Abstract
Purpose
The aim of this article is to define the place of new endovascular methods for the management of pulmonary embolisms (PE), on the basis of a multidisciplinary consensus.
Method and results
Briefly, from the recent literature, for high-risk PE presenting with shock or cardiac arrest, systemic thrombolysis or embolectomy is recommended, while for lowrisk PE, anticoagulation alone is proposed. Normo-tense patients with PE but with biological or imaging signs of right heart dysfunction constitute a group known as “at intermediate risk” for which the therapeutic strategy remains controversial. In fact, some patients may require more aggressive treatment in addition to the anticoagulant treatment, because approximately 10% will decompensate hemodynamically with a high risk of mortality. Systemic thrombolysis may be an option, but with hemorrhagic risks, particularly intra cranial. Various hybrid pharmacomechanical approaches are proposed to maintain the benefits of thrombolysis while reducing its risks, but the overall clinical experience of these different techniques remains limited. Patients with high intermediate and high risk pulmonary embolism should be managed by a multidisciplinary team combining the skills of cardiologists, resuscitators, pneumologists, interventional radiologists and cardiac surgeons. Such a team can determine which intervention – thrombolysis alone or assisted, percutaneous mechanical fragmentation of the thrombus or surgical embolectomy – is best suited to a particular patient.
Conclusions
This consensus document define the place of endovascular thrombectomy based on an appropriate risk stratification of PE.
Keywords: Thrombectomy, Pulmonary embolism, Thrombolytic therapy, Pharmacomechanical thrombectomy, Embolectomy
Thrombectomy; Pulmonary embolism; Thrombolytic therapy; Pharmacomechanical thrombectomy; Embolectomy.
1. Introduction
Currently, it is obvious that acute occlusion of coronary, cerebral or peripheral arteries generally calls for rapid action to restore the distal blood flow, most often using an endovascular approach. However, the guidelines on how to manage pulmonary embolism (PE) remain a matter of controversy. The lack of consensus is related to the numerous pharmacomechanical treatment options that have been developed, yet insufficiently validated. Indeed, unlike acute coronary syndrome, little clinical data are available on these techniques, and recommendations are generally provided by expert consensus based only on a limited number of cases.
The aim of this article is to outline the various interventional therapies described for pulmonary embolism and discuss their place in the management of this disease.
2. Pulmonary embolism: background
PE is the third leading cause of cardiovascular mortality in the United-States, after myocardial infarction and stroke [1, 2]. The estimated mortality is 9–11% and 9–17% at 30 and 90 days, respectively, with between 100,000 and 200,000 deaths annually in the United States [1, 3]. Furthermore, in 1–3% of cases, pulmonary embolism results in chronic thromboembolic pulmonary hypertension [4].
The management of PE is based on the severity of its haemodynamic, cardiac and respiratory consequences. A wide spectrum of symptoms are associated with acute PE, ranging from patients who are asymptomatic to those who present with sudden death. Patients who survive cardiac arrest or cardiogenic shock with hypotension represent about 3% of all PE patients and are considered as having high-risk PE (HRPE). These patients require immediate life-saving reperfusion to reduce morbidity and mortality. On the contrary, nearly 40% of patients experience low-risk PE defined by limited symptoms, a lack of haemodynamic or respiratory compromise, the absence of cardiorespiratory comorbidities or cancer, haemodynamic and respiratory stability, and no right ventricular dysfunction. The morbidity and mortality associated with low-risk PE is minimal and therefore conservative management by anticoagulant therapy alone is recommended [5].
However, most PE patients fall somewhere between these two extremes in the intermediate-risk (IHRPE) category (called “submassive” in the American Heart Association Scientific Statement). Patients in this group are normotensive but display at least one sign of right ventricular (RV) strain. Clinical practice guidelines distinguish two subgroups within this category. Patients with acute PE without haemodynamic instability but who display evidence both of RV strain on imaging and an elevated biomarker level are classified as intermediate-high-risk. Patients who display a single sign of RV dysfunction (either a biomarker or an imaging feature) or no signs but a Pulmonary Embolism Severity Index (PESI) ≥ 3 or simplified PESI (sPESI) > 0 belong to the intermediate-low-risk group [6].
Most risk stratification algorithms use multiple parameters to assess RV strain. Biomarker levels, such as troponin, B-type natriuretic peptide (BNP) and N-terminal pro BNP (NT-proBNP) provide evidence of myocardial injury. Echocardiography is used to assess RV dilation, global and local RV systolic dysfunction, tricuspid valve regurgitation, paradoxical interventricular septal motion, and blood pressure measurement in the pulmonary arteries.
Computed tomography (CT) is a key component in the diagnosis and assessment of the impact of PE, providing information on VR dilation and the shape of the septum. Furthermore, different post-treatment tools provide both functional and morphological evaluations of pulmonary thromboembolism. Compared to standard CT, latest generation (in particular, dual source CT systems) offers better image quality with reduced radiation dose, while still offering improved functional and quantitative analysis capacities [7].
However, assessment of the severity of PE using CT has not been found to correlate with morbidity and mortality. Various electrocardiographic changes, such as the inversion of T waves in the precordial (chest) leads, are indicative of RV strain.
Three scientific societies (American Heart Association, American College of Chest Physicians, European Society of Cardiology) [8, 9] have therefore included assessment of the PE-related risk in their guidelines, mainly based on haemodynamic instability and right ventricular dysfunction, and treatment is based on this stratification (Figure 1) [9].
Figure 1.
Risk stratification in PE. Patients with PE are classified according to haemodynamic consequences, biomarker levels and imaging findings. High-risk patients have acute haemodynamic failure, including cardiac arrest and cardiogenic shock. Patients with paradoxical bradycardia, ventricular tachycardia and relative hypotension (systolic blood pressure <90 mmHg) are included in this group. Normotensive patients with elevated cardiac biomarkers (troponin and natriuretic peptides) or CT/ultrasound evidence of right ventricular strain (dilation, interventricular septum bulging or right ventricular systolic dysfunction) are assigned to the intermediate-risk group. The low-risk group comprises patients without haemodynamic instability, with normal levels of RV strain biomarkers and low prognostic index scores (simplified pulmonary embolism severity index, sPESI).
All-cause mortality for intermediate-risk PE is approximately 1.9–2.9% at 7 days, 4.9–6.6% at 30 days and 14.5% at 90 days [10, 11]. The randomised PEITHO study that included over 1,000 intermediate-high risk PE patients reported a mortality of approximately 1.5% at 7 days and 2.8% at 30 days [12]. These estimated mortality rates have a significant impact on the decision algorithm because invasive procedures may also be associated with risks for the patient.
On the whole, the positive predictive value of ventricular strain alone is insufficient to predict haemodynamic deterioration or treatment intensification and justify invasive management of all intermediate-risk PE patients [3]. Nevertheless, some intermediate-risk PE patients may require urgent measures to restore stability, and about 10% of normotensive patients with RV strain decompensate suddenly [13].
Unfortunately, predictive factors that would help to prospectively identify such patients remain elusive and further research is required [3].
Although, gender-specific data are limited, there are several important differences between women and men in the clinical presentation and laboratory results that may influence the diagnosis and treatment of PE [14, 15] Overall, compared to men, women are older, with a higher severity, resulting in a poor prognosis. Men have hemoptysis, chest pain, fever, and pneumonia more often, and are less likely to experience cardiac decompensation early in the disease. Women have a significantly higher risk of major bleeding, suggesting the need for careful monitoring of anticoagulation. However, death and major bleeding rates at 6 months are similar in both sexes.
Using risk stratification markers, such as right ventricular dysfunction on TTE/CT, tachycardia, hypoxia and specific biomarker cutoff values, algorithms are able to predict adverse effects in both sexes (15) [6].
3. State of the art of reperfusion therapies
3.1. Systemic thrombolysis
Systemic thrombolysis remains the method of choice for severe high-risk pulmonary embolism. It accelerates the breakdown of clots in acute PE, lowering pulmonary artery pressure faster and reducing morbidity and premature mortality compared with anticoagulant therapy alone. However, it is associated with a higher risk of bleeding, especially intracranial haemorrhage (2–3%)—a fourfold increase compared with that of anticoagulant alone, particularly in patients aged over 65 years [16].
There are therefore various absolute or relative contraindications to systemic thrombolysis (Table 1).
Table 1.
Contraindications to thrombolytic therapy (American College of Chest Physicians).
| Absolute contraindications: |
| Structural intracranial abnormality |
| History of intracranial haemorrhage |
| Ischaemic stroke within 3 months |
| Active bleeding |
| Recent brain or spine surgery |
| Recent head trauma with fracture or brain damage |
| Active haemorrhagic syndrome |
| Relative contraindications: |
| Systolic blood pressure > 180 mmHg |
| Diastolic blood pressure > 110 mmHg |
| Recent bleeding (other than intracranial) |
| Recent surgery |
| Recent invasive procedure |
| Ischaemic stroke > 3 months |
| Oral anticoagulant therapy |
| Resuscitation following cardiopulmonary trauma |
| Pericarditis or pericardial effusion |
| Diabetic retinopathy |
| Advanced liver disease |
| Pregnancy |
| Age > 65 years, especially > 75 years |
3.2. Interventional therapies for pulmonary embolism
In theory, the invasive removal of a clot from an obstructed pulmonary artery should enhance reperfusion and restore distal flow earlier. A lung that is ventilated but not perfused is dead space. Recanalisation therefore improves oxygen levels by correcting the V/Q mismatch. It also reduces pulmonary vascular resistance, which lowers the RV afterload and helps the RV provide sufficient preload to the left ventricle and maintain cardiac output. The main trials performed for pulmonary embolism and the main general reviews on treatment options are summarized in Tables 2 and 3, respectively.
Table 2.
Main pulmonary embolism trials.
| Trial | N | Randomisation | Comparison | Follow-up time (days) | Intermediate-risk PE, n (%) | High-risk PE, n (%) | Efficacy |
|---|---|---|---|---|---|---|---|
| PEITHO, 2014 [10] Systemic fibrinolysis |
1006 | Tenecteplase, systemic (30–50 mg) | Heparin/LMWH Fondaparinux |
30 | 1005 (100) | 0 (0) | Mortality/Cardiac decompensation at d7: tenecteplase 2.6% vs placebo 5.6% (odds ratio: 0.44; 95% CI: 0.23–0.87; P = 0.02) |
| PERFECT, 2015 [22] Endovascular thrombolysis |
101 | tPA or urokinase + catheter-directed | Single arm | 30 | 73 (72) | 28 (28) | PASP 51.17 ± 14.06 to 37.23 ± 15.81 mmHg (P < 0.0001) |
| ULTIMA, 2013 [9] Ultrasound |
59 | tPA (20 mg) + ultrasound | Heparin | 90 | 59 (100) | 0 (0) | Reduction of RV/LV ratio from 1.28 ± 0.19 to 0.99 ± 0.17 at 24 h (P < 0.001) |
| SEATTLE II, 2015 [21] Ultrasound |
150 | tPA (24 mg) + ultrasound | Single arm | 30 | 119 (79) | 31 (21) | Reduction of RV/LV ratio from 1.55 to 1.13 at 48 h (P < 0.0001), reduction of PASP from 51.4 to 36.9 mmHg (P < 0.0001) at 48 h |
| OPTALYSE PE, 2018 [25] Ultrasound |
101 | tPA (8–24 mg) + ultrasound | 4 different protocols/tPA | 3 | 101 (100) | 0 (0) | Reduction of RV/LV ratio in both arms |
| FLARE, 2019 Mechanical [39] |
106 | FlowTriever Pulmonary Embolectomy |
Single arm | 30 | 104 (100) | 0 (0) | Reduction of RV/LV ratio from 1.53 to 1.15 at 48 h |
tPA: tissue plasminogen activator; LMWH: low molecular weight heparin; CI: confidence interval; PASP: pulmonary artery systolic pressure; RV: right ventricle; LV: left ventricle.
Table 3.
Main general reviews and meta-analyses on treatment options for pulmonary embolism.
| Authors, year | Period covered by review | Aim and main points of the review | Number of studies included |
|---|---|---|---|
| Kuo et al, 2009 [35] | January 1990–September 2008 | Non-comparative data meta-analysis Efficacy and safety of catheter-directed treatment for massive pulmonary embolism (thrombolysis, mechanical, aspiration, catheter-directed thrombolysis) |
N = 35 |
| Engelberger et al, 2014 [18] | ND | General review of efficacy and safety data on the use of ultrasound-assisted thrombolysis in acute pulmonary embolism. Pooled analysis (non-comparative data meta-analysis) of the data from the main studies | N = 7 |
| Ierardi et al, 2015 [40] | January 2000–June 2015 | General review of clinical experience worldwide with pharmacomechanical thrombolysis (AngioJet™) | N = 19 |
| Bajaj et al, 2016 [39] | 1966–September 2015 | Meta-analysis of the use of catheters for acute pulmonary embolism | N = 62 |
| Mostafa et al, 2016 [19] | 2008–September 2015 | Non-comparative data meta-analysis Efficacy and safety of ultrasound-assisted thrombolysis for acute pulmonary embolism |
N = 11 |
| Zarghouni et al, 2016 [41] | ND | General review of different catheter-directed methods to treat pulmonary embolism and results of the main studies in the literature according to the authors | N = 7 |
ND: not determined.
3.3. General concept
Once the decision to resort to an endovascular approach has been made, the first step is pulmonary artery catheterisation. The preferred route is generally femoral or jugular, but sometimes upper limb veins are used. Two catheters wire may be placed within the same vein if more than one catheter is required.
Right heart and pulmonary artery blood pressures must be measured to monitor the patient's haemodynamic response. Selective pulmonary angiography is generally performed to localise the target embolus and provides guidance for endovascular clearing of the obstruction.
Following treatment, patients are cared for in the intensive care unit to monitor their haemodynamic and respiratory status and detect any potential procedure-related complications, such as bleeding. All patients receive anticoagulant therapy (intravenous unfractionated heparin or low-molecular-weight heparin). Early echocardiography is helpful to assess the outcome.
It is important to emphasise that all catheter procedures require specific expertise in the endovascular field, with a resuscitation team trained to manage dysrhythmia and cardiogenic shock.
3.4. Endovascular treatment
The available treatment options are catheter-directed thrombolysis, thrombectomy or a combination of both. The aim is to reduce vascular occlusion and improve the patient's clinical status more rapidly than by peripheral venous administration of thrombolytics with a lower risk of bleeding. The main limiting factors are the size and volume of the clots, the age of the clots (older clots tend to reorganise and adhere to the vessel wall) and the patient's haemodynamic and respiratory status. The individual risks related to thrombolysis, cardiopulmonary instability and the time required for the procedure must also be taken into account.
Endovascular clot clearance methods can be classified into endovascular thrombolysis and mechanical or pharmacomechanical thrombectomy techniques (Table 4).
-
•
Catheter-directed thrombolysis (CDT):
Table 4.
Endovascular treatment options for pulmonary embolism.
| Devices | |
|---|---|
|
Endovascular thrombolysis | |
| Catheter-directed thrombolysis (CDT) |
|
| Thrombolysis and ultrasound | EkoSonic® (EKOS) |
| Pharmacomechanical thrombolysis |
AngioJet 6 F PE Power PulseTM (Boston Scientific), power injection of fibrinolytics (“pulse spray”) |
|
Mechanical thrombectomy | |
| Fragmentation |
|
| Direct aspiration |
|
| Aspirex rotational thrombectomy | Aspirex thrombectomy (Straub Medical) |
| Rotational thrombectomy (motorised rotating sinusoidal wire) | Cleaner Rotational Thrombectomy System (Argon Medical Devices) with or without fibrinolysis |
| Rheolytic thrombectomy (Venturi effect) | AngioJet 6 F PE® (Boston Scientific) with or without fibrinolysis |
| Other thrombectomy techniques |
|
Endovascular thrombolysis by placement of a catheter within the pulmonary artery offers the advantage of delivering a high local concentration of fibrinolytic agent to a larger surface of the clot. The dose used is much lower than with peripheral administration and its systemic fibrinolytic effect is therefore much reduced. A routine-use diagnostic angiography catheter or a specific multihole catheter (AngioDynamics) may be used to increase the concentration of the fibrinolytic agent (Figure 2). In theory, this enhances the efficiency and safety of fibrinolysis, thus reducing the risk of bleeding. Nevertheless, the guidelines issued by the American College of Chest Physicians recommend endovascular thrombolysis only for some specific patient groups, such as patients with a higher risk of bleeding, in addition to percutaneous thrombectomy in hypotensive patients, if systemic thrombolysis fails, or in patients with shock that could result in death before systemic thrombolysis is effective [17]. Typically, a multihole catheter is pushed through the clot in each pulmonary artery and infuses a fibrinolytic agent, such as tissue plasminogen activator (tPA), at a rate of 0.5–1 mg/h per catheter when 2 catheters are used, or 0.5–2 mg/h when only 1 catheter is used. Perfusion generally lasts 12–24 h, so the average total dose is 20–28 mg. Efficient systemic administration of heparin is continued throughout the endovascular fibrinolysis procedure.
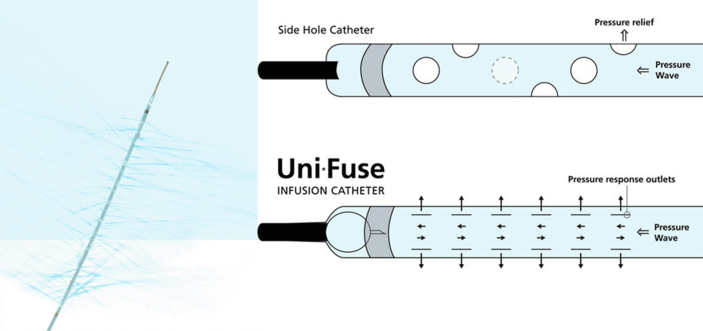
Figure 2.
Multihole infusion catheter (AngioDynamics, Inc.). Multihole infusion catheter with side holes for delivery of fibrinolytics within the thrombus using a simple wire that occludes the tip of the catheter in order to deliver the fluid through the side holes.
Despite the lack of a randomised trial comparing endovascular and systemic thrombolytic therapy, several extensive cost-benefit studies have been carried out. In a multicentre registry analysis, Bloomer et al. found that intracranial haemorrhage occurred in 1.5% and 0.35% of cases and major complications in 9.5% and 4.65% (generally transfusions) for massive and submassive PE, respectively [18]. Moreover, a national registry (National Readmission Database) consisting of 3,107 patients who received systemic thrombolysis and 1,319 patients treated with CDT, revealed that the rates of mortality (14.9% vs 6.12%), bleeding-related mortality (18.1% vs 8.4%) and readmission (10.6% vs 7.6%) were higher in the systemic thrombolysis group compared with the CDT group [19]. These results suggest that the risk of fatal bleeding complications is lower with CDT than with systemic thrombolysis. This is perhaps due to the approximately fourfold higher dose of fibrinolytics used during systemic administration. Nevertheless, these findings do not originate from randomised studies and should therefore be interpreted with caution. In addition, it should be noted that endovascular fibrinolysis is gradually being discontinued for stroke and acute coronary syndrome, due to the lack of evidence demonstrating its superiority over systemic fibrinolysis.
-
•
Ultrasound-assisted catheter-directed thrombolysis:
To improve the efficacy and speed of clot clearance, fibrinolysis can be combined with low-intensity ultrasound waves using a multihole catheter (EkoSonic Endovascular System, EKOS Corp) in an approach called ultrasound-assisted thrombolysis (Figure 3) [20, 21, 22]. Briefly, ultrasound changes the fibrin network structure and enhances thrombolysis.
Figure 3.
Ekos system. The Ekos system consists of a multihole infusion catheter, an ultrasonic core and a control unit (Ekos Corp.).
The randomised ULTIMA trial demonstrated that ultrasound-assisted thrombolysis resulted in a faster decrease of the RV/LV ratio in patients with acute intermediate-risk PE compared with anticoagulant therapy alone, and without any major bleeding. Yet, no significant change in the 90-day mortality was observed in this trial [23]. Similar results were reported in the prospective SEATTLE II trial, but with major bleeding in 10% of patients, although no fatal intracranial bleeds [24, 24]. Nevertheless, at present, there is no clear evidence demonstrating the benefit of ultrasound-enhanced thrombolysis over standard catheter-directed thrombolysis using the same dose of thrombolytics (PERFECT registry) [25]. On the contrary, the procedure and fluoroscopy times are significantly longer than for standard CDT [26]. Globally, compared with three randomised trials on systemic thrombolysis, mortality rates were similar but less major bleeding complications were observed [27, 28].
A recent meta-analysis of trials assessing this technique reported a large decrease in mean pulmonary artery pressure and a reduction in the RV/LV ratio in patients with high-risk PE (HRPE) and intermediate–high-risk PE (IHRPE) compared with heparin alone. In this meta-analysis, the rates of major and intracerebral bleeding were low (<0.5%) [28, 29].
-
•
Mechanical thrombectomy:
a. Fragmentation
Many techniques, including mechanical balloon fragmentation or motorised rotating pigtail catheters, have been used to break up thrombi in pulmonary arteries [25, 30]. The fragmentation of the thrombus and its migration into the distal pulmonary vascular system reduces global pulmonary vascular resistance and RV afterload. The increased contact surface of the thrombus fragments could enhance the efficacy of thrombolysis. Catheter aspiration is often combined with fragmentation to decrease the risk of distal embolisation by the fragmented thrombi, which could worsen the patient's clinical status or lead to chronic pulmonary hypertension [30].
Two specific fragmentation devices have been evaluated: a mechanical rotating thrombectomy device driven by compressed air or nitrogen (Helix Clot Buster, Ev3 Covidien), and a rotating thrombectomy system consisting of a motorised sinusoidal wire in a 6 or 7 F catheter (Cleaner Rotational Thrombectomy System, Argon Medical Devices).
Results with small numbers of patients confirm the feasibility of mechanical thrombectomy in high-risk PE, however it is difficult to assess their efficacy without data from controlled studies. Moreover, there have been several reports of haemodynamic deterioration following such procedures [30, 31].
b. Aspiration technique
-
-
Manual aspiration can be performed by placing catheters or large sheaths in the pulmonary arteries. One main limitation is the small catheter diameter compared with the size and volume of clots in patients with proximal PE. Specialised catheters, such as the Pronto thrombectomy catheter (Vascular Solutions) have been developed for manual aspiration.
-
-
The AngioVac Venous Drainage System (AngioDynamics) includes a funnel-shaped proximal balloon attached to a 22 F aspiration cannula (Figure 4). This system requires a 26 F introducer sheath in one vein and an 18 F reinfusion cannula in another. The 22 F cannula, inserted via the 26 F introducer sheath, is connected to a centrifugal pump and acts as an extracorporeal bypass by redirecting blood to the reinfusion cannula. The aspirated thrombi are retained on a filter and the blood reinfused. The AngioVac has had little success due to its unwieldy procedure and complications caused by the size and rigidity of the system, which is not easy to manoeuvre in the RV and pulmonary arteries [32].
-
-
The Indigo mechanical thrombectomy system (Penumbra) consists of an 8 F aspiration catheter connected to an aspiration pump and containing a metal wire with an olive-shaped distal tip that both maintains aspiration catheter permeability by facilitating aspiration and fragments the thrombus (Figure 5). Straight and curved catheters are available to enhance efficacy. Preliminary tests in a small number of proximal PE patients have shown promising results, with haemodynamic improvement and an 80% survival rate [33].
-
-
The FlowTriever System (Inari Medical) is a 3-component device consisting of a thrombectomy catheter with 3 nitinol disks, a retraction device for pushing and opening the disks into the clot, and a 20 F aspiration cannula (Figure 6). Access is provided via a 22 F introducer sheath and the catheter is pushed through the aspiration cannula into the thrombus. Once out of the cannula, the disks are deployed and facilitate clot aspiration by the aspiration cannula in a similar way to stent retriever thrombectomy for stroke treatment [34, 35, 36]. A recent prospective multicentre trial demonstrated the efficacy and safety of the FlowTriever system with a major complication rate of 3.8% and the need for adjunctive thrombolytics in only 2% of patients [36].
Figure 4.
AngioVac catheter (AngioDynamics) removes thrombi by pumping and filtering the patient's blood and then reinfusing it via a reperfusion circuit. This large-bore device is designed to be inserted via the femoral vein and used for removal by aspiration of large thrombi from the pulmonary arteries. The funnel-shaped balloon on its distal tip is used to occlude the vein in which it is placed.
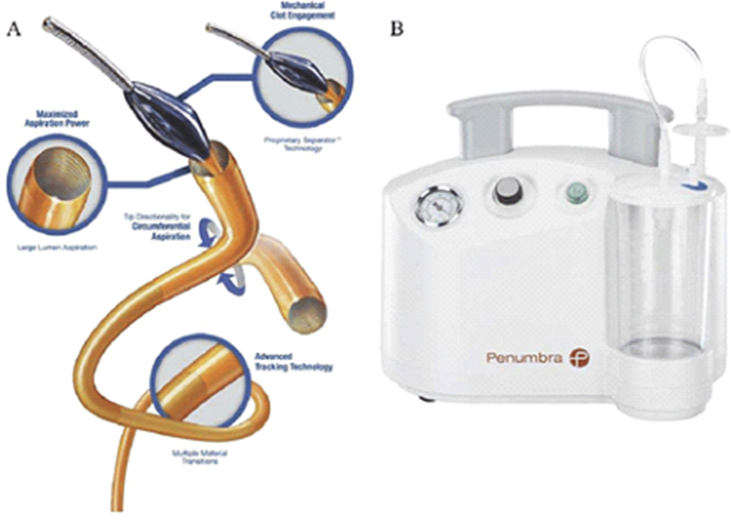
Figure 5.
Penumbra INDIGO system (Penumbra Inc.): Penumbra's Indigo CAT 8 system is a flexible 8 F aspiration catheter connected to a continuous vacuum system. The catheter's lumen contains a wire with an olive-shaped distal tip that enhances clot fragmentation and recovery.
Figure 6.
The FlowTriever device (Inari Medical) has 3 nitinol mesh disks that deploy into the thrombus and draw it into the catheter by manual aspiration.
c. Fragmentation with aspiration
-
-
The Aspirex thrombectomy catheter (Straub Medical AG) is widely used for peripheral embolism and creates negative pressure and suction using the Archimedes' screw principle via a rapidly rotating steel propeller within the catheter. The bevelled tip of the device dislocates the clot and fragments are removed through side holes on the catheter (Figure 7). However, so far, only few retrospective studies are available for this indication [37].
-
-
The AngioJet peripheral rheolytic thrombectomy catheter (Boston Scientific) is a pharmacomechanical thrombolysis device. It first enables catheter-directed thrombolysis by power-pulsed injection of thrombolytics into the thrombus to enhance its efficacy, then removes the thrombus mechanically. Its action is based on the high-speed injection of saline via the tip of the catheter. Retropulsion of pressurised saline through the distal catheter windows creates a vacuum by Bernoulli effect and the clot is fragmented, drawn into the catheter and removed via the return lumen (Figure 8). Although very efficient for peripheral venous thrombosis, a number of issues are associated with its use for PE [38]. Indeed, despite many reports of good clinical results, various serious complications have been described, in particular bradycardia, arrhythmia, hypotension and haemoglobinuria associated in rare cases with kidney failure [39, 40, 41]. These complications may result from the haemolysis caused by clot fragmentation which may trigger massive release within the pulmonary arteries of neurohormonal agents such as adenosine and bradykinin. This, combined with concomitant stretch-induced receptor activation in the pulmonary arteries, is considered to represent the main cause of bradyarrhythmia and hypotension observed during clot removal procedures. Another haemolysis-related problem is hyperkalaemia that can exacerbate electrical instability and result in serious ventricular arrhythmia, while haemoglobinuria further deteriorates kidney function which is generally already impaired by the low cardiac output that characterises shock. Mitigation measures to reduce such effects include temporary cardiac pacing, at the beginning or during the procedure, as well as the administration of drugs such as catecholamines and aminophylline.
It is important to note, however, that these results are somewhat dated, with a device and technique that have evolved with time [40, 41, 42].
Figure 7.
ASPIREX (Straub). Catheter with a worm screw beneath a side opening at its distal end. Thrombectomy is achieved by rotating the screw which draws the thrombus into the catheter.
Figure 8.
AngioJet rheolytic thrombectomy (Boston Scientific). Pressure-pulsed delivery of saline at the catheter's distal tip fragments the thrombus and creates a Venturi effect that aspirates and removes the fragments via a second lumen.
Besides endovascular methods, two other treatment options for PE are surgical embolectomy and extracorporeal membrane oxygenation (ECMO).
3.5. Surgical pulmonary embolectomy
For decades, surgical pulmonary embolectomy results were associated poor outcome as it is performed only as a lifesaving therapy. It reappears today, especially when other methods are contraindicated or fail and when the patient presents a relatively low surgical risk.
In practice, the results of surgical thrombectomy vary greatly depending on the selection of patients, the time of implementation and the experience of the surgical and medical teams. Surgical management can induce serious potential complications, including atrial fibrillation, tamponade, and right heart failure. In-hospital mortality rates of 2 recent studies with intermediate and high risk PE was range 6.6–11.7% [43, 44], and the 30-day mortality was only 4.6 % in a third study [45]. Thus, surgical embolectomy is useful to treat patients with large proximal or intracardiac thrombi with a risk of paradoxical embolism via a patent foramen ovale, in expert surgical centers.
3.6. Mechanical circulatory support
To provide temporary cardiopulmonary support to patients with acute cardiopulmonary failure prior to surgical or endovascular thrombectomy, extracorporeal veno-arterial membrane oxygenation (VA-ECMO) can be used [46]. Practically, the patient's venous blood is pumped through an oxygenator and then reinjected into the arterial system. It very quickly reduces the afterload on the failed RV and provides efficient cardiac output. A recent large retrospective multicenter HRPE-focused series suggested that standalone VA-ECMO are associated with higher mortality than ECMO plus reperfusion therapy (relative risk of death from all causes at 30 days 1.47, 95% confidence interval 0.98–2.20; P = 0.06).
With the percutaneous cannulation techniques and the improved integrated systems, ECMO is now easier and faster to perform, but it still requires specialized facilities and experienced ECMO physicians at the bedside, with a multidisciplinary team. Thus, given the high rate of complications (hemorrhage and infection, especially when used over longer periods), VA-ECMO can be considered in combination with endovascular or surgical thrombectomy in refractory circulatory collapse or cardiac arrest; it may be discussed in patients with HRPE or IHRPE who deteriorate and when thrombolysis is contraindicated or has failed. In the latest ESC recommendations of 2019, ECMO was classified as “may be considered” (class IIb and level of evidence C), but is derived from observational data and not supported by randomized trials [2].
3.7. A multidisciplinary approach to PE management
PE is at the intersection of several specialties, as for the management of stroke or acute coronary syndrome. Many hospitals have set up multidisciplinary interventional teams (Multidisciplinary PE Response Teams (PERTs) that include all the potentially involved specialties (cardiology, intensive care, interventional radiology and surgery), as well as specialists in haemostasis and imaging. These teams work in a consensual manner to optimise the management of patients with PE and hence overcome the flaws of risk stratification, the absence of a single optimal treatment for high-risk and intermediate-risk PE and the lack of data on innovative alternatives.
In addition, such teams can help in decisions about timing and type of reperfusion therapy. PERTs should work within an organized regional network of care with other hospitals, and in coordination with emergency transport services to help to diagnose and classify acute PE, as well as to select patients who should be transferred to the PE centre and/or require reperfusion and to decide on the optimal technique. Within a PE centre, all reperfusion therapies should be available with staff members trained and ableto deal with all of them and their complications around the clock. Consistently, the recent guidelines on PE support recommend the development of such PERTs and centres (grade IIa recommendation [6]).
As a whole, the current guidelines for the management of PE are as follows.
-
-
For high-risk PE, the treatment of choice is IV thrombolytic therapy (bolus and infusion) (class I, evidence level B). For high-risk PE, percutaneous mechanical thrombectomy has recently been classified as class IIa and level of evidence C (ESC 2019 [6] and AHA guidelines [9]), and class IIC in the ACCP guidelines [17]. This term encompasses all aspiration thrombectomy, thrombus fragmentation and rheolytic thrombectomy techniques which form a contrasted group.
-
-
For intermediate-risk PE, thrombolysis is only recommended in the event of haemodynamic deterioration during anticoagulant therapy (class I, level of evidence A). Surgical or endovascular thrombectomy should be considered in the event of haemodynamic deterioration during anticoagulant therapy (class IIa, level of evidence B). Routine use of thrombolysis is not recommended for intermediate-risk or low-risk PE (class III, level of evidence C).
Finally, the patient's quality of life could influence clinical strategy and must be evaluated. Thus, in order to allow a complete assessment of patients with a history of venous thromboembolism, health-related quality of life (HRQoL), using generic and disease-specific questionnaires, should be added in clinical trials. In a meta-analysis on the quality of life after PE, 584 patients, with a minimum follow-up of one year after PE, were compared to matched population norms, by using the Physical and the Mental Component Score (PCS and MCS) [43]. Although patients report a comparable quality of life (both generic and disease-specific) after a DVT event, patients with PE experience long-term physical health impairment compared to the general population. Persistent dyspnea after PE has been shown to be a predictor of decreased HRQoL.
This information could improve the ability of providers to educate patients about the potential long-term impact of venous thromboembolism events and their ability to weigh the risks and benefits of different prophylactic and treatment regimens.
4. Conclusions
Risk stratification is the first crucial step in PE management. For high-risk PE, systemic thrombolytic therapy is the appropriate first-line treatment [6]. If thrombolysis is contraindicated due to a high risk of bleeding, or if the patient's condition is too critical to wait for intravenous reperfusion of fibrinolytics (mainly cardiac arrest), then surgical or percutaneous mechanical thrombectomy may be considered to achieve haemodynamic stability quickly. Moreover, rescue surgical and percutaneous thrombectomy should be considered if intravenous thrombolysis fails.
The newly developed percutaneous techniques have promising potential in the care of such patients, as they provide quick and significant haemodynamic improvements with low rates of bleeding. There are many interventional strategies for acute PE, but for most only limited efficacy and safety data are available to date. Such studies are very often single-centre and based on a small number of cases. A few controlled, randomised trials have been performed, but with limited sample sizes and haemodynamic stability as the endpoint [5, 33, 43, 44, 45, 46].
By contrast, available data does not support the routine use of systemic thrombolytic therapy or endovascular procedures for patients with intermediate-high-risk PE. The decision to opt for interventional treatment should be made on a case-by-case basis and requires assessment of potential haemodynamic deterioration, RV dysfunction or respiratory impairment and the risk of complications. On the contrary, when the clinical condition deteriorates, some patients may be eligible for percutaneous thrombectomy.
Randomized studies to compare percutaneous thrombectomy and systemic thrombolysis using clinical criteria (mortality, bleeding complications, etc.) are therefore mandatory to guide clinicians. The target populations for such studies are unstable patients and/or patients at very high risk of deterioration. Future investigations should also include a cost-effectiveness analysis and the quality of life (taking into account the cost of human and hospital resources) of the expected benefits for patients. The results of these studies will help to better define the role of catheter-directed thrombectomy in pulmonary embolism.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
No data was used for the research described in the article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Park B., Messina L., Dargon P., Huang W., Ciocca R., Anderson F.A. Recent trends in clinical outcomes and resource utilization for pulmonary embolism in the United States: findings from the nationwide inpatient sample. Chest. 2009;136(4):983–990. doi: 10.1378/chest.08-2258. [DOI] [PubMed] [Google Scholar]
- 2.Konstantinides S.V., Meyer G., Becattini C., Bueno H., Geersing G.J., Harjola V.P. ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): the Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC) Eur. Respir. J. 2019;54(3) doi: 10.1183/13993003.01647-2019. 2019. [DOI] [PubMed] [Google Scholar]
- 3.Konstantinides S.V., Torbicki A., Agnelli G., Danchin N., Fitzmaurice D., Galie N. Corrigendum to: 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur. Heart J. 2015;36(39):2642. doi: 10.1093/eurheartj/ehu479. [DOI] [PubMed] [Google Scholar]
- 4.Kahn S.R., Houweling A.H., Granton J., Rudski L., Dennie C., Hirsch A. Long-term outcomes after pulmonary embolism: current knowledge and future research. Blood Coagul. Fibrinolysis. 2014;25(5):407–415. doi: 10.1097/MBC.0000000000000070. [DOI] [PubMed] [Google Scholar]
- 5.Abrahams-van Doorn P.J., Hartmann I.J., Cardiothoracic C.T. one-stop-shop procedure? Impact on the management of acute pulmonary embolism. Insights Imag. 2011;2(6):705–715. doi: 10.1007/s13244-011-0123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konstantinides S.V., Meyer G., Becattini C., Bueno H., Geersing G.J., Harjola V.P. ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS) Eur. Heart J. 2019;41(4):543–603. doi: 10.1093/eurheartj/ehz405. 2020. [DOI] [PubMed] [Google Scholar]
- 7.Lenga L., Trapp F., Albrecht M.H., Wichmann J.L., Johnson A.A., Yel I. Single- and dual-energy CT pulmonary angiography using second- and third-generation dual-source CT systems: comparison of radiation dose and image quality. Eur. Radiol. 2019;29(9):4603–4612. doi: 10.1007/s00330-018-5982-1. [DOI] [PubMed] [Google Scholar]
- 8.Dudzinski D.M., Giri J., Rosenfield K. Interventional treatment of pulmonary embolism. Circ. Cardiovasc. Interv. 2017;10(2) doi: 10.1161/CIRCINTERVENTIONS.116.004345. [DOI] [PubMed] [Google Scholar]
- 9.Jaff M.R., McMurtry M.S., Archer S.L., Cushman M., Goldenberg N., Goldhaber S.Z. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123(16):1788–1830. doi: 10.1161/CIR.0b013e318214914f. [DOI] [PubMed] [Google Scholar]
- 10.Jimenez D., de Miguel-Diez J., Guijarro R., Trujillo-Santos J., Otero R., Barba R. Trends in the management and outcomes of acute pulmonary embolism: analysis from the RIETE registry. J. Am. Coll. Cardiol. 2016;67(2):162–170. doi: 10.1016/j.jacc.2015.10.060. [DOI] [PubMed] [Google Scholar]
- 11.Kucher N., Rossi E., De Rosa M., Goldhaber S.Z. Massive pulmonary embolism. Circulation. 2006;113(4):577–582. doi: 10.1161/CIRCULATIONAHA.105.592592. [DOI] [PubMed] [Google Scholar]
- 12.Meyer G., Vicaut E., Danays T., Agnelli G., Becattini C., Beyer-Westendorf J. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N. Engl. J. Med. 2014;370(15):1402–1411. doi: 10.1056/NEJMoa1302097. [DOI] [PubMed] [Google Scholar]
- 13.Grifoni S., Olivotto I., Cecchini P., Pieralli F., Camaiti A., Santoro G. Short-term clinical outcome of patients with acute pulmonary embolism, normal blood pressure, and echocardiographic right ventricular dysfunction. Circulation. 2000;101(24):2817–2822. doi: 10.1161/01.cir.101.24.2817. [DOI] [PubMed] [Google Scholar]
- 14.Tanabe Y., Yamamoto T., Murata T., Mabuchi K., Hara N., Mizuno A. Gender differences among patients with acute pulmonary embolism. Am. J. Cardiol. 2018;122(6):1079–1084. doi: 10.1016/j.amjcard.2018.05.042. [DOI] [PubMed] [Google Scholar]
- 15.Keller K., Rappold L., Gerhold-Ay A., Hobohm L., Hasenfuss G., Konstantinides S.V. Sex-specific differences in pulmonary embolism. Thromb. Res. 2019;178:173–181. doi: 10.1016/j.thromres.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 16.Chatterjee S., Chakraborty A., Weinberg I., Kadakia M., Wilensky R.L., Sardar P. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. J. Am. Med. Assoc. 2014;311(23):2414–2421. doi: 10.1001/jama.2014.5990. [DOI] [PubMed] [Google Scholar]
- 17.Kearon C., Akl E.A., Ornelas J., Blaivas A., Jimenez D., Bounameaux H. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 18.Bloomer T.L., El-Hayek G.E., McDaniel M.C., Sandvall B.C., Liberman H.A., Devireddy C.M. Safety of catheter-directed thrombolysis for massive and submassive pulmonary embolism: results of a multicenter registry and meta-analysis. Cathet. Cardiovasc. Interv. 2017;89(4):754–760. doi: 10.1002/ccd.26900. [DOI] [PubMed] [Google Scholar]
- 19.Arora S., Panaich S.S., Ainani N., Kumar V., Patel N.J., Tripathi B. Comparison of in-hospital outcomes and readmission rates in acute pulmonary embolism between systemic and catheter-directed thrombolysis (from the national readmission Database) Am. J. Cardiol. 2017;120(9):1653–1661. doi: 10.1016/j.amjcard.2017.07.066. [DOI] [PubMed] [Google Scholar]
- 20.de Winter M.A., Hart E.A., van den Heuvel D.A.F., Moelker A., Lely R.J., Kaasjager K.A.H. Local ultrasound-facilitated thrombolysis in high-risk pulmonary embolism: first Dutch experience. Cardiovasc. Intervent. Radiol. 2019;42(7):962–969. doi: 10.1007/s00270-019-02200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engelberger R.P., Kucher N. Ultrasound-assisted thrombolysis for acute pulmonary embolism: a systematic review. Eur. Heart J. 2014;35(12):758–764. doi: 10.1093/eurheartj/ehu029. [DOI] [PubMed] [Google Scholar]
- 22.Mostafa A., Briasoulis A., Shokr M., Briasouli A.A., Panaich S., Grines C. Ultrasound Accelerated Thrombolysis in patients with acute pulmonary embolism: a systematic review and proportion meta-analysis. Int. J. Cardiol. 2016;211:27–30. doi: 10.1016/j.ijcard.2016.02.148. [DOI] [PubMed] [Google Scholar]
- 23.Kucher N., Boekstegers P., Muller O.J., Kupatt C., Beyer-Westendorf J., Heitzer T. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014;129(4):479–486. doi: 10.1161/CIRCULATIONAHA.113.005544. [DOI] [PubMed] [Google Scholar]
- 24.Piazza G., Hohlfelder B., Jaff M.R., Ouriel K., Engelhardt T.C., Sterling K.M. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. JACC Cardiovasc. Interv. 2015;8(10):1382–1392. doi: 10.1016/j.jcin.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 25.Kuo W.T., Banerjee A., Kim P.S., DeMarco F.J., Jr., Levy J.R., Facchini F.R. Pulmonary embolism Response to fragmentation, embolectomy, and catheter thrombolysis (PERFECT): initial results from a prospective multicenter registry. Chest. 2015;148(3):667–673. doi: 10.1378/chest.15-0119. [DOI] [PubMed] [Google Scholar]
- 26.Graif A., Grilli C.J., Kimbiris G., Agriantonis D.J., Chohan O.Z., Fedele C.R. Comparison of ultrasound-accelerated versus pigtail catheter-directed thrombolysis for the treatment of acute massive and submassive pulmonary embolism. J. Vasc. Intervent. Radiol. 2017;28(10):1339–1347. doi: 10.1016/j.jvir.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Kaymaz C., Akbal O.Y., Tanboga I.H., Hakgor A., Yilmaz F., Ozturk S. Ultrasound-assisted catheter-directed thrombolysis in high-risk and intermediate-high-risk pulmonary embolism: a meta-analysis. Curr. Vasc. Pharmacol. 2018;16(2):179–189. doi: 10.2174/1570161115666170404122535. [DOI] [PubMed] [Google Scholar]
- 28.Tapson V.F., Sterling K., Jones N., Elder M., Tripathy U., Brower J. A randomized trial of the optimum duration of acoustic pulse thrombolysis procedure in acute intermediate-risk pulmonary embolism: the OPTALYSE PE trial. JACC Cardiovasc. Interv. 2018;11(14):1401–1410. doi: 10.1016/j.jcin.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Pei D.T., Liu J., Yaqoob M., Ahmad W., Bandeali S.S., Hamzeh I.R. Meta-analysis of catheter directed ultrasound-assisted thrombolysis in pulmonary embolism. Am. J. Cardiol. 2019;124(9):1470–1477. doi: 10.1016/j.amjcard.2019.07.040. [DOI] [PubMed] [Google Scholar]
- 30.Nakazawa K., Tajima H., Murata S., Kumita S.I., Yamamoto T., Tanaka K. Catheter fragmentation of acute massive pulmonary thromboembolism: distal embolisation and pulmonary arterial pressure elevation. Br. J. Radiol. 2008;81(971):848–854. doi: 10.1259/bjr/93840362. [DOI] [PubMed] [Google Scholar]
- 31.Kumar N., Janjigian Y., Schwartz D.R. Paradoxical worsening of shock after the use of a percutaneous mechanical thrombectomy device in a postpartum patient with a massive pulmonary embolism. Chest. 2007;132(2):677–679. doi: 10.1378/chest.06-1082. [DOI] [PubMed] [Google Scholar]
- 32.Al-Hakim R., Park J., Bansal A., Genshaft S., Moriarty J.M. Early experience with AngioVac aspiration in the pulmonary arteries. J. Vasc. Intervent. Radiol. 2016;27(5):730–734. doi: 10.1016/j.jvir.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 33.De Gregorio M.A., Guirola J.A., Kuo W.T., Serrano C., Urbano J., Figueredo A.L. Catheter-directed aspiration thrombectomy and low-dose thrombolysis for patients with acute unstable pulmonary embolism: prospective outcomes from a PE registry. Int. J. Cardiol. 2019;287:106–110. doi: 10.1016/j.ijcard.2019.02.061. [DOI] [PubMed] [Google Scholar]
- 34.Tukaye D.N., McDaniel M., Liberman H., Burkin Y., Jaber W. Percutaneous pulmonary embolus mechanical thrombectomy. JACC Cardiovasc. Interv. 2017;10(1):94–95. doi: 10.1016/j.jcin.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 35.Wible B.C., Buckley J.R., Cho K.H., Bunte M.C., Saucier N.A., Borsa J.J. Safety and efficacy of acute pulmonary embolism treated via large-bore aspiration mechanical thrombectomy using the inari FlowTriever device. J. Vasc. Intervent. Radiol. 2019;30(9):1370–1375. doi: 10.1016/j.jvir.2019.05.024. [DOI] [PubMed] [Google Scholar]
- 36.Tu T., Toma C., Tapson V.F., Adams C., Jaber W.A., Silver M. A prospective, single-arm, multicenter trial of catheter-directed mechanical thrombectomy for intermediate-risk acute pulmonary embolism: the FLARE study. JACC Cardiovasc. Interv. 2019;12(9):859–869. doi: 10.1016/j.jcin.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 37.Bayiz H., Dumantepe M., Teymen B., Uyar I. Percutaneous aspiration thrombectomy in treatment of massive pulmonary embolism. Heart Lung Circ. 2015;24(1):46–54. doi: 10.1016/j.hlc.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 38.Dopheide J.F., Sebastian T., Engelberger R.P., Haine A., Kucher N. Early clinical outcomes of a novel rheolytic directional thrombectomy technique for patients with iliofemoral deep vein thrombosis. Vasa. 2018;47(1):56–62. doi: 10.1024/0301-1526/a000666. [DOI] [PubMed] [Google Scholar]
- 39.Kuo W.T., Gould M.K., Louie J.D., Rosenberg J.K., Sze D.Y., Hofmann L.V. Catheter-directed therapy for the treatment of massive pulmonary embolism: systematic review and meta-analysis of modern techniques. J. Vasc. Intervent. Radiol. 2009;20(11):1431–1440. doi: 10.1016/j.jvir.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Das S., Das N., Serota H., Vissa S. A retrospective review of patients with massive and submassive pulmonary embolism treated with AngioJet rheolytic thrombectomy with decreased complications due to changes in thrombolytic use and procedural modifications. Vascular. 2018;26(2):163–168. doi: 10.1177/1708538117722728. [DOI] [PubMed] [Google Scholar]
- 41.Latacz P., Simka M., Brzegowy P., Serednicki W., Konduracka E., Mrowiecki W. Treatment of high- and intermediate-risk pulmonary embolism using the AngioJet percutaneous mechanical thrombectomy system in patients with contraindications for thrombolytic treatment - a pilot study. Wideochir Inne Tech Maloinwazyjne. 2018;13(2):233–242. doi: 10.5114/wiitm.2018.75848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villalba L., Nguyen T., Feitosa R.L., Jr., Gunanayagam P., Anning N., Dwight K. Single-session catheter-directed lysis using adjunctive power-pulse spray with AngioJet for the treatment of acute massive and submassive pulmonary embolism. J. Vasc. Surg. 2019;70(6):1920–1926. doi: 10.1016/j.jvs.2019.03.038. [DOI] [PubMed] [Google Scholar]
- 43.Meneveau N., Guillon B., Planquette B., Piton G., Kimmoun A., Gaide-Chevronnay L. Outcomes after extracorporeal membrane oxygenation for the treatment of high-risk pulmonary embolism: a multicentre series of 52 cases. Eur. Heart J. 2018;39(47):4196–4204. doi: 10.1093/eurheartj/ehy464. [DOI] [PubMed] [Google Scholar]
- 44.Gayou E.L., Makary M.S., Hughes D.R., Hemingway J., Elliott E.D., Spain J.W. Nationwide trends in use of catheter-directed therapy for treatment of pulmonary embolism in medicare beneficiaries from 2004 to 2016. J. Vasc. Intervent. Radiol. 2019;30(6):801–806. doi: 10.1016/j.jvir.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 45.Pollak J.S. Catheter-based therapies for pulmonary emboli. Clin. Chest Med. 2018;39(3):651–658. doi: 10.1016/j.ccm.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 46.Jolly M., Phillips J. Pulmonary embolism: current role of catheter treatment options and operative thrombectomy. Surg. Clin. 2018;98(2):279–292. doi: 10.1016/j.suc.2017.11.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.