Abstract
Background: Up to 50% of chronic obstructive pulmonary disease (COPD) patients do not receive recommended care for COPD. To address this issue, we developed Proactive Integrated Care (Proactive iCare), a health care delivery model that couples integrated care with remote monitoring.
Methods: We conducted a prospective, quasi-randomized clinical trial in 511 patients with advanced COPD or a recent COPD exacerbation, to test whether Proactive iCare impacts patient-centered outcomes and health care utilization. Patients were allocated to Proactive iCare (n=352) or Usual Care ( =159) and were examined for changes in quality of life using the St George’s Respiratory Questionnaire (SGRQ), symptoms, guideline-based care, and health care utilization.
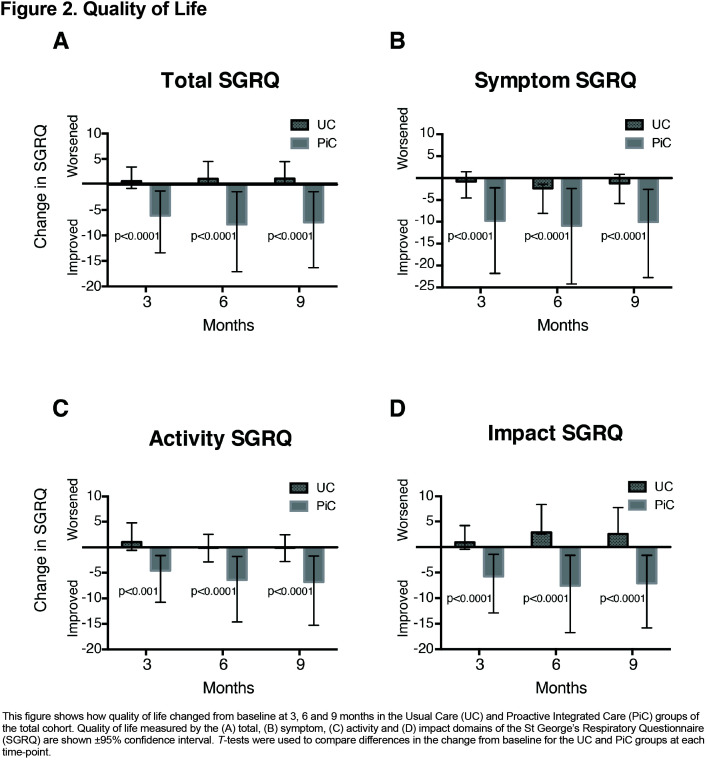
Findings: Proactive iCare improved total SGRQ by 7–9 units (p < 0.0001), symptom SGRQ by 9 units (p<0.0001), activity SGRQ by 6–7 units (p<0.001) and impact SGRQ by 7–11 units (p<0.0001) at 3, 6 and 9 months compared with Usual Care. Proactive iCare increased the 6-minute walk distance by 40 m (p<0.001), reduced annual COPD-related urgent office visits by 76 visits per 100 participants (p<0.0001), identified unreported exacerbations, and decreased smoking (p=0.01). Proactive iCare also improved symptoms, the body mass index-airway obstruction-dyspnea-exercise tolerance (BODE) index and oxygen titration (p<0.05). Mortality in the Proactive iCare group (1.1%) was not significantly different than mortality in the Usual Care group (3.8%; p=0.08).
Interpretation: Linking integrated care with remote monitoring improves the lives of people with advanced COPD, findings that may have been made more relevant by the coronavirus 2019 (COVID-19) pandemic.
Keywords: primary care, disease management, patient education, acute exacerbations of COPD, telemedicine, self-care
Introduction
This article contains supplemental material.
In the United States, chronic obstructive pulmonary disease (COPD) affects over 16 million people, costs $32 billion per year, and is the fourth leading cause of death.1-2-3-4 Exacerbations of COPD are among the most devastating complications of COPD, because they greatly increase the risk of death and account for 60% of COPD costs.3 Efforts have focused on standardizing recommendations for therapies that improve symptoms and limit exacerbations, identifying patients who might benefit from these treatments, and increasing compliance with recommended therapies.1,3 Surprisingly, only about 50% of COPD patients receive recommended therapies for COPD and COPD exacerbations,5-5-7 up to two-thirds of COPD exacerbations are not reported to health care providers,8-9-10 and only half of primary care physicians use COPD guidelines.11-12-13-14 Therefore, novel methods to improve health care delivery could bring substantial benefits, and may be particularly important given the disruption in health care due to the recent coronavirus 2019 (COVID-19) pandemic.15,16
Integrated care is a model of health care designed to improve delivery of care by increasing access to care and providing disease and self-care focused education. Enthusiasm for integrated care in patients with COPD was high after several studies showed that it decreased symptoms, improved health care delivery, enhanced quality of life and reduced health care utilization.17-18-19-20-21 But this excitement dampened after 2 well-designed clinical trials22,23 found that integrated care was unexpectedly associated with increased COPD-related hospitalizations, emergency department visits and all-cause mortality for reasons that remain unclear.24-25-26
Proactive iCare is a health care delivery model that couples traditional integrated care with remote monitoring by health care providers to enhance communication, education, recognition of exacerbations, and delivery of guideline-based care.27,28 It is designed to identify and solve problems early, because it does not solely depend on the ability of patients to recognize and act on chronic or impending health problems. We performed 2 studies of Proactive iCare in patients with advanced COPD. 27,28 The first study randomized 40 COPD patients to Proactive iCare or Usual Care for 3 months and showed that Proactive iCare improved quality of life and identified unreported exacerbations.27 The second study used a pre/post design to examine the impact of Proactive iCare on 100 rural COPD patients over a 3-month period, and showed that Proactive iCare improved quality of life, guideline-based care, recognition of unreported exacerbations and COPD-related health care utilization.28 The current study was designed to test the ability of Proactive iCare to improve patient-centered outcomes and health care utilization in a larger group of patients with advanced COPD from multiple sites over a 9-month period.
Materials and Methods
Study Design
We conducted a prospective, quasi-randomized clinical trial to determine the effect of Proactive iCare on patients with advanced COPD over a 22-month period between September 2006 and June 2008. The funding organization would not allow a traditional randomized, controlled clinical trial design for legislative reasons (i.e., Colorado Amendment 35),29 so a quasi-randomized design was used instead. The Colorado Multiple Institutional Review Board approved the study and all participants completed written, informed consent. This trial was registered at www.clinicaltrials.gov (NCT01044927).
Participants and Study Setting: Participants were recruited from primary care and pulmonary specialty clinics at the University of Colorado Hospital, Kaiser Permanente Colorado, the Denver Veterans Affairs Medical Center and primary care practices within the Colorado front-range urban corridor. Patients were also recruited from rural counties in part through collaboration with the High Plains Research Network.
Inclusion criteria required the patients to have:
Severe to very severe COPD (forced expiratory volume in 1 second [FEV1] < 50% predicted)30
or COPD with a FEV1 ≥ 50% predicted and a COPD exacerbation within the past year
Home access to a standard land telephone line
Legal U.S. and Colorado residency
Participants were excluded for:
A clinical diagnosis of asthma
Co-existing medical conditions that were likely to cause death within 2 years
Roentgenographic evidence of interstitial lung disease or other non-COPD related pulmonary diagnoses at the time of enrollment
Participation in another treatment study
Inability or unwillingness to cooperate with self-monitoring and reporting components
Inability to perform pulmonary function testing or a 6-minute walk
Prisoners, pregnant women, or institutionalized patients
Current alcohol or drug abuse
Non-English speakers
Inability to complete consent.
Interventions
Disease Management: Proactive iCare consisted of a 9-month disease management program that included: (1) COPD education, (2) exacerbation education, (3) direct communication with study coordinators, and (4) remote home monitoring.27 The monitoring equipment included a telecommunication platform (Bosch Health Buddy®, Robert Bosch Healthcare Palo Alto, California), a finger pulse oximeter (Onyx II Finger Pulse Oximeter 9550, Nonin Medical, Plymouth, Minnesota), a hand-held spirometer (Microlife #PF 100, Dunedin, Florida), and a pedometer (Health Measures SXC America on the Move, Stillwater, Minnesota).
COPD education was given to each participant during the 2-hour enrollment session, during weekday sessions with the Health Buddy®, and informally during phone calls with study coordinators.30 Participants were taught to recognize COPD exacerbations, the importance of early therapy with quick-relief medications, and the use of oral corticosteroids and/or antibiotics when needed. Patients were encouraged to call their health care provider anytime or study coordinator (9AM to 5PM Monday through Friday) if they suspected the onset of a COPD exacerbation.
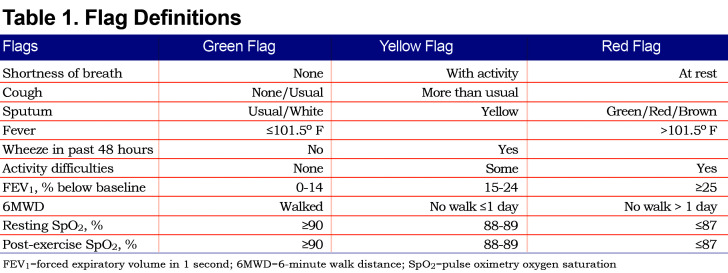
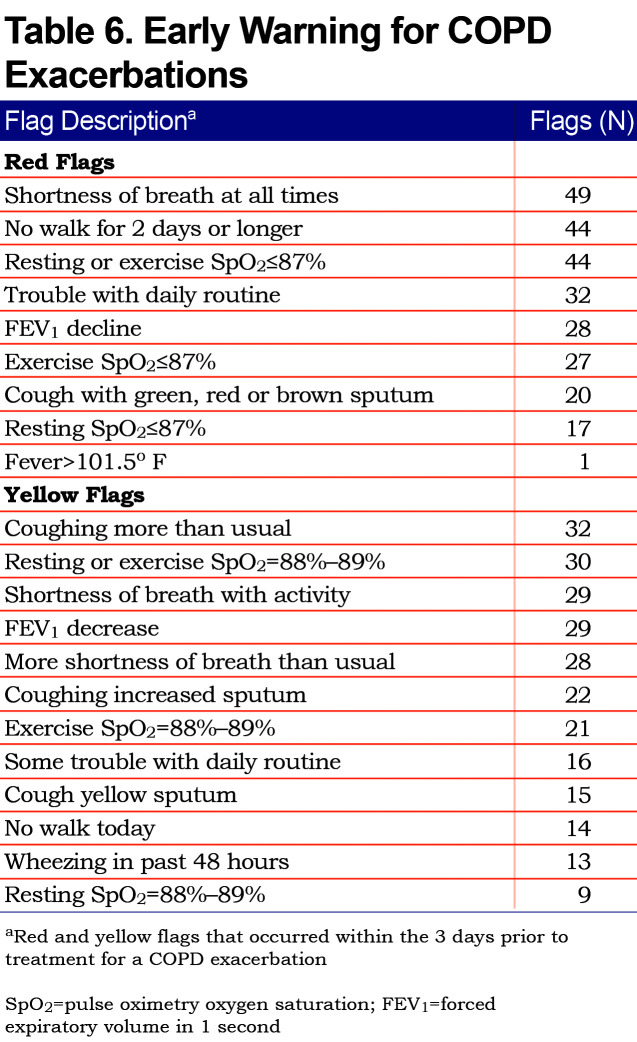
Each weekday, participants would participate in a Health Buddy® session at home lasting approximately 20 minutes. During this session they would receive COPD education30 and answer symptom-based questions. They would measure their FEV1 and oxygen saturation (SpO2) at rest. Participants would then walk for 6 minutes and measure the distance walked via a pedometer and the post-exertion SpO2. This information was entered into the Health Buddy, analyzed by predetermined algorithms, and categorized into 3 color-coded groups: green for stable, yellow advising caution and red indicating a possible decline in health status (Table 1). Data were transferred to a database overnight for coordinators to view the next weekday. Coordinators called all patients with red flags and used discretion in contacting patients who had persistent red or yellow flags. Coordinators helped resolve clinical problems directly or by calling the participant’s primary care provider.
Study coordinators called participants in the Proactive iCare Group at 3 and 6 months to administer the St George’s Respiratory Questionnaire (SGRQ) and collect data on health care utilization. Coordinators met with participants in person at 9 months to collect self-reported health care utilization, administer questionnaires, perform lung function testing and a 6-minute walk test (6MWT), and to retrieve equipment.
Usual Care: Participants enrolled into the Usual Care group continued to receive care by their health care provider. They did not receive COPD education or advice but were advised to inform their health care provider if they had a resting oxygen saturation £ 88% at enrollment. Study coordinators called Usual Care participants at 3 and 6 months to administer the SGRQ and collect data on health care utilization. Coordinators met with participants at 9 months in person to collect self-reported health care utilization, administer questionnaires, perform lung function testing and a 6MWT.
Outcomes: The primary outcome was health care costs with data coming from the University of Colorado and Kaiser Permanente. Quality of life was a key secondary outcome measured by the SGRQ at baseline, and 3, 6 and 9 months. Other secondary outcomes were measured at baseline and 9 months. These included respiratory symptoms (i.e., cough, sputum production and shortness of breath measured by the modified Medical Research Council [mMRC] Dyspnea scale), COPD and non-COPD-related health care utilization and the 6MWT. We also assessed use of guideline-based care and the number of COPD exacerbations. Self-reported COPD and non-COPD health care utilization was assessed for the previous 12 months at enrollment and compared to health care utilization at the study conclusion. A COPD exacerbation was defined as a worsening of a participant’s respiratory signs or symptoms that lead to a change in treatment, including quick-acting medications, and oral corticosteroids or antibiotics.
Randomization and Masking: Participants were allocated to experimental groups using a continuously rotating enrollment schedule with 4-day enrollment blocks. Participants were enrolled into the Proactive iCare group during the first 3 days of the block, and into the Usual Care group during the last day.
Sample Size
The study was originally powered to determine the effect of Proactive iCare on health care costs in urban patients with COPD, and quality of life in urban and rural patients compared to usual care after 1 year. The power calculation was based on a prior small study of Proactive iCare versus Usual Care.27 In this study, Proactive iCare reduced health care costs by $1,401±$10,717, compared with an increase of $1,709±12,570 in the Usual Care group.27 Assuming the same mean difference and standard deviation with a 2:1 allocation, a sample size of 243 in the Proactive iCare group and 121 in the Usual Care group would result in an 82% power to detect a difference using a 2-sided t-test with a Type I error rate=0.05. The study was also powered to determine the effect of Proactive iCare on quality of life measured by the SGRQ in the subgroup analyses. Prior work suggested that Proactive iCare improved the total SGRQ by 10.3±14.9 units compared to 0.6±12.3 in the Usual Care group.27 A sample size of 81 rural participants in the Proactive iCare group and 40 in the Usual Care group would yield a 96% power to detect a difference using a 2-sided t-test with a Type I error rate=0.05. Allowing for 10% attrition, recruitment of 400 intervention and 200 control participants would be sufficient to test the main hypotheses.
Statistical Methods
Statistical analyses were performed using SAS for Windows, version 9.2 (SAS Institute, Cary, North Carolina). Comparisons between study participants in the Proactive iCare and Usual Care groups were made using t-tests (or Wilcoxon tests if the distributions were skewed) for continuous variables and chi-square tests (or Fisher’s exact tests if cell sizes were small) for categorical variables. All tests were 2-sided with a significance level of P < 0.05. Changes from baseline in quality of life were determined for the SGRQ at 3, 6 and 9 months for the Proactive iCare and Usual Care groups. Differences between groups were then tested using t-tests. Linear mixed effects models for total SGRQ score including a quadratic time term were considered as an intention-to-treat analysis utilizing participants with incomplete data and compared with the analyses of participants with complete data.
Symptoms, guideline-based care, and 6MWT distances were compared between baseline and 9 months, and differences were tested using t-tests for continuous variables, and logistic regression including group by time interaction with generalized estimating equations (GEE) to account for the intra-class correlations arising from paired measurements for each patient for dichotomous variables (except chi-square tests when only 9-month measurements were considered). Self-reported COPD and non-COPD health care utilization was assessed for the previous 12 months at enrollment and compared to health care utilization at the study conclusion. Differences in annual health care utilization were generated and compared across Proactive iCare and Usual Care groups using Wilcoxon tests.
Protocol Changes
The study period was decreased from 12 to 9 months to allow more participants to be enrolled and complete the study by the mandatory study stop date. Enrollment sites were also added to the study, including the Denver Veterans Affairs Medical Center and primary care practices within the front-range urban corridor, including Denver, Colorado Springs and Pueblo. The primary outcome for the study was health care cost, which was to be derived from detailed records from the University of Colorado and Kaiser Permanente. Because the study expanded to multiple sites and systems, it was not feasible to obtain detailed cost information from all study sites, so cost estimates are presented instead.
Results
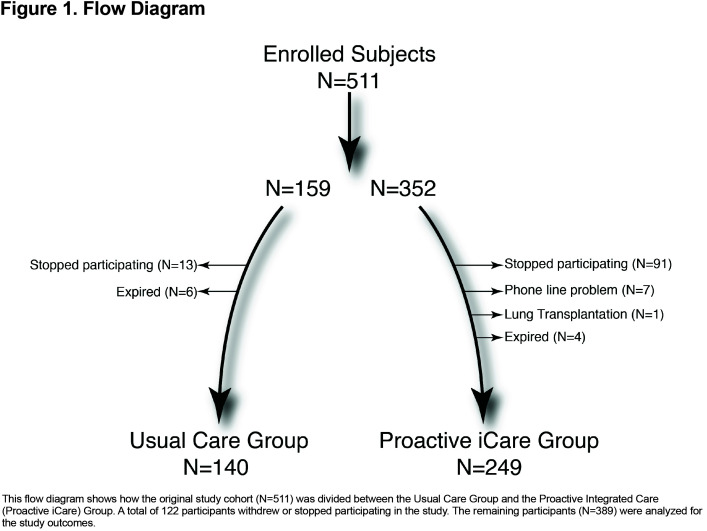
A total of 511 participants were enrolled in the study, including 352 in the Proactive iCare treatment group and 159 in the Usual Care control group (Figure 1). A total of 225 patients were enrolled from Kaiser Permanente Denver, 88 from the University of Colorado Hospital, 48 from the Denver VA Medical Center and 150 from private practices.
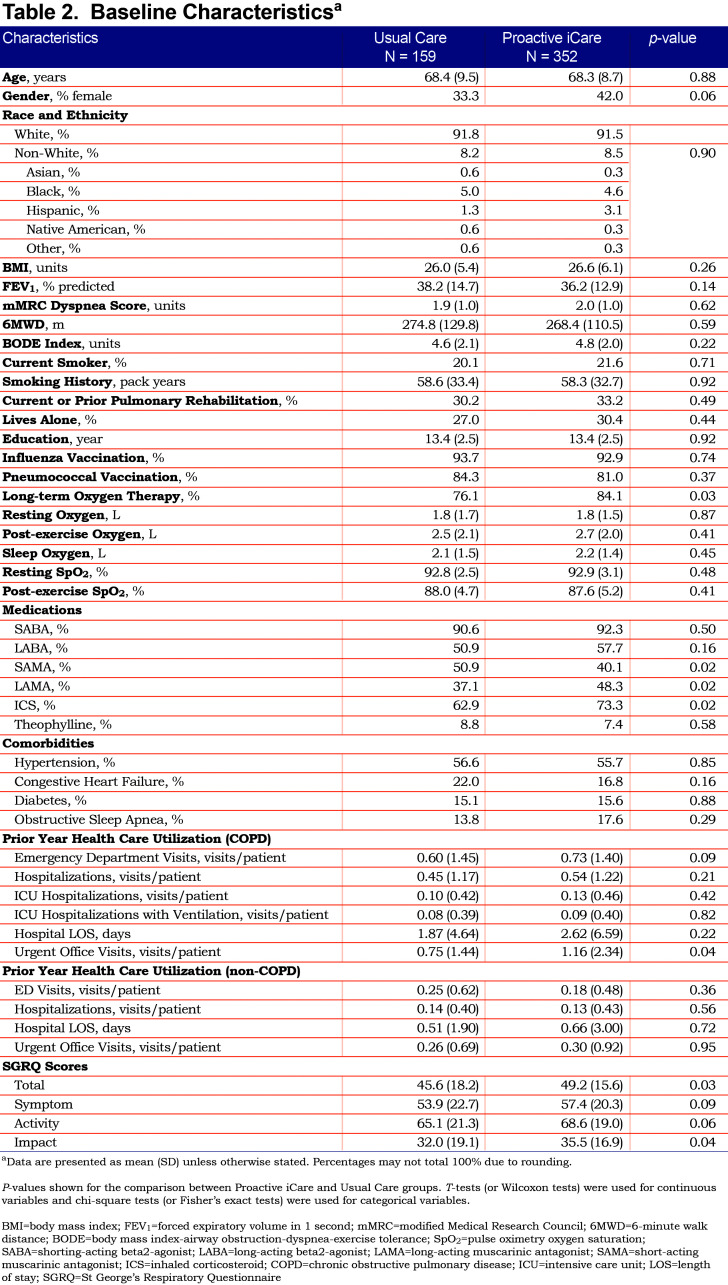
Overall, participants were 68 years old, predominantly male and had a pre-bronchodilator FEV1 of 37% predicted. Demographics and other patient characteristics at enrollment are summarized in Table 2. Usual Care and Proactive iCare groups were similar at baseline, with the exceptions of long-term oxygen therapy, use of short-acting muscarinic agonists (SAMAs), long-acting muscarinic agonists (LAMAs), and inhaled corticosteroids (ICSs), COPD-related urgent office visits within the previous 12 months, and the SGRQ total and impact scores (Table 2).
A total of 24% (N=122) of participants did not complete the study (Figure 1). In the Proactive iCare group, 19.3% of participants (N=68) dropped out of the study within the first 3 months, and an additional 10% (N=35) of participants dropped out in the last 6 months of the study. In the Usual Care group, 6.9% (N=11) participants dropped out of the study within the first 3 months, and another 5% (N=8) of participants dropped out in the last 6 months. Supplementary Table 1 in the online supplement (565.2KB, pdf) shows differences in baseline characteristics for completers (N=389) and non-completers (N=122). Non-completers were more likely to be current smokers, have a diagnosis of congestive heart failure and report more COPD-related health care usage within the prior year (Supplementary Table 1 in the online supplement (565.2KB, pdf) ). Non-completers also reported less use of pulmonary rehabilitation and years of education (Supplementary Table 1 in the online supplement (565.2KB, pdf) ). Supplementary Table 2 in the online supplement (565.2KB, pdf) shows differences in baseline characteristics for participants who completed the study. The groups remained balanced at baseline, except for differences in the use of SAMA, LAMA and ICS medications, and COPD-related emergency department and urgent office visits.
Quality of Life
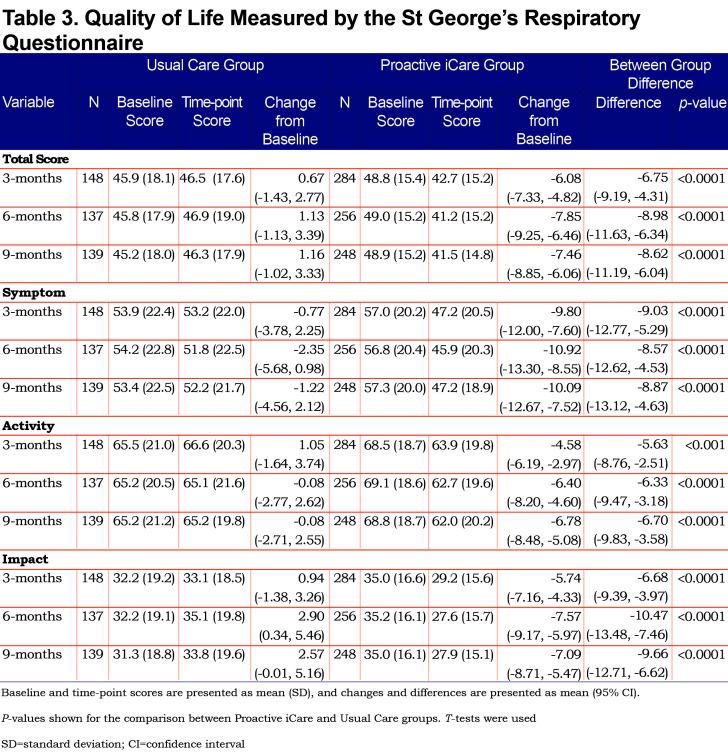
Patients randomized to receive Proactive iCare had large and consistent improvements (i.e., decreased scores exceeding the 4-point minimal clinically important difference) in the total SGRQ and SGRQ components (Table 3 and Figure 2). Using an intention-to-treat analysis, Proactive iCare improved the total SGRQ by 6.7 units, 9.5 units and 8.4 units (p < 0.0001) at 3, 6 and 9 months, respectively, compared with the Usual Care group. These improvements in the total SGRQ are similar to the results for participants with complete data in Table 3 and Figure 2.
Smoking Rates, Symptoms, Exercise Capacity and Guideline-based Care
After 9 months, participants in the Proactive iCare group reported less current smoking, cough, sputum, and breathlessness, and increased post-exercise oxygen liter flow and SpO2 compared with the Usual Care participants (Table 4). Participants in Proactive iCare were also able to walk 42m farther (i.e., a15% increase from baseline) during a 6MWT, whereas those randomized to Usual Care did not improve (i.e., a 1% increase). No differences were observed in the use of inhaled medications (data not shown).
Health Care Utilization
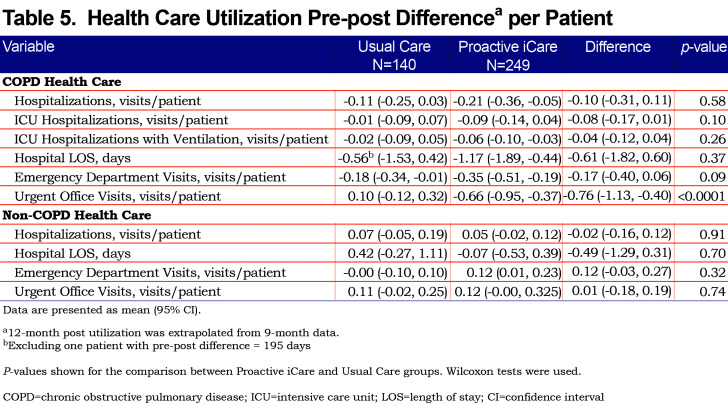
Self-reported COPD and non-COPD health care utilization was assessed for the previous 12 months at enrollment and compared to health care utilization at the study conclusion (extrapolating 12-month post utilization from 9-month data). Pre/post differences in health care utilization were generated for each group and then compared across Proactive iCare and Usual Care groups using Wilcoxon tests. This analysis shows increases or decreases in health care utilization across treatment and control groups. Proactive iCare substantially decreased COPD-related urgent office visits by 76 visits per 100 patients (p<0.0001), and non-significantly decreased COPD-related emergency departments visits (p=0.09) and intensive care unit (ICU) hospitalizations (p=0.10), compared with Usual Care (Table 5). In contrast, other measures of COPD- and non-COPD-related health care did not change.
Estimated Health Care Cost Savings
The potential impact of Proactive iCare versus Usual Care on health care costs was estimated by multiplying group differences in COPD-related health care use by costs for each type of health care. Costs for COPD hospitalizations, ICU hospitalizations, ICU hospitalizations requiring ventilation and emergency department visits were taken from studies that used the Premier PerspectiveÔ Database (2003-2008), which represented approximately 15% of all U.S. hospitalizations at the time.31-32-33 Costs for urgent COPD-related outpatient visits were taken from an additional reference that used a managed care claims database from 2006.34 This analysis found that Proactive iCare had the potential to decrease health care costs by $665,881 per 100 COPD patients enrolled, with the majority of health care savings coming from a reduction in ICU hospitalizations (Supplementary Table 3 in the online supplement (565.2KB, pdf) ). Yearly program costs were estimated to be approximately $230,000 per 100 enrollees, including a clinical coordinator ($90,000), supplies/expenses ($10,000), physician oversight ($10,000) and monitoring ($120,000). This results in an estimated cost savings of $4,359 per patient per year, and a return on investment of approximately 2.
Mortality
During the 9-month study, 4 of 352 participants (1.1%) died in the Proactive iCare Group versus 6 of 159 participants (3.8%) in the Usual Care group (p=0.08 Fisher’s exact test).
Subgroup Analyses
The effect of Proactive iCare was assessed in the urban (N=403) and rural cohorts (N=108). Baseline differences between the urban and rural cohorts are shown in Supplementary Table 4 in the online supplement (565.2KB, pdf) . Urban participants reported greater participation in pulmonary rehabilitation, a higher level of education, more vaccinations for influenza, higher post-exercise oxygen liter flow, higher use of shorting-acting beta2-agonists (SABAs), SAMAs, and more urgent office visits for non-COPD related problems, compared with rural participants. In contrast, rural participants were more likely to live alone.
Baseline characteristics in the overall urban cohort and in completers are shown in Supplementary Tables 5 and 6 in the online supplement (565.2KB, pdf) . Participants in the urban cohort treated with Proactive iCare showed large improvements in the total and component SGRQ scores across all 3 time-points (Supplementary Table 7 and Supplementary Figure 1 in the online supplement (565.2KB, pdf) ), compared with Usual Care. Proactive iCare was associated with significant improvements in dyspnea, sputum production, distance walked during the 6MWT, BODE index, and post exercise SpO2 % that mirrored results for the overall study (Supplementary Table 8 in the online supplement (565.2KB, pdf) ). Proactive iCare decreased COPD-related urgent office visits by 77 visits per 100 participants (p <0.0001), and non-significantly decreased COPD-related emergency departments visits (p=0.12) and hospitalizations (p=0.053), compared with Usual Care (Supplementary Table 9 in the online supplement (565.2KB, pdf) ). In the smaller rural cohort, we found no improvement in the SGRQ or in any component of health care utilization, but there were reductions in the number of current smokers (p=0.04), patients producing sputum (p=0.049), participants on long-term oxygen therapy (p=0.02) and post-exercise oxygen liter flow (p=0.004; data not shown).
Early Warning for COPD Exacerbations
A total of 352 participants in the Proactive iCare group had 210 COPD exacerbations during the study (i.e., 0.80 exacerbations/patient-year). A total of 262 red flags and 258 yellow flags occurred in the 3 days prior to an exacerbation (Table 6). The most common red and yellow flags were related to increasing shortness of breath, decreased physical activity, lower oxygen saturation and increased cough. Coordinator notes allowed for an accurate assessment of communication between participants, coordinators and medical providers for 110 exacerbations, and treatment choice for 195 exacerbations. The first exacerbation-related phone call was made from participant to medical provider (i.e., self-report) 21% of the time, from participant to coordinator (i.e., Proactive iCare assisted) 34% of the time, and from coordinator to participant (i.e., Proactive iCare identified) 45% of the time. Overall, the first exacerbation-related phone call involved a study coordinator 79% of the time. A total of 55% of exacerbations were treated with an oral corticosteroid, 64% were treated with an antibiotic and 33% were treated with a short-acting bronchodilator alone.
Discussion
The important findings of this study are that Proactive iCare improved quality of life, exercise capacity, identification of unreported exacerbations, and reduced urgent office visits for COPD. It may have also helped people stop smoking. These results suggest that coupling integrated care with remote monitoring may improve outcomes that are important for people with advanced COPD.
Multiple studies have evaluated the impact of integrated care on outcomes for COPD.17-18-19-20-21-22-23,35 Two Cochrane reviews concluded that integrated care/self-care programs improve quality of life and exercise tolerance for COPD, and decrease hospitalizations and hospital length of stay for COPD exacerbations.20,21 Both reviews included 2 important studies by Bourbeau et al and Rice et al.17,18 Bourbeau et al randomized 191 advanced COPD patients with a recent COPD exacerbation to a self-management program or usual care, and found that self-management decreased unscheduled office visits, emergency department visits and hospitalizations for COPD.17 Rice et al randomized 743 patients with severe COPD and a recent exacerbation or oxygen use to an integrated care program or usual care.18 They showed that integrated care decreased COPD-related hospitalizations and emergency department visits, and found that these improvements were related to the number of completed calls made by coordinators to patients.18
Unfortunately, not all studies of integrated care have been so positive.22,23,35 An important example is a well-designed study by Fan et al, which randomized COPD patients with a prior severe exacerbation tointegrated care versususual care.23 Unexpectedly, integrated care did not decrease COPD-related hospitalizations and was associated with increased mortality, causing the study to be stopped early. The reason(s) for increased mortality could not be determined, but the level of communication between coordinators and patients may be an important factor. For instance, there were 6 planned calls from coordinators to patients over a year in the Fan study versus 12 planned calls over the same period in the Bourbeau and Rice studies.17,18,23 Patients were prompted to call a coordinator during an exacerbation or if symptoms worsened in all 3 studies. However, only 4.5% of exacerbations in the treatment group were communicated to a coordinator in the Fan study,26 whereas 48% of exacerbations in the self-management group were communicated to a health care professional in the Bourbeau study.17 Similar data were not available from the Rice study.18 In response to a Letter to the Editor about this issue, Fan et al wrote, “Future studies are needed to help understand the role of symptoms and physiologic monitoring approaches in care management to correctly identify COPD exacerbations, assess need for evaluation by [the] health care team, and initiate appropriate and prompt treatment and follow-up care.”26 More recently, Aboumatar et al randomized 240 patients hospitalized for COPD to a program that combined transition of care and long-term self-management. Contrary to expectations, treatment resulted in more COPD-related emergency department visits and hospitalizations, without an improvement in quality of life.22
We found that Proactive iCare had positive effects on multiple patient-centered outcomes that replicate and extend results of past studies.27,28 For example, 2 smaller studies each conducted over 3 months suggested that Proactive iCare could improve the SGRQ by 10-12 units.27,28 In the current study, Proactive iCare improved the SGRQ by approximately 7 units at 3 months, which increased to approximately 9 units at 6 and 9 months, compared to Usual Care. These results were consistent in both the per-protocol and intention-to-treat analyses. Therefore, the effect of Proactive iCare on quality of life appears to be large, sustained, and greater than most other studies of integrated care for COPD.17,18,20,23,35 Proactive iCare also improved the 6MWD by 40m at 9 months compared to Usual Care, which is similar to the 45m improvement that was seen at 3 months in our rural study,28 and similar to other integrated care studies.20,21 We also saw improvements in self-reported cough and shortness of breath measured by the mMRC dyspnea scale that is similar to what was found in our rural study.28
Proactive iCare decreased urgent COPD-related outpatient visits by 76 visits per 100 participants. These results are similar to the Bourbeau study, which decreased unscheduled, COPD-related outpatient visits by approximately 60% in the self-management group.17 Separate studies using administrative data from the Health Buddy® Consortium Medicare Demonstration Project showed similar improvements in overall and COPD-related hospitalizations and health care costs, especially for those who engaged with the Health Buddy®.36-37-38 Many of the positive effects of Proactive iCare observed in the overall study were also present in the urban subgroup. On the other hand, fewer positive results were seen in the rural subgroup. This was surprising given the substantial effects of Proactive iCare in an earlier study of rural COPD patients,28 and may have been due to lower enrollment (N=83) that did not meet pre-specified goals.
Proactive iCare was designed to uncover and treat exacerbations that would otherwise go unreported or would be recognized at a later time. These goals are important because unreported exacerbations have negative effects,8-9-10 and early therapy improves outcomes.39 In past studies, Proactive iCare uncovered 47%–78% of red-flagged COPD exacerbations that were not self-reported to coordinators or providers.27,28 In the current study, coordinators initiated contact with participants for exacerbations 45% of the time, indicating that Proactive iCare can identify and treat exacerbations that may go unreported. Problems with shortness of breath, physical activity, cough, and oxygenation were the most common problems that preceded an exacerbation. These early indicators are similar to the results of 2 prior studies of Proactive iCare in COPD.27,28 For unclear reasons, significant proportions of COPD patients do not report exacerbations, hampering efforts to deliver early treatment.8-9-10 Integrated/self-care does not appear to fully resolve this problem, since 52% and 95% of exacerbations went unreported in studies by Bourbeau17 and Fan,23 respectively. Ultimately, these results may facilitate development of the next generation of remote, and preferably passive, monitoring tools that will quantitatively measure early exacerbation indicators, so that exacerbations can be identified and treated earlier.
During the course of the study, over twice the number of participants dropped out from the Proactive iCare group (i.e., 29%) compared to the Usual Care group (i.e., 11%). This is an important issue, because engagement with the Health Buddy® improves outcomes.36-37-38 The majority of the dropout participants dropped out from the Proactive iCare group during the first 3 months (i.e., 19%), with the remainder leaving the study between the 3rd and 9th month (i.e., 10%). In contrast, dropouts in the Usual Care group were fewer and occurred more evenly over the course of the study. The 3-month dropout rate of 19% in the Proactive iCare Group was very similar to the 18%, 3-month dropout rate that occurred during our rural Proactive iCare study.28 A small proportion of participants dropped out due to phone line problems or death, but the majority of participants stopped participating for unknown reasons. Anecdotally, the intensity of the intervention (approximately 20 minutes, 5 days per week) and difficulty adapting to new technology likely caused some people to drop out. Overall, participants who left the study were roughly twice as likely to be current smokers, to have congestive heart failure, and to report COPD-related emergency department, hospital and ICU health care utilization within the prior year. How or whether these baseline characteristics negatively impacted the willingness or ability of participants to remain engaged with the program is not known. But it is possible that patients with congestive heart failure may have needed different treatments, such as diuresis rather than a steroid. It may have also been difficult for patients who were frequently hospitalized to engage with an active monitoring program 5 days per week, either because of fatigue or cognitive deficits. Future studies will need to uncover and address barriers to implementation. They should also explore passive monitoring approaches that might make it easier for sicker patients to participate, such as wearable sensors that can detect motion or oxygen saturation, sensors integrated into a mattress that can measure heart or respiratory rate, or audio sensors in smart phones or smart speakers that can identify cough or wheezing.40-41-42
This study has several limitations including the quasi-randomized design, which can result in selection bias and imbalances of measured and unmeasured confounders. Despite this weakness, there were few imbalances in baseline characteristics between the Proactive iCare and Usual Care groups. We were not able to perform a rigorous health care cost analysis as originally planned, because participants came from different enrollment sites and systems. Instead, we reported the effects of Proactive iCare on pre-planned, patient-centered outcomes and health care utilization and estimated the effect on cost reductions. This analysis suggested that Proactive iCare has the potential to reduce costs by $4,359 per patient over a year, which is greater than the $3,110 cost reduction estimate used for the power analysis. Rigorous health care cost analyses should be performed when possible as Bourbeau et aldid on their self-care study.43 Health care utilization was self-reported and was not confirmed by obtaining medical records. To improve accuracy, coordinators contacted patients every 3 months to collect these results. Other outcomes were not dependent on patient recollection. Most outcomes were not assessed using an intention-to-treat analysis because of a lack of repeated outcome measures. In contrast, changes in the total SGRQ were assessed using both intention-to-treat and per-protocol analyses although most of the dropouts occurred within the first 3 months and a bias may still remain. The fact that this study was performed from 2006-2008 is also a limitation. With changes in communication technology and guidelines, more contemporaneous data are needed.
COPD is one of the most devastating diseases worldwide,1,2,4 yet recommended care for COPD remains suboptimal.5-6-7 New models of care, such as integrated care, hold the potential to improve patient-centered outcomes, but so far, results have been inconsistent.18,20,21,23,35 Methods to optimize delivery of care have become even more important during the COVID-19 pandemic, which has disrupted health care and increased our reliance on telehealth and telemonitoring.15,16 We hypothesized that coupling remote monitoring to integrated care would improve patient-centered outcomes and health care utilization by increasing delivery of guideline-based care and by acting as an early-warning system for COPD exacerbations. Our results suggest that Proactive iCare achieves these goals and does not worsen mortality. Future studies should also strive to understand barriers to implementation so that patient drop out is minimized, identify components of Proactive iCare that are most effective, and determine the optimal intervention intensity for different patient phenotypes. It will also be important to identify passive monitoring techniques and algorithms that identify exacerbations early and minimize the need for direct patient engagement. While these results are intriguing, they should be considered hypothesis generating until a randomized, controlled trial of Proactive iCare has been performed.
Abbreviations
Abbreviations: chronic obstructive pulmonary disease, COPD; Proactive Integrated Care, Proactive iCare; St George’s Respiratory Questionnaire, SGRQ; body mass index-airway obstruction-dyspnea-exercise tolerance, BODE; coronavirus-19, COVID-19; forced expiratory volume in 1 second, FEV1; pulse oximetry oxygenation, SpO2; 6-minute walk test, 6MWT; modified Medical Research Council dyspnea scale, mMRC; generalized estimating equations, GEE; long-term oxygen therapy, LTOT; short-acting beta2-agonist, SABA; inhaled corticosteroid, ICS; short-acting beta2-agonists, SABA; length of stay, LOS; intensive care unit, ICU; confidence interval, CI; standard deviation, SD
Funding Statement
This study was funded by the Colorado Cancer, Cardiovascular Disease and Chronic Pulmonary Disease Prevention, Early Detection and Treatment Program and the National Institutes of Health (HL129938-03).
References
- 1.National Heart, Lung and Blood Institute (NHLBI). COPD national action plan. NHLBI website. Published 2018. Updated 2019. Accessed September 2020. https://www.nhlbi.nih.gov/health-topics/all-publications-and-resources/copd-national-action-plan [Google Scholar]
- 2.Connors AF Jr,Dawson NV,Thomas C,et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments). Am J Respir Crit Care Med. 1996;154(4 Pt 1):959-967. doi: https://doi.org/10.1164/ajrccm.154.4.8887592 [DOI] [PubMed] [Google Scholar]
- 3.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease, 2019 report. GOLD website. Published November 2018. Accessed September 2020. https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf [Google Scholar]
- 4.Murphy SL,Xu J,Kochanek KD,Arias E. Mortality in the United States, 2017. NCHS Data Brief. 2018;(328):1-8. [PubMed] [Google Scholar]
- 5.McGlynn EA,Asch SM,Adams J,et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635-2645. doi: https://doi.org/10.1056/NEJMsa022615 [DOI] [PubMed] [Google Scholar]
- 6.Mularski RA,Asch SM,Shrank WH,et al. The quality of obstructive lung disease care for adults in the United States as measured by adherence to recommended processes. Chest. 2006;130(6):1844-1850. doi: https://doi.org/10.1378/chest.130.6.1844 [DOI] [PubMed] [Google Scholar]
- 7.Lindenauer PK,Pekow P,Gao S,Crawford AS,Gutierrez B,Benjamin EM. Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2006;144(12):894-903. doi: https://doi.org/10.7326/0003-4819-144-12-200606200-00006 [DOI] [PubMed] [Google Scholar]
- 8.Langsetmo L,Platt RW,Ernst P,Bourbeau J. Underreporting exacerbation of chronic obstructive pulmonary disease in a longitudinal cohort. Am J Respir Crit Care Med. 2008;177(4):396-401. doi: https://doi.org/10.1164/rccm.200708-1290OC [DOI] [PubMed] [Google Scholar]
- 9.Seemungal TA,Donaldson GC,Paul EA,Bestall JC,Jeffries DJ,Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1418-1422. doi: https://doi.org/10.1164/ajrccm.157.5.9709032 [DOI] [PubMed] [Google Scholar]
- 10.Vijayasaratha K,Stockley RA. Reported and unreported exacerbations of COPD: analysis by diary cards. Chest. 2008;133(1):34-41. doi: https://doi.org/10.1378/chest.07-1692 [DOI] [PubMed] [Google Scholar]
- 11.Barr RG,Celli BR,Martinez FJ,et al. Physician and patient perceptions in COPD: the COPD Resource Network Needs Assessment Survey. Am J Med. 2005;118(12):1415. doi: https://doi.org/10.1016/j.amjmed.2005.07.059 [DOI] [PubMed] [Google Scholar]
- 12.Davis KJ,Landis SH,Oh YM,et al. Continuing to Confront COPD International Physician Survey: physician knowledge and application of COPD management guidelines in 12 countries. Int J Chron Obstruct Pulmon Dis. 2015;10:39-55. doi: https://doi.org/10.2147/COPD.S70162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yawn BP,Wollan PC. Knowledge and attitudes of family physicians coming to COPD continuing medical education. Int J Chron Obstruct Pulmon Dis. 2008;3(2):311-317. doi: https://doi.org/10.2147/COPD.S2486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yawn BP,Wollan PC,Textor KB,Yawn RA. Primary care physicians', nurse practitioners' and physician assistants' knowledge, attitudes and beliefs regarding COPD: 2007 to 2014. Chronic Obstr Pulm Dis. 2016;3(3):628-635. doi: https://doi.org/10.15326/jcopdf.3.3.2015.0168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Filippo O,D'Ascenzo F,Angelini F,et al. Reduced rate of hospital admissions for ACS during Covid-19 outbreak in northern Italy. N Engl J Med. 2020;383(1):88-89. doi: https://doi.org/10.1056/NEJMc2009166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keesara S,Jonas A,Schulman K. Covid-19 and health care's digital revolution. N Engl J Med. 2020;382(23):e82. doi: https://doi.org/10.1056/NEJMp2005835 [DOI] [PubMed] [Google Scholar]
- 17.Bourbeau J,Julien M,Maltais F,et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch Intern Med. 2003;163(5):585-591. doi: https://doi.org/10.1001/archinte.163.5.585 [DOI] [PubMed] [Google Scholar]
- 18.Rice KL,Dewan N,Bloomfield HE,et al. Disease management program for chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care. 2010;182(7):890-896. doi: https://doi.org/10.1164/rccm.200910-1579OC [DOI] [PubMed] [Google Scholar]
- 19.Casas A,Troosters T,Garcia-Aymerich J,et al. Integrated care prevents hospitalisations for exacerbations in COPD patients. Eur Respir J. 2006;28(1):123-130. doi: https://doi.org/10.1183/09031936.06.00063205 [DOI] [PubMed] [Google Scholar]
- 20.Kruis AL,Smidt N,Assendelft WJ,et al. Integrated disease management interventions for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;(10):CD009437. doi: https://doi.org/10.1002/14651858.CD009437.pub2 [DOI] [PubMed] [Google Scholar]
- 21.Zwerink M,Brusse-Keizer M,van der Valk PD,et al. Self-management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;3:CD002990. doi: https://doi.org/10.1002/14651858.CD002990.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aboumatar H,Naqibuddin M,Chung S,et al. Effect of a hospital-initiated program combining transitional care and long-term self-management support on outcomes of patients hospitalized with chronic obstructive pulmonary disease: a randomized clinical trial. JAMA. 2019;322(14):1371-1380. doi: https://doi.org/10.1001/jama.2019.11982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan VS,Gaziano JM,Lew R,et al. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: a randomized, controlled trial. Ann Intern Med. 2012; 156(10):673-683. doi: https://doi.org/10.7326/0003-4819-156-10-201205150-00003 [DOI] [PubMed] [Google Scholar]
- 24.Rinne ST,Lindenauer PK,Au DH. Unexpected harm from an intensive COPD intervention. JAMA. 2019;322(14):1357-1359. doi: https://doi.org/10.1001/jama.2019.12976 [DOI] [PubMed] [Google Scholar]
- 25.Pocock SJ. Ethical dilemmas and malfunctions in clinical trials research. Ann Intern Med. 2012;156(10):746-747. doi: https://doi.org/10.7326/0003-4819-156-10-201205150-00015 [DOI] [PubMed] [Google Scholar]
- 26.Vandivier RW,Linderman DJ,Koff PB. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations. Ann Intern Med. 2012;157(7):530; author reply -1. doi: https://doi.org/10.7326/0003-4819-157-7-201210020-00018 [DOI] [PubMed] [Google Scholar]
- 27.Koff PB,Jones RH,Cashman JM,Voelkel NF,Vandivier RW. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J. 2009;33(5):1031-1038. doi: https://doi.org/10.1183/09031936.00063108 [DOI] [PubMed] [Google Scholar]
- 28.Linderman DJ,Koff PB,Freitag TJ,Min SJ,Vandivier RW. Effect of integrated care on advanced chronic obstructive pulmonary disease in high-mortality rural areas. Arch Intern Med. 2011;171(22): 2059-2061. [DOI] [PubMed] [Google Scholar]
- 29.Colorado Department of Public Health and Environment (CDPHE). Colorado amendment 35. CDPHE website. Published 2004. Updated 2020. Accessed September 2020. https://cdphe.colorado.gov/prevention-and-wellness/smoking-and-tobacco/tobacco-education-prevention-and-cessation-grant-0 [Google Scholar]
- 30.Rabe KF,Hurd S,Anzueto A,et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532-555. doi: https://doi.org/10.1164/rccm.200703-456SO [DOI] [PubMed] [Google Scholar]
- 31.Kiser TH,Allen RR,Valuck RJ,Moss M,Vandivier RW. Outcomes associated with corticosteroid dosage in critically ill patients with acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):1052-1064. doi: https://doi.org/10.1164/rccm.201401-0058OC [DOI] [PubMed] [Google Scholar]
- 32.Dalal AA,Shah M,D'Souza AO,Rane P. Costs of COPD exacerbations in the emergency department and inpatient setting. Respir Med. 2011;105(3):454-460. doi: https://doi.org/10.1016/j.rmed.2010.09.003 [DOI] [PubMed] [Google Scholar]
- 33.Lindenauer PK,Pekow PS,Lahti MC,Lee Y,Benjamin EM,Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA. 2010;303(23):2359-2367. doi: https://doi.org/10.1001/jama.2010.796 [DOI] [PubMed] [Google Scholar]
- 34.Dalal AA,Christensen L,Liu F,Riedel AA. Direct costs of chronic obstructive pulmonary disease among managed care patients. Int J Chron Obstruct Pulmon Dis. 2010;5:341-349. doi: https://doi.org/10.2147/COPD.S13771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalter-Leibovici O,Benderly M,Freedman LS,et al. Disease management plus recommended care versus recommended care alone for ambulatory COPD patients. Am J Respir Crit Care Med. 2018;197(12):1565-1574. doi: https://doi.org/10.1164/rccm.201711-2182OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baker LC,Johnson SJ,Macaulay D,Birnbaum H. Integrated telehealth and care management program for Medicare beneficiaries with chronic disease linked to savings. Health Aff. 2011;30(9):1689-97. doi: https://doi.org/10.1377/hlthaff.2011.0216 [DOI] [PubMed] [Google Scholar]
- 37.Baker LC,Macaulay DS,Sorg RA,Diener MD,Johnson SJ,Birnbaum HG. Effects of care management and telehealth: a longitudinal analysis using Medicare data. J Am Geriatr Soc. 2013;61(9):1560-1567. doi: https://doi.org/10.1111/jgs.12407 [DOI] [PubMed] [Google Scholar]
- 38.Au DH,Macaulay DS,Jarvis JL,Desai US,Birnbaum HG. Impact of a telehealth and care management program for patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2015;12(3):323-331. doi: https://doi.org/10.1513/AnnalsATS.201501-042OC [DOI] [PubMed] [Google Scholar]
- 39.Wilkinson TMA,Donaldson GC,Hurst JR,Seemungal TAR,Wedzicha JA. Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;169(12):1298-1303. doi: https://doi.org/10.1164/rccm.200310-1443OC [DOI] [PubMed] [Google Scholar]
- 40.Cheng Q,Juen J,Hsu-Lumetta J,Schatz B. Predicting transitions in oxygen saturation using phone sensors. Telemed J E Health. 2016;22(2):132-137. doi: https://doi.org/10.1089/tmj.2015.0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huffaker MF,Carchia M,Harris BU,et al. Passive nocturnal physiologic monitoring enables early detection of exacerbations in children with asthma. A proof-of-concept study. Am J Respir Crit Care Med. 2018;198(3):320-328. doi: https://doi.org/10.1164/rccm.201712-2606OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sugino A,Minakata Y,Kanda M,et al. Validation of a compact motion sensor for the measurement of physical activity in patients with chronic obstructive pulmonary disease. Respiration. 2012;83(4):300-307. doi: https://doi.org/10.1159/000330046 [DOI] [PubMed] [Google Scholar]
- 43.Bourbeau J,Collet J-P,Schwartzman K,Ducruet T,Nault D,Bradley C. Economic benefits of self-management education in COPD. Chest. 2006;130(6):1704-1711. doi: https://doi.org/10.1378/chest.130.6.1704 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article contains supplemental material.