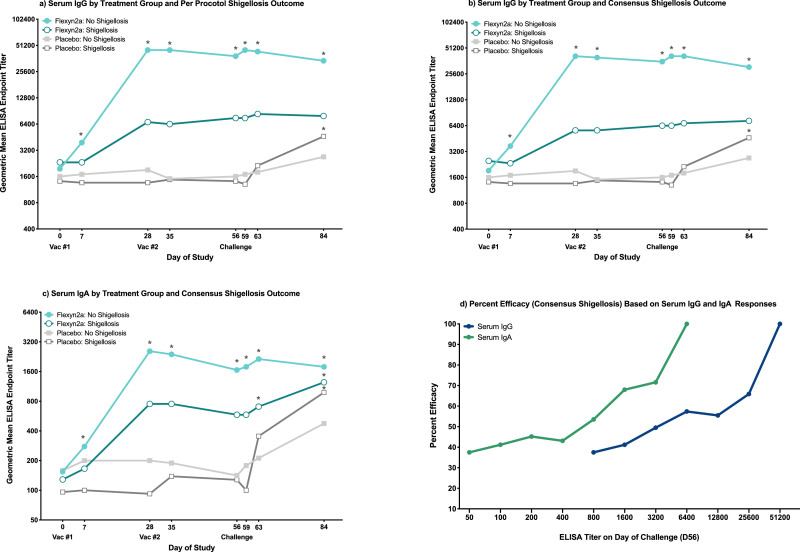
Fig. 1.
S. flexneri 2a LPS-Specific Serum IgG and IgA. (a) S. flexneri 2a LPS-specific serum IgG responses grouped by vaccinated subjects with or without per protocol shigellosis, and placebo subjects with or without per protocol shigellosis. (b) S. flexneri 2a LPS-specific serum IgG responses grouped by vaccinated subjects with or without consensus shigellosis, and placebo subjects with or without consensus shigellosis. (c) S. flexneri 2a LPS-specific serum IgA responses grouped by vaccinated subjects with or without consensus shigellosis, and placebo subjects with or without consensus shigellosis. * = significant difference as compared to baseline titres within the same treatment group/shigellosis outcome. Significance determined by repeated measures ANOVA of log-transformed titres with Bonferroni post-hoc test. (d) Percent efficacy against consensus shigellosis post-challenge in vaccinated subjects across increasing serum IgG and IgA ELISA endpoint titres.