Abstract
Background
Ovarian cancer remains the most lethal gynecologic malignancy with high recurrence rates. Because recurrence involves primarily the peritoneum, intraperitoneal chemotherapy is being evaluated as a new approach to treat microscopic peritoneal disease. One trial showed that cisplatin–paclitaxel intraperitoneal chemotherapy with intravenous paclitaxel improved survival but increased morbidity. Another trial reported a significant improvement in overall survival (OS) and disease-free survival (DFS) without increasing the morbidity (P = 0.76) or mortality rates (hazard ratio 0.67, P = 0.02) after adding hyperthermic intraperitoneal chemotherapy (HIPEC) to interval cytoreduction. The current trial aims to evaluate the impact of adding HIPEC to primary or interval cytoreductive surgery for epithelial ovarian cancer (EOC) on the efficacy, safety, treatment feasibility, and quality of life.
Patients and methods
This is an international, multicenter, open-label, randomized (1 : 1), two-arm, phase III clinical trial that will enroll 432 patients with newly diagnosed International Federation of Gynecology and Obstetrics (FIGO) stage III EOC. Patients are randomized to receive or not HIPEC with the standard of care. Inclusion criteria include patients with FIGO stage III EOC, Fallopian tube carcinoma or primary peritoneal cancer who undergo complete primary or interval cytoreduction. The primary objective is to assess DFS of the addition of HIPEC. Secondary objectives are the assessment of OS, safety, return to intended oncologic treatment, quality of life and the trade-off between efficacy and morbidity.
Conclusions
The results might help extend the indications of HIPEC to include patients undergoing primary cytoreduction, providing a standardized protocol for HIPEC in EOC management and reliable information on the quality of life after adding HIPEC.
Key words: ovarian cancer, HIPEC, overall survival, disease-free survival, quality of life
Highlights
-
•
Ovarian cancer remains the most lethal gynecologic cancer with high rates of recurrence involving primarily the peritoneum.
-
•
Intraperitoneal chemotherapy is being evaluated as a new therapeutic approach to treat microscopic peritoneal disease.
-
•
This trial evaluates the impact of adding HIPEC to primary or interval cytoreductive surgery for EOC.
-
•
This trial evaluates the efficacy, safety, treatment feasibility and quality of life after the addition of HIPEC.
-
•
This is an international, multicenter, open label, randomized, phase III clinical trial.
Introduction
Despite the 33% decrease in the overall mortality rate for ovarian cancer during the last four decades, it remains the most lethal gynecologic malignancy with high recurrence rates.1,2 More than two-thirds of patients present with advanced stage disease involving the abdominal cavity.3 Epidemiologic studies showed an improved 5-year survival for all epithelial ovarian cancer (EOC) stages (42% and 26% for FIGO stages III and IV, respectively), but no improvement in the 10-year overall survival (OS; 24% for all stages combined), reflecting a better disease control but no improvement in the long-term survival.1,4 Despite the revolutionary advances in the systemic treatment of advanced EOC, the majority of the patients (70%) diagnosed with advanced EOC will present a disease recurrence.5 A complete primary (PCS) or interval cytoreductive surgery (ICS) with carboplatin and paclitaxel-based chemotherapy with or without bevacizumab is the standard of care for EOC patients. Many clinical trials proved that the residual disease after surgery is an independent prognostic factor for EOC patients.2,3 However, because disease recurrence involves primarily the abdominopelvic peritoneal surface, new therapeutic approaches aiming to prevent peritoneal recurrence are being evaluated.
One of these approaches consist of associating a complete cytoreduction removing the entirety of macroscopic lesions (including extensive peritonectomy if required) with hyperthermic intraperitoneal chemotherapy (HIPEC) that is presumed to induce higher clearance of microscopic peritoneal lesions than with intravenous chemotherapy.6
The efficacy of associating HIPEC with optimal cytoreduction was proven and is the standard of care for treating certain peritoneal diseases such as pseudomyxoma peritonei,7 peritoneal mesothelioma8, 9, 10 and selected patients with colorectal carcinomatosis.11
However, in ovarian cancer treatment, the number of studies evaluating the impact of HIPEC on EOC treatment remains very scarce. In 2006, Armstrong et al.12 concluded in their randomized trial that the addition of cisplatin–paclitaxel-based intraperitoneal chemotherapy to intravenous paclitaxel is associated with an improved survival compared with cisplatin–paclitaxel intravenous chemotherapy only. This was, however, associated with severe morbidity. van Driel et al.6 published the only randomized trial (OVHIPEC) evaluating HIPEC in ovarian cancer treatment. Published in January 2018, OVHIPEC evaluated the impact of associating HIPEC with ICS. This trial indicated a significant improvement in OS and disease-free survival (DFS) after adding HIPEC, with no increase in the morbidity rate (P = 0.76) nor in the mortality rate reported [hazard ratio (HR) = 0.67, P = 0.02]. There are currently few other randomized trials evaluating the impact of HIPEC in ovarian cancer. Besides two ongoing trials in China and Korea, our trial is the first actively recruiting multicenter, randomized phase III trial aiming to evaluate the impact of HIPEC during the PCS and ICS for newly diagnosed ovarian cancer patients in France and Belgium. This trial started active recruitment in March 2019. The purpose of this trial is to evaluate the efficacy, safety, treatment feasibility and quality of life after adding HIPEC in the management of the global EOC population. The results of our trial might confirm and consolidate the previously published data of the only randomized controlled trial evaluating HIPEC in ICS.
Methods
Study design
CHIPPI-1808 (Chimiothérapie Hyperthérmique Intra-Péritonéale au cours d'une chirurgie Première ou Intervallaire) is an international, multicenter, open label, randomized (1 : 1), two-arm phase III clinical trial that will enroll 432 patients with newly diagnosed FIGO stage III EOC. This trial is registered in ClinicalTrials.gov (NCT03842982). The trial is organized and funded by the Oscar Lambret Cancer Center. It will take place in 16 tertiary cancer centers, 14 of which are in France and 2 of which are in Belgium. Institutional review board approval for the trial has been obtained. The inclusion and exclusion criteria are detailed in Appendix.
Appendix.
Inclusion and exclusion criteria
| Inclusion criteria |
| PRE-ELIGIBILITY CRITERIA TO BE CHECKED BEFORE SURGERY FOR PRE-REGISTRATION |
| Age ≥18 years and ≤76 years |
| HISTOLOGICALLY PROVEN PRIMARY EPITHELIAL OVARIAN CARCINOMA OR FALLOPIAN TUBE CARCINOMA OR PERITONEAL CARCINOMA (INCLUDING SEROUS PAPILLARY ADENOCARCINOMA, CLEAR-CELL CARCINOMA, MUCINOUS ADENOCARCINOMA AND ENDOMETRIOID CARCINOMA). IN CASE OF PRIMARY DEBULKING SURGERY, THE PATIENT CAN BE INCLUDED BASED ON AN EXTEMPORANEOUS DIAGNOSIS OF STAGE III INVASIVE CARCINOMA. |
| FIGO stage III |
Patient eligible for:
|
| World Health Organization (WHO) performance status ≤2 |
| PHYSICAL STATUS SCORE: AMERICAN SOCIETY OF ANESTHESIOLOGISTS (ASA) SCORE ≤2 or 3 if only related to a body mass index ≥40 KG/M2 OR TO MALIGNANT ASCITES |
Adequate bone marrow and renal function, as evidenced by the following tests carried out within 7 days prior to surgery:
|
| Negative serum pregnancy test within 7 days prior to surgery for women of childbearing age. |
| ABSENCE OF CONTRAINDICATION TO RECEIVE THE PRODUCTS USED IN THIS STUDY (CISPLATIN AND PRODUCTS USED IN NEOADJUVANT/ADJUVANT CHEMOTHERAPY) ACCORDING TO THE MOST RECENT SUMMARY OF PRODUCT CHARACTERISTICS OF THESE PRODUCTS13 |
| Patient is willing and able to comply with the protocol for the duration of the study including undergoing treatment and scheduled visits and examinations including follow-up |
| Signed, institutional review board-approved written informed consent |
| CRITERIA TO BE CHECKED PREOPERATIVELY FOR CONFIRMATION OF ENROLLMENT AND RANDOMIZATION |
| Residual disease after surgery CC0 (no macroscopic residue) or CC1 (residue < 2.5 mm) |
| Preoperative hemorrhage < 2.5 l |
| Strictly less than three digestive resections (other than appendectomy) carried out during surgery (a maximum of three digestive tract anastomosis). |
| Diuresis during surgery ≥1 ml/kg/h |
| Exclusion criteria |
| Benign disease, borderline disease, non-epithelial ovarian carcinoma or carcinosarcoma |
| Cirrhosis |
| Known hypersensitivity to any of the study drugs, study drug classes or excipients in the formulation |
| AUDITORY IMPAIRMENT (I.E. IF HEARING AID IS FITTED OR IF THE PATIENT IS COMPLAINING. IN CASES OF DOUBT, AN AUDIOGRAM SHOULD BE CARRIED OUT.) |
| Dehydration or intercurrent disease that contraindicates hyperhydration (including cardiorespiratory disease) |
| Other uncontrolled intercurrent disease including, but not limited to, diabetes; hypertension; symptomatic congestive heart or pulmonary failure; renal, hepatic or severe gastrointestinal (associated with diarrhea) chronic disease |
| ANY UNRESOLVED NATIONAL CANCER INSTITUTE COMMON TERMINOLOGY CRITERIA FOR ADVERSE EVENTS (NCI-CTCAE) GRADE ≥2 TOXICITY FROM PREVIOUS ANTICANCER THERAPY |
| Concomitant treatment with prophylactic phenytoin |
| Patients who received live attenuated vaccine, including yellow fever vaccine, within 30 days prior to inclusion (and, if patient is enrolled, up to 30 days after the last administration of study treatment) |
| Pregnant or breastfeeding woman |
| Psychiatric illness or social situation that would limit compliance with study requirement, substantially increase the risk of side-effects or compromise the ability of the patient to give written informed consent |
| Inability to comply with medical follow-up of the trial (geographical, social or psychic reasons) |
| Person under guardianship or curatorship |
Objectives
The primary objective of this trial is to assess the impact on DFS of adding HIPEC to the standard of care for EOC patients (chemotherapy and PCS or ICS) compared with the standard of care alone.
Secondary objectives consist of evaluating the impact of HIPEC on the OS, the safety, the feasibility of adjuvant treatment, the quality of life and evaluating the trade-off between efficacy and morbidity using the quality-adjusted time without symptoms or toxicity (Q-TWiST) approach.
Exploratory objectives consist of evaluating the impact of HIPEC on the count of residual viable cells (evaluated by flow cytometry) in abdominal drainage fluids (only for patients recruited in the Oscar Lambret Cancer Center) and establishing a biobank using tumor and blood samples for future translational research projects.
Interventions
The experimental arm (arm A) consists of the association of HIPEC with the standard of care (chemotherapy and cytoreductive surgery). The control arm (arm B) consists of the standard of care alone (chemotherapy and cytoreductive surgery). In both arms, and in the case of adjuvant or neoadjuvant chemotherapy, chemotherapy regimens follow the international guidelines. They consist of six cycles of platinum–paclitaxel-based regimens associated or not with bevacizumab and/or PARP inhibitors if indicated. Adjuvant chemotherapy is preferably started within the first 6 weeks following surgery. In case of neoadjuvant chemotherapy, surgery should be carried out in a time interval of 3-5 weeks in case of chemotherapy without bevacizumab, and in 4-6 weeks if bevacizumab is added. The patients remain eligible for the study if surgery is delayed beyond the recommended time interval (Figure 1).
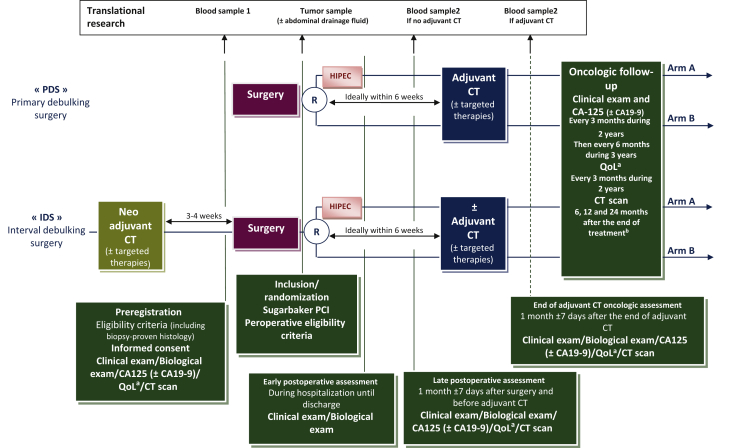
Figure 1.
The CHIPPI trial design.
The enrollment starts with a preregistration prior to surgery and a confirmation of enrollment and randomization during surgery. The experimental arm (arm A) involves the addition of HIPEC with the standard of care (chemotherapy and cytoreductive surgery). The control arm (arm B) consists of the standard of care alone (chemotherapy and cytoreductive surgery). Adjuvant or neoadjuvant chemotherapy regimens are associated or not with bevacizumab and PARP inhibitors. Adjuvant chemotherapy is preferably started within the first 6 weeks following surgery. Postoperative assessment starts immediately after surgery, is reassessed at 1 month after surgery before starting adjuvant chemotherapy and 1 month after achieving adjuvant chemotherapy. Patients will undergo physical examination with evaluation of tumor markers if informative (CA125 and/or CA19-9) every 3 months for 2 years, and then every 6 months until achieving 5 years of follow-up. Patients will also undergo CT scan imaging at 6, 12 and 24 months after achieving their treatment. Quality of life assessment will be carried out every 3 months for 2 years.
CT, computed tomography; EORTC, European Organization for Research and Treatment of Cancer; HIPEC, hyperthermic intraperitoneal chemotherapy; PCI, Peritoneal Cancer Index; QLQ-C30, Quality of Life Questionnaire-Score 30; QLQ-OV28, Quality of Life Questionnaire-Ovarian Cancer Module.
a Quality of life assessed by EORTC QLQ-C30 and QLQ-OV28 questionnaires.
b End of treatment is defined as the last day of adjuvant CT excluding targeted therapies or as the date of surgery if no adjuvant CT is administered.
Assignment of interventions
The enrollment in this trial is a two-step procedure with a preregistration prior to surgery and confirmation of enrollment and randomization during surgery.
The screening and the preregistration of the patients will be carried out during consultations when patients are deemed eligible for a cytoreductive surgery (PCS or ICS). All patients will undergo a complete work-up comprising imaging (thoraco-abdomino-pelvic computed tomography scan) associated with a biologic work-up including tumor markers' evaluation and a diagnostic laparoscopy to evaluate the extent and the resectability of the disease using the Peritoneal Carcinomatosis Index (PCI) or the Sugarbaker score. Based on this work-up, surgeons will assign patients to PCS or ICS.
Written informed consent is obtained from the patients before preregistration. Preregistration is carried out online prior to surgery.
All patients are operated with the intention to achieve complete cytoreduction (CC0). Patients are randomly assigned to receive HIPEC or not. The randomization takes place perioperatively, after achieving complete cytoreduction (CC0: no residual disease or CC1: residual nodules measuring <2.5 mm), assessing the patients and confirming that they fit the inclusion criteria, including the mandatory intraoperative checklist required for the enrollment (Appendix).
Randomization is carried out using an online centralized randomization software14 that ensures concealment. Randomization is carried out with a minimization program with a random factor set at 0.8 to obtain a balanced randomization (1 : 1) that is controlled for the treating center, the disease burden [estimated using the PCI evaluated preoperatively (PCI 0-10 versus 11-20 versus 21-30 versus 31-39)], postsurgery residue (CC0 versus CC1), timing of surgery (PCS versus ICS after <4 cycles versus ICS after >4 cycles) and histological type (high-grade serous carcinoma versus other types).
Randomization could not be stratified according to the type of adjuvant systemic treatment (with or without bevacizumab, with or without targeted treatment such as PARP inhibitors), as this decision is made based on postoperative histological analysis.
Participant timelines
Postoperative assessment starts immediately after surgery, is reassessed at 1 month after surgery before starting adjuvant chemotherapy and 1 month after achieving adjuvant chemotherapy (Figure 1). Oncologic follow-up will start at the end of treatment. Patients will undergo physical examination with evaluation of tumor markers if informative (CA125 and/or CA19-9) every 3 months for 2 years, and then every 6 months until achieving 5 years of follow-up. Patients will also undergo computed tomography scan imaging at 6, 12 and 24 months after achieving their treatment. Quality of life assessment will be carried out every 3 months for 2 years.
The end of treatment is defined as the last day of administration of conventional chemotherapy (excluding targeted therapy) or the date of surgery if no adjuvant chemotherapy is administered. The treatment can be terminated earlier if it causes unacceptable toxicity, intercurrent illness or in the case of disease progression.
The HIPEC procedure is carried out after the completion of cytoreduction and is based on the protocol described by van Driel et al.6 The procedure can be carried out using the open or closed technique and consists of infusing the peritoneal cavity with a heated saline infusion (40°C) mixed with cisplatin 100 mg/m2, with a maximal dose of 200 mg for patients with a body surface area >2 m2 and a perfusion time of 90 min. Simultaneously, patients receive an intravenous perfusion of sodium thiosulfate that is prolonged 6 h after the operation to prevent nephrotoxicity.6
Endpoints
DFS is defined as the time from randomization to the date of progression, relapse or death from any cause. Progression and relapses are confirmed by the local tumor board, based on Gynecologic Cancer Inter Group criteria in combination with clinical, biological and radiological assessments. For patients alive without progression or relapse, data are censored at the date of the last follow-up visit.
OS is defined as the time between randomization and death from any cause. For patients who are alive at the end of the study, data are censored at the date of last follow-up.
Adverse events (AEs) are evaluated from randomization up to 30 days after the end of treatment (surgery or chemotherapy), excluding AEs unequivocally related to the disease or its progression. AEs are classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE version 5.0). AEs of grade ≥3 (grade 3+) are considered severe.
The impact of HIPEC on adjuvant treatment feasibility is evaluated by the time interval between surgery and the start of adjuvant chemotherapy. Chemotherapy is considered delayed when this interval exceeds 6 weeks. In this case, the reasons for the delay are described. The impact of HIPEC is also evaluated by evaluating the total number of chemotherapy courses (neoadjuvant and adjuvant); if it is less than the six planned courses, the reasons are described.
Q-TWiST is computed from survival data (OS and DFS) and AE data (date of occurrence of grade 3+ AEs).
The quality of life is evaluated using the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire-Score 30 (QLQ-C30) and Quality of Life Questionnaire-Ovarian Cancer Module (QLQ-OV28).
In addition to the previously cited endpoints, blood and abdominal washing samples are taken after achieving HIPEC from the patients treated at the Oscar Lambret Cancer Center to carry out flow cytometry and evaluate the impact of HIPEC on the count of residual viable cells in the abdominal fluid drainage and the detection of free tumor DNA in peripheral blood.
Statistical analysis
We estimated the expected DFS in the control group (no HIPEC) assuming, based on a literature review, a mixed population (consisting of 33% PCS and 67% ICS), an exponential distribution of DFS time (constant risk over time) and a median DFS of 33 and 20 months in the PCS and ICS stratum, respectively, leading to lambda parameters of 0.0210/month and 0.0347/month, respectively. Based on these hypotheses and on the review of literature, we assumed a median DFS of 23 months from randomization (surgery) in the whole control (No HIPEC) group and 35 months in the experimental (HIPEC) group. We considered, based on the publication by van Driel et al.,6 that HIPEC would be of interest if it is associated with a 35% reduction in the risk of relapse (HR = 0.65), which is clinically meaningful and seems realistic. These survival estimates also consider the survival data after adding PARP inhibitors.6,15, 16, 17, 18, 19
Based on a log-rank test with a two-sided alpha of 5% and 90% power (beta 10%), the required number of events is 227. This number is achieved if 432 patients (216 per treatment arm) are accrued over 36 months, and the final analysis is carried out with a minimum follow-up of 14 months for the last patient.
An interim efficacy analysis of DFS using a Lan-DeMets Alpha-spending function based on an O'Brien and Fleming stopping rule will be carried out when ~90 events (40% of the total expected number of events) are observed. The early stopping rule allows a positive conclusion in case of a major benefit associated with HIPEC. Study duration and sample size may be re-evaluated and re-estimated during the trial course to ensure a sufficient power.
The main analysis will be carried out considering all patients in their treatment group allocated by randomization (intention to treat). A sensitivity analysis will be carried out excluding patients with a major protocol violation (per-protocol analysis). Major protocol violations will be defined by the Trial Management Committee before the final analysis. Safety analyses will be carried out on the ‘treated population’ considering the treatment actually received.
DFS and OS curves will be estimated using the Kaplan–Meier method and will be compared between treatment groups using the two-sided log-rank test. Treatment effect will be assessed by the HR of relapse or death (DFS), or death (OS) in Cox models. Treatment effect will also be estimated using the restricted mean survival time difference (rmstD)20 to estimate the absolute mean survival gain. Treatment effect will be estimated in each cohort separately (primary debulking surgery and interval debulking surgery). Heterogeneity of treatment effect between both cohorts will be evaluated graphically using a forest plot and tested using an interaction test.
Heterogeneity of treatment effect according to disease burden (Sugarbaker PCI), postsurgery residue (CC-0 versus CC-1) and histological type (high-grade serous carcinoma versus other) will also be evaluated graphically (forest plot) and tested using interaction tests.
A similar approach will be used to evaluate the effect of treatment according to the previous experience in HIPEC of the participating centers/surgeons and the experience within the trial (learning curve evaluated using an interaction term with the period of accrual), as well as the heterogeneity of treatment according to systemic treatment (with or without bevacizumab, with or without targeted therapy such as PARP inhibitors). This latter factor will be evaluated at the stage of the analysis and not as a stratification of the randomization because it will not be known when the patient is enrolled (the treatment may or not be started after surgery according postoperative patient results).
For the safety analysis, AEs will be described by the preferred term and then pooled by the system organ class. For each type of AE, the worst grade observed will be tabulated by treatment arm, and the percentages of grade 3+ cases will be provided. A butterfly plot will be used to illustrate the difference in proportion of patients experiencing AEs between treatment groups. Relative risks of severe AEs will be estimated with their 95% confidence intervals.
For the Q-TWiST analysis, each patient's OS will be partitioned into three mutually exclusive health states: time with severe AEs before progression (toxicity), time without symptoms of disease or grade 3+ toxicity of treatment (TWiST) and time after tumor progression or relapse. The time spent in each state will be weighted by a health-state utility associated with that state (0.5 for toxicity, 1 for TWiST and 0.5 for time after tumor progression or relapse) and summed to calculate the Q-TWiST. Q-TWiST will be compared between treatment groups using bootstrap samples.
Each dimension of QLQ-C30 and QLQ-OV28 will be described and analyzed using the time until definitive deterioration (TUDD). TUDD is defined as the time from randomization to the first observation of a definitive deterioration of QLQ-C30 or QLQ-OV28 score, or death.21 For each dimension of both questionnaires, TUDD will be estimated using the Kaplan–Meier method, compared between treatment groups (HIPEC/no HIPEC) using the Cox model to estimate the HR for quality of life deterioration and HRs for the different dimensions will be illustrated using a forest plot.
To limit the biases related to the open-label aspect of the trial, randomization will be carried out after completion of the cytoreductive surgery to minimize the surgical bias, and the postoperative follow-ups will be standardized in all the participating centers.
Discussion
Many studies that compared the outcomes of PCS and ICS found lower OS and DFS in ICS populations.22, 23, 24 Thus, despite CC, clinical outcomes of ICS are inferior to PCS. Besides the selection biases, and the presumed lighter tumor burden at diagnosis in the PCS group, a possible deleterious effect of initial chemotherapy cannot be ruled out. Recent molecular biology studies of high-grade serous carcinoma concluded four molecular subtypes: immunoreactive, mesenchymal, differentiated, and proliferative. Each of these subtypes presents a different impact on survival rates.25 the immunoreactive subtype is the most chemosensitive and is associated with the best prognosis.26,27 Another study, with extensive microanalysis of peritoneal and ovarian tumor implants, showed that several subtypes may be present in the same patient and in the same tumoral implant. Thus the previously enumerated molecular subtypes are not exclusive.28 Because the different tumoral clones present different chemosensitivity profiles, it may be that neoadjuvant chemotherapy induces a selection of the most chemoresistant clones while destroying the chemosensitive clones. This fact might explain the relatively higher recurrence and lower survival rates observed after ICS, despite adequate cytoreduction. Because PCS will excise both chemosensitive and chemoresistant clones, the remaining microscopic postoperative residual clones will also be a mixture of both clones. In this context, we can hypothesize that high-dose local chemotherapy associated with the synergistic effect of hyperthermia might destroy the chemosensitive clones and overcome the resistance of the chemoresistant clones.
Pharmacokinetic studies showed that intraperitoneal chemotherapy leads to a higher local intracellular drug concentration with a lower systemic toxicity. The addition of hyperthermia enhances the cytotoxic effect of the chemotherapeutic agents and increases the depth of drug tissue penetration.29,30 Furthermore, hyperthermia presents an independent cytotoxic effect, induces apoptosis, inhibits angiogenesis and most importantly induces an alteration in the BRCA2 gene, increasing the sensitivity to platinum drugs through the impairment of homologous recombination.31
The randomized OVHIPEC trial in 2018 demonstrated an enhanced OS and DFS after adding HIPEC for patients that underwent ICS for FIGO stage III EOC without increasing the morbidity. After a median follow-up of 4.7 years, survival data for patients in the HIPEC arm were better than those in the control group: 15 versus 11 months, respectively, for DFS (HR = 0.65; P = 0.003) and 48 versus 34 months, respectively, for OS (HR = 0.64; P = 0.01). The number of patients with grade 3-4 morbidity was similar in both groups.6 However, the OVHIPEC trial evaluated the impact of HIPEC on patients deemed not suitable for PCS. The CHIPPI trial is the first active phase III randomized trial evaluating the impact of HIPEC in both PCS and ICS setting as well as the impact of HIPEC on the quality of life and the risk–benefit ratio through the Q-TWIST approach. We acknowledge some limitations of this study; in particular, we may not be able to specifically address the issue of HIPEC treatment effect in patients receiving PARP inhibitors. However, the randomized design and the planned analysis of heterogeneity according to this factor will provide a treatment estimate in this setting. We expect that our findings will help to extend the indications of HIPEC to include patients undergoing primary cytoreduction for ovarian cancer, provide a standardized protocol for HIPEC in ovarian cancer management and provide reliable information on the quality of life related to the addition of HIPEC to the standard of care for EOC management.
Acknowledgments
Funding
None declared.
Disclosure
The authors have declared no conflicts of interests.
Data sharing
The data set used and/or analyzed during the current study are available from the corresponding author on reasonable request. Not all data are obtained yet since the study is ongoing.
Consent for publication
A signed informed consent is obtained from all patients included in the trial.
Footnotes
☆Note: The study has been submitted and approved by ethics committee (Committee for the Protection of Persons: 06/02/2019 (reference number: 10/19_1)). The study opened in March 2019. A written informed consent will be obtained from the study participants.
Supplementary data
References
- 1.Torre L.A., Trabert B., DeSantis C.E. Ovarian cancer statistics, 2018: ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horowitz N.S., Miller A., Rungruang B. Does aggressive surgery improve outcomes? Interaction between preoperative disease burden and complex surgery in patients with advanced-stage ovarian cancer: an analysis of GOG 182. J Clin Oncol. 2015;33(8):937–943. doi: 10.1200/JCO.2014.56.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delga B., Classe J.-M., Houvenaeghel G. 30 years of experience in the management of stage III and IV epithelial ovarian cancer: impact of surgical strategies on survival. Cancers. 2020;12(3):768. doi: 10.3390/cancers12030768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Timmermans M., Sonke G.S., Van de Vijver K.K., van der Aa M.A., Kruitwagen R.F.P.M. No improvement in long-term survival for epithelial ovarian cancer patients: a population-based study between 1989 and 2014 in the Netherlands. Eur J Cancer. 2018;88:31–37. doi: 10.1016/j.ejca.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 5.Ushijima K. Treatment for recurrent ovarian cancer—at first relapse. J Oncol. 2010;2010:497429. doi: 10.1155/2010/497429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Driel W.J., Koole S.N., Sikorska K. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378(3):230–240. doi: 10.1056/NEJMoa1708618. [DOI] [PubMed] [Google Scholar]
- 7.Chua T.C., Moran B.J., Sugarbaker P.H. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30(20):2449–2456. doi: 10.1200/JCO.2011.39.7166. [DOI] [PubMed] [Google Scholar]
- 8.Lutton N.J., Moran B.J. Clinical results of cytoreduction and HIPEC in pseudomyxoma peritonei. Cancer Treat Res. 2007;134:319–328. doi: 10.1007/978-0-387-48993-3_20. [DOI] [PubMed] [Google Scholar]
- 9.Mittal R., Chandramohan A., Moran B. Pseudomyxoma peritonei: natural history and treatment. Int J Hyperthermia. 2017;33(5):511–519. doi: 10.1080/02656736.2017.1310938. [DOI] [PubMed] [Google Scholar]
- 10.Helm J.H., Miura J.T., Glenn J.A. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: a systematic review and meta-analysis. Ann Surg Oncol. 2015;22(5):1686–1693. doi: 10.1245/s10434-014-3978-x. [DOI] [PubMed] [Google Scholar]
- 11.Elias D., Gilly F., Boutitie F. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2009;28(1):63–68. doi: 10.1200/JCO.2009.23.9285. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong D.K., Bundy B., Wenzel L. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354(1):34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 13.Base de Données Publique Des Médicaments http://base-donnees-publique.medicaments.gouv.fr/ Available at. Accessed March 17, 2021.
- 14.Ennov Clinical https://ecrf.ctd-cno.org/CSOnline/ Accessed March 17, 2021.
- 15.Burger R.A., Brady M.F., Bookman M.A. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365(26):2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Martin A., Gladieff L., Tholander B. Efficacy and safety results from OCTAVIA, a single-arm phase II study evaluating front-line bevacizumab, carboplatin and weekly paclitaxel for ovarian cancer. Eur J Cancer. 2013;49(18):3831–3838. doi: 10.1016/j.ejca.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Oza A.M., Cook A.D., Pfisterer J. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015;16(8):928–936. doi: 10.1016/S1470-2045(15)00086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oza A.M., Selle F., Davidenko I. Efficacy and safety of bevacizumab-containing therapy in newly diagnosed ovarian cancer. Int J Gynecol Cancer. 2017;27(1):50–58. doi: 10.1097/IGC.0000000000000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ray-Coquard I., Pautier P., Pignata S. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381(25):2416–2428. doi: 10.1056/NEJMoa1911361. [DOI] [PubMed] [Google Scholar]
- 20.Royston P., Parmar M.K.B. Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol. 2013;13:152. doi: 10.1186/1471-2288-13-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonnetain F., Fiteni F., Efficace F., Anota A. Statistical challenges in the analysis of health-related quality of life in cancer clinical trials. J Clin Oncol. 2016;34(16):1953–1956. doi: 10.1200/JCO.2014.56.7974. [DOI] [PubMed] [Google Scholar]
- 22.Mueller J.J., Zhou Q.C., Iasonos A. Neoadjuvant chemotherapy and primary debulking surgery utilization for advanced-stage ovarian cancer at a comprehensive cancer center. Gynecol Oncol. 2016;140(3):436–442. doi: 10.1016/j.ygyno.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kessous R., Laskov I., Abitbol J. Clinical outcome of neoadjuvant chemotherapy for advanced ovarian cancer. Gynecol Oncol. 2017;144(3):474–479. doi: 10.1016/j.ygyno.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 24.Luyckx M., Leblanc E., Filleron T. Maximal cytoreduction in patients with FIGO stage IIIC to stage IV ovarian, Fallopian, and peritoneal cancer in day-to-day practice: a retrospective French multicentric study. Int J Gynecol Cancer. 2012;22(8):1337–1343. doi: 10.1097/IGC.0b013e31826a3559. [DOI] [PubMed] [Google Scholar]
- 25.Cancer Genome Atlas Research Network Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verhaak R.G.W., Tamayo P., Yang J.-Y. Prognostically relevant gene signatures of high-grade serous ovarian carcinoma. J Clin Invest. 2013;123(1):517–525. doi: 10.1172/JCI65833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konecny G.E., Wang C., Hamidi H. Prognostic and therapeutic relevance of molecular subtypes in high-grade serous ovarian cancer. J Natl Cancer Inst. 2014;106(10):dju249. doi: 10.1093/jnci/dju249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leong H.S., Galletta L., Etemadmoghadam D. Efficient molecular subtype classification of high-grade serous ovarian cancer: molecular subtype classification of ovarian cancer. J Pathol. 2015;236(3):272–277. doi: 10.1002/path.4536. [DOI] [PubMed] [Google Scholar]
- 29.Detroz B., Laurent S., Honoré P., Blaffart F., Limet R., Meurisse M. Rationale for hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment or prevention of peritoneal carcinomatosis. Acta Chir Belg. 2004;104(4):377–383. [PubMed] [Google Scholar]
- 30.de Bree E., Rosing H., Filis D. Cytoreductive surgery and intraoperative hyperthermic intraperitoneal chemotherapy with paclitaxel: a clinical and pharmacokinetic study. Ann Surg Oncol. 2008;15(4):1183–1192. doi: 10.1245/s10434-007-9792-y. [DOI] [PubMed] [Google Scholar]
- 31.Koole S.N., Driel W.J., Sonke G.S. Hyperthermic intraperitoneal chemotherapy for ovarian cancer: the heat is on. Cancer. 2019;125(S24):4587–4593. doi: 10.1002/cncr.32505. [DOI] [PubMed] [Google Scholar]