Abstract
Roxarsone (Rox) is an organic arsenic compound used as a feed additive to promote animal growth. The release of Rox into the environment poses risks to human health. Rox demonstrated tumor-promoting and proangiogenic effects in xenograft models. Increasing studies revealed the tight relationship among angiogenesis, carcinogenesis, tumorigenesis, and glycolysis. Glycolysis, via hypoxia-inducible factor-1α (HIF-1α), controls vascular endothelial cell (VEC) growth. To date, there has been no literature report on the effect of Rox on HIF-1α-dependent glycolysis. Herein, we report that Rox promoted glycolysis in rat VECs, as shown by the increased adenosine triphosphate production, the lactic acid release, the activity and content of aldolase (ALD), and the expression levels of ALD A and glucose transporter 1 (GLUT1). Rox also increased the cellular levels of HIF-1α. Treatment with the HIF-1α inhibitor YC-1 reversed Rox-increased ALD A and GLUT1 levels and attenuated Rox-induced VEC viability, suggesting that Rox-induced HIF-1α contributes to the glycolytic and angiogenic effects of Rox. Rox also promoted tumor growth and angiogenesis and increased the levels of ALD A, GLUT1, and HIF-1α in the tumor tissue of a mouse xenograft model, whereas these effects were abolished using YC-1. Our findings indicated that Rox induces HIF-1α in VECs to promote glycolysis and angiogenesis thus enhancing the tumor growth.
Introduction
Angiogenesis, the formation of new blood vessels from pre-existing capillaries and circulating endothelial precursors, plays a pivotal role in the initiation of carcinogenesis and tumor progression.1 Endothelial cells (ECs) are inert blood vessel-lining materials and are active players in the formation of new blood vessels; therefore, EC metabolism is a key regulator of angiogenesis.2 Glycolysis, the main energy source in the endothelium, has distinct and essential roles during vessel formation, and ECs have higher rates of glycolysis.3,4 Hypoxia and hypoxia-inducible factor (HIF) signaling regulate multiple aspects of EC biology, including the cell survival, growth, invasion, and glucose metabolism, thus contributing to the induction of angiogenesis.5 Although the effect of hypoxia on cancer cell metabolism has received increased attention, the role of hypoxia and HIFs in EC metabolism remains poorly studied.5,6 With respect to the regulation of cellular metabolism, hypoxia-inducible factor-1α (HIF-1α) is the predominant subunit that induces glycolysis and other metabolic changes.6 Stabilization of HIF-1α results in a switch from oxidative phosphorylation toward glycolysis, by upregulating glycolytic enzymes, for example, aldolase (ALD) A and glycolysis-promoting proteins, such as glucose transporter 1 (GLUT1).5
Roxarsone (Rox), also known as 3-nitro-4-hydroxyphenyl-arsenic acid, is an organic arsenic additive that is used in animal feed to ensure proper weight gain, feed efficiency, and pigmentation in livestock and poultry.7 Although the usage of Rox as a feed additive is banned in the United States, Europe, and China, the compound is still used frequently in developing countries, such as Argentina, Brazil, and India.8 Only a small amount of Rox is absorbed by the animals; thus, the vast majority of Rox is excreted unchanged via the feces.9 This increases the level of arsenic in the environment and induces a risk of arsenic exposure in humans when the animal manure is used subsequently as an organic fertilizer.10 Arsenic has been identified as a type II carcinogen by the International Agency for Research on Cancer and is known to promote angiogenesis at low levels.11 Importantly, Rox was reported to be carcinogenic in male F344/N rats.12
Previously, we reported the tumor-promoting and proangiogenic effects of Rox in MCF7 and B16–F10 xenograft models and demonstrated that a proangiogenic mechanism might contribute to the tumor-promoting capability of Rox.8,13,14 However, currently, the role and mechanism of HIF-1α-mediated glycolysis in Rox-induced angiogenesis are not well understood. Therefore, in the present study, we evaluated the effects of treatment with, or repeated administration of, Rox on HIF-1α expression and glycolysis in primary cultured rat aorta ECs and in a xenograft model using the melanoma cell line B16–F10 in C57BL/6 mice. We also investigated whether pretreatment with the HIF-1α inhibitor YC-1 (Lificiguat, 3-(5′-hydroxymethyl-2′-furyl)-1-benzyl indazole) could rescue the Rox-induced effects.
Materials and Methods
Reagents
Rox (Cat. no. 46724, analytical standard) was purchased from Sigma-Aldrich (St. Louis, MO, USA), dissolved in 5 mL of methanol, and then diluted to 50 mL with deionized water to obtain a 1 mM stock solution. Then, 0.1, 1, and 10 μM Rox working solutions were made by further diluting the stock solution with incubation medium. Rox was diluted in phosphate-buffered saline (PBS) for administration to rats. YC-1 (Cat. no. S7958, purity = 99.94%) was obtained from Selleck (Houston, TX, USA). Other chemicals were purchased from local commercial sources and were of analytical grade.
Animals
All animal protocols were reviewed by the Committee for the Ethics of Animal Experiments of Yangzhou University. Experiments were carried out in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals in China and the EU Directive 2010/63/EU for animal experiments.
Male Wistar rats (weighing approximately 250 g) and C57BL/6 mice (weighing approximately 20 g) were obtained from the Comparative Medicine Center of Yangzhou University. The animals were housed in a room with controlled environmental conditions of a temperature of 22 °C, a relative humidity of 40–60%, and a 12 h light–dark cycle. Rats and mice had free access to standard diet and tap water.
Cell Isolation and Culture
The Wistar rats were anesthetized using 2% thiopental sodium and sacrificed. Vascular endothelial cells (VECs) were isolated from the thoracic aorta and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 15% (v/v) fetal bovine serum (FBS), 100 μg/mL of sodium heparin, 4 ng/mL of vascular endothelial growth factor (VEGF), and 100 U of penicillin/streptomycin at 37 °C in a 5% CO2 atmosphere, as reported previously.15 The cells were subcultured once they had formed a monolayer (after approximately 6 days of incubation). For subsequent assays, the rat VECs were digested with 2% trypsin, briefly centrifuged at 1000 rpm for 10 min, and resuspended at the required density in DMEM.
B16–F10 melanoma cells were a gift from the Research Group of Laboratory Animals of Yangzhou University and were maintained in DMEM containing 10% FBS and penicillin/streptomycin.
Measurement of Rat VEC Monolayer Bioimpedance
Real-time bioimpedance of rat VECs was measured using an xCELLigence RTCA Single Plate system (Agilent Technologies, Santa Clara, CA, USA). The results of the cellular impedance assay were reported as the unitless “cell index (CI)”. A 96-well E-plate was initially loaded with the trypsinized VECs (in 150 μL DMEM with approximately 5 × 103 cells per well), placed within the system sensor inside the cell incubator, and then allowed to settle overnight. On the following day, measurement of the CI was recorded just before the administration of 0–10 μM Rox. Data were analyzed using RTCA software V2.0 (Agilent).
ATP and Lactic Acid Assay
Rat VECs were seeded in a six-well plate at a density of 2 × 105 cells per well (in triplicate) and grown overnight in growth medium. The next day, 0–10 μM Rox was added. After incubation for 24 h, the cells were lysed using lysis buffer and intracellular ATP was quantified using an enhanced ATP assay kit. The luminescence was measured in a GloMax-Multi + Microplate Multimode Reader from Promega (Madison, WI, USA). In addition, the supernatant was collected for lactic acid (LD) release measurement using a lactic acid assay kit. The OD values were measured at 530 nm in a microplate reader.
Determination of ALD Activity and Content
Rat VECs were seeded in a six-well plate at a density of 2 × 105 cells per well (in triplicate) and grown overnight in a growth medium. The next day, 0–10 μM Rox was added. After incubation for 24 h, the cells were washed and lysed using three cycles of freeze–thawing. The lysate was centrifuged, and the supernatant was collected for ALD activity and content determination using a rat ALD activity/content enzyme-linked immunosorbent assay (ELISA) Kit. The OD values were measured at 450 nm in a microplate reader.
Determination of Cell Viability
Rat VECs suspended in growth medium were seeded in a 96-well plate at a density of 2 × 103 cells per well and grown overnight. The next day, cells were treated with PBS, 1 μM Rox, 50 μM YC-1, or 1 μM Rox plus 50 μM YC-1. After incubation for 24 h, each well was added with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) at a final concentration of 0.4 mg/mL in medium. After 4 h, reduced formazan was solubilized with 150 μL of dimethyl sulfoxide by shaking for 10 min. Cell viability was determined by measuring the optical density (OD) at 570 nm in a microplate reader. Six replica wells were analyzed for each treatment.
Mouse B16–F10 Xenograft Studies
B16–F10 cells (1 × 105) in 0.3 mL of nonsupplemented DMEM were injected subcutaneously into the right ribs of mice. The animals with visible tumors at 7 days after cell injection were intragastrically administered and/or intraperitoneally injected with PBS; 1, 5, and 25 mg/kg Rox; 15 mg/kg YC-1; or 5 mg/kg Rox plus 15 mg/kg YC-1 once a day for 1 week. During this week of administration, tumor measurement was conducted once a day. The length (L) and width (W) of the tumors were measured using vernier calipers, and the tumor volume was calculated using the formula: L × W2. The animals were sacrificed after 1 week of administration; the tumors were excised, weighed, and fixed overnight in neutral-buffered formalin; embedded in paraffin; and sectioned. The sections were then stained using hematoxylin–eosin (H&E) and photographed under a light microscope at 200× magnification.
Western Blotting
The treated VECs and tumor tissues were lysed in radioimmunoprecipitation assay lysis buffer with phenylmethylsulfonyl fluoride and a phosphatase inhibitor cocktail on ice. Then, the lysates were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis, with 20 μg of protein loaded per lane. The gels were transferred to nitrocellulose (NC) membranes using a Bio-Rad Transblot apparatus (Bio-Rad, Hercules, CA, USA). The membranes were blocked with 5% bovine serum albumin (BSA) in Tris-buffered saline with Tween 20 (TBST) for 2 h at room temperature and then incubated overnight with rabbit antibodies against HIF-1α (1:1000 dilution), ALD A (1:1000 dilution), GLUT1 (1:200 dilution), and β-actin (1:250 dilution). Blots were developed using the enhanced chemiluminescence detection system. The intensity of the immunoreactive protein bands on the blots was measured using ImageJ (NIH, Bethesda, MD, USA).
Statistical Analysis
Data are expressed as mean ± standard deviation. Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Dunnett post hoc test, or two-way ANOVA followed by Bonferroni post hoc test, for multiple comparisons.
Results
Rox Induces Proliferation of Rat VECs
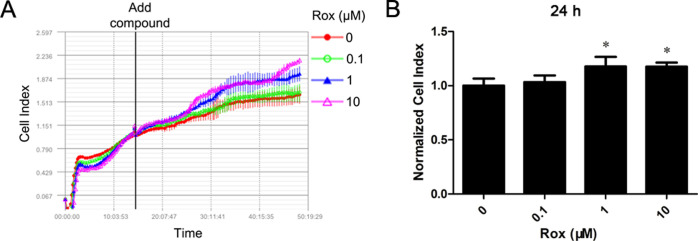
First, to observe the actions of Rox on primary cultured rat aorta VECs, cells were exposed to 0.1, 1, or 10 μM Rox, and then real-time bioimpedance measurements were carried out. We found that Rox increased the monolayer impedance (CI) of the rat VECs in a time- and concentration-dependent manner (Figure 1). As shown in Figure 1B, compared with that of the control group, treatment with 1 and 10 μM Rox increased the normalized CI significantly. The CI reflects a broad range of cell responses that affect electric current flow through the cell layer overlying an array of gold microelectrodes, including the effects on the cell number and shape, cell–cell junctions, cell–substrate adhesion, and ion channel activation.16 It has been shown that the CI value obtained on the RT-CES system correlates quantitatively with cell growth and cell numbers, and the RT-CES system has an efficiency similar to that of the MTT test for counting viable cells.17,18 The findings suggested that Rox induces the proliferation of rat VECs.
Figure 1.
Rox increases the monolayer impedance (CI) of rat vascular endothelial cells. Real-time bioimpedance of rat vascular endothelial cells treated with Rox (0–10 μM) for the indicated times was measured using the xCELLigence RTCA Single Plate system. *p < 0.05: significantly different from the control group.
Rox Induces Glycolysis in Rat VECs
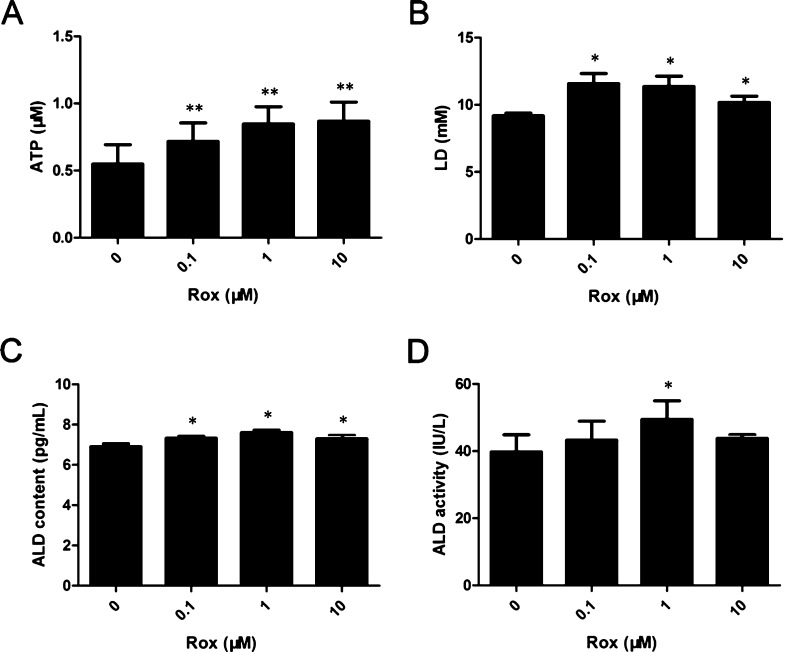
ECs have high rates of glycolysis, and glycolysis and glycolytic rates play a critical role in EC proliferation.19,20 Based on this, we hypothesized that Rox induced the proliferation of rat VECs by promoting intracellular glycolysis. To investigate the effects of Rox on glycolysis in VECs, cells were treated with 0.1, 1, or 10 μM Rox for 24 h. After Rox exposure, ATP production, LD release, and ALD activity and content were determined. Treatment with Rox dramatically increased intracellular ATP, LD, and ALD contents in a dose-dependent manner in primary cultured rat aorta VECs (Figure 2A–C). Compared with that of the control group, exposure to 1 μM Rox for 24 h increased the ALD activity significantly (Figure 2D). We also checked the levels of ALD A and GLUT1, the two glycolysis-related proteins, using western blotting. The data revealed that Rox upregulated the levels of these proteins in the cells (Figure 3). These findings indicated that Rox induces glycolysis in primary cultured rat aorta VECs.
Figure 2.
Rox induces ATP production, lactic acid (LD) release, and ALD activity and content in rat vascular endothelial cells. Cells were treated with 0–10 μM Rox for 24 h. The ATP (A) and LD (B) contents and the ALD activity (C) and content (D) were measured using ELISA. *p < 0.05, **p < 0.01: significantly different from the control group.
Figure 3.
Rox induces HIF-1α, ALD A, and GLUT1 protein expression in rat vascular endothelial cells. Cells were treated with Rox at indicated concentrations for 24 h. The protein levels of HIF-1α, ALD A, and GLUT1 were measured using western blotting. (A) Representative blots. (B) Blots for the indicated proteins subjected to semiquantitative analysis using NIH ImageJ. *p < 0.05, **p < 0.01: significantly different from the control group.
Rox Promotes Glycolysis by Inducing HIF-1α in Rat VECs
To understand the mechanism of Rox-induced glycolysis, we next determined the HIF-1α levels in rat VECs exposed to Rox. As shown in Figure 3, compared with that in the untreated group, Rox increased the HIF-1α levels in a concentration-dependent manner. To further confirm the role of HIF-1α induction in Rox-promoted glycolysis in VECs, we pretreated the rat VECs with YC-1 (an HIF-1α inhibitor) and then examined the HIF-1α, ALD A, and GLUT1 levels. The results showed that pretreatment with YC-1 attenuated the Rox-induced increases in the ALD A and GLUT1 levels in rat VECs (Figure 4A,B). We also evaluated cell viability using the MTT assay. Consistently, Rox increased the cell viability, whereas pretreatment with YC-1 reversed this effect (Figure 4C). Collectively, these data indicated that Rox induces glycolysis by increasing the HIF-1α levels in rat VECs.
Figure 4.
Inhibition of HIF-1α by pretreatment with YC-1 attenuates Rox-promoted HIF-1α, ALD A, and GLUT1 expression and cell viability in rat vascular endothelial cells. Cells were treated with or without Rox and with or without YC-1 for 24 h. Protein levels of HIF-1α, ALD A, and GLUT1 were measured using western blotting. (A) Representative blots. (B) Blots for the indicated proteins subjected to semiquantitative analysis using NIH ImageJ. (C) Cell viability detected using the MTT assay. *p < 0.05, **p < 0.01: significantly different from the control group; #p < 0.05 and ##p < 0.01: significantly different from the 1 μM Rox group.
Rox-Increased HIF-1α Levels Contribute to Glycolysis and Angiogenesis in a Mouse B16–F10 Xenograft Model
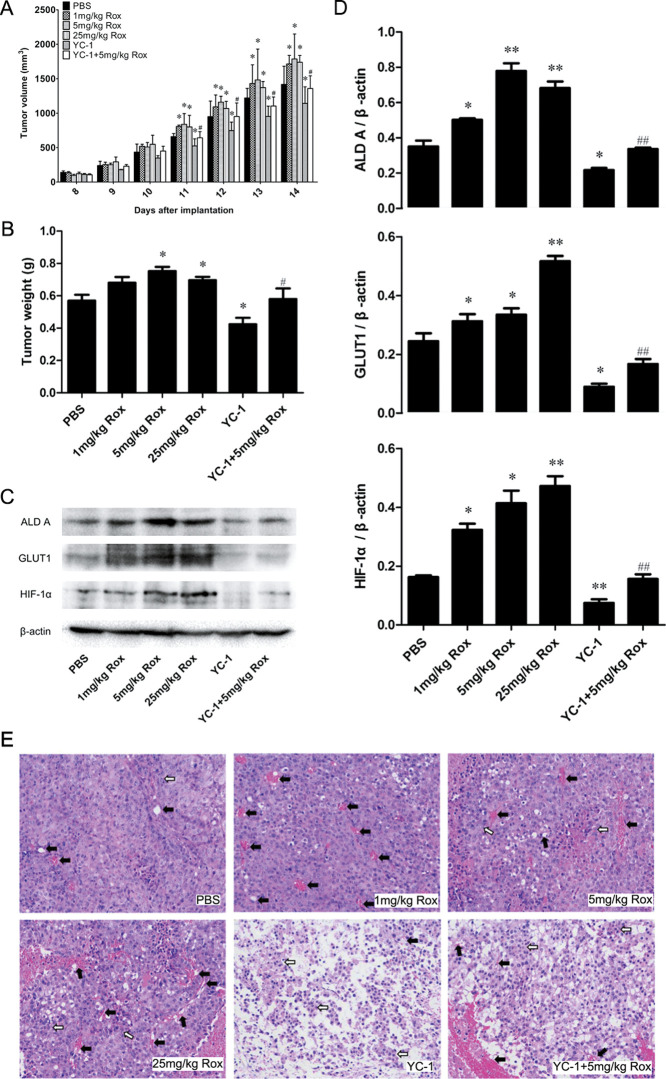
To further understand the role of HIF-1α-dependent glycolysis in angiogenesis and tumor development, we administered Rox, with or without YC-1, to B16–F10 xenograft-bearing C57BL/6 mice once a day for 7 days. The tumor size was measured using vernier calipers, and the tumor volume was calculated. Xenografts were then excised and weighed. As shown in Figure 5A,B, compared with mice administered with PBS, Rox administration (1, 5, and 25 mg/kg) increased the weight and volume of the tumors significantly. Compared with those in the 5 mg/kg Rox administration group, both the weight and volume of the xenografts were decreased significantly in the 5 mg/kg Rox with YC-1 group (Figure 5A,B). Then, ALD A, GLUT1, and HIF-1α levels were determined in the B16–F10 xenografts using western blotting. As expected, Rox increased the levels of ALD A, GLUT1, and HIF-1α, whereas YC-1 decreased the levels of these proteins (Figure 5C,D). Importantly, the protein levels in the 5 mg/kg Rox with YC-1 group were lower than those in the 5 mg/kg Rox-alone group (Figure 5C,D). Finally, changes in the growth pattern of tumor cells and blood vessel size were observed in the H&E-stained paraffin sections of the xenografts (Figure 5E). The B16–F10 cells in the xenograft tumors of untreated mice showed a typical diffuse pattern without growth, whereas the structure and organization of the xenograft tumors treated with 5 and 25 mg/kg Rox appeared tight, and the B16–F10 cells showed inward clustered growth centered on a blood vessel. In contrast, in the xenograft tumors that were treated with YC-1, the B16–F10 cells were typically diffuse, did not have a distinct growth pattern, and the numbers of B16–F10 cells and vessels were significantly lower than those in the control. The tight organization of the B16–F10 cells and increased vessels in the 5 mg/kg Rox group was clearly reversed in the group receiving Rox combined with YC-1, and there were fewer tumor cells around blood vessels in the combined treatment group. Fewer blood sinusoids were observed in the xenograft tumors treated with YC-1 + Rox than in the xenograft tumors treated with Rox alone. These findings indicated that Rox induces angiogenesis and tumor development by increasing HIF-1α-mediated glycolysis in B16–F10 xenograft-bearing C57BL/6 mice.
Figure 5.
Effects of Rox and/or YC-1 administration on the volume, weight, and expression of HIF-1α, ALD A, and GLUT1 and on angiogenesis of/in melanoma xenografts. B16–F10 tumor cells were implanted subcutaneously into the right ribs of C57BL/6 mice. After 7 days, the mice were intragastrically and/or intraperitoneally administered with PBS; 1, 5, and 25 mg/kg Rox; and/or YC-1 once a day for another 7 days, respectively. (A) The tumor size was measured using vernier calipers and the tumor volume was calculated. Data represent the average tumor volumes at the given day after implantation. (B) Xenograft tumors were excised and weighed. (C) Protein levels of HIF-1α, ALD A, and GLUT1 were measured using western blotting. Representative blots are shown. (D) Blots for the indicated proteins were subjected to semiquantitative analysis using NIH ImageJ. (E) Paraffin sections of xenografts were created and stained with H&E for histological analysis (100×). Representative images (of at least five mice per group) are shown. *p < 0.05, **p < 0.01: significantly different from the control group; #p < 0.05 and ##p < 0.01: significantly different from the 5 mg/kg Rox group.
Discussion and Conclusions
Angiogenesis, the process that leads to the formation of new blood vessels or neovascularization, is markedly perturbed in cancer.1 ECs form the inner lining of blood vessels and are essential for the normal function of the vascular system.21 Therefore, ECs are active players in the formation of new blood vessels.21 EC metabolism has only recently been recognized as a driving force of angiogenesis.22 Glycolysis is the main energy source in ECs.23 One of the main stimulators of angiogenesis is hypoxia.5 HIF-1α, a key protein in the hypoxia and HIF signaling system, plays a critical role in glucose metabolism regulation.6 Rox is an organic arsenic agent used as an animal feed additive for livestock and poultry to enhance weight gain and to improve feeding efficiency in developing countries.24 Arsenic has been reported to promote angiogenesis and has been identified as a type II carcinogen.25 Importantly, Rox has been reported as a carcinogenic agent for male F344/N rats.12 Previously, we indicated that the tumor-promoting effect of Rox might be attributed to its proangiogenic ability in MCF7 and B16–F10 xenograft models.8,13,14 However, there is little information on the underlying relationship between HIF-1α-mediated glycolysis and Rox-induced angiogenesis.
In the present study, we showed that Rox significantly induced rat VEC proliferation, as evidenced by the increased cell monolayer impedance in cells treated with Rox (Figure 1). This was consistent with the results of our previous study.15 Rox also increases Caco-2 cell proliferation.26 Besides, we found that ATP production and LD release were induced in cells exposed to Rox. In addition, the intracellular ALD content was increased by Rox. Consistent with the increased ALD content, intracellular ALD activity was enhanced. Importantly, western blotting revealed that Rox upregulated the levels of ALD A and GLUT1 in VECs (Figure 3). ALD A is one of the enzymes involved in glycolysis, and GLUT1 is a glycolysis-promoting protein.27,28 These results demonstrated that Rox induces proliferation and glycolysis in primary cultured rat aorta VECs.
Hypoxia and HIF signaling regulate glucose metabolism.5 The ALD family is one of the downstream target proteins of HIF-1α.29 In addition, hypoxia can induce the expression of GLUT1.30 To investigate the mechanism of Rox-induced glycolysis, we assessed the protein level of HIF-1α in rat VECs. The data revealed that Rox promoted the expression of HIF-1α, as demonstrated by the increased levels of the protein in the cells. Next, we utilized YC-1, an HIF-1α inhibitor, to verify the relationship between Rox-induced HIF-1α expression and glycolysis. The results showed that YC-1 pretreatment partially reversed Rox-increased intracellular ALD A and GLUT1 levels and cell viability in rat VECs (Figure 4). These findings demonstrated that Rox induces proliferation and glycolysis via increasing HIF-1α levels in rat VECs.
It has been shown that inhibition of HIF-1α decreases angiogenesis.31 We further administered Rox with or without YC-1 to B16–F10 xenograft-bearing C57BL/6 mice to investigate the role of HIF-1α-dependent glycolysis in angiogenesis and tumor development. The weight and volume of the tumors increased significantly after Rox administration. The weight and volume of the xenografts treated with YC-1 and Rox were lower than those in the xenografts treated with Rox alone. The protein levels of ALD A, GLUT1, and HIF-1α in the Rox with YC-1 group were lower than those in the Rox-alone group. Interestingly, H&E staining showed that the tight organization of B16–F10 cells and the increased number of vessels induced by the Rox group were clearly reversed by the combined Rox and YC-1 treatment, and there were fewer tumor cells around the blood vessels in the combined treatment group. Fewer blood sinusoids were observed in the xenograft tumors treated with YC-1 + Rox than in those treated with Rox alone. These results indicated that Rox induces angiogenesis and tumor development by increasing HIF-1α-mediated glycolysis in B16–F10 xenograft-bearing C57BL/6 mice.
Interestingly, in this study, it appears that some of the Rox effects are nonmonotonic. Arsenic is known to promote carcinogenesis at low concentrations and continual exposure but demonstrates antitumor effects at higher doses. For example, certain arsenic compounds have been approved by the Food and Drug Administration for the treatment of some blood cancers such as acute promyelocytic leukemia, and their efficacy has been evaluated in clinical trials of the treatment of multiple myelomas and a variety of solid tumors.32,33 Rox, as a kind of organoarsenic compound, may also exhibit the dual antitumor activity. It may be partially due to the biphasic impact of Rox on angiogenesis in that it promotes angiogenesis at low levels and inhibits angiogenesis at high levels. Definitely, further research should be conducted to address this issue.
In summary, we demonstrated that Rox induces proliferation and glycolysis in primary cultured rat aorta ECs. Rox increased the HIF-1α levels, leading to glycolysis and angiogenesis in vitro and in vivo. Our findings suggest that manipulation of HIF-1α expression could represent a potential approach to prevent Rox-induced angiogenesis.
Glossary
Abbreviations Used
- ALD
aldolase
- ANOVA
analysis of variance
- ATP
adenosine triphosphate
- BSA
bovine serum albumin
- CI
cell index
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
dimethyl sulfoxide
- ECL
enhanced chemiluminescence
- ELISA
enzyme-linked immunosorbent assay
- FBS
fetal bovine serum
- GLUT1
glucose transporter 1
- HIF-1α
hypoxia-inducible factor-1α
- LD
lactic acid
- MTT
thiazolyl blue tetrazolium bromide
- NC
nitrocellulose
- OD
optical density
- PBS
phosphate-buffered saline
- PMSF
phenylmethylsulfonyl fluoride
- Rox
roxarsone
- SD
standard deviation
- SDS
sodium dodecyl sulfate
- TBST
Tris-buffered saline with Tween 20
- VEGF
vascular endothelial growth factor
Author Contributions
∥ X.C. and M.Z. contributed equally to the work.
This work was financially supported by the grants from the Youth Science Foundation Project funded by National Natural Science Foundation of China (No. 31902324, Xin Chen) and by the project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
The authors declare no competing financial interest.
References
- Draoui N.; de Zeeuw P.; Carmeliet P. Angiogenesis revisited from a metabolic perspective: role and therapeutic implications of endothelial cell metabolism. Open Biol. 2017, 7, 170219. 10.1098/rsob.170219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlenova K.; Veys K.; Miranda-Santos I.; De Bock K.; Carmeliet P. Endothelial Cell Metabolism in Health and Disease. Trends Cell Biol. 2018, 28, 224–236. 10.1016/j.tcb.2017.10.010. [DOI] [PubMed] [Google Scholar]
- Akram M. Mini-review on glycolysis and cancer. J. Canc. Educ. 2013, 28, 454–457. 10.1007/s13187-013-0486-9. [DOI] [PubMed] [Google Scholar]
- Teuwen L.-A.; Draoui N.; Dubois C.; Carmeliet P. Endothelial cell metabolism. Curr. Opin. Hematol. 2017, 24, 240–247. 10.1097/moh.0000000000000335. [DOI] [PubMed] [Google Scholar]
- Wong B. W.; Marsch E.; Treps L.; Baes M.; Carmeliet P. Endothelial cell metabolism in health and disease: impact of hypoxia. EMBO J. 2017, 36, 2187–2203. 10.15252/embj.201696150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.; Lin D.; Taniguchi C. M. Hypoxia inducible factor (HIF) in the tumor microenvironment: friend or foe?. Sci. China Life Sci. 2017, 60, 1114–1124. 10.1007/s11427-017-9178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.; Wang X.; Wang G.; Wu C.; Li N. Genome-wide expression analysis of roxarsone-stimulated growth of broiler chickens (Gallus gallus). Comp. Biochem. Physiol. Genom. Proteonomics 2011, 6, 264–270. 10.1016/j.cbd.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Pang Y.; Wang K.; Wang Y.; Chenlin Z.; Lei W.; Zhang Y. Tumor-promoting and pro-angiogenic effects of roxarsone via VEGFR2/PLCγ/PKC signaling. Chem. Biol. Interact. 2018, 292, 110–120. 10.1016/j.cbi.2018.07.012. [DOI] [PubMed] [Google Scholar]
- Garbarino J. R.; Bednar A. J.; Rutherford D. W.; Beyer R. S.; Wershaw R. L. Environmental fate of roxarsone in poultry litter. I. Degradation of roxarsone during composting. Environ. Sci. Technol. 2003, 37, 1509–1514. 10.1021/es026219q. [DOI] [PubMed] [Google Scholar]
- Rutherford D. W.; Bednar A. J.; Garbarino J. R.; Needham R.; Staver K. W.; Wershaw R. L. Environmental fate of roxarsone in poultry litter. Part II. Mobility of arsenic in soils amended with poultry litter. Environ. Sci. Technol. 2003, 37, 1515–1520. 10.1021/es026222+. [DOI] [PubMed] [Google Scholar]
- Soucy N. V.; Ihnat M. A.; Kamat C. D.; Hess L.; Post M. J.; Klei L. R.; Clark C.; Barchowsky A. Arsenic stimulates angiogenesis and tumorigenesis in vivo. Toxicol. Sci. 2003, 76, 271–279. 10.1093/toxsci/kfg231. [DOI] [PubMed] [Google Scholar]
- NTP Toxicology and Carcinogenesis Studies of Roxarsone (CAS No. 121-19-7) in F344/N Rats and B6C3F1 Mice (Feed Studies). Natl. Toxicol. Progr. Tech. Rep. 1989, 345, 1–198. [PubMed] [Google Scholar]
- Wang Y.; Yin D.; Xu C.; Wang K.; Zheng L.; Zhang Y. Roxarsone induces angiogenesis via PI3K/Akt signaling. Cell Biosci. 2016, 6, 54. 10.1186/s13578-016-0119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Wang Y.; Lu Q.; Xin W.; Cui W.; Zhu J. Organoarsenic Roxarsone Promotes Angiogenesis In Vivo. Basic Clin. Pharmacol. Toxicol. 2016, 118, 259–270. 10.1111/bcpt.12501. [DOI] [PubMed] [Google Scholar]
- Zhu J.; Cui W.; Liu X.; Ying J.; Hu C.; Zhang Y. In vitro and ex vivo angiogenic effects of roxarsone on rat endothelial cells. Toxicol. Lett. 2013, 223, 175–182. 10.1016/j.toxlet.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Atienza J. M.; Zhu J.; Wang X.; Xu X.; Abassi Y. Dynamic monitoring of cell adhesion and spreading on microelectronic sensor arrays. J. Biomol. Screen 2005, 10, 795–805. 10.1177/1087057105279635. [DOI] [PubMed] [Google Scholar]
- Xing J. Z.; Zhu L.; Gabos S.; Xie L. Microelectronic cell sensor assay for detection of cytotoxicity and prediction of acute toxicity. Toxicol. Vitro 2006, 20, 995–1004. 10.1016/j.tiv.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Xing J. Z.; Zhu L.; Jackson J. A.; Gabos S.; Sun X.-J.; Wang X.-b.; Xu X. Dynamic monitoring of cytotoxicity on microelectronic sensors. Chem. Res. Toxicol. 2005, 18, 154–161. 10.1021/tx049721s. [DOI] [PubMed] [Google Scholar]
- Eelen G.; de Zeeuw P.; Simons M.; Carmeliet P. Endothelial cell metabolism in normal and diseased vasculature. Circ. Res. 2015, 116, 1231–1244. 10.1161/circresaha.116.302855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bock K.; Georgiadou M.; Schoors S.; Kuchnio A.; Wong B. W.; Cantelmo A. R.; Quaegebeur A.; Ghesquière B.; Cauwenberghs S.; Eelen G.; Phng L. K.; Betz I.; Tembuyser B.; Brepoels K.; Welti J.; Geudens I.; Segura I.; Cruys B.; Bifari F.; Decimo I.; Blanco R.; Wyns S.; Vangindertael J.; Rocha S.; Collins R. T.; Munck S.; Daelemans D.; Imamura H.; Devlieger R.; Rider M.; Van Veldhoven P. P.; Schuit F.; Bartrons R.; Hofkens J.; Fraisl P.; Telang S.; Deberardinis R. J.; Schoonjans L.; Vinckier S.; Chesney J.; Gerhardt H.; Dewerchin M.; Carmeliet P. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 2013, 154, 651–663. 10.1016/j.cell.2013.06.037. [DOI] [PubMed] [Google Scholar]
- Eelen G.; de Zeeuw P.; Treps L.; Harjes U.; Wong B. W.; Carmeliet P. Endothelial Cell Metabolism. Physiol. Rev. 2018, 98, 3–58. 10.1152/physrev.00001.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Sun X.; Carmeliet P. Hallmarks of Endothelial Cell Metabolism in Health and Disease. Cell Metab. 2019, 30, 414–433. 10.1016/j.cmet.2019.08.011. [DOI] [PubMed] [Google Scholar]
- Ganapathy-Kanniappan S.; Geschwind J.-F. H. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol. Canc. 2013, 12, 152. 10.1186/1476-4598-12-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris K. C.; Salazar J.; Quazi S.; Andra S. S.; Sarkar D.; Bach S. B. H.; Datta R. Controlling the fate of roxarsone and inorganic arsenic in poultry litter. J. Environ. Qual. 2008, 37, 963–971. 10.2134/jeq2007.0416. [DOI] [PubMed] [Google Scholar]
- Basu P.; Ghosh R. N.; Grove L. E.; Klei L.; Barchowsky A. Angiogenic potential of 3-nitro-4-hydroxy benzene arsonic acid (roxarsone). Environ. Health Perspect. 2008, 116, 520–523. 10.1289/ehp.10885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayse G. S.; Hammonds-Odie L. P.; Jackson K. M.; Tucker D. K.; Kirlin W. G. Permeation of roxarsone and its metabolites increases caco-2 cell proliferation. Adv. Biol. Chem. 2013, 03, 389–396. 10.4236/abc.2013.34041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.-C. Aldolase deployed for surveilling glucose. Nat. Rev. Mol. Cell Biol. 2020, 21, 714. 10.1038/s41580-020-00305-x. [DOI] [PubMed] [Google Scholar]
- Reckzeh E. S.; Waldmann H. Development of Glucose Transporter (GLUT) Inhibitors. Eur. J. Org Chem. 2020, 2020, 2321–2329. 10.1002/ejoc.201901353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierans S. J.; Taylor C. T. Regulation of glycolysis by the hypoxia-inducible factor (HIF): implications for cellular physiology. J. Physiol. 2021, 599, 23–37. 10.1113/jp280572. [DOI] [PubMed] [Google Scholar]
- Mamun A. A.; Hayashi H.; Yamamura A.; Nayeem M. J.; Sato M. Hypoxia induces the translocation of glucose transporter 1 to the plasma membrane in vascular endothelial cells. J. Physiol. Sci. 2020, 70, 44. 10.1186/s12576-020-00773-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepal M.; Gong Y.-D.; Park Y. R.; Soh Y. An activator of PHD2, KRH102140, decreases angiogenesis via inhibition of HIF-1α. Cell Biochem. Funct. 2011, 29, 126–134. 10.1002/cbf.1732. [DOI] [PubMed] [Google Scholar]
- Anderson K. C.; Boise L. H.; Louie R.; Waxman S. Arsenic trioxide in multiple myeloma: rationale and future directions. Canc. J. 2002, 8, 12–25. 10.1097/00130404-200201000-00003. [DOI] [PubMed] [Google Scholar]
- Zhou J. Arsenic trioxide: an ancient drug revived. Chin. Med. J. 2012, 125, 3556–3560. [PubMed] [Google Scholar]