Abstract
Aim
To investigate the rate of antibiotic resistance and its main risk factors in a population of patients with diabetic foot infection (DFI) during the COVID-19 pandemic, in comparison with the population of 2019.
Methods
Two hundred and twenty-five patients with DFI were admitted in a tertiary care center from January 2019 to December 2020. Antibiotic resistance was evaluated by microbiological examination of soft tissues’ or bone’s biopsy.
Results
Compared with 2019 group (n = 105), 2020 group (n = 120) had a significantly higher prevalence of antibiotic resistance [2019 vs 2020, 36% vs 63%, P <0.001] and more often was admitted with recent or current antibiotic therapy (18% vs 52%, P <0.001), which was frequently self-administered (5% vs 30%, P = 0.032). The risk of antibiotic resistance was also higher in 2020 group [OR 95% CI, 2.90 (1.68 to 4.99)]. Prior hospitalization, antibiotic self-administration and antibiotic prescription by general practitioners resulted as independent predictors of antibiotic resistance.
Conclusions
In a population of people with DFI admitted in a tertiary care center during the COVID-19 pandemic the prevalence of antibiotic resistance was higher than 2019. Previous hospitalization, antibiotic self-administration /prescription by general practitioners were related to higher risk of antibiotic resistant infections.
Keywords: Type 2 diabetes, Diabetic foot infection, Antibiotic resistance, Amputation, Diabetic foot ulcer
1. Introduction
Diabetes Mellitus (DM) affects more than 460 million people worldwide [1] and represents a major health care issue, given the high rate of long-term complications developed by diabetic people during their lifetime. Among these complications, diabetic foot ulcer (DFU) is the most common cause of non-traumatic lower limb amputation [2], with detrimental effects on mortality and quality of life. Moreover, DFU is responsible for a heavy burden on health care costs due to prolonged and recurrent hospitalization, infection and gangrene [3], [4].
Infections in DFU are capturing the attention of the clinicians because often related to severe discomfort, increased request of medical cares and high risk of healing failure, which may rapidly lead to hospitalization and lower extremities amputation [5]. The development of diabetic foot infection (DFI) is strictly associated with an open wound [6], but other risk factors include peripheral neuropathy and arteriopathy, diabetes-related immune dysfunction, renal impairment and the chronic course of the wound itself [6], [7]. DFI still remains a clinical challenge due to the complexity of medical and surgical treatments and the associated negative outcome. Hence, DFI diagnosis is essential in order to identify and properly treat the pathogens involved, with the ultimate goal of avoiding demolitive surgery or medical emergencies, such as wet gangrene or sepsis [8].
The treatment of infections, including DFI, is becoming increasingly difficult because of the massive consumption of antibiotic drugs which is heavily responsible for emerging antimicrobial resistances [9]. The World Health Organization has recognized the antibiotic resistance as one of the most concerning public health threat of the 21st century [10]; moreover, the risk of ineffective antibiotic administration may hinder the therapeutic target, compromising the healing process in patients with infectious diseases, including DFI. Therefore, the most effective management of both DFU and DFI appears strongly dependent on the continuous delivery of medical care by a multidisciplinary team which must involve several figures, such as diabetologists, surgeons, radiologists and infectious diseases specialists [11].
In this scenario, the sudden spread of coronavirus disease (COVID-19) infection has affected the health care department worldwide, determining an inevitable delay of the access to ordinary procedures and outpatients clinics [12]. After almost a year since the pandemic has been declared, recurrent lockdowns and interruption of daily activities have already caused profound changes in medical cares delivered to the people with chronic diseases, including DM and DFU. Moreover, we already reported a higher rate of lower extremities amputation in 2020, as compared with 2019 [12], as further confirmed by other authors [13], [14]. In our experience, amputation was significantly related to high rate of gangrene and emergency conditions, which were diagnosed during the first lockdown [12]. These circumstances have persisted over time, as well as the limitations we are all living with since the beginning of 2020. However, studies evaluating the risk of antibiotic resistance associated with the hospital admissions for DFI during the year of the pandemic are scanty.
The aim of the present study was: 1) to investigate the rate of antibiotic resistant infections and the associated risk factors in a population of patients with both DFU and DFI admitted to a tertiary care center during the 2020, in comparison with the population admitted with the same diagnoses in 2019; 2) to evaluate the risk of antibiotic resistance in 2020 vs 2019.
2. Material and methods
2.1. Patients
This is a retrospective study of people with DM and DFU admitted to the Division of Endocrinology and Metabolic Diseases at the Teaching Hospital of University of Campania “Luigi Vanvitelli” (Naples, Italy), from the 1st of January 2019 to the 31st of December 2020. In order to be included, the individuals had to have: 1) a diagnosis of diabetes resulting from medical records, 2) a hospital admission for DFU, 3) a microbiological diagnosis of DFI, according to Infectious Diseases Society of America/International Working Group on Diabetic Foot (IDSA/IWGDF) guidelines [15]. We excluded from the study patients who only attended the outpatient clinic, those with sterile cultural examination or with pre-ulcerative lesions.
2.2. Data collection
Baseline clinical features were collected from hospital medical records and organized into an internal electronic database. We registered age, sex, diabetes duration, whether patients were attending regular outpatient visits and were admitted for emergency conditions. HbA1c, renal function parameters [serum creatinine, estimated glomerular filtration rate (eGFR) and urinary albumin to creatinine ratio (UACR)], C-reactive protein (CRP) and White Blood Cells (WBC) count were recorded. Information about peripheral neuropathy and peripheral artery disease, history of previous ulcers was registered, as well as the duration of the current DFU, the presence of gangrene and osteomyelitis. The treatment assigned for the DFU, including revascularization and amputation, was also recorded. Moreover, information about the rate of mono- or polymicrobial infection, isolation of Gram-positive, Gram-negative or both was gathered. Furthermore, in order to investigate the prevalence of infections by antibiotic resistant microorganisms, we registered, among Gram-positive, Staphilococcus Aureus (S. Aureus) resistance to oxacillin, Corinebacterium Striatum (C. Striatum) resistance to both vancomycin and linezolid, Enterococcus Faecalis (E. Faecalis) resistance to ampicillin and Enterococcus Faecium (E. Faecium) resistance to vancomycin; among Gram-negative, single or multiple resistance to carbapenems, colistin, 3rd and 4th generation cephalosporins, piperacillin/tazobactam and quinolones was also evaluated and recorded. The main risk factors for antibiotic resistance, including prior hospitalization within 6 months before the admission and recent or current antibiotic therapy, were retrieved from patients’ clinical history. Specifically, we registered if the patients had taken any antibiotic therapy within 2 weeks from the hospital admission and whether this therapy was empirical or targeted according to any cultural examination; data about self-administration, prescription by general practitioners or specialists were also collected. This was a retrospective case notes analysis study and, as such, the local ethical committee was notified on data collection.
2.3. Clinical assessment
Peripheral artery disease was diagnosed with color doppler ultrasound, peripheral angio-CT or peripheral arteriography. Peripheral neuropathy was detected with the diabetic neuropathy index for somatic and autonomic neuropathy. The diagnosis of infection was performed by both clinical signs and soft tissues’ and/or bone’s biopsy. Moreover, all the patients underwent to X-ray examination of the foot; magnetic resonance imaging was performed if osteomyelitis was suspected. Revascularization was referred to both endovascular and surgical procedures. Amputation included the transverse removal of part of the lower limb above or below the ankle joint (both major and minor amputation) [16]. The University of Texas Classification System was used to classify the ulcers [17], whereas the IDSA/IWGDF classification was used to define the severity of the infections [15].
eGFR was determined by MDRD formula. Albuminuria was detected in fresh urine samples by immunonephelometry or immunoturbidimetry by calculating urinary albumin-to-creatinine ratio (UACR) in early-morning first-voided urine samples. The hospital’s chemistry laboratory also provided the assays for HbA1c, serum creatinine, CRP and WBC count, whereas the hospital’s microbiology laboratory was involved in the microbiological examination of the biopsies with the relative antibiograms.
2.4. Statistical analysis
Descriptive statistics was used to compare baseline features of patients admitted in 2019 vs those admitted in 2020. According to the sample distribution, data in tables and figures are presented as mean ± standard deviation or median and interquartile range. Differences between the populations were calculated by two-sided Student’s t-test or Wilcoxon-Mann-Whitney test, as appropriate. The χ2 test was used for comparing dichotomous variables. The odds ratio (OR) and 95% confidence interval of antibiotic resistance in 2020, as compared to 2019, was calculated from a logistic regression model, whereas a multivariate logistic regression analysis was used to evaluate the association between the antibiotic resistance and its main risk factors. The OR for antibiotic resistance and their respective 95% confidence interval were calculated. A P value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS software (version 14.2, SPSS, Chicago, IL, USA).
3. Results
The study population included 225 people, of whom 105 were admitted in 2019 and 120 in 2020. Baseline demographic and clinical characteristics are described in Table 1 . As compared with 2019, in 2020 the patients’ mean age was significantly lower [2019 vs 2020, median (IQR), 69.0 (62.0, 77.0) vs 65.0 (58.0, 74.0), P = 0.024]; moreover, a higher prevalence of men was found (54% vs 69%, P = 0.031). There were no differences between groups in biochemical parameters, except for UACR, CRP and WBC count, that were significantly higher in the cohort admitted in 2020. The prevalence of peripheral artery disease and peripheral neuropathy was similar in both groups; however, more patients in 2020 had history of previous ulcers, as compared with 2019 patients (50% vs 70%, P = 0.004). In 2020, the rate of individuals coming from regular outpatient visits was significantly lower (39% vs 25%, P = 0.048), whereas the number of patients who were admitted because of emergency conditions resulted significantly higher (37% vs 60%, P = 0.001), as compared with 2019 group. There were no differences between groups in ulcer duration, ulcer clinical features and rate of osteomyelitis, whereas the prevalence of gangrene was higher in 2020 (13% vs 38%, P <0.001). Moreover, there was no difference in the percentage of patients undergoing revascularization; the amputation rate was higher in 2020 (25% vs 40%, P = 0.022).
Table 1.
Baseline demographic and clinical characteristics of participants admitted in 2019 and 2020.
| Parameter | 2019 (n = 105) | 2020 (n = 120) | P |
|---|---|---|---|
| Age, years | 69.0 (62.0, 77.0) | 65.0 (58.0, 74.0) | 0.024 |
| Male/Female | 57/48 | 83/37 | 0.031 |
| Diabetes duration, years | 19.3 ± 10.9 | 21.3 ± 12.6 | 0.196 |
| HbA1c, % | 7.3 (6.7, 8.5) | 7.8 (6.8, 9.0) | 0.168 |
| HbA1c, mmol/mol | 56 (50, 69) | 62 (51, 75) | |
| Renal Fuction | |||
| Creatinine, mg/dl | 1.0 (0.8, 1.3) | 1.0 (0.8, 1.4) | 0.629 |
| eGFR, ml/min | 68.8 ± 29.7 | 67.1 ± 26.9 | 0.664 |
| UACR, mg/g Cr | 20.5 (5.5, 80.0) | 51.5 (25.0, 165.0) | <0.001 |
| CRP, mg/dl | 0.9 (0.3, 3.0) | 2.1 (0.8, 5.9) | <0.001 |
| WBC, nx10e3/ul | 8.3 (6.3, 10.0) | 9.6 (7.5, 11.7) | <0.001 |
| Peripheral Artery Disease, n (%) | 76 (72) | 90 (75) | 0.796 |
| Peripheral Neuropathy, n (%) | 78 (74) | 102 (85) | 0.066 |
| History of previous ulcer, n (%) | 53 (50) | 84 (70) | 0.004 |
| Ulcer duration, months | 5.0 (2.0, 12.0) | 4.0 (2.0, 8.0) | 0.195 |
| Outpatient patients, n (%) | 41 (39) | 31 (25) | 0.048 |
| Admitted in emergency, n (%) | 39 (37) | 72 (60) | 0.001 |
| TEXAS classification system, n (%) | |||
| Superficial wound | 27 (26) | 21 (18) | 0.181 |
| Affecting tendons/capsules | 25 (24) | 25 (21) | 0.708 |
| Affecting bone/joint | 53 (50) | 74 (62) | 0.120 |
| Osteomyelitis, n (%) | 53 (50) | 74 (62) | 0.120 |
| Gangrene, n (%) | 14 (13) | 46 (38) | <0.001 |
| Revascularization, n (%) | 14 (13) | 28 (23) | 0.080 |
| Amputation, n (%) | 26 (25) | 48 (40) | 0.022 |
Data are reported as mean ± SD, median (IQR) or number and percentage. CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; UACR, urinary albumin to creatinine ratio; WBC, white blood cells.
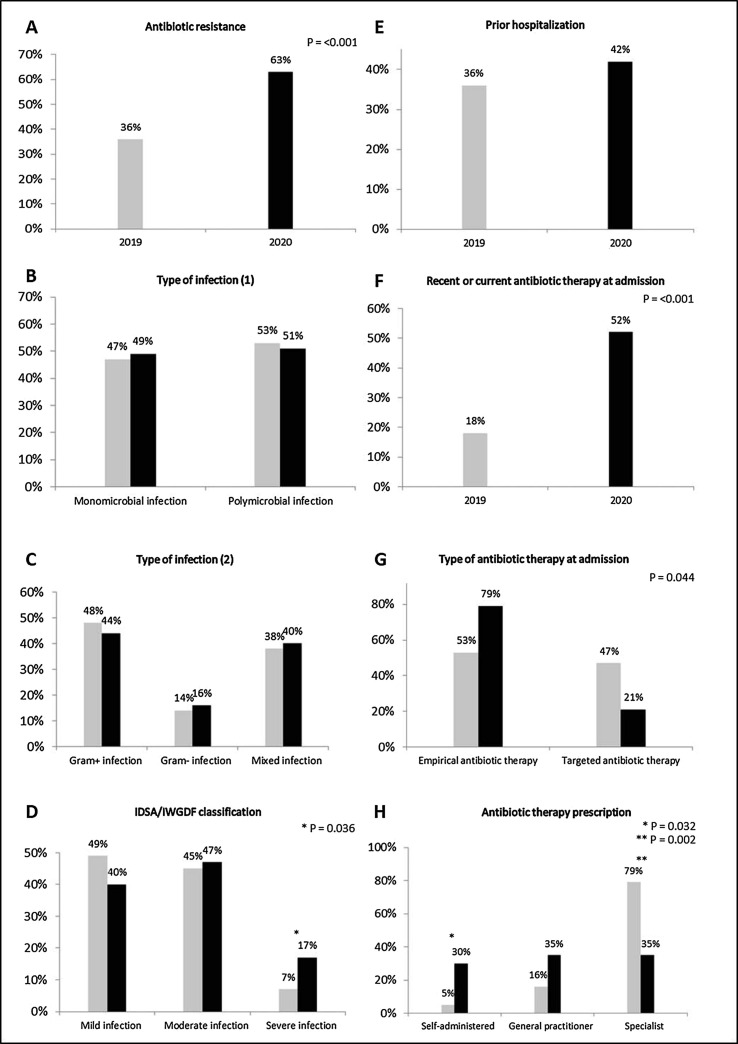
As compared with 2019, a higher rate of antibiotic resistance was detected in 2020 (36% vs 63%, P <0.001, Fig. 1 A). There were no differences between groups in the proportion of monomicrobial or polymicrobial infections (Fig. 1B), the type of pathogens involved (Gram-positive, Gram-negative or mixed) (Fig. 1C) and in the rate of prior hospitalization (Fig. 1E); according to the IDSA/IWGDF classification, the prevalence of severe infections was significantly higher in 2020 (7% vs 17%, P = 0.036) (Fig. 1D). Among the overall population, 19 patients of the 2019 group (18%) and 63 patients of the 2020 group (52%) were admitted with recent or current antibiotic therapy (P <0.001) (Fig. 1F). Within these subsets of patients, the rate of the administration of empirical antibiotics was higher in 2020 (53% vs 79%, P = 0.044) (Fig. 1G). Moreover, as compared with 2019, a higher rate of antibiotics self-administration (5% vs 30%, P = 0.032), associated with a significant reduction of prescriptions by specialists (79% vs 35%, P = 0.002), was found in 2020 (Fig. 1H).
Fig. 1.
Proportion of patients admitted in 2019 and 2020 according to: antibiotic resistance (A); monomicrobial or polymicrobial infection (B); Gram+, Gram- or mixed infection (C); diagnosis of mild, moderate or severe infection according to IDSA/IWGDF classification (D); history of prior hospitalization (within 6 months to the admission to our Division) (E); recent (within 2 weeks) or current antibiotic therapy at admission (F); empirical or targeted antibiotic therapy at admission (G); self-administration, general practitioners’ prescription or specialists’ prescription of antibiotic therapy at admission (H).
Table 2 describes antibiotic resistance features. There was no difference in the rate of resistance to single or multiple antibiotics. The most frequent Gram-positive pathogen isolated in both 2019 and 2020 was S. Aureus, whereas, among Gram-negative, P. Aeruginosa was detected more frequently in both cohorts (Supplementary Table S1). As compared with 2019, the prevalence of S. Aureus resistance to oxacillin (22% vs 41%, P = 0.022) and the prevalence of C. Striatum resistance to both vancomycin (70% vs 95%, P = 0.041) and linezolid (30% vs 68%, P = 0.031) were significantly higher in the 2020. No differences between groups were found in ampicillin resistance of E. Faecalis and vancomycin resistance of E. Faecium. Among Gram-negative pathogens, a significantly higher rate of resistance to carbapenems (15% vs 41%, P = 0.001), colistin (18% vs 49%, P = 0.010) and 3rd and 4th generation cephalosporins (25% vs 45%, P = 0.021) was observed in 2020; the prevalence of piperacillin/tazobactam and quinolones resistance was similar in both groups.
Table 2.
Antibiotic resistance characteristics and antibiograms of the participants admitted in 2019 and 2020.
| Parameter | 2019 (n = 105) | 2020 (n = 120) | P |
|---|---|---|---|
| Resistance to one/more antibiotics | 7/47 | 1/30 | 0.248 |
| Staphilococcus Aureus | 68 | 83 | |
| Oxacillin resistance, n (%) | 15 (22) | 34 (41) | 0.022 |
| Corinebacterium Striatum Vancomycin resistance, n (%) Linezolid resistance, n (%) |
20 14 (70) 6 (30) |
22 21 (95) 15 (68) |
0.041 0.031 |
| Enterococcus Faecalis | 20 | 13 | |
| Ampicillin resistance, n (%) | 2 (10) | 3 (23) | 0.360 |
| Enterococcus Faecium | 0 | 2 | |
| Vancomycin resistance, n (%) | 0 (0) | 0 (0) | 0.998 |
| Gram- pathogens | 67 | 75 | |
| Carbapenems resistance, n (%) | 10 (15) | 31 (41) | 0.001 |
| Colistin resistance, n (%) | 10 (18) | 37 (49) | 0.010 |
| Cephalosporins resistance, n (%) | 17 (25) | 34 (45) | 0.021 |
| Piperacillin/Tazobactam resistance, n (%) | 14 (21) | 21 (28) | 0.432 |
| Quinolones resistance, n (%) | 26 (39) | 41 (55) | 0.085 |
In the multiple logistic regression analysis (Table 3 ), prior hospitalization [OR, 95% CI, 2.09 (1.11 to 3.94), P = 0.02], antibiotic self-administration [23.77 (2.91 to 194.13), P = 0.00] and prescription by general practitioners [11.57 (2.95 to 45.37), P = 0.00] resulted as positive predictors of antibiotic resistance. The OR for antibiotic resistance was almost 3-fold higher in the 2020 group vs 2019 group [2.90 (1.68 to 4.99)].
Table 3.
Contribution of the main risk factors to antibiotic resistance based on multiple logistic regression.
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Age | 1.01 | 0.98 to 1.04 | 0.42 |
| Ulcer duration | 1.02 | 0.99 to 1.05 | 0.18 |
| Admission in emergency Yes vs No |
1.84 | 0.97 to 3.51 | 0.06 |
| Prior hospitalization Yes vs No |
2.09 | 1.11 to 3.94 | 0.02 |
| Antibiotic therapy Targeted vs Empirical |
0.54 | 0.16 to 1.91 | 0.34 |
| Antibiotic therapy prescription Self-administered By general practitioners By specialists |
23.77 11.57 1.89 |
2.91 to 194.13 2.95 to 45.37 0.69 to 5.15 |
0.00 0.00 0.21 |
4. Discussion
To the best of our knowledge, this is the first study evaluating the prevalence and the risk factors for antibiotic resistance in patients admitted in a tertiary care center for DFI during the COVID-19 pandemic. Our results show an almost 3-fold higher risk of antibiotic resistant infections in 2020, as compared with 2019. This finding was consistent with the higher rate of amputation performed and the more severe infections we detected at patients’ admission in 2020. Interestingly, antibiotic resistance did not seem related to the ulcer depth or the diagnosis of osteomyelitis; on the other hand, the rate of gangrene was significantly increased in 2020. Among antibiotic resistance risk factors, there was a higher rate of domiciliary antibiotic therapies, which were often self-administered in the 2020 group of patients. This was also confirmed by the logistic regression analysis, which identified history of prior hospitalization, antibiotic self-administration and antibiotic prescription by general practitioners as independent predictors of antibiotic resistance in these cohorts of patients.
The association of antibiotic resistance with the inappropriate use of antibiotics, in terms of administration and duration of treatments, was already described in patients with DFI [18]. Antimicrobial therapies are often prescribed by physicians or self-administered by patients, even without a certain diagnosis of infection, with the aim of reducing or preventing the bacterial colonization of the wound [8]. However, antibiotic treatments need to be considered as part of a multidisciplinary approach, which may require, at first, a proper diagnosis in association with an accurate debridement, drainage or resection of infected tissues [8]. Several studies have confirmed that the timing of interventions may improve DFI outcome and reduce the need or the duration of antibiotic therapies [19], [20]. On the other hand, the indiscriminate use of these drugs is strongly associated with the selection of resistant or multi-resistant pathogens [21], [22], [23], [24], determining healing impairment and additional demolitive surgery [5], [25]. Of note, the reduction of ulcer healing time together with the conservative approach may strongly improve the outcome of DFU, in terms of recurrence, amputation and mortality [26]. Moreover, antibiotic resistant infections usually require the administration of specific antibiotics and the hospitalization, which is itself a notorious risk factor for antibiotic resistance [27]. Furthermore, antibiotics may determine adverse effects or toxic reactions, which may be responsible for the worsening of the systemic clinical conditions and a dangerous reduction of patient’s compliance to the therapy [8].
Since the end of 2019, the COVID-19 pandemic emerged all over the world, making more challenging the ongoing management of people with chronic diseases [12], [28]. Although the spread of telehealth has represented an important mean of care delivery for several conditions, DFU often requires “face to face” visits with multiple specialists [13], which were abruptly interrupted or delayed. In addition, fragile patients have been dealing with the personal fear or the difficulty to reach hospitals and clinics, and the limitations in outpatient accesses in order to guarantee the mandatory social distancing. Moreover, the massive recruitment of infectious diseases specialists to COVID-19 dedicated wards have determined further issues in the attendance of specialists’ consultation. Therefore, it may be reasonable that the increased prevalence of antibiotic resistance in individuals with DFI is related to these circumstances, which were responsible for a higher rate of antibiotic self-administration and a reduction of prescription by specialists.
Several studies have investigated the bacteriological profile of patients affected by DFI, with controversial results due to different geographical areas, type of infections or method applied to get cultural samples [29], [30]. Our data, which are completely based on biopsies, confirm the role of S. Aureus and P. Aeruginosa as the main aerobic bacteria implicated in DFI, among Gram-positive and Gram-negative pathogens respectively [30]. Moreover, the prevalence of antibiotic resistance in the population of patients of 2019 is coherent with previous studies involving subjects with DFI [30]; on the other hand, the 2020 group has shown a significantly higher rate of antibiotic resistance, mainly concerning S. Aureus resistance to oxacillin, C. Striatum resistance to both vancomycin and linezolid and Gram-negative resistance to carbapenems, colistin and 3rd and 4th generations cephalosporins. Piperacillin/tazobactam and quinolones have remained effective against the majority of Gram-negative pathogens. Interestingly, there were no differences between groups in the rate of mono- or polymicrobial infections, which are usually responsible for a more severe DFI clinical course [31], but not directly involved in antibiotic resistance mechanisms. The isolation of multiple microorganisms may also be the result of a bacterial superinfection which is not primarily associated with the pathogenesis of DFU. Moreover, the antibiotic resistance of a single pathogen does not depend on the contemporary colonization by other microorganisms.
Our finding may help clinicians to deal with DFI and consider the risk of antibiotic resistance, which partially accounted for the increase of amputation rate over the year of the COVID-19 pandemic. Moreover, this study confirms the importance of stewardship programs, which aim to promote the awareness of antibiotics as precious but limited resources, and their inappropriate prescriptions as responsible for their own ineffectiveness [27], [32].
This study has limitations, mainly due to its retrospective design which does not allow to properly identify a cause-effect relationship between antibiotic resistance and the risk factors. Moreover, these results refer to a single-center experience, which may not be representative of the entire population with DFI. On the other hand, this study includes a quite large number of selected diabetic individuals, who underwent a high standard level of care according to both the expertise of a tertiary care center and the international practical guidelines. Another strength of the present study refers to the use of biopsies from bones or soft tissues to isolate pathogens.
In conclusion, in a population of patients with DFI admitted in a single tertiary care center, there was a 3-fold higher risk for antibiotic resistance in 2020, as compared with individuals admitted with the same diagnosis in 2019. Prior hospitalization, antibiotic self-administration or antibiotic prescription by general practitioners were predictors of antibiotic resistance in this population. Persisting the pandemic, clinicians should be aware that people with DFI may present more frequently antibiotic resistant infections; therefore, both a detailed pharmacological anamnesis and education of patients, together with stewardship programs may be helpful in the next future.
Acknowledgments
Acknowledgements
Funding
The authors received no funding from an external source.
Declaration of Competing Interest
M.I.M. received honoraria for speaking at meetings from Astra-Zeneca, Novo Nordisk, Sanofi-Aventis, Mundi Pharma, Merck. G.B. received honoraria for speaking at meetings for Roche and Novo Nordisk. K.E. received honoraria for speaking at meetings from Sanofi-Aventis, Lilly, AstraZeneca, Abbott, Boehringer Ingelheim, Novo Nordisk, Mundi Pharma. Other authors declare no conflict of interest.
Author contributions
P.C. and M.I.M. conceived the study and wrote the manuscript. M.M. contributed to the data analysis and in writing the manuscript. G.S. did the statistical analyses and contributed to the data analysis. L.C., L.S. and M.L. collected data and contributed to the data analysis. M.G. collected data and reviewed the manuscript for intellectual content. F.C., G.B. and N.C. contributed to the data analysis and reviewed the manuscript for intellectual content. K.E. conceived the study, contributed to the data analysis, reviewed and edited the manuscript. All authors approved the final version of the manuscript. K.E. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Saeedi P., Petersohn I., Salpea P., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 2.Apelqvist J., Larsson J. What is the most effective way to reduce incidence of amputation in the diabetic foot? Diabetes Metab Res Rev. 2000;16:S75–S83. doi: 10.1002/1520-7560(200009/10)16:1+<::aid-dmrr139>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Driver V.R., Fabbi M., Lavery L.A., Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Vasc Surg. 2010;52:17S–22S. doi: 10.1016/j.jvs.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Moss S.E., Klein R., Klein B.E. The prevalence and incidence of lower extremity amputation in a diabetic population. Arch Intern Med. 1992;152:610–616. [PubMed] [Google Scholar]
- 5.Noor S., Khan R.U., Ahmad J. Understanding Diabetic Foot Infection and its Management. Diabetes Metab Syndr. 2017;11149–156 doi: 10.1016/j.dsx.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 6.Chastain C.A., Klopfenstein N., Serezani C.H., Aronoff D.M. A Clinical Review of Diabetic Foot Infections. Clin Podiatr Med Surg. 2019;36:381–395. doi: 10.1016/j.cpm.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Akash M.S.H., Rehman K., Fiayyaz F., Sabir S., Khurshid M. Diabetes-associated infections: development of antimicrobial resistance and possible treatment strategies. Arch Microbiol. 2020;202:953–965. doi: 10.1007/s00203-020-01818-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbas M., Uçkay I., Lipsky B.A. In diabetic foot infections antibiotics are to treat infection, not to heal wounds. Expert Opin Pharmacother. 2015;16:821–832. doi: 10.1517/14656566.2015.1021780. [DOI] [PubMed] [Google Scholar]
- 9.Munita J.M., Arias C.A. Mechanisms of Antibiotic Resistance. Microbiol Spectr. 2016;4 doi: 10.1128/microbiolspec.VMBF-0016-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Antimicrobial resistance: global report on surveillance 2014. 2014. From http://www.who.int/drugresistance/documents/surveillancereport/en/, last accessed on 19th January, 2021.
- 11.Schaper N.C., van Netten J.J., Apelqvist J., Bus S.A., Hinchliffe R.J., Lipsky B.A., et al. update) Diabetes Metab Res Rev. 2019;2020(36) doi: 10.1002/dmrr.3266. [DOI] [PubMed] [Google Scholar]
- 12.Caruso P., Longo M., Signoriello S., Gicchino M., Maiorino M.I., Bellastella G., et al. Diabetic Foot Problems During the COVID-19 Pandemic in a Tertiary Care Center: The Emergency Among the Emergencies. Diabetes Care. 2020;43:e123–e124. doi: 10.2337/dc20-1347. [DOI] [PubMed] [Google Scholar]
- 13.Casciato D.J., Yancovitz S., Thompson J., Anderson S., Bischoff A., Ayres S., et al. Diabetes-related major and minor amputation risk increased during the COVID-19 pandemic. J Am Podiatr Med Assoc. 2020;3:20–224. doi: 10.7547/20-224. [DOI] [PubMed] [Google Scholar]
- 14.Rogers L.C., Snyder R.J., Joseph W.S. Diabetes-related Amputations: A Pandemic within a Pandemic. J Am Podiatr Med Assoc. 2020;3:20–248. doi: 10.7547/20-224. [DOI] [PubMed] [Google Scholar]
- 15.Lipsky B.A., Senneville É., Abbas Z.G., Aragón-Sánchez J., Diggle M., Embil J.M., et al. International Working Group on the Diabetic Foot (IWGDF). Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update) Diabetes Metab Res Rev. 2020;36:e3280. doi: 10.1002/dmrr.3280. [DOI] [PubMed] [Google Scholar]
- 16.Jones N.J., Harding K. 2015 International Working Group on the Diabetic Foot Guidance on the prevention and management of foot problems in diabetes. Int Wound J. 2015;12:373–374. doi: 10.1111/iwj.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavery L.A., Armstrong D.G., Harkless L.B. Classification of diabetic foot wounds. J Foot Ankle Surg. 1996;35:528–531. doi: 10.1016/s1067-2516(96)80125-6. [DOI] [PubMed] [Google Scholar]
- 18.Noor S., Borse A.G., Ozair M., Raghav A., Parwez I., Ahmad J. Inflammatory markers as risk factors for infection with multidrug-resistant microbes in diabetic foot subjects. Foot. 2017;32:44–48. doi: 10.1016/j.foot.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Dalla Paola L., Faglia E. Treatment of diabetic foot ulcer: an overview strategies for clinical approach. Curr Diabetes Rev. 2006;2:431–447. doi: 10.2174/1573399810602040431. [DOI] [PubMed] [Google Scholar]
- 20.Ruth Chaytor E. Surgical treatment of the diabetic foot. Diabetes Metab Res Rev. 2000;16:S66–S69. doi: 10.1002/1520-7560(200009/10)16:1+<::aid-dmrr140>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 21.Patterson J.E. Antibiotic utilization: is there an effect on antimicrobial resistance? Chest. 2001;119:426S–430S. doi: 10.1378/chest.119.2_suppl.426s. [DOI] [PubMed] [Google Scholar]
- 22.Goossens H., Ferech M., Vander Stichele R., Elseviers M. ESAC Project Group. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365:579–587. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 23.van de Sande-Bruinsma N., Grundmann H., Verloo D., Tiemersma E., Monen J., Goossens H., et al. European Antimicrobial Resistance Surveillance System Group; European Surveillance of Antimicrobial Consumption Project Group. Antimicrobial drug use and resistance in Europe. Emerg Infect Dis. 2008;14:1722–1730. doi: 10.3201/eid1411.070467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goossens H. Antibiotic consumption and link to resistance. Clin Microbiol Infect. 2009;15:12–15. doi: 10.1111/j.1469-0691.2009.02725.x. [DOI] [PubMed] [Google Scholar]
- 25.Hurlow J.J., Humphreys G.J., Bowling F.L., McBain A.J. Diabetic foot infection: A critical complication. Int Wound J. 2018;15:814–821. doi: 10.1111/iwj.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gazzaruso C., Gallotti P., Pujia A., Montalcini T., Giustina A., Coppola A. Predictors of healing, ulcer recurrence and persistence, amputation and mortality in type 2 diabetic patients with diabetic foot: a 10-year retrospective cohort study. Endocrine. 2021;71:59–68. doi: 10.1007/s12020-020-02431-0. [DOI] [PubMed] [Google Scholar]
- 27.Lipsky B.A. Diabetic foot infections: Current treatment and delaying the 'post-antibiotic era'. Diabetes Metab Res Rev. 2016;32:246–253. doi: 10.1002/dmrr.2739. [DOI] [PubMed] [Google Scholar]
- 28.Shin L., Bowling F.L., Armstrong D.G., Boulton A.J.M. Saving the Diabetic Foot During the COVID-19 Pandemic: A Tale of Two Cities. Diabetes Care. 2020;43:1704–1709. doi: 10.2337/dc20-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdulrazak A., Bitar Z.I., Al-Shamali A.A., Mobasher L.A. Bacteriological study of diabetic foot infections. J Diabetes Complications. 2005;19:138–141. doi: 10.1016/j.jdiacomp.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Citron D.M., Goldstein E.J., Merriam C.V., Lipsky B.A., Abramson M.A. Bacteriology of moderate-to-severe diabetic foot infections and in vitro activity of antimicrobial agents. J Clin Microbiol. 2007;45:2819–2828. doi: 10.1128/JCM.00551-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hitam S.A.S., Hassan S.A., Maning N. The Significant Association between Polymicrobial Diabetic Foot Infection and Its Severity and Outcomes. Malays J Med Sci. 2019;26:107–114. doi: 10.21315/mjms2019.26.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quilici M.T., Del Fiol Fde S, Vieira A.E., Toledo M.I. Risk Factors for Foot Amputation in Patients Hospitalized for Diabetic Foot Infection. J Diabetes Res. 2016;2016:8931508. doi: 10.1155/2016/8931508. [DOI] [PMC free article] [PubMed] [Google Scholar]