Abstract
OBJECTIVES
Moderate to vigorous physical activity is recommended to prevent hypertension according to the current guidelines. However, the degree to which the total physical activity (TPA) and its changes benefit normotensives and hypertensives is uncertain. We aimed to examine the effects of TPA and its changes on the incidence, progression, and remission of hypertension in the large-scale prospective cohorts.
METHODS
A total of 73,077 participants (55,101 normotensives and 17,976 hypertensives) were eligible for TPA analyses. During a mean follow-up of 7.16 years (394,038 person-years), 12,211 hypertension cases were identified. TPA was estimated as metabolic equivalents and categorized into quartiles. Cox proportional hazards regression and multivariable logistic regression were used to estimate associations of TPA and changes in TPA with incident hypertension and progression/remission of hypertension.
RESULTS
Compared with the lowest quartile of TPA, normotensives at the third and the highest quartile had a decreased risk of incident hypertension, with hazard ratios (HRs) of 0.86 [95% confidence interval (CI): 0.81−0.91] and 0.81 (95% CI: 0.77−0.86), respectively. Hypertensives at the highest quartile of TPA demonstrated a decreased risk of progression of hypertension [odds ratio (OR) = 0.87, 95% CI: 0.79−0.95], and an increased probability of hypertension remission (OR = 1.17, 95% CI: 1.05−1.29). Moreover, getting active from a sedentary lifestyle during the follow-up period could reduce 25% (HR = 0.75, 95% CI: 0.58−0.96) risk of incident hypertension, whereas those becoming sedentary did not achieve benefit from initially being active.
CONCLUSIONS
Our findings indicated that increasing and maintaining TPA levels could benefit normotensives, whereas higher TPA levels were needed to effectively control progression and improve remission of hypertension. Physical activity played undoubtedly an essential role in both primary and secondary prevention of hypertension.
Keywords: physical activity, incident hypertension, progression of hypertension, remission of hypertension, metabolic equivalent
High systolic blood pressure (SBP) has been identified as the leading risk factor of risk-attributable disability-adjusted life-years (DALYs), accounting for 10.4 million deaths worldwide according to the Global Burden of Disease Study 2017.[1] The number of hypertensive patients may increase to a total of 1.56 billion worldwide in 2025.[2] Unprecedented economic and technological improvements have resulted in great changes in lifestyle behaviors and increased prevalence of hypertension.[2,3] Thus, identifying modifiable risk factors and conducting behavioral interventions to mitigate the incidence and progression of hypertension should receive high priority.
Physical inactivity is one of the major controllable factors for cardiometabolic diseases, including hypertension.[4] Previous studies have reported the association between physical activity (PA) and incident hypertension, however, their results were inconsistent due to the different methods of the PA calculation.[5-7] A certain type of PA such as moderate to vigorous PA, leisure-time PA and occupational PA instead of the total physical activity (TPA) was considered in those studies, which could not provide comprehensive information on daily activities. Besides, most of the existing studies were conducted among Western populations whose lifestyles were different from Chinese populations.[6] It remained unclear about the exact effects of TPA and changes in TPA on the prevention of hypertension among general Chinese populations. More importantly, whether TPA could control the progression of hypertension and ameliorate hypertension was still needed to be illustrated since most studies were conducted among normotensives.[8,9] Considering the steep declines in TPA during the past decades, the health benefits from TPA and its changes on the incidence, progression, and remission of hypertension were highly needed to be comprehensively investigated.[10]
The purposes of the current study were to assess: (1) how great is the impact of TPA on incident hypertension among normotensives, (2) to what extent can hypertensives ameliorate through TPA, and (3) how much benefit can be expected from changes in TPA?
METHODS
Study Population
The current study population consisted of three Chinese prospective cohorts from the project of Prediction for Atherosclerotic Cardiovascular Disease Risk in China (China-PAR), including the China Multi-Center Collaborative Study of Cardiovascular Epidemiology (China MUCA-1998), the International Collaborative Study of Cardiovascular Disease in Asia (InterASIA), and the Community Intervention of Metabolic Syndrome in China and Chinese Family Health Study (CIMIC). The detailed designs for three cohorts have been illustrated elsewhere.[11] Briefly, the China MUCA-1998 and InterASIA cohorts were established in 1998 and 2000−2001, respectively. The first follow-up survey for these two cohorts was conducted from 2007 to 2008, and the second follow-up survey was conducted from 2012 to 2015. The CIMIC cohort was established in 2007−2008 and followed up in 2012−2015.
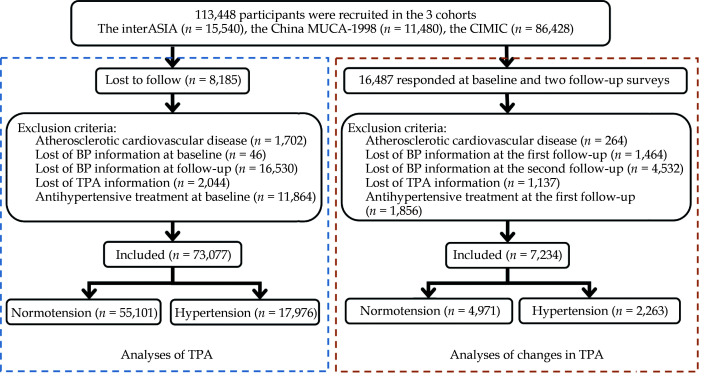
In total, 113,448 participants were enrolled at baseline and 105,263 participants (92.79%) had follow-up data. Of these participants followed up successfully, we excluded 1,702 participants with atherosclerotic cardiovascular disease (ASCVD), 46 participants with missing blood pressure (BP) information at baseline, 16,530 participants with missing BP information at follow-up, 2,044 participants with missing TPA information at baseline, and 11,864 participants with antihypertensive treatment at baseline. Finally, a total of 73,077 participants (55,101 normotensives and 17,976 hypertensives) were available for the analyses of TPA (Figure 1). A sample of 16,487 participants who responded to all three consecutive surveys from the baseline to the second follow-up survey were drawn from the China MUCA-1998 and InterASIA cohorts. With the similar exclusion criteria, a total of 4,971 normotensives and 2,263 hypertensives with TPA levels at baseline and the first follow-up survey served as the basis for analyses regarding the changes in TPA.
Figure 1.
Flow chart of study participants included and excluded in the analyses.
BP: blood pressure; China MUCA-1998: China Multi-Center Collaborative Study of Cardiovascular Epidemiology 1998; CIMIC: Community Intervention of Metabolic Syndrome in China and Chinese Family Health Study; InterASIA: International Collaborative Study of Cardiovascular Disease in Asia; TPA: total physical activity.
These preceding studies were approved by the Institutional Review Board at Fuwai Hospital in Beijing and participating institutions. Informed consent was obtained from each participant before data collection.
Data Collection
The protocols and questionnaires were similar for all cohorts at baseline and the follow-up surveys. A standardized questionnaire was used to collect information about demographic characteristics (age, gender, and educational level), personal medical history [ASCVD, hypertension, diabetes mellitus (DM), and dyslipidemia] and lifestyle risk factors (smoking status, drinking status, and TPA) by trained interviewers. A smoker was defined as someone who smoked more than 20 packs (or 500 grams of dry tobacco) during their lifetime. An alcohol drinker was defined as someone who drank at least once per week in the prior year. Physical examinations were also carried out among participants. Bodyweight and height were measured following a standard protocol with lightweight clothing and no shoes, and body mass index (BMI) was calculated as weight in kilograms divided by height in square meters (kg/m2). Additionally, overnight fasting blood samples were drawn from participants to measure serum lipid and glucose levels. DM was defined as having fasting glucose ≥ 126 mg/dL, and/or using insulin, and/or using oral hypoglycemic agents. Dyslipidemia was defined as having fasting cholesterol ≥ 240 mg/dL, and/or triglyceride (TG) ≥ 200 mg/dL, and/or high-density lipoprotein cholesterol (HDL-C) < 40 mg/dL, and/or low-density lipoprotein cholesterol (LDL-C) ≥ 160 mg/dL, and/or using lipid-lowing agents.
Blood Pressure Measurement
BP was measured by trained observers in the sitting position after at least a 5 min rest of each participant. An appropriate arm-cuff was chosen based on the circumference of the participant’s arm and placed on the right arm. Additionally, alcohol drinking, smoking, coffee/tea, and exercise were avoided for at least 30 min before the BP measurements. BP measurements were repeated 3 times by using a standard mercury sphygmomanometer or electronic BP monitor with a 30 s interval between each measurement and the average of 3 BP readings was used in the analyses. Hypertension was defined as SBP ≥ 140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg or using antihypertensive agents within the past two weeks. Consistent with previous studies,[12] the incident date of hypertension was identified as the date of the first diagnosis or initial use of antihypertensive agents. Briefly, for those who were not aware of their hypertension until the follow-up survey, the date of incident hypertension was defined as the date of the follow-up survey. For those using antihypertensive agents during follow-up, the date of incident hypertension was defined as the date of initial use of antihypertensive agents. Progression of hypertension was defined as ≥ 1 step increase in the BP stage or starting antihypertensive agents at follow-up. Remission of hypertension was defined as ≥1 step decrease in the BP stage without antihypertensive treatment at follow-up. Stage 1 hypertension was defined as 140 ≤ SBP < 159 mmHg and/or 90 ≤ DBP < 99 mmHg, stage 2 as 160 ≤ SBP < 179 mmHg and/or 100 ≤ DBP < 109 mmHg, and stage 3 as SBP ≥ 180 mm Hg and/or DBP ≥ 110 mm Hg. [13]
Physical Activity Assessment
A standardized questionnaire was used to collect information about the duration of time spent on vigorous-intensity, moderate-intensity, and light-intensity activities per day during the prior year.[14] Information on leisure-time PA, occupational PA, commuting to work, and household chores were collected for each participant. TPA was estimated as metabolic equivalents (METs)—a ratio of energy expended during an activity to the rate of energy expended at rest, and 1 MET is approximately equivalent to an oxygen uptake of 3.5 [mL·kg−1·min−1].[15] We quantified the types of PA following the 2011 Compendium of Physical Activities characterizing light-intensity PA (2.0 METs), moderate-intensity PA (4.0 METs), and vigorous-intensity PA (8.0 METs).[16] TPA was defined as the accumulation of light-intensity PA, moderate-intensity PA, and vigorous-intensity PA. Four TPA categories were established based on the quartiles of MET levels (supplemental Table 1S).
For analyses of changes in TPA, we compared incident hypertension and the progression/remission of hypertension among four groups: those sedentary at both baseline and the follow-up (stayed sedentary, being in the lowest 25%); those physically active at baseline and sedentary at follow-up (became sedentary, moved from highest 75% to lowest 25%); those sedentary at baseline and physically active at follow-up (became active, moved from lowest 25% to highest 75%); and those physically active at both visits (stayed active, being in the highest 75%).
Statistical Analysis
The baseline characteristics of the included participants were presented as mean ± SD for continuous variables and as percentages for categorical variables. Person-years of follow-up for each participant were calculated as the difference between the date of the baseline examination and the incident date of hypertension or the last follow-up interview.
In the current study, we used the Cox proportional hazards regression model stratified by cohort to assess the hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) for incident hypertension in relation to TPA or changes in TPA among participants without hypertension at baseline. Tests of the trend for HRs across TPA categories were performed by including the medium values of each category as a continuous variable in the Cox models. A sensitivity analysis was performed by excluding new-onset hypertension within one year after recruitment (n= 376) to assess the potential selection bias. Moreover, the multivariable logistic regression models were used to estimate odds ratios (ORs) and 95% CIs of progression/remission of hypertension in relation to TPA or changes in TPA among hypertensives. Sensitivity analyses with exclusion of participants receiving antihypertensive agents during follow-up were also performed.
Age, gender, BMI, region (north/south China), area (urban/rural area), educational level, smoking status, alcohol drinking status, blood glucose level, total cholesterol level, and baseline SBP were adjusted for analyzing the association between the TPA and hypertension. For analyses of changes in TPA, the total MET at baseline was further adjusted in the multivariate-adjusted analyses. The statistical analyses were performed using SAS 9.4 (SAS Institute Inc, Cary, NC). A two-sided P value < 0.05 was defined as statistical significance.
RESULTS
Baseline Characteristics
The baseline characteristics of the study population are presented in Table 1. The mean (SD) age of participants was 50.22 ± 11.65 years, 39.40% of them were males. The mean BMI was 23.57 ± 3.49 kg/m2, and the mean TPA was 36.45 ± 23.36 MET·h/d. Hypertensive participants were older, more likely to live in northern and rural areas, tended to have higher BMI, higher prevalence of DM and dyslipidemia, and lower TPA levels.
Table 1. Baseline characteristics of the study participants.
| Characteristic | Overall
(n= 73,077) |
Normotensive
(n= 55,101) |
Hypertensive
(n= 17,976) |
| Data are presented as n(%) or mean ± SD. BMI: body mass index; DBP: diastolic blood pressure; MET: metabolic equivalent; SBP: systolic blood pressure; TPA: total physical activity. | |||
| Age, yrs | 50.22 ± 11.65 | 48.72 ± 11.54 | 54.81 ± 10.77 |
| Male | 28,790 (39.40%) | 21,205 (38.48%) | 7,585 (42.20%) |
| BMI, kg/m2 | 23.57 ± 3.49 | 23.15 ± 3.31 | 24.85 ± 3.69 |
| Northern | 36,528 (49.99%) | 25,199 (45.73%) | 11,329 (63.02%) |
| Rural area | 65,607 (89.78%) | 49,133 (89.17%) | 16,474 (91.64%) |
| Less than a high school education | 62,375 (85.63%) | 46,336 (84.37%) | 16,039 (89.47%) |
| Smoker | 18,662 (25.62%) | 14,210 (25.88%) | 4,452 (24.84%) |
| Alcohol drinker | 13,496 (18.47%) | 9,581 (17.39%) | 3,915 (21.78%) |
| Diabetes mellitus | 3,414 (4.94%) | 2,135 (4.11%) | 1,279 (7.40%) |
| Dyslipidemia | 23,263 (31.85%) | 16,950 (30.78%) | 6,313 (35.13%) |
| SBP, mmHg | 125.13 ± 19.20 | 117.03 ± 11.49 | 149.94 ± 16.66 |
| DBP, mmHg | 77.70 ± 11.06 | 73.63 ± 7.96 | 90.19 ± 9.82 |
| TPA, MET·h/d | 36.45 ± 23.36 | 37.12 ± 23.33 | 34.38 ± 23.32 |
TPA and Incident Hypertension
During a total of 394,038 person-years of follow-up, 12,211 hypertension cases were identified (5,161 cases in men and 7,050 cases in women) from 55,101 normotensives. Participants in the third and the highest quartiles of TPA showed significant relative risk reductions of developing hypertension when compared with those in the lowest quartile, with the fully adjusted HRs (95% CIs) of 0.86 (0.81−0.91) and 0.81 (0.77−0.86), respectively (Table 2). In a sensitivity analysis, the estimations for the effects of TPA on incident hypertension were similar after excluding cases occurring in the first year of follow-up (supplemental Table 2S).
Table 2. Hazard ratios of incident hypertension according to quartiles of total physical activity.
| Variables | Quartile 1
(n= 13,482) |
Quartile 2
(n= 13,561) |
Quartile 3
(n= 13,783) |
Quartile 4
(n= 14,275) |
P-trend |
| Model 1: adjusted for age, gender, BMI, region, and area. Model 2: adjusted for covariates in model 1 plus educational level, smoking status, alcohol drinking status, blood glucose level and total cholesterol level.
Model 3: adjusted for covariates in model 2 plus baseline SBP. **P < 0.001 vs. the quartile 1 group. | |||||
| Cases | 3,519 | 3,228 | 2,699 | 2,765 | − |
| Person-years | 103,172.97 | 101,592.40 | 93,965.42 | 95,306.86 | − |
| Incidence rate (per 1000 person-years) | 34.10 | 31.77 | 28.72 | 29.01 | − |
| HR (95% CI) | |||||
| Model 1 | 1.00 | 1.01 (0.96−1.06) | 0.86 (0.81−0.91)** | 0.80 (0.76−0.84)** | < 0.001 |
| Model 2 | 1.00 | 1.01 (0.96−1.06) | 0.87 (0.82−0.92)** | 0.80 (0.76−0.85)** | < 0.001 |
| Model 3 | 1.00 | 1.01 (0.96−1.06) | 0.86 (0.81−0.91)** | 0.81 (0.77−0.86)** | < 0.001 |
TPA and Progression/Remission of Hypertension
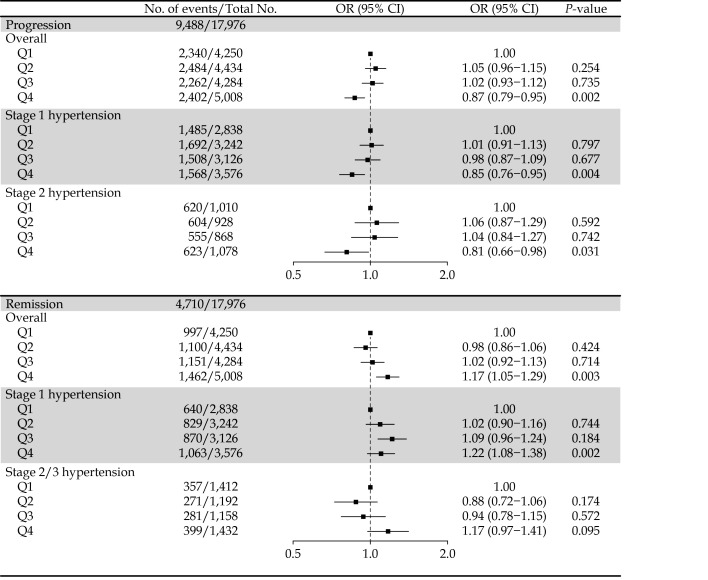
A total of 17,976 hypertensives were available for analyses of the progression of hypertension. Compared with those in the lowest quartile of the TPA group, hypertensive patients in the highest quartile had a significantly lower risk of the progression of hypertension, with the adjusted OR (95% CI) of 0.87 (0.79−0.95) (Figure 2). When stratified according to hypertension grade, the highest quartile of TPA was associated with a 15% (OR = 0.85; 95% CI: 0.76−0.95) and 19% (OR = 0.81; 95% CI: 0.66−0.98) lower risk of progression for stage 1 hypertensive patients and stage 2 hypertensive patients, respectively.
Figure 2.
Odd ratios (95% CI) of progression and remission of hypertension among hypertensive participants according to quartiles of total physical activity.
Model adjusted for age, gender, BMI, region, area, educational level, smoking status, alcohol drinking status, blood glucose level, total cholesterol level and baseline SBP. Q1: < 25 th percentile; Q2: 25th-50th percentile; Q3: 50th-75th percentile; Q4: >75 th percentile. BMI: body mass index; SBP: systolic blood pressure.
High levels of the TPA also had a protective effect on hypertension remission among hypertensive patients (Figure 2). Compared with patients in the lowest quartile, those in the highest quartile had a 17% (OR = 1.17; 95% CI: 1.05−1.29) higher probability of hypertension remission, and the effect was more pronounced among stage 1 hypertensive patients (OR = 1.22; 95% CI: 1.08−1.38). The protective effects of the TPA levels did not achieve statistical significance (OR = 1.17; 95% CI: 0.97−1.41) among stage 2/3 hypertensive patients.
Sensitivity analyses with exclusion of participants receiving antihypertensive agents during follow-up did not materially change the results (supplemental Figure 1S).
Changes in TPA and Hypertension
Among 4,971 normotensives in the China MUCA-1998 and InterASIA cohorts, a total of 734 (14.77%) participants became active and 729 (14.67%) became sedentary between baseline and the first follow-up survey (Table 3). Compared with participants who were sedentary at both visits, sedentary participants had significantly reduced risk of incident hypertension (HR = 0.75; 95% CI: 0.58−0.96) when they became active, whereas participants who stayed active during two visits had a 25% decrease in incident hypertension (HR = 0.75; 95% CI: 0.60−0.95). We also observed that those being a high level of TPA at the baseline but becoming sedentary at the follow-up period, did not achieve benefit from initially being active (HR = 0.98; 95% CI: 0.77−1.25).
Table 3. Hazard ratios of incident hypertension according to changes in total physical activity.
| Variables | Stayed Sedentary
(n= 482) |
Became Active
(n= 734) |
Became Sedentary
(n = 729) |
Stayed Active
(n= 3,026) |
| Model 1: adjusted for age, gender, BMI, region, area, and baseline total MET. Model 2: adjusted for covariates in model 1 plus educational level, smoking status, alcohol drinking status, blood glucose level, total cholesterol level. Model 3: adjusted for covariates in model 2 plus baseline SBP. *P < 0.05 vs. the stayed sedentary group. **P < 0.01 vs. the stayed sedentary group. BMI: body mass index; MET: metabolic equivalents; SBP: systolic blood pressure. | ||||
| Cases | 122 | 180 | 205 | 753 |
| Person-years | 2,566.72 | 4,125.83 | 3,834.62 | 16,250.84 |
| Incidence rate (per 1000 person-years) | 47.53 | 43.63 | 53.46 | 46.34 |
| HR (95% CI) | ||||
| Model 1 | 1.00 | 0.69 (0.54−0.88)** | 0.96 (0.76−1.21) | 0.73 (0.59−0.92)** |
| Model 2 | 1.00 | 0.73 (0.57−0.94)* | 0.96 (0.75−1.22) | 0.71 (0.56−0.89)** |
| Model 3 | 1.00 | 0.75 (0.58−0.96)* | 0.98 (0.77−1.25) | 0.75 (0.60−0.95)* |
Similar to the effect of changes in TPA on incident hypertension, both becoming active (OR = 1.23; 95% CI: 0.84−1.80) and staying active (OR = 1.32; 95% CI: 0.92−1.90) among hypertensives showed a higher probability of hypertension remission, although these findings did not reach significance due to the insufficient sample size (supplemental Figure 2S). We did not observe the significant protective effects of staying active (OR = 0.84; 95% CI: 0.59−1.19) and becoming active (OR = 0.97; 95% CI: 0.67−1.39) on the risk of progression among hypertensives. Sensitivity analysis excluding participants receiving antihypertensive agents during follow-up showed similar associations (supplemental Figure 3S).
DISCUSSION
In this large-scale prospective cohort study, we comprehensively examined the health benefits of TPA and its changes for the incidence, progression, and remission of hypertension. This study provided evidence that increased TPA levels could reduce the risk of incident hypertension among normotensives, and higher levels of the TPA could ameliorate hypertension among hypertensives. Moreover, getting active from a sedentary lifestyle rather than becoming sedentary from physically active reduced the risks of incident hypertension among normotensives. These results are of great public health significance and will allow us to conduct preventive interventions among the population.
Hypertension, an essential public-health challenge worldwide, remains inadequately controlled,[2,17] and PA was regarded as the nonpharmacological intervention for the prevention of hypertension in the guidelines.[18-20] Using the TPA, which integrated both the intensity and duration of PA and provided comprehensive information on daily activities, the current study confirmed the health benefits of increased TPA on the prevention of incident hypertension among normotensives. We observed the reduction in risks of incident hypertension in the third and the highest quartiles of TPA (more than 36 MET·h/day for males and 32 MET·h/day for females, corresponding to about 1 h of walking per day in addition to moderate-intensity occupational activities such as police, tailor, and baker). Be consistent with our findings, a prospective study in Japan suggested that increased daily life activity (more than 33.3 MET·h/day) was effective for the prevention of hypertension.[21] Another prospective study in Finland also observed the protective effects of TPA on developing hypertension though the TPA was not quantified.[22] However, the Trøndelag Health Study (HUNT), a longitudinal study including 26,539 participants in Norway did not observe statistically significant associations between the TPA and incident hypertension.[5] The controversial results might be due to the different methods of the TPA calculation. A weighted summary score of frequency, duration, and intensity of PA was used to assess the TPA in the HUNT Study, while the current study used the overall MET levels, which was more accurate because MET is an index of energy expenditure that quantifies the total amount of PA performed in a standardized manner across individuals and types of activities.[15]
Additionally, our results showed that a higher level of the TPA could reduce the risk of progression of hypertension and increase the probability of hypertension remission for hypertensives. The possible mechanisms of the antihypertensive effects included the reduction of vascular resistance, cardiac output, sympathetic nervous activity, plasma norepinephrine level and adiposity, and the improvement of insulin sensitivity, endothelial function, and energy balance.[23-27] The current guidelines from the European Society of Cardiology (ESC) prescribed a higher level of moderate-intensity PA for hypertensive individuals compared with the recommendations for healthy individuals.[28] Consistently, in the current study, the TPA level (the highest quartile, more than 56 MET·h/day for males and 48 MET·h/day for females) that hypertensive patients could achieve benefit from was higher than that for normotensives (the third quartile of TPA level) (supplemental material, Table 1S). Moreover, the protective effects of TPA on remission of hypertension did not reach significance among participants with stage 2/3 hypertension. This result supported the Scientific Statement from the American Heart Association (AHA), which recommended that PA combined with other nonpharmacological methods and antihypertensive medications were prescribed for patients with stage 2/3 hypertension.[19] Further investigation is warranted to determine the appropriate intensity and duration of PA for participants with different BP levels.
Changes in TPA have been related to cardiovascular diseases and cardiometabolic risk factors,[29-31] however, the effects of changes in the TPA on incident hypertension and progression/remission of hypertension are less known. Our results provided novel evidence that maintaining high TPA levels as well as getting active from a sedentary lifestyle during the follow-up period were associated with a lower risk of developing hypertension in the Chinese population. The Finnish Public Sector Study, including 15,634 cardio-metabolically healthy participants, found that there was a potential trend towards lower incident hypertension with increasing leisure-time and commuting PA over time, however, no significant association was found (OR = 0.70; 95% CI: 0.47−1.05).[31] Our study suggested that the TPA might take precedence over leisure-time PA with regard to the prevention of hypertension. More importantly, we did not found a sustained legacy effect of TPA among those being a high level of TPA at the baseline but becoming sedentary at the follow-up, which emphasized the importance of adherence to a physically active lifestyle in hypertension prevention. The evidence on the effects of changes in TPA on hypertension was limited in the current guidelines.[18,20,28] We recommended that becoming and staying active could reduce the risk of developing hypertension for normotensives. With regard to hypertensive patients, we provided clues to the health benefits from changes in TPA to the progression and remission of hypertension, though the protective effects of increasing TPA levels and maintaining high levels of TPA in the follow-up period on progression and remission of hypertension did not achieve statistical significance due to the insufficient sample size. Further research in large populations was needed to confirm the results.
The current study investigated the effects of the TPA and the changes in TPA on incident hypertension and progression/remission of hypertension based on nationwide ongoing large cohorts in China, with a high follow-up rate of 92.79%, and using the standardized questionnaire and review process across different cohorts. However, several limitations of the current study should be noted. First, since the TPA information was self-reported, memory bias was inevitable. Secondly, the time spent on specific activity was not available, and it was insufficient for us to calculate the total MET by MET values of the specific activity. Thirdly, the speed of progression and remission of hypertension was not analyzed in the current study due to the insufficient sample size. Finally, reverse causality might be another potential bias. The TPA levels were affected by the participants’ health conditions. However, participants with ASCVD at the baseline were excluded, and excluding new-onset hypertension within one year after recruitment did not considerably alter the results.
In summary, the current study advanced our understanding of the health benefits from TPA and its changes in both normotensives and hypertensives. We highlighted the importance of increased TPA in hypertension prevention among normotensives. Higher TPA levels could effectively ameliorate hypertension for hypertensives, whereas for patients with stage 2/3 hypertension, TPA combined with other nonpharmacological or pharmacological methods should be recommended. Importantly, getting active from a sedentary lifestyle during the follow-up period could reduce the risk of incident hypertension, while the protective effect was not observed among those giving up a physically active lifestyle. Our findings highlight that PA intervention is an important component of strategies for both primary and secondary prevention of hypertension. Considering the increasing tendency toward a sedentary lifestyle, greater efforts are needed to implement a national fitness program to reduce the increasing burden of hypertension.
ACKNOWLEDGMENTS
The authors gratefully acknowledge all the staffs and participants of the China-PAR project for their participation and contribution.
POTENTIAL CONFLICTS OF INTEREST
None.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
Funding Statement
This study was supported by the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (2019-I2M-2-003 and 2017-I2M-1-004), National Key Research & Development Program of China (2018YFE0115300 and 2017YFC0211700, and 2017YFC0908401), and the National Natural Science Foundation of China (91643208). The sources of funding had no role in study design, data collection, analyses, interpretation, and decision to submit the article for publication
Contributor Information
Xiao-Yang ZHOU, Email: xiaoyangzh@whu.edu.cn.
Xiang-Feng LU, Email: luxf@pumc.edu.cn.
References
- 1.GBD 2017 Risk Factor Collaborators Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kearney PM, Whelton M, Reynolds K, et al Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 3.Zang J, Ng SW Age, period and cohort effects on adult physical activity levels from 1991 to 2011 in China. Int J Behav Nutr Phys Act. 2016;13:40. doi: 10.1186/s12966-016-0364-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Global Recommendations on Physical Activity for Health; World Health Organization Press: Switzerland, 2010; 1-58.
- 5.Stenehjem JS, Hjerkind KV, Nilsen TIL Adiposity, physical activity, and risk of hypertension: prospective data from the population-based HUNT Study, Norway. J Hum Hypertens. 2018;32:278–286. doi: 10.1038/s41371-018-0042-5. [DOI] [PubMed] [Google Scholar]
- 6.Liu X, Zhang D, Liu Y, et al Dose-Response Association Between Physical Activity and Incident Hypertension: A Systematic Review and Meta-Analysis of Cohort Studies. Hypertension. 2017;69:813–820. doi: 10.1161/HYPERTENSIONAHA.116.08994. [DOI] [PubMed] [Google Scholar]
- 7.Diaz KM, Booth JN, Seals SR, et al Physical Activity and Incident Hypertension in African Americans: The Jackson Heart Study. Hypertension. 2017;69:421–427. doi: 10.1161/HYPERTENSIONAHA.116.08398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng L, Sun Z, Zhang X, et al Predictors of progression from prehypertension to hypertension among rural Chinese adults: results from Liaoning Province. Eur J Cardiovasc Prev Rehabil. 2010;17:217–222. doi: 10.1097/HJR.0b013e328334f417. [DOI] [PubMed] [Google Scholar]
- 9.Faselis C, Doumas M, Kokkinos JP, et al Exercise capacity and progression from prehypertension to hypertension. Hypertension. 2012;60:333–338. doi: 10.1161/HYPERTENSIONAHA.112.196493. [DOI] [PubMed] [Google Scholar]
- 10.Ng SW, Popkin BM Time use and physical activity: a shift away from movement across the globe. Obes Rev. 2012;13:659–680. doi: 10.1111/j.1467-789X.2011.00982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang X, Li J, Hu D, et al Predicting the 10-Year Risks of Atherosclerotic Cardiovascular Disease in Chinese Population: The China-PAR Project (Prediction for ASCVD Risk in China) Circulation. 2016;134:1430–1440. doi: 10.1161/CIRCULATIONAHA.116.022367. [DOI] [PubMed] [Google Scholar]
- 12.Huang K, Yang X, Liang F, et al Long-Term Exposure to Fine Particulate Matter and Hypertension Incidence in China. Hypertension. 2019;73:1195–1201. doi: 10.1161/HYPERTENSIONAHA.119.12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams B, Mancia G, Spiering W, et al 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 14.Han C, Liu F, Yang X, et al Ideal cardiovascular health and incidence of atherosclerotic cardiovascular disease among Chinese adults: the China-PAR project. Sci China Life Sci. 2018;61:504–514. doi: 10.1007/s11427-018-9281-6. [DOI] [PubMed] [Google Scholar]
- 15.Garber CE, Blissmer B, Deschenes MR, et al American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 16.Ainsworth BE, Haskell WL, Herrmann SD, et al 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 17.Lu J, Lu Y, Wang X, et al Prevalence, awareness, treatment, and control of hypertension in China: data from 1.7 million adults in a population-based screening study (China PEACE Million Persons Project) Lancet. 2017;390:2549–2558. doi: 10.1016/S0140-6736(17)32478-9. [DOI] [PubMed] [Google Scholar]
- 18.Eckel RH, Jakicic JM, Ard JD, et al 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S76–99. doi: 10.1161/01.cir.0000437740.48606.d1. [DOI] [PubMed] [Google Scholar]
- 19.Brook RD, Appel LJ, Rubenfire M, et al Beyond medications and diet: alternative approaches to lowering blood pressure: a scientific statement from the american heart association. Hypertension. 2013;61:1360–1383. doi: 10.1161/HYP.0b013e318293645f. [DOI] [PubMed] [Google Scholar]
- 20.Nerenberg KA, Zarnke KB, Leung AA, et al Hypertension Canada's 2018 Guidelines for Diagnosis, Risk Assessment, Prevention, and Treatment of Hypertension in Adults and Children. Can J Cardiol. 2018;34:506–525. doi: 10.1016/j.cjca.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 21.Nakanishi N, Suzuki K Daily life activity and the risk of developing hypertension in middle-aged Japanese men. Arch Intern Med. 2005;165:214–220. doi: 10.1001/archinte.165.2.214. [DOI] [PubMed] [Google Scholar]
- 22.Hu G, Barengo NC, Tuomilehto J, et al Relationship of physical activity and body mass index to the risk of hypertension: a prospective study in Finland. Hypertension. 2004;43:25–30. doi: 10.1161/01.HYP.0000107400.72456.19. [DOI] [PubMed] [Google Scholar]
- 23.Mancia G, Fagard R, Narkiewicz K, et al 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31:1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 24.Floras JS, Sinkey CA, Aylward PE, et al Postexercise hypotension and sympathoinhibition in borderline hypertensive men. Hypertension. 1989;14:28–35. doi: 10.1161/01.HYP.14.1.28. [DOI] [PubMed] [Google Scholar]
- 25.Cornelissen VA, Fagard RH Effects of endurance training on blood pressure, blood pressure-regulating mechanisms, and cardiovascular risk factors. Hypertension. 2005;46:667–675. doi: 10.1161/01.HYP.0000184225.05629.51. [DOI] [PubMed] [Google Scholar]
- 26.Cleroux J, Kouame N, Nadeau A, et al Aftereffects of exercise on regional and systemic hemodynamics in hypertension. Hypertension. 1992;19:183–191. doi: 10.1161/01.HYP.19.2.183. [DOI] [PubMed] [Google Scholar]
- 27.Arakawa K Antihypertensive mechanism of exercise. J Hypertens. 1993;11:223–229. doi: 10.1097/00004872-199303000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Pelliccia A, Sharma S, Gati S, et al 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2020;00:1–80. doi: 10.1093/eurheartj/ehaa735. [DOI] [PubMed] [Google Scholar]
- 29.Petersen CB, Gronbaek M, Helge JW, et al Changes in physical activity in leisure time and the risk of myocardial infarction, ischemic heart disease, and all-cause mortality. Eur J Epidemiol. 2012;27:91–99. doi: 10.1007/s10654-012-9656-z. [DOI] [PubMed] [Google Scholar]
- 30.Florido R, Kwak L, Lazo M, et al Six-Year Changes in Physical Activity and the Risk of Incident Heart Failure: ARIC Study. Circulation. 2018;137:2142–2151. doi: 10.1161/CIRCULATIONAHA.117.030226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leskinen T, Stenholm S, Heinonen OJ, et al Change in physical activity and accumulation of cardiometabolic risk factors. Prev Med. 2018;112:31–37. doi: 10.1016/j.ypmed.2018.03.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.