Abstract
Chronic obstructive pulmonary disease (COPD) often remains undiagnosed and untreated. To date, COPD screening/case finding has not been designed to identify clinically significant COPD, disease ready for therapies beyond smoking cessation. Herein, we describe the ongoing prospective, pragmatic cluster-randomized controlled trial to assess specificity and sensitivity of the COPD Assessment in Primary Care To Identify Undiagnosed Respiratory Disease and Exacerbation Risk (CAPTURE) tool consisting of 5 questions and peak expiratory flow. The tool is designed to identify clinically significant COPD (forced expiratory volume in 1 second [FEV1] to forced vital capacity [FVC] ratio <.70 plus FEV1% predicted <60% or increased risk for exacerbation) and the trial will explore the impact of CAPTURE-based screening on COPD diagnosis and treatment rates in primary care patients.
Of a total planned enrollment of 5000 English- or Spanish-speaking patients 45 to 80 years of age without a prior COPD diagnosis from 100 primary care practices, a total of 68 practices and 3064 patients have been enrolled in the study. Practices are centrally randomized to either usual care or clinician receipt of patient-level CAPTURE results. All clinicians receive basic COPD education with those in intervention practices also receiving CAPTURE interpretation education. In a single visit, patient participants complete a CAPTURE screening, pre- and post-bronchodilator spirometry and baseline demographic and health questionnaires to validate CAPTURE sensitivity, specificity, and predictive value of identifying undiagnosed, clinically significant COPD. One-year follow-up chart reviews and participant surveys assess the impact of sharing versus not sharing CAPTURE results with clinicians on clinical outcomes including level of respiratory symptoms and events and clinicians’ initiation of recommendation-concordant COPD care. This is one of the first U.S. studies to validate and assess impact of a simple COPD screening tool in primary care.
Keywords: copd, chronic obstructive pulmonary disease, primary care, screening, Case finding, practice-based research networks, tool validation, CAPTURE study
Introduction
This article contains supplemental material.
The COPD Assessment in Primary Care To Identify Undiagnosed Respiratory Disease and Exacerbation Risk (CAPTURE) Validation Study is a pragmatic cluster, randomized controlled trial to assess a screening approach to identify clinically significant chronic obstructive pulmonary disease (COPD) in primary care practices in the United States. COPD is the fourth leading cause of death in the United States and a major cause of morbidity, mortality, disability, hospitalizations, and health care expenditures.1-2-3 Within U.S. primary care settings, where the majority of people with COPD receive care, COPD is often under recognized and under diagnosed.4,5 This is similar to data reported from other countries.3,6-7-8
Undiagnosed COPD adversely affects patients,2-3-4,9-10-11-12 while recognition and diagnosis of COPD can facilitate improved patient outcomes through appropriate treatments.13,14 Limited data suggest that earlier detection of primary care patients with previously undiagnosed, yet clinically significant COPD, may improve short- and long-term patient outcomes and may be cost-effective.15,16
The U.S. Preventive Services Task Force recommends against screening asymptomatic adults for COPD17,18 but does not address the many adults who have unacknowledged and unaddressed respiratory symptoms and events.19,20 The CAPTURE tool was designed to identify those unrecognized symptoms and respiratory events.
Several COPD case-finding tools have been created based on existing epidemiologic literature or expert opinion.21-22-23-24-25 In general, these tools were designed without consideration of disease severity or exacerbation risk, resulting in the identification of a high proportion of patients with mild or minimally symptomatic disease.23,24,26-27-28-29-30-31 Most of the tools have only modest sensitivity and specificity.32 In addition, most of the case finding studies exclude people without a smoking history and the tools used rely on smoking as a primary risk factor, therefore, failing to identify non-smokers who constitute 25% of the COPD population.18,33 Even with these limitations, COPD screening/case finding in primary care using existing tools has been demonstrated to have a small but significant impact on increasing rates of diagnoses and physician’s clinical actions but no impact on patient outcomes.34-35-36
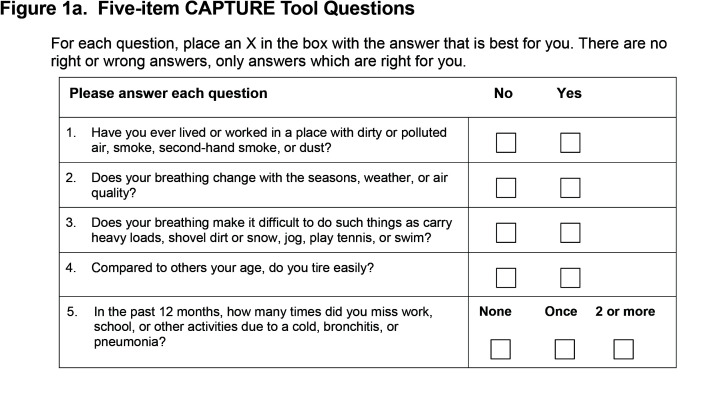
The CAPTURE tool was developed to overcome these limitations and designed to not a priori exclude never and former smokers. Using a novel approach, the selection of candidate items for the CAPTURE tool was based on 3 robust datasets.37 Machine learning, random forests analyses and qualitative work identified and validated variables most important in identifying patients with clinically significant COPD. A final 5-item questionnaire (Figure 1a) exhibited good operating characteristics for separating COPD cases from controls without COPD regardless of current or former smoking status and excellent operating characteristics to identify individuals with clinically significant COPD defined as an individual with spirometrically-confirmed obstruction (forced expiratory volume in 1 second [FEV1] to forced vital capacity [FVC] <0.70) plus FEV1 <60% of predicted or at high risk of exacerbation.38,39 The addition of sex-based peak expiratory flow (PEF) thresholds enhanced the operating characteristics of the 5-item questionnaire. Together the 5 questions and selective PEF constitute the CAPTURE approach with scores ranging from 0 to 6. (Figure 1b)
The primary goal of this study is to expand the generalizability and validation of the CAPTURE approach to include a wide range of primary care practices.39,40 The secondary goal is to assess the impact of sharing CAPTURE screening results with the primary care physicians and other clinicians.
Aims and Hypotheses
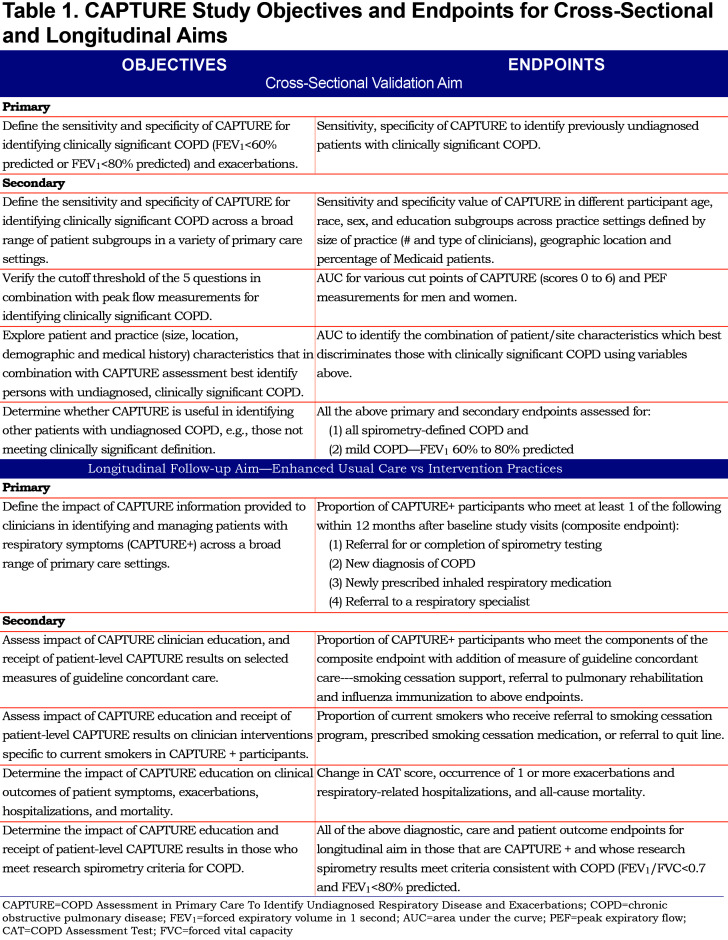
The CAPTURE Validation in Primary Care Study has cross-sectional validation and longitudinal follow-up aims (Table 1). The principal hypothesis is that the CAPTURE screening approach can identify primary care patients aged 45 to 80 years of age who have undiagnosed, clinically significant COPD with high sensitivity and specificity. The secondary hypothesis is that providing CAPTURE screening results to primary care clinicians will increase rates of COPD diagnosis and guideline concordant COPD care and improve patient outcomes. The primary and secondary objectives for the cross-sectional and longitudinal aims, as well as the endpoints to assess these goals, are enumerated in Table 1.
The study protocol was approved by the institutional review boards of Weill Cornell (central site), University of Michigan (data coordinating center [DCC]), each practice-based research network (PBRN) and the COPD Foundation. The CAPTURE Validation study is registered with the U.S. Clinical Trials Registration (NCTR NCT03581227) (cross-sectional validation aim), and NCT03583099 (longitudinal follow-up aim).
Methods/Design
Overview
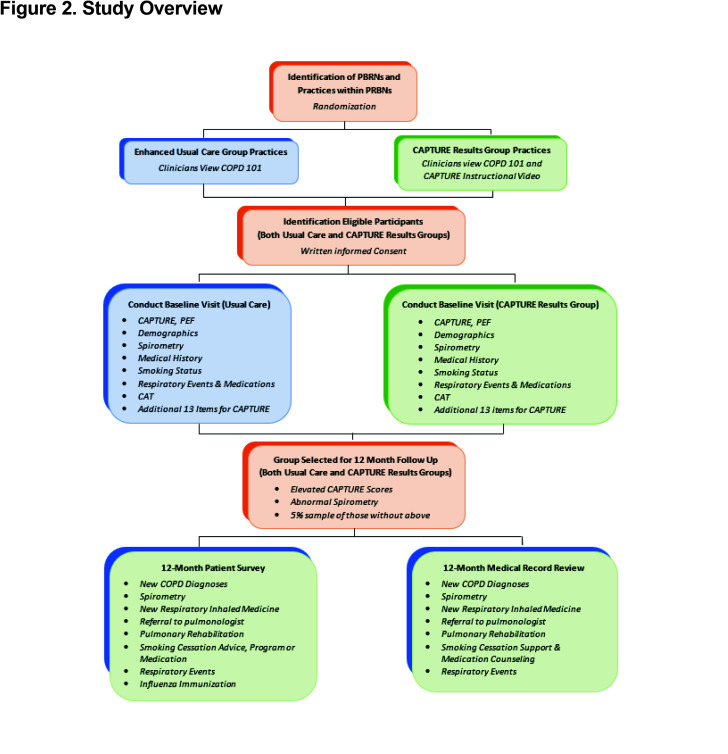
This is a prospective, pragmatic cluster randomized clinical trial (cRCT). Patient participants are recruited from 100 practices identified from 6 practice-based research networks (PBRNs). Randomization occurs at the practice level to either usual care or intervention defined as clinician receipt of patient level CAPTURE results. (Figure 2 and Table 1)
Recruitment of Practices and Patient Participants
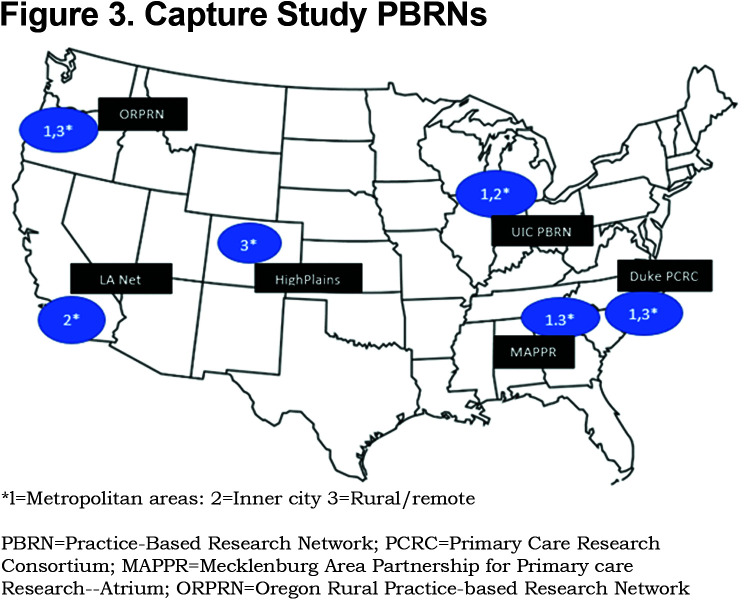
PBRNs: The 6 PBRNs were selected from a pool of PBRNs with prior experience in large scale clinical trials. Final selection was based on interest, availability, prior experience in respiratory-related clinical trials, geographic spread, and socioeconomic and racial/ethnic diversity of patients within the PBRN-affiliated practices. All expressed willingness to participate in a 5-year trial to enroll at least 20 practices and 1000 patients. (Figure 3)
Practices and Clinicians: Each PBRN identifies and enrolls practices based on interest and availability of practice space to complete the research visit and willingness of the practice’s clinicians to view the basic COPD educational video and for those randomized to the intervention, to also view the CAPTURE tool explanation and instructional video.
Patient Participants: Within each enrolled practice, potentially eligible patients of clinicians completing the required education are identified and invited to participate. Eligibility criteria are broad to allow generalizability to most primary care patients and, unlike many COPD screening or case finding studies, does not include restrictions on smoking status to facilitate identification of the group of people with COPD who have never smoked.
Inclusion criteria are:
Men or women between the ages of 45 and 80 years
Ability to read and complete visit in English or Spanish
Stated willingness to comply with all study procedures and availability for 1-year follow-up.
Exclusion criteria include:
Previous clinician diagnosis of COPD
Treated respiratory illness (with antibiotics and/or systemic steroids) in the 30 days prior to study visit
Unwilling or unable to complete all components of the single study visit
To ensure that participants can safely complete spirometry, patients are excluded if in the past 30 days they have undergone eye, chest or abdominal surgery, or experienced an acute myocardial infarction or stroke.
Randomization
Practices are randomized 1:1 centrally by the DCC. Randomization is stratified by PBRN, into 1 of the 2 arms:
Enhanced usual care arm with basic COPD education, or
the CAPTURE intervention arm with basic COPD education, plus CAPTURE education, and clinician receipt of patient level CAPTURE screening results.
The decision to randomize by practice rather than individual patient participant was done to limit cross contamination within a practice.
Practice-Related Study Procedures
The cross-sectional validation aimrequires that all enrolled patient participants complete the 5-item CAPTURE questions and perform 3 PEF maneuvers with the best PEF results selected for use. These data, along with the pre- and, when appropriate, post-bronchodilator spirometry, allow assessment of the operating characteristics of the CAPTURE tool to identify clinically significant COPD.
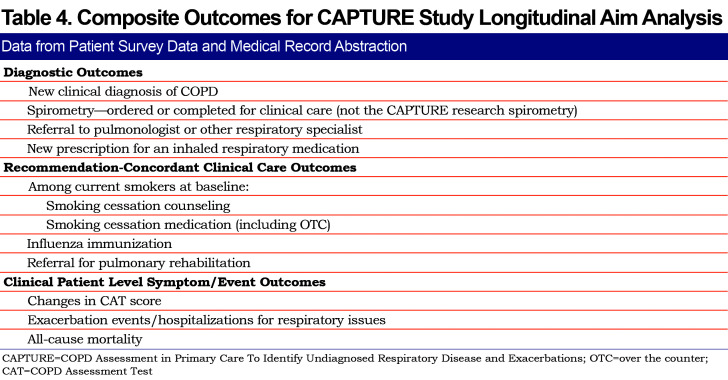
The longitudinal follow-up aim compares the rates of predefined outcomes (new COPD diagnosis, elements of COPD recommendation concordant care and patient-reported respiratory outcomes) between the 2 practice arms – enhanced usual care versus CAPTURE intervention. (Table 1)
Following randomization, all clinicians are invited to view the 30-minute basic COPD educational video developed to support optimal COPD management. Video content is based on recommendations from the 2017 update of the Global Initiative for Chronic Obstructive Lung Disease.41 Clinicians in practices randomized to the intervention group are additionally invited to view a brief (7-minute) video that provides information on interpretation and use of the CAPTURE tool in clinical care. Only patients of clinicians who have completed the videos for their study arm are eligible for enrollment from the practices.
Patient Participant-Related Study Procedures
Following review and signing of informed consent, patient participants complete the single study visit (V1) collecting the data summarized in Figure 3. A subset of enrolled patient participants is selected for a 12-month (longitudinal) follow-up. Follow-up is based on:
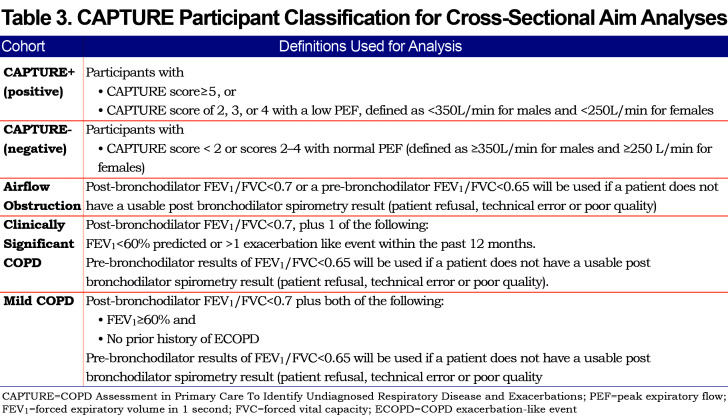
Participants who are CAPTURE +, previously defined as a CAPTURE score of 5 or 6 or CAPTURE score of 2, 3, or 4 with a low PEF, defined as <350 L/min for males and <250 L/min for females.
Participants with baseline post-bronchodilator FEV1/FVC < 0.7 and FEV1 < 80% predicted at baseline regardless of CAPTURE results. If a participant is unable to complete a post-bronchodilator spirometry (refusal, technical error on the part of coordinator, etc.), and the pre-bronchodilator FEV1/FVC is less than 0.65 and FEV <80% of predicted, the participant will be considered to have spirometrically-defined COPD for the purpose of follow-up in this study.
A random sample of approximately 5% of participants who do not meet criteria 1 or 2 above.
Follow-up data for this selected subset are collected from both medical record review and patient survey. (Figure 2)
Data Collected at Baseline During Visit 1
Demographic Data: Birth date, gender, race, ethnicity, education attained, current work status, living arrangement, source of health insurance, self-reported height and weight are collected.
CAPTURE Data: The CAPTURE tool (Figure 1a) is self-completed by all patient participants. Three PEF assessments using the Vitalograph® AsmaPlan® mechanical PEF meter with SafeTway® disposable mouthpieces (Vitalograph LTD, United Kingdom) are then completed. The highest PEF is recorded on the CAPTURE tool for CAPTURE scores of 2, 3 or 4. For patient participants in the intervention arm, the completed and scored CAPTURE tool and recommendations for next steps are provided to that patient’s clinician. (Figure 1b)
Medical History: Medical history is obtained by patient report and includes the items from the modified Charlson comorbidity score and month and year of most recent influenza immunization.42 While participants may not be familiar with all diagnoses queried, they are likely to recognize those for which they have received a diagnosis. (See Supplemental Table S1 in the online supplement (222.6KB, pdf) .)
COPD Assessment Test (CAT): The CAT isself-administered and is designed to provide an assessment of the presence and burden of respiratory symptoms on a 40-point scale.30,43
Other Respiratory Symptoms and Exacerbation-like Events: These arecollected using a tool adapted from the COPDGene study.44 Information includes frequency of respiratory or chest symptoms and phlegm (sputum/mucus) over the prior 12 months, the number of episodes of respiratory events for which the participant has received antibiotics or oral or intramuscular corticosteroids, number of respiratory-related hospitalizations and the “level 2” question from the modified Medical Research Council dyspnea scale, “walking slower than others their age.”45 The presence of 1 or more exacerbation-like events over the previous 12 months that were treated with antibiotics or systemic corticosteroids defines a patient as “at risk for exacerbation” based on evidence that a previous exacerbation predicts future exacerbations.14
Tobacco Smoke Exposure and Smoking Status: This information isself-reported based on questions from the Population Assessment of Tobacco and Health study46 including queries about ever smoking more than 100 cigarettes, age of smoking initiation, average number of cigarettes smoked per day, smoking status as of “one month ago” and presence and site of any second-hand smoke exposure.
Inhaled Respiratory Medication Use: This information is participant reported and facilitated by use of laminated pictures of all currently available prescription respiratory inhalers in the United States. Supplemental oxygen is also queried and recorded since oxygen may be used for conditions other than COPD that could impact responses to the CAPTURE questions.
Spirometry Testing: This testing is completed based on established American Thoracic Society/European Thoracic Society standards47 using the Easy One PC® Spirometer (ndd Medical Technologies Inc., Andover, Massachusetts). All patient participants complete pre-bronchodilatorassessment unlessthe participant reports use of a short- or long-acting bronchodilator within 2 hours of the study visit. When such a report is made, the spirometry test is considered a post-bronchodilator test. If pre-bronchodilator best FEV1/FVC is less than 0.70 or the best FEV1 % predicted is below 80%, a post-bronchodilator spirometry assessment is performed 15 to 20 minutes after inhalation of 2 puffs of albuterol 180 mcg HFA using an AeroChamber Plus* Flow-Vu® spacer with 1 minute between the first and the second inhalation.
Spirometry Results: These are centrally reviewed and adjudicated. Only results with quality grades of A, B, C, and D will be used in the data analysis for the study aims. Quality grades are based on:
≥ 3 acceptable tests, reproducible within 100 ml
≥ 3 acceptable tests, reproducible within 150 ml
≥ 2 acceptable tests, reproducible within 200 ml
1 or more acceptable tests, usable
1 or more acceptable tests, not usable
No acceptable tests
Presence of obstruction on post-bronchodilator spirometry is determined by the presence of an FEV1/FVC ratio less than 0.70. As mentioned earlier, if a patient participant is unable to complete a post-bronchodilator spirometry (refusal, technical error on the part of coordinator, etc.), and the pre-bronchodilator FEV1/FVC is less than 0.65, the patient is considered to have spirometrically- consistent obstruction (e.g., FEV1/FVC < 0.70) for the purpose of follow-up in this study.
Additional Items Related to CAPTURE: All participants complete a selection of 13 additional questions from the original CAPTURE potential question pool to allow further validation of the CAPTURE tool including consideration of potential changes in the current CAPTURE tool that might be required for a primary care patient population. (Supplemental Table S2—in the online supplement (222.6KB, pdf) .)
Contact information including address, phone numbers and email address are obtained to facilitate 12- month surveys in the follow-up cohort.
Sharing of Screening Results with Patient’s Clinician
CAPTURE results are returned to the patient’s clinician in the intervention arm as soon as possible after its completion (Figure 1b). Additional data, including spirometry results, are considered research data and are not shared with the patient’s clinical care team. For safety reasons, an FEV1 <30% of predicted is reported immediately to the patient’s clinician as required by the Data Safety Monitoring Board.
Sample Size Justification
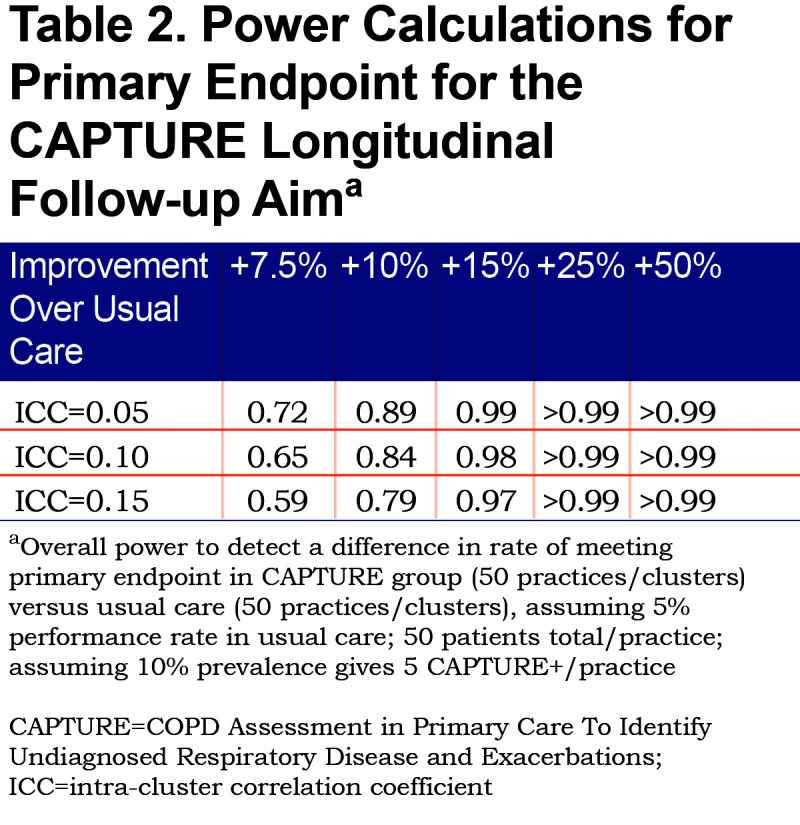
The primary analysis for the longitudinal follow-up aim drives the overall sample size requirements for the CAPTURE study. Power for this cluster randomized trial of 50 clusters (practices) per intervention arm assumes that we will identify approximately 5 CAPTURE positive (CAPTURE+) patients per cluster (practice) and that practices not randomized to the CAPTURE intervention will meet the composite endpoint in CAPTURE + patients 5% of the time. These assumptions are consistent with preliminary data observed in our initial small validation study.37 The sample size calculations allow for variability in the number of patients who are CAPTURE+ in each practice and the number of needed clusters (practices) is inflated by 11% to account for that.48 Because we have data specific to rates of CAPTURE+ patients from the initial validation study, we did not further modify those assumptions based on smoking rates for the specific study sites. Table 2 displays power for detecting a range of increased performance in reaching the combined endpoint for CAPTURE randomized practices, assuming a type I error of 5%. These calculations account for plausible intra-cluster correlation coefficient (ICC) values of 0.05, 0.10 and 0.15 that are typically seen in cluster randomized trials of behavioral interventions.49 Since there is variability in the actual number of CAPTURE+ patients identified in each practice, it is standard practice to inflate the number of needed clusters by 12% to account for this extra source of variability. Therefore, power estimates in Table 2 are conservatively based on 44 practices per study arm.
Outcomes and Data Analyses
Cross-Sectional Aim Validation
The principal analysis is assessment of sensitivity and specificity in identifying individuals with clinically significant COPD defined as individuals with post-bronchodilator FEV1/FVC < 0.70 in addition to either >1 COPD exacerbation-like event (ECOPD) within the past 12 months or an FEV1 < 60% predicted. Sensitivity is calculated among those participants who are CAPTURE+ and specificity is calculated among those who are and are not CAPTURE+ among those not classified as having spirometrically-defined clinically significant COPD.
Preliminary data from our initial small validation study suggested that our 5000 study participants would identify between 300 and 800 participants with previously undiagnosed clinically significant COPD (Supplemental Table S3 in the online supplement (222.6KB, pdf) ).37 Given the prior observation of 89.7% sensitivity and 93.1% specificity for identifying clinically significant COPD, the 95% confidence interval widths are given for true performance rates of 85%, 90% or 95% across a range of sample sizes.
CAPTURE tool sensitivity and specificity will also be assessed by sex, ethnic groups, rural and urban location, and educational level, as well as among individuals with all levels of spirometrically-defined COPD (Table 3)
Longitudinal Aim
For the subset of participants selected for the 12-month medical record review and patient survey, the primary analysis will compare the average cluster (per practice) sample proportion of the CAPTURE+ participants meeting composite clinical endpoints between usual care versus intervention arms (Table 4). A 2-sample test comparing average cluster sample proportions that explicitly accounts for correlation between individuals treated within the same practice will be used. A 2-sided p-value < 0.05 will be considered statistically significant. This analysis corresponds to a generalized estimating equation (GEE) regression analysis with:
individual-level binary composite outcome data,
the CAPTURE intervention group as a covariate,
practice id used to identify correlated outcomes,
an exchangeable correlation matrix that assumes similar correlation between all composite outcome results for patients within the same practice, and
a logistic link function.
Additional analyses will include variables for comorbid conditions with attention to previously diagnosed asthma and other obstructive lung diseases.
Secondary analyses on meeting the composite outcome for participants who are CAPTURE+ will employ the GEE analysis framework with patient participant (e.g., number of exacerbation events) and practice level predictors (e.g., number of clinicians, rural or non-rural practice location) in addition to the CAPTURE intervention group. Interactions will be assessed. We will also use the GEE framework to study and describe each of the individual components of the composite outcome as they relate to individual and practice level outcomes. In the subset of smoking patients, we will use GEE regression analysis to study additional binary outcomes, such as incidence of physician referral to a formal smoking cessation program and prescribed smoking cessation medication in participants who are smokers.
Exploratory Analyses Assess Data
In participants who are CAPTURE+, change in CAT score will be analyzed using mixed models with a random effect for practice. The average cluster sample proportion of patients who experience exacerbations during the 12-month follow-up period will be compared between intervention groups using methodology similar to that used for the primary endpoint. Survival analysis allowing for correlation of endpoints within cluster (practice) will be used to compare hospitalizations and mortality between intervention groups and perform multivariable analysis.
Model selection in GEE secondary analyses will be based on statistical significance at the 0.05 level using robust sandwich estimation of variability within clusters. Model fit will be checked by comparing observed versus predicted values within predefined subgroups of interest (sex, ethnic subgroups, rural and urban location, and educational status).
Strengths and Limitations
The rate and burden of unrecognized and undiagnosed COPD is significant. Current COPD screening and case finding programs often identify individuals with milder levels of disease who are only candidates for the universally recommended smoking interventions. In addition, many of those programs either exclude non-smokers or have a set of questions that rely heavily on smoking exposure to identify COPD. This study specifically does not exclude non-smokers allowing the opportunity to identify the 20% to 25% of people with COPD who have never smoked.
There is a need for a simple and acceptable COPD screening tool to identify individuals with clinically significant COPD who are immediate candidates for available therapeutic interventions. This is a pragmatic trial based in real world primary care practices with geographic, economic, racial and health system diversity. The patient and practice study burden are low as would be required for any screening program that is to be carried out in the context of usual practice.
We include a 12-month follow-up to assess the impact of the screening among those with elevated CAPTURE scores considered to identify individuals at increased risk of having clinically significant COPD, those with spirometrically-defined COPD regardless of CAPTURE score and a 5% sample of those without either abnormality to assess clinical impact of the screening. Follow-up is done using a combination of patient-reported and medical record review outcomes. Our sample size is based on findings from our prior validation study, inflating the number of practices and patients enrolled to account for the likely variability of COPD prevalence across the country and from our previous study.
Our definition of at risk for exacerbations does not include events that may have been mild exacerbations. This may, therefore, not include all individuals who are at risk for future exacerbations but identifies those with the most clearly recognized exacerbation events. Mild exacerbations may be difficult for both patients and clinicians to recognize and report.14
While a geographically diverse group of PBRNs are included, they may not reflect all primary care practices or actions that would be taken following screening. The screening results are not always presented during a patient visit requiring patients and clinicians to deal with results outside the clinical setting. While this is not ideal, it is not unusual for clinicians to receive test or other results requiring interaction with a patient after a visit. The requirements for study participation are minimal, however, not all patients who are invited agree to participate, limiting the generalizability of results to all primary care patients.
Abbreviations
Abbreviations: chronic obstructive pulmonary disease, COPD; COPD Assessment in Primary Care To Identify Undiagnosed Respiratory Disease and Exacerbation Risk study, CAPTURE; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; peak expiratory flow, PEF; data coordinating center, DCC; practice-based research networks, PBRN; cluster, randomized clinical trial, cRCT; single study visit, V1; COPD Assessment Test, CAT; CAPTURE positive, CAPTURE+; intra-cluster correlation coefficient, ICC; COPD exacerbation-like event, ECOPD; general estimating equation, GEE
Funding Statement
This study is funded by the National Heart, Lung and Blood Institute (HL136682, Fernando Martinez and Meilan Han, Co-PIs). Additional support was provided via unrestricted grants to the COPD Foundation from AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Sunovion and TEVA.
References
- 1.Sullivan J,Pravosud V,Mannino DM,Siegel K,Choate R,Sullivan T. National and state estimates of COPD morbidity and mortality - United States, 2014-2015. Chronic Obstr Pulm Dis. 2018;5(4):324-333. doi:https://doi.org/10.15326/jcopdf.5.4.2018.0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miravitlles M,Soriano J,Garcia-Rio F,et al. Prevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activities. Thorax. 2009;64(10):863-868. doi:https://doi.org/10.1136/thx.2009.115725 [DOI] [PubMed] [Google Scholar]
- 3.Labonte LE,Tan WC,Li PZ,et al. The Canadian Research Network the CanCOLD Collabrative Research Group . Undiagnosed chronic obstructive pulmonary disease contributes to the burden of health care use. Data from the CanCOLD Study. Am J Respir Crit Care Med. 2016;194(3):285-298. doi:https://doi.org/10.1164/rccm.201509-1795OC [DOI] [PubMed] [Google Scholar]
- 4.Martinez CH,Mannino DM,Jaimes FA,et al. Undiagnosed obstructive lung disease in the United States. Associated factors and long-term mortality. Ann Am Thorac Soc. 2015;12(12):1788-1795. doi:https://doi.org/10.1513/AnnalsATS.201506-388OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Make B,Dutro MP,Paulose-Ram R,Marton JP,Mapel DW. Undertreatment of COPD: a retrospective analysis of US managed care and Medicare patients. Int J Chron Obstruct Pulmon Dis. 2012;7:1-9. doi:https://doi.org/10.2147/COPD.S27032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindberg A,Jonsson AC,Ronmark E,Lundgren R,Larsson LG,Lundback B. Prevalence of chronic obstructive pulmonary disease according to BTS, ERS, GOLD and ATS criteria in relation to doctor's diagnosis, symptoms, age, gender, and smoking habits. Respiration. 2005;72(5):471-479. doi:https://doi.org/10.1159/000087670 [DOI] [PubMed] [Google Scholar]
- 7.Lamprecht B,Soriano JB,Studnicka M,et al. Determinants of underdiagnosis of COPD in national and international surveys. Chest. 2015;148(4):971-985. doi:https://doi.org/10.1378/chest.14-2535 [DOI] [PubMed] [Google Scholar]
- 8.Hill K,Goldstein RS,Guyatt GH,et al. Prevalence and underdiagnosis of chronic obstructive pulmonary disease among patients at risk in primary care. CMAJ. 2010;182(7):673-678. doi:https://doi.org/10.1503/cmaj.091784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez F,Han M,Allinson J. At the root: defining and halting progression of early chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197(12):1540-1551. doi:https://doi.org/10.1164/rccm.201710-2028PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy DE,Panos RJ. Diagnosis of COPD and clinical course in patients with unrecognized airflow limitation. Int J Chron Obstruct Pulmon Dis. 2013;8:199-208. doi:https://doi.org/10.2147/COPD.S39555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mapel DW,Robinson SB,Dastani HB,Shah H,Phillips AL,Lydick E. The direct medical costs of undiagnosed chronic obstructive pulmonary disease. Value Health. 2008;11(4):628-636. doi:https://doi.org/10.1111/j.1524-4733.2007.00305.x [DOI] [PubMed] [Google Scholar]
- 12.Akazawa M,Halpern R,Riedel AA,Stanford RH,Dalal A,Blanchette CM. Economic burden prior to COPD diagnosis: a matched case-control study in the United States. Respir Med. 2008;102(12):1744-1752. doi:https://doi.org/10.1016/j.rmed.2008.07.009 [DOI] [PubMed] [Google Scholar]
- 13.Qaseem A,Snow V,Shekelle P,et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2007;147(9):633-638. doi:https://doi.org/10.7326/0003-4819-147-9-200711060-00008 [PubMed] [Google Scholar]
- 14.Criner GJ,Bourbeau J,Diekemper RL,et al. Executive summary: prevention of acute exacerbation of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest. 2015;147(4):883-893. doi:https://doi.org/10.1378/chest.14-1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rennard SI,Drummond MB. Early chronic obstructive pulmonary disease: definition, assessment, and prevention. Lancet. 2015;385(9979):1778-1788. doi:https://doi.org/10.1016/S0140-6736(15)60647-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Decramer M,Miravitlles M,Price D,et al. New horizons in early stage COPD--improving knowledge, detection and treatment. Respir Med. 2011;105(11):1576-1587. [DOI] [PubMed] [Google Scholar]
- 17.USPSTF. Screening for chronic obstructive pulmonary disease US Preventive Services Task Force Recommendation statement. JAMA. 2016;315(13):1372-1377. doi:https://doi.org/10.1001/jama.2016.2638 [DOI] [PubMed] [Google Scholar]
- 18.Guirguis-Blake J,Senger C,Webber E,Mularski R,Whitlock E. Screening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315(13):1378-1393. doi:https://doi.org/10.1001/jama.2016.2654 [DOI] [PubMed] [Google Scholar]
- 19.Martinez FJ,O'Connor GT. Screening, case-finding, and outcomes for adults with unrecognized COPD. JAMA. 2016;315(13):1343-1344. doi:https://doi.org/10.1001/jama.2016.3274 [DOI] [PubMed] [Google Scholar]
- 20.Yawn B,Martinez F. POINT: can screening for COPD improve outcomes? Yes. Chest. 2020;157(1):7-9. doi:https://doi.org/10.1016/j.chest.2019.05.034 [DOI] [PubMed] [Google Scholar]
- 21.Calverley PM,Nordyke RJ,Halbert RJ,Isonaka S,Nonikov D. Development of a population-based screening questionnaire for COPD. COPD. 2005;2(2):225-232. doi:https://doi.org/10.1081/COPD-57594 [PubMed] [Google Scholar]
- 22.van Schayck CP,Halbert RJ,Nordyke RJ,Isonaka S,Maroni J,Nonikov D. Comparison of existing symptom-based questionnaires for identifying COPD in the general practice setting. Respirology. 2005;10(3):323-333. doi:https://doi.org/10.1111/j.1440-1843.2005.00720.x [DOI] [PubMed] [Google Scholar]
- 23.Yawn BP,Mapel DW,Mannino DM,et al. Development of the lung function questionnaire (LFQ) to identify airflow obstruction. Int J Chron Obstruct Pulmon Dis. 2010;5:1-10. [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez FJ,Raczek AE,Seifer FD,et al. Development and initial validation of a self-scored COPD Population Screener Questionnaire (COPD-PS). COPD. 2008;5(2):85-95. doi:https://doi.org/10.1080/15412550801940721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han MK,Steenrod AW,Bacci ED,et al. Identifying patients with undiagnosed COPD in primary care settings: insight from screening tools and epidemiologic studies. Chronic Obstr Pulm Dis. 2015;2(2):103-121. doi:https://doi.org/10.15326/jcopdf.2.2.2014.0152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freeman D,Nordyke RJ,Isonaka S,et al. Questions for COPD diagnostic screening in a primary care setting. Respir Med. 2005;99(10):1311-1318. doi:https://doi.org/10.1016/j.rmed.2005.02.037 [DOI] [PubMed] [Google Scholar]
- 27.Price DB,Tinkelman DG,Halbert RJ,et al. Symptom-based questionnaire for identifying COPD in smokers. Respiration. 2006;73(3):285-295. doi:https://doi.org/10.1159/000090142 [DOI] [PubMed] [Google Scholar]
- 28.Price DB,Tinkelman DG,Nordyke RJ,Isonaka S,Halbert RJ. Group CQS; Scoring system and clinical application of COPD diagnostic questionnaires. Chest. 2006;129(6):1531-1539. doi:https://doi.org/10.1378/chest.129.6.1531 [DOI] [PubMed] [Google Scholar]
- 29.Raghavan N,Lam YM,Webb KA,et al. Components of the COPD Assessment Test (CAT) associated with a diagnosis of COPD in a random population sample. COPD. 2012;9(2):175-183. doi:https://doi.org/10.3109/15412555.2011.650802 [DOI] [PubMed] [Google Scholar]
- 30.Kotz D,Nelemans P,van Schayck CP,Wesseling GJ. External validation of a COPD diagnostic questionnaire. Eur Respir J. 2008;31(2):298-303. doi:https://doi.org/10.1183/09031936.00074307 [DOI] [PubMed] [Google Scholar]
- 31.Haroon S,Jordan R,Takwoingi Y,Adab P. Diagnostic accuracy of screening tests for COPD: a systematic review and meta-analysis. BMJ Open. 2015;5(10):e008133. doi:https://doi.org/10.1136/bmjopen-2015-008133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sogbetun F,Eschenbacher W,Welge J,Panos R. A comparison of five surveys that identify individuals at risk for airflow obstruction and chronic obstructive pulmonary disease. Respir Med. 2016;120:1-9. doi:https://doi.org/10.1016/j.rmed.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 33.Chilvers E,Lomas D. Diagnosing COPD in non-smokers: splitting not lumping. Thorax. 2010;65(6):465-466. doi:https://doi.org/10.1136/thx.2009.128421 [DOI] [PubMed] [Google Scholar]
- 34.Jordan RE,Adab P,Sitch A,et al. Targeted case finding for chronic obstructive pulmonary disease versus routine practice in primary care (TargetCOPD): a cluster-randomised controlled trial. Lancet Respir Med. 2016;4(9):720-730. doi:https://doi.org/10.1016/S2213-2600(16)30149-7 [DOI] [PubMed] [Google Scholar]
- 35.Yawn BP,Duvall K,Peabody J,et al. The impact of screening tools on diagnosis of chronic obstructive pulmonary disease in primary care. Am J Prev Med. 2014;47(5):563-575. doi:https://doi.org/10.1016/j.amepre.2014.07.030 [DOI] [PubMed] [Google Scholar]
- 36.Bertens LC,Reitsma JB,van Schayck Y,et al. COPD detected with screening: impact on patient management and prognosis. Eur Respir J. 2014;44(6):1571-1578. doi:https://doi.org/10.1183/09031936.00074614 [DOI] [PubMed] [Google Scholar]
- 37.Leidy NK,Kim K,Bacci ED,et al. Identifying cases of undiagnosed, clinically significant COPD in primary care: qualitative insight from patients in the target population. NPJ Prim Care Respir Med. 2015;25:15024. doi:https://doi.org/10.1038/npjpcrm.2015.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leidy N,Malley K,Steenrod A,et al. Insight into best variables for COPD case identification: a random forests analysis. Chronic Obstr Pulm Dis. 2016;3(1):406-418. doi:https://doi.org/10.15326/jcopdf.3.1.2015.0144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez FJ,Mannino D,Leidy NK,et al. A new approach for identifying patients with undiagnosed chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195(6):748-756. doi:https://doi.org/10.1164/rccm.201603-0622OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eremenco SL,Cella D,Arnold BJ. A comprehensive method for the translation and cross-cultural validation of health status questionnaires. Eval Health Prof. 2005;28(2):212-232. doi:https://doi.org/10.1177/0163278705275342 [DOI] [PubMed] [Google Scholar]
- 41.Vogelmeier CF,Criner GJ,Martinez FJ,et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557-82. [DOI] [PubMed] [Google Scholar]
- 42.Hall WH,Ramachandran R,Narayan S,Jani AB,Vijayakumar S. An electronic application for rapidly calculating Charlson comorbidity score. BMC Cancer. 2004;4(94):94. doi:https://doi.org/10.1186/1471-2407-4-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones PW,Harding G,Berry P,Wiklund I,Chen W-H,Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648-654. doi:https://doi.org/10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 44.Regan EA,Hokanson JE,Murphy JR,et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32-43. doi:https://doi.org/10.3109/15412550903499522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Launois C,Barbe C,Bertin E,et al. The modified Medical Research Council scale for the assessment of dyspnea in daily living in obesity: a pilot study. BMC Pulm Med. 2012;12:61. doi:https://doi.org/10.1186/1471-2466-12-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.National Institutes of Health National Institute on Drug Abuse (NIDA). Population Assessment of Tobacco and Health study. NIDA website. . Updated June 2020. Accessed August 2020. https://www.drugabuse.gov/research/nida-research-programs-activities/population-assessment-tobacco-health-path-study [Google Scholar]
- 47.Miller MR,Hankinson J,Brusasco V,et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319-338. [DOI] [PubMed] [Google Scholar]
- 48.van Breukelen GJ,Candel MJ. Efficiency loss due to varying cluster sizes in cluster randomized trials and how to compensate for it: comment on You et al.(2011). . Clin Trials. 2012;9(1):125; author reply 6-7. doi:https://doi.org/10.1177/1740774511428649 [DOI] [PubMed] [Google Scholar]
- 49.University of Aberdeen Health Services Unit. Research tools—study sample size calculator. University of Aberdeen website.Published May 1999. Accessed August 2020. https://www.abdn.ac.uk/hsru/what-we-do/tools/index.php#panel177 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article contains supplemental material.