Summary
Slow neurobiological rhythms, such as the circadian secretion of glucocorticoid (GC) hormones, modulate a variety of body functions. Whether and how endocrine fluctuations also exert an influence on perceptual abilities is largely uncharted. Here, we show that phasic increases in GC availability prove beneficial to auditory discrimination. In an age-varying sample of N = 68 healthy human participants, we characterize the covariation of saliva cortisol with perceptual sensitivity in an auditory pitch discrimination task at five time points across the sleep-wake cycle. First, momentary saliva cortisol levels were captured well by the time relative to wake-up and overall sleep duration. Second, within individuals, higher cortisol levels just prior to behavioral testing predicted better pitch discrimination ability, expressed as a steepened psychometric curve. This effect of GCs held under a set of statistical controls. Our results pave the way for more in-depth studies on neuroendocrinological determinants of sensory encoding and perception.
Subject areas: Biological Sciences, Neuroscience, Sensory Neuroscience, Endocrinology
Graphical abstract
Highlights
-
•
Cortisol and auditory perception in N = 68 human adults at 5 times of day
-
•
Higher momentary cortisol levels predicted better pitch discrimination
-
•
This glucocorticoid effect held under a set of statistical control models
-
•
Paves way for studies on neuroendocrinological constraints of sensation and perception
Biological Sciences; Neuroscience; Sensory Neuroscience; Endocrinology
Introduction
Most physiological functions in humans exert circadian rhythmicity (Dibner et al., 2010). That is, bodily homeostatic functions oscillate with a period of about 24 hr and are vital in adapting the organism to its environment (Spiga et al., 2014). These functions are regulated through the endogenous circadian clock system with the suprachiasmatic nucleus (SCN) as a pacemaker, synchronizing subordinate tissue clocks located throughout the body (Dibner et al., 2010).
While sensory, perceptual, and cognitive functions all have been shown to also be subject to—much faster—rhythmicity and to covary with brain states at the sub-second (“neural oscillations”, e.g. Henry and Obleser, 2012) or seconds-to-minutes scale (e.g., Park et al., 2014; Rebollo et al., 2018), a potential circadian role of the endocrine system in the regulation of sensation and perception, in particular, has received much less attention.
The endocrine system, with glucocorticoids (GCs) as the main effector of the hypothalamic-pituitary-adrenal (HPA) axis, exhibits also prominent circadian rhythmicity (Spencer and Deak, 2017). Cortisol as the major human endogenous GC is recognized in psychophysiological research primarily as a stress hormone, reactive to physical and emotional stress (Katsu and Iguchi, 2016). More important to the current investigation, however, blood cortisol levels, approximated well by the saliva cortisol level lagging it (Kirschbaum and Hellhammer, 1989), are lowest in the late afternoon up to midnight and begin to rise up again during the second half of the night to peak during the early morning (Pruessner et al., 1997) (Figure 1A). It has been a long-standing hypothesis that GCs and their circadian dynamics are linked to cognitive function. There is evidence of a cortisol influence on different cognitive phenomena such as attention and executive functions in general (Roberts et al., 1998).
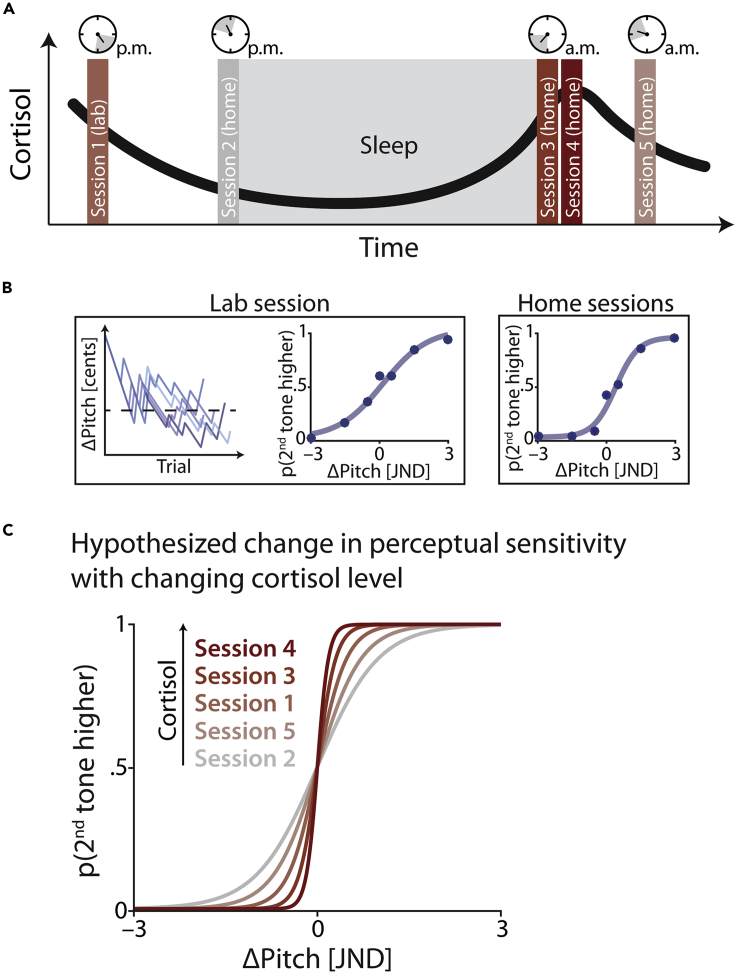
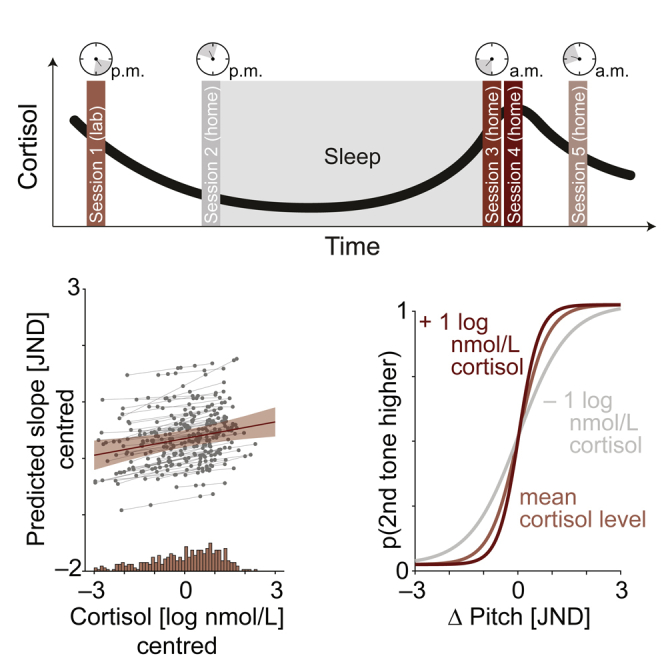
Figure 1.
Experimental design and hypothesis
(A) Design. In five sessions, participants were asked to take saliva samples, from which their cortisol levels were measured. After a first laboratory session (in the afternoon), participants were asked to perform the other four sessions at home. To capture circadian differences in cortisol levels (black curve), these “home” sessions were timed to align with the individual participant's sleep-wake cycle such that sessions 2 and 3 had to be completed immediately before going to sleep and immediately after wake-up, respectively. Two further sessions (4 and 5) were performed 30 and 120 min after wake-up.
(B) Psychophysical testing. In addition to the collection of saliva samples, participants performed a pitch discrimination task in each session. In the lab session, we first assessed individual participants' pitch discrimination thresholds (just-noticeable difference; JND) using five separate staircases (see methods for details). These individual JNDs were then used in an online experiment, which participants performed in all five sessions. Psychometric functions (shown in blue) were fit to the data obtained in each session. The slope of the psychometric function served as a measure of perceptual sensitivity.
(C) Hypothesis. Increased levels of GC availability should result in steeper psychometric functions, reflecting higher perceptual sensitivity. Note that here sessions are not ordered chronologically but by cortisol level. All illustrations in (A)–(C) are schematic, visualizing the hypothesized results of the current study.
Understanding better this potential association between central levels of GC hormones and sensory-cognitive performance has implications for the notorious relation of stress-related and hearing disorders (Canlon et al., 2013). It can also further our understanding of how healthy variations in the central availability of stress hormones like cortisol might help regulate sensory and cognitive function.
Generally, we do not know much about how strongly, and in which direction, GC availability impacts cognitive function due to a large dynamic range and a variety of pathways by which GCs can act upon central nervous processes (Di et al., 2009; Hall et al., 2015; Lupien and Lepage, 2001; Wolf et al., 2002). Previous results have been mixed: for instance, high baseline cortisol levels have been associated with impaired memory, executive functions, and visual perception (Echouffo-Tcheugui et al., 2018) but also with improved attention and sensory performance in dichotic hearing (Al’Absi et al., 2002). Dijckmans and colleagues (Dijckmans et al., 2017) reported better performance in high cognitive function tasks for participants exhibiting larger variation of cortisol levels throughout the day. An earlier peak and greater magnitude of the typical cortisol awakening response (CAR, a cortisol peak 30–45 min post wake-up) has been shown to be predictive of relatively better executive function-related performance (Evans et al., 2011, 2012).
More generally, it is assumed that a decrease in the dynamic range of circadian GC secretion, either due to an attenuated CAR or due to a slowed elimination of stress-induced cortisol spikes, is associated with cognitive impairment in elderly subjects (Beluche et al., 2010; Evans et al., 2011; Stawski et al., 2011). Importantly, little is known on how and to what extent circadian changes in GC availability can influence perceptual processes directly. Visual sensitivity has been reported to fluctuate with time of day (Echouffo-Tcheugui et al., 2018; Stolz et al., 1987; Tassi and Pins, 2009). Clinically, as part of their seminal studies on individuals presenting with GC insufficiency (e.g. characteristic of Addison disease), Henkin and colleagues observed a systematic pattern of lowered perceptual thresholds (i.e. better detection) paired with lowered discrimination thresholds (Henkin, 1970) and in the case of audition, a generally lowered dynamic range (Henkin and Daly, 1968). Not least, the systemic administration of synthetic corticosteroids has become a mainstay in treating various hearing disorders, assuming a protective effect of GCs in the inner ear (Trune and Canlon, 2012).
Most directly pertaining to the present study, there is little evidence on how physiological endocrine fluctuations along the circadian cycle influence perception. First evidence with respect to a possible involvement of the circadian system in auditory function is given by the existence of a molecular circadian clock in the cochlea (Meltser et al., 2014) as well as in the inferior colliculus (Park et al., 2016). In addition, Meltser and colleagues (Meltser et al., 2014) reported higher auditory sensitivity, both on molecular and behavioral levels, at specific times of the day (for review, see Basinou et al., 2017).
Note that a direct impact of cortisol on auditory perception is physiologically plausible: first, experimental cortisol exposure stimulates the auditory system but leads to damages in the long term (Al-Mana et al., 2008). Second, GC receptors are expressed in the inner ear, especially in the cochlea (Rarey and Curtis, 1996), as well as in brainstem nuclei involved in auditory processing (Jennes and Langub, 2000). Thus, the SCN-controlled fluctuations in GC availability can impact auditory function at the sensory level directly (Cederroth et al., 2019) and at different levels of the auditory pathway (Canlon et al., 2007).
In the current study, we focus on the impact of the circadian variation of GC availability on auditory perceptual sensitivity in discriminating (i.e., not in detecting the presence of) sounds. We used a psychophysical method, a two-alternative forced-choice (2AFC) task, to describe individual sensitivity for pitch discrimination. Our lead hypothesis here is that GC levels (as proxied by saliva cortisol) impact perceptual performance above and beyond expected drivers such as sex or chronological age: higher levels of saliva cortisol just prior to performing a challenging pitch discrimination task should lead to a steeper psychometric curve, indicating a state of elevated perceptual sensitivity and, thus, better auditory discrimination abilities. As auxiliary hypotheses, we expected older participants (i) to show less perceptual sensitivity in auditory pitch discrimination (e.g., Clinard et al., 2010) and (ii) to present with lower levels of saliva cortisol (Evans et al., 2011). The current design allowed us to control for potential confounds of cross-sectional age differences when studying GCs and auditory perception.
In a large, age-varying sample of participants (N = 68), we investigated the relationship of saliva cortisol and perceptual performance at the state (i.e., within individuals) and trait level (i.e., between individuals). In detail, we tested a cohort of healthy young adults (age range: 19–30 years) and a cohort of middle-aged to older participants (age range 50–70 years; see Table S5) at five different measurements covering a time interval of approximately 18 hr (see Figure 1A).
We recorded individual sleep duration and aligned cortisol sampling and behavioral testing relative to the sleep-wake cycle to optimally capture the post-awakening rise and subsequent drop in GC levels (Clow et al., 2010; Wilhelm et al., 2007).
Results
We investigated the impact of human circadian variation in cortisol levels on perceptual sensitivity in a challenging auditory pitch discrimination task. Task difficulty was titrated based on the individual just-noticeable difference (JND). We used separate linear mixed-effects models (i) to test how salivary cortisol secretion changes as a function of time relative to the sleep-wake cycle and age cohort, and (ii) to understand how the observed fluctuation in cortisol levels, in turn, predict perceptual discrimination sensitivity, represented by the slope of the psychometric function. Each model also tested for effects of additional, potential confounders such as sex or sleep duration.
Explaining momentary states of saliva cortisol
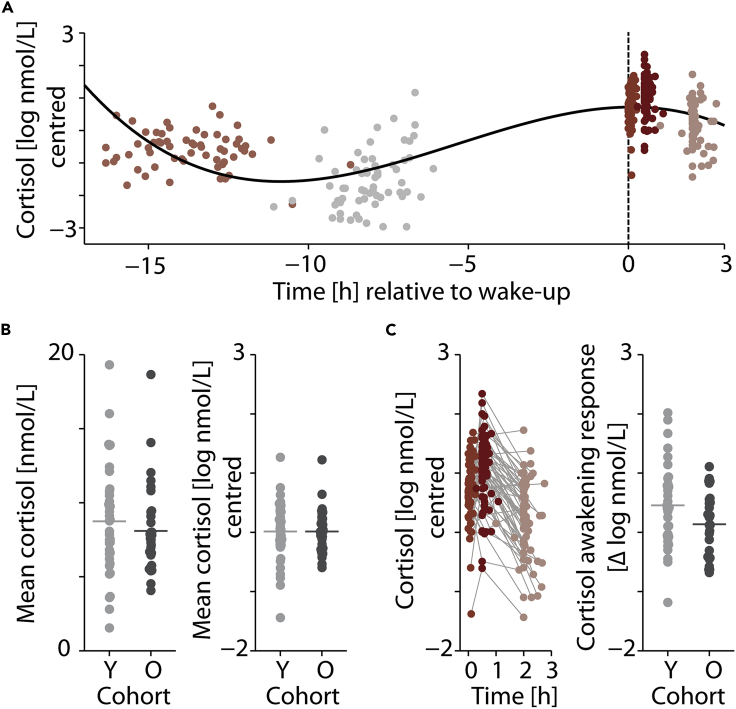
As revealed by model comparison, the momentary level of salivary cortisol was well accounted for by the daytime of measurement (expressed relative to the individual wake-up time and modeled using polynomial regressors of first, second, and third order) and total sleep duration between measurements (conditional R2 = .75; see Table S1 for full model details). Increased sleep duration was associated with overall lower levels of cortisol (β = −.16, standard error [SE] = .04, p = .001, log Bayes factor10 (logBF) = 3.2) while changes in cortisol over time were best described by a cubic trend (β = −.15, SE = .07, p = .025, logBF = −.13). As shown in Figure 2A, this cubic trend captures the decline in cortisol levels from afternoon to late evening and the characteristic cortisol awakening response (see Figure 2C). The considerable improvement of model fit by the inclusion of session-specific random intercepts further attests to the impact of daytime on cortisol level (likelihood ratio test; χ21 = 69.5, p < .001, logBF = 31.9). Overall levels of cortisol did not differ significantly between the younger and older cohort (χ21 = 2.05, p = .15, logBF = −1.9; see Figure 2B). Neither did the cortisol awakening response exhibit a clear effect of age cohort (Figure 2C). The inclusion of participants' sex did not improve model fit, either (χ21 = 1.0, p = .31, logBF = −2.4).
Figure 2.
Momentary states of glucocorticoid levels (salivary cortisol)
(A) Changes in individual salivary cortisol concentration measured in log nmol/L across five experimental sessions. Cortisol levels are mean centered across all N = 68 participants. Sessions are grouped by color and aligned by wake-up time (dashed vertical line). Black curve shows the cubic trend of time that was modeled using polynomial regression.
(B) Left image: individual mean cortisol levels [nmol/L] across sessions shown separately for the younger (Y, light gray) and older (O, dark gray) age cohort. Dots represent individual mean values (N = 68); horizontal lines show the respective group average. Right image: individual mean cortisol levels per cohort after log-transformation and mean centering for statistical analysis.
(C) Left image: trajectory of individual cortisol levels [log nmol/L] following wake-up. Time is expressed relative to wake-up time. Note the rise in cortisol levels 30 min after wake-up (session 4, dark red). Right image: individual cortisol awakening response (CAR) expressed as the difference in cortisol levels [log nmol/L, centered] 30 min after wake-up relative to wake-up shown separately for the younger (Y, light gray) and older (O, dark gray) age cohort. Horizontal lines indicate the group mean.
Saliva cortisol predicts perceptual discrimination sensitivity
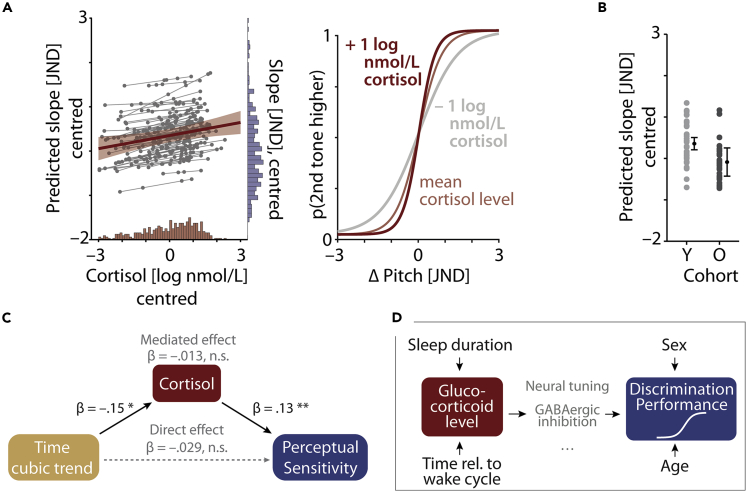
As the main analysis (Figure 3), we probed the predictive power of cortisol levels measured just prior to performing a challenging pitch discrimination task on participants' perceptual discrimination sensitivity. As indicated by the best-fitting linear mixed-effects model, perceptual sensitivity, operationalized as the slope of the psychometric function, was significantly influenced by the momentary level of cortisol, age cohort, and sex (conditional R2 = .47; see Table S2 for full model details).
Figure 3.
Salivary cortisol predicts perceptual discrimination sensitivity
(A) Left image: change in perceptual sensitivity (operationalized by the slope of psychometric function) as predicted by cortisol. Predicted group-level fixed-effect (red slope) with 95% confidence interval (CI) error band is shown along with the estimated subject-specific random slopes (thin gray lines) and single-subject, single-session predictions (gray dots). Note that subject-specific random slopes did not improve the model fit and were added for illustrative purposes only. Histograms on the bottom and right side of the plot display the distribution of log-transformed cortisol and raw slope values, respectively. Right image: illustration of how variation in cortisol level impacts the steepness of the psychometric curve.
(B) Difference in perceptual sensitivity between age groups. Colored dots (light gray, young [Y] cohort; dark gray, older [O] cohort) show single-subject predicted slope values based on the best-fitting linear mixed-effects model. Black dots represent the fixed-effect group-level prediction and 95% CI.
(C) Results of causal mediation analysis. Formally accounting for the potentially mediating role of cortisol does not lead to a significant change in the effect of the cubic trend of time on perceptual sensitivity. ∗p < .05; ∗∗p < .01.
(D) Summary of effects observed. The panel summarizes observed (black solid arrows) and statistically excluded (absence of arrows) effects. Intervening (i.e., mediating) effects of how GCs can act upon resulting perceptual outcomes must obviously exist but remain subject to future experimentation. For illustration only, viable paths via a sharpening of neural tuning and/or increased levels of GABAergic inhibition are shown in gray.
See also Figures S1–S3 and Tables S1–S4.
In line with our hypotheses, increased levels of cortisol led to heightened perceptual sensitivity (β = .13, SE = .04, p = .004, logBF = 1.4). More specifically, as illustrated in Figure 3A (right panel), an increase in cortisol by one unit log(nmol/L) steepened the slope of the psychometric curve by one 10th of the JND. To interpret this effect with respect to the measured log cortisol levels, we examined the range of cortisol levels recorded per individual. On average, the individual cortisol levels changed by 2.71 nmol/L (±sd 0.85 nmol/L) across the course of the experiment. Importantly, as shown by subject-specific slopes added for illustrative purposes in Figure 3A (left panel), the relationship of cortisol and perceptual sensitivity was consistently observable across individual participants. An additional analysis including separate regressors for the state- (i.e., within-subjects) and trait-level (i.e., between-subjects) effect of cortisol on perceptual sensitivity provided additional support for cortisol-driven changes in perceptual sensitivity at the level of the individual participant (within-subject effect of cortisol: β = .13, SE = .04, p = .004, logBF = 1.2; between-subject effect cortisol: β = .13, SE = .23, p = .56, logBF = −2.7; see methods and Table S3 for full model details).
As expected, we observed a significant decrease in perceptual sensitivity for the older compared to the younger cohort (β = −.52, SE = .18, p = .005, logBF = 1.3; see Figure 3B). More precisely, we observed shallower slopes for older participants with an overall difference in the slopes of younger and older participants of nearly half a JND. Participant‘s sex proved to be an additional significant predictor with females showing overall lower perceptual sensitivity (β = −.36, SE = .18, p = .049, logBF = –.84; see Figure S1). Participants' sleepiness (assessed via the Karolinska Sleepiness Scale; see methods) or response bias (indicated by the point of subjective equality [PSE] on the psychometric function), however, did not influence behavioral performance. The inclusion of these predictors did not significantly improve model fit (likelihood ratio tests, all p > .067, all logBFs < −1.2).
Lastly, we investigated whether changes in cortisol would differentially impact perceptual sensitivity across the two age groups, despite overall comparable levels of cortisol observed for younger and older adults. However, the inclusion of the respective interaction term did not improve the model fit (χ21 = .91, p = .34, logBF = −2.4).
Cortisol does not predict response bias
To investigate whether the association with momentary cortisol levels was specific to perceptual sensitivity, we ran a control model probing for their effect on response bias. We found a significant increase in PSE for older participants (β = .44, SE = .2, p = .027, logBF = −.34). Importantly, however, circadian fluctuations in cortisol did not significantly predict changes in response bias (β = .001, SE = .04, p = .98, logBF = −2.9, see Figure S3 and Table S4).
Ruling out confounding effects of task proficiency
One concern we aimed to target is the obvious repetition of the pitch discrimination task in close succession, especially in the morning of the second testing day (i.e., three times of testing within approximately two hours). Reassuringly, however, no training or time-of-day effects on our main outcome measure of pitch discrimination perceptual sensitivity were evident (Figure S2). The inclusion of session (χ21 = 1.6, p = .21, logBF = −2.1) or time (linear, quadratic, or cubic trend) did not improve model fit (all p > .4, logBFs < −2.5).
Cortisol is not simply mediating an effect of daytime on perceptual sensitivity
An additional control analysis considered the possibility that the observed link between cortisol and perceptual discrimination sensitivity could reflect an indirect effect of daytime on perceptual sensitivity. While the absence of any systematic changes in the slope of the psychometric function with time (see above) rendered this scenario unlikely, we still formally tested this possibility using causal mediation analysis. As shown in Figure 3C, the comparison of the estimated total and direct effect of time (cubic trend) on perceptual sensitivity showed a comparably small and non-significant change (−.042, 95% confidence interval [CI] [–.13, .04] vs. −.029, CI[–.13, .07]) when accounting for the indirect influence via cortisol (−.013, CI[–.07, .04]). In other words, the observed increase in perceptual sensitivity with increasing levels of salivary cortisol does provide evidence for their potentially causal relationship.
Discussion
Does the momentary availability of glucocorticoids (GCs) predict changes in perceptual abilities, and if so, to what degree? The current study set out to gather decisive data on this seemingly simple question. In a mixed between- and within-participants design using multiple saliva cortisol samples and multiple associated behavioral assessments of perceptual sensitivity throughout the circadian cycle, we here have indeed provided evidence for a link between GC level and auditory perceptual discrimination ability.
A first result lending overall credibility to our approach is the circadian modulation of saliva cortisol. A highly consistent pattern of relative cortisol-level displacement dependent on daytime of measurement was observable (Figure 2A), which in concert with the individual duration of sleep (taking place between measurements 2 and 3) could explain the observed GC variance to a large degree.
Second, as the main result of our study, saliva cortisol levels just prior to performing the pitch discrimination task were predictive of perceptual sensitivity. Statistically, dissecting the influence of trait-level (i.e., person to person) versus state-level (i.e., session to session) variation in cortisol showed that it was the momentary cortisol level just prior to behavioral testing that covaried with perceptual sensitivity. The robustness and size of this effect is illustrated in Figure 3A: a change of one's own cortisol level by one unit log(nmol/L) steepens one's psychometric curve by approximately 1/10 of the JND in pitch. Essentially, all participants showed this positive relationship of momentary saliva cortisol levels with the steepness of the psychometric curve in pitch discrimination. Lastly, a series of control analyses underscores the directness and putative causality of the effect of cortisol on auditory discrimination performance.
This result fills various gaps in our knowledge on how the endocrine system impacts perception and behavior. We will discuss potential mechanisms, limitations, and implications below.
Potential mechanisms: How could GC levels act upon perceptual sensitivity?
The present results imply that, within normal levels of a healthy endocrine system, relative increases in centrally available GCs are accompanied by an objective improvement in the ability to discriminate sounds. This is broadly in line with a view of stress hormones and activity of the HPA axis as preparing the body for action (Habib et al., 2001). Enhanced discrimination abilities certainly fit into this view.
What are mechanistic pathways by which GCs could bring such an improved discrimination about and how specific to auditory discrimination might these pathways be? We here aim to briefly cover two mechanistically conceivable paths—one relating to the established circadian dependence of GC and GC receptor dynamics in the inner ear and auditory system, and one relating to improved neural tuning via potentially GC-related GABAergic signaling.
First, there is ample evidence by now for a causal role of GC dynamics in the healthy as well as stress-related malfunction of the auditory system (e.g., Canlon et al., 2007; Canlon et al., 2013; Hossain et al., 2008). The immunosuppressive and anti-inflammatory effect of GCs requires binding to GC receptors, which are expressed not only in the structures of the HPA axis but also in the auditory system (Canlon et al., 2007). Also, the fine-tuned balance of GC receptors with mineralocorticoid receptor activity is well documented (e.g., de Kloet et al., 2018; Singer et al., 2018). Thus, the GC dynamics in the inner ear are under circadian control, which has also been demonstrated for the auditory system in the rodent (Cederroth et al., 2019). Accordingly, protective effects of GCs against hearing damage have been proposed. The effects of GC availability on perceptual discrimination abilities observed here might in part reflect such slow-acting variations in peripheral or central auditory function (Cederroth et al., 2019).
Second, GCs have been shown to facilitate inhibitory GABAergic synaptic input (and to concomitantly suppress excitatory glutamatergic drive) at least in hypothalamic neurons, part of the HPA axis (Di et al., 2009). It remains speculative at this point whether such a combined effect of GCs might also tip other brain areas toward inhibition, with concomitant improvements of discrimination abilities both at the neural and behavioral level. This poses a testable pathway: recent neurophysiology work using optogenetic stimulation of layer 6 cortical neurons in rodents has provided compelling evidence for a dissociation and rapid switching of detection-optimal versus discrimination-optimal configurations at neural and behavioral levels (Guo et al., 2017; Linden, 2017).
Both peripheral effects of GC and central effects at various stages need to be considered and tested in detail in future studies. However, a 2AFC discrimination task such as the present one requires the system to detect equally well two stimuli and to arbitrate between the two with respect to one task-relevant dimension (here, tone frequency). In signal detection theoretic terms, one stimulus (here, the one higher in tone frequency) is considered as “signal plus some noise” and should be chosen by the listener, while the other is considered “only noise”. Thus, improving sensitivity in such a task requires a mechanism that is able to improve the “signal to noise” ratio—either at the level of neural encoding (inner ear, midbrain, auditory cortex) or at the level of decision-making (auditory cortex and beyond), or both.
A concept viable at all these levels is neural tuning, the degree to which a neuron or neuronal population is selectively responsive to a certain range along a given featural dimension, here, pitch or sound frequency. Neural tuning in the auditory cortex is known to be highly adaptive to task demands in any given listening situation (Atiani et al., 2009; Holdgraf et al., 2016; Jiang et al., 2018). Additionally, improved discriminability of tones is a phenomenon with a clear auditory-cortical contribution (Christensen et al., 2019). Recent work in humans also underscores that ongoing neural population dynamics, which should be especially amenable to endocrine modulation, can flexibly (i.e., from trial to trial) affect behavioral sensitivity (e.g., Gelbard-Sagiv et al., 2018; Waschke et al., 2019).
It is worth noting that an inhibition- and tuning-related mechanism has received at least circumstantial support by the seminal clinical observations of Henkin and colleagues in the late 1960s. Primary GC insufficiency as observed in Addison disease was found accompanied by paradoxically improved detection thresholds but a decrease in discrimination abilities (Henkin and Daly, 1968; Henkin et al., 1967). Thus, there lies great promise in better understanding the differential GC susceptibility of detection and discrimination processes in the auditory system.
An obvious next step should be to manipulate GC availability in the human listener directly. In healthy participants, a relatively unspecific but carefully titrated administration of synthetic GC analogs can easily be used to obtain experimental control over GC levels within the normal dynamic range of HPA axis activity (Born et al., 1987). Not least, this would bring the field back to a promising lead that it seemingly left behind half a century ago: namely, the seminal work by Henkin and others on mechanistic pathways of how adrenocortical hormones control the detection and integration of sensory signals (Henkin, 1970). In clinical patients with primary GC insufficiencies (e.g. Addison disease) or GC hyper-availability (i.e., Cushing’s syndrome), it will be fruitful to build on pioneering but technically limited work linking better thresholds (e.g., hyper-sensibility in detection) but lowered discrimination abilities in the auditory domain to cortisol states (e.g., Henkin and Daly, 1968).
Limitations of the study
As summarized in Figure 3D, the current work helps us rule out two potential (i.e., theoretically plausible) confounders. Namely, both participants' age and participants' sex were indeed predictive of perceptual performance (with younger and male participants outperforming older and female participants, respectively). However, they are both highly unlikely to confound the observed effect of GCs on this performance, as neither of the two could account for momentary cortisol levels (note the absence of arrows from sex or age into GC in Figure 3D).
Not shown in Figure 3D, but reported in detail above, other more global “state” variables such as time of day (recall that most data here were acquired either just prior to bedtime or immediately after waking up) or total duration of sleep were good predictors of the momentary cortisol level. These, however, failed to account for any meaningful variance in the behavioral outcome (note the absence of arrows from “time relative to wake cycle” and “sleep duration” to “discrimination performance”). Thus, it was not the case that, for example, participants who had slept more were overall providing higher perceptual sensitivity across all testing instances or vice versa. Neither did testing in the evening yield lower discrimination performance, all other things being equal.
Unsurprisingly, the mediation analysis we performed for the sake of completeness (see Results) also did not provide any evidence for a potential mediation (i.e., daytime ➝ cortisol ➝ performance). Instead, our results expose a more direct link from momentary salivary cortisol level to sensitivity in perceptual discrimination.
Note that we made a set of design choices (e.g., cortisol sampling always directly preceding the behavioral test) that help to rule out a conceivable, reverse causal relationship (i.e., worse performance in the behavioral task leading to perceived stress and, thus, to higher cortisol level). Such a hypothesis, however, is rendered unlikely on two grounds. First, a previous study has found no effect of task effort or hearing status on cortisol as a marker of stress (Zekveld et al., 2019). Second, the present data themselves invalidate this notion, as higher cortisol levels just prior to testing were accompanied by better not worse performance at test.
This leaves us with one potential unobserved confound, namely, arousal. Could elevated levels of arousal have led to higher cortisol levels and, hence, to better behavioral performance? Arousal is generally assumed to establish an inverted u-shaped impact on performance (i.e., the “Yerkes-Dodson law”; Gelbard-Sagiv et al., 2018; Waschke et al., 2019; Yerkes and Dodson, 1908). However, the fact that we did not observe such a pattern does not necessarily rule out a confounding influence of arousal as our paradigm might have captured only activity along the “rising” flank of such an inverted u.
Nevertheless, we deem a confounding influence of autonomic arousal on both, GC levels and performance, unlikely on various grounds. First, the relationship of stress on individual cortisol levels is not as clear-cut as often assumed (Kirschbaum et al., 1995). Second, autonomic arousal markers, such as heart rate and blood pressure on the one hand and cortisol on the other, have been shown to exert a dissociable, that is, sufficiently independent impact on performance in a verbal memory task (Schwabe et al., 2008). Third, even if we assume a mechanistic link between arousal and cortisol, it is hard to imagine why autonomic arousal should have covaried so consistently across our diverse cohorts of young and old adults and time of day in order to yield the consistent behavioral effects. The Karolinska Sleepiness Scale, assessed here as a control measure and arguably a proxy of arousal, did not indicate any systematic covariation with behavioral outcome.
Nevertheless, a future study co-registering pupil dilation (as an established proxy of arousal) or physiological markers such as skin conductance and heart rate, ideally in a setting where GC levels are manipulated experimentally, should help to illuminate the causal links between arousal, cortisol, and discrimination performance.
Implications
We here have shown that a main neurobiological circadian rhythm in the human body, the secretion of GCs (here, captured as saliva cortisol), covaries with the individual fluctuation of auditory perceptual abilities (here, captured as pitch discrimination sensitivity immediately after taking the saliva sample). We have demonstrated that momentary GC levels show the expected circadian change and that these within-individual fluctuations of GC levels directly predict perceptual discrimination sensitivity.
This result opens at least two new research avenues: first, experimental control and manipulation of endocrine modulators such as the GC system can help to constrain future research into the organization of the auditory system. Second, our study opens new paths to improving or restoring discrimination abilities, a particularly vulnerable aspect of auditory function in both aging generally and age-related hearing loss specifically.
Resource availability
Lead contact
Jonas Obleser, Dept. of Psychology, University of Lübeck, Ratzeburger Allee 160, 23,562 Lübeck, Germany, Telephone +49 451 3101 3620, jonas.obleser@uni-luebeck.de.
Materials availability
No materials other than data and code apply.
Data and code availability
All data and scripts for data analysis are available at the Open Science Framework (OSF; https://osf.io/ns26m/).
Methods
All methods can be found in the accompanying transparent methods supplemental file.
Acknowledgments
This research has been supported by the European Research Council (ERC-CoG-2014-646696 “audadapt” to J.O.) and the Deutsche Forschungsgemeinschaft (DFG OS353-10/1 to H.O.). Franziska Scharata and Anne Hermann helped acquire the data.
Author contributions
Conceptualization, J.O., J.K., R.V., and H.O.; methodology, J.O., J.K., R.V., S.T., and H.O.; investigation, R.V., F.L., C.D., J.K., S.T., and J.O.; writing – original draft, J.O., J.K., R.V., and S.T.; writing – review & editing, J.O., J.K., R.V., F.L., C.D., S.T., and H.O.; funding acquisition, J.O. and H.O.; resources, J.O. and H.O.; supervision, J.O., J.K., S.T., and H.O.
Declaration of interests
The authors declare no competing interests.
Published: April 23, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102345.
Supplemental information
References
- Al-Mana D., Ceranic B., Djahanbakhch O., Luxon L.M. Hormones and the auditory system: a review of physiology and pathophysiology. Neuroscience. 2008;153:881–900. doi: 10.1016/j.neuroscience.2008.02.077. [DOI] [PubMed] [Google Scholar]
- Al’Absi M., Hugdahl K., Lovallo W.R. Adrenocortical stress responses and altered working memory performance. Psychophysiology. 2002;39:95–99. doi: 10.1017/S0048577202001543. [DOI] [PubMed] [Google Scholar]
- Atiani S., Elhilali M., David S.V., Fritz J.B., Shamma S.A. Task difficulty and performance induce diverse adaptive patterns in gain and shape of primary auditory cortical receptive fields. Neuron. 2009;61:467–480. doi: 10.1016/j.neuron.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basinou V., Park J.S., Cederroth C.R., Canlon B. Circadian regulation of auditory function. Hear. Res. 2017;347:47–55. doi: 10.1016/j.heares.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beluche I., Carrière I., Ritchie K., Ancelin M.L. A prospective study of diurnal cortisol and cognitive function in community-dwelling elderly people. Psychol. Med. 2010;40:1039–1049. doi: 10.1017/S0033291709991103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born J., Kern W., Fehm-Wolfsdorf G., Fehm H.L. Cortisol effects on attentional processes in man as indicated by event-related potentials. Psychophysiology. 1987;24:286–292. doi: 10.1111/j.1469-8986.1987.tb00297.x. [DOI] [PubMed] [Google Scholar]
- Canlon B., Meltser I., Johansson P., Tahera Y. Glucocorticoid receptors modulate auditory sensitivity to acoustic trauma. Hear. Res. 2007;226:61–69. doi: 10.1016/j.heares.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Canlon B., Theorell T., Hasson D. Associations between stress and hearing problems in humans. Hear. Res. 2013;295:9–15. doi: 10.1016/j.heares.2012.08.015. [DOI] [PubMed] [Google Scholar]
- Cederroth C.R., Park J.S., Basinou V., Weger B.D., Tserga E., Sarlus H., Magnusson A.K., Kadri N., Gachon F., Canlon B. Circadian regulation of cochlear sensitivity to noise by circulating glucocorticoids. Curr. Biol. 2019;29:2477–2487 e2476. doi: 10.1016/j.cub.2019.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen R.K., Linden H., Nakamura M., Barkat T.R. White noise background improves tone discrimination by suppressing cortical tuning curves. Cell Rep. 2019;29:2041–2053 e2044. doi: 10.1016/j.celrep.2019.10.049. [DOI] [PubMed] [Google Scholar]
- Clinard C.G., Tremblay K.L., Krishnan A.R. Aging alters the perception and physiological representation of frequency: evidence from human frequency-following response recordings. Hear. Res. 2010;264:48–55. doi: 10.1016/j.heares.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clow A., Hucklebridge F., Stalder T., Evans P., Thorn L. The cortisol awakening response: more than a measure of HPA axis function. Neurosci. Biobehav. Rev. 2010;35:97–103. doi: 10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- de Kloet E.R., Meijer O.C., de Nicola A.F., de Rijk R.H., Joels M. Importance of the brain corticosteroid receptor balance in metaplasticity, cognitive performance and neuro-inflammation. Front. Neuroendocrinol. 2018;49:124–145. doi: 10.1016/j.yfrne.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Di S., Maxson M.M., Franco A., Tasker J.G. Glucocorticoids regulate glutamate and GABA synapse-specific retrograde transmission via divergent nongenomic signaling pathways. J. Neurosci. 2009;29:393–401. doi: 10.1523/JNEUROSCI.4546-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner C., Schibler U., Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- Dijckmans B., Tortosa-Martínez J., Caus N., González-Caballero G., Martínez-Pelegrin B., Manchado-Lopez C., Clow A. Does the diurnal cycle of cortisol explain the relationship between physical performance and cognitive function in older adults? Eur. Rev. Aging Phys. Activity. 2017;14:6. doi: 10.1186/s11556-017-0175-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echouffo-Tcheugui J.B., Conner S.C., Himali J.J., Maillard P., DeCarli C.S., Beiser A.S., Seshadri S. Circulating cortisol and cognitive and structural brain measures: the Framingham Heart Study. Neurology. 2018;91:1961–1970. doi: 10.1212/WNL.0000000000006549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P., Hucklebridge F., Loveday C., Clow A. The cortisol awakening response is related to executive function in older age. Int. J. Psychophysiol. 2012;84:201–204. doi: 10.1016/j.ijpsycho.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Evans P.D., Fredhoi C., Loveday C., Hucklebridge F., Aitchison E., Forte D., Clow A. The diurnal cortisol cycle and cognitive performance in the healthy old. Int. J. Psychophysiol. 2011;79:371–377. doi: 10.1016/j.ijpsycho.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Gelbard-Sagiv H., Magidov E., Sharon H., Hendler T., Nir Y. Noradrenaline modulates visual perception and late visually evoked activity. Curr. Biol. 2018;28:2239–2249 e2236. doi: 10.1016/j.cub.2018.05.051. [DOI] [PubMed] [Google Scholar]
- Guo W., Clause A.R., Barth-Maron A., Polley D.B. A corticothalamic circuit for dynamic switching between feature detection and discrimination. Neuron. 2017;95:180–194 e185. doi: 10.1016/j.neuron.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib K.E., Gold P.W., Chrousos G.P. Neuroendocrinology of stress. Endocrinol. Metab. Clin. North Am. 2001;30:695–728. doi: 10.1016/s0889-8529(05)70208-5. [DOI] [PubMed] [Google Scholar]
- Hall B.S., Moda R.N., Liston C. Glucocorticoid mechanisms of functional connectivity changes in stress-related neuropsychiatric disorders. Neurobiol. Stress. 2015;1:174–183. doi: 10.1016/j.ynstr.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkin R.I. The effects of corticosteroids and ACTH on sensory systems. Prog. Brain Res. 1970;32:270–294. doi: 10.1016/S0079-6123(08)61545-9. [DOI] [PubMed] [Google Scholar]
- Henkin R.I., Daly R.L. Auditory detection and perception in normal man and in patients with adrenal cortical insufficiency: effect of adrenal cortical steroids. J. Clin. Invest. 1968;47:1269–1280. doi: 10.1172/JCI105819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkin R.I., McGlone R.E., Daly R., Bartter F.C. Studies on auditory thresholds in normal man and in patients with adrenal cortical insufficiency: the role of adrenal cortical steroids. J. Clin. Invest. 1967;46:429–435. doi: 10.1172/JCI105544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M.J., Obleser J. Frequency modulation entrains slow neural oscillations and optimizes human listening behavior. Proc. Natl. Acad. Sci. U S A. 2012;109:20095–20100. doi: 10.1073/pnas.1213390109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdgraf C.R., de Heer W., Pasley B., Rieger J., Crone N., Lin J.J., Knight R.T., Theunissen F.E. Rapid tuning shifts in human auditory cortex enhance speech intelligibility. Nat. Commun. 2016;7:13654. doi: 10.1038/ncomms13654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain A., Hajman K., Charitidi K., Erhardt S., Zimmermann U., Knipper M., Canlon B. Prenatal dexamethasone impairs behavior and the activation of the BDNF exon IV promoter in the paraventricular nucleus in adult offspring. Endocrinology. 2008;149:6356–6365. doi: 10.1210/en.2008-0388. [DOI] [PubMed] [Google Scholar]
- Jennes L., Langub M.C. Humana Press; 2000. Endocrine Targets in the Brain BT - Neuroendocrinology in Physiology and Medicine. [Google Scholar]
- Jiang X., Chevillet M.A., Rauschecker J.P., Riesenhuber M. Training humans to categorize monkey calls: auditory feature- and category-selective neural tuning changes. Neuron. 2018;98:405–416 e404. doi: 10.1016/j.neuron.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsu Y., Iguchi T. Handbook of Hormones. Academic Press; 2016. Cortisol. Part III: Lipophilic Hormones in Vertebrates, 533– 595 –532. [Google Scholar]
- Kirschbaum C., Hellhammer D.H. Salivary cortisol in psychobiological research: an overview. Neuropsychobiology. 1989;22:150–169. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C., Prussner J.C., Stone A.A., Federenko I., Gaab J., Lintz D., Schommer N., Hellhammer D.H. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosom. Med. 1995;57:468–474. doi: 10.1097/00006842-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Linden J.F. Timing is everything: corticothalamic mechanisms for active listening. Neuron. 2017;95:3–5. doi: 10.1016/j.neuron.2017.06.032. [DOI] [PubMed] [Google Scholar]
- Lupien S.J., Lepage M. Stress, memory, and the hippocampus: can’t live with it, can’t live without it. Behav. Brain Res. 2001;127:137–158. doi: 10.1016/s0166-4328(01)00361-8. [DOI] [PubMed] [Google Scholar]
- Meltser I., Cederroth C.R., Basinou V., Savelyev S., Lundkvist G.S., Canlon B. TrkB-mediated protection against circadian sensitivity to noise trauma in the murine cochlea. Curr. Biol. 2014;24:658–663. doi: 10.1016/j.cub.2014.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.D., Correia S., Ducorps A., Tallon-Baudry C. Spontaneous fluctuations in neural responses to heartbeats predict visual detection. Nat. Neurosci. 2014;17:612–618. doi: 10.1038/nn.3671. [DOI] [PubMed] [Google Scholar]
- Park J.S., Cederroth C.R., Basinou V., Meltser I., Lundkvist G., Canlon B. Identification of a circadian clock in the inferior colliculus and its dysregulation by noise exposure. J. Neurosci. 2016;36:5509–5519. doi: 10.1523/JNEUROSCI.3616-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner J.C., Wolf O.T., Hellhammer D.H., Buske-Kirschbaum A., Auer K.V., Jobst S., Kirschbaum C. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61:2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Rarey K.E., Curtis L.M. Receptors for glucocorticoids in the human inner ear. Otolaryngol. Head Neck Surg. 1996;115:38–41. doi: 10.1016/S0194-5998(96)70133-X. [DOI] [PubMed] [Google Scholar]
- Rebollo I., Devauchelle A.D., Beranger B., Tallon-Baudry C. Stomach-brain synchrony reveals a novel, delayed-connectivity resting-state network in humans. eLife. 2018;7:e33321. doi: 10.7554/eLife.33321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A.C., Robbins T.W., Weiskrantz L., Royal Society (Great Britain). Discussion Meeting . Oxford University Press; 1998. The Prefrontal Cortex : Executive and Cognitive Functions. [Google Scholar]
- Schwabe L., Bohringer A., Chatterjee M., Schachinger H. Effects of pre-learning stress on memory for neutral, positive and negative words: different roles of cortisol and autonomic arousal. Neurobiol. Learn. Mem. 2008;90:44–53. doi: 10.1016/j.nlm.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Singer W., Kasini K., Manthey M., Eckert P., Armbruster P., Vogt M.A., Jaumann M., Dotta M., Yamahara K., Harasztosi C. The glucocorticoid antagonist mifepristone attenuates sound-induced long-term deficits in auditory nerve response and central auditory processing in female rats. FASEB J. 2018;32:3005–3019. doi: 10.1096/fj.201701041RRR. [DOI] [PubMed] [Google Scholar]
- Spencer R.L., Deak T. A users guide to HPA axis research. Physiol. Behav. 2017;178:43–65. doi: 10.1016/j.physbeh.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiga F., Walker J.J., Terry J.R., Lightman S.L. HPA axis-rhythms. Compr. Physiol. 2014;4:1273–1298. doi: 10.1002/cphy.c140003. [DOI] [PubMed] [Google Scholar]
- Stawski R.S., Almeida D.M., Lachman M.E., Tun P.A., Rosnick C.B., Seeman T. Associations between cognitive function and naturally occurring daily cortisol during middle adulthood: timing is everything. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2011;66:71–81. doi: 10.1093/geronb/gbq094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz G., Aschoff J.C., Aschoff J., Born J. Circadian variation in the visual evoked potential (VEP) Electroencephalogr. Clin. Neurophysiol. Suppl. 1987;40:279–283. [PubMed] [Google Scholar]
- Tassi P., Pins D. Diurnal rhythmicity for visual sensitivity in humans? Chronobiol. Int. 2009;14:35–48. doi: 10.3109/07420529709040540. [DOI] [PubMed] [Google Scholar]
- Trune D.R., Canlon B. Corticosteroid therapy for hearing and balance disorders. Anat. Rec. (Hoboken) 2012;295:1928–1943. doi: 10.1002/ar.22576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waschke L., Tune S., Obleser J. Local cortical desynchronization and pupil-linked arousal differentially shape brain states for optimal sensory performance. eLife. 2019;8:e51501. doi: 10.7554/eLife.51501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm I., Born J., Kudielka B.M., Schlotz W., Wust S. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology. 2007;32:358–366. doi: 10.1016/j.psyneuen.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Wolf O.T., Convit A., Thorn E., Leon M.J. Salivary cortisol day profiles in elderly with mild cognitive impairment. Psychoneuroendocrinology. 2002;27:777–789. doi: 10.1016/s0306-4530(01)00079-8. [DOI] [PubMed] [Google Scholar]
- Yerkes R.M., Dodson J.D. The relation of strength of stimulus to rapidity of habit-formation. J. Comp. Neurol. Psychol. 1908;18:459–482. [Google Scholar]
- Zekveld A.A., van Scheepen J.A.M., Versfeld N.J., Veerman E.C.I., Kramer S.E. Please try harder! the influence of hearing status and evaluative feedback during listening on the pupil dilation response, saliva-cortisol and saliva alpha-amylase levels. Hear. Res. 2019;381:107768. doi: 10.1016/j.heares.2019.07.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and scripts for data analysis are available at the Open Science Framework (OSF; https://osf.io/ns26m/).