Abstract
Gastrointestinal (GI) symptoms have been described in a conspicuous percentage of coronavirus disease 2019 (COVID-19) patients. This clinical evidence is supported by the detection of viral RNA in stool, which also supports the hypothesis of a possible fecal-oral transmission route. The involvement of GI tract in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is corroborated by the theoretical assumption that angiotensin converting enzyme 2, which is a SARS-CoV-2 target receptor, is present along the GI tract. Studies have pointed out that gut dysbiosis may occur in COVID-19 patients, with a possible correlation with disease severity and with complications such as multisystem inflammatory syndrome in children. However, the question to be addressed is whether dysbiosis is a consequence or a contributing cause of SARS-CoV-2 infection. In such a scenario, pharmacological therapies aimed at decreasing GI permeability may be beneficial for COVID-19 patients. Considering the possibility of a fecal-oral transmission route, water and environmental sanitation play a crucial role for COVID-19 containment, especially in developing countries.
Keywords: COVID-19, SARS-CoV-2, Gastrointestinal symptoms, Gut microbiome, Dysbiosis, Zonulin, Fecal-oral transmission
Core Tip: Coronavirus disease 2019 (COVID-19) patients may suffer from gastrointestinal symptoms that are associated with gastrointestinal dysbiosis. Even though the exact role of gut microbiome perturbation as a either a cause or a consequence of the disease is still to be elucidated, pharmacological interventions aimed at containing intestinal permeability may be of support in COVID-19 patients.
INTRODUCTION
In December 2019, in Wuhan, one of the largest cities in the Chinese province of Hubei, a new coronavirus appeared that was responsible for a pneumonia epidemic that was named by the World Health Organization (WHO) on February 11, 2020 as coronavirus disease 2019 (COVID-19), and declared as a dangerous threat to public health and an international health emergency[1,2]. Indeed, COVID-19 infections rapidly spread and was declared a pandemic by the WHO one month later on March 11, 2020. In a short time, the pandemic involved all continents, and at the time of writing this review, accounts for more than 100 million confirmed cases worldwide, also becoming a serious threat to the global economy[3,4].
At the beginning of January 2020, the new coronavirus responsible for this pandemic was named “severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)” by the International Committee on Taxonomy of Viruses[5]. It is a single-stranded RNA virus with positive polarity and is closely related to the B-line of beta-coronaviruses, which are typically responsible for severe and lethal respiratory syndromes[2,6]. Phylogenetic analysis showed that the viral sequence of this new coronavirus has a strong similarity (89%) with two other coronaviruses similar to SARS and deriving from bats, namely bat-SL-CoVZC45 (GenBank access n. MG772933.1) and bat-SL-CoVZXC21 (GenBank access n. MG772934.1). Similarities were also found with SARS-CoV, the virus that caused the SARS pandemic in 2002/2003, and the Middle East Respiratory Syndrome (MERS-CoV) pandemic of 2012, with which SARS-CoV-2, shares sequence homologies (79% with SARS-CoV and 50% with MERS-CoV)[7-9] as well as zoonotic transmission and several clinical features.
MECHANISM OF INFECTION
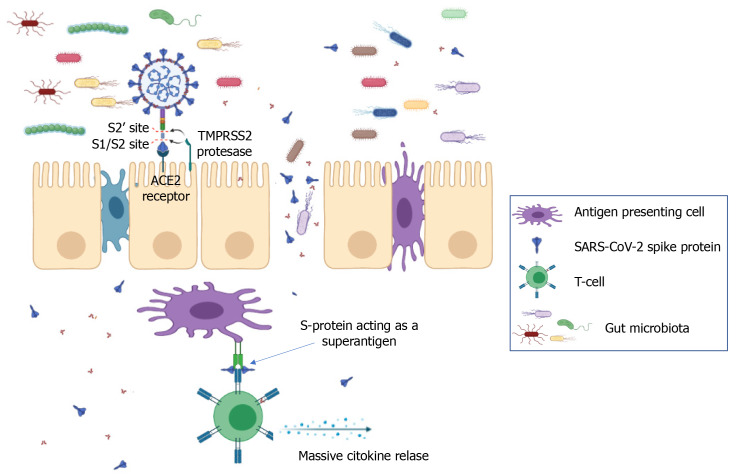
Studies carried out so far suggest that the human angiotensin converting enzyme 2 (ACE2) is the main target receptor for the virus to gain access into human host cells. Binding occurs between the ACE2 binding domain located at the C-terminal end of the viral spike protein and the ACE2 target receptor located on the surface of the host cell membrane[10]. After binding to ACE2 receptors on human cells[11], the SARS-CoV-2 spike protein is cleaved by two mucosa-specific serine proteases, namely TMPRSS2 and TMPRSS4, to facilitate viral cellular infection[12].
Tissues with the highest expression of ACE2, and therefore a high sensitivity to infection are present in both the respiratory and the gastrointestinal (GI) tracts. The receptor is present in the pulmonary epithelium, in type 2 pneumocytes that are present in the alveoli where the exchange between oxygen and carbon dioxide takes place, in the nose, mouth, stratified epithelial cells in the upper esophagus, and absorbent enterocytes of the ileum and colon. Other receptors that are known to allow entry of SARS-CoV-2 are DC-SIGN (CD209), CD147 and L-SIGN (CD209L)[13-15]. Transmission of the virus can take place through direct contact with respiratory droplets from infected subjects or indirectly from contaminated objects and surfaces[16].
CLINICAL SYMPTOMS
Clinical manifestations associated with COVID-19 infection range from complete absence of symptoms (asymptomatic subjects) to serious and even fatal infections. In most cases, the disease is mild to moderate, so that symptoms can be managed safely at home without the need for hospitalization. The main target of the infection is the respiratory tract, and therefore, the clinical manifestations associated with the disease are mostly respiratory. The most common symptoms are similar to those of seasonal flu and include fever, dry cough, dyspnea, sore throat, headache, muscle aches, and asthenia. Severe respiratory distress, chest pain, pneumonia, and pulmonary insufficiency can also be found in severe cases[17-19]. In SARS-CoV-2 pneumonia, lung computed tomography scans shows bilateral, subpleural ground-glass opacity lesions[20-22]. In addition to pulmonary clinical manifestations, heart damage can also occur in infected patients. Shi et al[23] in a study conducted in Wuhan on 400 patients, found that about one-fifth experienced heart disease as a risk factor for worse outcome. Heart damage essentially consists of sudden and severe myocardial inflammation leading to heart failure and arrhythmias[24]. For this reason, hypertensive patients, and in general all individuals with a positive history of cardiovascular disease, are more at risk of experiencing a poor prognosis than other patients[25]. SARS-CoV-2 can also damage the central nervous system, with symptoms that range from mild (anosmia, dysgeusia) to severe (viral encephalitis). Moreover, COVID-19 infection, like other pulmonary infectious diseases, may represent a risk factor for acute cerebrovascular events. The observed D-dimer elevation in COVID-19 patients may indicate additional risk of cerebrovascular events[26]. Kidney and liver damage have also been reported. Proteinuria, hematuria, increased aspartate transaminase (AST, also known as aspartate aminotransferase or glutamic oxaloacetic transaminase), alanine aminotransferase (ALT, also known as alanine transaminase or glutamic-pyruvic transaminase), and bilirubin have been found in about half of infected patients[27,28].
The virulence of microorganisms determines the damage to host cells before the microorganisms are cleared by host immunity[29]. Yet, the virulence of the infective microorganism does not fully account for the virulence of the disease, the majority of which results from inflammation produced by the immune response[29]. That has been demonstrated for many infective diseases and is particularly true for SARS-CoV-2. Most viral and bacterial infections in humans are self-limiting[29,30], and infecting microorganisms become commensal if they can coexist with the human host. The SARS-CoV-2 virus infection is no different[31], and that could explain why most COVID-19 cases are asymptomatic or mild.
COVID-19 AND THE GI TRACT
Although, as reported, SARS-CoV-2 infection mainly affects the respiratory system[32], leading to breathing difficulties, dry cough, and nasal congestion to respiratory failure, this novel coronavirus can be found in the GI tract as well[33]. Furthermore, SARS-CoV-2 RNA has been isolated from stools. Viral nucleocapsid protein can be observed in duodenal and rectal glandular epithelial cells by laser scanning confocal microscopy. The available results provide evidence of the activity of this virus in the GI tract[34].
GI SYMPTOMATOLOGY
GI symptoms are present in approximately 50% of COVID-19 patients, consisting mainly of diarrhea, nausea, vomiting, and abdominal discomfort[35]. Moreover, several studies have shown that SARS-CoV-2 is able to interact with ACE2 receptors on ileal enterocytes and colon epithelial cells, so that a trophism for the GI tract has been hypothesized as well[27,36]. Further evidence supporting the possibility that both the small and large intestines may be susceptible to SARS-CoV-2 infection, was the isolation of viral RNA from the GI epithelium and staining of the viral nucleocapsid protein in these cells[34]. In addition, viral RNA was also isolated in stool samples from COVID-19 patients, and it was presumed that the viral components originated from infected enterocytes[34,36]. Considering the overall evidence, several reports have expressed concerns of possible fecal-oral transmission of SARS-CoV-2[37,38]. Nevertheless, the findings were not unexpected as other well-known coronaviruses, including those causing the MERS and SARS epidemics, have shown to infect enterocytes, leading to several GI symptoms[39].
Several reports have focused on the GI symptomatology in COVID-19 patients, underlining clinical outcomes that varied widely in terms of frequency. Nine of those studies that included a total of 4177 patients found that the incidence of GI symptoms varied from 4.9% to 61.3%. The most frequent symptoms were diarrhea (8.4%) and lack of appetite (7.4%); 4.1% of patients complained about nausea-related disorders, while vomiting was reported in 2.4% and abdominal pain in 1.7% of the cases[40-48]. Interestingly, the frequency of diarrhea, nausea, and abdominal pain in COVID-19 patients was shown to be higher among women than men[49]. Finally, Yang et al[50] examined 50 COVID-19 patients by dividing them in two groups by the presence of either initial pulmonary or initial GI symptoms. They found that patients with GI manifestations as initial symptoms had more severe clinical outcomes, leading to longer hospitalization.
As stated above, and similar to SARS-CoV-1, SARS-CoV-2 infection is mediated by ACE2, which allows the virus to enter the cells and replicate by taking advantage of the host synthesis apparatus[51]. A recent study has shown that the binding affinity of the spike protein of SARS-CoV-2 is about 10-20 times higher than that of SARS-CoV-1[52]. ACE2 receptors are widely expressed in the epithelia of both the respiratory and GI tracts. A recent multiomic in vitro study has shown that expression of these membrane enzymes is higher in intestinal cell lines than lung cell lines[36].
The pathophysiology of GI symptoms is not entirely clear, but it seems that they originate as a result of several phenomena. ACE2 performs an essential function in the intestine by regulating amino acid homeostasis and microbiome balance[53]. It is possible that the binding of SARS-CoV-2 to these membrane receptors reduces their availability, giving rise to an alteration of physiological function that induces a dysbiosis that leads to diarrhea, one of the most frequent symptoms reported during SARS-CoV2 infection[54]. The development of a cytokine storm could be indeed the cause of damage to the GI tract. Autopsies of SARS patients found increased expression of proinflammatory cytokines in ACE2-expressing cells compared with those that do not express this enzyme on their membranes[55]. It is fair to think that this happens following SARS-CoV-2 infection. Inflammation could also stimulate the gut microbiota to release molecules that increase the inflammatory state of the intestine and subsequently spread from the intestine into the circulatory system to cause systemic damage with more severe consequences than the viral infection itself[56]. In addition to locally occurring phenomena, the gut-lung axis seems to have a pivotal role in the development of GI symptoms as well. This axis represents a link between two organs that share a common mucous immune system[57]. Its influence has been hypothesized as a result of studies that have described COVID-19 patients with GI symptoms but without SARS-CoV-2 RNA-positive stool samples[54].
The impact of GI comorbidities on the development of symptoms in COVID-19 patients is yet to be defined. While the relationship between disorders such as diabetes, cardiovascular diseases, and hypertension and COVID-19-related severity was proven by several studies[58]. There are not yet sufficient studies to demonstrate a causal link between the presence of previous GI conditions and the onset of COVID-19-related symptomatology.
Indeed, existing studies do not provide indisputable results. A study carried out in China found that pre-existing GI disorders represent a third comorbidity in COVID-19 patients[59]. Another study carried out in Wuhan reported a relatively high percentage of COVID-19 patients with GI disorders[60]. Conversely, other studies carried out in the United States[61] and in Italy[62] have not found a significant correlation between SARS-CoV-2 infection and the presence of inflammatory bowel disease. On the contrary, Papa et al[63] observed a better clinical outcome in patients presenting with digestive comorbidities, as presumably they do not hinder the immune response against SARS-CoV-2. However, the authors point out that the number of cases observed was small[63].
FECAL-ORAL TRANSMISSION OF SARS-COV-2
A potential fecal-oral transmission route of SARS-CoV-2, in addition to respiratory droplets, person-to-person contact, or indirect infection following contact with contaminated surfaces, has been proposed by several authors[37,38,64,65]. The presence of common GI symptoms in the COVID-19 patients mentioned above is evidence that the GI tract is involved in SARS-CoV-2 infection.
Viral RNA has been detected in esophagus, stomach, duodenum, and rectum tissue from patients with severe SARS-CoV-2 infections[66]. Notably, the first COVID-19 patient in the United States tested positive for SARS-CoV-2 in loose stool specimens by real-time reverse transcription polymerase chain reaction[67]. Several studies report detection of viral RNA in stool samples in adults[34,68-72] and children[73-75] diagnosed with COVID-19. Interestingly, two studies confirmed that SARS-CoV-2 was detectable in fecal samples even after negative testing of respiratory swabs[70,72], and in patients who did not present GI symptoms[66,69,71]. In addition to SARS-CoV-2 RNA detection in stool samples, positive staining of viral nucleocapsid protein in gastric, duodenal, and rectal epithelia might demonstrate the infection of GI glandular epithelial cells[34]. Wang et al[68] found a high content of viral RNA in fecal specimens from COVID-19 patients, and electron microscopy confirmed the presence of living viral particles in those samples. Wölfel et al[69] could not isolate living SARS-CoV-2 from stool samples despite the presence of high concentrations of viral RNA, but did detect cells in a few stools that contained subgenomic mRNA, which suggests active replication of the virus in the intestinal tract. The presence of viral subgenomic mRNA indicates actively infected cells because such mRNA is transcribed only in infected cells and is not found packaged into virions[69]. As in adults, SARS-CoV-2 in children might persist in the GI tract longer than in the respiratory system. Actually, a high percentage of children diagnosed of COVID-19 tested positive on rectal or anal swabs, and some were positive after nasopharyngeal or throat swabs became negative[73-75].
Other evidence that supports an alternative fecal-oral transmission of COVID-19 is the expression of SARS-CoV-2 entry-genes in the cells of intestinal tissues. Mucosal cells of the lower intestine can be infected by other coronaviruses, resulting in diarrhea and other enteric symptoms. In this case, SARS-CoV-2 could be carried by saliva and secretions into the digestive tract, where it would infect the ACE2-expressing enterocytes. It has been confirmed that SARS-CoV-2 can infect enterocytes present in human small intestine organoid models, which supports virus replication and a GI route of infection[76]. However, another study demonstrated that human GI secretions were able to inactivate SARS-CoV-2, which suggests that although virus infection and pathogenesis is enhanced in the intestine, it is not clear if it would be a primary site of infection and possible route of transmission[77]. An alternative route of transmission that acts together with the respiratory route might explain the rapid spread of disease.
GUT MICROBIOME ROLE IN COVID-19 SEVERITY
COVID-19 symptomatology is strictly related to both a physiological and an aberrant activation of immune system, leading in very severe cases to a cytokine storm[78]. Based on this premise, it is not surprising that the gut microbiome, which is closely linked to the immune status of the host, may play a role in COVID-19 severity. A gut microbiome imbalance has been associated in COVID-19 patients with elevated concentrations of inflammatory cytokines, and blood markers, including C-reactive protein (CRP), lactate dehydrogenase, and aspartate aminotransferase[79]. Yeoh et al[79] examined blood and fecal samples from 100 young (mean age of about 36 years) confirmed positive COVID-19 patients in two Hong Kong hospitals. They also analyzed samples from patients for up to 30 days after clearance of SARS-CoV-2. All patients had slight or moderate symptomatology, with only 5% with severe and 3% with critical conditions. Compared with a group of healthy adult controls, COVID-19 patients had higher fecal populations of Ruminococcus gnavus, Ruminococcus torques, and Bacteroides dorei species. This imbalance seemed not to depend on the pharmacological therapy administered. The same investigators reported a stratification of gut microbiota composition associated with disease severity in hospitalized COVID-19 patients. Cytokine (IL-10 and tumor necrosis factor-), enzyme (AST, -glutamyl transferase, lactate dehydrogenase), CRP, N-terminal proB-type natriuretic peptide levels, and erythrocyte sedimentation rate were previously reported as associated with increased COVID-19-related symptomatology[80-82]. Those parameters were also significantly associated with microbiota composition in COVID-19 patients. Remarkably, the levels of those molecules increased in parallel with changes in the microbiota composition, representing more severe disease states[79]. Overall, the results suggest that the gut microbiota composition might be linked to the extent of the immune response to COVID-19 and subsequent tissue damage, thus playing a role in regulating disease severity. The persistence of gut microbiota dysbiosis even 30 days after clearance of SARS-CoV-2 might also be linked to the persistence of COVID-19 related symptoms, such as fatigue, dyspnea, and joint pain.
Moreover, Yu[83] suggested that nutritional disorders impair immunity, causing hyperinflammation and leading to an overload of cytokines in COVID-19 patients. Autophagy induced by restrictive eating could be an efficient strategy to prevent COVID-19-related symptomatology. Indeed, it inhibits overgrowth of the microbiota and partially reduces excessive nutrition for replication. Autophagy also attenuates inflammation. On that basis, Yu[83] recommended restoration of good nutritional status together with restriction of food intake, especially in older subjects, to reduce the risk of developing severe symptomatology if infected by COVID-19.
On the other hand, as inflammation can be considered as a physiological response of the immune system to damaged tissues[84], it is a protective reaction to remove the injurious stimuli and to initiate the healing of damaged tissues[84]. Nutrition excess slows tissue healing, indeed nutrition derived from the degradation of the damaged tissue coupled with excess nutrition intake will be mostly turned into lipid intermediates and deposited in nonadipose tissue, lipotoxicity and further tissue damage. Thus, in a state of overnutrition, inflammation can become chronic. In this regard, undernutrition might be beneficial in fighting viral infection, as anorexia nervosa patients seem to be free of common viral infections[85,86].
ROLE OF THE GI TRACT IN COVID-19 COMPLICATIONS
Multisystem inflammatory syndrome in children (MIS-C) is defined by the Centers for Disease Control and Prevention[87] as a severe illness requiring hospitalization that occurs in individuals < 21 years of age with evidence of current or recent SARS-CoV-2 infection, or recent exposure to an individual with COVID-19. Children with MIS-C have fever, laboratory evidence of inflammation, and multiorgan involvement without an alternative plausible diagnosis. Ninety-two percent of children with MIS-C report GI symptoms[88] and 80% develop cardiac pathology. MIS-C is characterized by a superantigen-mediated hyperinflammatory response with a unique T-cell profile and multiple autoantigens[89-91]. It has been recently shown that the spike protein of SARS-CoV-2 contains sequence and structural motifs similar to a Staphylococcal Enterotoxin B superantigen[92], and superantigen-induced skewing of the T-cell repertoire in MIS-C patients with expansion of the T-cell receptor beta variable gene correlates with the cytokine storm and hyperinflammation seen in MIS-C[91]. Inflamed expansion of antibodies for multiple non-COVID-19 pathogens, including common coronaviruses, influenza and respiratory syncytial virus[93] plus numerous self-antigens[89,90] also correlates with the severe cardiac complications of MIS-C. Immune complexes stimulate monocytes/macrophages, eliciting phagocytosis, macrophage activation and cytokine storm[94,95] (Figure 1). The instigating factor for MIS-C has not been elucidated, but prominent GI symptoms suggest an intestinal source. The upper or lower respiratory tract are unlikely sources of antigens triggering this hyperinflammatory response, given the relative lack of respiratory symptoms in MIS-C, low/undetectable viral load in respiratory secretions[93], and the 2-4 wk delay between initial infection/exposure and development of MIS-C. Rather, ongoing antigen exposure is much more likely to be from the GI tract as has been shown in Kawasaki disease (KD), a similar illness with a hyperinflammatory profile leading to fever, rash, elevated inflammatory markers resulting in vasculitis, coronary aneurysms, and cardiac pathology. In KD, increased intestinal permeability secondary to upregulation of zonulin, a molecule that regulates paracellular molecule trafficking, has been associated with increased circulating immunoglobulin A and cardiac complications in children with KD[96].
Figure 1.
Gut dysbiosis induced by the immune response to severe acute respiratory syndrome coronavirus 2 in the gastrointestinal tract increases gut permeability, which in turn increases spike protein trafficking. Spike protein was reported to act as a superantigen that links the T-cell receptor and major histocompatibility complex II on antigen-presenting cells. ACE2: Angiotensin converting enzyme 2; S-protein: Spike protein; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
PHARMACOLOGICAL IMPLICATIONS OF FECAL-ORAL TRANSMISSION
A study by Yeoh et al[79] was not designed to determine whether dysbiosis is the consequence of patient immune response rather than a contributing cause in disease onset and severity. Nevertheless, the reported association seems to be sufficiently robust to support the benefit of pharmacological intervention aimed at decreasing gut permeability for COVID-19 GI symptoms management, and potentially to reduce the risk of its complications, including MIS-C. Such a strategy could both reduce GI tract inflammation and help accelerate naturally occurring dysbiosis correction. Larazotide acetate, a synthetic octapeptide designed as a specific zonulin receptor antagonist, was successfully and safely used to modulate gut permeability and the related inflammation[97,98]. Di Micco et al[99] recently proposed an additional role for larazotide in COVID-19 patients. Based on an in-silico evaluation, they reported the ability of larazotide to inhibit the N3 protease of SARS-CoV-2. As larazotide was specifically designed as an oral drug able to reach the lower GI tract, it might be useful both for its direct anti-SARS-CoV-2 effect and to prevent antigen trafficking of SARS-CoV-2-derived molecules, including spike protein from the gut lumen to the bloodstream, which could trigger a cytokine storm leading to MIS-C.
CONCLUSION
In summary, whether digestive symptoms are a direct response to SARS-CoV-2 infection of the GI tract, or a secondary outcome of COVID-19, is still to be completely understood. The presence of viral RNA in the GI tract might partially explain GI symptoms even if it does not mean that there is infection in the intestine. In either case, robust evidence supports GI tract involvement in COVID-19 as well as a potential fecal-oral transmission route. The GI seems to be not only a reservoir of SARS-CoV-2, but also an active battlefield in which virus, innate and adaptive immune system responses, epithelial cells, and gut microbiota play critical roles. The interventions for managing GI-related issues should be twofold. From a patient perspective, pharmacological treatment to reduce the severity of GI symptoms and support a quick re-establishment of eubiosis should be evaluated. Moreover, interventions to ensure water sanitation should be added to the current policies for COVID-19 pandemic control. Considering that many people worldwide lack access to clean drinking water and good sanitation, the possible containment of COVID-19 through water treatment should be carefully taken into account to reduce the actual and future magnitude of the pandemic.
Footnotes
Conflict-of-interest statement: Authors declare that there are no conflicts of interest.
Manuscript source: Invited manuscript
Peer-review started: February 1, 2021
First decision: February 27, 2021
Article in press: March 19, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Merrett ND S-Editor: Gao CC L-Editor: Filipodia P-Editor: Wang LL
Contributor Information
Jacopo Troisi, Metabolomics Section, Theoreo srl - Spin-off Company of the University of Salerno, Montecorvino Pugliano 84090, SA, Italy; Department of Chemistry and Biology “A. Zambelli”, University of Salerno, Fisciano 84084, SA, Italy; European Biomedical Research Institute of Salerno, Salerno 84125, SA, Italy. troisi@theoreosrl.com.
Giorgia Venutolo, European Biomedical Research Institute of Salerno, Salerno 84125, SA, Italy.
Meritxell Pujolassos Tanyà, Metabolomics Section, Theoreo srl - Spin-off Company of the University of Salerno, Montecorvino Pugliano 84090, SA, Italy.
Matteo Delli Carri, Metabolomics Section, Theoreo srl - Spin-off Company of the University of Salerno, Montecorvino Pugliano 84090, SA, Italy; Department of Chemistry and Biology “A. Zambelli”, University of Salerno, Fisciano 84084, SA, Italy.
Annamaria Landolfi, Department of Medicine, Surgery and Dentistry “Scuola Medica Salernitana”, University of Salerno, Baronissi 84081, SA, Italy.
Alessio Fasano, European Biomedical Research Institute of Salerno, Salerno 84125, SA, Italy; Mucosal Immunology and Biology Research Center and Center for Celiac Research, Harvard Medical School, Massachusetts Gen Hosp Children, Mucosal Immunology and Biology Research Center, Boston, MA 02114, United States.
References
- 1.Wu YC, Chen CS, Chan YJ. The outbreak of COVID-19: An overview. J Chin Med Assoc. 2020;83:217–220. doi: 10.1097/JCMA.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin Y, Yang H, Ji W, Wu W, Chen S, Zhang W, Duan G. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses. 2020;12 doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Coronavirus Disease (COVID-19) Pandemic, 2020. [cited January 26, 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 .
- 5.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S, Yuen KY. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang S, Du L, Shi Z. An emerging coronavirus causing pneumonia outbreak in Wuhan, China: calling for developing therapeutic and prophylactic strategies. Emerg Microbes Infect. 2020;9:275–277. doi: 10.1080/22221751.2020.1723441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren LL, Wang YM, Wu ZQ, Xiang ZC, Guo L, Xu T, Jiang YZ, Xiong Y, Li YJ, Li XW, Li H, Fan GH, Gu XY, Xiao Y, Gao H, Xu JY, Yang F, Wang XM, Wu C, Chen L, Liu YW, Liu B, Yang J, Wang XR, Dong J, Li L, Huang CL, Zhao JP, Hu Y, Cheng ZS, Liu LL, Qian ZH, Qin C, Jin Q, Cao B, Wang JW. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J (Engl) 2020;133:1015–1024. doi: 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol. 2020;94 doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; 181: 271-280. :e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao HL, Zhao LX, Yang J, Tong N, An L, Liu QT, Xie MR, Li CS. Association between ACE2/ACE balance and pneumocyte apoptosis in a porcine model of acute pulmonary thromboembolism with cardiac arrest. Mol Med Rep. 2018;17:4221–4228. doi: 10.3892/mmr.2018.8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Kang Z, Gong H, Xu D, Wang J, Li Z, Cui X, Xiao J, Meng T, Zhou W, Liu J, Xu H. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. 2020 Preprint. bioRxiv. 2020.01.30.927806 [Google Scholar]
- 16.Lotfi M, Hamblin MR, Rezaei N. COVID-19: Transmission, prevention, and potential therapeutic opportunities. Clin Chim Acta. 2020;508:254–266. doi: 10.1016/j.cca.2020.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A COVID-19 Lombardy ICU Network. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lovato A, de Filippis C, Marioni G. Upper airway symptoms in coronavirus disease 2019 (COVID-19) Am J Otolaryngol. 2020;41:102474. doi: 10.1016/j.amjoto.2020.102474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rettner R. How Does the New Coronavirus Compare with the Flu? Sci Am . 2020 [Google Scholar]
- 20.Song F, Shi N, Shan F, Zhang Z, Shen J, Lu H, Ling Y, Jiang Y, Shi Y. Emerging 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Radiology. 2020;295:210–217. doi: 10.1148/radiol.2020200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavallo AU, Troisi J, Forcina M, Mari PV, Forte V, Sperandio M, Pagano S, Cavallo P, Floris R, Garaci F. Texture Analysis in the Evaluation of Covid-19 Pneumonia in Chest X-Ray Images: a Proof of Concept Study. Curr Med Imaging. 2021 doi: 10.2174/1573405617999210112195450. [DOI] [PubMed] [Google Scholar]
- 22.Cui N, Zou X, Xu L. Preliminary CT findings of coronavirus disease 2019 (COVID-19) Clin Imaging. 2020;65:124–132. doi: 10.1016/j.clinimag.2020.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu H, Rhee JW, Cheng P, Waliany S, Chang A, Witteles RM, Maecker H, Davis MM, Nguyen PK, Wu SM. Cardiovascular Complications in Patients with COVID-19: Consequences of Viral Toxicities and Host Immune Response. Curr Cardiol Rep. 2020;22:32. doi: 10.1007/s11886-020-01292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rizzo P, Vieceli Dalla Sega F, Fortini F, Marracino L, Rapezzi C, Ferrari R. COVID-19 in the heart and the lungs: could we "Notch" the inflammatory storm? Basic Res Cardiol. 2020;115:31. doi: 10.1007/s00395-020-0791-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, Liu C, Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cha MH, Regueiro M, Sandhu DS. Gastrointestinal and hepatic manifestations of COVID-19: A comprehensive review. World J Gastroenterol. 2020;26:2323–2332. doi: 10.3748/wjg.v26.i19.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsen CP, Bourne TD, Wilson JD, Saqqa O, Sharshir MA. Collapsing Glomerulopathy in a Patient With COVID-19. Kidney Int Rep. 2020;5:935–939. doi: 10.1016/j.ekir.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levin BR, Antia R. Why we don't get sick: the within-host population dynamics of bacterial infections. Science. 2001;292:1112–1115. doi: 10.1126/science.1058879. [DOI] [PubMed] [Google Scholar]
- 30.Levin BR, Baquero F, Ankomah PP, McCall IC. Phagocytes, Antibiotics, and Self-Limiting Bacterial Infections. Trends Microbiol. 2017;25:878–892. doi: 10.1016/j.tim.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Azkur AK, Akdis M, Azkur D, Sokolowska M, van de Veen W, Brüggen MC, O'Mahony L, Gao Y, Nadeau K, Akdis CA. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75:1564–1581. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel KP, Patel PA, Vunnam RR, Hewlett AT, Jain R, Jing R, Vunnam SR. Gastrointestinal, hepatobiliary, and pancreatic manifestations of COVID-19. J Clin Virol. 2020;128:104386. doi: 10.1016/j.jcv.2020.104386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H . Gastroenterology 2020; 158: 1831-1833. :e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du M, Cai G, Chen F, Christiani DC, Zhang Z, Wang M. Multiomics Evaluation of Gastrointestinal and Other Clinical Characteristics of COVID-19. Gastroenterology 2020; 158: 2298-2301. :e7. doi: 10.1053/j.gastro.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gu J, Han B, Wang J. COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission. Gastroenterology. 2020;158:1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51:843–851. doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leung WK, To KF, Chan PK, Chan HL, Wu AK, Lee N, Yuen KY, Sung JJ. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011–1017. doi: 10.1016/j.gastro.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ai JW, Zi H, Wang Y, Huang Q, Wang N, Li LY, Pei B, Ji J, Zeng XT. Clinical Characteristics of COVID-19 Patients With Gastrointestinal Symptoms: An Analysis of Seven Patients in China. Front Med (Lausanne) 2020;7:308. doi: 10.3389/fmed.2020.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao C, Chen M, He L, Xie J, Chen X. Clinical features and outcomes of COVID-19 patients with gastrointestinal symptoms. Crit Care. 2020;24:340. doi: 10.1186/s13054-020-03034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferm S, Fisher C, Pakala T, Tong M, Shah D, Schwarzbaum D, Cooley V, Hussain S, Kim SH. Analysis of Gastrointestinal and Hepatic Manifestations of SARS-CoV-2 Infection in 892 Patients in Queens, NY. Clin Gastroenterol Hepatol 2020; 18: 2378-2379. :e1. doi: 10.1016/j.cgh.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, Hao SR, Jia HY, Cai H, Zhang XL, Yu GD, Xu KJ, Wang XY, Gu JQ, Zhang SY, Ye CY, Jin CL, Lu YF, Yu X, Yu XP, Huang JR, Xu KL, Ni Q, Yu CB, Zhu B, Li YT, Liu J, Zhao H, Zhang X, Yu L, Guo YZ, Su JW, Tao JJ, Lang GJ, Wu XX, Wu WR, Qv TT, Xiang DR, Yi P, Shi D, Chen Y, Ren Y, Qiu YQ, Li LJ, Sheng J, Yang Y. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Redd WD, Zhou JC, Hathorn KE, McCarty TR, Bazarbashi AN, Thompson CC, Shen L, Chan WW. Prevalence and Characteristics of Gastrointestinal Symptoms in Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection in the United States: A Multicenter Cohort Study. Gastroenterology 2020; 159: 765-767. :e2. doi: 10.1053/j.gastro.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Remes-Troche JM, Ramos-de-la-Medina A, Manríquez-Reyes M, Martínez-Pérez-Maldonado L, Lara EL, Solís-González MA. Initial Gastrointestinal Manifestations in Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection in 112 Patients From Veracruz in Southeastern Mexico. Gastroenterology. 2020;159:1179–1181. doi: 10.1053/j.gastro.2020.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang H, Liao YS, Gong J, Liu J, Xia X, Zhang H. Clinical characteristics of coronavirus disease (COVID-19) patients with gastrointestinal symptoms: A report of 164 cases. Dig Liver Dis. 2020;52:1076–1079. doi: 10.1016/j.dld.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang L, Mei Q, Li L, Ye C, Huang Y, Wang Y, Tong F, Gao Y, Pan A. [Analysis of gastrointestinal symptoms in 80 patients with coronavirus disease 2019] Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020;32:412–416. doi: 10.3760/cma.j.cn121430-20200406-00411. [DOI] [PubMed] [Google Scholar]
- 48.Zheng T, Yang C, Wang HY, Chen X, Yu L, Wu ZL, Sun H. Clinical characteristics and outcomes of COVID-19 patients with gastrointestinal symptoms admitted to Jianghan Fangcang Shelter Hospital in Wuhan, China. J Med Virol. 2020;92:2735–2741. doi: 10.1002/jmv.26146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sierpiński R, Pinkas J, Jankowski M, Zgliczyński WS, Wierzba W, Gujski M, Szumowski Ł. Sex differences in the frequency of gastrointestinal symptoms and olfactory or taste disorders in 1942 nonhospitalized patients with coronavirus disease 2019 (COVID-19) Pol Arch Intern Med. 2020;130:501–505. doi: 10.20452/pamw.15414. [DOI] [PubMed] [Google Scholar]
- 50.Yang TY, Li YC, Wang SC, Dai QQ, Jiang XS, Zuo S, Jia L, Zheng JB, Wang HL. Clinical characteristics of patients with COVID-19 presenting with gastrointestinal symptoms as initial symptoms: Retrospective case series. World J Clin Cases. 2020;8:2950–2958. doi: 10.12998/wjcc.v8.i14.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kotfis K, Skonieczna-Żydecka K. COVID-19: gastrointestinal symptoms and potential sources of SARS-CoV-2 transmission. Anaesthesiol Intensive Ther. 2020;52:171–172. doi: 10.5114/ait.2020.93867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R, Lipinski S, Wild B, Camargo SM, Singer D, Richter A, Kuba K, Fukamizu A, Schreiber S, Clevers H, Verrey F, Rosenstiel P, Penninger JM. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ye Q, Wang B, Zhang T, Xu J, Shang S. The mechanism and treatment of gastrointestinal symptoms in patients with COVID-19. Am J Physiol Gastrointest Liver Physiol. 2020;319:G245–G252. doi: 10.1152/ajpgi.00148.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He L, Ding Y, Zhang Q, Che X, He Y, Shen H, Wang H, Li Z, Zhao L, Geng J, Deng Y, Yang L, Li J, Cai J, Qiu L, Wen K, Xu X, Jiang S. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J Pathol. 2006;210:288–297. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Villapol S. Gastrointestinal symptoms associated with COVID-19: impact on the gut microbiome. Transl Res. 2020;226:57–69. doi: 10.1016/j.trsl.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, Hugenholtz P, Hansbro PM. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. 2017;15:55–63. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- 58.Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of Underlying Diseases in Hospitalized Patients with COVID-19: a Systematic Review and Meta-Analysis. Arch Acad Emerg Med. 2020;8:e35. [PMC free article] [PubMed] [Google Scholar]
- 59.Wu J, Liu J, Zhao X, Liu C, Wang W, Wang D, Xu W, Zhang C, Yu J, Jiang B, Cao H, Li L. Clinical Characteristics of Imported Cases of Coronavirus Disease 2019 (COVID-19) in Jiangsu Province: A Multicenter Descriptive Study. Clin Infect Dis. 2020;71:706–712. doi: 10.1093/cid/ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.CDC COVID-19 Response Team. Preliminary Estimates of the Prevalence of Selected Underlying Health Conditions Among Patients with Coronavirus Disease 2019 - United States, February 12–March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Istituto Superiore di Sanità. Characteristics of COVID-19 patients dying in Italy. Epicentro. [cited January 26, 2021]. In: Istituto Superiore di Sanità [Internet]. Available from: https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-analysis-of-deaths .
- 63.Papa A, Covino M, Pizzolante F, Miele L, Lopetuso LR, Bove V, Iorio R, Simeoni B, Vetrone LM, Tricoli L, Mignini I, Schepis T, D'Alessandro A, Coppola G, Nicoletti T, Visconti E, Rapaccini G. Gastrointestinal symptoms and digestive comorbidities in an Italian cohort of patients with COVID-19. Eur Rev Med Pharmacol Sci. 2020;24:7506–7511. doi: 10.26355/eurrev_202007_21923. [DOI] [PubMed] [Google Scholar]
- 64.Ding S, Liang TJ. Is SARS-CoV-2 Also an Enteric Pathogen With Potential Fecal-Oral Transmission? Gastroenterology. 2020;159:53–61. doi: 10.1053/j.gastro.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heller L, Mota CR, Greco DB. COVID-19 faecal-oral transmission: Are we asking the right questions? Sci Total Environ. 2020;729:138919. doi: 10.1016/j.scitotenv.2020.138919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin L, Jiang X, Zhang Z, Huang S, Fang Z, Gu Z, Gao L, Shi H, Mai L, Liu Y, Lin X, Lai R, Yan Z, Li X, Shan H. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 67.Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Gerber SI, Kim L, Tong S, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK Washington State 2019-nCoV Case Investigation Team. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, Hoelscher M, Bleicker T, Brünink S, Schneider J, Ehmann R, Zwirglmaier K, Drosten C, Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 70.Wu Y, Guo C, Tang L, Hong Z, Zhou J, Dong X, Yin H, Xiao Q, Tang Y, Qu X, Kuang L, Fang X, Mishra N, Lu J, Shan H, Jiang G, Huang X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang J, Wang S, Xue Y. Fecal specimen diagnosis 2019 novel coronavirus-infected pneumonia. J Med Virol. 2020;92:680–682. doi: 10.1002/jmv.25742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang W, Du RH, Li B, Zheng XS, Yang XL, Hu B, Wang YY, Xiao GF, Yan B, Shi ZL, Zhou P. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xing YH, Ni W, Wu Q, Li WJ, Li GJ, Wang WD, Tong JN, Song XF, Wing-Kin Wong G, Xing QS. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J Microbiol Immunol Infect. 2020;53:473–480. doi: 10.1016/j.jmii.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu CLH, Raval M, Schnall JA, Kwong JC, Holmes NE. Duration of Respiratory and Gastrointestinal Viral Shedding in Children With SARS-CoV-2: A Systematic Review and Synthesis of Data. Pediatr Infect Dis J. 2020;39:e249–e256. doi: 10.1097/INF.0000000000002814. [DOI] [PubMed] [Google Scholar]
- 75.Xu Y, Li X, Zhu B, Liang H, Fang C, Gong Y, Guo Q, Sun X, Zhao D, Shen J, Zhang H, Liu H, Xia H, Tang J, Zhang K, Gong S. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J, Breugem TI, Ravelli RBG, Paul van Schayck J, Mykytyn AZ, Duimel HQ, van Donselaar E, Riesebosch S, Kuijpers HJH, Schipper D, van de Wetering WJ, de Graaf M, Koopmans M, Cuppen E, Peters PJ, Haagmans BL, Clevers H. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369:50–54. doi: 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zang R, Gomez Castro MF, McCune BT, Zeng Q, Rothlauf PW, Sonnek NM, Liu Z, Brulois KF, Wang X, Greenberg HB, Diamond MS, Ciorba MA, Whelan SPJ, Ding S. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abc3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yeoh YK, Zuo T, Lui GC, Zhang F, Liu Q, Li AY, Chung AC, Cheung CP, Tso EY, Fung KS, Chan V, Ling L, Joynt G, Hui DS, Chow KM, Ng SSS, Li TC, Ng RW, Yip TC, Wong GL, Chan FK, Wong CK, Chan PK, Ng SC. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70:698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shen B, Yi X, Sun Y, Bi X, Du J, Zhang C, Quan S, Zhang F, Sun R, Qian L, Ge W, Liu W, Liang S, Chen H, Zhang Y, Li J, Xu J, He Z, Chen B, Wang J, Yan H, Zheng Y, Wang D, Zhu J, Kong Z, Kang Z, Liang X, Ding X, Ruan G, Xiang N, Cai X, Gao H, Li L, Li S, Xiao Q, Lu T, Zhu Y, Liu H, Guo T. Proteomic and Metabolomic Characterization of COVID-19 Patient Sera. Cell 2020; 182: 59-72. :e15. doi: 10.1016/j.cell.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gao L, Jiang D, Wen XS, Cheng XC, Sun M, He B, You LN, Lei P, Tan XW, Qin S, Cai GQ, Zhang DY. Prognostic value of NT-proBNP in patients with severe COVID-19. Respir Res. 2020;21:83. doi: 10.1186/s12931-020-01352-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Henry BM, Aggarwal G, Wong J, Benoit S, Vikse J, Plebani M, Lippi G. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis. Am J Emerg Med. 2020;38:1722–1726. doi: 10.1016/j.ajem.2020.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu L. Restoring Good Health in Elderly with Diverse Gut Microbiome and Food Intake Restriction to Combat COVID-19. Indian J Microbiol. :2021: 1–4. doi: 10.1007/s12088-020-00913-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Costantini S. The Value of the Cytokinome Profile. In: Sharma A. Inflammatory Diseases. Rijeka: IntechOpen, 2011. [Google Scholar]
- 85.Omodei D, Pucino V, Labruna G, Procaccini C, Galgani M, Perna F, Pirozzi D, De Caprio C, Marone G, Fontana L, Contaldo F, Pasanisi F, Matarese G, Sacchetti L. Immune-metabolic profiling of anorexic patients reveals an anti-oxidant and anti-inflammatory phenotype. Metabolism. 2015;64:396–405. doi: 10.1016/j.metabol.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 86.Nova E, Samartín S, Gómez S, Morandé G, Marcos A. The adaptive response of the immune system to the particular malnutrition of eating disorders. Eur J Clin Nutr. 2002;56 Suppl 3:S34–S37. doi: 10.1038/sj.ejcn.1601482. [DOI] [PubMed] [Google Scholar]
- 87.Centers for Disease Control and Prevention. Information for Healthcare Providers about Multisystem Inflammatory Syndrome in Children (MIS-C). [cited January 27, 2021]. In: Centers for Disease Control and Prevention [Internet]. Available from: https://www.cdc.gov/mis-c/hcp/
- 88.Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, Newburger JW, Kleinman LC, Heidemann SM, Martin AA, Singh AR, Li S, Tarquinio KM, Jaggi P, Oster ME, Zackai SP, Gillen J, Ratner AJ, Walsh RF, Fitzgerald JC, Keenaghan MA, Alharash H, Doymaz S, Clouser KN, Giuliano JS Jr, Gupta A, Parker RM, Maddux AB, Havalad V, Ramsingh S, Bukulmez H, Bradford TT, Smith LS, Tenforde MW, Carroll CL, Riggs BJ, Gertz SJ, Daube A, Lansell A, Coronado Munoz A, Hobbs CV, Marohn KL, Halasa NB, Patel MM, Randolph AG Overcoming COVID-19 Investigators; CDC COVID-19 Response Team. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Consiglio CR, Cotugno N, Sardh F, Pou C, Amodio D, Rodriguez L, Tan Z, Zicari S, Ruggiero A, Pascucci GR, Santilli V, Campbell T, Bryceson Y, Eriksson D, Wang J, Marchesi A, Lakshmikanth T, Campana A, Villani A, Rossi P CACTUS Study Team. Landegren N, Palma P, Brodin P. The Immunology of Multisystem Inflammatory Syndrome in Children with COVID-19. Cell 2020; 183: 968-981. :e7. doi: 10.1016/j.cell.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gruber CN, Patel RS, Trachtman R, Lepow L, Amanat F, Krammer F, Wilson KM, Onel K, Geanon D, Tuballes K, Patel M, Mouskas K, O'Donnell T, Merritt E, Simons NW, Barcessat V, Del Valle DM, Udondem S, Kang G, Gangadharan S, Ofori-Amanfo G, Laserson U, Rahman A, Kim-Schulze S, Charney AW, Gnjatic S, Gelb BD, Merad M, Bogunovic D. Mapping Systemic Inflammation and Antibody Responses in Multisystem Inflammatory Syndrome in Children (MIS-C). Cell 2020; 183: 982-995. :e14. doi: 10.1016/j.cell.2020.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Porritt RA, Paschold L, Rivas MN, Cheng MH, Yonker LM, Chandnani H, Lopez M, Simnica D, Schultheiß C, Santiskulvong C, Van Eyk J, Fasano A, Bahar I, Binder M, Arditi M. Identification of a unique TCR repertoire, consistent with a superantigen selection process in Children with Multi-system Inflammatory Syndrome. bioRxiv. 2020:Preprint. [Google Scholar]
- 92.Cheng MH, Zhang S, Porritt RA, Noval Rivas M, Paschold L, Willscher E, Binder M, Arditi M, Bahar I. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation. Proc Natl Acad Sci USA. 2020;117:25254–25262. doi: 10.1073/pnas.2010722117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yonker LM, Neilan AM, Bartsch Y, Patel AB, Regan J, Arya P, Gootkind E, Park G, Hardcastle M, St John A, Appleman L, Chiu ML, Fialkowski A, De la Flor D, Lima R, Bordt EA, Yockey LJ, D'Avino P, Fischinger S, Shui JE, Lerou PH, Bonventre JV, Yu XG, Ryan ET, Bassett IV, Irimia D, Edlow AG, Alter G, Li JZ, Fasano A. Pediatric Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Clinical Presentation, Infectivity, and Immune Responses. J Pediatr 2020; 227: 45-52. :e5. doi: 10.1016/j.jpeds.2020.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Carter MJ, Fish M, Jennings A, Doores KJ, Wellman P, Seow J, Acors S, Graham C, Timms E, Kenny J, Neil S, Malim MH, Tibby SM, Shankar-Hari M. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat Med. 2020;26:1701–1707. doi: 10.1038/s41591-020-1054-6. [DOI] [PubMed] [Google Scholar]
- 95.Pierce CA, Preston-Hurlburt P, Dai Y, Aschner CB, Cheshenko N, Galen B, Garforth SJ, Herrera NG, Jangra RK, Morano NC, Orner E, Sy S, Chandran K, Dziura J, Almo SC, Ring A, Keller MJ, Herold KC, Herold BC. Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.abd5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Noval Rivas M, Wakita D, Franklin MK, Carvalho TT, Abolhesn A, Gomez AC, Fishbein MC, Chen S, Lehman TJ, Sato K, Shibuya A, Fasano A, Kiyono H, Abe M, Tatsumoto N, Yamashita M, Crother TR, Shimada K, Arditi M. Intestinal Permeability and IgA Provoke Immune Vasculitis Linked to Cardiovascular Inflammation. Immunity 2019; 51: 508-521. :e6. doi: 10.1016/j.immuni.2019.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Troisi J, Venutolo G, Terracciano C, Carri MD, Di Micco S, Landolfi A, Fasano A. The therapeutic use of the zonulin inhibitor AT-1001 (Larazotide) for a variety of acute and chronic inflammatory diseases. Curr Med Chem. 2021 doi: 10.2174/0929867328666210104110053. [DOI] [PubMed] [Google Scholar]
- 98.Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. 2011;91:151–175. doi: 10.1152/physrev.00003.2008. [DOI] [PubMed] [Google Scholar]
- 99.Di Micco S, Musella S, Scala MC, Sala M, Campiglia P, Bifulco G, Fasano A. In silico Analysis Revealed Potential Anti-SARS-CoV-2 Main Protease Activity by the Zonulin Inhibitor Larazotide Acetate. Front Chem. 2020;8:628609. doi: 10.3389/fchem.2020.628609. [DOI] [PMC free article] [PubMed] [Google Scholar]