Abstract
Purpose
Frontline health care workers (HCWs) must wear a standard N95 or FFP2 respirator during worldwide pandemics of respiratory diseases including COVID-19 to protect against airborne infectious pathogens when performing care activities. This study aimed to quantitatively investigate the fit of most of the common FFRs used during the COVID-19 pandemic in Iran.
Methods
A total of 37 volunteers were fit tested in 20 selected FFRs in a randomized order. The selected FFRs were underwent quantitative fit testing by PortaCount® model 8038. To determine the effects of face sizes on respirator fit, the participants’ facial dimensions were measured using a digital caliper.
Results
The rate of passing fit tests for the studied FFRs were surprisingly low with 11 out of 20 FFRs having less than 10% passing fit tests and the best performers having only 43% and 27% passing fit tests (brands 2 and 20, respectively). Cup-shaped respirators provided significantly greater fit than the vertical flat-fold ones (p < 0.001). A significantly different FFs were found among the respirator brands (F = 13.60, p < 0.001).
Conclusion
Overall, unacceptably low fit factors were obtained from the studied FFRs. The main reasons for this are suspected to single size and style for each studied FFR. It confirms the importance and requirement of the proper respirator selection in that way fitted optimally into facial dimensions, appropriate usage, and properly performing the fit testing procedure. A unique fit test panel should be developed to guide respirator wearers in selecting the appropriate FFR for their specific face sizes.
Keywords: Coronavirus (COVID-19). Filtering face-piece respirators. Quantitative fit test. Respiratory protection program. Respirator characteristics. Subject features
Introduction
There has been considerable concern about providing optimal respiratory protection against transmissible infectious pathogens in health care workers (HCWs) during worldwide pandemics such as influenza and the current COVID-19 pandemic. The HCWs are required to don FFP2 based on the EN149 standard [1] or N95 according to the National Institute for Occupational Safety and Health (NIOSH 42 CFR 84) [2], to protect themselves against pathogenic or infectious aerosols in healthcare setting; ultimately, against the COVID-19 during aerosol-generating procedures (AGPs) such as tracheal intubation, noninvasive ventilation, tracheotomy, cardiopulmonary resuscitation, manual ventilation before intubation, and bronchoscopy, among others [3, 4].
To assure respirators are capable of providing the necessary level of protection for wearers, it is required firstly that the respirators meet the filtration efficiency requirements and secondly that acceptable respirator fitting into the wearers’ facial dimension. “Filtration efficiency” pertains to the capability of the filter media to capture or filter out the dangerous airborne particles before entering to a subject’s breathing zone and inhaling by his/her respiratory system. “Respirator fitting” determines the capability of fitting a respirator with a specific make, model, style, and size into the wearers’ facial dimensions in order to protect respiratory system against the air contaminants. In addition to the filtration efficiency, the capability of respirator fitting shall not be ignored [5–7].
NIOSH has developed the total inward leakage (TIL) performance requirements for all classes of respirators [6, 8]. The TIL value is a function of filter penetration through filter media (FP) and leakage through the face-seal and exhalation valves. In other words, the TIL value means the realistic protection level obtained by the respirator while considering the roles of all penetration paths which can be obtained from the inverse of the fit factor (FF) measured during quantitative fit testing [9, 10]. Fit testing procedures determine whether acceptable fit is obtained by a specific respirator on a specific wearer. Respirator fit testing is classified into the qualitative fit testing (QLFT) and quantitative fit testing (QNFT) according to the respiratory protection standards [9–12]. QLFT relies on the subjective detection of a test agent by the respirator wearer. Test agents such as Bitrex™, saccharin, isoamyl acetate, and irritant smoke are presented to the respirator wearer while performing standardized fit test exercises. The respirator wearer provides a positive response if the test agent is detected during any exercise of the fit test. QNFT uses an instrument to objectively quantify the fit of a respirator to a wearer. The most commonly QNFT procedure using ambient aerosol (TSI PortaCount®) measures the concentration of an aerosol challenge agent outside the respirator (Cout) and compares to the aerosol concentration inside the respirator (Cin) with the presumption that aerosol present within the respirator is primarily due to leakage of the respirator and not penetration of particles through the filter media. The ratio of Cout to Cin is used to calculate the “fit factor” (FF) [12].
Generally, the selection of the fit test methods depend on the several criteria like the severity of the respiratory hazards [13], numbers of the organization’s workers [13], ability to use [14], skill and understanding on how to conduct the fit test [14], take preventive maintenance and care [14], check for system leakage [15], system calibration [15], suitability of the test method for the respirator [14], workers’ abilities to recognize the challenge agent [14], cost and benefits of the fit test method [14], and applicable legal requirements [14]. Noticeably, various factors influencing the respirator fit including the respirator design (style, brand, model, size, etc.) [16], facial features (face size, face shape, facial hair) [16], any changes in physical features such as facial deformity, dental changes, cosmetic surgery, substantial weight loss or gain [16], and also training [16].
Apart from the numerous studies were performed regarding the filtration efficiency [17–22]; several studies were conducted on the QNFT procedure [23–29]. For instance, Spies et al. stated more than one model and size of the RPE are required to provide for the respiratory protection program (RPP) [30]. Another study by Lawrence et al., concluded that the QLFT had lower pass rates than the TSI PortaCount® Plus with the N95-Companion; thus, it would be contributed to the most of the subjects retested, which in turns increased the required time and cost for the QLFT procedure [31]. In addition, another research focused that the user seal checks (USCs) could not be as a substitution for the QNFT, due to its low accuracy, sensitivity, and predictive value [32]. During the COVID-19 pandemic, numerous brands of imported and domestic respirators are being used by Iranian HCWs with primary attention paid to filtration efficiency. While an essential aspect of respiratory protection, this emphasis has inadvertently neglected the role of respirator fit. Surprisingly, the QNFT has not been investigated in Iran until this study, although several studies have examined the QLFT procedure on a few numbers of respirators [33–37]. Accordingly, this study, for the first time, was aimed to examine the quantitative fitting characteristics of the most well-known and available FFRs with various makes, models, and styles being used during the COVID-19 pandemic in Iran.
Materials and methods
Study design
This cross-sectional study was conducted on the volunteers of the School of Public Health, Shiraz University of Medical Sciences, Iran in 2020.
Inclusion criteria
Subjects who were not able to medically wear the respirators according to the OSHA 29CFR 1910.134 were not included in the study. For example, volunteers with cardiovascular, high blood pressure, or respiratory diseases (asthma, pneumonia, shortness of breath, dyspnea); facial hair (stubble and beard); facial deformity, acnes, or scars; facial surgery; and plastic or rhinoplasty surgery were excluded from the study [38, 39]. Meanwhile, if the study participants exhibited difficulty in breathing during the tests, we stopped the test and request them to remove the respirator.
Participants
Thirty-seven participants consisted of 25 (67.60%) females and 12 (32.40%) males with a mean age of 24.6 ± 4.2 years were tested in the Industrial Safety laboratory of the School of Health. Before undertaking the investigation, participants were given a description of their responsibilities during the test procedure including the USCs and QNFT procedures.
Respirators
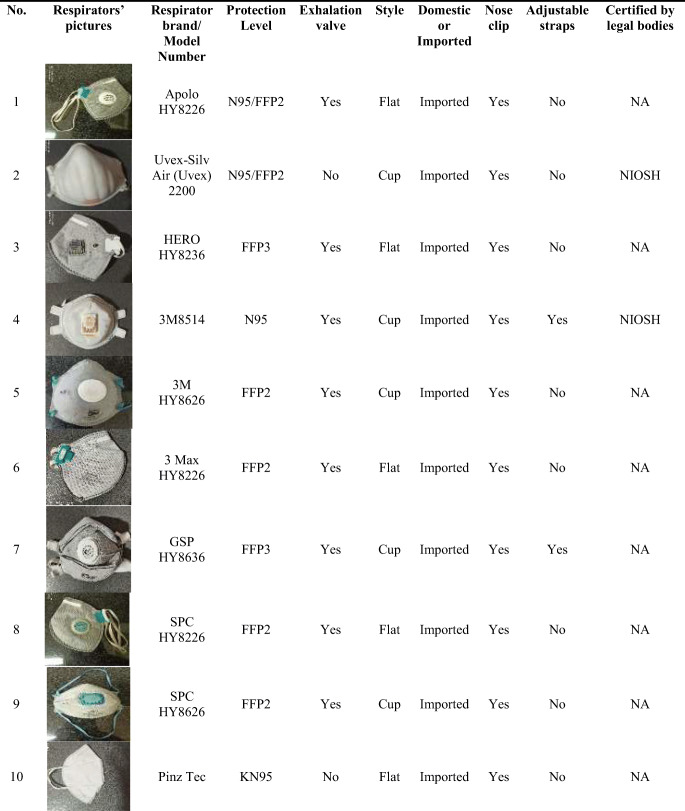
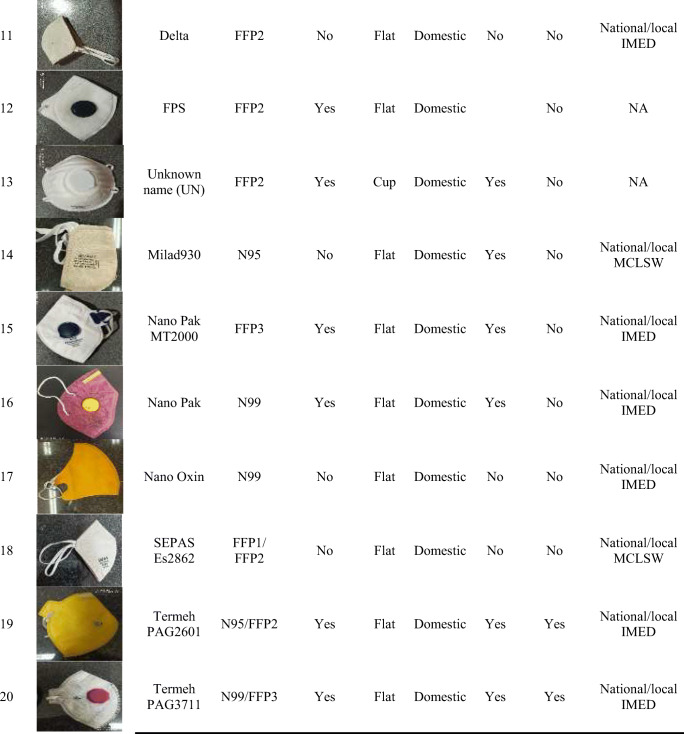
All participants were fit tested in each of the 20 selected FFRs available in the Iranian market (10 domestic and 10 imported). These 20 FFRs consisted of 6 cup-shaped and 14 vertical flat-fold respirators that are being used during the COVID-19 pandemic in Iran. Participants were fit tested in each respirator in a randomized order. Each respirator was assigned a number from 1 to 20 and randomized. Subjects were fit tested in each respirator in the randomized order using the Latin Square Design (LSD). The details of the studied FFRs are presented in Table 1.
Table 1.
Characteristics of the studied filtering face-piece respirators (FFRs) used in the current study a
NA Not Accessible
NIOSH US National Institute for Occupational Safety and Health
IMED Iranian National Medical Device Directorate
MCLSW Ministry of Cooperatives Labor and Social Welfare
a One Size Fits All (OSFA)
As shown in Table 1, 6 out of the 20 studied respirators were designated by the respirator manufacturer as FFP3 or N99 FFRs. The remaining respirators were N95 or N95/FFP2 designation. Five FFRs had exhalation valves. Six FFRs were cup-shaped and 14 FFRs were vertical flat-fold styles. A total of 10 brands of imported and 10 brands of domestic respirators were assessed in the study.
Instruments
A NaCl particle generator (model 8026, TSI Inc., Shoreview, MN, USA) was utilized to enhance the ambient aerosol levels for fit testing. The PortaCount® respirator fit tester (model 8038, TSI Inc., Shoreview, MN, USA) was employed to perform ambient aerosol fit testing procedures and the probe kit (model 8025-N95, TSI Inc., Shoreview, MN, USA) was used to insert a test probe into each respirator for testing.
Set up and experiments
The experiment was set up to comply with OSHA 29 CFR 1910.134 requirements. A small room approximately 4 m × 4 m, without operating air conditioning systems was selected for conducting the test. All participants abstained from drinking, eating, chewing gum, and smoking cigarettes or cigars for at least 30 min before starting the study to obtain reliable measurements.
Particle generation
A-non-hazardous test aerosol such as salt (NaCl) solution was used as the challenge agent in order to measure the leakage rate of the face-seal leakage of the respirator being fit tested. To produce the required particle concentration for ambient of fit testing room, all manufacturer’s instructions were followed; most notably, a 2% NaCl solution with the count median diameter (CMD) of 0.04 μm and geometric standard deviation (GSD) of 2.2 μm was used and the particle generator was placed at least 1.80 m away from the respirator fit tester during operation. The particle generator was only used sparingly; before beginning fit tests to build up particle concentration in the test room (~8000 particles/cc, for respirator with ≥99% efficiencies and ~ 800 particles/cc, for respirators with <99% efficiencies) and during testing only if the particle concentration fell below the minimum concentration of the fit tester which required for the respirator being tested.
Fit testing protocol
Before performing fit tests on participants, the fit tester “Daily Checks” were conducted to ensure full function of the device and sufficient aerosol count within the test room. QNFTs were conducted in Stand-Alone mode (not controlled by the fit test software). A test probe was inserted into each respirator between the participants’ nose and mouth and participants were instructed how to properly don and doff each respirator type, perform the USCs (negative and positive pressure seal checks) while wearing the respirator for at least 5 minutes before conducting the fit test. These procedures ensured ambient particles were purged from inside the respirator and permitted participants to ascertain if the respirator was seated correctly and comfortably.
Fit tests were carried out according to the OSHA eight exercise protocol (normal breathing; deep breathing; head side to side; head up and down; talking out loud; grimace; bending over; and normal breathing). Each test exercise was conducted for 1 min, except for the grimace (15 s). The harmonic mean of each exercise’s fit factor was used to calculate the “overall fit factor” by the PortaCount® respirator fit tester. The overall fit factor was reported and recorded at the end of each fit test. The overall fit factor was compared to the minimum required FF for FFRs (FF ≥ 100). If the overall fit factor was greater than or equal to the required FF, then the wearer would be approved to use that respirator for occupational protection (Fig. 1) [40].
Fig. 1.
The participant while performing the QNFT procedure using the PortaCount® fit tester model 8038
NIOSH developed the total inward leakage (TIL) performance requirements for all classes of respirators [6, 8]. The TIL value is a function of particle penetration through filter media (FP) and leakage through the face-seal and exhalation valves. In other words, the TIL tests the realistic protection level obtained by the respirator while considering the roles of all leakage/ penetration paths. The filtration efficiency (FE) is obtained from the FP which means that the ratio of test aerosol concentration inside the respirator to its concentration outside the respirator, which are represented by Eqs. 1 and 2, respectively:
| 1 |
| 2 |
The TIL value for each tested respirator was calculated based on the obtained overall fit factor in which an inverse relationship exists between the TIL value and overall fit factor, according to Eq. 3 [9, 10]:
| 3 |
Facial measurements
In the final stage, to determine the participants’ face sizes able to attain acceptable fit from each tested FFR, the participants’ bivariate facial dimensions (face length and face width) were measured using a calibrated digital caliper (model HB-101-111, Guanglu® Digital Caliper Manufacturer Co., Ltd., China) in accordance with ISO/TS 16976–2:2010. As a result, the participants’ face sizes were categorized into three groups: small (cells 1–3), medium (cells 4–7), or large face size (cells 8–10) according to the NIOSH bivariate fit test panel [41]. Overall, 18.9%, 75.7%, and 5.4% of the participants fell into the small, medium, and large face size cells, respectively, according to the NIOSH bivariate fit test panel.
Statistical analysis
Since subjects were measured repeatedly twenty times, the repeated measurement analysis of variance (ANOVA) was used to compare the FFs while controlling the factors such as age, gender, and participants’ face size as confounding variables. However, the mentioned confounding variables would be removed if they were non-significance. For each respirator, we tested differences between males and females by FFs using an independent sample T-test. To investigate the statistical effects of the participants’ face sizes on the FFs, the analysis of variance ANOVA was used with the small, medium, and large designations as the categorical variable of facial dimension [42].
On the other hand, in order to manage the correlation between the repeated measures by a more sophisticated analysis, the linear mixed model (LMM) was utilized in the way that the unconstructed covariance pattern was considered as the covariance pattern for the baseline (full) model; then, the simpler structure was used via the Likelihood Ratio Test (LTR) method to assess if a simpler covariance structure could be replaced to be reduced model. It should be mentioned that the consecutive LRTs selected in Heterogeneous Compound Symmetry (HCS) method as the simplest and most representative covariance pattern. Statistical differences were considered to be significant at the 95% level (p < 0.05). Statistical analysis was conducted using SPSS version 22.0 software.
Results
As seen in Table 2, the fit test passing rates varied amongst the studied FFRs. The overall fit test passing rate was low. Only 11 out of the 20 studied respirators had more than 10% of passing fit test values. Respirators 2, 4, and 20 had the highest fit test passing rates of all studied respirators (43%‚ 27%, and 27%), respectively.
Table 2.
The QNFT results of studied respirators compared to the brand 2 by the repeated measurements
| Respirator code | QNFT result (n = 37) | Mean ± SD of FFs |
95% CI for Mean of FFs | 95th Percentile of FFs | 95% CI for 95th Percentile of FFs | pa | Overall p |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Passed N (%) |
Failed N (%) |
|||||||||
| Lower | Upper | Lower | Upper | |||||||
| 1 | 1(2.70) | 36(97.30) | 23.24 ± 27.33 | 16.41 | 34.24 | 61 | 34 | 170 | <0.001 | <0.001 |
| 2 | 16(43.20) | 21(56.80) | 110.49 ± 72.20 | 87.94 | 133.78 | 200 | 200 | 200 | – | |
| 3 | 6(16.22) | 31(83.78) | 62.62 ± 39.33 | 50.59 | 74.38 | 151 | 101.01 | 160 | >0.05 | |
| 4 | 10(27) | 27(73.0) | 59.84 ± 52.51 | 43.32 | 76.64 | 180 | 140 | 180 | >0.05 | |
| 5 | 2(5.41) | 35(94.59) | 26.76 ± 24.09 | 20.16 | 35.03 | 100 | 50.60 | 100 | <0.001 | |
| 6 | 3(8.11) | 34(91.89) | 31.05 ± 30.46 | 22.16 | 41.51 | 104 | 54 | 140 | <0.001 | |
| 7 | 6(16.22) | 31(83.78) | 53.51 ± 47.21 | 39.52 | 70.24 | 155 | 101 | 200 | >0.05 | |
| 8 | 5(13.51) | 32(86.49) | 42.70 ± 44.01 | 29.24 | 56.81 | 144 | 100.40 | 180 | <0.001 | |
| 9 | 2(5.40) | 35(94.59) | 30.51 ± 28.28 | 22.17 | 40.51 | 106 | 60 | 115 | <0.001 | |
| 10 | 2(5.40) | 35(94.59) | 11.39 ± 22.21 | 5.76 | 19.23 | 100 | 20 | 100 | <0.001 | |
| 11 | 3(8.10) | 34(91.89) | 18.43 ± 27.60 | 10.73 | 27.43 | 102 | 25.50 | 120 | <0.001 | |
| 12 | 3(8.10) | 34(91.89) | 13.51 ± 26.17 | 5.89 | 23.40 | 100 | 10 | 100 | <0.001 | |
| 13 | 0 | 37 (100) | 4.84 ± 2.46 | 4.08 | 5.62 | 9.10 | 8.10 | 10 | <0.001 | |
| 14 | 4(10.81) | 33(89.19) | 18.38 ± 29.04 | 10.27 | 28.08 | 100 | 23.50 | 100 | <0.001 | |
| 15 | 4(10.81) | 33(89.19) | 24.70 ± 31.68 | 15.94 | 35.57 | 120 | 39 | 120 | <0.001 | |
| 16 | 0 | 37(100) | 6.48 ± 2.51 | 5.07 | 7.30 | 12 | 9.0 | 12 | <0.001 | |
| 17 | 0 | 37(100) | 5.08 ± 2.16 | 4.43 | 5.79 | 9.10 | 7.30 | 10 | <0.001 | |
| 18 | 3(8.10) | 34(91.89) | 21.40 ± 25.87 | 14.19 | 30.37 | 100.50 | 34 | 105 | <0.001 | |
| 19 | 6(16.22) | 31(83.78) | 29.40 ± 36.46 | 18.38 | 41.75 | 106.50 | 100 | 120 | <0.001 | |
| 20 | 10(27.0) | 27(73.0) | 110.73 ± 207.04 | 51.62 | 186.92 | 670.90 | 464.90 | 760 | >0.05 | |
QNFT Quantitative Fit Test
FF Fit Factor
SD Standard Deviation
CI Confidence Interval
a Mean FFs of all studied respirators compared to that of the brand 2 (as the best fitting respirator evaluated in this study)
Respirators 2 and 20 had the highest mean FF and [95CI%] at 110.5 [87–133] and 110.7 [51–187], respectively. The 95th percentile of FFs for respirators 2 and 20 respirators were also highest of all (200 and 670.90, respectively) contrasted against the three worst performing FFRs whose 95th percentile FFs were 9, 9, and 12. It is noteworthy that no statistically significant differences were found between the best fitting FFR (respirator 2) and four other studied FFRs (respirators 3, 4, 7, and 20).
Table 3 compares the mean FFs obtained from the studied FFRs by respirator style (cup vs. flat-fold). Overall, the cup-shaped FFRs had significantly higher mean FFs than flat-fold ones (48 vs. 30), p < 0.001.
Table 3.
Comparison of the QNFT results of the studied respirators by respirator style
| Respirator style | Numbers of studied respirators (%) |
Mean ± SD for FFs |
95% CI for Mean of FFs |
Overall p |
|
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Cup | 6 (30) | 47.66 ± 54.85 | 41.27 | 55.50 | <0.001 |
| Flat-fold | 14 (70) | 30.13 ± 67.99 | 24.38 | 36.04 | |
SD Standard Deviation
FF Fit Factor
CI Confidence Interval
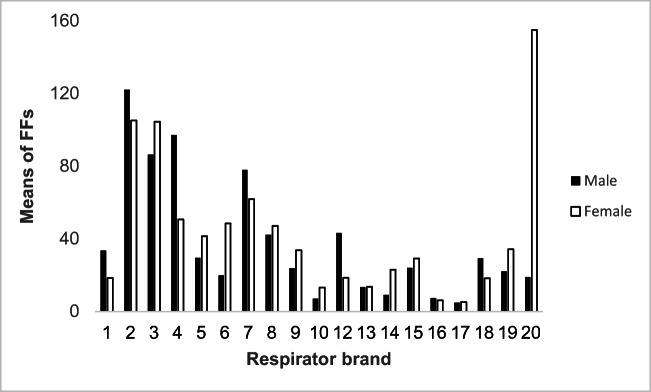
The mean FFs of the studied FFRs compared across gender are illustrated in Fig. 2. In line with our prediction, the overall mean of FFs were not significantly different between males and females, except for one of the best fitting respirators (brand 20) which showed a tremendous difference in FF between male and female test subjects (19 vs.155), p < 0.05. Moreover, no statistically significant differences were found between the participants’ face sizes and proportions of passing fit testing.
Fig. 2.
The mean fit factors (FFs) of the studied FFRs between male and female participants (refer to Table 1 for FFR features)
The factors significantly affecting the FFs obtained from the QNFT were noted in Table 4. As observed, the LMM noted that there were significantly different FFs among the respirator brands (F = 13.60, p < 0.001). Meantime, studied respirator styles were significantly different by the FFs during the QNFT procedure (F = 4.31, p < 0.05).
Table 4.
The factors influencing on the FFs obtained from the QNFT by the LMM
| Variable | F | p |
|---|---|---|
| Respirator brand | 13.60 | p < 0.001 |
| Style | 4.31 | 0.04 |
| Face size | −2.688 | 0.07 |
| Gender | 0.125 | 0.72 |
| Age | 2.411 | 0.12 |
Discussion
The purpose of the present study was to evaluate the quantitative fitting characteristics of the FFRs utilized during the COVID-19 pandemic in Iran. Several findings were obtained from this study which are explored in greater detail below. First of all, most of the studied respirators had low rates of passing fit tests (less than 10% of passed tests) and low FFs in general (<40). This finding is similar to previous studies [43–47]. Among the imported FFRs, one likely explanation is limited size and style options of the studied FFRs which came in only one size and one style. A further point, all studied FFRs were designed and developed for populations from foreign countries; in other words, these respirators were made to fit the facial anthropometry of a population different from the test group (Iranian people).
Even the domestic respirators in this study also had low proportions of passing fit tests (an average of 8.9%). Again, it seems the most likely reason is the molds used during the production of the domestic FFRs were based on facial anthropometries different from the study group. Additionally, no unique fit test panel was developed to determine/guide the appropriate respirator size for Iranian wearers. Moreover, no requirements regarding respirator fit testing and filtration efficiency were established to apply during the designing and manufacturing of the FFRs in Iran. It should be mentioned that the manufacturers shall develop a unique fit test panel by measuring the Iranian subjects’ facial dimensions and design the respirators based on the panel to ensure the respirators would provide optimal protection for the wearers. Also, it is critically required to develop the filtration efficiency and fit testing regulations as soon as possible to optimize the domestic respirators’ designs. At the very least, having domestic and imported respirators, total inward leakage testing, and developing a fit test panel will guide wearers on the face shape suited to the particular respirator. Meanwhile, a system for continuously verifying the quality of the respirators should be established. This could be accomplished through randomized spot-checking and yearly re-certification of approved respirators. In the future, manufacturers should account for both filtration efficiency and face fit for the end-user population to ensure adequate respiratory protection.
Seo et al. found there was low passing fit testing (21%) among the HCWs. Also, no statistically significant differences among the studied N95 respirators. Cameron et al., demonstrated that 6.2% of the Australian HCWs failed the first four masks and 1.6% failed all studied masks. It confirms that several masks are required to test on the HCWs to find the mask with optimal fitting [48]. The study by Coffey et al., concluded that fit testing using ambient aerosol (TSI PortaCount® Plus) and corn oil generated aerosol methods identified poorly fitting respirators better than two qualitative fit test methods (Bitrex and Sacchrin) and the TSI PortaCount® Plus coupled with the TSI N95-Companion aerosol generator [47]. Furthermore, Lawrence et al. concluded that the fit test passing rate for qualitative fit testing was lower than the TSI PortaCount® Plus coupled with TSI N95-Companion and this lead to more retesting; which in turn increases the required time and overall cost for performing fit tests [31]. Chadyiwa et al. compared the and QLFT and QNFT procedures among the 99 HCWs in South Africa. The proportions of passing QLFT were higher than the QNFT (89.2% vs. 45.9%); however, there were not significantly different in fit testing results. Considerably, only 45% of the passing both fit tests, was below the required value of 95% by the American National Standards Institute (ANSI). It should be taken into account that safety principles for implementation of fit testing are required to prevent and control the COVID-19 pandemic [49]. Grinshpun et al. assessed the performance of the developed AccuFIT 9000 apparatus compared to the PortaCount® fit tester (as a reference). The study stated the AccuFIT 9000 could be determined the poorly fitting respirators in which met the ANSI requirement≥0.95 (a sensitivity of 0.95 and specificity of 0.97); therefore, use of the novel apparatus could be acceptable for quantitative fit testing [50]. A recent study addressed that there was a significant inverse relation between the achieved respirator fit and facial hair [51]. But other researches indicated that high passing rates obtained from the quantitative fit testing [48, 52–54].
Additionally, another study stressed that only 59% and 18% of the participants failed the fit test after passing the USCs and that only after failure of the respirator such as strap slipping. That study concentrated on USCs and strap slipping despite participants passing the primary fit test because the researcher wanted subjects to attain the best possible respiratory protection for HCWs during aerosol generating procedures such as cardiopulmonary resuscitation (CPR) [55]. Other research has pointed out that the USCs should not be a replacement for fit testing [23, 45, 56]. Meanwhile, Viscusi et al. stated that the USCs might be partially beneficial during the respirator donning process for users who have previously passed a fit test for some models of the FFRs [57]. Lam et al. remarked that the USCs could not identify any gross leakage during respirator donning; however, it would promote the proper donning procedures [58]. O’Kelly et al. evaluated the accuracy of fit checking of the N95 and KN95 respirators compared to the QNFT procedures of those respirators, surgical, and cloth masks. The study presented that the N95 respirator offered more protection than the KN95 respirator. All non-N95 respirators had low FFs. There was a correlation between the results from the fit checking and FFs; thus‚ fit checking could not be determined or substituted for the respirator/mask fit testing [59].
This study showed that none of the 20 tested FFRs had high rates of passing fit tests and the two best performing FFRs, one imported (respirator 2) and one domestic (respirator 20) only provided passing QNFT about 43% and 27%, respectively. In addition, the study by De-Yñigo-Mojado et al., concentrated that the FFP3 had higher FFs than the surgical masks and other ones (FFP1, FFP2, etc.) used by the HCWs (40.7 ± 37.8 vs. 3.2 ± 5.0) [60]. Buckley et al. assessed the quantitative fit testing on the various masks/respirators with different filter media. Among all, the N95 respirator had highest FFs (FF = 83). The FFs for the HensNest (HEPA, 3 ply) was 3 times than that of the HensNest (HEPA, 1 ply) (23 vs. 8) [61]. Ballard et al. investigated the substitutions of a surgical mask and N95 respirator by surgical N95 respirators in Thailand. The FFs for two unsealed surgical masks ranged 4–5; while, for surgical masks which sealed with 3 M micro-pore tape were 33–38. Also, the FFs of four unsealed masks and respirators ranged from 11 to 95 after sealing increased to ≥199. To provide pleasant respiratory protection for the HCWs against the COVID-19 pandemic, it is mandatory to instruct regarding the proper donning and doffing of respirators and fit testing procedures as essential component of the RPP [62].
In this study, no statistically significant differences were found between the best fitting FFRs (respirator 2) and four other studied FFRs including 3, 4, 7, and 20. Contrasting our results to the high passing rates from the above discussed studies, our results indicate the facial dimensions of the study participants are poorly suited to the tested FFRs. Within this study, these results indicate some FFRs were better fitted to the facial anthropometries of our (Iranian) test subjects. Thus, it seems these selected respirators (2, 3, 4, 7, and 20) were more adequately fitted to the facial dimensions of the participants and more likely to provide the needed protection against COVID-19 in HCW settings; therefore, these five respirators could provide appropriate protection for some HCWs more frequently than the other studied FFRs. Another view, with such a poorly fitting selection of FFRs available to the Iranian HCWs, it is even more vital to perform QNFT to ensure the selection of an adequate FFR. The main cause of this finding could be attributed to the following reasons: Firstly, the respirator 2 was certified by NIOSH and respirator 20 was certified by the Iranian local/national regulatory bodies. Secondly, these respirators underwent TIL testing and demonstrated TIL values below 1% (equivalent to FF >100) which is one of the NIOSH’s criteria for certifying respirators [8]. Thirdly, the design of those respirators by consideration of the respirator characteristics and subject features and verifying a certification program regarding the TIL requirements. Some common design characteristics are believed to be better fit of these five respirators: four-point head band attachment, flexible nose clip, nose foam and a sealing lip on the respirators 2, 4, 7, and 20 led to the lower leakage across the nose bridge. O’Kelly et al. utilized some modifications (fit hacks such as placement of two brands of pantyhose, tightly binding the mask on the face by a rolled first-aid gauze, knotting ear loops, filling of visible gaps by a first aid gauze, or sealing the edges of the mask using a cloth tape) on the masks to improve the fit of KN95 and surgical masks. The applied fit hacks decreased the gaps between the face and the edges of the masks; as a result, the wearers protected against the airborne particles [63]. Runde et al. examined the effects of rubber band mask brace on a surgical mask during the quantitative fit testing procedure (TSI PortaCount Pro Model 8038). All studied subjects passed the test while the mask brace was anchored on a face shield or with a paperclip. It is drawn to the conclusion that all the surgical mask with brace could improve the protection during the COVID-19 pandemic [64].
In the current study, the repeated measurements exhibited that statistically significant differences were determined among the respirator brands by the FFs which consistent with the previous studies [44, 65–69]. The LMM was also represented the similar findings. The result of this research provides valuable insight into respirator fit testing; in total, face seal leakages result from several factors such as respirator size or style, incorrect face-piece size or shape, imperfect sealing lip, beard growth, perspiration or facial oils would lead to the slippage of the face-piece, user failure to use all the head straps, improper positioning and adjusting of a face-piece on a wearer’s face, incorrect head strap tension or position, improper maintenance of the respirators, and respirator damage [70]. Another implication, training on proper donning and doffing of the respirators would improve the protection for the wearers based on the study by Kim et al. [71].
In this study, cup-shaped respirators compared to the flat-fold ones had modestly higher mean FFs (47 vs. 38; p < 0.001). It seems that fitting characteristics for the design of the cup-shaped respirators were more suited to our population than the flat-fold ones. Better fit of cup-shaped FFR was not consistent with earlier studies [72, 73]. However, another study demonstrated no significant differences between the cup-shaped and flat-fold respirators by the FFs [74, 75]. We believe that various sizes for the tested respirators would improve the fit for wearers, based upon the large difference in male and female results in respirator 20.
Overall, there were no statistically significant differences in the mean FFs between males and females. This finding was similar to previous studies [26, 30, 65, 69, 76, 77]. On the contrary, some studies reported mean FFs of males were higher than those of females in most cases [45, 68, 73, 78]. Our observation of no significant differences is likely due to the overall poor fit (low FF scores and high fit variability) of the tested FFRs. This study was limited to FFRs currently being used by HCWs in Iran during the COVID-19 pandemic, other FFRs from different manufacturers, models, sizes, and styles may have different results.
Conclusion
Taken together, the overall rate of passing fit tests for all studied FFRs during the COVID-19 pandemic was low. It confirms the importance and requirement of the proper respirator selection (make, model, style, and size) in that way fitted optimally into wearers’ facial dimensions (face size and face shape), appropriate usage (donning and doffing), and properly performing the fit testing procedure; particularly for the HCWs exposed to the patients with a confirmed or suspected COVID-19. One important reason for these findings might be that all studied FFRs came in a one-size-fits-all. To satisfy with safety and ergonomics aspects of the respirator fitting requirements, it is required to provide various respirators with different makes, models, styles, and sizes in order. Furthermore, no unique fit test panel was developed or recommended to guide respirator wearers in selecting the FFR appropriate to their specific face sizes. Although manufacturers are already required to account for user anthropometry in their designs, this is a new rule that is still being implemented. To ensure manufacturers are complying with the new rules, the national legal bodies should certify respiratory protection products for both filtration efficiency and total inward leakage specific to the Iranian workforce. Meanwhile, an optimal fit test panel should also be developed for the Iranian people which determines the appropriate face sizes and shapes for selection of the respirators’ sizes and styles. At the very least, respirator manufacturers should identify which cells within an established fit test panel will fit their products.
Acknowledgements
The authors’ thank are due to the participants’ contribution in conducting the study tests.
Code availability
Not applicable.
Authors’ contributions
Anahita Fakherpour and Mehdi Jahangiri conceptualized and designed the research. Anahita Fakherpour, Mehdi Jahangiri, Hossein Charkhand, Siamak Abbaspour administrated the project and acquired the data. Mozhgan Seif conducted the statistical analyses and interpreted the data. Anahita Fakherpour and Mehdi Jahangiri prepared the first draft. Mehdi Jahangiri and Evan L. Floyd validated the accuracy of the work. All authors read and approved the final manuscript.
Funding
The present research was financially supported by Shiraz University of Medical Sciences, Shiraz, Iran (Grant numbers 99–01–42-22861).
Data availability
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
Ethical approval for the study was obtained by the Research Ethics Committee of Shiraz University of Medical Sciences (approval code IR.SUMS.REC.1399.678). Before commencing the study, all participants provided verbal and written informed consent which included the approval code, consent statement, study purposes and procedures, and right to confidentiality and withdrawal. After the study participants were initially trained about the study procedures, they signed a written informed consent form.
Consent to participate
Both verbal and written consents were taken from participants who voluntarily participated in the study.
Consent for publication
Both verbal and written consents were taken from participant who presented his picture during fit testing procedure in the manuscript.
Competing interests
No potential conflict of interest was reported by the authors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Anahita Fakherpour, Email: anahita.f00@gmail.com.
Mehdi Jahangiri, Email: jahangiri_m@sums.ac.ir.
Mozhgan Seif, Email: m_seif@sums.ac.ir.
Hossein Charkhand, Email: hossein.charkhand@aryasasol.com.
Siamak Abbaspour, Email: abbaspoursi@aryasasol.com.
Evan L. Floyd, Email: Evan-Floyd@ouhsc.edu
References
- 1.EN149 . Respiratory protective devices — Filtering half masks to protect against particles —Requirements, testing, marking. 2009. [Google Scholar]
- 2.NIOSH . The National Institute for Occupational Safety and Health. NIOSH Guide to the Selection and Use of Particulate Respirators. 1996. [Google Scholar]
- 3.World Health Organization (WHO) Rational use of personal protective equipment for coronavirus disease (COVID-19) and considerations during severe shortages. 2020. [Google Scholar]
- 4.World Health Organization (WHO) Advice on the use of masks in the community, during home care and in health care settings in the context of the novel coronavirus (2019-nCoV) outbreak. 2020. [Google Scholar]
- 5.Mueller W, Horwell CJ, Apsley A, Steinle S, McPherson S, Cherrie JW, et al. The effectiveness of respiratory protection worn by communities to protect from volcanic ash inhalation. Part I: Filtration efficiency tests. Int J Hyg Environ Health. 2018;221:967–976. doi: 10.1016/j.ijheh.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Rengasamy S, Walbert GF, Newcomb WE, Faulkner K, Rengasamy MM, Brannen JJ, et al. Total inward leakage measurement of particulates for N95 filtering facepiece respirators—a comparison study. Ann Occup Hygiene. 2014;58:206–216. doi: 10.1093/annhyg/met054. [DOI] [PubMed] [Google Scholar]
- 7.Fakherpour A, Jahangiri M. The respirator fitting characteristics: the emerging but negligent issues influencing optimal respiratory protection against new coronavirus (Covid-19). Int J Occup Hygiene. 2020;12:176–9.
- 8.NIOSH . Program concept for total inward leakage (TIL) performance requirements and test methods. 2004. [Google Scholar]
- 9.ISO16975-3 . International Organization for Standardization, Respiratory protective devices-Selection, use and maintenance- Part 3: Fit Testing procedures. 2017. [Google Scholar]
- 10.ANSI-Z88.10 . American National Standards Institute - respirator fit testing methods. New York: American Industrial Hygiene Association; 2010. [Google Scholar]
- 11.BS EN 529 . Respiratory protective devices – Recommendations for selection, use, care and maintenance – Guidance document European Committee for Standardization, British Standards Institution (BSI). 2005. [Google Scholar]
- 12.OSHA . Occupational Safety and Health Administration Title 29 CFR.1910.134 app a. respiratory protection program standards- fit testing procedures (mandatory) Washington: Occupational Safety & Health Administration (OSHA), government publishing Office; 2016. [Google Scholar]
- 13.Pritchard JA. A Guide to industrial respiratory protection: Los Alamos Scientific Laboratory; 1976.
- 14.Colton CE. Respirator fit testing: choosing the best method. Met Finish. 2001;99:53–55. doi: 10.1016/S0026-0576(01)81436-7. [DOI] [Google Scholar]
- 15.3M . 3M Respirator Fit Testing. 2004. [Google Scholar]
- 16.Rajhans GS, Pathak BP. Practical guide to respirator usage in industry 2ed. Woburn: Butterworth-Heinemann; 2002. [Google Scholar]
- 17.Faridi S, Nodehi RN, Sadeghian S, Tajdini M, Hoseini M, Yunesian M, et al. Can respirator face masks in a developing country reduce exposure to ambient particulate matter? J Exposure Sci Environ Epidemiol. 2020;30:606–617. doi: 10.1038/s41370-020-0222-6. [DOI] [PubMed] [Google Scholar]
- 18.Viscusi DJ, Bergman M, Sinkule E, Shaffer RE. Evaluation of the filtration performance of 21 N95 filtering face piece respirators after prolonged storage. Am J Infect Control. 2009;37:381–386. doi: 10.1016/j.ajic.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ntlailane MGL, Wichmann J. Effectiveness of N95 respirators for nanoparticle exposure control (2000–2016): a systematic review and meta-analysis. J Nanopart Res. 2019;21:1–15. doi: 10.1080/15459624.2013.877590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.B-g Y, Wang Y-x, X-y Y, Zhang F, Y-l P. Impact of structural features on dynamic breathing resistance of healthcare face mask. Sci Total Environ. 2019;689:743–753. doi: 10.1016/j.scitotenv.2019.06.463. [DOI] [PubMed] [Google Scholar]
- 21.Rengasamy S, Miller A, Eimer BC. Evaluation of the filtration performance of NIOSH-approved N95 filtering facepiece respirators by photometric and number-based test methods. J Occup Environ Hyg. 2011;8:23–30. doi: 10.1080/15459624.2010.515556. [DOI] [PubMed] [Google Scholar]
- 22.Rengasamy S, Eimer BC, Shaffer RE. Comparison of nanoparticle filtration performance of NIOSH-approved and CE-marked particulate filtering facepiece respirators. Ann Occup Hygiene. 2009;53:117–128. doi: 10.1093/annhyg/men086. [DOI] [PubMed] [Google Scholar]
- 23.Danyluk Q, Hon CY, Neudorf M, Yassi A, Bryce E, Janssen B, et al. Health care workers and respiratory protection: is the user seal check a surrogate for respirator fit-testing? J Occup Environ Hygiene. 2011;8:267–270. doi: 10.1080/15459624.2011.566016. [DOI] [PubMed] [Google Scholar]
- 24.Skretvedt OT, Loschiavo JG. Effect of facial hair on the face seal of negative-pressure respirators. Am Ind Hyg Assoc J. 1984;45:63–66. doi: 10.1080/15298668491399389. [DOI] [PubMed] [Google Scholar]
- 25.Janssen LL, Luinenburg DM, Mullins HE, Nelson TJ. Comparison of three commercially available fit-test methods. AIHA J. 2002;63:762–767. doi: 10.1080/15428110208984767. [DOI] [PubMed] [Google Scholar]
- 26.Lee K, Slavcev A, Nicas M. Respiratory protection against mycobacterium tuberculosis: quantitative fit test outcomes for five type N95 filtering-facepiece respirators. J Occup Environ Hyg. 2004;1:22–28. doi: 10.1080/15459620490250026. [DOI] [PubMed] [Google Scholar]
- 27.Myong J-P, Byun J, Cho Y, Seo H-K, Baek J-E, Koo J-W, et al. The education and practice program for medical students with quantitative and qualitative fit-test for respiratory protective equipment. Industrial Health. 2015. 10.2486/indhealth.2015-0072. [DOI] [PMC free article] [PubMed]
- 28.Hon C-Y, Danyluk Q, Bryce E, Janssen B, Neudorf M, Yassi A, et al. Comparison of qualitative and quantitative fit-testing results for three commonly used respirators in the healthcare sector. J Occup Environ Hygiene. 2017;14:175–179. doi: 10.1080/15459624.2016.1237030. [DOI] [PubMed] [Google Scholar]
- 29.Mullins H, Danisch S, Johnston A. Development of a new qualitative test for fit testing respirators. Am Ind Hyg Assoc J. 1995;56:1068–1073. doi: 10.1080/15428119591016278. [DOI] [PubMed] [Google Scholar]
- 30.Spies A, Wilson KS, Ferrie R. Respirator fit of a medium mask on a group of south Africans: a cross-sectional study. Environ Health. 2011;10:17. doi: 10.1186/1476-069X-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawrence RB, Duling MG, Calvert CA, Coffey CC. Comparison of performance of three different types of respiratory protection devices. J Occup Environ Hyg. 2006;3:465–474. doi: 10.1080/15459620600829211. [DOI] [PubMed] [Google Scholar]
- 32.Lam S, Lee J, Yau S, Charm C. Sensitivity and specificity of the user-seal-check in determining the fit of N95 respirators. J Hosp Infect. 2011;77:252–256. doi: 10.1016/j.jhin.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fakherpour A, Jahangiri M, Seif M. Qualitative fitting characteristics of filtering face-piece respirators on Iranian people. J Environ Health Sci Eng. 1:11. 10.1007/s40201-020-00484-x. [DOI] [PMC free article] [PubMed]
- 34.Honarbakhsh M, Jahangiri M, Ghaem H, Ghorbani M, Omidvari F, Khorasani MA, et al. Qualitative fit testing of medium-size N95/FFP2 respirators on iranian health care workers. Health Scope. 2018;7. 10.5812/jhealthscope.62884.
- 35.Jahangiri MMAH. Assessment of fitting half-facepiece respirators in IRANIAN workers of a petrochemical industry. Hormozgan Med J. 1388.
- 36.Fakherpour A, Jahangiri M, Yousefinejad S, Seif M, Banaee S. Assessment of aloe vera for qualitative fit testing of particulate respirators: a logistic regression approach. Ind Health. 2020;58:46–53. doi: 10.2486/indhealth.2109-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fakherpour A, Jahangiri M, Yousefinejad S, Seif M. Feasibility of replacing homemade solutions by commercial products for qualitative fit testing of particulate respirators: a mixed effect logistic regression study. MethodsX. 2019;6:1313–1322. doi: 10.1016/j.mex.2019.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.HSE 282/28. Health and Safety Executive (HSE). Guidance on respiratory protective equipment (RPE) fit testing. 2012.
- 39.Nancy J, Bolinger RHS. NIOSH Guide to Industrial Respiratory Protection. U.S. Department of Health and Human Services: Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health (NIOSH); 1987.
- 40.TSI . Portacount® Pro 8030 And Portacount® Pro+ 8038 Respirator Fit Testers. Shoreview: TSI; 2015. [Google Scholar]
- 41.ISO/TS16976-2:2010(en). International Organization for Standardization, Respiratory protective devices — Human factors (ISO/TS 16976-2:2010(en)) — Part 2: Anthropometrics. 2010.
- 42.Kutner MH, Nachtsheim CJ, Neter J, Li W. Applied linear statistical models. New York: McGraw-Hill Irwin; 2005. [Google Scholar]
- 43.Brosseau LM. Fit testing respirators for public health medical emergencies. J Occup Environ Hyg. 2010;7:628–632. doi: 10.1080/15459624.2010.514782. [DOI] [PubMed] [Google Scholar]
- 44.Pauli U, Karlen S, Summermatter K. The importance of fit-testing particulate filtering facepiece respirators! Appl Biosafety. 2014;19:184–192. doi: 10.1177/153567601401900402. [DOI] [Google Scholar]
- 45.Lam SC, Lee JKL, Lee LYK, Wong KF, Lee CNY. Respiratory protection by respirators: the predictive value of user seal check for the fit determination in healthcare settings. Infect Control Hospital Epidemiol. 2011;32:402–403. doi: 10.1086/659151. [DOI] [PubMed] [Google Scholar]
- 46.Manganyi J, Wilson KS, Rees D. Quantitative respirator fit, face sizes, and determinants of fit in south African diagnostic laboratory respirator users. Ann Work Exposures Health. 2017;61:1154–1162. doi: 10.1093/annweh/wxx077. [DOI] [PubMed] [Google Scholar]
- 47.Clapham SJ, Stephenson DJ, Wallace DO, Lillquist DR, Suruda AJ. Comparison of N95 disposable filtering facepiece fits using Bitrex qualitative and TSI Portacount® quantitative fit testing. Int J Occup Environ Health. 2000;6:50–54. doi: 10.1179/oeh.2000.6.1.50. [DOI] [PubMed] [Google Scholar]
- 48.Cameron S, Cheung W, Cronin N, Griffiths K, Hunt R, Innes L, et al. Quantitative fit testing with limited supplies of respirator masks in hospital personnel during the COVID-19 pandemic. Australian Health Rev. 2020;44:542–543. doi: 10.1071/AH20154. [DOI] [PubMed] [Google Scholar]
- 49.Chadyiwa M, Van Wyk R. Qualitative versus quantitative fit-testing of two commonly used respirators in resource-limited healthcare facilities. 2020. [Google Scholar]
- 50.Grinshpun SA, Yermakov M, Kano M. Evaluation of AccuFIT 9000: a novel apparatus for quantitative fit testing of particulate respirators. Ann Work Exposures Health. 2020. 10.1093/annweh/wxaa116. [DOI] [PMC free article] [PubMed]
- 51.Sandaradura I, Goeman E, Pontivivo G, Fine E, Gray H, Kerr S, et al. A close shave? Performance of P2/N95 respirators in health care workers with facial hair: results of the BEARDS (Adequate Respiratory DefenceS) study. J Hospital Infect. 2020. 10.1016/j.jhin.2020.01.006. [DOI] [PubMed]
- 52.Winski T, ErgHF RGC. Proportion of the UK working population tested who achieved an effective fit with the 3M 8835+ FFR. 2016. [Google Scholar]
- 53.Winski TA, Mueller WA, Graveling RA. If the mask fits: facial dimensions and mask performance. Int J Ind Ergon. 2019;72:308–310. doi: 10.1016/j.ergon.2019.05.011. [DOI] [Google Scholar]
- 54.Floyd EL, Henry JB, Johnson DL. Influence of facial hair length, coarseness, and areal density on seal leakage of a tight-fitting half-face respirator. J Occup Environ Hyg. 2018;15:334–340. doi: 10.1080/15459624.2017.1416388. [DOI] [PubMed] [Google Scholar]
- 55.Hwang SY, Yoon H, Yoon A, Kim T, Lee G, Jung KY, et al. N95 filtering facepiece respirators do not reliably afford respiratory protection during chest compression: A simulation study. Am J Emerg Med. 2020;38:12–17. doi: 10.1016/j.ajem.2019.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Derrick J, Chan Y, Gomersall C, Lui S. Predictive value of the user seal check in determining half-face respirator fit. J Hosp Infect. 2005;59:152–155. doi: 10.1016/j.jhin.2004.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Viscusi DJ, Bergman MS, Zhuang Z, Shaffer RE. Evaluation of the benefit of the user seal check on N95 filtering facepiece respirator fit. J Occup Environ Hyg. 2012;9:408–416. doi: 10.1080/15459624.2012.683757. [DOI] [PubMed] [Google Scholar]
- 58.Lam SC, Lui AK, Lee LY, Lee JK, Wong K, Lee CN. Evaluation of the user seal check on gross leakage detection of 3 different designs of N95 filtering facepiece respirators. Am J Infect Control. 2016;44:579–586. doi: 10.1016/j.ajic.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O'Kelly E, Arora A, Pirog S, Ward J, Clarkson PJ. Comparing the Fit of N95, KN95, Surgical, and Cloth Face Masks and Assessing the Accuracy of Fit Checking. medRxiv. 2020. 10.1101/2020.08.17.20176735. [DOI] [PMC free article] [PubMed]
- 60.De-Yñigo-Mojado B, Madera-García J, Becerro-de-Bengoa-Vallejo R, Losa-Iglesias ME, Rodríguez-Sanz D, San-Antolín M, et al. Fit factor of masks used by physicians in clinical settings. Int J Med Sci. 2020;17:2696. doi: 10.7150/ijms.50657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buckley J, Gladle M, Murray K, Sample W. SUBJECT: Quantitative Respirator Fit Testing of HensMask. 2020. [Google Scholar]
- 62.Ballard DH, Jammalamadaka U, Meacham KW, Hoegger MJ, Burke BA, Morris JA, et al. Quantitative fit tested N95 respirator-alternatives generated with CT imaging and 3D printing: a response to potential shortages during the COVID-19 pandemic. Acad Radiol. 2020. 10.1016/j.acra.2020.11.005. [DOI] [PMC free article] [PubMed]
- 63.O'Kelly E, Arora A, Pirog S, Ward J, Clarkson PJ. Face Mask Fit Hacks: Improving the Fit of KN95 Masks and Surgical Masks with Fit Alteration Techniques. medRxiv. 2020. 10.1101/2020.10.28.20221895. [DOI] [PMC free article] [PubMed]
- 64.Runde DP, Harland KK, Van Heukelom P, Faine B, O'Shaughnessy P, Mohr NM. The “double eights mask brace” improves the fit and protection of a basic surgical mask amidst COVID-19 pandemic. J Am College Emerg Physicians Open 10.1002/emp2.12335. [DOI] [PMC free article] [PubMed]
- 65.Oestenstad RK, Elliott LJ, Beasley TM. The effect of gender and respirator brand on the association of respirator fit with facial dimensions. J Occup Environ Hyg. 2007;4:923–930. doi: 10.1080/15459620701709619. [DOI] [PubMed] [Google Scholar]
- 66.Coffey CC, Lawrence RB, Zhuang Z, Duling MG, Campbell DL. Errors associated with three methods of assessing respirator fit. J Occup Environ Hyg. 2006;3:44–52. doi: 10.1080/15459620500455398. [DOI] [PubMed] [Google Scholar]
- 67.Coffey CC, Lawrence RB, Zhuang Z, Campbell DL, Jensen PA, Myers WR. Comparison of five methods for fit-testing N95 filtering-facepiece respirators. Appl Occup Environ Hyg. 2002;17:723–730. doi: 10.1080/10473220290107002. [DOI] [PubMed] [Google Scholar]
- 68.Kim H, Han DH, Roh YM, Kim K, Park YG. Facial anthropometric dimensions of Koreans and their associations with fit of quarter-mask respirators. Ind Health. 2003;41:8–18. doi: 10.2486/indhealth.41.8. [DOI] [PubMed] [Google Scholar]
- 69.Foereland S, Robertsen O, Hegseth MN. Do various respirator models fit the Workers in the Norwegian Smelting Industry? Saf Health Work. 2019;10:370–376. doi: 10.1016/j.shaw.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Centers for Disease Control . NIOSH recommended guidelines for personal respiratory protection of Workers in Health-care Facilities Potentially Exposed to tuberculosis. 1992. [Google Scholar]
- 71.Kim H, Lee J, Lee S, Oh J, Kang B, Lim TH, et al. Comparison of fit factors among healthcare providers working in the Emergency Department Center before and after training with three types of N95 and higher filter respirators. Medicine (Baltimore) 2019;98:e14250-e. doi: 10.1097/MD.0000000000014250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee S, Kim H, Lim T, Oh J, Kang H, Ahn C, et al. Simulated workplace protection factors for respirators with N95 or higher filters for health care providers in an emergency medical centre: a randomized crossover study. Hong Kong J Emerg Med. 2017;24:282–289. doi: 10.1177/1024907917735088. [DOI] [Google Scholar]
- 73.Huh YJ, Jeong HM, Lim J, Park HY, Kim MY, Oh HS, et al. Fit characteristics of N95 filtering facepiece respirators and the accuracy of the user seal check among Koreans. Infect Control Hospital Epidemiol. 2018;39:104–107. doi: 10.1017/ice.2017.271. [DOI] [PubMed] [Google Scholar]
- 74.Niezgoda G, Kim J-H, Roberge RJ, Benson SM. Flat fold and cup-shaped N95 filtering facepiece respirator face seal area and pressure determinations: a stereophotogrammetry study. J Occup Environ Hyg. 2013;10:419–424. doi: 10.1080/15459624.2013.801246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhuang Z, Coffey CC, Ann RB. The effect of subject characteristics and respirator features on respirator fit. J Occup Environ Hyg. 2005;2:641–649. doi: 10.1080/15459620500391668. [DOI] [PubMed] [Google Scholar]
- 76.Seo H, B-k K, Y-i K. Fit Testing for Domestic N95 Medical Masks. J Korean Soc Occup Environ Hygiene. 2020;30:124–133. doi: 10.15269/JKSOEH.2020.30.2.124. [DOI] [Google Scholar]
- 77.Oberg T, Brosseau LM. Surgical mask filter and fit performance. Am J Infect Control. 2008;36:276–282. doi: 10.1016/j.ajic.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Han DH. Fit factors for quarter masks and facial size categories. Ann Occup Hygiene. 2000;44:227–234. doi: 10.1016/j.ajem.2019.03.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request.