Abstract
Leclercia adecarboxylata is a motile, gram negative bacillus in the Enterobacteriaceae family that is a rarely isolated cause of disease, despite being ubiquitous in nature. A 2019 review article identified only 74 reported cases, most often in immunocompromised patients [1]. The organism is generally susceptible to most antibiotics although multiantibiotic resistant strains have been reported. We report a case of a 62-year-old Caucasian man with multiple co-morbidities treated for L. adecarboxylata endocarditis with intravenous ceftriaxone.
Keywords: Leclercia adecarboxylata, Endocarditis, Opportunistic pathogen
Introduction
Leclercia adecarboxylata is a motile, gram negative bacillus in Enterobacteriaceae family [2]. The pathogen was first described by Leclerc as Escherichia adecarboxylata in 1962. In 1986 Leclercia, named in honor of Leclerc, was recognized as a new genus in the family Enterobacteriaceae [3]. The reclassification was enabled by the emergence of more sensitive testing methods such as DNA hybridization and computer identification studies [4]. The organism is widely distributed in nature, to include water and as a commensal in the gut [4]. Rarely, Leclercia is isolated from immunocompetent patients, but is primarily found to cause infection in the immunocompromised [5]. Due to limited reports of human infection, L. adecarboxylata was previously thought to be an opportunistic pathogen. However, this may reflect misdiagnosis, as the organism shares many biochemical features with Escherichia coli. As with other atypical species, mass spectrometry and automated identification systems have allowed more frequent and accurate identification [3]. Previous searches of the literature have only revealed two other reports of L. adecarboxylata endocarditis [6]. We report a case of L. adecarboxylata isolated from a Peripheral Inserted Central Catheter (PICC) and subsequent eustachian valve endocarditis.
Case report
A 62 year old Caucasian man with a history of three-vessel coronary artery bypass in 2016, sick sinus syndrome status post Micra pacemaker, hypertension, hyperlipidemia, colo-cutaneous fistula with colostomy bag and a ventral incision with wound vacuum-assisted closure (VAC), on Total Peripheral Nutrition (TPN) for bowel rest through a left arm PICC, presented to the Emergency Department with a 3 day history of progressively worsening dyspnea, high fever up to 39 °C, chills, vomiting, diaphoresis and generalized weakness. He had a previous history of three PICC associated blood stream infections, with his most recent infection in October 2019 due to Staphylococcus epidermis. He stated that his current episode felt like his previous bloodstream infections.
On admission, he was febrile with a temperature 38.1 °C, tachycardic with heart rate of 130 beats per minute (bpm), hypotensive with blood pressure of 77/52 mmHg, and respiratory rate of 18 cycles per minute (cpm). Cardiac examination revealed a tachycardic rate of 130 s bpm with a regular rhythm and no murmur. Breath sounds were clear to auscultation and percussion bilaterally with no wheezes, rales or rhonchi. His abdomen was soft and nontender, and the wound VAC covering ventral incision present and ostomy bags covering enterocutaneous fistulae were clean and intact with no surrounding erythema or induration. There were no signs of conjunctival hemorrhage, splinter hemorrhages, Janeway lesions, or Osler nodes. After administration of 2 L of normal saline, his heart rate improved to about 100 bpm, and the patient reported feeling significantly better. However, he still required norepinephrine 0.15mcg/kg/min to maintain a mean arterial pressure greater than 65 mmHg. The patient was started on intravenous (IV) vancomycin and cefepime as empiric antibiotic therapy for septic shock.
Laboratory findings showed white cell count of 6400/mm3, hemoglobin of 10.2 mg/dL, and platelet level of 76,000/mm3. Blood cultures were drawn from both forearm venipuncture and from the patient’s PICC. Chest x-ray done at time of presentation was normal, urine analysis was normal, and an abdominal/pelvis Computed tomography (CT) scan revealed no acute changes or signs of an infectious process. On day 2, two of four blood cultures drawn in aerobic and anaerobic bottles from the PICC was positive, showing gram negative rods on gram staining. The plate cultures grew two plus (++) gram-negative non lactose fermenting bacilli, that were later identified as L. adecarboxylata using, Matrix Assisted Laser Desorption/Ionization Time of Flight (MALDI-TOF), while blood cultures drawn from venipuncture sites were negative. The organism was pan-susceptible as shown in Table 1. The patient’s PICC was removed to achieve source control, and IV vancomycin and cefepime were discontinued in favor of cefazolin 2 mg IV every 8 h based on sensitivities shown in Table 1.
Table 1.
Microbiology culture and susceptibility report.
|
Leclercia adecarboxylata |
||
|---|---|---|
| Drug | MINT | MDIL |
| Amoxicillin/Clavulanate | S | < = 2 |
| Ampicillin | S | < = 2 |
| Ampicillin/Sulbactam | S | < = 2 |
| Cefazolin | S | < = 4 |
| Cefepime | S | < = 1 |
| Ceftriaxone | S | < = 1 |
| Ciprofloxacin | S | < = 0.25 |
| Ertapenem | S | < = 0.5 |
| Gentamicin | S | < = 1 |
| Imipenem | S | < = 0.25 |
| Tobramycin | S | < = 1 |
| Trimethoprim/Sulfamethoxazole | S | < = 20 |
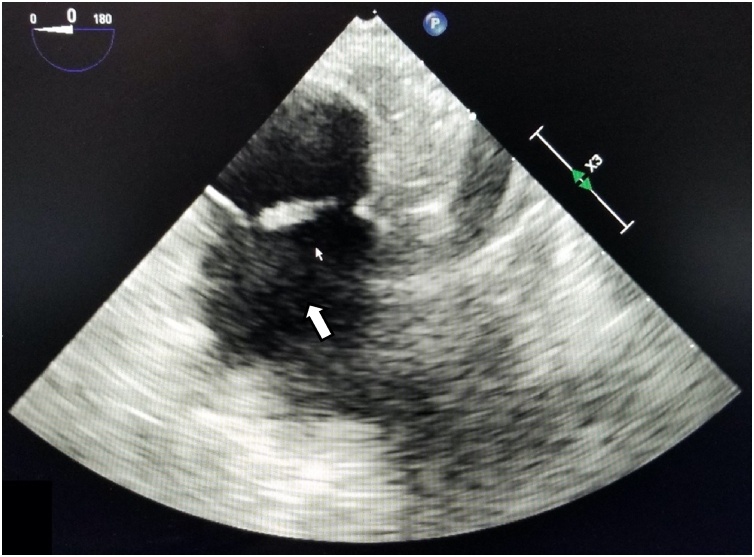
A transthoracic echocardiogram (TTE) was performed to assess for valvular changes or vegetations to indicate endocarditis; however, the imaging study was limited by the patient’s body habitus and was deemed poor quality. A subsequent transesophageal echocardiogram (TEE) revealed a “12 mm × 4 mm mobile echodensity attached to the Eustachian valve” that was visualized moving freely and independently (Fig. 1). Upon review with the cardiologist, the valvular mass was visualized and confirmed to be freely mobile independent of the eustachian valve. The diagnosis of L. adecarboxylata infective endocarditis was subsequently made. A new PICC was placed after 6 days of negative blood cultures in preparation for outpatient parenteral antibiotic therapy (OPAT). His discharge antibiotic regimen was switched to IV ceftriaxone 2 g daily for 6 weeks for easy of dosing.
Fig. 1.
Transesophageal echocardiogram. Arrow pointing to vegetation on Eustachian valve.
Discussion
Leclercia adecarboxylata is ubiquitous in the environment. It is found in food, water and other environmental sources [5]. However, it is rarely isolated from clinical samples and is not known as a frequent cause of infection. As such, it was initially thought to be an opportunistic pathogen in immunocompromised hosts [7]. This paucity of reported cases may reflect misdiagnosis at least in part, as the organism shares many biochemical features with E. coli. Advancements in microbiology techniques have led to accurate identification and segregation of L. adecarboxylata from E. coli and an increase in diagnosis [4]. These techniques include mass spectrometry and other automated identification systems [3]. In particular, Matrix Assisted Laser Desorption/Ionization Time of Flight (MALDI-TOF) mass spectrometry is preferred for precise species identification over conventional methods [3].
Since the first case reported in 1991, approximately 74 cases have been reported in English-language journals as of July 2020 [8]. Cases have been described both in the immunocompetent, and more frequently in the immunocompromised, with risk factors including central catheter use, malignancy, immunodeficiency, and invasive procedures [6]. In the immunocompetent, infections are primarily in the soft tissue and found in association with an aquatic environment [5]. It has also been isolated from a blood sample from a healthy donor [9], and implicated in several cases of bacteremia in the immunocompetent. In the immunocompromised, Leclercia adecarboxylata is implicated in cases which involve endocarditis [6], catheter-related bacteremia [10], bacteremia and cellulitis [4] and spontaneous bacterial peritonitis [3]. Our patient was deemed to be relatively immunocompromised based upon his co-morbidities including gastrointestinal surgeries with dependence on TPN. Literature search revealed two other reports of L. adecarboxylata endocarditis [6,12]. The diagnosis of endocarditis was based upon the modified Duke criteria with one major criteria and three minor criteria (fever, predisposing heart condition, and positive blood cultures) [11]. As reports of Leclercia adecarboxylata endocarditis are rare, there are not established treatment regimens and antibiotic durations readily available [10]. As the organism isolated was pan-sensitive, we treated the patient with IV ceftriaxone 2 g daily for 6 weeks for ease of home dosing.
To conclude, we report a case of eustachian valve endocarditis caused by Leclercia adecarboxylata. Although it is quite rare, it may be an emerging cause of infection as advanced diagnostic technology allows its more frequent identification [3,4,7]. Clinicians should be aware of this pathogen, particularly in patients that are immunocompromised, have central catheters and pre-existing heart conditions.
Author contribution
Kashif Malik — case report design, writing.
Ryann Davie — data collection, writing.
Allison Withers — data collection, writing.
Mohammad Faisal — data collection, writing.
Folake Lawal — case report design, data collection, final review.
Funding statement
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Contributorship statement
All authors were involved in the design and conception of this manuscript.
Data sharing
All data pertaining to this research article are included within the manuscript as written.
Declaration of Competing Interest
The authors report no declarations of interest.
References
- 1.Spiegelhauer M.R. Leclercia adecarboxylata: a case report and literature review of 74 cases demonstrating its pathogenicity in immunocompromised patients. Infect Dis (Lond) 2019;51(3):179–188. doi: 10.1080/23744235.2018.1536830. [DOI] [PubMed] [Google Scholar]
- 2.Kashani A. Leclercia adecarboxylata bacteremia in a patient with ulcerative colitis. Case Rep Gastrointest Med. 2014;2014 doi: 10.1155/2014/457687. p. 457687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adapa S. Peritonitis from Leclercia adecarboxylata: an emerging pathogen. Clin Case Rep. 2019;7(4):829–831. doi: 10.1002/ccr3.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anuradha M. Leclercia adecarboxylata isolation: case reports and review. J Clin Diagn Res. 2014;8(12):DD03–DD04. doi: 10.7860/JCDR/2014/9763.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keren Y. Is Leclercia adecarboxylata a new and unfamiliar marine pathogen? J Clin Microbiol. 2014;52(5):1775–1776. doi: 10.1128/JCM.03239-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee B. A case of Leclercia adecarboxylata endocarditis in a woman with endometrial cancer. Am J Med Sci. 2009;337(2):146–147. doi: 10.1097/MAJ.0b013e31817bf997. [DOI] [PubMed] [Google Scholar]
- 7.Stock I., Burak S., Wiedemann B. Natural antimicrobial susceptibility patterns and biochemical profiles of Leclercia adecarboxylata strains. Clin Microbiol Infect. 2004;10(8):724–733. doi: 10.1111/j.1469-0691.2004.00892.x. [DOI] [PubMed] [Google Scholar]
- 8.Merza N. Leclercia adecarboxylata cholecystitis with septic shock in immunocompetent patient. Case Rep Crit Care. 2019;2019 doi: 10.1155/2019/5057071. p. 5057071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davenport P., Land K.J. Isolation of Leclercia adecarboxylata from the blood culture of an asymptomatic platelet donor. Transfusion. 2007;47(10):1816–1819. doi: 10.1111/j.1537-2995.2007.01397.x. [DOI] [PubMed] [Google Scholar]
- 10.De Mauri A. Leclercia adecarboxylata and catheter-related bacteraemia: review of the literature and outcome with regard to catheters and patients. J Med Microbiol. 2013;62(Pt 10):1620–1623. doi: 10.1099/jmm.0.059535-0. [DOI] [PubMed] [Google Scholar]
- 11.Li J.S. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30(4):633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 12.Dudkiewicz B., Szewczyk E. Etiology of bacterial endocarditis in materials from cardiology and cardiac surgery clinics of the Lodz academy. Med Dosw Mikrobiol. 1993;45:357–359. [PubMed] [Google Scholar]