Abstract
Anogeissus leiocarpus (Combretaceae) is a medicinal plant used in Togo to treat diabetes mellitus and others diseases. The present study was undertaken to evaluate the antihyperlipidemic and antioxidant activities of total extract and fractions of roots of Anogeissus leiocarpus.
The antihyperlipidemic activity of the total extract and the supernatant was performed in vivo by the fructose overload test in ICR mice. Antioxidant potential was determined in vitro by methods based on scavenging of DPPH∗, total antioxidant capacity and reducing power. After the screening, phenolic compounds and flavonoids were evaluated by the well–known colorimetric assay using respectively Folin–Ciocalteu reagent and aluminium chloride.
The results obtained showed that the total extract and the supernatant significantly reduced the serum and liver levels of triglycerides and hence the level of VLDL-Cholesterol compared to hyperlipidemic mice. In vitro the total extract and fractions had the ability to scavenge free radicals, to reduce metal and possessed strong total antioxidant activity. Phytochemical screening revealed the presence of polyphenols, flavonoids, alkaloids, tannins and saponosides in the extract and fractions. And the supernatant fraction contained more polyphenolic compounds than others.
From this study, it is concluded that the total extract and fraction of Anogeissus leiocarpus possessed strong antihyperlipidemic, antioxidant properties and were riched in polyphenols, which can be used in the treatment of diabetes mellitus’ complications. Hence, the supernatant fraction was the most biologically active.
Keywords: Anogeissus leiocarpus, Supernatant, Antihyperlipidemic, Antioxidant, Polyphenols
Anogeissus leiocarpus, Supernatant, Antihyperlipidemic, Antioxidant, Polyphenols.
1. Introduction
Diabetes is a serious, long-term sate that occurs when the body cannot produce any or enough insulin or cannot effectively use the insulin [1].
In diabetics, chronic hyperglycemia which is a source of oxidative stress, remains a very critical condition because of its nature to generate serious life-threatening health complications such as cardiovascular diseases, neuropathy, retinopathy, nephropathy [2]. In fact, wide range of cardiovascular conditions comprise the largest cause of both morbidity and mortality for people living with diabetes [3]. According to the prevision of International Diabetes Federation, the number of deaths resulting from diabetes and its complications in 2019 was estimated to be 4.2 million [4].
Despite the numerous antidiabetic drugs available associated to side effect, most of the population treat many diseases with plants. In diabetes, herbal alternatives have proven to provide symptomatic relief and assist in the prevention of the complications of the disease [5]. Therefore, till the last few years, different extracts from different traditional medicinal plants have been studied which helpful for management of diabetes mellitus [6].
Ethnopharmacological survey of plants carried out by [7] reported that Anogeissus leiocarpus, is one of the plants (Combretaceae) used traditionally in the treatment of diabetes mellitus. Our previous studies had revealed the antihyperglycemic activity of the total extract and fractions (supernatant and pellet) of roots of Anogeissus leiocarpus. Administered at a dose of 500 mg/kg, the hydro alcoholic extract of A. leiocarpus lowered hyperglycemia in ICR mice with carbohydrate overload but did not show any significant effect on basal glycemia; comparing the fractions, the supernatant fraction is the most biologically active at the dose of 100 mg/kg [8].
The present preliminary study was carried out to evaluate the in vivo antihyperlipidemic and antioxidant activities in vitro of total extract and fractions of roots of Anogeissus leiocarpus.
2. Material and methods
2.1. Drugs and chemicals
Commercial reagent kits for determination of Total cholesterol (Chol), HDL, LDL were purchased from Biolabo S.A. (Paris, France).
1,1-diphenyl-2-picryl hydrazyl hydrate(DPPH), aluminum chloride, gallic acid, ascorbic acid, rutin, iron chloride, polyvinylpolypyrrolidone, Folin Ciocalteu reagent, aluminium chloride, acetate Sodium were purchased from Sigma-Aldrich (St. Louis,MO, USA). All other unlabelled chemicals and reagents were available commercially.
2.2. Animals
ICR mice (35 ± 5 g) were housed in standard environmental conditions (temperature 24–25 °C, relative humidity and a 12t/12 h light-dark cycle) and fed with standard rat diet and water ad libitum. The study was approved by the Ethics Committee of the University of Lome, a branch of the National Ethics Committee for control and supervision of experiments on animals (NSBM/UL/14/NS0004).
2.3. Plant material
Roots of Anogeissus leiocarpus (a deciduous tree) were collected from Tsévié, Zio (TOGO) in the month of July 2018. The plants have been identified in Botany and Plant Ecology Laboratory of Faculty of Science (University of Lome), where voucher specimen was deposited in the herbarium under the number TOGO 15483.
Roots of Anogeissus leiocarpus were cleaned out with water, cut into small pieces, dried at the Animal Physiology laboratory at 22 °C and then reduced into powder with the mill Thomas Scientific™.
2.4. Extraction and fractionation
About 400 g of Roots of Anogeissus leiocarpus were extracted in water/ethanol (5:5) for 72 h. The crude extract was filtered on Whatman paper and evaporated in vacuum at 45 °C using a rotary evaporator (Buchi R120). The yield of the dry extract was 5.68 %.
About 30g of hydroethanolic extract obtained was suspended in frozen ethanol 75% within 24 h. Supernatant was separated from pellet by centrifugation at 2500 rpm and evaporated in vacuum at 45 °C using a rotary evaporator. Pellet was then concentrated to dryness [8].
2.5. Effects of total extract and supernatant of roots of Anogeissus leiocarpus on hyperlipidemic mice
2.5.1. Experimental design
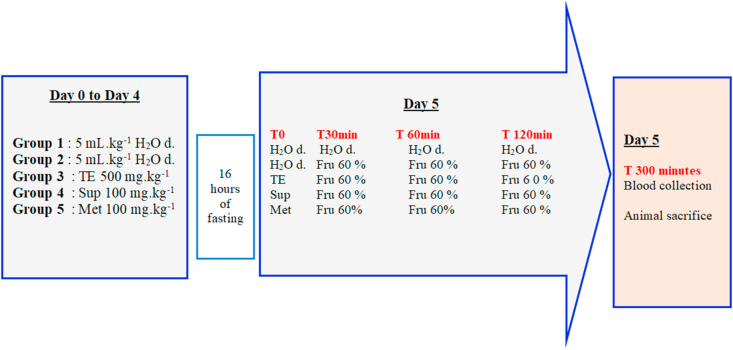
In order to evaluate the effect of the total extract on triglyceride synthesis, the fructose overload test in mice was adopted according to the method of [9] with slight modifications. Thirty normal healthy mice were kept separately in 5 random groups (n = 6) (Figure 1).
Figure 1.
Experimental design of fructose 60% overload test. H2O d: distillated water, TE: total extract, Sup: supernatant fraction, Met: metformin, min: minutes, Fru: Fructose.
Blood samples taken in anticoagulant-free tubes were centrifuged at 3000 rpm for 10 min. The serum were separated, stored at -20 °C until usage. The serum was analyzed for Triglyceride (TG), Total Cholesterol (T-Chol), lipoproteins (HDL, LDL, VLDL), using an automatic analyzer (Rayto Chemray-120). The animals were then euthanized by cervical dislocation and the liver of each animal was removed and kept in the freezer for triglyceride extraction.
2.5.2. Extraction of triglycerides
Triglycerides were extracted from mice liver according to the method described by [10].
0.5 g sample of liver was homogenized in 500 μL of buffer composed of: NaCl (2 mM), EDTA (20 mM) and phosphate buffer (50 mM pH 7.4). To 10 μL of homogenate, 10 μL of tert-butyl alcohol and 5 μL of a mixture of Triton X-100/methanol (1:1, v/v) were added. The triglyceride determination was performed using an assay kit (Pharmalab Innovus).
2.6. In vitro antioxidant assays
2.6.1. DPPH∗ radical scavenging activity
The stable 1,1-diphenyl-2- picrylhydrazyl (DPPH∗) free radical scavenging activity, was performed by the method described of [11] with slight modifications.
1.5 mL DPPH∗ solution (100 μmol.L−1) and 0.25 mL methanol or 0.25 mL methanolic extract and fraction solution were mixed. The absorbance was determined at 517 nm, after 30 min of incubation. Ascorbic acid at different concentrations served as a standard.
| The percentage inhibition activity was calculated from [(A0–A1)/A0] ∗100, |
where A0 is the absorbance of the control, and A1 is the absorbance of the extract or fraction/standard.
2.6.2. Reducing power
The reducing ability was determined by the method of [12]. Different concentrations of total extract and fractions (1 mL) were mixed with phosphate Buffer (2.5 mL) and potassium ferricyanide at 1% (2.5 mL). This mixture was kept at 50 °C in water bath for 20 min. After cooling, 2.5 mL of 10% trichloroacetic acid was added to stop the reaction, The upper layer of solution (2.5 mL) was mixed with distilled water (2.5 mL) and 0.1% of ferric chloride solution (0.5 mL) after 10 min of centrifugation at 3000 rpm. Ascorbic acid served as standard. The absorbance was measured at 700 nm.
2.6.3. Total antioxidant capacity
The antioxidant activity of the extracts was evaluated by the phosphomolybdenum method according to the method of [11].
3 mL of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate) was added to 0.3 mL of total extract and fractions. The tubes containing the reaction solution were incubated at 95 °C for 90 min. Ascorbic acid served as standard. After cooling to room temperature, the absorbance of the solution was measured at 695 nm against blank. The antioxidant activity is expressed as the number of equivalents of ascorbic acid.
2.7. Phytochemical study
2.7.1. Preliminary phytochemical analysis
The phytochemical analysis was performed for detection of active phyto-constituents present in the extract and fractions using standard procedure of [13] and [14].
2.7.2. Determination of total phenolic compounds and tannins
Total phenolic compound contents were firstly estimated using Folin-Ciocalteu 10%, according to the method of [15]. All the phenolic compounds of the extract and fractions were oxidized by the Folin-Ciocalteu reagent (a mixture of phosphotungstic acid and phosphomolybdic acid) which is reduced during the oxidation of the phenols in a mixture of blue oxides of tungsten and molybdenum which can be determined spectroscopically at 735 nm.
In other to determine the amount of tannins, A second dosage was performed after the fixation of tannins by Polyvinylpyrrolidone (PVP). The difference between the first and the second dosage expresses the total amount of tannins according to the method of [16]. Gallic acid was used as standard at different concentrations (0–200 μg mL−1). The absorbance of the reaction was measured at 735 nm using US/VIS Spectrophotometer Wavelength. Total phenols and tannins values were expressed in term of gallic acid equivalent mg.g −1 of extract and fractions.
2.7.3. Total flavonoids content
Flavonoid content in the extract and fractions was determined according to the method used by [17]. Briefly, to 2 mL of extract/rutin at different concentrations (5–100 μg mL −1), 2 mL of aluminum chloride (20 mg mL −1) and 6 mL of sodium acetate (50 mg mL −1) were added. Absorbance was read at 440 nm after 150 min of incubation. Flavonoids values were expressed in term of rutin equivalent mg.g−1 of extract.
2.7.4. Determination of polysaccharides
Firstly, samples were treated by the method of [18] in order to remove cell walls that contain insoluble fibers such as pectin, inorganic salts, and low, molecular, weight compounds of <8000 Da, including proteins. According to the method of [19], 200 μL of a 5% (w/v) aqueous phenol solution and 1 mL of concentrated sulphuric acid were added to 200 μL of the samples (total extract, fractions, standard range, control). This method is an based on sulfuric acid hydrolysis of complex polysaccharides. After homogenization, the mixture was heated in a water bath at 100 °C for 5 min and cooled in darkness for 30 min. Glucose at different concentrations (50–200 μg mL−1) served as standard. The absorbance was determined at 480 nm using US/VIS Spectrophotometer Wavelength.
2.8. Statistical analysis
Results were expressed as mean ± standard error of the mean (SEM). Statistical analysis was performed by two-way analysis of variance (ANOVA) followed by Dunnett's test to evaluate significant differences between groups. The level of significance was set at p < 0.05 and statistical analysis were carried out using Graph Pad Prism 7.0.
3. Results
3.1. Effect of total extract and supernatant on hyperlipidemia
Fructose overload caused significant increase of serum triglycerides and VLDL cholesterol in hyperlidemic mice compared to normals (Table 1). The administration of the total extract and supernatant significantly decreased serum triglycerides and VLDL cholesterol levels compared to positif control, respectively. The decrease was more pronounced in the liver than in the serum. Indeed, in the liver, the administration of the total extract at the dose of 100 mg.Kg−1, and the supernatant fraction (100 mg.Kg−1) significantly (p < 0.0001) decreased triglyceride levels compared to the hyperlipidemic controls. A similar effect was also noted with VLDL cholesterol levels. Supernatant was found to be highly potent as shown in Table 1.
Table 1.
Effect of total extract and supernatant on lipid profil.
| NC | HC | TE 500 | Sup 100 | Met 100 | |
|---|---|---|---|---|---|
|
Serum | |||||
| TG (g.L−1) | 0.36 ± 0.04 | 1.08 ± 0.05#### | 0.73 ± 0.04∗ | 0.41 ± 0.07∗∗∗∗ | 0.83 ± 0.11 |
| Chol-T (g.L−1) | 1.29 ± 0.06 | 1.59 ± 0.13 | 1.55 ± 0.18 | 1.17 ± 0.07 | 1.18 ± 0.09 |
| HDL-C (g.L−1) | 1.01 ± 0.05 | 0.92 ± 0.06 | 1.26 ± 0.10∗ | 1.10 ± 0.09 | 0.93 ± 0.03 |
| LDL-C (g.L−1) | 0.28 ± 0.04 | 0.69 ± 0.11# | 0.36 ± 0.14 | 0.14 ± 0.04∗∗ | 0.23 ± 0.08∗ |
| VLDL-C (mg.dL−1) |
7.2 ± 0.9 |
21.6 ± 1.1#### |
14.8 ± 1.0∗ |
8.2 ± 1.6∗∗∗∗ |
16.6 ± 2.2 |
|
Liver | |||||
| TG (g.L−1) | 0.43 ± 0.04 | 1.15 ± 0.11#### | 0.49 ± 0.03∗∗∗∗ | 0.30 ± 0.03∗∗∗∗ | 0.67 ± 0.05∗∗∗ |
| VLDL-C (mg.dL−1) | 9.26 ± 0.88 | 23.02 ± 2.37#### | 11.13 ± 1.28∗∗∗∗ | 5.26 ± 0.55∗∗∗∗ | 13.36 ± 1.05∗∗∗ |
The NC and HC groups received distilled water and the groups (TE 500, Sup 100 and Met 100) were treated respectively with total extract (500 mg.Kg−1), supernatant (100 mg.Kg−1) and metformin (100 mg.Kg−1) for 4 days. The results are treated with Anova one way and represent the mean ± ESM. ∗p < 0.05 ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001: Compared to hyperlipidemic control (HC); ####p < 0.0001; #p < 0.05: Compared to normal control (NC).
3.2. In vitro antioxidant assays
3.2.1. In vitro DPPH∗ radical scavenging and total antioxidant activity of total extract and fractions of roots of A. leiocarpus
Table 2 shows that the total extract and fractions scavenge the DPPH∗ radical in dose dependent manner. Compared to ascorbic acid, the supernatant fraction has the highest DPPH∗ radical scavenging capacity.
Table 2.
In vitro DPPH∗ radical scavenging and total antioxidant activity of total extract and fractions of roots of A. leiocarpus.
| IC 50 (μg.mL−1) | Total antioxidant (mg AA.g−1) | |
|---|---|---|
| Ascorbic acid | 46.9 ± 0.15 | - |
| Total extract | 48.7 ± 0.15 | 233 ± 1.5 |
| Supernatant | 23.3 ± 0.35 | 237 ± 1.5 |
| Pellet | 72.7 ± 0.70 | 141 ± 2.5 |
The total antioxidant were expressed in mg/g of Ascorbic Acid. The results represent the means ± SEM. N = 3.
While increasing the concentration of the extract and fractions, the antioxidant capacity increases and is correlated to the increase of absorbance. As shown in Table 2, The Total antioxydant capacity value represent all antioxidant compounds present in the extract as equivalence of ascorbic acid which act in hydrophilic and lipophilic part. In comparison, supernatant fractions possesses higher total antioxidant capacity.
3.2.2. Reducing power of the total extract and fractions of A. leiocarpus
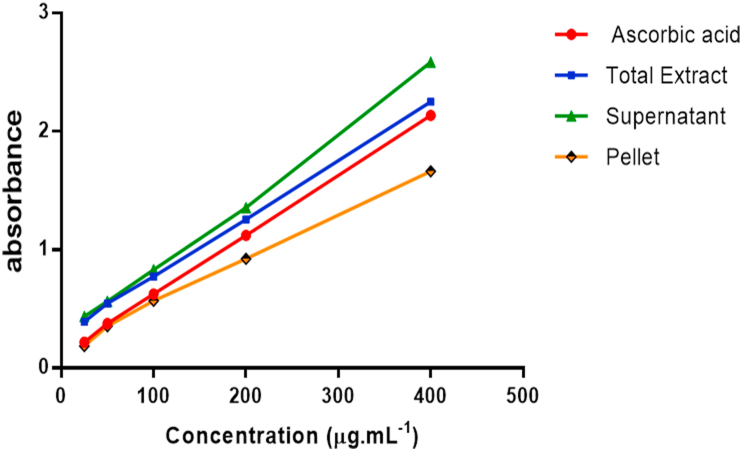
The reducing power of the total extract and fractions was concentration dependent and significantly different from that of ascorbic acid (Figure 2).
Figure 2.
Reducing power of total extract and fractions of roots of A. leiocarpus. Ascorbic acid was used as a positive control. Each point represents the mean ± ESM (N = 3).
Compared to ascorbic acid (positive control), the supernatant has a higher reducing power according to the following diagram: Supernatant > Total extract > Ascorbic acid > pellet.
3.3. Phytochemical study
3.3.1. Identification of the major chemical groups in the total extract and fractions of Anogeissus leiocarpus’ roots
Phytochemical screening of the total extract and fractions of A. leiocarpus revealed the presence of alkaloids, saponins and polyphenolic compounds (Table 3).
Table 3.
Phytochemical screening of total extract and fractions of roots of A. leiocarpus.
| Compounds | Total extract | Supernatant | Pellet |
|---|---|---|---|
| Alkaloids | + | + | + |
| Saponosids (triterpens) | + | + | + |
| Phenolic compounds | + | + | + |
| Flavonoids | + | + | + |
| Condensed Tannins | + | + | + |
| Anthracene (Quinones) | + | + | + |
| Oses | + | + | + |
+: presence.
3.3.2. Dosage of total phenols, tannins, flavonoids and polysaccharides
Table 4 shows that the amount of phenolic compounds, tannins, flavonoids and polysaccharides were significantly high in the supernatant followed by the total extract and the pellet of Anogeissus leiocarpus.
Table 4.
Total phenols, tannins, flavonoids and polysaccharides amount of total extract and fractions of roots of A. leiocarpus.
| Compounds | Total extract | Supernatant | Pellet |
|---|---|---|---|
| Total Phenols (mg AG.g−1) | 74.5 ± 0.003 | 75.5 ± 0.008 | 73 ± 0.011 |
| Tannins (mg AG.g−1) | 63.5 ± 0.003 | 70.5 ± 0.007 | 62.5 ± 0.007 |
| Flavonoids (mg R.g−1) | 42.5 ± 0.005 | 57 ± 0.005 | 48.5 ± 0.003 |
| Polysaccharides (mg GLU.g−1) | 297 ± 0.008 | 331 ± 0.002 | 281 ± 0.007 |
Total phenols and tannins are expressed in mg Gallic Acid Equivalent/g extract. Flavonoids are expressed in mg Rutin Equivalent/g extract. Polysaccharides are expressed in mg Glucose Equivalent/g extract. The results represent the mean ± SEM. N = 3.
4. Discussion
Several factors of hyperlipidemia include a high fat dietary, physical inactivity, obesity smoking and smoking [20]. Diabetes combined with chronic hyperlipidemia could accelerate the progress of atherosclerosis and increase the incidence of cardiovascular disease [21]. In fact, patients live with hyperlipidemia have usually a high levels of total cholesterol, triglycerides, and LDL-cholesterol; as well as a decrease in HDL-cholesterol [22]. Elevated LDL-cholesterol and triglycerides lead to diseases such as biliary obstruction, type 2 diabetes mellitus, coronary artery disease and artherosclerosis which are main cause of cardiovascular disease worldwide [20] in which elevated oxidative stress plays a pivotal role [23]. Therefore, the control of total cholesterol, lipoproteins and triglycerides, as well as reducing of oxidative stress will be a great importance in the management of the disease.
This present study was undertaken to evaluate in vivo the antihyperlipidemic property and the antioxidant activities of total extract and fractions of roots of Anogeissus leiocarpus.
Hyperlipidemia in mice was induced by a 60% fructose overload. Indeed, studies in animals have shown that a rich diet in fructose can lead to hyperglycemia and hypertriglyceridemia followed by insulin resistance ([24, 25]). The results showed a significant increase in serum triglycerides and LDL-C levels in hyperlipidemic controls. The administration of the total extract and the supernatant caused a significant decrease of triglycerides and LDL-C levels compared to the hyperlipidemic control groups. A significant increase of triglycerides level was also observed in the hyperlipidemic mice when triglyceride were extracted. This triglycerides level was reduced significantly in the liver of groups treated with total extract and supernatant. The in vivo potential antihyperlipidemic was also found by [26] on the leaves of Anogeissus leiocarpus on diabetic model of rats. The antihyperlipidemic activity of the total extract and especially supernatant may be due to the enhance of triglycerides catabolism. As reported by many researchers, the restoration of the catabolism of triglycerides could be due to the stimulation of the lipolytic activity of plasma lipoprotein lipase [27]. These mecanisms of action of A. leiocarpus were similar to antihyperlipidemic agents like Statins and fibrates whose enhance LDL-cholesterol clearance and decrease in hepatic VLDL-cholesterol production [28]. A significant (p < 0.05) increased in HDL-cholesterol in treated groups prove that A. leiocarpus can be used to prevent artherosclerosis and reduce oxidative stress. As the matter of fact, HDL-cholesterol inhibit the artherosclerosis formation and remains an important marker of oxidative stress in cholesterol metabolism. The reduction of oxidative stress was in accordence with antioxidant activity of A. leiocarpus.
The method of fractionation of the hydroalcoholic extract of the roots of Anogeissus leiocarpus [8] has allowed two fractions to be obtained; the supernatant fraction and the pellet fraction. With phytochemical screening, the presence of alkaloids, saponins and polyphenolic compounds were revealed in the different fractions as well as in the total extract, in accordance to the work of [29]. Quantitative tests confirmed the presence of these phenolic compounds. However, the supernatant fraction contained more phenolic compounds at concentrations of 75.5 and 70.5 mg equivalent of AG.g−1 respectively fo for total phenol and tannins, and 57 mg equivalent of Routine/g for flavonoids. The supernatant is more richer in polysaccharides than the total extract and pellet.
Nowadays, many studies demonstrated that plants contained polyphenolic compounds and polysaccharides were able to eliminate free radicals and could therefore be categorized as natural antioxidants [30]. Thus, the antioxidant activities of the total extract and fractions were evaluated in vitro through the DPPH∗ radical scavenging capacity, the reducing power and the total antioxidant capacity.
It is well known that the antioxidant activity of plant extracts containing polyphenol components is due to their capacity to be donors of hydrogen atoms or electrons and to capture the free radicals. DPPH∗ test is used to prove the capacity of the total extract and fractions of roots of Anogeissus leiocarpus to act as donors of hydrogen atoms. The results have shown the free radical DPPH∗ scavenging ability of the fractions and total extract and fractions in dose dependent manner. In comparison, the supernatant has the higher capacity to scavenge the DPPH∗ radical. Iron and other metals (copper, chromium, cobalt, vanadium, arsenic….) promote oxidation by acting as catalysts in the formation of free radicals through the transfer of free electrons during the reaction [31]. Likewise, iron in the free state is a powerful electron donor that reacts notably with hydrogen peroxide (H2O2) to generate highly reactive hydroxyl radicals (HO−, HO°) according to the Fenton reaction [32]. The results showed that the total extract and fractions have reduced in concentration dependent manner the Fe3+ to the Fe2+. In other hands, the fractions and total extract have shown a dose-dependent antioxidant capacity. Furthermore Compared to ascorbic acid (reference), The supernatant showed high level of radical scavenging activity, reduced more the metals with higher antioxidant activities than the total extract and pellet fractions. Hence, the supernatant presented the most considerable total antioxidant capacity.
The antihyperlipidemic and antioxidant activities of the total extract and supernatant may be due to the polyphenolic compounds and related compounds present in them. The supernatant fraction contains more bioactive compounds than other. This could explain the most pronounced in vivo antihyperlipidemic effect and antioxidant activities of this fraction.
5. Conclusion
It concluded that the total extract and fractions of roots of Anogeissus leiocarpus have shown remarquable and strong antihyperlipidemic and antioxidant properties. Such activities may be due to the phytochemical compounds present in the total extract and fractions. It may be helpful in preventing the macrovascular complications associated with diabetes mellitus and this knowledge could be used for future investigation as novel natural antioxydant and antihyperlipidemic drugs.
Declarations
Author contribution statement
Aku Enam Motto: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Povi Lawson-Evi; Kwashie Eklu-Gadegbeku: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Batomayena Bakoma: Analyzed and interpreted the data; Wrote the paper.
Kodjo Aklikokou: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.American Diabetes Association 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Supplement 1):S13–S28. doi: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- 2.Ighodaro O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018;108:656–662. doi: 10.1016/j.biopha.2018.09.058. [DOI] [PubMed] [Google Scholar]
- 3.Hussain M.S., Jahan N., Rashid M.M.O., Hossain M.S., Chen U., Rahman N. Antihyperlipidemic screening and plasma uric acid reducing potential of Momordica charantia seeds on Swiss albino mice model. Heliyon. 2019;5(5) doi: 10.1016/j.heliyon.2019.e01739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Diabetes Federation (IDF) ninth ed. 2019. IDF Diabetes Atlas.https://www.diabetesatlas.org/en/ [Google Scholar]
- 5.Karigidi K.O., Olaiya C.O. Antidiabetic activity of corn steep liquor extract of Curculigo pilosa and its solvent fractions in streptozotocin-induced diabetic rats. J. Tradit. Compl. Med. 2020;10(6):555–564. doi: 10.1016/j.jtcme.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konda P.Y., Dasari S., Konanki S., Nagarajan P. In vivo antihyperglycemic, antihyperlipidemic, antioxidative stress and antioxidant potential activities of Syzygium paniculatum Gaertn. in Streptozotocin-induced diabetic rats. Heliyon. 2019;5(3) doi: 10.1016/j.heliyon.2019.e01373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kpodar M.S., Lawson-Evi P., Bakoma B., Eklu-Gadegbeku K., Agbonon A., Aklikokou K., Gbeassor M. Ethnopharmacological survey of plants used in the treatment of diabetes mellitus in south of Togo (Maritime Region) J. Herb. Med. 2015;5(3):147–152. [Google Scholar]
- 8.Motto E.A., Lawson-Evi P., Kantati Y., Eklu-Gadegbeku K., Aklikokou K., Gbeassor M. Antihyperglycemic activity of total extract and fractions of Anogeissus leiocarpus. J. Drug Deliv. Therapeut. 2020;10(3):107–113. [Google Scholar]
- 9.Kadébé Z.T., Metowogo K., Bakoma B., Lawson-Evi S.P., Eklu-Gadegbeku K., Aklikokou K., Gbeassor M. Antidiabetic activity of Plumeria alba Linn (apocynaceae) root extract and fractions in streptozotocin-induced diabetic rats. Trop. J. Pharmaceut. Res. 2016;15(1):87–94. [Google Scholar]
- 10.Zhou K., Xia W., Zhang C., Yu L.L. In vitro binding of bile acids and triglycerides by selected chitosan preparations and their physico-chemical properties. LWT-Food Sci. Technol. 2006;39(10):1087–1092. [Google Scholar]
- 11.Kumaran A., Karunakaran R.J. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT-Food Sci. Technol. 2007;40(2):344–352. [Google Scholar]
- 12.Oyaizu M. Studies on products of browning reaction. Jpn. J. Nutr. Diet. 1986;44(6):307–315. [Google Scholar]
- 13.Evans W.C. Elsevier Health Sciences; 2009. Trease and evans' Pharmacognosy E-Book. [Google Scholar]
- 14.Karumi Y., Onyeyili P.A., Ogugbuaja V.O. Identification of active principles of M. balsamina (Balsam Apple) leaf extract. J. Med. Sci. 2004;4(3):179–182. [Google Scholar]
- 15.Naczk M., Shahidi F. The effect of methanol-ammonia-water treatment on the content of phenolic acids of canola. Food Chem. 1989;31(2):159–164. [Google Scholar]
- 16.Maksimović Z., Malenčić Đ., Kovačević N. Polyphenol contents and antioxidant activity of Maydis stigma extracts. Bioresour. Technol. 2005;96(8):873–877. doi: 10.1016/j.biortech.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Mimica-Dukic N. University of Novi Sad; 1992. Investigation on Secondary Biomolecules in Some Mentha-Species; p. 150. Thesis. [Google Scholar]
- 18.Eberendu A.R., Luta G., Edwards J.A., Mcanalley B.H., Davis B., Rodriguez S., Ray Henry C. Quantitative colorimetric analysis of aloe polysaccharides as a measure of Aloe vera quality in commercial products. J. AOAC Int. 2005;88(3):684–691. [PubMed] [Google Scholar]
- 19.Dubois M., Gilles K.A., Hamilton J.K., Rebers P.T., Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28(3):350–356. [Google Scholar]
- 20.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133(2):187–225. doi: 10.1161/CIRCULATIONAHA.115.018585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan D., Li L., Li Z., Zhang Y., Ma X., Wu L., Qin G. Effect of hyperlipidemia on the incidence of cardio-cerebrovascular events in patients with type 2 diabetes. Lipids Health Dis. 2018;17(1):102. doi: 10.1186/s12944-018-0676-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song D.X., Jiang J.G. Hypolipidemic components from medicine food homology species used in China: pharmacological and health effects. Arch. Med. Res. 2017;48(7):569–581. doi: 10.1016/j.arcmed.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Incalza M.A., D'Oria R., Natalicchio A., Perrini S., Laviola L., Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018;100:1–19. doi: 10.1016/j.vph.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Zavaroni I., Chen Y.D.I., Reaven G.M. Studies of the mechanism of fructose-induced hypertriglyceridemia in the rat. Metabolism. 1982;31(11):1077–1083. doi: 10.1016/0026-0495(82)90155-x. [DOI] [PubMed] [Google Scholar]
- 25.Brun T., Bartley C., Maechler P. Exposure of beta-cells to chronic fructose potentiates glucose-stimulated insulin secretion through ATP signaling. Rev. Med. Suisse. 2019;15(638):390–392. [PubMed] [Google Scholar]
- 26.Onoja U.S., Ugwu C.C., Uzor P.F., Nweze I.E., Omeje E.O., Nnamani P.O., Effiong E.J. Effect of Anogeissus leiocarpus Guill and Perr Leaf on Hyperglycaemia and Associated Dyslipidaemia in Alloxan-induced Diabetic Rats. Dhaka Univ. J. Pharm. Sci. 2018;17(1):65–72. [Google Scholar]
- 27.Dallinga-Thie G.M., Kroon J., Borén J., Chapman M.J. Triglyceride-rich lipoproteins and remnants: targets for therapy? Curr. Cardiol. Rep. 2016;18(7):67. doi: 10.1007/s11886-016-0745-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fiévet C., Staels B. Combination therapy of statins and fibrates in the management of cardiovascular risk. Curr. Opin. Lipidol. 2009;20(6):505. doi: 10.1097/MOL.0b013e328332e9ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orlando G., Ferrante C., Zengin G., Sinan K.I., Bene K., Diuzheva A., Chiavaroli A. Qualitative chemical characterization and multidirectional Biological Investigation of Leaves and Bark Extracts of Anogeissus leiocarpus (DC.) Guill. & Perr.(Combretaceae) Antioxidants. 2019;8(9):343. doi: 10.3390/antiox8090343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong X., Li X., Xia Y., Xu J., Li Q., Zhang C., Li M. Effects of phytochemicals from plant-based functional foods on hyperlipidemia and their underpinning mechanisms. Trends Food Sci. Technol. 2020;103(September 2020):304–320. [Google Scholar]
- 31.Končić M.Z., Barbarić M., Perković I., Zorc B. Antiradical, chelating and antioxidant activities of hydroxamic acids and hydroxyureas. Molecules. 2011;16(8):6232–6242. doi: 10.3390/molecules16086232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winterbourn C.C. Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol. Lett. 1995;82:969–974. doi: 10.1016/0378-4274(95)03532-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.