Abstract
Background/Aims:
Semiannual hepatocellular carcinoma (HCC) surveillance is recommended in patients with cirrhosis; however, recent studies have raised questions over its utility. We investigated the impact of surveillance on early detection and survival in a nationally-representative database.
Methods:
We included patients with cirrhosis and HCC from the Optum database (2001–2015) with >6 months of follow-up between cirrhosis and HCC diagnoses. Surveillance adherence was defined as proportion of time covered (PTC), with each six-month period after abdominal imaging defined as “covered”. To determine the association between surveillance and mortality, we compared PTC between fatal and non-fatal HCC.
Results:
Of 1,001 patients with cirrhosis and HCC, 256 died with median follow-up 30 months. Median PTC by any imaging was greater in early-stage vs. late-stage HCC (43.6 vs. 37.4%, p = 0.003) and non-fatal vs. fatal HCC (40.8 vs. 34.3%, p = 0.001). In multivariable analyses, each 10% increase in PTC was associated with increased early HCC detection (OR 1.07, 95% CI 1.01–1.12) and decreased mortality (HR 0.95; 95% CI 0.90–1.00). On subgroup analysis, PTC by CT/MRI was associated with early tumor detection and decreased mortality; however, PTC by ultrasound was only associated with early-detection but not decreased mortality. These findings were robust across sensitivity analyses.
Conclusions:
In a US cohort of privately-insured HCC patients, PTC by any imaging modality was associated with increased early detection and decreased mortality. Continued evaluation of HCC surveillance strategies and effectiveness is warranted.
Keywords: screening, liver cancer, Optum
LAY SUMMARY
Liver cancer is a major cause of cancer-related death. Patients with cirrhosis are at high risk for developing liver cancer. While screening for liver cancer among patients with cirrhosis is recommended, there has been controversy recently about how useful screening use. Here, we used a large insurance claims database with >150,000,000 people to investigate whether prior liver cancer screening improves outcomes in patients with cirrhosis and liver cancer. We found that liver cancer screening is associated with improved survival and detection of cancer at an early stage.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fourth-leading cause of cancer death worldwide.1 In contrast to trends with other common malignancies, HCC incidence and mortality are increasing in the United States, largely due to an increase in non-alcoholic fatty liver disease and peak in hepatitis C virus-related cirrhosis.2–4 Unfortunately, HCC prognosis is poor with median survival under two years after diagnosis, which in part can be attributed to underuse of early detection strategies and limited effectiveness of therapies for patients with advanced stage disease.5
Several professional societal guidelines recommend HCC surveillance in at-risk populations, including those with cirrhosis, using ultrasound (US) with or without alpha-fetoprotein (AFP).6–8 However, HCC surveillance in patients with cirrhosis does not have level I evidence and has been primarily supported by cohort studies demonstrating an association with earlier stage detection, greater likelihood of receiving curative therapy, and improved survival.9–11 These studies have notable limitations including potential for lead time bias, length time bias, and residual confounding.12 It is well recognized that US and AFP can have limited sensitivity for early stage HCC detection in clinical practice, with a recent meta-analysis reporting a sensitivity of only 63% for early-stage HCC detection when using the two tests in combination.13 Studies have also suggested high rates of false positive or indeterminate results leading to potential screening-related harms, such as additional diagnostic imaging and/or biopsy.14,15 Other limitations of surveillance include poor surveillance adherence and appropriate treatment for HCC patients detected at an early stage, related to both physician and patient factors.16,17 These prevalent failures in the HCC screening process have led to increasing controversy about the value of surveillance in patients with cirrhosis.18
This controversy was recently brought to light after a case-control study from the Veterans Affairs system failed to show an improvement in overall survival with HCC surveillance.12 The authors of this study found no difference in surveillance receipt between patients with fatal HCC and a matched cohort of patients with cirrhosis. However, it is unclear if these results are generalizable to a non-Veterans Affairs population and warrant validation, particularly as prior studies have suggested large site-level and physician-level variations in HCC surveillance receipt and effectiveness.
Therefore, we aimed to characterize the association between HCC surveillance receipt and overall survival in a large nationally representative cohort of privately-insured patients with cirrhosis.
METHODS
Cohort
We conducted a secondary analysis of the Optum database (2001–2015), a claims database including over 150 million privately-insured patients in the United States. We included patients with cirrhosis, defined by ≥2 previously-validated ICD-9 codes19 and HCC (≥2 ICD-9 codes of 155.0 or 155.2). We required two ICD-9 codes for cirrhosis and HCC to maximize the positive predictive value for both conditions. Exclusion criteria included any extrahepatic cancer diagnoses other than non-melanoma skin cancer, history of liver transplantation prior to first HCC diagnosis, and <6 months of follow-up between cirrhosis diagnosis and HCC diagnosis (Fig. 1).
Figure 1:
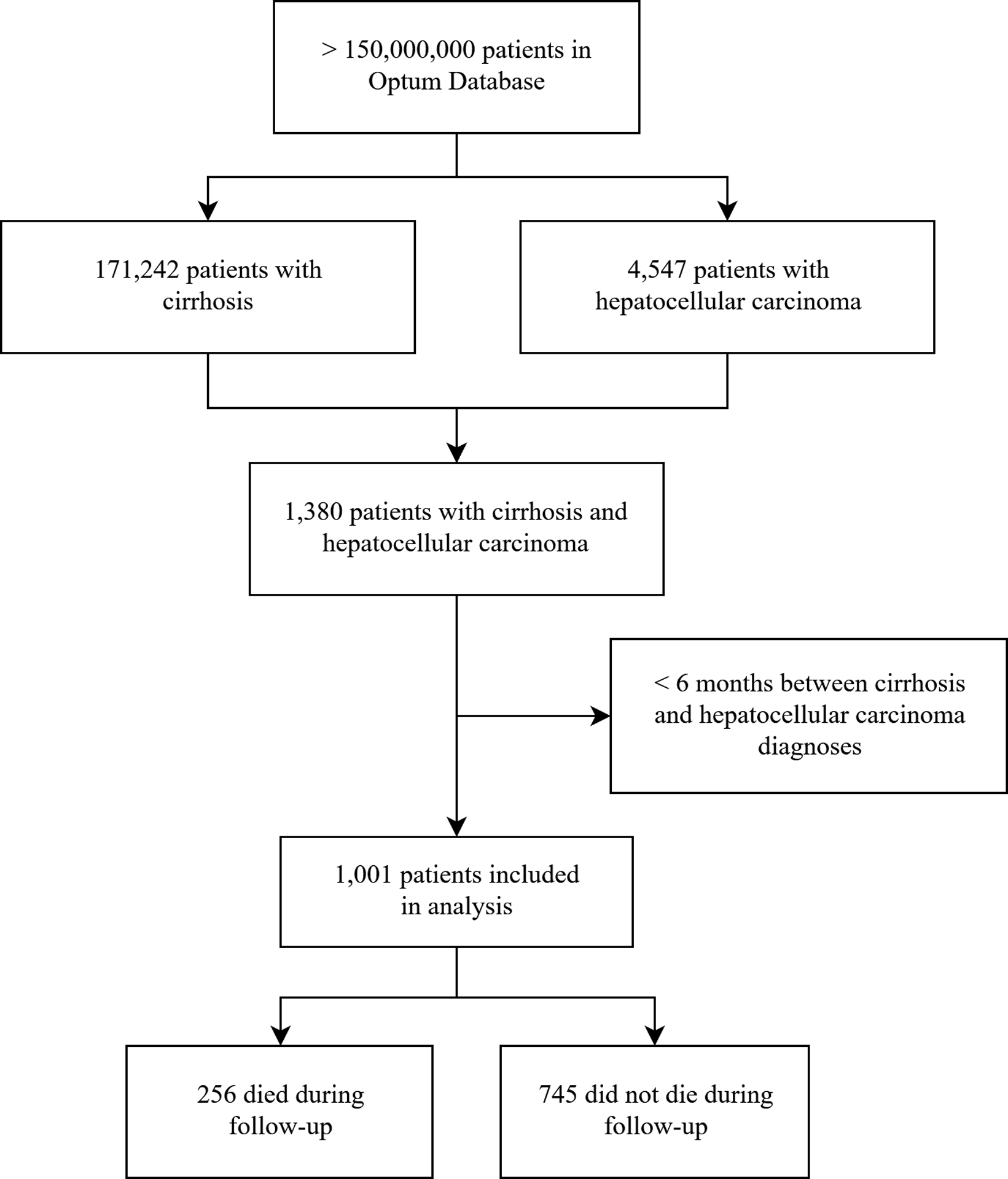
Study design
Definitions
We classified cirrhosis as compensated or decompensated, with decompensated cirrhosis diagnosis based on a history of ascites, hepatic encephalopathy, or variceal bleeding. Ascites was diagnosed based on relevant diagnosis codes, plus use of diuretics (loop diuretics and/or mineralocorticoid receptor antagonists), receipt of paracentesis, or receipt of transjugular intrahepatic portosystemic shunt placement. Hepatic encephalopathy was diagnosed using relevant diagnosis codes plus use of lactulose or rifaximin. Disease etiology was based on diagnosis codes: viral disease was defined as presence of at least two diagnostic codes for chronic hepatitis B or C infection, alcoholic liver disease based on presence of at least two codes for alcohol misuse, combined alcoholic and viral disease based on presence of at least two codes for both viral hepatitis and alcoholic liver disease, and non-viral non-alcoholic disease based on one or no codes for either viral or alcoholic liver disease. Diagnostic and procedure codes are summarized in Supp. Table 1.
Adherence to surveillance
Adherence to surveillance was measured by the proportion of time “covered” (PTC), i.e. time up-to-date with HCC surveillance.20 Each six-month period after abdominal imaging including ultrasound, contrast-enhanced CT, and contrast-enhanced MRI was defined as “covered.” All imaging studies could have been done with or without AFP, but presence of AFP was not sufficient when used alone given insufficient sensitivity for early HCC detection. Although imaging studies may not have been conducted for diagnostic purposes, we considered patients covered after any adequate study because these studies obviated the need for repeat surveillance testing; however, we did not include studies which were inadequate for diagnosis or surveillance such as Doppler ultrasound or non-contrast-enhanced cross-sectional imaging. PTC was measured as time up-to-date, divided by the total follow-up period between date of the first cirrhosis diagnosis code and the date of HCC diagnosis. We excluded the time frame between any CT or MRI obtained within six months of HCC diagnosis from the PTC numerator and denominator to adjust for delays between HCC diagnosis on imaging and placement of HCC diagnostic codes.
Statistical analysis
Continuous variables were depicted as mean ± standard deviation or median (interquartile range [IQR]), and categorical variables were represented as proportions (%). Normally-distributed variables were compared using t tests and non-normally distributed variables were compared using the rank-sum test. Chi-square tests were used to compare categorical variables.
The primary outcome of our study was the association between PTC and patient survival and a secondary outcome was the association between PTC and early stage HCC detection facilitating curative treatment receipt. For the association between survival and PTC, we used three methods. First, we used a Wilcoxon rank-sum test to compare PTC based on survival status as a binary variable (i.e. deceased or alive). Next, we performed multivariable logistic regression to compare adjusted PTC (adjusted for age, sex, race, region, cirrhosis diagnosis year, decompensated liver disease at cirrhosis diagnosis, and disease etiology) based on survival status. Finally, we used a Cox proportional hazards model based on time-to-event analysis; patients were censored at loss to follow-up or liver transplantation. In the multivariable Cox model, PTC was the primary independent variable; covariates were age, sex, and all other non-redundant factors associated with mortality at P < 0.10 in univariable analyses. We also performed sensitivity analysis with adjustment for lead-time bias by assuming sojourns of 3, 6, or 9 months in patients who had PTC below the median.10
In a secondary analysis, we also performed multivariable logistic regression to define correlates of curative treatment receipt, defined as receiving liver transplantation, surgical resection, or local ablation as the first HCC treatment. For this analysis, PTC was the primary independent variable; covariates were age, sex, and all other non-redundant factors associated with early-stage diagnosis at P < 0.10 in univariable analyses.
For both analyses, we first defined PTC using receipt of any imaging (ultrasound, contrast CT, or contrast MRI). We then performed subgroup analyses to assess association between PTC and both outcomes among (1) those who received abdominal ultrasound and (2) those who received contrast-enhanced CT or MRI. Finally, we performed several sensitivity analyses: (1) requiring either 9 or 12 months of follow-up between cirrhosis and HCC diagnoses, (2) excluding patients with decompensated cirrhosis, and (3) excluding inpatient imaging studies.
For all analyses, statistical significance was defined as a two-tailed P value < 0.05. All statistical analyses were performed using R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria) with the tidyverse,21 survival,22 and survminer23 packages.
RESULTS
Patient characteristics
We identified 171,242 individuals with cirrhosis, of whom 1,001 had HCC (Fig. 1). Among those with HCC, 256 died during follow-up. Median time between cirrhosis and HCC diagnoses was 37.3 months (IQR 20.9–62.0 months) and median follow-up after HCC diagnosis was 30.4 months (IQR 12.8–49.7 months). The etiology of disease was combined alcoholic and viral disease in in 42%, viral disease alone in 28%, alcoholic liver disease alone in 17%, and non-viral non-alcoholic in 13%. Approximately 57% and 24% of patients with chronic hepatitis B or C, respectively, received antiviral therapy during follow-up. Among patients with HCC who died during follow-up, median survival was 9.4 months (IQR 3.0–22.1 months). Patients with fatal HCC were older, more frequently had history of alcohol use, and were less frequently from the Pacific region of the United States than those with non-fatal HCC (Table 1). Prior to HCC diagnosis, most patients (54%) had imaging using a combination of ultrasound, CT, and MRI; 28% had imaging exclusively with US; 11% of patients exclusively with CT/MRI; and 8% of patients had not received any surveillance prior to HCC diagnosis.
Table 1:
Characteristics of patients with fatal and non-fatal hepatocellular carcinoma
| Trait | Overall | Fatal N = 256 | Non-fatal N = 745 | P value |
|---|---|---|---|---|
| Age | 58.4 ± 10.6 | 60.9 ± 10.5 | 57.5 ± 10.5 | < 0.001 |
| Year of HCC diagnosis | 2012 (2009–2013) | 2010 (2008–2013) | 2012 (2010–2014) | < 0.001 |
| % Male | 67.6% | 70.7% | 66.6% | < 0.001 |
| Race | ||||
| Asian | 6.6% | 3.9% | 7.5% | 0.057 |
| Black | 7.4% | 9.8% | 6.6% | |
| Hispanic | 17.9% | 15.2% | 18.8% | |
| White | 61.2% | 65.4% | 59.9% | |
| Other/unknown | 6.9% | 5.9% | 7.2% | |
| Subspecialty care before cancer diagnosis | ||||
| Gastroenterologist | 89.7% | 91.0% | 89.3% | 0.41 |
| Hepatology (subset of gastroenterologists) | 29.3% | 23.1% | 31.4% | 0.011 |
| Number of imaging studies before cancer diagnosis | ||||
| Ultrasound | 2 (1–4) | 2 (1–4) | 2 (1–5) | 0.88 |
| Computed tomography | 1 (0–3) | 2 (1–3) | 1 (0–2) | 0.004 |
| Magnetic resonance imaging | 0 (0–2) | 0 (0–1) | 0 (0–2) | 0.006 |
| Computed tomography or magnetic resonance imaging | 2 (1–4) | 2 (1–4) | 2 (1–4) | 0.57 |
| Any imaging | 5 (3–9) | 5 (3–8) | 5 (2–9) | 0.84 |
| Region | ||||
| Mountain | 4.0% | 4.7% | 3.8% | 0.003 |
| Midwest | 16.5% | 19.9% | 15.3% | |
| Northeast | 7.1% | 8.2% | 6.7% | |
| Pacific | 17.8% | 9.8% | 20.5% | |
| Southeast | 34.2% | 38.3% | 32.8% | |
| Southwest | 20.5% | 19.1% | 20.9% | |
| Hepatitis C virus | 67.3% | 64.1% | 68.5% | 0.20 |
| Hepatitis B virus | 14.3% | 9.8% | 15.8% | 0.017 |
| Alcohol history | 59.0% | 69.9% | 55.3% | < 0.001 |
| Complications at cirrhosis diagnosis | ||||
| Ascites | 6.7% | 4.7% | 7.4% | 0.10 |
| Encephalopathy | 2.7% | 3.5% | 2.4% | 0.39 |
| Variceal bleed | 8.3% | 8.6% | 8.2% | 0.84 |
| Any decompensation | 15.4% | 15.2% | 15.4% | 0.94 |
| Alpha-fetoprotein measurement | 62.3% | 52.9% | 65.5% | < 0.001 |
Proportion time covered
Overall median PTC by any abdominal imaging was 38.7%, by US 13.1%, and by CT or MRI 25.2%. Factors associated with higher PTC included younger age, Asian race, region, later year of cirrhosis diagnosis, subspecialty hepatology care, combined viral-alcohol disease etiology, and history of hepatic decompensation (Supp. Tables 2–4).
Survival analysis
On primary analysis, PTC by any imaging was higher in patients with non-fatal HCC than fatal HCC: 40.8% vs. 34.4%, p = 0.001 (Table 2). On subgroup analysis, PTC by CT/MRI was significantly higher in patients with non-fatal HCC, but PTC by US alone did not differ between fatal and non-fatal HCC (Table 2). Results were consistent across sensitivity analyses as detailed in Table 2.
Table 2:
Comparison of proportion of time covered between fatal and non-fatal hepatocellular carcinoma
| Period between cirrhosis and HCC diagnosis | Proportion time covered by | Fatal HCC | Non-fatal HCC | P value |
|---|---|---|---|---|
| >6 months All patients N = 745 (non-fatal), N = 256 (fatal) |
Any imaging | 34.3% (16.7–52.1%) | 40.8% (19.1–64.6%) | 0.001 |
| Ultrasound | 21.4% (7.2–43.7%) | 27.0% (6.9–49.7%) | 0.161 | |
| Computed tomography or magnetic resonance imaging | 11.6% (0–27.9%) | 14.7% (0–42.9%) | 0.030 | |
| >9 months All patients N = 692 (non-fatal), N = 240 (fatal) |
Any imaging | 32.3% (16.0–49.1%) | 40.4% (19.4–62.4%) | < 0.001 |
| Ultrasound | 20.3% (6.8–39.3%) | 26.1% (7.4–47.0%) | 0.050 | |
| Computed tomography or magnetic resonance imaging | 11.6% (0–27.1%) | 15.1% (0–41.9%) | 0.005 | |
| >12 months All patients N = 646 (non-fatal), N = 228 (fatal) |
Any imaging | 31.6% (15.7–47.9%) | 39.4% (19.2–62.1%) | < 0.001 |
| Ultrasound | 19.8% (7.0–38.5%) | 25.4% (7.7–45.1%) | 0.060 | |
| Computed tomography or magnetic resonance imaging | 11.7% (0–26.3%) | 15.2% (0–39.8%) | 0.005 | |
| >6 months, excluding those with decompensated cirrhosis N = 630 (non-fatal), N = 217 (fatal) |
Any imaging | 32.1% (15.5–49.0%) | 38.8% (17.1–61.8%) | 0.003 |
| Ultrasound | 19.8% (5.8–42.0%) | 24.2% (5.6–46.9%) | 0.24 | |
| Computed tomography or magnetic resonance imaging | 11.3% (0–27.7%) | 12.6% (0–40.2%) | 0.14 |
HCC, hepatocellular carcinoma.
In adjusted analysis, there was no difference in PTC by any imaging or PTC by US between fatal and non-fatal HCC (Supp. Table 5). However, PTC by CT/MRI was significantly higher among patients with non-fatal than fatal HCC (difference = −4.9%; 95% confidence interval −8.9 to −1.0%; P = 0.015). The association between survival and adjusted PTC by CT/MRI remained significant across most sensitivity analyses except among those with compensated cirrhosis (Supp. Table 5).
Predictors of survival
On univariable Cox analysis, greater PTC by any modality was associated with decreased mortality: hazard ratios (HR) per 10% change in PTC were 0.91 (95% CI 0.87–0.95) for any imaging, 0.95 (0.90–0.99) for US, and 0.90 (0.85–0.95) for CT/MRI (Fig. 2 and Table 3). After adjustment for lead-time bias, the association between any imaging and decreased mortality remained significant (Supp. Fig. 1). In multivariable Cox analysis (Methods and Supp. Table 6), adjusted PTC by any imaging remained significantly associated with survival (HR 0.94; 95% CI 0.90–0.99 per 10% change in PTC) (Table 3). In subgroup analysis, PTC by CT or MRI but not ultrasound was associated with survival (Table 3). On sensitivity analysis where only outpatient imaging studies were included, adjusted PTC by any imaging or US was not significantly associated with survival, while PTC by CT/MRI remained significant (Supp. Table 7). When stratified by modality, adjusted PTC by MRI alone was associated with decreased mortality (HR 0.69, 0.52–0.93, P = 0.01), but not PTC by CT (HR 0.92, 0.85–1.00, P = 0.06).
Figure 2:
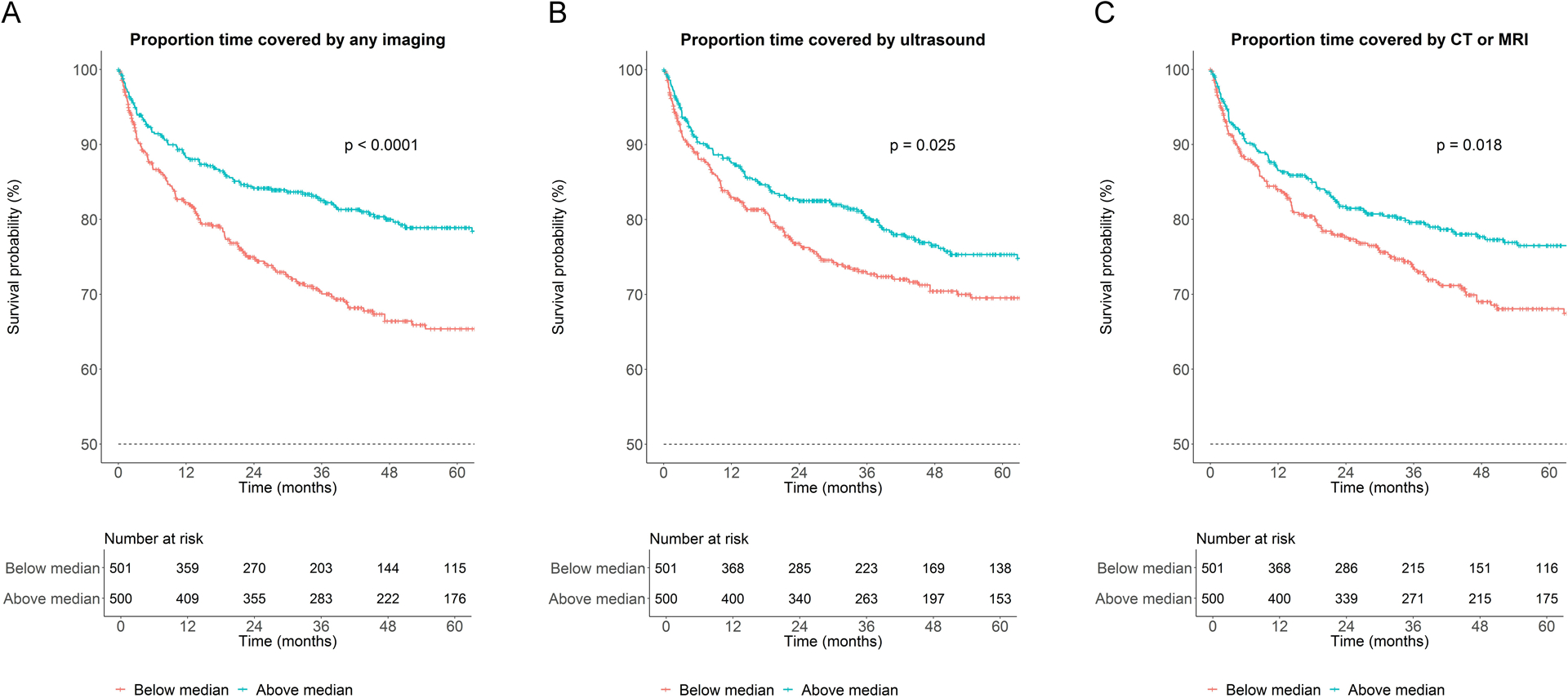
Survival based on proportion time covered by surveillance. Kaplan-Meier curves depicting survival based on proportion time covered by (A) any imaging, (B) ultrasound, or (C) computed tomography (CT) or magnetic resonance imaging (MRI).
Table 3:
Association between proportion time under surveillance and mortality
| Proportion time under surveillance (per 10%) | Unadjusted hazard ratio | P value | Adjusted hazard ratio* | P value |
|---|---|---|---|---|
| Any imaging | 0.91 (0.87–0.95) | < 0.001 | 0.94 (0.90–0.99) | 0.026 |
| Ultrasound | 0.95 (0.90–0.99) | 0.016 | 0.99 (0.94–1.04) | 0.80 |
| Computed tomography or magnetic resonance imaging | 0.90 (0.85–0.95) | < 0.001 | 0.92 (0.87–0.97) | < 0.001 |
Adjusted for age, sex, race, region, year of cirrhosis diagnosis, disease etiology, and history of decompensation at time of cirrhosis diagnosis. Hazard ratio was not adjusted for treatment type as that is itself associated with proportion time under surveillance.
Early diagnosis and multivariable analysis
We used receipt of curative therapy (ablation, resection, or liver transplantation) as a proxy for early HCC diagnosis. Patients with early-stage HCC who underwent curative treatment had higher PTC by any imaging compared to those with later-stage HCC (43.6% vs. 37.4%, P = 0.003) (Supp. Table 8), which was consistent across sensitivity analyses. On subgroup analysis, PTC by CT/MRI was also greater in patients with early-stage HCC who underwent curative treatment; however, there was no difference in PTC by US except in a sensitivity analysis among those with compensated cirrhosis (Supp. Table 8).
In univariable logistic regression, PTC by any imaging was associated with increased probability of early detection and curative treatment receipt: OR 1.08 (1.03–1.14), P = 0.001 (Table 4). After adjustment for other factors associated with early-stage diagnosis (Supp. Table 9), PTC by any imaging or CT/MRI but not by US were associated with early detection and curative treatment receipt (Table 4). Some patients with early-stage disease may have received no therapy or only locoregional therapy. We conducted sensitivity analyses to account for this possibility by assuming that (in addition to patients receiving curative therapy) 20% of patients receiving locoregional therapy, no therapy, or either locoregional therapy or no therapy had early-stage disease. PTC by any imaging remained significantly associated with increased early diagnosis in univariable analysis and most multivariable analyses (Supp. Table 10).
Table 4:
Association between proportion time under surveillance and diagnosis at an early stage
| Proportion time under surveillance (per 10%) | Unadjusted odds ratio | P value | Adjusted odds ratio* | P value |
|---|---|---|---|---|
| Any imaging | 1.08 (1.03–1.14) | 0.001 | 1.08 (1.03–1.13) | 0.002 |
| Ultrasound only | 1.05 (1.00–1.10) | 0.033 | 1.04 (1.00–1.09) | 0.068 |
| Computed tomography or magnetic resonance imaging | 1.05 (1.01–1.10) | 0.026 | 1.05 (1.00–1.10) | 0.030 |
Adjusted for age, sex, and disease etiology.
DISCUSSION
In a large privately insured cohort of patients with HCC, PTC by any imaging was associated with decreased mortality and increased early HCC detection on both unadjusted and adjusted analyses. On subgroup analysis based on imaging type, unadjusted and adjusted PTC by CT/MRI were associated with early diagnosis and decreased mortality. While unadjusted PTC by US was associated with early diagnosis and decreased mortality, the association was no longer significant in multivariable models.
This study adds to the literature about HCC surveillance utility by suggesting that surveillance is beneficial. Our study results and methodology differ from the recent Veterans Affair study by Moon et al. in several ways.12 Our definition of surveillance is continuous and accounts for differences in frequency of imaging studies. In order to be effective, surveillance should be conducted at regular intervals, and a single imaging study does not constitute surveillance.6 In contrast, the Moon et al. study did not clearly define surveillance frequency, but rather reported the proportion of patients undergoing imaging within a prolonged period of up to four years. It also included AFP-only surveillance which has not shown to be an effective surveillance strategy. In addition, our study used far less restrictive criteria for follow-up duration before HCC diagnosis, which is less likely to yield a biased cohort. Finally, the cohort in our study is more representative of the overall HCC population than that in the Veterans Affairs study in which no patients underwent liver transplantation and <17% received curative therapy.
Subgroup analyses showed that while PTC by CT/MRI was consistently associated with improved survival and early diagnosis, PTC by US was only consistently associated with early diagnosis. There are several possible explanations for the lack of association between PTC by US and survival. First, US may lack sufficient sensitivity for early stage disease detection: US sensitivity is decreased by obesity, liver nodularity, or severe steatosis, which are common features in Western patients with cirrhosis.9,13 This is especially notable given that combined use of US and AFP was low in the Optum database with median 0% PTC by US plus AFP in the overall cohort. Second, there may be a “threshold” PTC by US that is adequate for identifying early-stage vs. intermediate-stage disease, and if that threshold is not reached then the benefit of US surveillance is not significant. Randomized studies suggest that short HCC surveillance intervals (3–4 months) are required to achieve greater detection of very early-stage vs. early-stage HCC,24,25 and perhaps an analogous threshold exists between early- and intermediate-stage HCC. While the distinction between very early- and early-stage HCC is important,26 the distinction between early- and intermediate-stage disease may be more meaningful as patients with intermediate-stage disease are frequently ineligible for curative therapy.27,28
CT and MRI are more considerably sensitive for HCC than US, but whether CT or MRI are appropriate and cost-effective as screening modalities is not well-established.29 A recent prospective cohort study comparing HCC surveillance by MRI vs. US in Korean patients primarily with viral hepatitis showed superior HCC detection rates with MRI, although most tumors detected on MRI alone were very early stage.30 Cost-effectiveness analyses of surveillance strategies incorporating cross-sectional imaging have yielded mixed results.31–33 Further, these analyses did not require inclusion of AFP in screening strategies; in our cohort, use of AFP was low, so we were unable to assess the association between AFP and prognosis or early diagnosis. Our study suggests that US-only surveillance may not improve prognosis and that a strategy incorporating CT and/or MRI may be more effective. However, further prospective studies on cross-sectional imaging for routine HCC surveillance is required to address whether this approach is valid.
PTC was low among individuals with HCC in our study and the median PTC by any imaging of 39% corresponds approximately to an imaging study every 15 months. This value is similar to what was previously reported in other analyses of commercial insurance claims database (i.e. Truven) and systematic reviews.17,20 Disparities in healthcare utilization and delivery exist based on race, insurance type, geography (e.g. urban vs. rural), and treatment setting (e.g. academic vs. community) among patients with HCC.34–36 In addition, patients often have misconceptions about HCC and surveillance, and patient-perceived barriers to HCC surveillance have been associated with lower HCC surveillance rates.37 Previous studies found that seeing a non-gastroenterology provider, greater age, compensated cirrhosis, non-Caucasian race, and lower socioeconomic status are associated with decreased adherence to HCC surveillance.20,38,39 Among patients with HCC in the Optum database, younger age, decompensated disease, and subspecialty hepatology care were associated with increased HCC surveillance; however, it was Asian patients who had the highest surveillance rates.
Our study has several limitations that warrant discussion. First, there is a risk of confounding by indication based on imaging modality: CT or MRI may have been more frequently obtained due to symptoms or to follow indeterminate nodules. However, presence of symptoms at HCC diagnosis is associated with a poorer prognosis,40 and patients with higher PTC were more likely to have liver decompensation (data not shown), so one would expect that this confounding by indication from symptomatic HCC would produce an association between greater PTC by CT/MRI and poorer prognosis. We also excluded CT or MRI obtained within 6 months of HCC diagnosis to account for delays between HCC diagnosis and diagnostic code entry, to decrease the risk of confounding by indication. Second, we were not able to determine whether a study was obtained for surveillance or for another indication. We attempted to account for this by separately analyzing outpatient studies, which are presumably more likely to be performed for surveillance than are inpatient or emergency department studies. We also note that in practice any adequate imaging study would serve as surveillance, regardless of the original indication for the study. Third, we could not distinguish between prevalent and incident cirrhosis diagnoses, and patients with an existing cirrhosis diagnosis on entry into the Optum database may have undergone surveillance studies we could not measure. If this is the case, though, we expect that this misclassification would have tended to decrease the measured impact of surveillance. Fourth, we did not have data on tumor stage, and our use of receipt of curative therapy as a proxy for early diagnosis is limited by the possibility for disparities in healthcare delivery/access and non-use of potentially-curative treatment modalities in patients with more advanced liver disease. In addition, it can be difficult to determine with administrative databases whether treatment was administered with curative intent, especially with patients undergoing transarterial therapy with the aim of downstaging to meet criteria for transplant.41 Our cohort included only a small number of patients of Asian or African ancestry, which is a notable limitation given the racial disparities in HCC care described in the previous paragraph.35,36,42 Finally, there is risk for ascertainment bias as patients may have lost commercial insurance following their HCC diagnosis, and patients who subsequently died may have been more likely to have lost insurance due to functional decline. It is unlikely this ascertainment bias would have differentially affected patients based on PTC under the null hypothesis of no effect of surveillance.
In conclusion, we found that in a large insurance claims database, HCC surveillance as measured by PTC by any imaging or by CT/MRI was associated with improved survival and diagnosis at an earlier stage, but PTC by abdominal US was not associated with survival. Our study highlights the need for further study of optimal surveillance strategies for patients with cirrhosis and brings further question to the effectiveness of US-based surveillance.
Supplementary Material
Financial support:
V.L.C. was supported in part by a University of Michigan Training in Basic and Translational Digestive Sciences T32 grant (5T32DK094775). A.S, N.P., and E.T. are in part supported by grants NCI R01 CA222900.
Abbreviations:
- AFP
alpha-fetoprotein
- CT
computed tomography
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- IQR
interquartile range
- MRI
magnetic resonance imaging
- PTC
percentage time covered
- US
ultrasound
Footnotes
Conflicts of interest:
Vincent Chen: none
Amit Singal Consulting: Eisai, BMS, Bayer, Exelixis, Roche, Glycotest, Exact Sciences. Advisory committee review board: TARGET.
Elliott Tapper: Consulting: Novartis, Allergan. Advisory board or review panel: Bausch Health.
Neehar Parikh: Consultant: Bristol-Myers Squibb, Exelixis, Eli Lilly, Freenome; Advisory Board: Eisai, Bayer, Exelixis, Wako Diagnostics; Research Grants: Bayer, Target Pharmasolutions, Exact Sciences
References
- 1.Bertuccio P, Turati F, Carioli G, et al. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol. 2017;67(2):302–309. [DOI] [PubMed] [Google Scholar]
- 2.Njei B, Rotman Y, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology. 2015;61(1):191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanwal F, Kramer JR, Duan Z, Yu X, White D, El-Serag HB. Trends in the Burden of Nonalcoholic Fatty Liver Disease in a United States Cohort of Veterans. Clin Gastroenterol Hepatol. 2016;14(2):301–308 e301–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: observational study. BMJ. 2018;362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies. Liver Int. 2009;29(4):502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. [DOI] [PubMed] [Google Scholar]
- 7.Galle PR, Forner A, Llovet JM, et al. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. Journal of Hepatology. 2018;69(1):182–236. [DOI] [PubMed] [Google Scholar]
- 8.Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130(7):417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi DT, Kum HC, Park S, et al. Hepatocellular Carcinoma Screening Is Associated With Increased Survival of Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2019;17(5):976–987 e974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen VL, Yeh ML, Le AK, et al. Anti-viral therapy is associated with improved survival but is underutilised in patients with hepatitis B virus-related hepatocellular carcinoma: real-world east and west experience. Aliment Pharmacol Ther. 2018;48(1):44–54. [DOI] [PubMed] [Google Scholar]
- 12.Moon AM, Weiss NS, Beste LA, et al. No Association Between Screening for Hepatocellular Carcinoma and Reduced Cancer-related Mortality in Patients with Cirrhosis. Gastroenterology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tzartzeva K, Obi J, Rich NE, et al. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology. 2018;154(6):1706–1718.e1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atiq O, Tiro J, Yopp AC, et al. An assessment of benefits and harms of hepatocellular carcinoma surveillance in patients with cirrhosis. Hepatology. 2017;65(4):1196–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konerman MA, Verma A, Zhao B, Singal AG, Lok AS, Parikh ND. Frequency and Outcomes of Abnormal Imaging in Patients With Cirrhosis Enrolled in a Hepatocellular Carcinoma Surveillance Program. Liver Transplantation. 2019;25(3):369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devaki P, Wong RJ, Marupakula V, et al. Approximately one-half of patients with early-stage hepatocellular carcinoma meeting Milan criteria did not receive local tumor destructive or curative surgery in the post-MELD exception era. Cancer. 2014;120(11):1725–1732. [DOI] [PubMed] [Google Scholar]
- 17.Zhao C, Jin M, Le RH, et al. Poor adherence to hepatocellular carcinoma surveillance: A systematic review and meta-analysis of a complex issue. Liver Int. 2018;38(3):503–514. [DOI] [PubMed] [Google Scholar]
- 18.Kansagara D, Papak J, Pasha AS, et al. Screening for Hepatocellular Carcinoma in Chronic Liver Disease: A Systematic ReviewScreening for Hepatocellular Carcinoma in Chronic Liver Disease. Annals of Internal Medicine. 2014;161(4):261–269. [DOI] [PubMed] [Google Scholar]
- 19.Nehra MS, Ma Y, Clark C, Amarasingham R, Rockey DC, Singal AG. Use of administrative claims data for identifying patients with cirrhosis. Journal of clinical gastroenterology. 2013;47(5):e50–e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldberg DS, Valderrama A, Kamalakar R, Sansgiry SS, Babajanyan S, Lewis JD. Hepatocellular Carcinoma Surveillance Among Cirrhotic Patients With Commercial Health Insurance. J Clin Gastroenterol. 2016;50(3):258–265. [DOI] [PubMed] [Google Scholar]
- 21.Wickham H tidyverse: Easily Install and Load the ‘Tidyverse’. R package version 1.2.1. https://CRAN.R-project.org/package=tidyverse. Published 2017. Accessed March 1, 2019. [Google Scholar]
- 22.Therneau T A Package for Survival Analysis in S version 2.38. https://CRAN.R-project.org/package=survival. Published 2015. Accessed March 1, 2019.
- 23.Kassambara A, Kosinski M. survminer: Drawing Survival Curves using ‘ggplot2’. R package version 0.4.3. https://CRAN.R-project.org/package=survminer. Published 2018. Accessed March 1, 2019. [Google Scholar]
- 24.Trinchet JC, Chaffaut C, Bourcier V, et al. Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3- and 6-month periodicities. Hepatology. 2011;54(6):1987–1997. [DOI] [PubMed] [Google Scholar]
- 25.Wang JH, Chang KC, Kee KM, et al. Hepatocellular carcinoma surveillance at 4- vs. 12-month intervals for patients with chronic viral hepatitis: a randomized study in community. Am J Gastroenterol. 2013;108(3):416–424. [DOI] [PubMed] [Google Scholar]
- 26.Yang JD. Detect or not to detect very early stage hepatocellular carcinoma? The western perspective. Clin Mol Hepatol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciria R, Lopez-Cillero P, Gallardo AB, et al. Optimizing the management of patients with BCLC stage-B hepatocellular carcinoma: Modern surgical resection as a feasible alternative to transarterial chemoemolization. Eur J Surg Oncol. 2015;41(9):1153–1161. [DOI] [PubMed] [Google Scholar]
- 28.Zhaohui Z, Shunli S, Bin C, et al. Hepatic Resection Provides Survival Benefit for Selected Intermediate-Stage (BCLC-B) Hepatocellular Carcinoma Patients. Cancer Res Treat. 2019;51(1):65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanna RF, Miloushev VZ, Tang A, et al. Comparative 13-year meta-analysis of the sensitivity and positive predictive value of ultrasound, CT, and MRI for detecting hepatocellular carcinoma. Abdominal Radiology. 2016;41(1):71–90. [DOI] [PubMed] [Google Scholar]
- 30.Kim SY, An J, Lim YS, et al. MRI With Liver-Specific Contrast for Surveillance of Patients With Cirrhosis at High Risk of Hepatocellular Carcinoma. JAMA Oncol. 2017;3(4):456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lima PH, Fan B, Berube J, et al. Cost-Utility Analysis of Imaging for Surveillance and Diagnosis of Hepatocellular Carcinoma. AJR Am J Roentgenol. 2019:1–9. [DOI] [PubMed] [Google Scholar]
- 32.Andersson KL, Salomon JA, Goldie SJ, Chung RT. Cost effectiveness of alternative surveillance strategies for hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2008;6(12):1418–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim HL, An J, Park JA, Park SH, Lim YS, Lee EK. Magnetic Resonance Imaging Is Cost-Effective for Hepatocellular Carcinoma Surveillance in High-Risk Patients With Cirrhosis. Hepatology. 2019;69(4):1599–1613. [DOI] [PubMed] [Google Scholar]
- 34.Hoehn RS, Hanseman DJ, Jernigan PL, et al. Disparities in care for patients with curable hepatocellular carcinoma. HPB (Oxford). 2015;17(9):747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong RJ, Devaki P, Nguyen L, Cheung R, Nguyen MH. Ethnic disparities and liver transplantation rates in hepatocellular carcinoma patients in the recent era: results from the Surveillance, Epidemiology, and End Results registry. Liver Transpl. 2014;20(5):528–535. [DOI] [PubMed] [Google Scholar]
- 36.Sonnenday CJ, Dimick JB, Schulick RD, Choti MA. Racial and geographic disparities in the utilization of surgical therapy for hepatocellular carcinoma. J Gastrointest Surg. 2007;11(12):1636–1646; discussion 1646. [DOI] [PubMed] [Google Scholar]
- 37.Farvardin S, Patel J, Khambaty M, et al. Patient-reported barriers are associated with lower hepatocellular carcinoma surveillance rates in patients with cirrhosis. Hepatology. 2017;65(3):875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan D, Yopp A, Beg MS, Gopal P, Singal AG. Meta-analysis: underutilisation and disparities of treatment among patients with hepatocellular carcinoma in the United States. Aliment Pharmacol Ther. 2013;38(7):703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singal AG, Yopp A, C SS, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27(7):861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen VL, Le AK, Kim NG, et al. Effects of Cirrhosis on Short-term and Long-term Survival of Patients With Hepatitis B-related Hepatocellular Carcinoma. Clin Gastroenterol Hepatol. 2016;14(6):887–895 e881. [DOI] [PubMed] [Google Scholar]
- 41.Yao FY, Mehta N, Flemming J, et al. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology. 2015;61(6):1968–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu JC, Neugut AI, Wang S, et al. Racial and insurance disparities in the receipt of transplant among patients with hepatocellular carcinoma. Cancer. 2010;116(7):1801–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


