis occurs, this balance is disrupted, and the higher cell activity determines whether the bone metastasis is osteogenic or osteolytic. Osteogenic bone metastasis is the result of the activation of osteoblasts and the inhibition of osteoclasts. Conversely, osteolytic bone metastasis involves the activation of osteoclasts and the inhibition of osteoblasts (Wood and Brown, 2020).
Extracellular vesicles (EVs) are bi-layered membrane vesicles with a diameter of 30–100 nm secreted into the microenvironment via exocytosis by multiple cells. EVs are rich in many components, including cell-specific proteins, lipids, and RNA (i.e., mRNA, miRNA, and other non-coding RNA) (Dai et al., 2020; Yang E. et al., 2020). These vesicles are secreted by multiple cell types (e.g., tumor cells, macrophages, and fibroblasts) and are widely distributed in the blood, urine, ascites, synovial fluid, breast milk, and other body fluids. EVs carry and transmit important signaling molecules that affect the physiological and pathological state of their target cells (Cheng et al., 2020; LeBleu and Kalluri, 2020). Studies have shown that EVs participate in intercellular communication, immune responses, angiogenesis, and tumor cell growth (Yi et al., 2020). They have now been discovered in multiple cell types. Indeed, tumor cell-derived EVs have become a hot research area in the field of cancer (Tamura et al., 2020), and there have been many reports on EVs in cancer. For instance, EVs may release self-carrying cytokines to enhance the occurrence, development, proliferation, and migration of tumors and make tumors drug-resistant (Vasconcelos et al., 2019; Wortzel et al., 2019). In addition, the anticancer activity of miR-124 delivered by BM-MSC-associated EVs can act on the proliferation, epithelial-mesenchymal transition, and chemotherapy sensitivity of pancreatic cancer cells (Xu Y. et al., 2020). Endometrial cancer cells can promote M2-like macrophage polarization by delivering exosomal miRNA-21 under hypoxia conditions (Xiao et al., 2020). Furthermore, exosomal miR-92a-3p derived from highly metastatic cancer cells promotes the epithelial-mesenchymal transition and the metastasis of low-metastatic cancer cells by regulating the PTEN/Akt pathway in hepatocellular carcinoma (Yang B. et al., 2020). Moreover, neuroblastoma-secreted EVs carrying miR-375 promote osteogenic differentiation of bone marrow mesenchymal stromal cells (Colletti et al., 2020).
In recent years, many studies have demonstrated that tumor-derived EVs are an important component of the microenvironment of bone tumors. This article reviews the roles of tumor-derived EVs in bone metastasis and their potential molecular mechanisms and provides new insights for inhibiting bone metastasis.
Generation, Composition, and Major Biological Functions of Extracellular Vesicles
The formation of EVs mainly involves four processes, including sprouting, invagination, multivesicular body formation, and secretion (Mathew et al., 2020; Zhang Y. et al., 2020). EVs are generated in endosomes. Invagination of the cell membrane results in the formation of early endosomes, and late endosomes sprout inward to form luminal vesicles that are transformed into multivesicular bodies (MVBs) with multiple small vesicles. After the MVBs fuse with the cell membrane, the inner vesicles sink again, and granular vesicles sprout and are released out of the cell as EVs (Li et al., 2020; Naseri et al., 2020). Upon reaching recipient cells, the EVs release their contents in specific cells through ligand binding, phagocytosis, and fusing with the cell membrane to change the physiological state and function of cells (Huyan et al., 2020).
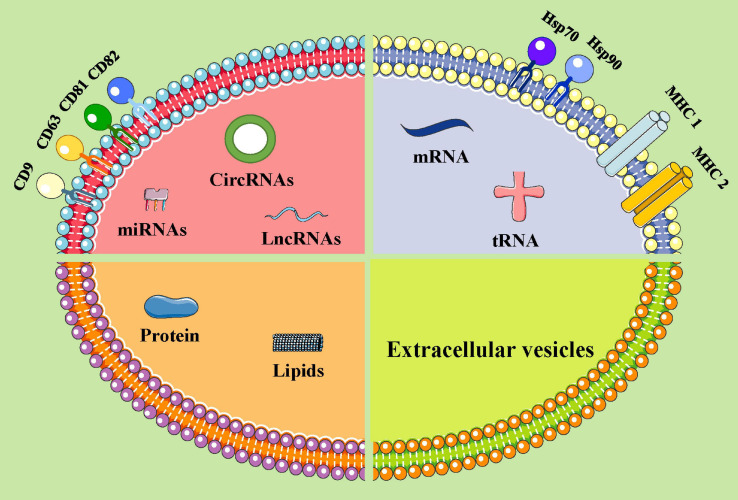
Extracellular vesicles are mainly composed of proteins, nucleic acids, and lipids (Kalluri and LeBleu, 2020; Zhao X. et al., 2020). All EVs express membrane-bound proteins, such as tetraspanin (CD9, CD63, CD81, and CD82), which may be used as biomarkers and are associated with the biogenetic derivation of EVs (Mathieu et al., 2019; Pegtel and Gould, 2019). EVs derived from different cells express specific proteins, such as ALG-2 interacting protein X (ALix), Wilms tumor type 101 protein (TSG101), and heat shock protein HSP70, and are associated with specific cell functions. EVs also include nucleic acids, such as mRNA, miRNA, and lncRNA (Mathew et al., 2020; Zhang Y. et al., 2020). These nucleic acids fuse with target cells and regulate protein expression and signaling in recipient cells. The lipids in the EVs include cholesterol, diacylglycerol, and phospholipid (Mathew et al., 2020; Zhang Y. et al., 2020). The lipids not only participate in the formation and maintenance of EV morphology but also participate in intercellular communication as signal molecules. These contents are transported to target cells via body fluids and are implicated in angiopoiesis and the occurrence, development, and metastasis of tumors (Kalluri and LeBleu, 2020; Slomka et al., 2020; Zhao X. et al., 2020; Zhang Y. et al., 2020; Figure 1). Potential exosomal biomarkers and clinical targets are displayed in Table 1.
FIGURE 1.
The components of extracellular vesicles (ideograph). EVs can be secreted by various types of cells. EVs carry a variety of proteins, lipids, DNA, mRNA, and non-coding RNAs. Moreover, EV surface proteins contain the following substances: MHC class I/II molecules, heat shock proteins (HSP70, HSP90), and four transmembrane family proteins (CD63, CD9, CD81, and CD82, etc.). miRNAs, microRNAs; lncRNAs, long non-coding RNAs; circRNAs, circular RNAs; mRNAs, messenger RNA; tRNA, transfer RNA.
TABLE 1.
Potential exosomal biomarker and targets in a clinical setting.
| Cargo | Type | Signaling pathway | EVs function | References |
| TGFβ1 | Protein | Activating ERK, Akt, and anti-apoptotic pathway | Promotes tumor growth | Raimondo et al., 2015 |
| E-cadherin | Protein | Activating β-catenin and NF-kB signaling pathway | Angiogenesis | Tang et al., 2018 |
| miR-23a | miRNA | Targeted to ZO1 | Increases vascular permeability and cancer migration | Hsu et al., 2017 |
| miRNA-191, miR-21, miR-451a | miRNAs | / | Biomarkers for pancreatic cancer | Goto et al., 2018 |
| miR-17-5p, miR-92a-3p | miRNAs | / | Biomarkers for colon cancer | Fu et al., 2018 |
| Glypican-1 | Protein | / | Biomarkers for pancreatic cancer | Melo et al., 2015 |
| MET | Protein | Activating MET signaling | Priming premetastatic niches | Peinado et al., 2012 |
| miR-9 | miRNA | / | Increasing cancer growth | Baroni et al., 2016 |
| miR-21 | miRNA | Regulating PTEN/PI3K/AKT pathway | Inhibits apoptosis and increase drug resistance | Zheng et al., 2017 |
| miR-105, miR-181c, miR-200 | miRNAs | / | Increases metastasis | Le et al., 2014; Zhou et al., 2014; Tominaga et al., 2015 |
| ZFAS1 | lncRNA | Regulating MAPK signal and EMT | Increases cancer growth and metastasis | Pan et al., 2017 |
Extracellular vesicles are important carriers for intercellular communication, immunoregulation, and disease diagnosis and prognostic markers (Melo et al., 2015; Tang et al., 2018). Circulating EVs can be used as non-invasive biomarkers and liquid biopsies for early detection, diagnosis, and treatment of cancer (Kalluri, 2016; Hsu et al., 2017; Pan et al., 2017; Fu et al., 2018; Goto et al., 2018). For example, EVs derived from chronic myelogenous leukemia cells contain the cytokine TGFβ1, which binds to the TGFβ1 receptor on leukemia cells, thereby promoting tumor growth by activating ERK, Akt, and anti-apoptotic pathways (Raimondo et al., 2015). Many tumor types are dependent on mitochondrial metabolism by triggering adaptive mechanisms to optimize their oxidative phosphorylation with respect to substrate supply and energy demands. Exogenous EVs may induce metabolic reprogramming through the recovery of cancer cell respiration and the suppression of tumor growth (Tomasetti et al., 2017). Lugini et al. (2016) found that EVs from human colorectal cancer induce a tumor-like behavior in colonic mesenchymal stromal cells, which may be involved in cancer progression or interfere with cancer. Cossetti et al. (2014) discovered that somatic RNA is transferred to sperm cells, which can therefore act as the final recipients of somatic cell-derived information.
Exosomal miRs involved in the modulation of cancer metabolism may potentially optimize diagnosis and therapy (Le et al., 2014; Zhou et al., 2014; Tominaga et al., 2015; Baroni et al., 2016; Zheng et al., 2017). Moreover, tumor-derived exosomes (TDEs) are implicated in the formation and progression of different cancer processes, including tumor microenvironment (TME) remodeling, angiogenesis, metastasis, invasion, and drug resistance (Mashouri et al., 2019). In recent years, the study of EVs has become a new direction for further applied research. For glioblastoma patients, an invasive blood sample is used to diagnose and follow the response to therapy. The protein cargo of plasma GBM EVs can be used to detect a tumor, characterize its molecular profile, and tailor treatment (Osti et al., 2018). In addition, high levels of EVs in the plasma of melanoma patients represent a method for clinical management through the expression of CD63 and caveolin-1 (Logozzi et al., 2009). Rodríguez Zorrilla et al. (2019) also found CD63 expression induced by a dramatic reduction in plasmatic EV after surgical treatment in oral squamous cell carcinoma (OSCC) patients. Lower plasma EV levels correlated with a better life expectancy in OSCC patients (Rodríguez Zorrilla et al., 2019). Expression of both CD81 and PSA are high only in prostate cancer patients, and the levels of tumor biomarkers (e.g., PSA-EVs) may represent a prostate cancer diagnosis (Logozzi et al., 2017). Logozzi et al. (2019a) found that plasma EVs expressing PSA (Exo-PSA) could distinguish prostate cancer patients from healthy individuals both in specificity and sensitivity (Logozzi et al., 2019a).
Roles and Mechanisms of the Bone Microenvironment in Bone Metastasis
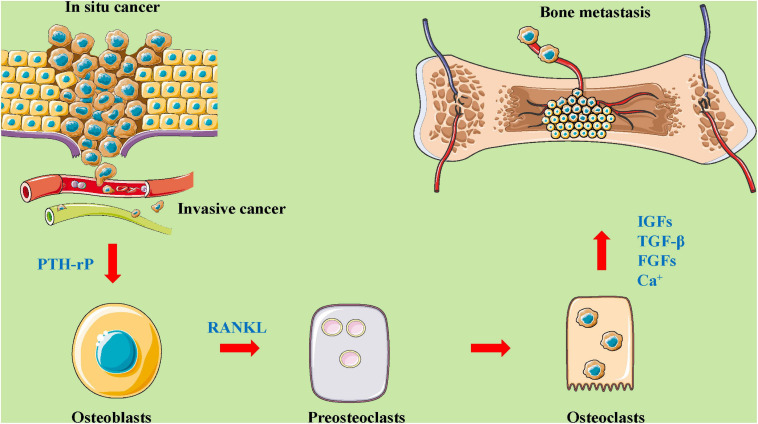
The tumor microenvironment plays an important role in tumor development by establishing interactions between host components and the tumor cells. Factors produced by tumor cells can alter the microenvironment at distant organs, generating pre-metastatic niches for subsequent metastasis. Pre-metastatic niche formation occurs as a sequence of events generated by the tumor cells that effectually prime the target site of disease for arrival, metastasis, and survival. Importantly, the existence of a pre-metastatic niche implies that metastasis to a particular organ is a predetermined event. Endothelial growth factor plays a critical role in the formation of the pre-metastatic niche. The bone microenvironment is rich with different cell types, including osteoblasts, osteoclasts, bone marrow stromal cells, immune cells, and vascular endothelial cells (Browne et al., 2014; Waning and Guise, 2014; Coleman et al., 2020). Osteoblasts and osteoclasts are key cell players in this microenvironment that induce osteolytic, osteogenic, or mixed bone metastasis (Esposito and Kang, 2014; Croucher et al., 2016; Coleman et al., 2020). However, bone marrow stromal cells exert inhibitory effects on bone metastasis through the integrins (αvβ3, α2β1, α4β1), TGFβ family members, bone resident proteins (BSP, OPG, SPARC, OPN), RANKL, and PTHrP (Lipton, 2006; Esposito and Kang, 2014). Moreover, immune surveillance, immune killing, the formation of pre-metastatic lesions, the cooperation of osteoclasts and immune cells, and the bone nutrient supply of vascular endothelial cells are important during bone metastasis (Guise et al., 2006; Quayle et al., 2015; Yao et al., 2020). During this metastatic process, tumor cells interact with cells in the bone microenvironment (Coleman et al., 2020; Mukaida et al., 2020; Figure 2).
FIGURE 2.
The roles and mechanisms of the bone microenvironment in tumor metastasis to bone. A complex and abnormal cycle of bone metastasis involving mutual interactions between tumor cells, bone cells (osteoclasts and osteoblasts), and the bone matrix. As shown in this figure, in situ cancer cells enter the blood vessels, causing proliferation, migration, and invasion. Then, these cells act on osteoblasts by regulating PTH-rP, which affects preosteoclasts and osteoclasts through the mediation of a related protein (such as RANKL). Osteoblasts and osteoclasts affect bone metastases by mediating the expression and secretion of factors (for example, IGFs, TGF-β, FGFs, CA2+). FGFs, fibroblast growth factors; IGFs, insulin-like growth factors; PTH-rP, parathyroid hormone-related protein; RANKL, receptor activator for nuclear factor-κB ligand; TGF-β, transforming growth factor-β.
Cancer acidity has a major role in inducing increased EV release by human cancer cells. Previous studies demonstrated that low pH was a sign of tumor malignancy that could influence EV release and uptake by human cancer cell lines of different histotypes, particularly prostate cancer (Parolini et al., 2009; Logozzi et al., 2018). Besides, the acquisition of a osteoblastic/osteolytic phenotype, such as CAIX, has been detected both in vitro, increased by the low pH condition, and in the plasma of patients, where a clear enzymatic activity, together with a reduced intraluminal pH of EVs was seen (Logozzi et al., 2019b). Tumor acidity and EV levels are strongly related and contribute to the malignant tumor. Buffering, alkalinization, or anti-acidic treatment reduces EV levels in cancer cells. Furthermore, plasma EV levels allow for an early diagnosis of the disease. Therapeutic strategies are being actively pursued to counteract the immunosuppressive and tumor-promoting activities of EVs (Logozzi et al., 2019c).
Autoregulation of Tumor Cells
The autoregulation of tumor cells is important for determining whether they maintain a latent, dormant state within the bone microenvironment long-term and is a key mechanism against autoimmunity and chemical drugs (Aguirre Ghiso, 2002; Sosa et al., 2014). When tumor cells encounter severe conditions (e.g., hypoxia, hypoglycemia, high acid environment), they may change the modification of proteins in the endoplasmic reticulum. In addition to inhibiting self-proliferation, tumor cells also control their quantity via autophagy (Aguirre Ghiso et al., 1999; Liu et al., 2002). The autophagy of tumor cells is mainly associated with the mammalian target of rapamycin (mTOR) (Malladi et al., 2016). In the case of cellular deficiency, mTORC1 kinase activity is reduced, which induces the autophagy of tumor cells, and they enter into a quiescent period. Tumor cells can also enter a Sox-dependent stem-like state and silence the Wnt signaling pathway, which are important for bone metastasis (Malladi et al., 2016). The autoregulation of tumor cells is a major mode of tumor cell cycle quiescence. When studying the influence of the bone microenvironment on metastatic factors, attention should be paid to the relationship between the bone microenvironment and the intrinsic factors of tumor cells.
Osteoblasts and Tumor Cells
The interaction between tumor cells and osteoblasts is mainly reflected in the promotion of tumor cell adhesion and colonization by osteoblasts. With continuous research on bone metastasis, more and more experiments have put forth the concept of “pre-metastatic lesions;” that is, after tumor cells reach the target organ, they become infiltrating tumor cells and enter a dormant period (i.e., no proliferation or activation in the short term, Ki67-negative) (Ren et al., 2015; Wang et al., 2018). However, in bone metastasis, the colonization of disseminated tumor cells (DTC) is closely associated with osteoblasts (Grudowska et al., 2017). In addition, Wang et al. (2015) showed that cell activation in early bone metastasis is associated with N–E cadherin and E–E cadherin on the cell membranes of osteoblasts. The binding of osteoblastic N-cadherin to N-cadherin on tumor cells activates the mTOR signaling pathway to enhance the activation and proliferation of dormant tumor cells. Moreover, osteoblasts are involved in the proliferation process following the activation of the quiescent tumor cells. Thus, osteoblasts participate in a “vicious cycle” between tumor cells and the bone microenvironment, whereby the destruction of the bone microenvironment and tumor cell proliferation promote each other by releasing vascular endothelial growth factor-(VEGF), matrix metalloproteinases (MMPs), thrombospondin, and inflammatory and coagulation factors (Hirshberg et al., 2014; Wang et al., 2015). Recent studies have shown that osteoblasts assist tumor cell colonization through receptors in the early stage of bone metastasis and mainly participate in the “vicious cycle” to promote tumor proliferation in the late stage of bone metastasis (Weilbaecher et al., 2011; Hirshberg et al., 2014).
Osteoclasts and Tumor Cells
Osteoclasts are an important promoting factor during bone metastasis. At the early stage of bone metastasis, vascular cell adhesion molecule-1 (VCAM1) and the integrin family are important molecules mediating the relationship between tumor cells and osteoclasts (Lu et al., 2011). High VCAM1 levels in tumor cells interact with osteoclast-expressed α4β1 integrin, which may recruit osteoclast precursor cells, initiate the osteoclast process, and ultimately induce the osteolytic clinical manifestations of bone metastasis (Lu et al., 2011). The “vicious cycle” in the bone microenvironment starts with the beginning of the osteoclast process. Current research has demonstrated that osteoclasts are important in bone metastasis and remodeling. As important effector cells, specific inhibition of the tumor-osteoclast process greatly improves the quality of life of patients with bone metastasis (Kelly et al., 2005). The biomarker PTHrP(12–48) can stimulate the expression of cleaved caspase 3 in OCLs and their precursors, causing apoptosis (Kamalakar et al., 2017). PCAT7 is a potential therapeutic target against bone metastasis of PCa via the TGF-β signaling pathway (Lang et al., 2020). Zoledronate enhances osteoclast differentiation through the IL-6/RANKL signal pathway in osteocyte-like MLO-Y4 cells (Kim et al., 2019).
Bone Marrow Stromal Cells and Tumor Cells
After tumor cells infiltrate the bone marrow, bone marrow stromal cells are important for tumor cell dormancy (Dormady et al., 2000). A recent study indicated that bone marrow stromal cells have an inhibitory effect on tumor cells. It is often necessary to overcome these inhibitory processes to activate dormant tumor cells (Kobayashi et al., 2011). This is also a key target for researchers to design anti-tumor cell drugs to inhibit tumor metastasis and growth.
Immune Cells and Tumor Cells
An important relationship has been observed between tumor cells as special antigens in vivo and autoimmune cells. After tumor cells reach the bone microenvironment, they promote the growth of CD56+CD8+ T cells and memory CD4+ T cells in the bone microenvironment (Feuerer et al., 2001). There is a significant association between CD8+ T cells and the incubation period of tumor metastasis as CD8+ T cells, as important cells for adaptive immunity, directly kill tumor cells. However, studies have shown that infiltrating dendritic plasma cells continuously release Th2 signals, inhibit CD8+ T cells, and promote the maturation of regulatory T cells and dormant tumor growth (Feuerer et al., 2001). A reduction in PDC slows down the activation process for bone metastasis. Depletion of CD8+ and CD4+ T cells induces tumors to come out of dormancy and apoptosis and induces an increase in tumor cell Ki67, which increases their proliferation and activation (Sawant et al., 2012). Immune cells also participate in the activation of tumor cell dormancy by changing the process of bone remodeling (Sawant et al., 2012). With the rise of tumor immunotherapy, more attention is being paid to bone metastasis and immune cells. However, most of the current bone metastasis animal models are immunodeficient. Researchers should pay attention to the relationship between immune cells and bone metastasis (Kianercy and Pienta, 2016; Wu et al., 2016).
Vascular Endothelial Cells and Tumor Cells
After tumor cells reach the bone microenvironment, neovascularization becomes an important source of the nutrient supply. Endothelial cells surrounding the neovascularization secrete TGF-β1 and periostin to induce the further proliferation of tumors (Mulcrone et al., 2017). VEGF-α is an important angiogenesis factor that plays an essential role in promoting the activation of tumor cell dormancy (Mulcrone et al., 2017). Nutrient supply is an important resource for cell growth. The study of nutrient supply channels is of great significance for not only bone metastasis but also for tumor metastasis in general and tumor growth in situ. In addition, it is essential to study vascular endothelial cells during bone metastasis.
Extracellular Vesicles and Bone Metastasis
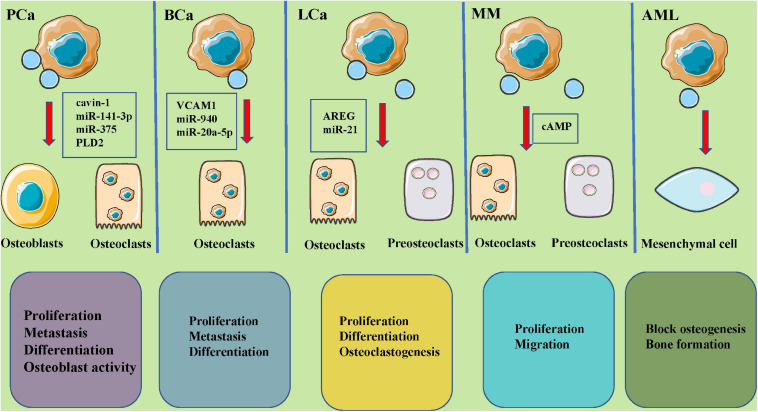
Recent studies have indicated that tumor-derived EVs play important roles in the microenvironment of bone tumors (Table 2 and Figure 3).
TABLE 2.
Studies reporting the roles of tumor-derived extracellular vesicles in the development and progression of bone metastases.
| Tumor type | References | Origin of Evs | Isolation of Evs | Characterization of Evs | Exosomal RNAs | How to find | Validation of RNAs | Target cells | Underlying functions |
| Prostate cancer | Inder et al., 2014 | Prostate cancer PC3 cells | Ultracentrifugation | / | Cavin-1/PTRF | / | Western blot | Osteoblast and osteoclast | Induce osteoblast proliferation, and osteoclast differentiation |
| Ye et al., 2017 | MDA PCa 2b cells | Ultracentrifugation | TEM, NAT, western blot (CD63, GM130) | miR-141-3p | / | RT-qPCR | Osteoblasts | Promote osteoblastic metastasis | |
| Li et al., 2019 | LNCap cells | Ultracentrifugation | TEM, NTA, western blot (CD9, HSP70, Alix) | miR-375 | Small RNA sequencing | RT-qPCR | Osteoblasts (hFOB1.19) | Promote osteoblasts differentiation | |
| Borel et al., 2020 | C4-2B cells | Ultracentrifugation | NTA, TEM, western blot (Alix, CD9, CLN3) | PLD2 | / | / | Osteoblasts | Enhance osteoblast activity | |
| Breast cancer | Kang, 2016 | Breast cancer cells | / | / | VCAM1 | Gene expression profiling | / | Osteoclasts | Mediate the recruitment of pre-osteoclasts and promote their differentiation to mature osteoclasts |
| Hashimoto et al., 2018 | MDA-MB-231 cell | Ultracentrifugation | NTA, TEM, western blot (CD9, TSG101) | miR-940 | Microarray analysis | RT-qPCR | / | Induces extensive osteoblastic lesions | |
| Guo et al., 2019 | MDA-MB-231 cell | Ultracentrifugation | TEM, western blot (CD63, TSG101) | miR-20a-5p/SRCIN1 | / | RT-qPCR | Osteoclasts | Enhances the proliferation and differentiation of osteoclasts | |
| Lung cancer | Taverna et al., 2017 | CRL-2868 cells | Ultracentrifugation | TEM, western blot (Alix, TSG101, CD63) | AREG | / | / | Osteoclasts | Enhance the proliferation and differentiation of osteoclasts |
| Xu et al., 2018 | A549 cells | Exosomes isolation kit | TEM, western blot (CD63, TSG101) | miR-21/PDCD4 | / | RT-qPCR | Preosteoclasts | Facilitate osteoclastogenesis | |
| Multiple myeloma | Raimondi et al., 2015 | Multiple myeloma cells | Ultracentrifugation, sucrose purification | DLS, western blot (Alix, CD63) | / | / | / | Preosteoclast | Modulate pre-osteoclast migration and pro-differentiative role |
| Garimella et al., 2014 | 143B cells | Differential ultracentrifugation | NTA, TEM | cAMP | / | / | Osteoclasts | Contain pro-osteoclastic cargo | |
| Acute myelocytic leukemia | Kumar et al., 2018 | AML cells | Ultracentrifugation | NTA, western blot (TSG101, CD63) | Rab27a | / | / | Mesenchymal | Block osteogenesis and bone formation |
| Melanoma | Mannavola et al., 2019 | Melanoma cells | Ultracentrifugation | NTA, TEM, western blot (CD81, TSG101, CANX), flow cytometry | CXCR4/CXCR7 | / | RT-qPCR | / | Reprogram the innate osteotropism of melanoma |
| Peinado et al., 2012 | Peripheral blood of melanoma subjects | Ultracentrifugation, sucrose purification | TEM, western blot (TYRP2, VLA-4, HSP70, HSP90 HSC70) | MET receptor | / | / | / | Induce vascular leakage at pre-metastatic lesions |
CAF, cancer-associated fibroblast; PAF, paracancer-associated fibroblast; AML, acute myeloid leukemia; TEM, transmission electron microscope; NTA, NanoSight NS300 particle size analyzer; DLS, dynamic light scattering.
FIGURE 3.
The roles of tumor-derived extracellular vesicles in bone metastases and potential molecular mechanisms. EVs, secreted by PCa (cavin-1, miR-142-3p, miR-375, PLD2), BCa (VCAM1, miR-940, miR-20a-5p), LCa (AREG, miR-21), MM (cAMP), and AML tumors could carry different and abundant content and play a key role in osteoclastic and osteoblastic cycling, leading to various metastatic lesions. PCa, prostate cancer; BCa, breast cancer; LCa, lung cancer; MM, multiple myeloma; AML, acute myelocytic leukemia; TGFB2, transforming growth factor beta 2; AXL, AXL receptor tyrosine kinase; PLD2, phospholipase D2; VCAM1, vascular cell adhesion molecule 1; AREG, amphiregulin.
Extracellular Vesicles and Prostatic Cancer Metastasis to Bone
Prostatic cancer is the most common malignant tumor and an important cause of death, with gradually higher incidences and poor efficacies (Archer et al., 2020). Current therapies for prostatic cancer bone metastases continue to focus on reducing symptoms and improving quality of life in these patients. Bone metastases can result in many complications, such as refractory bone pain, hypercalcemia, pathologic fractures, and spinal cord compression, and can even cause more serious complications, such as permanent paralysis (Boucher et al., 2020; Xue et al., 2020). Therefore, an effective treatment strategy to reduce the rate of bone metastases and the associated complications is urgently needed in clinical practice to improve patient survival rates and quality of life.
In recent years, EVs have been reported to be essential for the metastasis of prostatic cancer to bones. Inder et al. (2014) showed that PC3-EVs could induce osteoblast proliferation and osteoclast differentiation. These effects have been shown to be reduced by cavin-1 expression in PC3 cells, which is an important discovery since cavin-1 has also been demonstrated to be a tumor suppressor in caveolin-1-positive prostate cancer. However, a critical question remains, which concerns how cavin-1 expression selectively reduces EV levels in some molecules, including cargo, structural, and functional proteins, and miRNAs. Moreover, Ye et al. (2017) conducted a series of in vivo and in vitro studies and found that the MDA prostatic carcinoma (PCa) 2b cell-derived exosomal miR-141-3p could transfer from EVs to osteoblasts and promote osteoblastic metastasis by regulating exosomal miR-141-3p levels. These studies indicated that miRNAs could mediate cancer cell-to-osteoblast communication, which is important for the formation of bone metastases and osteogenic damage in PCa. Li et al. (2019) confirmed that high miR-375 expression was observed in LNCap-derived EVs that could preferentially reach osteoblasts and enhance osteoblast miR-375 levels. In addition, exosomal miR-375 might be associated with significantly higher osteoblast activity. Further investigations should be performed concerning the mechanisms underlying bone metastasis in PCa patients, and the molecular mechanisms underlying exosomal miR-375 involvement in the activation and differentiation of osteoblasts. Dai et al. (2019) confirmed that primary PCa cells could educate bone marrow to create a pre-metastatic niche via primary PCa EV-mediated transfer of PKM2 into bone marrow stem cells (BMSCs) with the subsequent upregulation of CXCL12. Furthermore, Borel et al. (2020) showed that phospholipase D2 (PLD2) stimulated EV secretion and enhanced osteoblast activity in PCa cell models. Therefore, PLD2 could be considered a potential player in establishing PCa bone metastases by acting through tumor cell-derived-EVs. EVs, released from prostate tumor cells, decreased DC-STAMP, TRAP, cathepsin K, and MMP-9 expression and thus decreased established markers for osteoclast fusion and differentiation. These findings suggest that tumor cell-derived microvesicles play an important role in cancer progression and disease aggressiveness (Terese et al., 2016).
Extracellular Vesicles and Breast Cancer Metastasis to Bone
Breast cancer is currently one of the most common malignant tumors in women worldwide, and the incidence of this disease has been rising in recent years. Breast cancer patients develop tumor metastasis during the advanced stages of disease. Bone tissue is one of the most common sites for metastases in patients with advanced breast cancer, and the metastatic ratio is much higher than that of the liver, lung, and kidney (Park et al., 2020; Zhang D. et al., 2020). Breast cancer metastasis to bones not only affects patient quality of life but also results in anemia, fractures, paraplegia, hypercalcemia, pain, cachexia, and increased mortality (Fico and Santamaria-Martinez, 2020). When breast tumors metastasize to bone, the balance between osteoblasts and osteoclasts is damaged. Osteoclasts are continuously activated, as manifested by higher osteoclast activity, resulting in osteolytic diseases with osteolysis and structural bone damage (Zhao Z. et al., 2020). Multiple bone growth factors are activated and released during bone resorption and remodeling, which provide an appropriate microenvironment for tumor cell growth, invasion, and metastasis (Zhao Z. et al., 2020). Once breast cancer cells have migrated to bone, the unique bone microenvironment could help to exchange biological information from the tumor cells to osteoblasts and osteoclasts, breaking the balance between osteolysis or osteogenesis during bone remodeling, which further results in fractures and pain and finally leads to death (Zhang D. et al., 2020; Pang et al., 2021). Although the tumors can be excised in clinical settings, tumor cells have often already spread and metastasized to bone, resulting in osteolytic lesions.
Previous studies have shown that EVs play key roles in breast cancer metastasis to bone. Lim et al. (2011) showed that the transfer of miRNAs from bone marrow stroma to breast cancer cells could involve the dormancy of bone marrow metastases. The results indicated that MDA-MB-231 and T47D breast cancer cells were arrested in the G0 phase of the cell cycle during co-culture with bone marrow stromal cells, and several miRNAs involved in cell proliferation were identified by analyzing miRNA expression profiles. These miRNAs included miR-127, miR-197, miR-222, and miR-223, that target CXCL12. Meanwhile, CXCL12-specific miRNAs were transported from bone marrow stroma to breast cancer cells via gap junctions, resulting in lower CXCL12 levels and reduced cell proliferation. Stroma-derived EVs containing miRNAs also contributed to breast cancer cell quiescence to a lesser degree than miRNAs transmitted via gap junctions. Kang (2016) showed that higher vascular cell adhesion molecule 1 (VCAM1) expression in disseminated breast tumor cells mediated the recruitment of pre-osteoclasts and promoted the differentiation of these cells to mature osteoclasts during bone metastasis formation. Bendinelli et al. (2017) clarified the control of the hepatocyte growth factor/mesenchymal-epithelial transition factor gene (HGF/Met) axis using DNA methylation and found that this axis was significantly involved in the supportive cell-metastatic cell nexus and metastatic outgrowths. This translational research focused on the effects of the microenvironment on breast cancer cell phenotypes and the formation of a pre-metastatic niche, and the colonization of these cells in bone. The results suggested the importance of targeting tumor microenvironments by blocking epigenetic mechanisms that control critical colonization events, such as the HGF/Met axis and the WW domain-containing oxidoreductase (Wwox) gene, and therapies targeting bone metastases. Hashimoto and his colleagues suggested that miRNAs secreted by cancer cells in the bone microenvironment induced bone metastasis phenotypes (Hashimoto et al., 2018). Interestingly, the implantation of miR-940-overexpressing MDA-MB-231 cells induced extensive osteoblastic lesions in the resultant tumors by facilitating osteogenic differentiation of host mesenchymal cells, even though MDA-MB-231 breast cancer cells have commonly been considered an osteolytic-inducing cancer cell line. Tiedemann et al. (2019) identified that extracellular L-plastin and peroxiredoxin 4 (PRDX4) played a specific role in stimulating osteoclastogenesis, thus promoting osteolysis during tumor metastasis to bone, which suggested that information regarding the expression of these proteins might be especially useful in the treatment of cancers that frequently metastasize to bone. Another study demonstrated that miR-20a-5p, transferred from breast cancer cell-derived EVs, enhanced the proliferation and differentiation of osteoclasts by targeting SRCIN1, thereby laying scientific foundations for the development of EV- or miR-20a-5p-targeted therapeutic interventions in patients with breast cancer progression (Guo et al., 2019). Breast cancer cell-derived EVs, associated with the formation of a pre-metastatic niche via the transfer of miR-21 and the regulation of PDCD4 protein levels, play an important role in promoting breast cancer bone metastasis (Yuan et al., 2021). Moreover, breast cancer cell (MDA-MB-231) EVs inhibited osteoblast differentiation and reduced cell numbers and activities by increasing osteoclast formation in a RANKL-independent manner. In osteoblasts, breast cancer cell EVs were shown to enhance transcription and increase angiogenesis and osteoclastogenesis (Loftus et al., 2020).
Extracellular Vesicles and Lung Cancer Metastasis to Bone
Lung cancer has currently one of the highest incidences of malignancy worldwide. Bone is a distant metastatic site for lung cancer, and the most common sites for lung cancer bone metastases include the spine, chest, and pelvis (Tam et al., 2020). The main routes of bone metastases occur through blood and direct local infiltration with rich blood supplies in the marrow cavity and higher adhesion molecule expression in malignant tumor cells, and a large number of growth factors in bone (Xu S. et al., 2020). After metastatic bone disease, patients develop a series of bone-related events, including pain, hypercalcemia, dyskinesia, spinal cord compression, and pathologic fractures, all of which severely affect patient quality of life (Huang et al., 2020). Taverna et al. (2017) observed that non-small-cell lung cancer (NSCLC) EVs that activated the amphiregulin (AREG)-induced epidermal growth factor (EGFR) pathway in pre-osteoclasts, in turn, caused higher RANKL expression in osteocytes. RANKL could also induce the expression of proteolytic enzymes involved in osteoclastogenesis and thereby trigger a vicious cycle in the process of osteolytic bone metastasis (Taverna et al., 2017). Xu et al. (2018) showed that lung adenocarcinoma-derived exosomal miR-21 showed promise as a facilitator of osteoclastogenesis and thus as a potential therapeutic target of bone metastasis. Understanding new advances in the diagnosis and treatment of lung cancer metastasis to bone holds great significance for prolonging survival and improving therapeutic effects in patients with lung cancer with metastasis to bone.
Extracellular Vesicles and Multiple Myeloma
Multiple myeloma (MM) is characterized as a proliferation of malignant clonal plasma cells that can infiltrate bone marrow and replace marrow cells. This malignancy also causes the mass production of abnormal immunoglobulins and destruction of bone, producing a series of clinical symptoms and signs that seriously threaten the quality of life and lifespan of middle-aged and elderly adults (Garces et al., 2020; Jeon et al., 2020). At present, the primary therapies include combined chemotherapies and stem cell transplants (Coffey et al., 2019). Since the proliferation ratio of tumor cells and formation of multiple resistant strains is low in MM, the therapeutic efficacy of clinical treatments remains less than ideal. Thus, finding a novel therapeutic target is urgently needed.
There are many factors that cause MM bone disease, high osteoclastic activity is the main reason, although many other factors are involved (Abdi et al., 2013; Rossi et al., 2013; Hameed et al., 2014). The proliferation of MM is markedly related to the increase in the number and activity of osteoclasts. This relationship maintains a balance between the abnormal cycle of bone destruction and tumor cell survival. A report by Raimondi et al. (2015) stated that EVs derived from MM were also able to regulate the migration and differentiation of osteoclasts. Additionally, MM-derived EVs have been shown to have two primary functions. One, it can promote the appearance of osteoclasts, and two, it can help improve bone resorption of mature osteoclast-like cells. Moreover, Garimella et al. (2014) reported that highly invasive and metastatic osteosarcoma 143B cells could produce EVs by using ionomycin and forskolin to actively mobilize intracellular calcium or increase cyclic adenosine monophosphate (cAMP) levels. Raimondo et al. (2019) reported that AREG was specifically enriched in EVs from MM cells and that EV-derived AREG participated in MM-induced osteoclastogenesis by activating EGFR ligands in pre-osteoclasts. The above studies have proven that EVs are the main factors of communication between MM cells and the bone marrow microenvironment. More importantly, the above conclusions support the role of tumor cell-derived microvesicles in disease aggressiveness and cancer progression.
Extracellular Vesicles and Acute Myelocytic Leukemia
Acute myelocytic leukemia (AML) is a heterogeneous clonal hematopoietic stem cell disease mainly characterized by the severe blockage of myeloid cell differentiation, rapid clonal cell proliferation, and the infiltration of other organs by immature myeloid bone marrow cells (Lu et al., 2017; Zhou et al., 2020). The incidence of AML increases with age. AML is considered the most common acute leukemia in adults, with a low incidence in children, only accounting for 15%∼20% of childhood leukemias (Zhou et al., 2020). Recently, Kumar et al. (2018) analyzed a new AML mouse model and demonstrated the niche transformation model. The study results indicated that the hematopoietic microenvironment could be altered with the help of EV transfer (through the Dickkopf WNT signaling pathway inhibitor 1 (DKK1) gene). It was also found that normal niches could be turned into malignant niches. In addition, mesenchymal progenitor cells increased, and osteogenesis and bone formation were blocked after injecting AML-derived EVs into mice. The proliferation rate of AML cells was also shown to be accelerated by the AML-derived EVs. However, AML-derived EVs could be destroyed by Rab27a targeting, which provided inhibitory effects that could significantly delay the appearance of leukemia (Kumar et al., 2018). In addition, DKK1 was found to belong to a normal hematopoietic and osteogenic inhibitory factor. Therefore, related reports stated that bone cell loss was due to the stimulating effects of AML-derived EVs on DKK1 (Kumar et al., 2018). Thus, targeting EVs could represent a new strategy for cancer treatments. The effective suppression of the hematopoietic microenvironment might be a crucial new direction for the control of malignant blood cell proliferation.
Extracellular Vesicles and Melanoma Metastasis to Bone
Malignant melanoma (Mm) is a highly malignant melanocyte tumor that accounts for 90% of skin malignancies causing death (Broggini et al., 2020; Mannavola et al., 2020). Mm is formed from the malignant transformation of melanocytes located at the epidermal basement membrane. This tumor is more common on the face and heels and primarily progresses from the malignant transformation of nevi or pigmented spots (Wilson et al., 2020). Mm is highly malignant and vulnerable to lymphatic and blood tract metastases in the early stages. Mm often metastasizes to the liver, lung, kidney, and brain, and a few reports have demonstrated metastasis to the gastrointestinal tract and ovaries. However, metastases to bone marrow have only rarely been reported (Wilson et al., 2020). Mannavola et al. (2019) demonstrated that tumor-derived EVs reprogrammed the innate osteotropism of melanoma cells in vitro by upregulating membrane CXCR7. These results provide the possible identification of targets for future drug development to skeletal metastases of malignant melanoma (Mannavola et al., 2019). Peinado et al. (2012) indicated that EVs from highly metastatic melanoma increased the metastatic behaviors of primary tumors by permanently “educating” bone marrow progenitors via the MET receptor. Melanoma-derived EVs also induced vascular leakage in pre-metastatic lesions and reprogrammed bone marrow progenitors toward a c-Kit+Tie2+MET+ pro-vasculogenic phenotype (Peinado et al., 2012). Lower MET expression in EVs weakened the pro-metastatic behaviors of bone marrow cells (Peinado et al., 2012). All of these studies implied that melanoma-derived EVs could facilitate bone metastases using some factors, such as MET.
Conclusion and Outlook
Bone metastasis, as the major complication in patients with advanced tumors, characterized by osteolytic lesion, often occurring in chest bones. Since metastasized cancer cells can damage bone marrow hematopoiesis and bone structure, bone metastasis becomes the major symptom in advanced diseases, as well as the leading cause of death (Coleman et al., 2020). The destruction of the hemopoietic system by tumor cells mainly results in anemia and an increased infection tendency. Excessive bone growth causes local pain, fracture, and spinal cord compression; even paraplegia can be seen with severe bone damage and not only accelerates the death of patients but also seriously reduces quality of life (Mukaida et al., 2020). The discovery of novel biomarkers and targets to control metastatic bone disease is urgently needed. EVs have been reported to be biomarkers for cancer diagnoses and targets for novel therapies. EVs, as carriers of protein, RNAs, and other bioactive molecules, serve as a tool for cell-to-cell communications and could be effective in regulating the signaling pathways or biological behaviors of recipient cells. This review summarizes the roles of tumor-derived EVs in bone metastasis and concludes that proteins and RNAs in EVs derived from tumor cells could enhance tumor invasion and metastasis and might be useful as targets for cancer therapy and the inhibition of bone metastases.
Although research has proven a vital role for EVs in tumor metastasis to bones, many problems still need to be investigated in future studies. These include the roles of tumor-derived EV compositions (including RNA and DNA) in determining bone-specific metastases, whether tumor metastasis could be prevented by inhibiting the secretion of tumor-derived EVs, especially in bone metastases and other organs that damage bodily functions, and if clinical transformations could be carried out using targeted therapies for tumor metastasis to bone based on discovered molecular mechanisms. Solving these issues will help highlight the underlying mechanisms of bone metastases. Future therapeutic strategies could involve a combination of several drugs that might block multiple targets or pathways simultaneously to improve quality of life, prolong survival, and provide greater therapeutic benefits for patients with bone metastases.
Author Contributions
SL and WW contributed to original draft preparation, allocation, revision, supplement, and edition. Both authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the generous support of the Liaoning Cancer Hospital & Institute (Shenyang) and China Medical University (Shenyang). We also thank Chaonan Sun for her numerous constructive comments in the process of writing this manuscript.
Abbreviations
- EV
extracellular vesicle
- miRNAs
micro RNAs
- circRNAs
circular RNAs
- lncRNAs
long non-coding RNAs
- FGFs
fibroblast growth factors
- IGFs
insulin-like growth factors
- PTH-rP
parathyroid hormone-related protein
- RANKL
receptor activator for nuclear factor- κ B ligand
- TGF- β
transforming growth factor- β
- DTC
disseminated tumor cells
- PCa
prostate cancer
- BCa
breast cancer
- LCa
lung cancer
- MM
multiple myeloma
- AML
acute myelocytic leukemia
- PLD2
phospholipase D2
- VCAM1
vascular cell adhesion molecule 1
- AREG
amphiregulin
- cAMP
adenosine monophosphate
- EGFR
epidermal growth factor receptor ligands
- MVB
multivesicular body
- TSG101
Wilms tumor type 101 protein
- Alix
ALG-2 interacting protein X
- TDEs
tumor-derived exosomes
- TME
tumor microenvironment
- mTOR
mammalian target of rapamycin.
Footnotes
Funding. This work was supported by the Natural Science Foundation of Liaoning Province (2020-MS-058) and the Shenyang Young and Middle-Aged Scientific and Technological Innovation Talent Support Plan (RC190456).
References
- Abdi J., Chen G., Chang H. (2013). Drug resistance in multiple myeloma: latest findings and new concepts on molecular mechanisms. Oncotarget 4 2186–2207. 10.18632/oncotarget.1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre Ghiso J. A. (2002). Inhibition of FAK signaling activated by urokinase receptor induces dormancy in human carcinoma cells in vivo. Oncogene 21 2513–2524. 10.1038/sj.onc.1205342 [DOI] [PubMed] [Google Scholar]
- Aguirre Ghiso J. A., Kovalski K., Ossowski L. (1999). Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J. Cell Biol. 147 89–104. 10.1083/jcb.147.1.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer M., Dogra N., Kyprianou N. (2020). Inflammation as a driver of prostate cancer metastasis and therapeutic resistance. Cancers (Basel) 12:2984. 10.3390/cancers12102984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroni S., Romero-Cordoba S., Plantamura I., Dugo M., D’Ippolito E., Cataldo A., et al. (2016). Exosome-mediated delivery of miR-9 induces cancer-associated fibroblast-like properties in human breast fibroblasts. Cell Death Dis. 7:e2312. 10.1038/cddis.2016.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendinelli P., Maroni P., Matteucci E., Desiderio M. A. (2017). Epigenetic regulation of HGF/Met receptor axis is critical for the outgrowth of bone metastasis from breast carcinoma. Cell Death Dis. 8:e2578. 10.1038/cddis.2016.403 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Borel M., Lollo G., Magne D., Buchet R., Brizuela L., Mebarek S. (2020). Prostate cancer-derived exosomes promote osteoblast differentiation and activity through phospholipase D2. Biochim. Biophys. Acta Mol. Basis Dis. 1866:165919. 10.1016/j.bbadis.2020.165919 [DOI] [PubMed] [Google Scholar]
- Boucher J., Balandre A. C., Debant M., Vix J., Harnois T., Bourmeyster N., et al. (2020). Cx43 present at the leading edge membrane governs promigratory effects of osteoblast-conditioned medium on human prostate cancer cells in the context of bone metastasis. Cancers (Basel) 12:3013. 10.3390/cancers12103013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broggini T., Piffko A., Hoffmann C. J., Ghori A., Harms C., Adams R. H., et al. (2020). Ephrin-B2-EphB4 communication mediates tumor-endothelial cell interactions during hematogenous spread to spinal bone in a melanoma metastasis model. Oncogene 39 7063–7075. 10.1038/s41388-020-01473-y [DOI] [PubMed] [Google Scholar]
- Browne G., Taipaleenmaki H., Stein G. S., Stein J. L., Lian J. B. (2014). MicroRNAs in the control of metastatic bone disease. Trends Endocrinol. Metab. 25 320–327. 10.1016/j.tem.2014.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., Meng J., Zhu L., Peng Y. (2020). Exosomal noncoding RNAs in Glioma: biological functions and potential clinical applications. Mol. Cancer 19:66. 10.1186/s12943-020-01189-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey D. G., Wu Q. V., Towlerton A. M. H., Ornelas S., Morales A. J., Xu Y., et al. (2019). Ultradeep, targeted sequencing reveals distinct mutations in blood compared to matched bone marrow among patients with multiple myeloma. Blood Cancer J. 9:77. 10.1038/s41408-019-0238-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R. E., Croucher P. I., Padhani A. R., Clezardin P., Chow E., Fallon M., et al. (2020). Bone metastases. Nat. Rev. Dis. Primers 6:83. 10.1038/s41572-020-00216-3 [DOI] [PubMed] [Google Scholar]
- Colletti M., Tomao L., Galardi A., Paolini A., Di Paolo V., De Stefanis C., et al. (2020). Neuroblastoma-secreted exosomes carrying miR-375 promote osteogenic differentiation of bone-marrow mesenchymal stromal cells. J. Extracell. Vesicles 9:1774144. 10.1080/20013078.2020.1774144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossetti C., Lugini L., Astrologo L., Saggio I., Fais S., Spadafora C. (2014). Soma-to-germline transmission of RNA in mice xenografted with human tumour cells: possible transport by exosomes. PLoS One 9:e101629. 10.1371/journal.pone.0101629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croucher P. I., McDonald M. M., Martin T. J. (2016). Bone metastasis: the importance of the neighbourhood. Nat. Rev. Cancer 16 373–386. 10.1038/nrc.2016.44 [DOI] [PubMed] [Google Scholar]
- Dai J., Escara-Wilke J., Keller J. M., Jung Y., Taichman R. S., Pienta K. J., et al. (2019). Primary prostate cancer educates bone stroma through exosomal pyruvate kinase M2 to promote bone metastasis. J. Exp. Med. 216 2883–2899. 10.1084/jem.20190158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J., Su Y., Zhong S., Cong L., Liu B., Yang J., et al. (2020). Exosomes: key players in cancer and potential therapeutic strategy. Signal. Transduct. Target Ther. 5:145. 10.1038/s41392-020-00261-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormady S. P., Zhang X. M., Basch R. S. (2000). Hematopoietic progenitor cells grow on 3T3 fibroblast monolayers that overexpress growth arrest-specific gene-6 (GAS6). Proc. Natl. Acad. Sci. U.S.A. 97 12260–12265. 10.1073/pnas.97.22.12260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M., Kang Y. (2014). Targeting tumor-stromal interactions in bone metastasis. Pharmacol. Ther. 141 222–233. 10.1016/j.pharmthera.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerer M., Rocha M., Bai L., Umansky V., Solomayer E. F., Bastert G., et al. (2001). Enrichment of memory T cells and other profound immunological changes in the bone marrow from untreated breast cancer patients. Int. J. Cancer 92 96–105. [PubMed] [Google Scholar]
- Fico F., Santamaria-Martinez A. (2020). TGFBI modulates tumour hypoxia and promotes breast cancer metastasis. Mol. Oncol. 14 3198–3210. 10.1002/1878-0261.12828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu F., Jiang W., Zhou L., Chen Z. (2018). Circulating exosomal miR-17-5p and miR-92a-3p predict pathologic stage and grade of colorectal cancer. Transl Oncol 11 221–232. 10.1016/j.tranon.2017.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garces J. J., Simicek M., Vicari M., Brozova L., Burgos L., Bezdekova R., et al. (2020). Transcriptional profiling of circulating tumor cells in multiple myeloma: a new model to understand disease dissemination. Leukemia 34 589–603. 10.1038/s41375-019-0588-4 [DOI] [PubMed] [Google Scholar]
- Garimella R., Washington L., Isaacson J., Vallejo J., Spence M., Tawfik O., et al. (2014). Extracellular membrane vesicles derived from 143B osteosarcoma cells contain pro-osteoclastogenic cargo: a novel communication mechanism in osteosarcoma bone microenvironment. Transl. Oncol. 7 331–340. 10.1016/j.tranon.2014.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T., Fujiya M., Konishi H., Sasajima J., Fujibayashi S., Hayashi A., et al. (2018). An elevated expression of serum exosomal microRNA-191, – 21, -451a of pancreatic neoplasm is considered to be efficient diagnostic marker. BMC Cancer 18:116. 10.1186/s12885-018-4006-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grudowska A., Czaplinska D., Polom W., Matuszewski M., Sadej R., Skladanowski A. C. (2017). Tetraspanin CD151 mediates communication between PC3 prostate cancer cells and osteoblasts. Acta Biochim. Pol. 64 135–141. 10.18388/abp.2016_1356 [DOI] [PubMed] [Google Scholar]
- Guise T. A., Mohammad K. S., Clines G., Stebbins E. G., Wong D. H., Higgins L. S., et al. (2006). Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin. Cancer Res. 12(Pt 2) 6213s–6216s. 10.1158/1078-0432.CCR-06-1007 [DOI] [PubMed] [Google Scholar]
- Guo L., Zhu Y., Li L., Zhou S., Yin G., Yu G., et al. (2019). Breast cancer cell-derived exosomal miR-20a-5p promotes the proliferation and differentiation of osteoclasts by targeting SRCIN1. Cancer Med. 8 5687–5701. 10.1002/cam4.2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameed A., Brady J. J., Dowling P., Clynes M., O’Gorman P. (2014). Bone disease in multiple myeloma: pathophysiology and management. Cancer Growth Metastasis 7 33–42. 10.4137/CGM.S16817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K., Ochi H., Sunamura S., Kosaka N., Mabuchi Y., Fukuda T., et al. (2018). Cancer-secreted hsa-miR-940 induces an osteoblastic phenotype in the bone metastatic microenvironment via targeting ARHGAP1 and FAM134A. Proc. Natl. Acad. Sci. U.S.A. 115 2204–2209. 10.1073/pnas.1717363115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga T. (2019). Bone metastasis: Interaction between cancer cells and bone microenvironment. J. Oral Biosci. 61 95–98. 10.1016/j.job.2019.02.002 [DOI] [PubMed] [Google Scholar]
- Hirshberg A., Berger R., Allon I., Kaplan I. (2014). Metastatic tumors to the jaws and mouth. Head Neck Pathol. 8 463–474. 10.1007/s12105-014-0591-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y. L., Hung J. Y., Chang W. A., Lin Y. S., Pan Y. C., Tsai P. H., et al. (2017). Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene 36 4929–4942. 10.1038/onc.2017.105 [DOI] [PubMed] [Google Scholar]
- Huang L., Jiang X. L., Liang H. B., Li J. C., Chin L. H., Wei J. P., et al. (2020). Genetic profiling of primary and secondary tumors from patients with lung adenocarcinoma and bone metastases reveals targeted therapy options. Mol. Med. 26:88. 10.1186/s10020-020-00197-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyan T., Li H., Peng H., Chen J., Yang R., Zhang W., et al. (2020). Extracellular vesicles – advanced nanocarriers in cancer therapy: progress and achievements. Int. J. Nanomedicine 15 6485–6502. 10.2147/IJN.S238099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inder K. L., Ruelcke J. E., Petelin L., Moon H., Choi E., Rae J., et al. (2014). Cavin-1/PTRF alters prostate cancer cell-derived extracellular vesicle content and internalization to attenuate extracellular vesicle-mediated osteoclastogenesis and osteoblast proliferation. J Extracell Vesicles 3:23784. 10.3402/jev.v3.23784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon H. L., Oh I. S., Baek Y. H., Yang H., Park J., Hong S., et al. (2020). Zoledronic acid and skeletal-related events in patients with bone metastatic cancer or multiple myeloma. J. Bone Miner. Metab. 38 254–263. 10.1007/s00774-019-01052-6 [DOI] [PubMed] [Google Scholar]
- Kalluri R. (2016). The biology and function of exosomes in cancer. J. Clin. Invest. 126 1208–1215. 10.1172/JCI81135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R., LeBleu V. S. (2020). The biology, function, and biomedical applications of exosomes. Science 367:eaau6977. 10.1126/science.aau6977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamalakar A., Washam C. L., Akel N. S., Allen B. J., Williams D. K., Swain F. L., et al. (2017). PTHrP(12-48) modulates the bone marrow microenvironment and suppresses human osteoclast differentiation and lifespan. J. Bone Miner. Res. 32 1421–1431. 10.1002/jbmr.3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y. (2016). Dissecting tumor-stromal interactions in breast cancer bone metastasis. Endocrinol. Metab. (Seoul) 31 206–212. 10.3803/EnM.2016.31.2.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayazi Atici O., Govindrajan N., Lopetegui-Gonzalez I., Shemanko C. S. (2020). Prolactin: a hormone with diverse functions from mammary gland development to cancer metastasis. Semin. Cell Dev. Biol. 10.1016/j.semcdb.2020.10.005 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Kelly T., Suva L. J., Huang Y., Macleod V., Miao H. Q., Walker R. C., et al. (2005). Expression of heparanase by primary breast tumors promotes bone resorption in the absence of detectable bone metastases. Cancer Res. 65 5778–5784. 10.1158/0008-5472.CAN-05-0749 [DOI] [PubMed] [Google Scholar]
- Kianercy A., Pienta K. J. (2016). Positive feedback loops between inflammatory, bone and cancer cells during metastatic niche construction. Adv. Exp. Med. Biol. 936 137–148. 10.1007/978-3-319-42023-3_7 [DOI] [PubMed] [Google Scholar]
- Kim H. J., Kim H. J., Choi Y., Bae M. K., Hwang D. S., Shin S. H., et al. (2019). Zoledronate enhances osteocyte-mediated osteoclast differentiation by IL-6/RANKL Axis. Int. J. Mol. Sci. 20:1467. 10.3390/ijms20061467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A., Okuda H., Xing F., Pandey P. R., Watabe M., Hirota S., et al. (2011). Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J. Exp. Med. 208 2641–2655. 10.1084/jem.20110840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzeszinski J. Y., Wan Y. (2015). New therapeutic targets for cancer bone metastasis. Trends Pharmacol. Sci. 36 360–373. 10.1016/j.tips.2015.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B., Garcia M., Weng L., Jung X., Murakami J. L., Hu X., et al. (2018). Acute myeloid leukemia transforms the bone marrow niche into a leukemia-permissive microenvironment through exosome secretion. Leukemia 32 575–587. 10.1038/leu.2017.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang C., Dai Y., Wu Z., Yang Q., He S., Zhang X., et al. (2020). SMAD3/SP1 complex-mediated constitutive active loop between lncRNA PCAT7 and TGF-β signaling promotes prostate cancer bone metastasis. Mol. Oncol. 14 808–828. 10.1002/1878-0261.12634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laranga R., Duchi S., Ibrahim T., Guerrieri A. N., Donati D. M., Lucarelli E. (2020). Trends in bone metastasis modeling. Cancers (Basel) 12:2315. 10.3390/cancers12082315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le M. T., Hamar P., Guo C., Basar E., Perdigao-Henriques R., Balaj L., et al. (2014). miR-200-containing extracellular vesicles promote breast cancer cell metastasis. J. Clin. Invest. 124 5109–5128. 10.1172/JCI75695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBleu V. S., Kalluri R. (2020). Exosomes as a multicomponent biomarker platform in cancer. Trends Cancer 6 767–774. 10.1016/j.trecan.2020.03.007 [DOI] [PubMed] [Google Scholar]
- Li M., Li S., Du C., Zhang Y., Li Y., Chu L., et al. (2020). Exosomes from different cells: Characteristics, modifications, and therapeutic applications. Eur. J. Med. Chem. 207:112784. 10.1016/j.ejmech.2020.112784 [DOI] [PubMed] [Google Scholar]
- Li S. L., An N., Liu B., Wang S. Y., Wang J. J., Ye Y. (2019). Exosomes from LNCaP cells promote osteoblast activity through miR-375 transfer. Oncol. Lett. 17 4463–4473. 10.3892/ol.2019.10110 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lim P. K., Bliss S. A., Patel S. A., Taborga M., Dave M. A., Gregory L. A., et al. (2011). Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 71 1550–1560. 10.1158/0008-5472.CAN-10-2372 [DOI] [PubMed] [Google Scholar]
- Lipton A. (2006). Future treatment of bone metastases. Clin. Cancer Res. 12(Pt 2) 6305s–6308s. 10.1158/1078-0432.CCR-06-1157 [DOI] [PubMed] [Google Scholar]
- Liu D., Aguirre Ghiso J., Estrada Y., Ossowski L. (2002). EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Cancer Cell 1 445–457. 10.1016/s1535-6108(02)00072-7 [DOI] [PubMed] [Google Scholar]
- Liu Z., Wang L., Xu H., Du Q., Li L., Wang L., et al. (2020). Heterogeneous responses to mechanical force of prostate cancer cells inducing different metastasis patterns. Adv. Sci. (Weinh) 7:1903583. 10.1002/advs.201903583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus A., Cappariello A., George C., Ucci A., Shefferd K., Green A., et al. (2020). Extracellular vesicles from osteotropic breast cancer cells affect bone resident cells. J. Bone Miner. Res. 35 396–412. 10.1002/jbmr.3891 [DOI] [PubMed] [Google Scholar]
- Logozzi M., Angelini D. F., Giuliani A., Mizzoni D., Di Raimo R., Maggi M., et al. (2019a). Increased plasmatic levels of PSA-expressing exosomes distinguish prostate cancer patients from benign prostatic hyperplasia: a prospective study. Cancers 11:1449. 10.3390/cancers11101449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logozzi M., Angelini D. F., Iessi E., Mizzoni D., Fais S. (2017). Increased psa expression on prostate cancer exosomes in in vitro condition and in cancer patients. Cancer Lett. 403 318–329. 10.1016/j.canlet.2017.06.036 [DOI] [PubMed] [Google Scholar]
- Logozzi M., Capasso C., Di Raimo R., Del Prete S., Mizzoni D., Falchi M., et al. (2019b). Prostate cancer cells and exosomes in acidic condition show increased carbonic anhydrase IX expression and activity. J. Enzyme Inhibit. Med. Chem. 34 272–278. 10.1080/14756366.2018.1538980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logozzi M., Milito A. D., Lugini L., Borghi M., Calabrò L., Spada M., et al. (2009). High levels of exosomes expressing cd63 and caveolin-1 in plasma of melanoma patients. PLoS One 4:e5219. 10.1371/journal.pone.0005219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logozzi M., Mizzoni D., Angelini D. F., Di Raimo R., Falchi M., Battistini L., et al. (2018). Microenvironmental pH and exosome levels interplay in human cancer cell lines of different histotypes. Cancers(Basel) 10:370. 10.3390/cancers10100370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logozzi M., Spugnini E., Mizzoni D., Di Raimo R., Fais S. (2019c). Extracellular acidity and increased exosome release as key phenotypes of malignant tumors. Cancer Metastasis Rev. 38 93–101. 10.1007/s10555-019-09783-8 [DOI] [PubMed] [Google Scholar]
- Lu J. W., Hsieh M. S., Hou H. A., Chen C. Y., Tien H. F., Lin L. I. (2017). Overexpression of SOX4 correlates with poor prognosis of acute myeloid leukemia and is leukemogenic in zebrafish. Blood Cancer J. 7:e593. 10.1038/bcj.2017.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Mu E., Wei Y., Riethdorf S., Yang Q., Yuan M., et al. (2011). VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer Cell 20 701–714. 10.1016/j.ccr.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugini L., Valtieri M., Federici C., Cecchetti S., Meschini S., Condello M., et al. (2016). Exosomes from human colorectal cancer induce a tumor-like behavior in colonic mesenchymal stromal cells. Oncotarget 7 50086–50098. 10.18632/oncotarget.10574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R. Y., Zhang H., Li X. F., Zhang C. B., Selli C., Tagliavini G., et al. (2020). Monocyte-derived macrophages promote breast cancer bone metastasis outgrowth. J. Exp. Med. 217:e20191820. 10.1084/jem.20191820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malladi S., Macalinao D. G., Jin X., He L., Basnet H., Zou Y., et al. (2016). Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell 165 45–60. 10.1016/j.cell.2016.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannavola F., Mandala M., Todisco A., Sileni V. C., Palla M., Minisini A. M., et al. (2020). An italian retrospective survey on bone metastasis in melanoma: impact of immunotherapy and radiotherapy on survival. Front. Oncol. 10:1652. 10.3389/fonc.2020.01652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannavola F., Tucci M., Felici C., Passarelli A., D’Oronzo S., Silvestris F. (2019). Tumor-derived exosomes promote the in vitro osteotropism of melanoma cells by activating the SDF-1/CXCR4/CXCR7 axis. J. Transl. Med. 17:230. 10.1186/s12967-019-1982-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazzi F., Orlandi A., Manfrida S., Masiello V., Di Leone A., Massaccesi M., et al. (2020). Diagnosis and treatment of bone metastases in breast cancer: radiotherapy, local approach and systemic therapy in a guide for clinicians. Cancers (Basel) 12:2390. 10.3390/cancers12092390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashouri L., Yousefi H., Aref A. R., Ahadi A. M., Molaei F., Alahari S. K. (2019). Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer 18:75. 10.1186/s12943-019-0991-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew M., Zade M., Mezghani N., Patel R., Wang Y., Momen-Heravi F. (2020). Extracellular vesicles as biomarkers in cancer immunotherapy. Cancers (Basel) 12:2825. 10.3390/cancers12102825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu M., Martin-Jaular L., Lavieu G., Thery C. (2019). Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 21 9–17. 10.1038/s41556-018-0250-9 [DOI] [PubMed] [Google Scholar]
- Melo S. A., Luecke L. B., Kahlert C., Fernandez A. F., Gammon S. T., Kaye J., et al. (2015). Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 523 177–182. 10.1038/nature14581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaida N., Zhang D., Sasaki S. I. (2020). Emergence of cancer-associated fibroblasts as an indispensable cellular player in bone metastasis process. Cancers (Basel) 12:2896. 10.3390/cancers12102896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcrone P. L., Campbell J. P., Clement-Demange L., Anbinder A. L., Merkel A. R., Brekken R. A., et al. (2017). Skeletal colonization by breast cancer cells is stimulated by an osteoblast and beta2AR-dependent neo-angiogenic switch. J. Bone Miner. Res. 32 1442–1454. 10.1002/jbmr.3133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseri M., Bozorgmehr M., Zoller M., Ranaei Pirmardan E., Madjd Z. (2020). Tumor-derived exosomes: the next generation of promising cell-free vaccines in cancer immunotherapy. Oncoimmunology 9:1779991. 10.1080/2162402X.2020.1779991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osti D., Del Bene M., Rappa G., Santos M., Matafora V., Richichi C., et al. (2018). Clinical significance of extracellular vesicles in plasma from glioblastoma patients. Clin. Cancer Res. 25 266–276. 10.1158/1078-0432.CCR-18-1941 [DOI] [PubMed] [Google Scholar]
- Pan L., Liang W., Fu M., Huang Z. H., Li X., Zhang W., et al. (2017). Exosomes-mediated transfer of long noncoding RNA ZFAS1 promotes gastric cancer progression. J. Cancer Res. Clin. Oncol. 143 991–1004. 10.1007/s00432-017-2361-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y., Su L., Fu Y., Jia F., Zhang C., Cao X., et al. (2021). Inhibition of furin by bone targeting superparamagnetic iron oxide nanoparticles alleviated breast cancer bone metastasis. Bioact. Mater. 6 712–720. 10.1016/j.bioactmat.2020.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. B., Hwang K. T., Chung C. K., Roy D., Yoo C. (2020). Causal Bayesian gene networks associated with bone, brain and lung metastasis of breast cancer. Clin. Exp. Metastasis 37 657–674. 10.1007/s10585-020-10060-0 [DOI] [PubMed] [Google Scholar]
- Parolini I., Federici C., Raggi C., Lugini L., Palleschi S., De Milito A., et al. (2009). Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 284 34211–34222. 10.1074/jbc.M109.041152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegtel D. M., Gould S. J. (2019). Exosomes. Annu. Rev. Biochem. 88 487–514. 10.1146/annurev-biochem-013118-111902 [DOI] [PubMed] [Google Scholar]
- Peinado H., Aleckovic M., Lavotshkin S., Matei I., Costa-Silva B., Moreno-Bueno G., et al. (2012). Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 18 883–891. 10.1038/nm.2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quayle L., Ottewell P. D., Holen I. (2015). Bone metastasis: molecular mechanisms implicated in tumour cell dormancy in breast and prostate cancer. Curr. Cancer Drug Targets 15 469–480. 10.2174/1568009615666150506092443 [DOI] [PubMed] [Google Scholar]
- Raimondi L., De Luca A., Amodio N., Manno M., Raccosta S., Taverna S., et al. (2015). Involvement of multiple myeloma cell-derived exosomes in osteoclast differentiation. Oncotarget 6 13772–13789. 10.18632/oncotarget.3830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondo S., Saieva L., Corrado C., Fontana S., Flugy A., Rizzo A., et al. (2015). Chronic myeloid leukemia-derived exosomes promote tumor growth through an autocrine mechanism. Cell Commun. Signal. 13:8. 10.1186/s12964-015-0086-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondo S., Saieva L., Vicario E., Pucci M., Toscani D., Manno M., et al. (2019). Multiple myeloma-derived exosomes are enriched of amphiregulin (AREG) and activate the epidermal growth factor pathway in the bone microenvironment leading to osteoclastogenesis. J. Hematol. Oncol 12:2. 10.1186/s13045-018-0689-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G., Esposito M., Kang Y. (2015). Bone metastasis and the metastatic niche. J. Mol. Med. (Berl.) 93 1203–1212. 10.1007/s00109-015-1329-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez Zorrilla S., Pérez-Sayans M., Fais S., Logozzi M., Gallas Torreira M., García García A. (2019). A pilot clinical study on the prognostic relevance of plasmatic exosomes levels in oral squamous cell carcinoma patients. Cancers 11:429. 10.3390/cancers11030429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M., Pitari M. R., Amodio N., Di Martino M. T., Conforti F., Leone E., et al. (2013). miR-29b negatively regulates human osteoclastic cell differentiation and function: implications for the treatment of multiple myeloma-related bone disease. J. Cell Physiol. 228 1506–1515. 10.1002/jcp.24306 [DOI] [PubMed] [Google Scholar]
- Sawant A., Hensel J. A., Chanda D., Harris B. A., Siegal G. P., Maheshwari A., et al. (2012). Depletion of plasmacytoid dendritic cells inhibits tumor growth and prevents bone metastasis of breast cancer cells. J. Immunol. 189 4258–4265. 10.4049/jimmunol.1101855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimeca M., Trivigno D., Bonfiglio R., Ciuffa S., Urbano N., Schillaci O., et al. (2020). Breast cancer metastasis to bone: from epithelial to mesenchymal transition to breast osteoblast-like cells. Semin. Cancer Biol. 10.1016/j.semcancer.2020.01.004 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Slomka A., Mocan T., Wang B., Nenu I., Urban S. K., Gonzales-Carmona M., et al. (2020). EVs as potential new therapeutic Tool/target in gastrointestinal cancer and HCC. Cancers (Basel) 12:3019. 10.3390/cancers12103019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa M. S., Bragado P., Aguirre-Ghiso J. A. (2014). Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat. Rev. Cancer 14 611–622. 10.1038/nrc3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z., Yang Z., Xu Y., Chen Y., Yu Q. (2015). Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol. Cancer 14:48. 10.1186/s12943-015-0321-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam A. H., Schepers A. J., Qin A., Nachar V. R. (2020). Impact of extended-interval versus standard dosing of zoledronic acid on skeletal events in non-small-cell lung cancer and small-cell lung cancer patients with bone metastases. Ann. Pharmacother. 10.1177/1060028020967629 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Tamura T., Yoshioka Y., Sakamoto S., Ichikawa T., Ochiya T. (2020). Extracellular vesicles in bone metastasis: key players in the tumor microenvironment and promising therapeutic targets. Int. J. Mol. Sci. 21:6680. 10.3390/ijms21186680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M. K. S., Yue P. Y. K., Ip P. P., Huang R. L., Lai H. C., Cheung A. N. Y., et al. (2018). Soluble E-cadherin promotes tumor angiogenesis and localizes to exosome surface. Nat. Commun. 9:2270. 10.1038/s41467-018-04695-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna S., Pucci M., Giallombardo M., Di Bella M. A., Santarpia M., Reclusa P., et al. (2017). Amphiregulin contained in NSCLC-exosomes induces osteoclast differentiation through the activation of EGFR pathway. Sci. Rep. 7:3170. 10.1038/s41598-017-03460-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terese K., Marie L., Anders W., Emma P., Benedetta B. (2016). Tumor cell-derived exosomes from the prostate cancer cell line tramp-c1 impair osteoclast formation and differentiation. PLoS One 11:e0166284. 10.1371/journal.pone.0166284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedemann K., Sadvakassova G., Mikolajewicz N., Juhas M., Sabirova Z., Tabaries S., et al. (2019). Exosomal release of L-plastin by breast cancer cells facilitates metastatic bone osteolysis. Transl. Oncol. 12 462–474. 10.1016/j.tranon.2018.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasetti M., Lee W., Santarelli L., Neuzil J. (2017). Exosome-derived microRNAs in cancer metabolism: possible implications in cancer diagnostics and therapy. Exp. Mol. Med. 49:e285. 10.1038/emm.2016.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga N., Kosaka N., Ono M., Katsuda T., Yoshioka Y., Tamura K., et al. (2015). Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat. Commun. 6:6716. 10.1038/ncomms7716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos M. H., Caires H. R., Abols A., Xavier C. P. R., Line A. (2019). Extracellular vesicles as a novel source of biomarkers in liquid biopsies for monitoring cancer progression and drug resistance. Drug Resist. Updat. 47:100647. 10.1016/j.drup.2019.100647 [DOI] [PubMed] [Google Scholar]
- Vicent S., Perurena N., Govindan R., Lecanda F. (2015). Bone metastases in lung cancer. Potential novel approaches to therapy. Am. J. Respir. Crit. Care Med. 192 799–809. 10.1164/rccm.201503-0440SO [DOI] [PubMed] [Google Scholar]
- Wang H., Tian L., Liu J., Goldstein A., Bado I., Zhang W., et al. (2018). The osteogenic niche is a calcium reservoir of bone micrometastases and confers unexpected therapeutic vulnerability. Cancer Cell 34 823–839.e7. 10.1016/j.ccell.2018.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Yu C., Gao X., Welte T., Muscarella A. M., Tian L., et al. (2015). The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell 27 193–210. 10.1016/j.ccell.2014.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Xia F., Wei Y., Wei X. (2020). Molecular mechanisms and clinical management of cancer bone metastasis. Bone Res. 8:30. 10.1038/s41413-020-00105-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waning D. L., Guise T. A. (2014). Molecular mechanisms of bone metastasis and associated muscle weakness. Clin. Cancer Res. 20 3071–3077. 10.1158/1078-0432.CCR-13-1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weilbaecher K. N., Guise T. A., McCauley L. K. (2011). Cancer to bone: a fatal attraction. Nat Rev Cancer 11 411–425. 10.1038/nrc3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. A., Zhong J., Johannet P., Lee Y., Masub N., Wechter T., et al. (2020). Preliminary analysis of distinct clinical and biologic features of bone metastases in melanoma. Melanoma Res. 30 492–499. 10.1097/CMR.0000000000000691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S. L., Brown J. E. (2020). Personal medicine and bone metastases: biomarkers, micro-RNAs and bone metastases. Cancers (Basel) 12:2109. 10.3390/cancers12082109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortzel I., Dror S., Kenific C. M., Lyden D. (2019). Exosome-mediated metastasis: communication from a distance. Dev. Cell 49 347–360. 10.1016/j.devcel.2019.04.011 [DOI] [PubMed] [Google Scholar]
- Wu A. C., He Y., Broomfield A., Paatan N. J., Harrington B. S., Tseng H. W., et al. (2016). CD169(+) macrophages mediate pathological formation of woven bone in skeletal lesions of prostate cancer. J. Pathol. 239 218–230. 10.1002/path.4718 [DOI] [PubMed] [Google Scholar]
- Xiao L., He Y., Peng F., Yang J., Yuan C. (2020). Endometrial cancer cells promote M2-like macrophage polarization by delivering exosomal miRNA-21 under hypoxia condition. J. Immunol. Res. 2020:9731049. 10.1155/2020/9731049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Cao S., Geng J., Wang C., Meng Q., Yu Y. (2020). High prognostic nutritional index (PNI) as a positive prognostic indicator for non-small cell lung cancer patients with bone metastasis. Clin. Respir. J. 15 225–231. 10.1111/crj.13288 [DOI] [PubMed] [Google Scholar]
- Xu Y., Liu N., Wei Y., Zhou D., Lin R., Wang X., et al. (2020). Anticancer effects of miR-124 delivered by BM-MSC derived exosomes on cell proliferation, epithelial mesenchymal transition, and chemotherapy sensitivity of pancreatic cancer cells. Aging (Albany NY) 12 19660–19676. 10.18632/aging.103997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Liu X., Wang H., Li J., Dai L., Li J., et al. (2018). Lung adenocarcinoma cell-derived exosomal miR-21 facilitates osteoclastogenesis. Gene 666 116–122. 10.1016/j.gene.2018.05.008 [DOI] [PubMed] [Google Scholar]
- Xue P., Wu Z., Wang K., Gao G., Zhuang M., Yan M. (2020). Oncological outcome of combining cytoreductive prostatectomy and metastasis-directed radiotherapy in patients with prostate cancer and bone oligometastases: a retrospective cohort study. Cancer Manag. Res. 12 8867–8873. 10.2147/CMAR.S270882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Feng X., Liu H., Tong R., Wu J., Li C., et al. (2020). High-metastatic cancer cells derived exosomal miR92a-3p promotes epithelial-mesenchymal transition and metastasis of low-metastatic cancer cells by regulating PTEN/Akt pathway in hepatocellular carcinoma. Oncogene 39 6529–6543. 10.1038/s41388-020-01450-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E., Wang X., Gong Z., Yu M., Wu H., Zhang D. (2020). Exosome-mediated metabolic reprogramming: the emerging role in tumor microenvironment remodeling and its influence on cancer progression. Signal. Transduct. Target Ther. 5:242. 10.1038/s41392-020-00359-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao D., Huang L., Ke J., Zhang M., Xiao Q., Zhu X. (2020). Bone metabolism regulation: Implications for the treatment of bone diseases. Biomed. Pharmacother. 129:110494. 10.1016/j.biopha.2020.110494 [DOI] [PubMed] [Google Scholar]
- Ye Y., Li S. L., Ma Y. Y., Diao Y. J., Yang L., Su M. Q., et al. (2017). Exosomal miR-141-3p regulates osteoblast activity to promote the osteoblastic metastasis of prostate cancer. Oncotarget 8 94834–94849. 10.18632/oncotarget.22014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi M., Xu L., Jiao Y., Luo S., Li A., Wu K. (2020). The role of cancer-derived microRNAs in cancer immune escape. J. Hematol. Oncol. 13:25. 10.1186/s13045-020-00848-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J. J., Pollock C. B., Kelly K. (2005). Mechanisms of cancer metastasis to the bone. Cell Res. 15 57–62. 10.1038/sj.cr.7290266 [DOI] [PubMed] [Google Scholar]
- Yuan X., Qian N., Ling S., Li Y., Li Y. (2021). Breast cancer exosomes contribute to pre-metastatic niche formation and promote bone metastasis of tumor cells. Theranostics 11 1429–1445. 10.7150/thno.45351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Iwabuchi S., Baba T., Hashimoto S. I., Mukaida N., Sasaki S. I. (2020). Involvement of a transcription factor, Nfe2, in breast cancer metastasis to bone. Cancers (Basel) 12:3003. 10.3390/cancers12103003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Bado I., Wang H., Lo H. C., Zhang X. H. (2019). Bone metastasis: find your niche and fit in. Trends Cancer 5 95–110. 10.1016/j.trecan.2018.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Bi J., Huang J., Tang Y., Du S., Li P. (2020). Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 15 6917–6934. 10.2147/IJN.S264498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Ren Y., Lu Z. (2020). Potential diagnostic and therapeutic roles of exosomes in pancreatic cancer. Biochim. Biophys. Acta Rev. Cancer 1874:188414. 10.1016/j.bbcan.2020.188414 [DOI] [PubMed] [Google Scholar]
- Zhao Z., Pi Y., Jiang L., Xiang Y., Wei J., Yang P., et al. (2020). Deep neural network based artificial intelligence assisted diagnosis of bone scintigraphy for cancer bone metastasis. Sci. Rep. 10:17046. 10.1038/s41598-020-74135-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P., Chen L., Yuan X., Luo Q., Liu Y., Xie G., et al. (2017). Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J. Exp. Clin. Cancer Res. 36:53. 10.1186/s13046-017-0528-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Fong M. Y., Min Y., Somlo G., Liu L., Palomares M. R., et al. (2014). Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 25 501–515. 10.1016/j.ccr.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Liang S., Zhan Q., Yang L., Chi J., Wang L. (2020). HSPG2 overexpression independently predicts poor survival in patients with acute myeloid leukemia. Cell Death Dis. 11:492. 10.1038/s41419-020-2694-7 [DOI] [PMC free article] [PubMed] [Google Scholar]