Abstract
Background
Programmed death 1/ligand 1 (PD-1/L1) inhibitors have acceptable antitumor activity in patients with platinum-resistant urothelial cancer (UC). However, the reliability and comparability of the antitumor activity, safety profiles and survival outcomes of different immune checkpoint inhibitors are unknown. Our objective was to compare the clinical efficacy and safety of anti–PD-1/PD-L1 therapies in platinum-resistant UC patients.
Methods
We reviewed the published trials from the PubMed, Embase and Cochrane Library databases up to August 2020. A well-designed mirror principle strategy to screen and pair trial characteristics was used to justify indirect comparisons. The primary end point was the objective response rate (ORR). The safety profile and survival outcomes were also evaluated. The restricted mean survival time (RMST) up to 12 months was calculated.
Results
Eight studies including 1,666 advanced or metastatic UC patients (1,021 patients with anti–PD-L1 treatment and 645 patients with anti–PD-1 treatment) met the study criteria. The ORRs of anti–PD-1 and PD-L1 therapy were 22% (95% CI, 18%–25%) and 15% (95% CI, 13%–17%) with all studies combined. The proportions of the treated population with a confirmed objective response (I2 = 0; P = 0.966; HR, 1.60; 95% CI, 1.23–2.07; P < 0.001) and disease control (I2 = 30.6%; P = 0.229; HR, 1.35; 95% CI, 1.10–1.66; P = 0.004) were higher with anti–PD-1 therapy than with anti–PD-L1 therapy. The treatment-related adverse events (AEs) (I2 = 78.3%; P = 0.003; OR, 1.09; 95% CI, 0.65–1.84; P = 0.741) and grade 3–5 treatment-related AEs (I2 = 68.5%; P = 0.023; OR, 1.69; 95% CI, 0.95–3.01; P = 0.074) of anti–PD-1 therapy were comparable to those of anti–PD-L1 therapy. The RMST values at the 12-month follow-up were 9.4 months (95% CI,: 8.8–10.0) for anti–PD-1 therapy and 9.3 months (95% CI, 8.8–9.7) for anti–PD-L1 therapy (z = 0.26, P = 0.794). There was no significant difference between patients in the anti–PD-1 and anti–PD-L1 groups (12-month overall survival (OS): 43% versus 42%, P = 0.765. I2 = 0; P = 0.999; HR, 0.95; 95% CI, 0.83–1.09; P = 0.474).
Conclusions
The results of our systematic comparison suggest that anti–PD-1 therapy exhibits better antitumor activity than anti–PD-L1 therapy, with comparable safety profiles and survival outcomes. These findings may contribute to enhanced treatment awareness in patients with platinum-resistant UC.
Keywords: immunotherapy, urologic neoplasms, review, programmed cell death 1 receptor, programmed cell death 1 ligand
Introduction
Advanced or metastatic urothelial cancer (UC) patients have a poor prognosis (1, 2), and platinum-based first-line chemotherapy is the standard treatment option for these patients (1, 3–5). However, the median overall survival (OS) of UC patients who benefit from combination chemotherapy regimens is only 14 to 15 months (1). When first-line chemotherapy resistance occurs, other regimens have limited efficacy, and these patients have an OS of approximately 6 months (3, 6, 7).
Immune checkpoint inhibitors including programmed death 1 (PD-1) and programmed death 1 programmed death ligand 1 (PD-L1) treatment represent a breakthrough in the treatment of advanced or metastatic UC (8, 9), and the safety and activity of anti–PD-1/PD-L1 therapy for advanced or metastatic UC patients have been confirmed (1). In a multicenter, phase 3 randomized trial (KEYNOTE-045), pembrolizumab showed encouraging survival benefits over chemotherapy in advanced/metastatic, platinum-refractory UC (10, 11). Atezolizumab was also confirmed to have a clinical benefit, as the survival of the immunotherapy group was higher than that of the chemotherapy group (12–15).
Studies have confirmed that the mechanism divergence in the inhibitory pathway influences the clinical effects of PD-1 and PD-L1 therapy (16–18). However, the differences between anti–PD-1 and anti–PD-L1 in advanced or metastatic UC patients have raised uncertainties. A network meta-analysis that compared the survival of patients with PD-1 versus PD-L1 blockade included only two studies, limiting the amount of data available for analysis (19).
In this meta-analysis, we used a well-designed mirror principle strategy that included screening and pairing trial characteristics to adjust for indirect comparisons (20). The durable response rates, survival, and tolerability of anti–PD-1/PD-L1 therapy in patients with platinum-resistant UC were strictly assessed.
Methods
Search Strategy
Literature Search Strategy
Following the Preferred Reporting Items for Systematic Review and Meta- Analyses (PRISMA) statement, the strategy of the study was determined in advance and uploaded to the PROSPERO online platform. PubMed, Embase, and the Cochrane Library were searched for studies published up to August 2020. The following medical subject heading (MeSH) terms and their combinations were searched in the [Title/Abstract] field: PD-1, PD-L1, programmed death receptor 1, programmed death receptor ligand 1, immune checkpoint inhibitors, and urothelial carcinoma.
Inclusion and Exclusion Criteria
Eligible studies included the following: (1) patients with advanced or metastatic UC; (2) anti–PD-1/PD-L1 treatment; (3) patients with platinum-resistant disease; (4) clinical trials (phase I, II or III); (5) ≥20 patients who reported responses; and (6) published in the English language. Studies including anti–PD-/PD-L1 treatment with other immunotherapies, anti–PD-/PD-L1 neoadjuvant treatment, and anti–PD-/PD-L1 as maintenance treatment and retrospective studies were excluded. For duplicate publications, only the most recent and complete publication was included.
Data Extraction
Xueying Li and Zaishang Li independently extracted and summarized the information. A senior researcher (Hui Han) served as the adjudication author and resolved any disagreements. Disagreements among all authors were resolved via discussion. The following information was extracted from the studies: first author, year of publication, phase of trials, National Clinical Trial number, clinical trial name, treatments, number of patients, sex, age, physical condition score, follow-up time, objective response rate (ORR), progression-free survival (PFS), OS, and adverse events (AEs). The level of evidence for the evaluated studies was assessed according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system and the Oxford system (21, 22).
Statistical Analysis
The mirror principle was applied to compare anti–PD-1 and anti–PD-L1 therapies (20). The studies were matched based on characteristics including immunotherapy drugs, therapeutic schedule, clinical trial phase, previous treatments, lines of treatment, PD-L1 expression level, Eastern Cooperative Oncology Group (ECOG) performance status, and sex ratio. Studies lacking a relevant variable, for example, the IMvigor130 trial, were also eligible for this study. Only successfully matched studies were further analyzed.
The primary outcome was the ORR. The secondary outcomes were the disease control rate (DCR), AEs, and OS. The AEs were evaluated according to human body systems. Survival data were reconstructed with Engauge software for direct comparisons (19, 23). Reconstructed survival data meta-analysis methods were used to estimate the restricted mean survival time (RMST) up to 12 months and assessed using the method described by Grambsch and Therneau (24–26). RMST analyses were conducted with R software (survRM2 and metafor packages).
The survival rate of affected patients was expressed as the hazard ratio (HR), and the presence of lymph node metastasis was expressed as an odds ratios (OR) (27). Stata version 12 (Stata Corp, College Station, TX, USA) was used for comparisons of the HR or OR and their 95% confidence intervals (CIs). Specified subgroup analyses were conducted for studies that included immune checkpoint inhibitors and other lines of treatment. Statistical heterogeneity between studies was assessed using the chi-square test with a random-effects model if the P value was <0.10; otherwise, a fixed-effects model was used. Sensitivity analyses were performed for high-quality studies. Funnel plots, and Begg’s and Egger’s tests were used to screen for potential publication bias.
Results
Study Selection and Characteristics
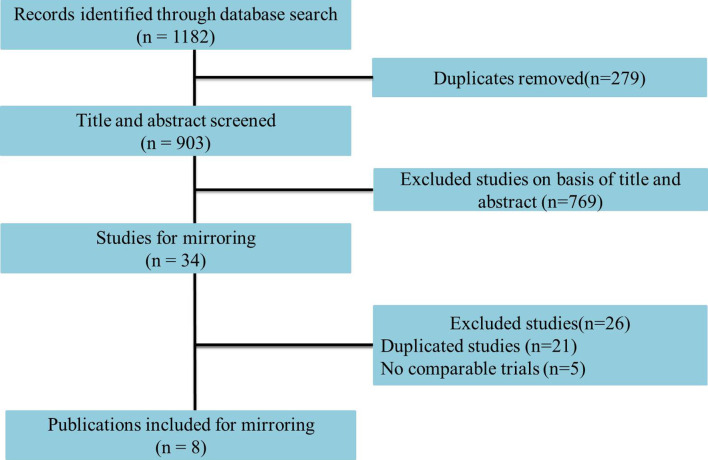
Eight studies including 1,666 advanced or metastatic UC patients (1,021 patients with anti–PD-L1 treatment and 645 patients with anti–PD-1 treatment) met the inclusion criteria ( Figure 1 ). Detailed characteristics are summarized in Table 1 . The JAVELIN Solid trial (dose-expansion cohort) (34), in which 90% of patients with ≥1% PD-L1 expression were given avelumab, was matched with the KEYNOTE-012 trial (30). The level of evidence according to the GRADE and Oxford systems for the evaluated studies is shown in Table 1 . Table 2 shows the matched outcomes using the mirror principle (32).
Figure 1.
Flowchart of study selection.
Table 1.
The characteristics of included trials.
| Author | Year | NCT Number | RCT name | Clinical Trial Phase | Intervention | Total (n) | Median Age(range) | Male n(%) | ECOGPS=0 n(%) | MPs Median, months (95% CI) | mOS Median, months (95% CI) | Follow-up (month) Median, months (range or IQR) | Level of evidence according to the GRADE system | Level of evidence according to the Oxford System |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fradet et al. (11) | 2019 | NCT02256436 | KEYNOTE-045 | III | Pembrolizumab | 270 | 67 (29–88) | 200 (74) | 119 (44) | 2.1 (2.0–2.2) | 10.1 (8.0–12.3) | 27.7 (median) | Grade 1 | 1b |
| Powles et al. (28) | 2018 | NCT02302807 | IMvigor211 | III | Atezolizumab | 467 | 67 (43–88) | 81 (70) | 61 (53) | NA | 8.6 (7.8–9.6) | 17·3 (range, 0–24·5) | Grade 1 | 1b |
| Ohyama et al. (29) | 2019 | NCT 02387996 | CheckMate 275 | II | Nivolumab | 270 | 66 (38–90) | 211 (78) | 145 (54) | 1.9 (1.9–2.3) | 8.6 (6.1–11.3) | 33.7 (minimum) | Grade 2 | 2b |
| Rosenberg et al. (30) | 2016 | NCT02108652 | IMvigor210 (Cohort 2) | II | Atezolizumab | 310 | 66 (32–91) | 241 (78) | 117 (38) | 2.7 (2.1–3.9) | 7.9 (6.6–9.3) | 11.7 (IQR, 11·4–12·2) | Grade 2 | 2b |
| Sharma et al. (31) | 2016 | NCT01928394 | CheckMate 032 | I/II | Nivolumab | 78 | 66 (31–85) | 54 (69) | 42 (54) | NA | 9.7 (7.3–16.2) | 15.2 (IQR, 12·9–16·8) | Grade 2 | 2b |
| Powles et al. (32) | 2017 | NCT01693562 | Study 1108 | I/II | Durvalumab | 191 | 67 (34–88) | 136 (71) | 64 (34) | 1.5 (1.4–1.9) | 18.2 (8.1-NE) | 5.8 (range, 0.4–25.9) | Grade 2 | 2b |
| Plimack et al. (33) | 2017 | NCT01848834 | KEYNOTE-012 | Ib | Pembrolizumab | 33 | 70 (44–85) | 23 (70) | 9 (27) | 2 (2–4) | 13 (5–20) | 13 (IQR, 5–23) | Grade 2 | 2b |
| Apolo et al. (34) | 2017 | NCT01772004 | JAVELIN Solid (dose-expansion cohort) | Ib | Avelumab | 44 | 68 (63–73) | 30 (68) | 19 (43) | 2.9 (1.5–4.4) | 13.7 (8.5-NE) | 16.5 (IQR, 15.8–16.7) | Grade 2 | 2b |
IQR, interquartile range; HR, hazard ratio; NA, not available, ICI, immune checkpoint inhibitor; Ctl, control.
Table 2.
The indirect comparison of selected studies based on the mirror principle.
| Matched groups | RCT name | Immunotherapy drug | Therapeutic schedule | Clinical trial Phase | Therapeutic schedule | Lines of Treatment | PD-L1 status | ECOG-PS | Male (%) ± 2% |
|---|---|---|---|---|---|---|---|---|---|
| 1 | KEYNOTE-045 | PD-L1 | Pembrolizumab | III | Platinum-based chemotherapy | ≤3 | All | 0–1** | 72% (+2%) |
| IMvigor211 | PD-1 | Atezolizumab | III | Platinum-based chemotherapy | ≤3 | All | 0–1 | 72% (−2%) | |
| 2 | CheckMate 275 | PD-L1 | Nivolumab | II | Platinum-based chemotherapy | 2 | All | 0–1*** | 78% (0) |
| IMvigor210 (Cohort 2) | PD-1 | Atezolizumab | II | Platinum-based chemotherapy | 2 | All | 0–1 | 78% (0) | |
| 3 | CheckMate 032 | PD-L1 | Nivolumab | I/II | Platinum-based chemotherapy | 2 | All | 0–1 | 70% (−1%) |
| Study 1108 | PD-1 | Durvalumab | I/II | Platinum-based chemotherapy | 2 | All | 0–1 | 70% (+1%) | |
| 4 | KEYNOTE-012 | PD-L1 | Pembrolizumab | Ib | Previous treatment, including platinum-based therapy | ≥1 | ≥1% PD-L1 expression | 0–1 | 69% (+1%) |
| JAVELIN Solid (dose-expansion cohort) | PD-1 | Avelumab | Ib | Platinum-based chemotherapy | ≥1 | ≥1% PD-L1 expression* | 0-1 | 69% (-1%) |
*90% patients with ≥1% PD-L1 expression; **only 2 (0.7%) patients with ECOG performance status of 2; ***only one (0.4%) patient had ECOGperformance status of 3.
The studies included two anti–PD-1 drugs (two studies on pembrolizumab and two studies on nivolumab) and three anti–PD-1 trials (two studies on atezolizumab, one study on avelumab, and one study on durvalumab). The sensitivity analysis, Begg’s test and Egger’s test showed that no bias existed in the selected studies.
Antitumor Activity
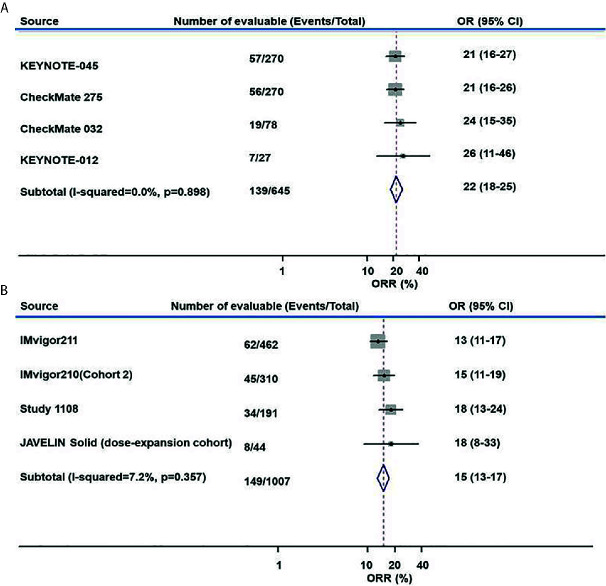
The ORR of anti–PD-L1 therapy was 15% (95% CI, 13%–17%) for all studies combined ( Figure 2A ). The combined ORR of anti–PD-1 therapy was 22% (95% CI, 18%–25%) ( Figure 2B ).
Figure 2.
Meta-analysis of pooled odds ratios of an objective response to anti–PD-1 and anti–PD-L1 therapy. (A) anti–PD-1, (B) anti–PD-L1.
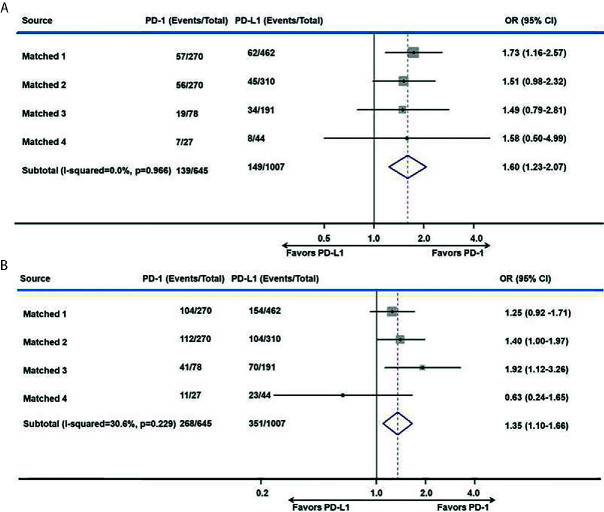
After matching, the proportion of the treated population with a confirmed objective response was higher with anti–PD-1 therapy than with anti–PD-L1 therapy (I2 = 0; P = 0.966; HR, 1.60; 95% CI, 1.23–2.07; P < 0.001) among advanced or metastatic UC patients after progression on platinum-based chemotherapy ( Figure 3A ). According to the subgroup analysis, the ORR was also higher with anti–PD-1 therapy than with anti–PD-L1 therapy in studies with a sample size >50 (I2 = 0%; P = 0.875; HR, 1.60; 95% CI, 1.22–2.08; P < 0.001).
Figure 3.
Meta-analysis of pooled odds ratios of a tumor response to anti–PD-1 versus anti–PD-L1 therapy. (A) ORR, (B) DCR.
The DCR was also higher with anti–PD-1 therapy than with anti–PD-L1 therapy in all included studies (I2 = 30.6%; P = 0.229; HR, 1.35; 95% CI, 1.10–1.66; P = 0.004. Figure 3B ) and in studies with a sample size >50 (I2 = 0%; P = 0.404; HR, 1.40; 95% CI, 1.13–1.72; P = 0.002).
However, the progressive disease rate of the anti–PD-1 group was comparable to that of the anti–PD-L1 group (I2 = 41.7%; P = 0.162; HR, 0.82; 95% CI, 0.62–1.10; P = 0.189). The investigator-assessed antitumor activity and median duration of response are shown in Table 3 .
Table 3.
Adverse events that occurred during the trial period.
| Variable | Matched 1 | Matched 2 | Matched 3 | Matched 4 | ||||
|---|---|---|---|---|---|---|---|---|
| anti–PD-L1 | anti–PD-1 | anti–PD-L1 | anti–PD-1 | anti–PD-L1 | anti–PD-1 | anti–PD-L1 | anti–PD-1 | |
| Objective response | ||||||||
| No. objective response (n/N) | 62/462 | 57/270 | 45/310 | 56/270 | 34/191 | 19/78 | 8/44 | 7/27 |
| ORR (%) | 13 (11–17) | 21 (16–27) | 15 (11–19) | 21 (16–26) | 18 (13–24) | 24 (15–35) | 18 (8–33) | 26 (11–46) |
| DCR n (%) | 154 (33) | 104 (39) | 104 (34) | 112 (42) | 70 (37) | 41 (53) | 23 (52) | 11 (41) |
| CR n (%) | 16 (3) | 25 (9) | 15 (5) | 18 (7) | 7 (4) | 5 (6) | 5 (11) | 3 (1) |
| PR n (%) | 46 (10) | 32 (12) | 30 (10) | 38 (14) | 27 (14) | 14 (18) | 3 (7) | 4 (2) |
| SD n (%) | 92 (20) | 47 (17) | 59 (19) | 56 (21) | 36 (19) | 22 (28) | 15 (34) | 4 (2) |
| PD n (%) | 240 (52) | 131 (49) | 159 (51) | 111 (41) | 88 (46) | 30 (38) | 15 (34) | 14 (52) |
| Median duration of response | 21.7 (13.0–21.7) | NE (1.6–30.0) | NE (2.0–13.7) | 20.3 (11.5-31.3) | NE (0.9-19.9) | 9.4 (5.7–12.5) | NE (3-NE) | 10 (4–22) |
| Adverse events | ||||||||
| Treatment-related AEs | 319 (69) | 165 (62) | 215 (69) | 187 (69) | 116 (61) | 63 (81) | 29 (66) | 20 (61) |
| Treatment-related AEs (3–5 grade) | 91 (20) | 44 (17) | 50 (16) | 67 (25) | 13 (7) | 17 (22) | 3 (7) | 5 (15) |
| Treatment-related serious AEs | 72 (16) | 32 (12) | 34 (11) | NA | 9 (5) | 8 (10) | 2 (5) | 3 (9) |
| Treatment-related AEs leading to treatment discontinuation | 16 (3) | 18 (7) | 11 (4) | 27 (10) | 9 (5) | 2 (3) | 4 (9) | 2 (6) |
| Treatment-related AEs lead to death | 4 (1) | 4 (2) | 0 | 3 (1) | 2 (1) | 1 (1) | 0 | 0 |
| AEs with incidence ≥1% | ||||||||
| Asthenia | 51 (43) | 17 (5) | 21 (8) | 19 (6) | 0 | 0 | 5 (11) | 0 |
| Circulatory | 0 | 0 | 8 (3) | 0 | 3 (2) | 0 | 0 | 0 |
| Decreased appetite | 56 (18) | 25 (9) | 36 (13) | 26 (8) | 18 (9) | 0 | 2 (5) | 0 |
| Fatigue | 116 (25) | 37 (14) | 93 (34) | 52 (17) | 37 (19) | 28 (36) | 9 (20) | 6 (18) |
| Pyrexia | 40 (9) | 0 | 28 (10) | 17 (5) | 15 (8) | 0 | 0 | 0 |
| Dermatological | 120 (26) | 84 (32) | 54 (20) | 120 (39) | 33 (17) | 39 (50) | 8 (18) | 2 (6) |
| Endocrine | 53 (45) | 19 (5) | 0 | 46 (15) | 9 (5) | 5 (6) | 4 (9) | 0 |
| Gastrointestinal | 161 (35) | 78 (29) | 87 (32) | 106 (34) | 29 (15) | 11 (14) | 9 (20) | 0 |
| Hematogenous | 29 (15) | 8 (10) | 9 (3) | 30 (10) | 8 (4) | 27 (35) | 10 (23) | 1 (3) |
| Hepatic | 0 | 0 | 10 (4) | 15 (5) | 25 (13) | 5 (6) | 3 (7) | 2 (6) |
| Renal | 0 | 0 | 0 | 5 (2) | 1 (1) | 1 (1) | 0 | 0 |
| Respiratory | 35 (11) | 8 (3) | 17 (6) | 14 (5) | 0 | 7 (9) | 1 (2) | 0 |
| Others | 49 (11) | 36 (14) | 0 | 0 | 11 (6) | 10 (13) | 0 | 10 (30) |
(1) Circulatory: atrial fibrillation, cardiorespiratory arrest, hypertension, hypotension, myocarditis (2); dermatological: alopecia, dermatitis acneiform, dry mouth, maculopapular, mucosal inflammation, skin reactions, pruritus, rash, stomatitis, tumor flare, uveitis (3); endocrine:adrenal disorder, diabetes, hypothyroidism, hypophysitis, hyperthyroidism, hypersensitivity, hyperglycemia, pituitary disorder, rheumatoid arthritis, thyroid disorder; (4) gastrointestinal: abdominal pain, colitis, constipation, diarrhea, intestinal perforation, increased amylase, nausea, pancreatitis, vomiting (5); hematogenous: anemia, blood alkaline phosphatase increased, creatine phosphokinase, dehydration, hyponatremia, increased blood ALP level, Infusion-related reaction, leukocyte count decreased, lipase elevated, lymphocyte count decreased, neutropenia, thrombocytopenia (6); hepatic: alanine aminotransferase increased, amylase increased, aspartate aminotransferase increased, blood bilirubin increased, hepatitis (7); renal: nephritis, renal failure, urinary tract obstruction (8); respiratory: cough, dyspnea, interstitial lung disease, pneumonitis, respiratory tract infection, respiratory failure, wheezing (9); others: arthralgia, dysgeusia, edema peripheral, muscle spasms, myalgia, myositis, neuromyopathy, pain, paresthesia, peripheral sensory neuropathy, peripheral sensory neuropathy, rhabdomyolysis, toxic encephalopathy.
Safety Analysis
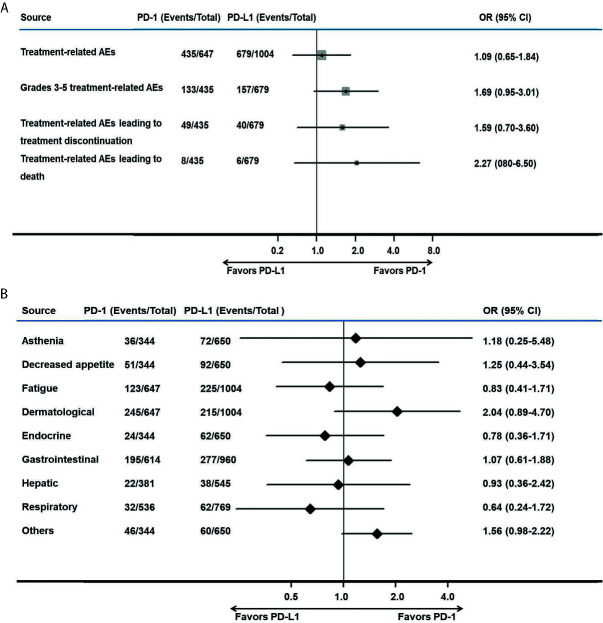
The treatment-related AEs (I2 = 78.3%; P = 0.003; OR, 1.09; 95% CI, 0.65–1.84; P = 0.741) and grade 3–5 treatment-related AEs (I2 = 68.5%; P = 0.023; OR, 1.69; 95% CI, 0.95–3.01; P = 0.074) associated with anti–PD-1 therapy were comparable to those of anti–PD-L1 therapy. Various AEs are shown in Figure 4 and Table 3 .
Figure 4.
Meta-analysis of pooled odds ratios of adverse events of anti–PD-1 versus anti–PD-L1 therapy. (A) adverse events with anti–PD-1 versus anti–PD-L1 therapy, (B) adverse events with an incidence ≥1% for anti–PD-1 versus anti–PD-L1 therapy.
No significant difference was found among treatment-related AEs leading to treatment discontinuation (I2 = 58.1%; P = 0.067; OR, 1.59; 95% CI, 0.70–3.60; P = 0.264) and AEs leading to death (I2 = 0; P = 0.524; OR, 2.27; 95% CI, 0.80–6.50; P = 0.127), as shown in Figure 4A . Among AEs with an incidence ≥1%, there were no AEs that occurred more frequently in the PD-1 group ( Figure 4B ).
Survival
The median OS times of patients with anti–PD-L1 therapy and anti–PD-1 therapy were 8.4 months (95% CI, 7.7–9.2), and 9.8 months (95% CI, 8.3–11.4), respectively, in all studies combined.
In an analysis that considered time separately, the number of anti–PD-1–treated patients at risk of death was similar to that of the anti–PD-L1-treated group at 6 months (I2 = 91.2%; P = 0; OR, 1.65; 95% CI, 0.76–3.57; P = 0.204). At 12 months, the number of anti–PD-1–treated patients at risk of death was higher than that of anti–PD-L1–treated patients (I2 = 87.8%; P = 0; OR, 2.17; 95% CI, 1.04–4.44; P = 0.033).
However, the RMST values at the 12-month follow-up were 9.4 months (95% CI, 8.8–10.0) for anti–PD-1 therapy and 9.3 months (95% CI, 8.8–9.7) for anti–PD-L1 therapy (z = 0.26, P = 0.794).
The reconstructed survival data were highly consistent with the published data. The Kaplan-Meier (KM) curves of patients in the anti–PD-1 and anti–PD-L1 groups were reconstructed at the common maximum follow-up time (12 months) for direct comparison ( Figure 5A ). No significant difference was observed between patients in the anti–PD-1 and anti–PD-L1 groups (12-month OS: 43% versus 42%, P = 0.765. I2 = 0; P = 0.999; HR, 0.95; 95% CI, 0.83–1.09; P = 0.474, Figure 5B ).
Figure 5.
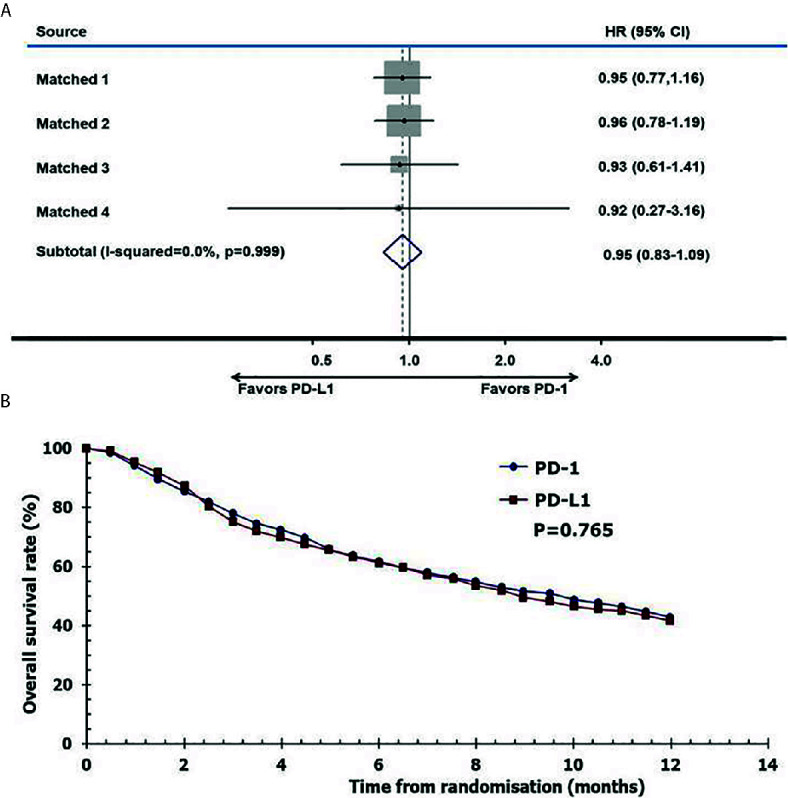
Pooled hazard ratio of survival. (A) meta-analysis of pooled hazard ratios of overall survival outcomes of anti–PD-1 versus anti–PD-L1 therapy, (B) overall survival of patients with immune checkpoint inhibitors using reconstructed survival data.
Discussion
In this study, we first used a well-designed mirror principle strategy that involved screening and pairing trial characteristics to minimize the potential bias in patients with platinum-resistant UC (20). The systematic review and meta-analysis was first adjusted for direct comparisons of anti–PD-1 and anti–PD-L1 therapy. The results suggested that anti–PD-1 therapy exhibited better antitumor activity than anti–PD-L1 therapy, with an acceptable safety profile.
Immune checkpoint inhibitors have remarkable clinical effects after progression with platinum-based chemotherapy (13, 33, 34). A meta-analysis showed that the ORR of immune-checkpoint inhibitors was 17.7% (95% CI, 16%–20%) (35). In another meta-analysis, the ORR of second-line or later treatment with immune checkpoint inhibitors was 18% (95% CI, 15%–22%) (8). Due to lack of head-to-head research or appropriate statistical methods, the difference in ORRs between patients treated with anti–PD-1 and anti–PD-L1 therapy has not been examined. Our results showed that the ORR of anti–PD-L1 therapy was 15% (95% CI, 15%–17%), and the ORR of anti–PD-1 therapy was 22% (95% CI, 18%–25%). Patient characteristics were matched using the mirror principle to minimize potential bias, and this method has been shown to be effective (20). With the mirror principle, the overall results showed that anti–PD-1 therapy exhibited better antitumor activity than anti–PD-L1 therapy in terms of the ORR and DCR.
The safety profiles of both therapies were acceptable. These immune checkpoint inhibitors were well tolerated in previous trials (13, 28, 31, 36). Grade 1–2 AEs were the most frequent treatment-related AEs and were manageable with expectant treatment (1, 15). In this analysis, the treatment-related AEs of anti–PD-1 therapy were comparable to those of anti–PD-L1 therapy. A network meta-analysis that included only two studies showed that pembrolizumab had advantages over atezolizumab in terms of serious AEs (9). However, we found that the incidence of treatment-related AEs and grade 3–5 treatment-related AEs associated with anti–PD-1 therapy were comparable to those of anti–PD-1 therapy.
In a previous study, immunotherapies had more obvious survival benefits than chemotherapy (9, 35). Even for monotherapy, immune checkpoint inhibitors also has delightful prognosis. The IMvigor130 trail provided evidence to support that anti–PD-L1 therapy plus chemotherapy can prolong the PFS of urothelial carcinoma patients (15). The Keynote-045 and IMvigor211 studies also demonstrated that the OS rate for pembrolizumab treatment was higher than that of chemotherapy in patients with platinum-resistant UC (11, 29). There were only two trials, which had insufficient power, that indicated no significant differences between PD-1 and PD-L1 blockade (19, 20). Using the same statistical methods, the median OS times with anti–PD-L/PD-L1 therapy were 8.4 months and 9.8 months, respectively, in all studies combined. At the common maximum follow-up time (12 months), although the rate of death of anti–PD-1–treated patients was lower, the OS of anti–PD-1–treated patients was comparable to that of anti–PD-L1–treated patients.
Differences exist in the mechanism of action between PD-1 and PD-L1 (16, 37, 38), which might explain the clinical differences in theory. PD-1 antibodies can bind to PD-1 to its ligands (PD-L1 and PD-L2), however, the interaction of PD-1 and PD-L2 remains intact, which may inhibit activation of T cells in PD-L1 antibodies (20). Therefore, the tumor might escape antitumor immune response through the PD-1/PD-L2 axis when being treated with anti–PD-L1, which may explain why patients receiving anti–PD-1 therapy had a better response rate than anti–PD-L1 therapy. Studies are ongoing and some patients had subsequent therapies that impact on survival outcomes. The results of this study may provide a reference for clinical studies that contributes to enhancing treatment awareness in patients with platinum-resistant UC.
Several limitations of this study should be noted. 1) Due to the lack of clinical trials, the trials included in this study were relatively limited after matching. However, this illustrates the importance and necessity of this research. 2) Because the details of all studies are not available, the study lacks individual patient data creates an important handicap when comparing and matching. The method used was an indirect comparison. However, the statistical methods were also confirmed (19, 20, 25). This study screened the research through the mirror matching method, which led to the inability of matching analysis of some cancer data. All analyses may be considered exploratory rather than hypothesis-tested in our study. 3) We could not distinguish the differences among various types of drugs. 4) Some information was incomplete. For example, AEs were classified into different systems instead of being analyzed individually. However, the main endpoint of ORR was a better comparison of the outcome than AEs in this study. For a few studies, a subgroup analysis of metastases or PD-L1 expression was not performed. We suggest that subgroup analyses including metastases or PD-L1 expression will provide information for future studies.
Conclusion
In summary, the results of our systematic comparison suggest that anti–PD-1 therapy exhibits better antitumor activity than PD-L1 therapy in patients with platinum-resistant UC. The safety profiles and survival outcomes for PD-1 and PD-L1 treatment were comparable. These findings may contribute to enhancing treatment awareness in patients with platinum-resistant UC treatment.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
FZ and KX had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study conception and design: ZL, XL, HH, and FZ. Acquisition of data: ZL, XL, and HH. Analysis and interpretation of data: all authors. Drafting of the manuscript: ZL and XL. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: ZL, XL, FZ, and HH. Obtaining funding: ZL. Administrative, technical, or material support: FZ. Supervision: FZ. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81902610) and Science and Technology Planning Project of Shenzhen Municipality (CN) (JCYJ20190807145409328).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to the authors of the trials, the enrolled patients, their families, and the referring physicians.
Abbreviations
AE, adverse event; CI, confidence interval; CR, complete remission; DCR, disease control rate; HR, hazard ratio; OR, odds ratios; ORR, objective response rate; OS, overall survival; PD, progressive disease; PD-1/L1, programmed death 1/ligand 1; PFS, progression-free survival; RMST, restricted mean survival time; PR, partial response; SD, stable disease; UC, urothelial cancer.
References
- 1. Flaig TW, Spiess PE, Agarwal N, Bangs R, Boorjian SA, Buyyounouski MK, et al. Bladder Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2020) 18(3):329–54. 10.6004/jnccn.2020.0011 [DOI] [PubMed] [Google Scholar]
- 2. Nakagawa T, Taguchi S, Kanatani A, Kawai T, Ikeda M, Urakami S, et al. Oncologic Outcome of Metastasectomy for Urothelial Carcinoma: Who Is the Best Candidate? Ann Surg Oncol (2017) 24(9):2794–800. 10.1245/s10434-017-5970-8 [DOI] [PubMed] [Google Scholar]
- 3. Kaufman D, Raghavan D, Carducci M, Levine EG, Murphy B, Aisner J, et al. Phase II trial of gemcitabine plus cisplatin in patients with metastatic urothelial cancer. J Clin Oncol Off J Am Soc Clin Oncol (2000) 18(9):1921–7. 10.1200/JCO.2000.18.9.1921 [DOI] [PubMed] [Google Scholar]
- 4. Loehrer PJ Sr, Einhorn LH, Elson PJ, Crawford ED, Kuebler P, Tannock I, et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol Off J Am Soc Clin Oncol (1992) 10(7):1066–73. 10.1200/JCO.1992.10.7.1066 [DOI] [PubMed] [Google Scholar]
- 5. Scher HI. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Urol (1992) 148(5):1625–6. [PubMed] [Google Scholar]
- 6. Galsky MD, Mironov S, Iasonos A, Scattergood J, Boyle MG, Bajorin DF. Phase II trial of pemetrexed as second-line therapy in patients with metastatic urothelial carcinoma. Invest New Drugs (2007) 25(3):265–70. 10.1007/s10637-006-9020-9 [DOI] [PubMed] [Google Scholar]
- 7. Vaughn DJ, Broome CM, Hussain M, Gutheil JC, Markowitz AB. Phase II trial of weekly paclitaxel in patients with previously treated advanced urothelial cancer. J Clin Oncol Off J Am Soc Clin Oncol (2002) 20(4):937–40. 10.1200/JCO.2002.20.4.937 [DOI] [PubMed] [Google Scholar]
- 8. Tafuri A, Smith DD, Cacciamani GE, Cole S, Shakir A, Sadeghi S, et al. Programmed Death 1 and Programmed Death Ligand 1 Inhibitors in Advanced and Recurrent Urothelial Carcinoma: Meta-analysis of Single-Agent Studies. Clin Genitourin Cancer (2020) 351–60. 10.1016/j.clgc.2020.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang H, Liu J, Fang K, Ke C, Jiang Y, Wang G, et al. Second-line treatment strategy for urothelial cancer patients who progress or are unfit for cisplatin therapy: a network meta-analysis. BMC Urol (2019) 19(1):125. 10.1186/s12894-019-0560-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vaughn DJ, Bellmunt J, Fradet Y, Lee JL, Fong L, Vogelzang NJ, et al. Health-Related Quality-of-Life Analysis From KEYNOTE-045: A Phase III Study of Pembrolizumab Versus Chemotherapy for Previously Treated Advanced Urothelial Cancer. J Clin Oncol Off J Am Soc Clin Oncol (2018) 36(16):1579–87. 10.1200/JCO.2017.76.9562 [DOI] [PubMed] [Google Scholar]
- 11. Fradet Y, Bellmunt J, Vaughn DJ, Lee JL, Fong L, Vogelzang NJ, et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of >2 years of follow-up. Ann Oncol (2019) 30(6):970–6. 10.1093/annonc/mdz127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Petrylak DP, Powles T, Bellmunt J, Braiteh F, Loriot Y, Morales-Barrera R, et al. Atezolizumab (MPDL3280A) Monotherapy for Patients With Metastatic Urothelial Cancer: Long-term Outcomes From a Phase 1 Study. JAMA Oncol (2018) 4(4):537–44. 10.1001/jamaoncol.2017.5440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pal SK, Hoffman-Censits J, Zheng H, Kaiser C, Tayama D, Bellmunt J. Atezolizumab in Platinum-treated Locally Advanced or Metastatic Urothelial Carcinoma: Clinical Experience from an Expanded Access Study in the United States. Eur Urol (2018) 73(5):800–6. 10.1016/j.eururo.2018.02.010 [DOI] [PubMed] [Google Scholar]
- 14. Perez-Gracia JL, Loriot Y, Rosenberg JE, Powles T, Necchi A, Hussain SA, et al. Atezolizumab in Platinum-treated Locally Advanced or Metastatic Urothelial Carcinoma: Outcomes by Prior Number of Regimens. Eur Urol (2018) 73(3):462–8. 10.1016/j.eururo.2017.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Galsky MD, Arija JAA, Bamias A, Davis ID, De Santis M, Kikuchi E, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet (2020) 395(10236):1547–57. 10.1016/S0140-6736(20)30230-0 [DOI] [PubMed] [Google Scholar]
- 16. Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity (2007) 27(1):111–22. 10.1016/j.immuni.2007.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest (2015) 125(9):3384–91. 10.1172/JCI80011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yearley JH, Gibson C, Yu N, Moon C, Murphy E, Juco J, et al. PD-L2 Expression in Human Tumors: Relevance to Anti-PD-1 Therapy in Cancer. Clin Cancer Res (2017) 23(12):3158–67. 10.1158/1078-0432.CCR-16-1761 [DOI] [PubMed] [Google Scholar]
- 19. Niglio SA, Jia R, Ji J, Ruder S, Patel VG, Martini A, et al. Programmed Death-1 or Programmed Death Ligand-1 Blockade in Patients with Platinum-resistant Metastatic Urothelial Cancer: A Systematic Review and Meta-analysis. Eur Urol (2019) 76(6):782–9. 10.1016/j.eururo.2019.05.037 [DOI] [PubMed] [Google Scholar]
- 20. Duan J, Cui L, Zhao X, Bai H, Cai S, Wang G, et al. Use of Immunotherapy With Programmed Cell Death 1 vs Programmed Cell Death Ligand 1 Inhibitors in Patients With Cancer: A Systematic Review and Meta-analysis. JAMA Oncol (2019) 375–84. 10.1001/jamaoncol.2019.5367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ (2004) 328(7454):1490. 10.1136/bmj.328.7454.1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schunemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ (2008) 336(7653):1106–10. 10.1136/bmj.39500.677199.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials (2007) 8:16. 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grambsch P, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika (1994) 81(3):515–26. 10.2307/2337123 [DOI] [Google Scholar]
- 25. Le CT, Grambsch PM, Louis TA. Association between survival time and ordinal covariates. Biometrics (1994) 50(1):213–9. [PubMed] [Google Scholar]
- 26. Smith CT, Williamson PR, Marson AG. Investigating heterogeneity in an individual patient data meta-analysis of time to event outcomes. Stat Med (2005) 24(9):1307–19. 10.1002/sim.2050 [DOI] [PubMed] [Google Scholar]
- 27. Hartung J, Knapp G, Sinha BK. Statistical Meta-analysis With Applications. Hoboken, NJ: John Wiley & Sons; (2008). 10.1002/9780470386347 [DOI] [Google Scholar]
- 28. Powles T, O’Donnell PH, Massard C, Arkenau HT, Friedlander TW, Hoimes CJ, et al. Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma: Updated Results From a Phase 1/2 Open-label Study. JAMA Oncol (2017) 3(9):e172411. 10.1001/jamaoncol.2017.2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ohyama C, Kojima T, Kondo T, Naya Y, Inoue T, Tomiya Y, et al. Nivolumab in patients with unresectable locally advanced or metastatic urothelial carcinoma: CheckMate 275 2-year global and Japanese patient population analyses. Int J Clin Oncol (2019) 24(9):1089–98. 10.1007/s10147-019-01450-w [DOI] [PubMed] [Google Scholar]
- 30. Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet (2016) 387(10031):1029–20. 10.1016/S0140-6736(16)00561-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sharma P, Callahan MK, Bono P, Kim J, Spiliopoulou P, Calvo E, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol (2016) 17(11):1590–8. 10.1016/S1470-2045(16)30496-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Powles T, Duran I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet (2018) 391(10122):748–57. 10.1016/S0140-6736(17)33297-X [DOI] [PubMed] [Google Scholar]
- 33. Plimack ER, Bellmunt J, Gupta S, Berger R, Chow LQ, Juco J, et al. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. Lancet Oncol (2017) 18(2):212–20. 10.1016/S1470-2045(17)30007-4 [DOI] [PubMed] [Google Scholar]
- 34. Apolo AB, Infante JR, Balmanoukian A, Patel MR, Wang D, Kelly K, et al. Avelumab, an Anti-Programmed Death-Ligand 1 Antibody, In Patients With Refractory Metastatic Urothelial Carcinoma: Results From a Multicenter, Phase Ib Study. J Clin Oncol Off J Am Soc Clin Oncol (2017) 35(19):2117–24. 10.1200/JCO.2016.71.6795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sternberg CN, Loriot Y, James N, Choy E, Castellano D, Lopez-Rios F, et al. a Multinational Single-arm Safety Study of Atezolizumab Therapy for Locally Advanced or Metastatic Urothelial or Nonurothelial Carcinoma of the Urinary Tract. Eur Urol (2019) 76(1):73–81. 10.1016/j.eururo.2019.03.015 [DOI] [PubMed] [Google Scholar]
- 36. Chang SS. Re: Atezolizumab in Patients with Locally Advanced and Metastatic Urothelial Carcinoma Who Have Progressed following Treatment with Platinum-Based Chemotherapy: A Single-Arm, Multicentre, Phase 2 Trial. J Urol (2016) 196(6):1637–8. 10.1016/j.juro.2016.09.025 [DOI] [PubMed] [Google Scholar]
- 37. Di Nunno V, De Luca E, Buttigliero C, Tucci M, Vignani F, Gatto L, et al. Immune-checkpoint inhibitors in previously treated patients with advanced or metastatic urothelial carcinoma: A systematic review and meta-analysis. Crit Rev Oncol Hematol (2018) 129:124–32. 10.1016/j.critrevonc.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 38. Galsky MD, Saci A, Szabo PM, Han GC, Grossfeld G, Collette S, et al. Nivolumab in Patients with Advanced Platinum-resistant Urothelial Carcinoma: Efficacy, Safety, and Biomarker Analyses with Extended Follow-up from CheckMate 275. Clin Cancer Res (2020) 5120–8. 10.1158/1078-0432.CCR-19-4162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fessas P, Lee H, Ikemizu S. Janowitz T. A molecular and preclinical comparison of the PD-1-targeted T-cell checkpoint inhibitors nivolumab and pembrolizumab. Semin Oncol (2017) 44(2):136–40. 10.1053/j.seminoncol.2017.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, et al. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front Pharmacol (2017) 8:561. 10.3389/fphar.2017.00561 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.