Abstract
Background
Metabolic diseases are risk factors for severe Coronavirus disease (COVID-19), which have a close relationship with metabolic dysfunction-associated fatty liver disease (MAFLD).
Aims
To evaluate the presence of MAFLD and fibrosis in patients with COVID-19 and its association with prognosis.
Methods
Retrospective cohort study. In hospitalized patients with COVID-19, the presence of liver steatosis was determined by computed tomography scan (CT). Liver fibrosis was assessed using the NAFLD fibrosis score (NFS score), and when altered, the AST to platelet ratio index (APRI) score. Mann-Whitney U, Student´s t-test, logistic regression analysis, Kaplan-Meier curves and Cox regression analysis were used.
Results
432 patients were analyzed, finding steatosis in 40.6%. No differences in pulmonary involvement on CT scan, treatment, or number of days between the onset of symptoms and hospital admission were found between patients with and without MAFLD. The presence of liver fibrosis was associated with higher severity scores, higher levels of inflammatory markers, requirement of mechanical ventilation, incidence of acute kidney injury (AKI), and higher mortality than patients without fibrosis.
Conclusion
The presence of fibrosis rather than the presence of MAFLD is associated with increased risk for mechanical ventilation, development of AKI, and higher mortality in COVID-19 patients.
Keywords: liver steatosis, computed tomography, SARS-CoV-2, prognosis
1. Introduction
Coronavirus disease (COVID-19) caused by the SARS-CoV-2 virus, was first reported in December 2019 and the initial cases were reported in Wuhan, China; currently, this pandemic is seen all over the world, affecting more than 92.1 million people globally, from which more than 1 973 000 patients have died to date [1]. The mortality rate among patients with severe COVID-19 ranges from 21% to 30%, varying according the population studied [2]. The known risk factors associated with the development of complications and mortality in patients with COVID-19 include the presence of obesity, hypertension, type 2 diabetes mellitus (T2DM), cardiovascular diseases, chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), as well as other diseases causing immunosuppression (human immunodeficiency virus (HIV), transplantation, malignancy, chemotherapy), although the role of immunosuppression is still controversial [3], [4], [5]. No curative treatment is available to date, the only drug that has proven a decrement in mortality is dexamethasone [6]. Predicting which patients will develop a severe disease based on the known risk factors (as well as the upcoming predictors), is highly important to timely allocate the available resources to the patients at the higher risk, and possibly, modify their outcome.
On the other hand, non-alcoholic fatty liver disease (NAFLD), recently renamed metabolic dysfunction-associated fatty liver disease (MAFLD), is the main cause of liver disease globally, and due to its close relationship with features of the metabolic syndrome, including obesity and insulin resistance, it is becoming one of the main etiologies of chronic liver disease in the world [7], [8], [9], [10]. A common factor in the pathophysiology with other metabolic diseases, including MAFLD, is the presence of systemic chronic inflammation [11], that in turn promotes the development and progression of liver fibrosis. In terms of liver fibrosis, according to the National Health and Nutrition Examination Survey, up to 10% of the patients with MAFLD have advanced fibrosis [12], and importantly, its presence is associated with the presence of concomitant diseases (e.g. infections), and increases the risk of both liver related and non-liver related adverse clinical outcomes [13]. (Alejandro WJG) Regarding this, pneumonia is one of the most common infections in patients with advanced liver fibrosis, and in fact, respiratory virus are detected in up to 20% of cirrhotic patients admitted in critical care units, exhibiting a higher mortality rate [14].
The poor outcome in patients with COVID-19 and metabolic disorders could be a consequence of an “acute on chronic inflammation” process, where perhaps the chronic basal inflammation milieu of patients with metabolic disorders such as MAFLD and even more in those with MAFLD and liver fibrosis could increase the risk of a hyperinflammatory response in patients with COVID-19. Recognizing an additional risk factor for adverse outcomes among patients with metabolic diseases could help to accurately allocate those with known MAFLD and those with high risk of MAFLD and liver fibrosis into a closer monitoring and early treatment. Therefore, the aim of the study was to evaluate the prevalence of MAFLD and liver fibrosis in patients with COVID-19 and its association with the development of complications, inflammatory markers, higher values in severity scores, increased risk of requirement mechanical ventilation, and overall mortality.
2. Materials and methods
This was a retrospective cohort study performed in a tertiary care center in Mexico City (Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán), from March to May 2020. The study was conducted according to the Declaration of Helsinki and was approved by the Institutional Ethics and Research Committees (Ref. Number 3405).
2.1. Patients
All patients admitted to our center from March 1st to May 19th, 2020, older than 18 years old, any gender, with a confirmed diagnosis of SARS-CoV-2 infection by real-time polymerase chain reaction (RT-PCR) [15] were included in the study. Only patients with severe disease requiring treatment with oxygen were included. Patients transferred from or to another hospital, those who solicited voluntary discharge or those lacking follow-up data were not included. Patients with known liver disease at admission, or positive for viral hepatitis or relevant alcohol intake were excluded from the analysis.
2.2. Biochemical tests
Upon admission, every patient underwent a blood draw and the following measurements were done: complete blood count, glucose, creatinine, serum electrolytes, acute phase reactants (ferritin, C-reactive protein, lactate dehydrogenase (LDH)), liver function tests, creatine phosphokinase (CPK), arterial blood gases, D-dimer, high-sensitivity troponin I (hsTpI), and fibrinogen. HIV test and viral hepatitis panel (HBV, HCV) were also performed. All tests were performed according to the standards of the Institutional central laboratory which is accredited by the College of American Pathologists (CAP).
2.3. Computed tomography
Upon admission, a non-contrast thorax CT scan was performed in all patients to evaluate the extent of lung damage. For the purposes of the study, only CT scans with images from the liver at the level of the right portal vein branch and from the upper pole of the spleen to the splenic hilum were included. All CT scans were performed with the same device (CT, Revolution EVO, General Electric Healthcare, Waukesha, WI, USA); the employed protocol was low dose CT for thorax assessment and includes the following parameters: helicoidal acquisition, acquisition field from the thoracic outlet to the L1 vertebral body, 120 kV voltage, 2-6 mAs, 1.5 pitch, 0.4 s rotation time, and 5 mm helicoidal thickness.
A single highly trained radiologist blinded to the patients´ status evaluated the CT scans, aiming to quantitatively detect the presence of liver steatosis, according to the following criteria: a) attenuation coefficient ≤ 40 Hounsfield units (HU), in an area of 20cm2 between the segments VII and VIII in the liver; and b) attenuation coefficient ≥10 HU in an area of 5 cm2 in the splenic parenchyma than in the area previously described in the liver; to better illustrate the described evaluation, representative images are shown in Supplementary Figure 1. Furthermore, a qualitative assessment was also performed, evaluating the presence of liver steatosis by comparing the density of the liver versus the spleen; when the liver attenuation was lower that the splenic attenuation was considered as liver steatosis.
To assess the degree of fatty liver infiltration, the liver/spleen ratio (L/S ratio) <0.70 was used as a cutoff value to discriminate between patients with or without severe liver steatosis, as described previously [16].
2.4. Estimation of liver fibrosis
In order to estimate the presence of liver fibrosis, a bi-step approach was done in patients with diagnosis of liver steatosis by CT scan, using as a first evaluation the NAFLD fibrosis score (NFS) [17]. The participants with values > -1.455 – 0.675 (indeterminate) or >0.675 (severe fibrosis F3,F4) were further analyzed by the AST to Platelet Ratio Index (APRI) [18], and when the result in this index was >1.0, the individuals were finally classified as high-risk of severe liver fibrosis.
2.5. Statistical analysis
The sample size was estimated according to a previous study in patients with COVID-19, where mortality in the non-diabetic population admitted to hospital was 17.5%, vs 29.6% in people with diabetes [19]. At our center, the general mortality is around 20% in COVID patients. We assumed an increase in mortality of 15% in those patients with MAFLD. Finally, with α y β error of 0.05 and 0.2, the final number was 151 patients per group (302 patients in total).
The normality of the data was evaluated using Shapiro-Wilk test. Data is presented as mean ± SD, median (P25-P75), or absolute frequencies. Results at baseline and final evaluations in each group (paired data) were analyzed with the Wilcoxon signed-rank test. For comparisons between groups, Mann-Whitney U or Student´s t-test were used. Logistic regression analysis was used to assess clinical outcomes, and time dependent survival analysis including Kaplan-Meier curves and Cox regression analysis was performed to assess overall mortality. To ensure that the patients in the analysis had proportional risks, those patients with in-hospital stay longer than 28-days (n=5) were excluded, survival analysis both by Kaplan-Meier and by multivariate analysis (Cox-proportional hazards regression) was conducted for 28-day prognosis.
Statistical analysis was carried out with the package software SPSS version 20.0 (IBM, Armonk, NY, USA).
3. Results
Hospital records from March to May 2020, showed 547 patients with the diagnosis of COVID-19, from which 53 were excluded for lack of complete follow-up or negative RT-PCR. From the eligible patients 19 did not have an appropriate CT scan (showing artifacts, post-surgical findings, or not reaching the proposed level at the liver and spleen), therefore we included and collected data from 475 patients. To ensure the validity of the data we eliminated records from patients with known or recent diagnosis of liver disease different from MAFLD (e.g. autoimmune liver diseases, alcohol, hepatitis C or B infections, history of liver transplantation) and those with cancer, HIV or use of drugs that could cause fatty liver, thus analyzing 432 patients.
The baseline characteristics of the total population and according to the presence or absence of MAFLD are shown in Table 1 . In total, 432 patients were analyzed, from which 40.6% had fatty liver by CT scan assessment (Supplementary Figure 2). Most of the patients were men, with an average age of 51 ± 13 years. When classified by body mass index (BMI), 44.6% of the population had obesity of some degree. The prevalence of other features of the metabolic syndrome was high, with 24% of T2DM and 27.8% of hypertension. The presence of other comorbidities (CKD, COPD, immunosuppression, etc.) was <5% in the total population with the same distribution among the groups. Severity scores, as well as markers of inflammation, were increased in total population, where 80.8% of the patients had a moderate or severe pulmonary involvement on CT scan.
Table 1.
Baseline characteristics of the total population and according to MAFLD presence.
| All(n=432) | No MAFLD (n=256) | MAFLD (n=176) | p value | |
|---|---|---|---|---|
| Demographic features | ||||
| Sex (% Male / Female) | 64.6 / 35.4 | 61.2 / 38.8 | 69.5 / 30.5 | 0.083 |
| Age | 51 ± 13 | 52 ± 14 | 48 ± 12 | 0.000 |
| BMI | 29.4 (26.7 – 33) | 28.3 (25.3 – 31.4) | 30.5 (28.2 – 34.3) | 0.000 |
| Comorbidities (n / %) | ||||
| Malnutrition | 12 (2.9) | 8 (3.3) | 6 (3.5) | 0.000 |
| Normal Weight | 55 (13.3) | 49 (20.2) | 4 (2.4) | |
| Overweight | 162 (39.2) | 98 (40.3) | 64 (37.6) | |
| Obesity G1 | 120 (29.1) | 58 (23.9) | 62 (36.5) | |
| Obesity G2 | 43 (10.4) | 22 (9.1) | 21 (12.4) | |
| Obesity G3 | 21 (5.1) | 8 (3.3) | 13 (7.6) | |
| T2DM | 104 (24) | 50 (19.5) | 54 (30.5) | 0 .008 |
| Hypertension | 121 (27.8) | 66 (25.7) | 55 (31.1) | 0.232 |
| Chronic Kidney disease | 8 (1.8) | 5 (1.9) | 3 (1.7) | 1.000 |
| Pulmonary obstructive disease | 3 (0.7) | 0 (0) | 3 (1.7) | 0.67 |
| Autoimmune disease | 7 (1.6) | 3 (1.2) | 4 (2.3) | |
| Immunosuppression | 2 (0.5) | 2 (0.8) | 0 (0) | |
| Use of steroids | 7 | 3 (1.7) | 4 (2.3) | 0.307 |
| Metabolic syndrome | 152 (35.1) | 69 (26.9) | 83 (47.2) | 0.000 |
| Prognostic scores | ||||
| qSOFA | 1 (0 – 1) | 1 (0 – 1) | 1 (0 – 1) | 0.672 |
| SOFA | 2 (1 – 2) | 2 (1 – 2) | 2 (1 – 3) | 0.016 |
| NEWS | 7 (5 – 8) | 7 (5 – 8) | 7 (5 – 8) | 0.252 |
| PSI/PORT | 62 (50 – 81) | 65 (51 – 81) | 59 (48 – 74) | 0.34 |
| SMART COP | 3 (2 – 4) | 3 (2 – 4) | 3 (2 – 4) | 0.317 |
| Bello-Chavolla et al. score | 7 (6 – 7) | 7 (6 – 7) | 7 (6 – 8) | 0.419 |
| Biochemical values | ||||
| CRP (Ref. value: 0 - 1mg/dL) |
13.2 (6.4 – 20.1) | 12.8 (6.3 – 19.6) | 13.7 (6.5 – 21.5) | 0.166 |
| Ferritin (Ref. value: 11 – 306.8ng/mL) |
578 (286 – 997) | 515 (261 – 938) | 672 (334 – 1048) | 0.054 |
| D-dimer (Ref. value: 0- 500ng/mL) |
647 (420 – 1102) | 665 (417 – 1138) | 605 (420 – 997) | 0.645 |
| LDH (Ref. value: 120 - 246U/L) |
348 (267 – 458) | 342 (257 – 455) | 363 (291 – 472) | 0.068 |
| Troponins (Ref. value: <15pg/mL) |
4.9 (3.2 – 9.4) | 4.8 (3.2 – 10.6) | 4.9 (3.2 – 7.1) | 0.469 |
| CPK (Ref. value: 30 -233U/L) |
108 (59 – 237) | 97 (54 – 197) | 141 (73 – 320) | 0.000 |
| Bilirubin (Ref. value: 0/3- 1mg/dL) |
0.5 (0.4 – 0.7) | 0.5 (0.4 – 0.8) | 0.6 (0.5 – 0.8) | 0.191 |
| ALT (Ref. value: 7-52U/L) |
37 (25 – 54) | 33 (22 – 52) | 42 (29 – 60) | 0.000 |
| AST (Ref. value: 13 - 39U/L) |
42 (30 – 60) | 40 (27 – 57) | 45 (32 – 66) | 0.009 |
| Globulins (Ref. value: 1.9 – 3.7g/dL) |
3.3 ± 0.4 | 3.3 ± 0.4 | 3.2 ± 0.4 | 0.945 |
| Albumin (Ref. value: 3.5-5.7g/dL) |
3.4 ± 0.5 | 3.7 ± 0.5 | 3.8 ± 0.4 | 0.004 |
| ALP (Ref. value: 34-104U/L) |
85 (70 – 109) | 86 (70 – 113) | 85 (67 – 105) | 0.216 |
| Creatinine (Ref. value: 0.6 0 1.2mg/dL) |
0.9 (0.7 – 1.1) | 0.9 (0.8 – 1.0) | 0.9 (0.7 – 1.1) | 0.773 |
| Glucose (Ref. value: 70-99 mg/dL) |
116 (101-143) | 112 (99 - 132) | 123 (105-175) | 0.001 |
| Leukocytes (Ref. value: 4-12 × 10^3/uL) |
7.3 (5.5 – 9.6) | 7.1 (5.4 – 9.6) | 7.6 (5.7 – 10) | 0.375 |
| Lymphocytes (Ref. value: 1 – 3.9 × 10^3/uL) |
811 (615 – 1058) | 781 (577 – 1020) | 875 (653 – 1139) | 0.020 |
| Platelets (Ref. value: 150 - 450K/uL) |
215 (174 - 277) | 221 (176 – 284) | 207 (172 – 268) | 0.081 |
| 25 (HO) vitamin D (Ref. value: 30 - 100ng/mL) |
21 (16 – 27) | 21 (16 – 26) | 21 (15 – 28) | 0.788 |
| Triglycerides (Ref. value: <150mg7dL) |
147 (114 – 189) | 142 (114 – 190) | 150 (115 – 189) | 0.930 |
| CT results | ||||
| Mild (Ref <20%) | 83 (19.2) | 51 (19.9) | 32 (18.1) | |
| Moderate (20 – 50%) | 161 (37.2) | 97 (37.9) | 64 (36.2) | |
| Severe (>50%) | 189 (43.6) | 108 (42.2) | 81 (45.8) | |
| Treatment n(%) | ||||
| Antibiotics | 422 (97.7) | 252 (98.8) | 170 (96) | |
| Antimalarials | 146 (33.6) | 81 (31.4) | 65 (36.7) | |
| Tocilizumab | 56 (12.9) | 31 (12) | 25 (14.1) | |
| Remdesivir | 2 (0.5) | 2 (0.8) | 0 (0) | |
| Other | ||||
| PaO2/FiO2 ratio | 233 (155-286) | 240 (171-289) | 220 (133-276) | 0.032 |
| Neutrophil/Lymphocyte ratio | 7.1 (4.4-11.8) | 7.3 (4.6-12.3) | 6.6 (4.0-10.6) | 0.156 |
| Days between the beginning of symptoms and hospitalization | 7 (5 – 10) | 8 (5 – 10) | 7 (5 – 10) | 0.179 |
BMI, body mass index; T2DM, type 2 diabetes mellitus; CRP, c-reactive protein; LDH, lactate dehydrogenase; CPK, creatine phosphokinase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase.
When the population was analyzed according to the presence of fatty liver on CT scan, patients with MAFLD were found to be younger, with higher BMI, and a higher proportion of grade 2 or 3 obesity, metabolic syndrome and T2DM. Regarding severity scores, only the SOFA score was statistically different in patients with MAFLD, although this result probably has no clinical relevance. In general, inflammatory markers showed a trend towards higher levels in patients with MAFLD, however, only CPK showed statistical significance. The PaO2/FiO2 ratio was lower in patients with MAFLD. Finally, there were no differences in the distribution of pulmonary involvement on CT scan, treatment, or the number of days between the onset of symptoms and hospital admission.
In order to fully assess the severity of the disease in patients with MAFLD by CT scan, a newly proposed cutoff of <0.7 in the liver/spleen ratio was set to classify patients as severe fatty liver infiltration [16]. This resulted in 108 (61.4%) patients being classified as severe fatty liver infiltration and 68 (38.6%) as mild-moderate fatty liver.
On the other hand, the presence of liver fibrosis was addressed by a successive evaluation using the NFS score first, and when altered, APRI score was calculated. The probability of liver fibrosis according to NFS score in patients with liver steatosis was low in 8.5% (n=15), intermediate in 32.4% (n=57), and high in 54% (n=95), 9 patients had no BMI data, therefore the score was not calculated. When the APRI score was calculated at the same time, 22.7% of patients (n=40) were classified as high probability of liver fibrosis. And when the successive approach above mention was used, where only patients with intermediate and high probability of fibrosis by NFS had APRI calculated, 21% of patients (n=37) met the criteria for high risk of liver fibrosis.
In MAFLD patients, the presence of liver fibrosis was associated with higher values in severity scores such as NEWS score, and the recently developed population-specific Bello-Chavolla et al. score [20] as well as higher levels of inflammatory markers including LDH, CPK, fibrinogen, and ferritin, and higher levels of transaminases and leukocytes; interestingly, vitamin D levels were lower in the group of patients with fibrosis. Also, patients with fibrosis required mechanical ventilation more frequently, had a higher incidence of acute kidney injury (AKI) and had higher mortality. (Table 2 )
Table 2.
Characteristics and outcomes in patients with and without liver fibrosis in the MAFLD group.
| No fibrosis (n=139) | Severe fibrosis (n=37) | p value | |
|---|---|---|---|
| Demographic features | |||
| Age (years) | 47.4 ± 12 | 50.7± 12 | 0.153 |
| BMI (kg/m2) | 31.3 ± 4.6 | 33.3 ± 8.7 | 0.196 |
| Fibrosis scores | |||
| NFS | 0.49 ± 1.56 | 2.34 ± 1.58 | 0.000 |
| APRI | 0.57 ± 0.59 | 1.47± 0.84 | 0.000 |
| Prognostic scores | |||
| qSOFA | 1.0 (0-1) | 1 (1-1) | 0.346 |
| SOFA | 2 (1-2) | 2 (1-3) | 0.202 |
| NEWS | 7 (5-8) | 8 (6-9) | 0.033 |
| PSI/PORT | 59 (48-74) | 65 (50-76) | 0.572 |
| SMART COP | 3 (3-4) | 3 (2-4) | 0.590 |
| Bello-Chavolla et al. score | 6 (5-7) | 7 (6-8) | 0.026 |
| Biochemical values | |||
| CRP (ref: 0-1mg/dl) | 15.0±10.3 | 14.5± 8.1 | 0.743 |
| Ferritin (ref: 11- 306.8ng/ml) | 755 ± 641 | 936 ± 721 | 0.157 |
| D-dimer (ref: 0-500ng/ml) | 1606 ± 7055 | 1173 ± 2183 | 0.732 |
| LDH (ref: 120 - 246u/l) | 380 ± 148 | 470 ± 200 | 0.004 |
| Troponins (ref:<15pg/ml) | 8.5 ± 18.4 | 14.0±26.9 | 0.199 |
| CPK (ref: 30-223u/l) | 224 ± 271 | 526 ± 738 | 0.032 |
| Bilirubin (ref: mg/dl) | 0.66 ± 0.22 | 0.71± 0.36 | 0.169 |
| ALT (ref: 7-52u/l) | 45.8 ± 40.3 | 67.7 ± 38.2 | 0.005 |
| AST (ref:13-39u/l) | 48.2± 38.3 | 83.0 ± 30.3 | 0.000 |
| Globulins (ref: 1.9-3.7g/dl) | 3.2 ± 0.4 | 3.3 ± 0.4 | 0.669 |
| Albumin (ref:3.5 -5.7g/dl) | 3.7 ± 0.4 | 3.8 ± 0.4 | 0.888 |
| ALP (ref: 34-104u/l) | 90±35 | 94±39 | 0.522 |
| Creatinine (ref: 0.6-1.2mg/dl) | 0.98 ± 0.47 | 0.99 ± 0.33 | 0.917 |
| Glucose (ref:70-99 mg/dl) | 123 (105-165) | 125 (104-188.5) | 0.802 |
| Leukocytes (ref: 4- 12 × 10^3/ul) | 8.4 ± 3.5 | 7.1 ± 2.5 | 0.021 |
| Lymphocytes (ref: 3.9 × 10^3/ul) | 930±435 | 926±377 | 0.956 |
| Platelets (ref: 150-450k/ul) | 238±76 | 160±53 | 0.000 |
| 25 oh vitamin D (ref: 30-100ng/ml) | 22.3 ± 8.5 | 19.2 ± 6.8 | 0.078 |
| Triglycerides (ref:<150mg7dl) | 162±124 | 172±70 | 0.698 |
| PaO2/FiO2 ratio | 224 (137-276) | 191 (112-277) | 0.435 |
| Neutrophil/Lymphocyte ratio | 7.0 (4.1-12.1) | 6.1 (3.9-9.0) | 0.191 |
| Other (n / %) | |||
| Metabolic syndrome | 63 (45.3) | 20 (54.1) | 0.529 |
| Severe COVID-19 | 100 (72.5) | 26 (78.8) | 0.459 |
| Admission to ICU | 32 (22.9) | 13 (39.4) | 0.051 |
| Discharge from ICU | 15 (46.9) | 5 (38.5) | 0.607 |
| Acute kidney injury | 28 (20.1) | 11 (33.3) | 0.104 |
| Thrombotic event | 1 (0.7) | 1 (3.0) | 0.346 |
| Death | 21(15.0) | 10(32.3) | 0.024 |
| Days between the beginning of symptoms and hospitalization | 7 (5-9) | 8 (6-10) | 0.287 |
| Length of hospital stay (days) | 8 (4-12) | 9 (6-16) | 0.297 |
| Days in ICU | 12 (7-23) | 10 (4-12) | 0.061 |
| Days between the beginning of hospitalization and death | 8 (5-20) | 8 (6-14) | 0.919 |
| Days between ICU requirement and death | 5 (3-8) | 7 (6-12) | 0.264 |
BMI, body mass index; NFS, NAFLD fibrosis score; APRI, AST to platelet ratio index; CRP, c-reactive protein; LDH, lactate dehydrogenase; CPK, creatine phosphokinase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; COVID-19, coronavirus disease 2019; ICU, Intensive care unit.
With the aim of delimiting the specific role of liver fibrosis in the different outcomes of patients with COVID-19 and MAFLD, we constructed different logistic regression models, including demographic and biochemical variables as well as prognostic scores and markers and a combined model. Fibrosis by NFS/APRI was associated with the need for orotracheal intubation independently of demographic characteristics [OR: 2.59 (1.18-5.66)], biochemical markers [OR: 2.86 (1.18-6.97)], severity scores [OR: 2.60 (1.11-6.08)] and in the combined model [OR: 3.24 (1.35-7.76)]. With regards to the development of AKI, several variables were associated with the development of AKI, including age, gender, LDH, systolic blood pressure and PaO2/FiO2 ratio, while fibrosis showed statistical significance in all the constructed models. (Table 3 )
Table 3.
Logistic regression analysis to evaluate the association of fibrosis with clinical outcomes.
| Endotracheal intubation – demographic variables | ||||
|---|---|---|---|---|
| OR | CI 95% | β | p value | |
| Fibrosis APRI/NFS | 2.595 | 1.187 - 5.662 | 0.954 | 0.017 |
| Gender (Female) | 0.478 | 0.202 – 1.131 | -0.738 | 0.093 |
| Age | 0.980 | 0.969 – 0.991 | -0.020 | 0.001 |
| BMI > 30 kg/m2 | 0.788 | 0.409 – 1.519 | -0.238 | 0.477 |
| -2 log likelihood →block 0: 245.37, block 1:192.90; Cox & Snell R2: 0.257; Nagelkerke R2: 0.342; Hosmer and Lemeshow: 0.420 | ||||
| Endotracheal intubation – Biochemical variables | ||||
| OR | CI 95% | β | p value | |
| Fibrosis APRI/NFS | 2.869 | 1.181 – 6.970 | 1.054 | 0.020 |
| LDH | 1.000 | 0.997 – 1.002 | 0.001 | 0.889 |
| CRP | 1.038 | 0.993 – 1.086 | 0.038 | 0.099 |
| CPK | 1.000 | 0.999 – 1.001 | 0.000 | 0.514 |
| Total lymphocytes | 0.998 | 0.997 – 0.999 | -0.002 | 0.000 |
| -2 log likelihood →block 0: 227.35, block 1:195.675; Cox & Snell R2: 0.302; Nagelkerke R2: 0.402; Hosmer and Lemeshow: 0.202 | ||||
| Endotracheal intubation –Severity scores and markers | ||||
| OR | CI 95% | β | p value | |
| Fibrosis APRI/NFS | 2.601 | 1.112 – 6.084 | 0.956 | 0.027 |
| PSI/PORT score | 0.992 | 0.980 – 1.004 | -0.008 | 0.185 |
| qSOFA score | 3.288 | 1.380 – 7.836 | 1.190 | 0.007 |
| PaO2/FiO2 ratio | 0.992 | 0.989 - 0.995 | -0.008 | 0.000 |
| NLR | 1.003 | 0.973 – 1.035 | 0.003 | 0.827 |
| -2 log likelihood →block 0: 238.44, block 1:169.73; Cox & Snell R2: 0.329; Nagelkerke R2: 0.439; Hosmer and Lemeshow: 0.359 | ||||
| Endotracheal intubation – Combined model | ||||
| OR | CI 95% | β | p value | |
| Fibrosis APRI/NFS | 3.243 | 1.355 – 7.760 | 1.176 | 0.008 |
| Age | 0.974 | 0.953 - 0.995 | -0.026 | 0.017 |
| Total lymphocytes | 1.000 | 0.999 – 1.001 | 0.000 | 0.690 |
| qSOFA | 5.067 | 2.002 – 12.825 | 1.623 | 0.001 |
| PaO2/FiO2 ratio | 0.994 | 0.990 – 0.997 | -0.006 | 0.001 |
| -2 log likelihood →block 0: 239.82, block 1:163.66; Cox & Snell R2: 0.356; Nagelkerke R2: 0.475; Hosmer and Lemeshow: 0.154 | ||||
| Acute kidney injury - Demographic variables | ||||
| OR | CI 95% | β | p value | |
| Fibrosis APRI/NFS | 2.522 | 1.123 – 5.665 | 0.925 | 0.025 |
| Gender (Female) | 0.281 | 0.102 – 0.773 | -1.268 | 0.014 |
| Age | 0.982 | 0.971 – 0.994 | -0.018 | 0.002 |
| BMI > 30 kg/m2 | 0.622 | 0.314 – 1.231 | -0.476 | 0.173 |
| -2 log likelihood →block 0: 243.98, block 1:179.58; Cox & Snell R2: 0.307; Nagelkerke R2: 0.409; Hosmer and Lemeshow: 0.868 | ||||
| Acute kidney injury –Biochemical variables | ||||
| OR | CI 95% | β | p value | |
| Fibrosis APRI/NFS | 2.634 | 1.031 - 6.732 | 0.968 | 0.043 |
| LDH | 1.004 | 1.001 – 1.007 | 0.004 | 0.019 |
| CRP | 1.039 | 0.990 – 1.091 | 0.093 | 0.118 |
| CPK | 1.000 | 0.999 – 1.001 | 0.000 | 0.994 |
| Glucose | 1.002 | 0.997 – 1.007 | 0.002 | 0.413 |
| SBP | 0.967 | 0.955 – 0.980 | -0.033 | 0.000 |
| -2 log likelihood →block 0: 221.80, block 1: 138.47; Cox & Snell R2: 0.406; Nagelkerke R2: 0.541; Hosmer and Lemeshow: 0.176 | ||||
| Acute kidney injury - Severity scores and markers | ||||
| OR | CI 95% | β | p value | |
| Fibrosis APRI/NFS | 2.640 | 1.077 – 6.470 | 0.971 | 0.034 |
| SOFA | 1.169 | 0.949 – 1.440 | 0.156 | 0.143 |
| Bello-Chavolla score | 0.973 | 0.865 – 1.095 | -0.027 | 0.655 |
| PaO2/FiO2 ratio | 0.991 | 0.987 – 0.994 | -0.009 | 0.000 |
| NLR | 1.021 | 0.991 – 1.051 | 0.021 | 0.168 |
| -2 log likelihood →block 0: 232.89, block 1: 152.32; Cox & Snell R2: 0.381; Nagelkerke R2: 0.508; Hosmer and Lemeshow: 0.285 | ||||
| Acute kidney injury – Combined model | ||||
| OR | CI 95% | β | p value | |
| Fibrosis APRI/NFS | 2.511 | 1.000 – 6.304 | 0.921 | 0.050 |
| Gender | 0.397 | 0.130 – 1.206 | -0.925 | 0.103 |
| Age | 1.014 | 0.981 – 1.049 | 0.014 | 0.405 |
| PaO2/FiO2 ratio | 0.995 | 0.991 – 0.999 | 0.005 | 0.028 |
| LDH | 1.004 | 1.001 – 1.007 | 0.004 | 0.007 |
| SBP | 0.979 | 0.961 – 0.997 | 0.021 | 0.024 |
| -2 log likelihood →block 0: 230.12, block 1: 140.95; Cox & Snell R2: 0.416; Nagelkerke R2: 0.554; Hosmer and Lemeshow: 0.247 | ||||
APRI, AST to platelet ratio index; NAFLD fibrosis score; BMI, body mass index; LDH, lactate dehydrogenase; CRP, c-reactive protein; CPK, creatine phosphokinase; NLR, Neutrophil/Lymphocyte ratio, SBP, systolic blood pressure.
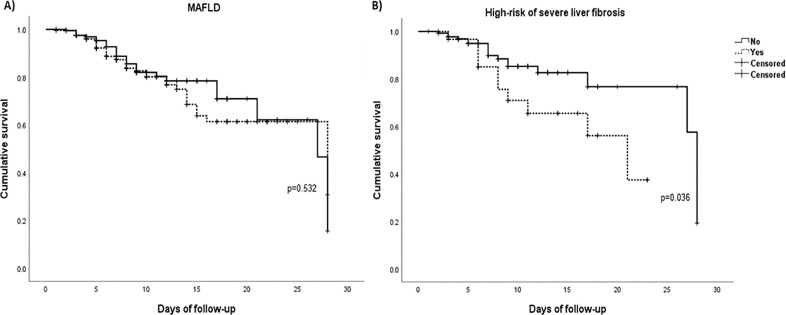
Finally, to further assess the implications of liver disease in the prognosis of COVID-19 patients accounting for time-dependence, we conducted survival analysis in which first Kaplan-Meier curves were created to evaluate the effect of both MAFLD and liver fibrosis on the survival of patients with MAFLD and COVID-19, where fibrosis rather than MAFLD was significantly associated with 28-day mortality (p=0.036) (Fig. 1 ). Then a Cox regression analysis was performed, specifically to evaluate if the role of fibrosis in mortality was truly independent of other variables; Table 4 , shows both univariate and multivariate analysis where four different models were created to avoid collinearity and saturation of the models. These results show fibrosis by NFS/APRI remained independently associated with mortality independently of demographic characteristics [HR: 2.33 (1.07-5.25)] and severity scores and markers [HR: 2.90 (1.14-7.37)], as well as in the combined model [HR: 2.54 (1.14-5.63)], however it lost statistical significance in the model with AKI and endotracheal intubation, where only the last two remained statistically significant associated with mortality.
Fig. 1.
Kaplan–Meier curves for survival in patients with MAFLD (A) and in patients with high-risk of severe fibrosis by NFS/APRI (B). Mean survival time: 21.07 ± 0.9 days (non-MAFLD) and 21.95 ± 1.11 days (MAFLD; and 23.5 ± 1.1 days (no fibrosis) and 16.7 ± 1.5 days (fibrosis). MAFLD, metabolic associated fatty liver disease.
Table 4.
Cox regression analysis for survival in patients with and without fibrosis.
| SURVIVAL – demographic variables | ||||
|---|---|---|---|---|
| HR | CI 95% | β | p value | |
| Fibrosis APRI/NFS | 2.332 | 1.077 – 5.049 | 0.847 | 0.032 |
| Gender (Female) | 0.424 | 0.154 – 1.171 | -0.858 | 0.098 |
| Age | 1.035 | 1.002 – 1.070 | 0.035 | 0.040 |
| BMI | 1.087 | 1.029 – 1.147 | 0.083 | 0.003 |
| -2 log likelihood →block 0: 245.43, block 1: 228.41, Chi-square:18.999, df:4, sig.: 0.001 | ||||
| Survival – severity scores and markers | ||||
|---|---|---|---|---|
| HR | CI 95% | β | p value | |
| Fibrosis APRI/NFS | 2.901 | 1.141 – 7.370 | 1.065 | 0.025 |
| NLR | 1.014 | 0.977 – 1.053 | 0.014 | 0.457 |
| LDH | 1.002 | 1.000 – 1.004 | 0.002 | 0.053 |
| PSI-PORT | 1.024 | 1.007 – 1.041 | 0.023 | 0.005 |
| CRP | 1.062 | 1.015– 1.111 | 0.060 | 0.010 |
| qSOFA | 0.693 | 0.254 – 1.890 | -0.367 | 0.474 |
| PaO2/FiO2 ratio | 1.001 | 0.998 – 1.004 | 0.001 | 0.468 |
| -2 log likelihood →block 218.91:, block 1:187.18, Chi-square:35.72, df:7, sig.:0.000 | ||||
| Survival – combined model | ||||
|---|---|---|---|---|
| HR | CI 95% | β | p value | |
| Fibrosis APRI/NFS | 2.543 | 1.147 - 5.637 | 0.933 | 0.022 |
| PSI-PORT | 1.017 | 1.000 - 1.034 | 0.017 | 0.050 |
| CRP | 1.070 | 1.025 - 1.118 | 0.068 | 0.002 |
| Age | 1.018 | 0.982 – 1.055 | 0.018 | 0.336 |
| BMI | 1.086 | 1.025-1.150 | 0.082 | 0.005 |
| -2 log likelihood →block 0:235.13, block 1: 203.17, Chi-square:35.06, df:5, sig.: 0.000 | ||||
| Survival – other outcomes | ||||
|---|---|---|---|---|
| HR | CI 95% | β | p value | |
| Fibrosis APRI/NFS | 1.655 | 0.749 – 3.661 | 0.504 | 0.213 |
| AKI | 3.022 | 1.181 – 7.735 | 1.106 | 0.021 |
| Endotracheal intubation | 3.441 | 1.226 – 9.661 | 1.236 | 0.019 |
| -2 log likelihood →block 247:, block 1: 181:, Chi-square:36.58, df:3, sig.:0.000 | ||||
APRI, AST to platelet ratio index; NAFLD fibrosis score; BMI, body mass index; T2DM, type 2 diabetes mellitus; LDH, lactate dehydrogenase; AKI, acute kidney injury; CRP, C-reactive protein; NLR, neutrophil/lymphocyte ratio.
4. Discussion
In the present study from a tertiary care center, reconverted for the care of COVID-19 patients, we present the outcomes of patients with COVID-19 and MAFLD diagnosed by CT scan. The overall prevalence of MAFLD was 40.6%, which is similar to the prevalence in the Hispanic population, thus perhaps the presence of MALFD per se does not imply an increased risk of hospitalization in patients with COVID-19 [21]. Likewise, we did not find significant differences in the outcomes of hospitalized patients with MAFLD and those without MAFLD. However, we did find a significant increase in the risk of mechanical ventilation requirement, acute kidney injury, and mortality in patients with MAFLD and advanced liver fibrosis diagnosed using the combined approach of high NFS and APRI. The role of MAFLD in the outcomes of patients with COVID-19 is still controversial, with some studies reporting unfavorable outcomes in all patients with MAFLD, while others, like the present study, report worse outcomes only when liver fibrosis is present [22], [23], [24].
In a recently published retrospective study where the outcome of patients with chronic liver disease and COVID-19 was evaluated, MAFLD was found in 15% of the population, and was independently associated with an increase in ICU admission (OR 2.3) and mechanical ventilation (OR 2.1), and the presence of cirrhosis was an independent predictor of mortality (OR 12.5) [22]. Another study conducted at four sites in Zhejiang Province, China, between January and February 2020, evaluated 310 patients hospitalized with COVID-19 finding 30.3% patients with MAFLD. The presence of MAFLD was diagnosed by CT scan and the presence of fibrosis was evaluated using the originally validated cut-points for fibrosis-4 (FIB-4) index and the NFS score. This study found that patients with MAFLD and fibrosis had an increased risk of having severe COVID-19 illness, irrespective of other metabolic comorbidities [23]. In another study reported by Dong Ji et al. patients with MAFLD had a higher risk of disease progression, higher likelihood of abnormal liver tests, and longer viral shedding time compared to patients without MAFLD [24].
Our results are coherent with the fact that, in general, the prognosis of MALFD is determined by the severity of liver fibrosis rather than by the presence of steatosis or steatohepatitis, which it is seen as well in the context of COVID-19 [12,25]. This could be explained by a more pronounced baseline systemic inflammation profile in patients with liver fibrosis influencing different organs and systems and the interaction between them, leading to further inflammation and activation of the immune response, contributing to higher inflammation when SARS-CoV-2 is added [26].
In our cohort, patients with MALFD were younger and had a higher BMI with a higher proportion of grade 2 or 3 obesity, and a higher proportion of T2DM. In a previous report by K.I. Zheng et al., the risk of severe disease in MAFLD patients with co-existing obesity was six times greater after adjustment for confounders, suggesting a synergistic effect between MAFLD and obesity when assessing the risk of severe COVID-19 [27].
The information in the present study was collected before the release of the results of the RECOVERY trial (ref), therefore no changes in survival are attributed to steroid use.
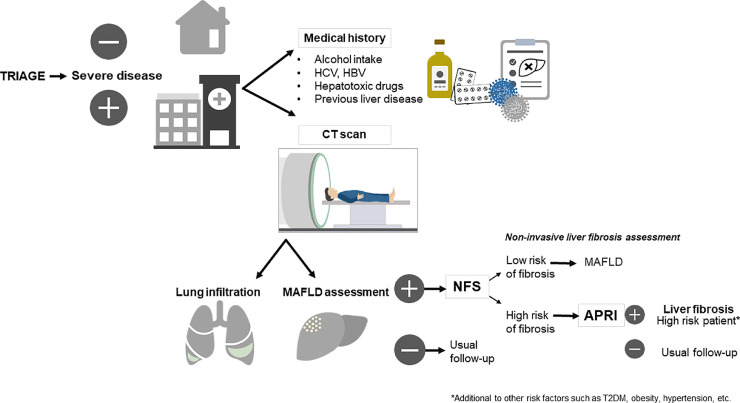
This study has several limitations, the first is the retrospective nature of the study, and the fact that liver steatosis was diagnosed by CT scan, and fibrosis by non-invasive scores which are not the standard methods for diagnosing these entities. However, given the high risk of SARS-CoV-2 transmission to healthcare workers, this approach is safer than exposing them to perform an additional study, such as transient elastography. Another noteworthy aspect is that COVID-19 patients often have elevated transaminases, which could affect the diagnostic precision of the fibrosis predictive scores, since most of them use these values to predict liver fibrosis, increasing the risk of over-diagnosis of advanced fibrosis in our cohort. It is not clear whether COVID-19 is solely responsible for the development of liver injury, or whether liver injury is a consequence of the systemic inflammation caused by the virus or by drug-induced liver injury [28]. The main advantage of this study is that it was conducted in a country with one of the highest prevalence of MAFLD, and with a different genetic background also accounting for higher prevalence of other metabolic diseases including T2D and obesity; therefore are able to evaluate a good proportion of these patients and the statistical approach that was carried out allows for solid results. Finally, based in the findings of the present study, we propose a sequential approach to identify patients with MAFLD and high risk of advanced fibrosis, emphasizing the fact that an adequate diagnosis can be done with the studies performed upon admittance (i.e. chest CT scan, history and biochemical tests). (Fig. 2 )
Fig. 2.
Proposed assessment of MAFLD and liver fibrosis in hospitalized patients with COVID-19. This diagnostic approach highlights the importance of liver fibrosis in patients with MAFLD and COVID-19 as an additional risk factor for adverse clinical outcomes. For individuals admitted for inpatient medical care, three points are critical in the proposed assessment: 1) To investigate risk factors related to liver disease different from MAFLD; 2) The assessment of liver steatosis with the already available lung CT scan, to avoid unnecessary exposure to radiation and to expedite the assessment; and 3) To sequentially assess the risk of severe liver fibrosis with the NAFLD fibrosis score (NFS) and then with the AST to platelet ratio index (APRI). This approach for detecting patients with MAFLD and liver fibrosis among those with COVID-19 requiring inpatient care, provides a reliable algorithm using already available resources (CT scan and biochemical tests), and therefore accelerating the diagnostic time, limiting the costs and the exposure to radiation, as well as limiting the contact with healthcare staff. MAFLD, metabolic associated fatty liver disease; NFS, NAFLD fibrosis score; APRI, AST to platelet ratio index; HCV, hepatitis C virus; HBV, hepatitis B virus.
In conclusion, the presence of fibrosis rather than the presence of MAFLD has an impact on the risk of mechanical ventilation requirement, development of acute kidney injury, and higher mortality in patients with COVID-19.
Declaration of Competing Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgments
Acknowledgments
The authors would like to thank all the staff of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán for their collaboration in the medical care of these patients in order to be able to carry out this work.
Financial disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
FJC and YAN are supported by: German Research Foundation (SFB/TRR57/P04, SFB 1382-403224013/A02 and DFG NE 2128/2-1) (to YAN), the MINECO Retos by MINECO Retos (SAF2017-87919R to YAN) and (SAF2016-78711 to FJC), and the EXOHEP-CM S2017/BMD-3727, NanoLiver-CMY2018/NMT-4949, ERAB Ref. EA 18/14, AMMF 2018/117, UCM-25-2019 and COST Action CA17112 (to YAN and FJC). YAN and FJC are Ramón y Cajal Researchers RYC2015-17438 and RyC2014-15242, respectively. FJC is a Gilead Liver Research Scholar.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.dld.2021.01.019.
Appendix. Supplementary materials
References
- 1.Johns Hopkins Coronavirus Resource Center . 2020. COVID-19 Map. Johns Hopkins Coronavirus Resour Cent. [Google Scholar]
- 2.Tian W., Jiang W., Yao J., Nicholson C.J., Li R.H., Sigurslid H.H. Predictors of mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.26050. jmv.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA - J Am Med Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao Y., Chen Y., Liu M., Shi S., Tian J. Impacts of immunosuppression and immunodeficiency on COVID-19: a systematic review and meta-analysis. J Infect. 2020;81:e93. doi: 10.1016/j.jinf.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Certain Medical Conditions and Risk for Severe COVID-19 Illness | CDC n.d.
- 6.Horby P., Lim W.S., Emberson J., Mafham M., Bell J., Linsell L. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. MedRxiv. 2020 doi: 10.1101/2020.06.22.20137273. 2020.06.22.20137273. [DOI] [Google Scholar]
- 7.Eslam M., Sanyal A.J., George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999–2014. doi: 10.1053/j.gastro.2019.11.312. e1. [DOI] [PubMed] [Google Scholar]
- 8.The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245–266. doi: 10.1016/S2468-1253(19)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Younossi Z., Anstee Q.M., Marietti M., Hardy T., Henry L., Eslam M. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 10.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 11.Furman D., Campisi J., Verdin E., Carrera-Bastos P., Targ S., Franceschi C. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25:1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le M.H., Devaki P., Ha N.B., Jun D.W., Te H.S., Cheung R.C. Prevalence of non-alcoholic fatty liver disease and risk factors for advanced fibrosis and mortality in the United States. PLoS One. 2017;12 doi: 10.1371/journal.pone.0173499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mantovani A., Scorletti E., Mosca A., Alisi A., Byrne C.D., Targher G. Complications, morbidity and mortality of nonalcoholic fatty liver disease. Metabolism. 2020 doi: 10.1016/j.metabol.2020.154170. [DOI] [PubMed] [Google Scholar]
- 14.Bajpai V., Gupta E., Mitra L.G., Kumar H., Maiwall R., Soni K.D. Spectrum of respiratory viral infectionsin liver disease patients with cirrhosis admitted in critical care unit. J Lab Physicians. 2019;11:356–360. doi: 10.4103/jlp.jlp_6_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K.W. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwasaki M., Takada Y., Hayashi M., Minamiguchi S., Haga H., Maetani Y. Noninvasive evaluation of graft steatosis in living donor liver transplantation. Transplantation. 2004;78:1501–1505. doi: 10.1097/01.TP.0000140499.23683.0D. [DOI] [PubMed] [Google Scholar]
- 17.Angulo P., Hui J.M., Marchesini G., Bugianesi E., George J., Farrell G.C. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 18.Wai C.T., Greenson J.K., Fontana R.J., Kalbfleisch J.D., Marrero J.A., Conjeevaram H.S. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 19.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bello-Chavolla O.Y., Bahena-López J.P., Antonio-Villa N.E., Vargas-Vázquez A., González-Díaz A., Márquez-Salinas A. Predicting mortality due to SARS-CoV-2: a mechanistic score relating obesity and diabetes to COVID-19 outcomes in Mexico. J Clin Endocrinol Metab. 2020;105 doi: 10.1210/clinem/dgaa346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Browning J.D., Szczepaniak L.S., Dobbins R., Nuremberg P., Horton J.D., Cohen J.C. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 22.Hashemi N., Viveiros K., Redd W.D., Zhou J.C., McCarty T.R., Bazarbashi A.N. Impact of chronic liver disease on outcomes of hospitalized patients with COVID-19: a multicentre United States experience. Liver Int Off J Int Assoc Study Liver. 2020 doi: 10.1111/liv.14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Targher G., Mantovani A., Byrne C.D., Wang X.-B., Yan H.-D., Sun Q.-F. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020;69:1545–1547. doi: 10.1136/gutjnl-2020-321611. [DOI] [PubMed] [Google Scholar]
- 24.Ji D., Qin E., Xu J., Zhang D., Cheng G., Wang Y. Non-alcoholic fatty liver diseases in patients with COVID-19: a retrospective study. J Hepatol. 2020;73:451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Angulo P., Kleiner D.E., Dam-Larsen S., Adams L.A., Bjornsson E.S., Charatcharoenwitthaya P. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–397. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trebicka J., Amoros A., Pitarch C., Titos E., Alcaraz-Quiles J., Schierwagen R. Addressing profiles of systemic inflammation across the different clinical phenotypes of acutely decompensated cirrhosis. Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hussain A., Vasas P., El-Hasani S. Letter to the Editor: obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108 doi: 10.1016/j.metabol.2020.154256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alqahtani S.A., Orn J., Schattenberg M. Liver injury in COVID-19: The current evidence Key points n.d. doi: 10.1177/2050640620924157. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.