Abstract
OBJECTIVE
Hybrid coronary revascularization (HCR) combines a minimally invasive surgical approach to the left anterior descending (LAD) artery with percutaneous coronary intervention (PCI) for non-LAD diseased coronary arteries. It is associated with shorter hospital lengths of stay and recovery times than conventional coronary artery bypass surgery, but there is little information comparing it to isolated PCI for multivessel disease. Our objective is to compare long-term outcomes of HCR and PCI for patients with multivessel disease.
METHODS
This cohort study used data from New York’s cardiac surgery and PCI registries in 2010−2016 to examine mortality and repeat revascularization rates for patients with multivessel coronary artery disease who underwent HCR and PCI. Cox proportional hazards methods were used to reduce selection bias. Patients were followed for a median of four years.
RESULTS
There was a total of 335 HCR patients (1.2%) and 25,557 PCI patients (98.8%) after exclusions. There was no difference in 6-year risk adjusted survival between HCR and PCI patients (83.17% vs. 81.65%, adjusted hazard ratio (aHR) = 0.90 (95% CI: 0.67−1.20). However, HCR patients were more likely to be free from repeat revascularization in the LAD artery (91.13% vs. 83.59%, aHR = 0.51 (95% CI: 0.34−0.77)).
CONCLUSIONS
For patients with multi-vessel coronary artery disease, HCR is rarely performed. There are no differences in mortality rates after four years, but HCR is associated with lower repeat revascularization rates in the LAD artery, presumably due to better longevity in left arterial mammary grafts.
For most patients with multivessel disease coronary artery disease, either coronary artery bypass graft (CABG) surgery or percutaneous coronary intervention (PCI) is the recommended option. The advantage of CABG surgery is generally the durability of the bypass grafts, and CABG surgery is recommended especially among the highest risk patients (e.g., three vessel disease, left main (LM) disease, multivessel disease with proximal left anterior descending artery (LAD) disease).[1-4] Nevertheless, an advantage of CABG surgery is the superior outcomes achieved with left internal mammary artery (LIMA) grafts to the LAD for patients with LAD disease.[5-7]
Hybrid coronary revascularization (HCR) is an approach that has been developed to combine the main advantages of both CABG surgery and PCI. It consists of using a LIMA anastomosis to the LAD via a minimally invasive CABG surgery approach (no sternotomy) in addition to PCI for other diseased coronary arteries. The rationale for using this approach in lieu of using PCI for all diseased coronary arteries is the potential for more durability of the LAD revascularization as a result of the LIMA to LAD anastomosis. Several studies have compared HCR to CABG surgery, but they are limited with respect to sample size, number of institutions represented, duration, and inability to capture population-based practice.[8-24] Multi-center studies comparing HCR with PCI, which are arguably more relevant since these two alternatives are the least invasive ones, are extremely limited.[25,26]
The purposes of this study are to: (1) describe the use of HCR and the characteristics of patients undergoing HCR vs. PCI in a population-based setting, and (2) compare short- and medium-term outcomes for HCR and PCI for patients with multi-vessel coronary artery disease accompanied by LAD disease using New York’s clinical cardiac registries.
METHODS
This study did not require Institutional Review Board approval because it was a retrospective study with encrypted patient identifiers. Patients and the public were not involved in the design or conduct of the study.
Databases
Patients in the study were identified using the New York State Department of Health’s Cardiac Surgery Reporting System (CSRS) and Percutaneous Coronary Interventions Reporting System (PCIRS) registries. These registries contain all patients who undergo CABG surgery (as well as other cardiac surgery) and PCI, respectively, in non-federal hospitals in the state. The registries were linked using patient identifiers, hospital identifiers, and dates of admission, procedure and discharge to the New York State Vital Statistics death registry to obtain deaths that occurred after discharge. Also, the CSRS and PCIRS were linked to one another to obtain information on procedures that were undergone by each of the patients receiving HCR and to obtain information on repeat revascularizations.
Study Population
Patients were initially eligible for the study if: (1) they had multivessel disease (at least 70% stenosis in two or more major epicardial coronary arteries) that included a diseased LAD artery (≥ 70% stenosis); and (2) they underwent either minimally invasive CABG surgery (no sternotomy) or PCI in the LAD artery between 1/10/10 and 11/30/16; and (3) they underwent elective PCI in one or more other diseased arteries within 60 days before or after the LAD procedure without any other concomitant major cardiac surgery (e.g., valve surgery). HCR patients were identified by first finding multivessel disease patients in the CSRS registry who underwent isolated CABG surgery performed on the LAD artery with minimally invasive surgery, and then querying the PCIRS registry to link PCI procedures performed within 60 days before or after the CABG surgery in non-LAD vessels. PCI patients in the study who underwent a staged PCI (coded in the index admission as intended to be staged, and who underwent a second non-emergency PCI in a different vessel that was coded as the second part of a staged procedure) within 60 days of the index PCI were collapsed into a single admission so that the staged procedure would not be regarded as a different patient.
Initially, 33,954 patients were candidates for the study. Patients were then sequentially excluded for the following reasons: hemodynamic instability or shock (496), previous organ transplant (171), prior cardiac surgery (1,979), emergency and salvage patients (30), out-of-state patients (1,421), and patients with invalid social security numbers (1,965). Out-of-state patients were excluded because deaths after discharge could not be identified using New York’s vital statistics data, and patients with invalid social security numbers were excluded because of the difficulty of linking them to vital statistics data. The remaining 335 HCR patients and 27,557 PCI patients were subjects of the study.
Outcomes
The primary outcome was all-cause mortality. Patients were followed from the date of their index revascularization (the first of the hybrid or PCI procedures performed) through December 31, 2017. We also examined differences in repeat revascularization in the LAD artery, defined as any unstaged revascularization (PCI or CABG surgery) in the LAD artery after the index HCR procedure or PCI. The median follow-up time for outcomes was four years.
Statistical Analysis
Because this is an observational study with substantial differences in patient characteristics between the two interventions, we used Cox proportional hazards models (one for mortality and one for repeat revascularization in the LAD artery) to adjust for patient risk factors for adverse outcomes and to reduce treatment selection bias. All patient characteristics in the registry were used as independent variables, the adverse outcome (mortality or repeat revascularization in the LAD artery) was used as the dependent variable, and treatment type (HCR, PCI) was the study variable. Significance was identified using the adjusted hazard ratio from the Cox models, and adjusted survival curves were created using the corrected group prognosis method described by Ghali, et al.[27] Numerous pre-selected patient subgroups, including the highest volume HCR hospitals and surgeons, were also tested for differences in mortality.
All reported P-values are two-sided, and all analyses were performed using SAS, version 9.4 (SAS Institute, Cary, N.C.). Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
RESULTS
Characteristics of the Study Population
There was a total of 335 HCR (1.20%) patients and 25,557 PCI patients (98.80%) after the exclusion criteria were applied. The percentage of HCRs performed did not vary greatly from year to year, with lows of 0.91% in 2010, and a high of 1.55% in 2014. HCR was performed in a total of 16 of the 41 hospitals in which cardiac surgery was performed during the study period. However, 85% of all HCRs were performed in six of those hospitals. Also, the surgical part of HCR was performed by 21 of the 211 surgeons performing isolated CABG during the study period, but 81% of all HCRs were performed by six of those surgeons. Of the HCRs performed in the study period, PCI and CABG were performed in the same admission 44% of the time, CABG was performed in an earlier admission 18% of the time, and PCI was performed in an earlier admission 38% of the time. Of the 335 HCR cases, 320 underwent off-pump surgery, and another five underwent off-pump surgery followed by on-pump surgery. The remaining 10 patients underwent on-pump surgery.
Table 1 presents baseline characteristics of HCR and PCI patients. As indicated, HCR patients were less likely to be women, had lower body mass indices, and lower prevalence of myocardial infarctions. They also had a higher likelihood of having a history of congestive heart failure, and were more likely to have three vessel disease, proximal LAD disease, and left main disease.
Table 1. Baseline characteristics of hybrid coronary revascularization and PCI patients: New York, 2010-2016.
| Prevalence in HCR patients
(n = 335) |
Prevalence in PCI patients
(n = 27,557) |
P-value for univariate
analysis |
|
| Data are presented as n (%). BMI: body mass index; LAD: left anterior descending; PCI: percutaneous coronary intervention. | |||
| Demographic | |||
| Patient age, yrs | 0.28 | ||
| 18−44 | 8 (2.39%) | 933 (3.39%) | |
| 45−54 | 56 (16.72%) | 4083 (14.82%) | |
| 55−64 | 97 (28.96%) | 8028 (29.13%) | |
| 65−74 | 108 (32.24%) | 7987 (28.98%) | |
| 75−84 | 55 (16.42%) | 5066 (18.38%) | |
| ≥ 85 | 11 (3.28%) | 1460 (5.30%) | |
| Sex | 0.0042 | ||
| Male | 263 (78.51%) | 19,676 (71.40%) | |
| Female | 72 (21.49%) | 7881 (28.60%) | |
| BMI | 0.030 | ||
| < 18.5 kg/m 2 | 4 (1.19%) | 219 (0.79%) | |
| ≥ 18.5 and < 25 kg/m 2 | 89 (26.57%) | 5755 (20.88%) | |
| ≥ 25 and < 30 kg/m 2 | 127 (37.91%) | 10,657 (38.67%) | |
| ≥ 30 and < 35 kg/m 2 | 81 (24.18%) | 6702 (24.32%) | |
| ≥ 35 and < 40 kg/m 2 | 25 (7.46%) | 2736 (9.93%) | |
| ≥ 40 kg/m2 | 9 (2.69%) | 1488 (5.40%) | |
| Ventricular function | |||
| Ejection fraction | 0.068 | ||
| Ejection fraction 50% or greater | 250 (74.63%) | 19,807 (71.88%) | |
| Ejection fraction 40%−49% | 55 (16.42%) | 4319 (15.67%) | |
| Ejection fraction 30%−39% | 25 (7.46%) | 2048 (7.43%) | |
| Ejection fraction 20%−29% | 4 (1.19%) | 1142 (4.14%) | |
| Ejection fraction less than 20% | 1 (0.30%) | 241 (0.87%) | |
| Pre−procedural MI | 0.0037 | ||
| STEMI in 24 h | 32 (9.55%) | 3459 (12.55%) | |
| NSTEMI in 24 h | 8 (2.39%) | 1561 (5.66%) | |
| MI with or without ST elevation 1−20 days | 57 (17.01%) | 5323 (19.32%) | |
| No MI within 20 days | 238 (71.04%) | 17,214 (62.47%) | |
| Patient history | |||
| Previous PCI procedure | 57 (17.01%) | 4708 (17.08%) | 0.97 |
| Comorbidities | |||
| Congestive heart failure | 0.042 | ||
| Current | 26 (7.76%) | 1954 (7.09%) | |
| Past | 16 (4.78%) | 721 (2.62%) | |
| None | 293 (87.46%) | 24,882 (90.29%) | |
| Chronic lung disease | 16 (4.78%) | 1576 (5.72%) | 0.46 |
| Cerebrovascular disease | 34 (10.15%) | 2377 (8.63%) | 0.32 |
| Peripheral vascular disease | 28 (8.36%) | 2014 (7.31%) | 0.46 |
| Diabetes requiring medication | 119 (35.52%) | 9383 (34.05%) | 0.57 |
| Renal Failure | 0.71 | ||
| Creatinine ≤ 1.2 mg/dL | 249 (74.33%) | 19,890 (72.18%) | |
| Creatinine > 1.2 and ≤ 1.5 mg/dL | 63 (18.81%) | 5357 (19.44%) | |
| Creatinine > 1.5 and ≤ 2.0 mg/dL | 11 (3.28%) | 1222 (4.43%) | |
| Creatinine > 2.0 mg/dL or Require renal dialysis | 12 (3.58%) | 1088 (3.95%) | |
| Vessels | |||
| 3-vessel disease | 174 (51.94%) | 9694 (35.18%) | < 0.0001 |
| Proximal LAD disease | 232 (69.25%) | 11,710 (42.49%) | < 0.0001 |
| LMT disease | 45 (13.43%) | 665 (2.41%) | < 0.0001 |
Outcomes
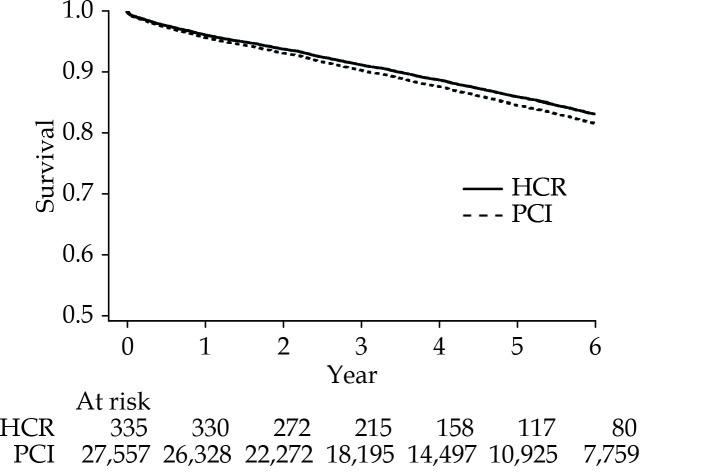
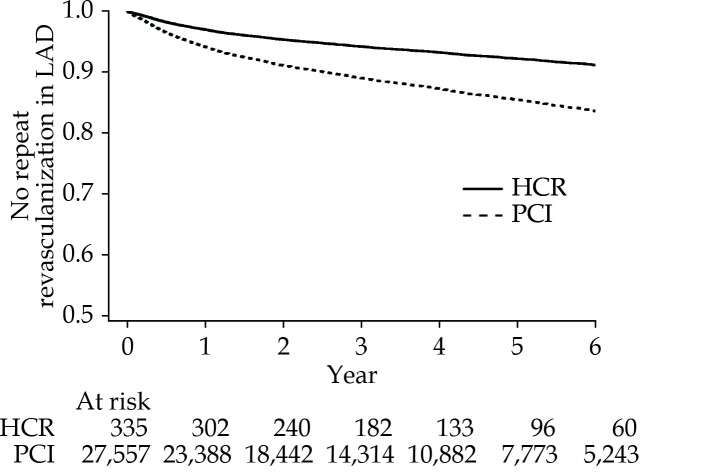
Figure 1 presents a survival curve for risk-adjusted mortality, and Figure 2 presents a survival curve for risk-adjusted avoidance of repeat revascularization in the LAD artery. The median follow-up time was 3.81 years patients undergoing HCR and 4.20 years for patients undergoing PCI.
Figure 1.
Survival curve for HCR vs. PCI.
HCR: hybrid coronary revascularization; PCI: percutaneous coronary intervention.
Figure 2.
Repeat Revascularization in LAD Artery for HCR vs. PCI.
HCR: hybrid coronary revascularization; LAD: left anterior descending; PCI: percutaneous coronary intervention.
There was no difference in survival between HCR and PCI (83.17% vs. 81.65%, P = 0.46, adjusted hazard ratio (aHR) = 0.90; 95% CI: 0.67−1.20), see Figure 1 and Table 2. When the study was limited to the six highest volume HCR hospitals, there was still no significant mortality difference: (aHR = 0.87; 95% CI: 0.61−1.23), P-value for interaction between mortality and highest volume hospitals = 0.90. An examination of pre-selected subgroups of patients indicates that there was a significant interaction between revascularization strategy and mortality for body mass index, with PCI associated with better relative results among patients in the 25 kg/m2−35 kg/m2 range than among patients with higher and lower body mass indices (see Table 2).
Table 2. Comparison of four-year mortality and repeat revascularization in left anterior descending artery for hybrid coronary revascularization and percutaneous coronary intervention for pre-selected patient subgroups: New York, 2010−2016.
| Subgroup | Prevalence | Mortality | Repeat revascularization in LAD | |||||
| In HCR | In PCI | Hazard ratio
(95% CI) of HCR/PCI |
P-value for interaction | Hazard ratio
(95% CI) of HCR/PCI |
P-value for interaction | |||
| BMI: body mass index; HCR: hybrid coronary revascularization; LAD: left anterior descending; MI: myocardial infarction; PCI: percutaneous coronary interventions. | ||||||||
| All cases | 100.00% | 100.00% | 0.90 (0.67−1.20) | 0.51 (0.34−0.77) | ||||
| Age ≥ 75 yrs | 19.70% | 23.68% | 1.03 (0.67−1.59) | 0.52 | 0.80 (0.33−1.94) | 0.36 | ||
| Age < 75 yrs | 80.30% | 76.32% | 0.82 (0.55−1.22) | 0.49 (0.31−0.76) | ||||
| Female | 21.49% | 28.60% | 0.54 (0.27−1.08) | 0.07 | 0.68 (0.32−1.44) | 0.43 | ||
| Male | 78.51% | 71.40% | 1.08 (0.78−1.49) | 0.48 (0.30−0.77) | ||||
| BMI < 25 kg/m 2 | 27.76% | 21.67% | 0.63 (0.36−1.12) | 0.003 | 0.49 (0.20−1.18) | 0.90 | ||
| BMI ≥ 25 and < 35 kg/m 2 | 62.09% | 62.99% | 1.42 (1.00−2.03) | 0.54 (0.33−0.90) | ||||
| BMI ≥ 35 kg/m2 | 10.15% | 15.33% | 0.30 (0.10−0.93) | 0.52 (0.19−1.40) | ||||
| Ejection fraction ≤ 50% | 25.37% | 28.12% | 0.81 (0.50−1.32) | 0.39 | 0.70 (0.35−1.41) | 0.36 | ||
| Ejection fraction > 50% | 74.63% | 71.88% | 0.94 (0.65−1.36) | 0.46 (0.28−0.75) | ||||
| MI within 20 days | 28.96% | 37.53% | 0.76 (0.44−1.32) | 0.44 | 0.54 (0.24−1.20) | 0.97 | ||
| No MI within 20 days | 71.04% | 62.47% | 0.93 (0.66−1.32) | 0.53 (0.33−0.84) | ||||
| Previous PCI procedure, yes | 17.01% | 17.08% | 1.21 (0.64−2.28) | 0.39 | 0.39 (0.15−1.05) | 0.49 | ||
| Previous PCI procedure, no | 82.99% | 82.92% | 0.83 (0.60−1.16) | 0.56 (0.36−0.86) | ||||
| Congestive heart failure, yes | 12.54% | 9.71% | 0.63 (0.35−1.12) | 0.24 | NA (NA−NA) | 0.88 | ||
| Congestive heart failure, no | 87.46% | 90.29% | 1.01 (0.72−1.42) | 0.62 (0.42−0.93) | ||||
| Chronic lung disease, yes | 4.78% | 5.72% | 0.53 (0.23−1.19) | 0.21 | 0.43 (0.06−3.09) | 0.88 | ||
| Chronic lung disease, no | 95.22% | 94.28% | 1.00 (0.73−1.37) | 0.53 (0.35−0.80) | ||||
| Cerebrovascular disease, yes | 10.15% | 8.63% | 1.14 (0.62−2.10) | 0.37 | 0.43 (0.11−1.73) | 0.74 | ||
| Cerebrovascular disease, no | 89.85% | 91.37% | 0.84 (0.60−1.17) | 0.52 (0.34−0.79) | ||||
| Peripheral vascular disease, yes | 8.36% | 7.31% | 0.76 (0.38−1.55) | 0.87 | 0.23 (0.03−1.64) | 0.35 | ||
| Peripheral vascular disease,no | 91.64% | 92.69% | 0.92 (0.66−1.26) | 0.57 (0.37−0.85) | ||||
| Diabetes requiring medication, yes | 35.52% | 34.05% | 0.64 (0.39−1.05) | 0.10 | 0.45 (0.24−0.84) | 0.59 | ||
| Diabetes requiring medication, no | 64.48% | 65.95% | 1.10 (0.76−1.58) | 0.60 (0.35−1.01) | ||||
| Creatinine > 1.5 mg/dL or require renal dialysis | 6.86% | 8.38% | 1.22 (0.72−2.09) | 0.42 | NA (NA−NA) | 0.88 | ||
| Creatinine ≤ 1.5 mg/dL | 93.14% | 91.62% | 0.83 (0.58−1.17) | 0.58 (0.39−0.86) | ||||
| 3-vessel disease, yes | 51.94% | 35.18% | 0.73 (0.49−1.08) | 0.15 | 0.39 (0.21−0.74) | 0.19 | ||
| 3-vessel disease, no | 48.06% | 64.82% | 1.16 (0.76−1.79) | 0.68 (0.40−1.15) | ||||
| Proximal LAD disease, yes | 69.25% | 42.49% | 0.97 (0.69−1.38) | 0.19 | 0.54 (0.33−0.87) | 0.81 | ||
| Proximal LAD disease, no | 30.75% | 57.51% | 0.73 (0.42−1.27) | 0.51 (0.24−1.08) | ||||
| LMT disease, yes | 13.43% | 2.41% | 1.02 (0.55−1.91) | 0.36 | 0.35 (0.11−1.11) | 0.50 | ||
| LMT disease, no | 86.57% | 97.59% | 0.85 (0.61−1.18) | 0.56 (0.37−0.87) | ||||
As demonstrated by Figure 2 and Table 2, there was a difference in freedom from repeat revascularization in the LAD artery after a median 4-year follow-up period (91.13% vs. 83.59%, P = 0.001, aHR = 0.51, 95% CI: 0.34−0.77). When the study was limited to the six highest volume HCR hospitals, there was a larger, repeat revascularization difference: (aHR = 0.42, 95% CI: 0.26−0.69, P-value for interaction between repeat revascularization and highest volume hospitals = 0.01. An examination of pre-selected subgroups of patients indicates that no patient subgroups had significant interactions between revascularization strategy and repeat revascularization in the LAD artery (see Table 2).
DISCUSSION
In our study that compared outcomes of HCR and PCI for patients with multi-vessel disease, we found that HCR was rarely used as an alternative to PCI. Only 1.20% of all patients studied received HCR, and more than 80% of all HCRs were limited to six surgeons and six hospitals. Also, the percentage of HCRs did not increase substantially during the study. Although our study yielded the same mortality conclusions as earlier studies of HCR vs. PCI (no difference in mortality), it is notable that unlike earlier studies, we examined rates of repeat revascularization in the LAD artery (the primary difference in the two approaches) as a separate outcome, and we found a difference between the two alternatives. HCR patients were less likely to experience repeat revascularization in the LAD artery than PCI patients (91.13% vs. 83.59%, P = 0.001, aHR = 0.51 (95% CI: 0.34−0.77)) after a median follow-up of four years.
Hybrid coronary revascularization potentially combines the best attributes of CABG surgery with PCI to achieve outcomes with a minimally invasive approach that are better than outcomes achieved using only PCI. The rationale is that the LAD is the most important of the three main coronary branches, and a LIMA to LAD bypass has been demonstrated to be superior to PCI with respect to event-free survival, angina relief, and long-term patency.[25,28,29] Nevertheless, potential disadvantages of HCR include the risk of adverse events in the interval between the two procedures, the risks (compared to CABG surgery for all vessels) associated with dual antiplatelet therapy, the combination of complications associated with PCI and CABG surgery, and the added difficulty of performing minimally invasive surgery.[25] Although there are numerous (albeit small and mostly single-center) studies that compare HCR with conventional CABG surgery,[8-24] there are very few multi-center studies that compare HCR with PCI, the other minimally invasive alternative.[25,26]
Lowenstern, et al.[26] used data from the National Cardiovascular Data Registry CathPCI database to examine the utilization of HCR and to compare risk-adjusted in-hospital mortality of HCR vs. multivessel PCI between 2009 and 2017. They found that only 0.2% of patients undergoing either HCR or PCI underwent HCR. The adjusted in-hospital mortality rates of HCR and PCI were not significantly different (OR (HCR/PCI) = 1.54 (95% CI: 0.92−2.59)).[26] Limitations of the study acknowledged by the authors are that staged procedures could not be captured, and more importantly, the only outcomes that could be captured were at the time of discharge from the primary hospitalization.
Puskas, et al.[25] used an observational multi-institutional study to compare 200 HCR patients and 98 PCI patients in 11 centers. The authors found that propensity weighted MACCE (death, stroke, myocardial infarction, repeat revascularization) rates were similar between the two groups at 12 months follow-up (HR (HCR/PCI) = 0.87; 95% CI: 0.56−1.36) during a median follow-up period of 17.6 months. The MACCE rates were also similar after 12-month post-intervention (HR = 1.06, 95% CI: 0.67−1.70). The authors concluded that a randomized trial with longer-term outcomes is needed to definitively compare the two treatment options. The authors acknowledge that longer follow-up would have provided a better understanding of the relative benefits of HCR and PCI.
In summary, there are very few existing comparisons of HCR vs. PCI outcomes for patients with multivessel disease, and these studies are limited with respect to follow-up time and/or number of patients. Also, although a randomized trial was initiated in 2014 (https://clinicaltrials.gov/ct2/show/NCT03089398), unfortunately this trial was recently terminated due to the inability to recruit enough patients.
Although not a randomized trial, our study has a few advantages in comparison to the earlier studies comparing HCR and PCI. First, it presents information on the multi-institutional use of HCR and the comparative outcomes of HCR and conventional CABG surgery in a large population-based region. Although the recent study using CathPCI data referenced above was larger, that study only had access to in-hospital outcomes and did not contain information on staged procedures. Also, our study has a median follow-up of nearly four years, whereas the Puskas, et al.[25] study had an 18-month follow-up time (and noted that the survival curves were beginning to diverge in favor of HCR). Longer follow-up is particularly important because it has been shown that minimally invasive CABG surgery is associated with LAD artery durability than PCI. Third, our study examined differences in repeat revascularization rates in the LAD artery as well as differences in mortality rates.
Furthermore, despite the fact that only 1.2% of the cases are HCR cases in our study, it contains the most current HCR data there are and documenting that HCRs are still rare is an important finding in itself. In addition, we had enough statistical power to demonstrate that HCR has a significantly lower repeat revascularization rate than PCI. Although there was no difference in mortality rates, the 95% CI was not so large and the point estimate was not so far from 1.0 that it appeared that low statistical power was a major reason for the conclusion (aHR = 0.90, 95% CI: 0.67−1.20).
Limitations
There are several limitations of this study. It is an observational study, and it is therefore subject to selection bias because of its non-randomized nature. We attempted to minimize this bias by Cox proportional hazards models to control for differences in patient risk factor among patients undergoing HCR and PCI. Nevertheless, there are inevitably factors related to outcomes that were not available to us, and unmeasured confounding is undoubtedly present.
For two-stage procedures, there is the possibility of survival bias since only patients who survive long enough to undergo the second procedure are included in the study. We were not able to confirm that the second stages of hybrid procedures were truly planned, although we did require that they were not performed on the same vessel as the first procedure and they were not done on an emergency basis. Because we used New York State vital statistics data to capture mortality after discharge and the New York cardiac registries to capture repeat revascularization, we were unable to capture events that occurred in other states. To minimize the probability that these events could have occurred out-of-state, we limited the study to New York State patients. Nevertheless, New York patients could have died, been admitted for an MI or stroke, or undergone repeat revascularization out-of-state, and this would have been missed by our study. However, there is no reason why there should be a bias in favor of either type of treatment with respect to missed patients, and an earlier study demonstrated that there was no bias in this regard.[30] Another limitation is that HCR involves a group of heterogeneous procedures and we were unable to capture the adjunctive pharmacologic therapies used in between and after the procedures, or how their utilization affected outcomes.
Conclusion
HCR is rarely performed as an alternative to PCI for patients with coronary artery disease involving the LAD artery and at least one other major epicardial artery. There are no differences in mortality in a median follow-up of four years, but HCR is associated with lower repeat revascularization than PCI. This may be an important consideration in choosing a treatment alternative. A randomized controlled trial would be helpful to confirm these findings, but that may not be possible unless the utilization of HCR increases.
References
- 1.Park SJ, Ahn JM, Kim YH, et al Trial of everolimus-eluting stents or bypass surgery for coronary disease. N Engl J Med. 2015;372:1204–1212. doi: 10.1056/NEJMoa1415447. [DOI] [PubMed] [Google Scholar]
- 2.Farkouh ME, Domanski M, Sleeper LA, et al Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–2384. doi: 10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- 3.Head SJ, Davierwala PM, Serruys PW, et al Coronary artery bypass grafting vs. percutaneous coronary intervention for patients with three-vessel disease: final five-year follow-up of the SYNTAX trial . Eur Heart J. 2014;35:2821–2830. doi: 10.1093/eurheartj/ehu213. [DOI] [PubMed] [Google Scholar]
- 4.Gaudino M, Hameed I, Farkouh ME, et al Overall and cause-specific mortality in randomized clinical trials comparing percutaneous coronary interventions with coronary bypass surgery: A meta-analysis. JAMA Intern Med. 2020:e204748. doi: 10.1001/jamainternmed.2020.4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavalcante R, Sotomi Y, Zeng Y, et al Coronary bypass surgery versus stenting in multivessel disease involving the proximal left anterior descending coronary artery. Heart. 2017;103:428–433. doi: 10.1136/heartjnl-2016-309720. [DOI] [PubMed] [Google Scholar]
- 6.Boylan MJ, Lytle BW, Loop FD, et al Surgical treatment of isolated left anterior descending coronary stenosis: Comparison of left internal mammary artery and venous autograft at 18 to 20 years of follow-up. J Thorac Cardiovasc Surg. 1994;107:657–662. doi: 10.1016/S0022-5223(94)70320-5. [DOI] [PubMed] [Google Scholar]
- 7.Hannan EL, Racz M, Holmes DR, et al Comparison of coronary artery stenting outcomes in the eras before and after the introduction of drug-eluting stents. Circulation. 2008;117:2071–2078. doi: 10.1161/CIRCULATIONAHA.107.725531. [DOI] [PubMed] [Google Scholar]
- 8.de Cannière D, Jansens JL, Goldschmidt-Clermont P, et al Combination of minimally invasive coronary bypass and percutaneous transluminal coronary angioplasty in the treatment of double-vessel coronary disease: two-year follow-up of a new hybrid procedure compared with “on-pump” double bypass grafting. Am Heart J. 2001;142:563–570. doi: 10.1067/mhj.2001.118466. [DOI] [PubMed] [Google Scholar]
- 9.Kon ZN, Brown EN, Tran R, et al Simultaneous hybrid coronary revascularization reduces postoperative morbidity compared with results from conventional off-pump coronary artery bypass. J Thorac Cardiovasc Surg. 2008;135:367–375. doi: 10.1016/j.jtcvs.2007.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen L, Hu S, Wang H, et al One-stop hybrid coronary revascularization versus coronary artery bypass graft and percutaneous coronary intervention for the treatment of multivessel coronary artery disease: three-year follow-up results from a single institution. J Am Coll Cardiol. 2013;61:2525–2533. doi: 10.1016/j.jacc.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Patel NC, Hemli JM, Kim MC, et al Short- and intermediate-term outcomes of hybrid coronary revascularization for double-vessel disease. J Thorac Cardiovasc Surg. 2018;156:1799–1807. doi: 10.1016/j.jtcvs.2018.04.078. [DOI] [PubMed] [Google Scholar]
- 12.Halkos ME, Liberman HA, Devireddy C, et al Early clinical and angiographic outcomes after robotic-assisted coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2014;147:179–185. doi: 10.1016/j.jtcvs.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Rosenblum JM, Harskamp RE, Hoedemaker N, et al Hybrid coronary revascularization versus coronary artery bypass surgery with bilateral or single internal mammary artery grafts. J Thor Cardiovasc Surg. 2016;151:1081–1089. doi: 10.1016/j.jtcvs.2015.10.061. [DOI] [PubMed] [Google Scholar]
- 14.Adams C, Burns DJ, Chu MW, Jones PM, Shridar K, Teefy P, et al Single-stage hybrid coronary revascularization with long-term follow-up. Eur J Cardiothorac Surg. 2014;45:438–442. doi: 10.1093/ejcts/ezt390. [DOI] [PubMed] [Google Scholar]
- 15.Harskamp RE, Vassiliades TA, Mehta RH, et al Comparative effectiveness of hybrid coronary revascularization vs coronary artery bypass grafting. J Am Coll Surg. 2015;221:326–334. doi: 10.1016/j.jamcollsurg.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Xia Y, Katz AN, Forest SJ, et al Hybrid coronary revascularization has improved short-term outcomes but worse mid-term reintervention rates compared to CABG: a propensity-matched analysis. Innovations (Phila) 2017;12:174–179. doi: 10.1097/imi.0000000000000376. [DOI] [PubMed] [Google Scholar]
- 17.Giambruno V, Hafiz A, Fox SA, et al Is the future of coronary arterial revascularization a hybrid approach? The Canadian experience across three centers. Innovations (Phila) 2017;12:82–86. doi: 10.1097/imi.0000000000000355. [DOI] [PubMed] [Google Scholar]
- 18.Tajstra M, Hrapkowicz T, Hawranek M, et al Hybrid coronary revascularization in selected patients with multivessel disease. J Am Coll Cardiol Interv. 2018;11:847–852. doi: 10.1016/j.jcin.2018.01.271. [DOI] [PubMed] [Google Scholar]
- 19.Gasior M, Zembala MO, Tajstra M, et al Hybrid revascularization for multivessel coronary artery disease. J Am Coll Cardiol Interv. 2014;7:1277–1284. doi: 10.1016/j.jcin.2014.05.025. [DOI] [Google Scholar]
- 20.Harskamp RE, Brennan JM, Xian Y, et al Practice patterns and clinical outcomes after hybrid coronary revascularization in the United States: an analysis from the Society of Thoracic Surgeons adult cardiac database. Circulation. 2014;130:872–879. doi: 10.1161/CIRCULATIONAHA.114.009479. [DOI] [PubMed] [Google Scholar]
- 21.Harskamp RE, Bagai A, Halkos ME, et al Clinical outcomes after hybrid coronary revascularization versus coronary artery bypass surgery: a meta-analysis of 1,190 patients. Am Heart J. 2014;167:585–592. doi: 10.1016/j.ahj.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Zhu P, Zhou P, Sun Y, et al Hybrid coronary revascularization versus coronary artery bypass grafting for multivessel coronary artery disease: systematic review and meta-analysis. J Cardiothorac Surg. 2015;10:63. doi: 10.1186/s13019-015-0262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu FB, Cui LQ Short-term clinical outcomes after hybrid coronary revascularization versus off-pump coronary artery bypass for the treatment of multivessel or left main coronary artery disease: a meta-analysis. Coron Artery Dis. 2015;26:526–534. doi: 10.1097/MCA.0000000000000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phan K, Wong S, Wang N, et al Hybrid coronary revascularization versus coronary artery bypass surgery: systematic review and meta-analysis. Int J Cardiol. 2015;179:484–488. doi: 10.1016/j.ijcard.2014.11.061. [DOI] [PubMed] [Google Scholar]
- 25.Puskas JD, Halkos ME, DeRose JJ, et al Hybrid coronary revascularization for the treatment of multivessel coronary artery disease: a multicenter observational study. J Am Coll Cardiol. 2016;68:356–365. doi: 10.1016/j.jacc.2016.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowenstern A, Wu J, Bradley SM, et al Current landscape of hybrid revascularization: A report from the NCRD CathPCI Registry. Am Heart J. 2019;215:167–177. doi: 10.1016/j.ahj.2019.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghali WA, Quan H, Brant R, et al Comparison of two methods of calculating adjusted survival curves from proportional hazards models. JAMA. 2001;286:1494–1497. doi: 10.1001/jama.286.12.1494. [DOI] [PubMed] [Google Scholar]
- 28.Diegeler A, Thiele H, Falk V, et al Comparison of stenting with minimally invasive bypass surgery for stenosis of the left anterior descending coronary artery. N Engl J Med. 2002;347:561–566. doi: 10.1056/NEJMoa013563. [DOI] [PubMed] [Google Scholar]
- 29.Loop FD Internal-thoracic artery grafts: biologically better coronary arteries. N Engl J Med. 1996;334:263–265. doi: 10.1056/NEJM199601253340411. [DOI] [PubMed] [Google Scholar]
- 30.Hannan EL, Racz MJ, McCallister BD, et al A comparison of three-year survival after coronary artery bypass graft surgery and percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1999;33:63–72. doi: 10.1016/S0735-1097(98)00540-3. [DOI] [PubMed] [Google Scholar]