Abstract
The present work reports the electrochemical sensing of acrylamide (AM) using a poly(methylene blue)-modified glassy carbon electrode (PMB/GCE) where PMB functions as an electrochemical reporter. PMB was prepared by electrochemical polymerization of methylene blue. Electrochemical sensing of AM was facilitated by the interaction between AM and PMB. Further the interaction between AM and PMB was investigated using ultraviolet–visible (UV–vis) spectroscopy and Raman analysis. The surface morphology was confirmed by atomic force microscopy (AFM) and field emission scanning electron microscopy (FESEM) analyses. PMB/GCE was further characterized by X-ray photoelectron spectroscopy (XPS), and the electrochemical performance was assessed using cyclic voltammetry and differential pulse voltammetry. Cyclic voltammetry analysis showed a decrease in current at the redox center of PMB upon addition of AM. The association constant and binding number of AM with PMB/GCE were calculated using differential pulse voltammetry and found to be 8.9 × 106 M–1 and 0.64 (∼1), respectively. The results indicated a strong interaction of AM on the PMB/GCE surface. Further, chronocoulometry analysis of PMB/GCE in the presence of AM showed a decrease in charge due to the interaction of AM with PMB. Under optimized conditions, PMB/GCE exhibited a decrease in current proportional to the concentration of AM in the range of 0.025–16 μM with sensitivity and detection limit 0.252 μA nM–1 and 0.13 nM, respectively. Real sample analysis was carried out by the standard addition method using the solution extracted from potato chips.
1. Introduction
Acrylamide (AM) is classified as a class 2A carcinogen containing an allylic group with a resonance-stabilized amide group. It has been observed that AM imparts potential genetic, neuro, and reproductive toxicity to humans.1 AM has a wide range of applications in various industries such as food packaging, paper, and textile and is also used in the separation of proteins.2−5 Deep-fried foods (≥120 °C) contain AM as a result of the Maillard reaction between the reducing sugar and amino acid present in food during food processing/cooking. It has been found that AM is predominantly formed by two pathways, i.e., Strecker synthesis (Schiff base as intermediate) and acrolein (decarboxylation of organic acids).6,7 It has been estimated that exposure humans to AM through food and modern industrialization has reached a range of 0.2–1.9 μg/kg. It has been estimated that concentration of AM in fried potatoes, bakery products, and coffee corresponds to 272–570, 75–1044, and 229–890 mg/kg, respectively. AM toxicity arises due to the formation of adducts with biomolecules such as DNA, neuronal protein, hemoglobin, etc.8−10 AM belongs to the category of soft electrophiles, which may react with soft nucleophiles such as amino acids (cysteine, lysine, and histidine) and the N-terminal amine of the protein moiety.11 Owing to the toxic nature of AM formed in food products, the determination of AM becomes highly important and necessary. The conventional methods for the determination of AM include capillary electrophoresis, enzyme-linked immunosorbent assay (ELISA), and chromatographic techniques such as high-performance liquid chromatography (HPLC), gas chromatography–mass spectrometry (GC–MS), liquid chromatography–MS (LC-MS), etc.12−16 But all of these techniques involve tedious sampling procedures, complex sample pretreatment, high operational cost, and require trained personnel. It is important to mention that electrochemical methods were found to be advantageous over other methods in terms of time and cost with the added advantage of allowing on-site detections. Recently, hemoglobin-modified electrodes have been developed for the detection of AM due to their adduct-forming ability with AM and thereby inhibiting electrochemical signature. Because of the impaired conductive nature of hemoglobin, electrode modifications using conductive materials such as multi-walled carbon nanotubes (MWCNTs) and ionic liquids have been attempted16−19 Usually, a hemoglobin-modified electrode detects AM through formation of adduct Hb-Fe(II)-AM, and this leads to an increase of distance from the electrode surface. Consequently, a decline in peak current is observed, which is proportional to the AM concentration. Several electrochemical sensors for AM have been reported based on hemoglobin (Hb/DDAB/CP,11 Hb/SWCNT/GC,20 Hb/AuNPs,21 Hb/cMWCNT/CuNPs/polyaniline/pencil graphite,22 Hb/cMWCNT/Fe3O4/chitosan/Au electrode,23 and Hb-DDAB/PtAuPd/Ch-IL/MWCNTsIL/GCE),7 DNA (DNA/GO/GCE24 and ssDNA/Au electrode),25 and cell-based sensing (pheochromocytoma cell-AuNPs/GO electrode10 and potentiometric sensors based on a Pseudomonas aeruginosa-modified electrode).12 All of these methods require a surface modification step, which is an important step in deciding the sensitivity and robustness of the detection protocol. In the present work, methylene blue has been used as an electrode modifier for sensing of AM. Methylene blue belongs to a class of phenothiazine redox dyes, which exhibits electrochemical polymerization ability and redox nature on the modified surface. Poly(methylene blue) (PMB) is a well-known electroactive polymer, exhibiting its oxidation and reduction peaks at a low potential, which can be utilized for sensing applications. Also, the preparation of PMB via electropolymerization is quite simple and rapid. PMB-modified electrodes have been developed and employed for sensing of various analytes such as H2O2,26 4-nitrophenol,27 dopamine,28 hemoglobin,29 N-acetylcysteine,30 NADH,31 catechin,32 vitamin B6,33 etc. PMB was fabricated on a glassy carbon electrode through electropolymerization of methylene blue (MB) to reduce the issues related to electrode modification. The electrochemical sensing of AM on PMB/glassy carbon electrode (GCE) was facilitated due to the surface interactions of AM with PMB. Further, this method was found to be rapid, cost-effective, and reproducible under optimized conditions.
2. Result and Discussion
2.1. Electrochemical Polymerization of MB on GCE
Figure 1a shows the electrochemical polymerization of MB on a GC electrode for 50 cycles. The first cycle exhibits two oxidation peaks at −0.12 and 1.0 V, which correspond to monomer oxidation and irreversible oxidation attributed to the radical cation formation (Figure S1). An additional oxidation peak at 0.01 V with a broad feature and quasi-reversible nature was noted after a few cycles of electropolymerization. The observed peak exhibits a positive shift in potential compared to that of the monomer. Radical cation formation occurs at a high potential due to the presence of a tertiary amino substituent in the ring. The formation of radical cation initiates the polymerization by reacting with MB monomers available in the vicinity of the electrode–electrolyte interface. The formed unstable cation radical binds covalently with another aromatic ring of the monomer through the ortho position to amino groups, since the carbon atom adjacent to the amino group is more electronegative. The electrochemical polymerization of methylene blue proceeds via direct ring-to-ring coupling or through nitrogen bridges.34 A similar kind of observation was reported for other phenazine polymerization.35 The surface coverage concentration (Γ) and the thickness of PMB on the GC electrode were measured to be 15.882 × 10–10 mol cm–2 (using Γ = Q/nFA) and 0.63 nm (using d = v × Γ), respectively.30
Figure 1.
Electrochemical polymerization of MB (0.1 mM) in (NH4)2SO4 (45 mM) with a scan rate of 50 mVs–1 for 50 cycles (a); cyclic voltammograms of PMB/GCE (blue, red) and GCE (black, yellow) in the absence (blue, black) and presence (red, yellow) of 100 μM AM at pH 5 (b). Raman spectra of PMB in the absence (black) and presence (red) of AM (c).
2.2. Characterization and Electroanalytical Ability of the PMB/GCE Electrode
Figure 1b shows the cyclic voltammogram of PMB/GCE in the absence and presence of AM. From the voltammogram, it was observed that PMB/GCE exhibited a decrease in peak current in the presence of AM, which might be attributed to the interaction of AM with PMB. The interaction between AM and PMB has been probed further using Raman and ultraviolet–visible (UV–vis) analyses. Raman analysis was used to understand the kind of interaction between AM and PMB on the electrode surface. The Raman spectrum (Figure 1c) of PMB was recorded in the absence of AM, and the following peaks were obtained (1037, 1061, 1304, 1332, 1395, 1433, 1477, 1501, and 1624 cm–1). The peak at 1037 cm–1 corresponds to the aromatic stretching frequency of the C–S bond, and the peaks at 1395 and 1433 cm–1 arise due to the bending vibration of −N(CH3)2 in PMB. The stretching frequency of the phenyl ring in PMB was observed at 1624 cm–1, and addition of AM to PMB/GCE led to a decrease in the peak intensity. A notable decrease in peak intensity was observed at 1433 and 1624 cm–1, which was attributed to the chemical interaction of AM with PMB at the −N(CH3)2 site and thereby disturbing the phenyl ring stretching. Also, appearance of a new peak at 808 cm–1 was observed, which correspond to the C–C bond formation. In addition to this, a peak at 770 cm–1 corresponding to the in-plane bending of the C–H group was observed in the absence of AM. In the presence of AM, the peak decreased substantially (Figure 1c). Further, to understand the interaction between PMB and AM, UV–vis analysis was performed. The molecular interaction of AM with MB (monomer of PMB) was observed. MB showed absorbance at 616 nm (shoulder peak) and 664 nm (λmax) corresponding to the cationic form of the monomer and formation of dimer. Upon interaction with AM, a hypochromic shift was noted on increasing the AM concentration (Figure S2). From the results, it was evident that AM affects the chromophore site of MB, which resulted in a hypochromic shift. From UV–vis and Raman analyses, we conclude that a covalent interaction between AM and PMB would be possible and hence a decrease in current was observed upon addition of AM (Scheme 1).
Scheme 1. Proposed Pathway of Interaction between AM and PMB.
X-ray photoelectron spectroscopy (XPS) of PMB/GCE (Figure 2a–d) was used to probe the PMB-modified GCE. From the survey spectrum, peaks corresponding to carbon, sulfur, and nitrogen were detected. The core-level spectra of C 1s reveal the presence of three peaks at 284.4, 285.1, and 287.5 eV corresponding to the C=C, C=N, and C–N bonds. The core-level S 2p spectrum exhibited four peaks at 164.3 and 165.7 eV corresponding to the C–S–C bond and other two peaks at 167.5 and 168.8 eV corresponding to adsorbed sulfate anions on the PMB surface. The deconvolution spectrum of N 1s shows two peaks at 399.7 and 402.4, which correspond to the C–N=C and C=N+(CH3)2 functional groups, respectively. Figure 3 shows the surface morphology of bare GCE and PMB/GCE. From the analysis, it was observed that a network of PMB was formed on the electrode surface. This clearly indicates the adhesion of PMB, as the condensed aromatic structure adsorbed onto the electrode surface and underwent polymerization.36 Topographic information of bare GC and PMB/GCE was obtained using atomic force microscopy (AFM) analysis. From the analysis, the formation of PMB on the GCE was clearly visible in the two-dimensional (2D) images (Figure S3) compared to that of bare GCE. The average roughness values for bare GCE and PMB/GCE were found to be 42 and 69 nm, respectively.
Figure 2.
XPS survey spectrum of PMB/GCE (a) and XPS core-level spectra of C 1s (b), N 1s (c), and S 2p (d).
Figure 3.
Field emission scanning electron microscopy (FESEM) of bare GCE (a) and PMB/GCE (b).
2.3. Optimization of Electrochemical AM Sensing
PMB/GCE was tested at various pH values (from 3 to 10) to understand the electrochemical behavior. Figure 5a clearly shows quasi-reversible nature of PMB, with increasing pH, and a shift in potential to a negative side accompanied by the broadening of peak and disappearance of one of the redox peaks. From the results, it can be inferred that proton concentration plays an important role in the redox activity of PMB. The voltammetric curves of PMB/GCE exhibit a quasi-reversible nature for the two redox peaks, and a shift in potential toward the negative direction was noted for each increase in pH without addition of AM. As the proton concentration decreased, the voltammetric peaks became broader (Figure 4a,b). Upon addition of AM, a decrease in current was noted irrespective of pH change. The maximum decrease in current was observed at pH 5 compared to all other pH values. The observation may be substantiated by considering AM as an electrophile at acidic pH, and so a strong interaction between AM and PMB has been expected. As a result, pH 5 was chosen for the analysis of AM (Figure 4c). As the pH increases from 3 to 8, the oxidation potential of PMB decreases linearly, and the corresponding regression equation can be given as follows: Epa (V) = −0.0412 pH + 0.4004 (R2 = 0.9741) (Figure 4d). From the slope value, it can be known that two oxidation routes may be possible, i.e., 1e–/1H+ and 2e–/1H+. From the Nernst equation Epa = E – [(2.303mRT)/(nF)]pH, the ratio of m/n was found to be ∼2/3, where m and n represent the numbers of protons and electrons involved in the electrochemical oxidation process and R and T have their usual meanings.
Figure 5.
Effect of polymerization cycles (10 [black], 30 [red], 50 [blue], 70 [pink], and 100 [green]) toward sensing of 100 μM AM at a scan rate of 50 mVs–1 (a). Effect of scan rate (10–1000 mVs–1) in the presence of 100 μM AM using PMB/GCE (b). Plot of log ν vs log Ipa (c). Chronocoulometry of PMB/GCE in the absence of AM (black) and in the presence of 50 μM AM (red). The inset is the plot of charge vs root of time (d).
Figure 4.
Cyclic voltammograms of PMB/GCE at pH 3–9 in the absence (a) of AM and in the presence (b) of 100 μM AM. Ipa vs pH (c) and Epa vs pH (d) [absence of AM (solid line) and presence of AM (dotted line)] at a scan rate of 50 mVs–1.
It is important to note that the polymer film thickness is affected
by the polymerization cycles. Also, it is known that the higher the
number of polymerization cycle, the greater the polymer thickness
at the electrode surface, thereby hindering the ionic/electronic conductivity
at the electrode–electrolyte interface. Few cycles of polymerization
may leave the GCE surface uncovered, leading to exposure of the GCE
surface without polymer formation, so interaction of the analyte with
the electrode becomes less and affects the electroanalytical performance
of the electrode. To ascertain the optimum polymer film thickness
that exhibits better response toward AM, the effect of polymerization
cycle (Figure 5a) was studied in the presence of AM in the
range of 10–100 cycles. Among these, PMB formed after 50 cycles
exhibited the maximum decrease in current upon addition of AM compared
to other cycles (Figure S4). The effect
of scan rate (Figure 5b) for PMB/GCE with addition of AM (100 μM) at pH 5 was studied
at different scan rates in the range of 10–1000 mVs–1. The double log plot of oxidation peak current and log ν
(Figure 5c) clearly
indicates that the process comprises both diffusion- and adsorption-controlled
processes, log Ipa (μA) =
0.841 log ν (mVs–1) – 0.690
(R2 = 0.9930). The observed two oxidation
and reduction peaks might be attributed to the oxidation/reduction
and doping/dedoping process of PMB, respectively. The number of electrons
transferred in the electrode process was evaluated to be 2 using the
following equation: I = nFQv/4RT. From the plot of Epa vs
log ν, the electron-transfer rate constant (ks) and charge transfer coefficient were calculated using
the Laviron equation 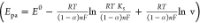 (>150 mVs–1). From the
equation, the electrode kinetic parameters ks and α were found to be 0.2 and 0.14 s–1, respectively.
(>150 mVs–1). From the
equation, the electrode kinetic parameters ks and α were found to be 0.2 and 0.14 s–1, respectively.
Figure 5d shows the chronocoulometry responses of PMB/GCE in the presence and absence of AM at pH 5 as the background electrolyte. The diffusion coefficient (D) of AM at the modified electrode can be calculated using Q = ((2nFACD1/2t1/2)/π1/2) + Qads. The plot of charge (Q) vs square root of time (t1/2) exhibits a linear relationship (linear regression equation Q = 4.043 × 10–5 + 4.4579 × 10–7, R2 = 0.9973). By substituting n = 2, A = 0.07 cm2, and c = 50 μM in the above equation, we obtained a diffusion coefficient of 4.8 × 10–8 cm2 s–1. Also, the adsorption capacity (Γs) of PMB/GCE toward AM was calculated to be 6.86 × 10–10 mol cm–2 using the equation Qads = nFAΓs. From the values, it is clear that PMB/GCE exhibits good adsorption property toward AM.
2.4. Differential Pulse Voltammetry Studies
After optimizing the pH of electrolyte medium and number of polymerization cycles, the electrochemical sensing ability of PMB/GCE toward different concentrations of AM in the range of 25 nM to 16 μM was studied using cyclic voltammetry. The observed decrease in current from the voltammograms upon addition of AM also includes the capacitive current arisen due to charges residing on the electrode–electrolyte interface (Figure S5). To circumvent the capacitive current, differential pulse voltammetry (DPV) was chosen as the electroanalytical technique for sensing of AM using PMB/GCE in the range of 25 nM to 16 μM (Figure 6a). A decrease in current proportional to the concentration of AM was observed. Figure 6b clearly shows that the calibration plot follows an “adsorption model”-like plot rather than a linear one; this reveals that the electrode reaction undergoes a surface binding process rather than an electrochemical process. From the observation, we infer that the electrode process undergoes a binding process. PMB/GCE acts as an electrochemical reporter, without any labels for additional output signals, i.e., a decrease in redox peaks of PMB under optimized conditions has been found to be proportional to the AM concentration. The corresponding log calibration plot shows a linear equation in the concentration range of 0.025–17.5 μM (inset Figure 6b) with the regression equation ΔI = 0.252 log[AM] + 0.040 (R2 = 0.9884). The sensitivity and LOD for AM exhibited by PMB/GCE were calculated to be 0.252 μA nM–1 and 0.13 nM, respectively.
Figure 6.
DPV plot for PMB/GCE in the absence and presence of 0.025, 0.075, 0.32, 0.8, 1.768, 6.587, 16.179, and 30363 μM concentrations of AM (a); plot of ΔI vs [AM] (b), and inset is the plot of ΔI vs log [AM]; association plot of PMB/GCE with AM (c); and the effect of interference with various amino acids and ions (d).
Table 1 compares the electroanalytical performances of PMB/GCE with other reported electrodes. Comparison with other modified electrodes clearly reveals that PMB/GCE exhibits better limit of detection. Also, it is important to note that PMB is electropolymerized on the GCE surface, which gives additional advantage of better adherence onto the electrode surface. It has been reported that AM forms an adduct with the guanine base.37 Similarly, PMB/GCE can also interact with AM to form an adduct. The formation of adduct can be studied using the equation
The plot of log [ΔI/(ΔImax – ΔI)] vs log[AM] tends to be linear if adduct formation occurs. From Figure 6c, a good linear regression equation was obtained, log[ΔI/(ΔImax – ΔI)] = 0.5432 log[AM]– 1.04, with a correlation coefficient of R2 = 0.9888. The binding number (m) and association equilibrium constant (Ka) were found to be 0.5432 and 8.9 × 106 M–1, respectively. From the results, it can be concluded that the interaction of AM and PMB occurs via covalent bond formation, since the association constant is found to be high compared to that of DNA-based AM sensing.25 Further, the evidence of covalent bond formation between PMB and AM is also observed from UV–vis and Raman analyses.
Table 1. Comparison of Electrochemical Sensing of AM with Reported Worksa.
| electrode | modifiers | limit of detection (nM) | linear range (μM) | references |
|---|---|---|---|---|
| ITO | Hb/GNPS/ITO | 40 | 0.01–10 | (21) |
| pencil graphite electrode | MWCNT/CuNP/PANI | 0.2 | 0.005–75000 | (22) |
| glassy carbon electrode | DNA/GO | 50 | 0.05–1000 | (24) |
| gold electrode | ssDNA | 8.1 | 0.4–200 | (25) |
| screen-printed electrode | COOH-SWCNT | 30 | 10–200 | (38) |
| glassy carbon electrode | PMB | 0.13 | 0.025–16 | this work |
SWCNT, single-walled carbon nanotube; DDAB, didodecyldimethylammonium bromide; Hb, hemoglobin; AuNP, gold nanoparticle; ssDNA, single-stranded deoxyribonucleic acid; MWCNT, multi-walled carbon nanotube; ITO, indium tin oxide; CuNP, copper nanoparticle; and PANI, poly(aniline).
2.5. Interference, Reproducibility, and Real Sample Analysis
Electrochemical sensing of AM using PMB/GCE was subjected to interference analysis in the presence of various amino acids such as proline, l-leucine, phenylalanine, threonine, arginine, asparagine, glycine, valine, alanine, methionine, tryptophan, and histidine and common ions such as sodium, potassium, dihydrogen phosphate, hydrogen phosphate, acetate, chloride, sulfate, etc. From the analysis, we infer that no interference was observed from amino acids (Figure 6d). This might be attributed to the formation of positive charge on amino acids under the studied pH and hence an electrostatic repulsion at PMB/GCE has been anticipated. The reproducibility of PMB/GCE was investigated using 100 μM AM at pH 5, and after six consecutive measurements, RSD was found to be 1.2%, which clearly shows the reproducibility of PMB/GCE. After 2 days of PMB/GCE storage (ambient) in the background electrolyte, the electrode exhibited a response of 98% compared to that of fresh electrode, and for the next consecutive 50 cycles, the electrode exhibited a reproducible signal of 89% compared to the first cycle in the presence of AM (Figure S6). PMB/GCE does not require any controlled conditions for storage like DNA/hemoglobin-modified electrodes employed for AM determination. From the analysis, PMB/GCE shows prominence in electrochemical sensing of AM compared to other reported electrodes because of its ease and control in preparation via electropolymerization. Ex situ analysis of PMB/GCE after AM sensing was carried out using XPS and FESEM analyses (Figure S7). From XPS analysis, we found that the chemical environment of N 1s and S 2p had been changed dramatically; this clearly infers the surface interaction of AM with PMB/GCE. In addition to this, FESEM analysis after AM sensing was performed and surface adsorption of AM on PMB/GCE was observed. These results were consistent with that of XPS analysis.
Real sample analysis was carried out by extracting AM from potato chips using the procedure reported in ref (39). AM was extracted from commercially available potato chips and spiked with 10, 15, and 20 μM concentrations of AM (Figures S7, S8). The experiments were carried out using the cyclic voltammetric technique under optimized conditions. The recovery values obtained using potato chip solutions were 104.4, 102.1, and 102% (with a RSD of 2.8%) for each respective concentration of AM. From the analysis, it was clear that the electrochemical platform developed using PMB exhibits good sensing ability for AM even in real samples. This presents the prospects of developing PMB-based electrochemical sensors for AM in deep-fried foods.
3. Conclusions
In this work, PMB/GCE was prepared using the electropolymerization route, which is rapid and reproducible. The surface of PMB/GCE was characterized using FESEM and XPS analyses. The PMB/GCE facilitated the electrochemical sensing of AM through surface interactions because of which selective sensing of AM was possible with better anti-interferent ability. Also, the interaction between AM and PMB was confirmed based on the observed spectroscopic signatures using UV–vis and Raman spectroscopy. PMB/GCE exhibited detection ability in a wide range of concentrations (0.025–16 μM; 1000 fold) with a detection limit of 0.13 nM. Also, the developed electrochemical sensor platform exhibited acceptable real-time sensing ability toward potato chips as the model matrix. The platform presents a prospect of electrochemical sensing of other AM congeners with suitable modifications.
4. Experimental Section
4.1. Materials
All reagents were of analytical grade and used without further purification. All of the reagents were prepared using Millipore water (18.2 MΩ·cm). Proline (99%), l-leucine (98%), phenylalanine (98%), threonine (98%), arginine (98%), asparagine (98%), glycine (99%), valine (98%), alanine (98%), methionine (98%), tryptophan (98%), histidine (98%), acrylamide (99%), NaOH, CH3COONa·3H2O (99.5%), Na2HPO4·H2O (99%), NaH2PO4·2H2O (99%), and KCl (99%) were purchased from Merck. Methylene blue monohydrate (96%) was purchased from Acros; HCl (33–37%) and (NH4)2SO4 (99%) were purchased from Fisher Scientific.
4.2. Procedure
All of the reagents were prepared using Millipore water with resistivity of 18.2 MΩ·cm–1. Solutions of pH 3–5 (0.1 M) were prepared by mixing CH3COOH (0.1 M) and CH3COONa (0.1 M) in appropriate ratios. Solutions of pH 6–8 were prepared by mixing appropriate amounts of Na2HPO4 (0.1 M) and NaH2PO4 (0.1 M). Solutions of pH 9 and 10 were prepared by adjusting the pH of Na2HPO4 (0.1 M) solution using NaOH (0.1 M). Stock solutions (100 mM) of MB, (NH4)2SO4, and AM were prepared and stored in the dark, when not in use. The concentrations of (NH4)2SO4 and MB used for electropolymerization were 45 mM and 0.1 mM, respectively.
4.3. Instruments
The absorption spectrum of MB at pH 5 was recorded using a Thermo Scientific Evolution 300 UV–vis spectrophotometer. The surface of PMB/GCE was analyzed using an X-ray photoelectron spectroscopy ESCALAB 250XI base system with an XR6 microfocused monochromator (Al Kα, hν = 1486.6 eV) as the probe. Raman spectroscopy (HORIBA LabRAM HR Evolution with LabSpec software) was used to analyze the surface of PMB/GCE. The surface morphology of PMB was examined using FESEM (SUPRA 55VP, Gemini Column). Electrochemical polymerization was carried out by cyclic voltammetry in the potential range of −0.4 to 1.2 with a scan rate of 50 mVs–1 for 50 cycles using a three electrode system: PMB/GCE acts as the working electrode and the saturated silver/silver chloride electrode and platinum mesh electrode act as the reference and counter electrodes, respectively. Cyclic voltammetry studies were carried out using CHI electrochemical analyzer (CHI 1000A). AFM was carried out using Agilent Technologies 5500 series scanning probe microscope (noncontact mode). Differential pulse voltammetry and chronocoulometry analyses were recorded in CHI electrochemical analyzer (CHI 9000B). Differential pulse voltammetry (DPV) was carried out in the potential range of −0.4 to 0.6 V with an amplitude of 0.05 V, an increment potential of 4 mV, a quiet time of 2 s, a pulse width of 0.05 s, a pulse period of 0.5 s, and a sampling width of 0.01 s. Chronocoulometry was carried using the potential range −0.3 to 0.3 V with the number of steps 2, pulse width 0.25 s, and sample interval 2 ms.
Acknowledgments
The authors wish to acknowledge the Director, CSIR-CECRI, for continuous support and encouragement. A.E. wishes to acknowledge the financial support from UGC-JRF award (Dec 2018, 109376). The authors acknowledge the staff members of CECRI-CIF for the characterization facility.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c06315.
Electrochemical polymerization of MB (0.1 mM) in (NH4)2SO4 (45 mM) with a scan rate of 50 mVs–1 for 1, 10, 20, 30 cycles (Figure S1); UV–vis spectrum of methylene blue (red line) with various concentrations of AM (100–600 nM) (Figure S2); AFM images of the bare glassy carbon electrode and PMB/GCE (Figure S3); effect of polymerization cycles on AM sensing using PMB/GCE (Figure S4); cyclic voltammograms of various concentrations of AM (50–11 665 nM) using PMB/GCE at a scan rate of 50 mVs–1 (Figure S5); cyclic voltammogram of PMB/GCE at pH 5 for consecutive 50 cycles at a scan rate of 50 mVs–1 (Figure S6); XPS C 1s, N 1s, and S (2p) spectra and FESEM image of AM-PMB/GCE (Figure S7); calibration plot of concentration vs ΔI obtained for real sample analysis using the standard addition experiment (Figure S8); GC–MS data for 10 ppm AM and potato chips; extract spiked with 10 ppm AM (Figure S9) (PDF)
The authors declare no competing financial interest.
Notes
CECRI/PESVC/PUBS./2020–58
Supplementary Material
References
- Zyzak D. V.; Sanders R. A.; Stojanovic M.; Tallmadge D. H.; Eberhart B. L.; Ewald D. K.; Gruber D. C.; Morsch T. R.; Strothers M. A.; Rizzi G. P.; Villagran M. D. Acrylamide Formation Mechanism in Heated Foods. J. Agric. Food Chem. 2003, 51, 4782–4787. 10.1021/jf034180i. [DOI] [PubMed] [Google Scholar]
- Zhang P.; Pan H.; Wang J.; Liu X.; Hu X. Telomerase Activity-Independent Function of Telomerase Reverse Transcriptase Is Involved in Acrylamide-Induced Neuron Damage. Biotech. Histochem. 2014, 89, 327–335. 10.3109/10520295.2013.855323. [DOI] [PubMed] [Google Scholar]
- Zamora R.; Delgado R. M.; Hidalgo F. J. Model Reactions of Acrylamide with Selected Amino Compounds. J. Agric. Food Chem. 2010, 58, 1708–1713. 10.1021/jf903378x. [DOI] [PubMed] [Google Scholar]
- Yaylayan V. A.; Wnorowski A.; Perez Locas C. Why Asparagine Needs Carbohydrates to Generate Acrylamide. J. Agric. Food Chem. 2003, 51, 1753–1757. 10.1021/jf0261506. [DOI] [PubMed] [Google Scholar]
- Roder D.; Fountoulakis M. Effect of protein application mode and acrylamide concentration on the resolution of protein spots separated by two-dimensional gel electrophoresis. Electrophoresis 1997, 2085–2090. 10.1002/elps.1150181135. [DOI] [PubMed] [Google Scholar]
- Yogeswaran U.; Chen S.-M. Multi-Walled Carbon Nanotubes with Poly(Methylene Blue) Composite Film for the Enhancement and Separation of Electroanalytical Responses of Catecholamine and Ascorbic Acid. Sens. Actuators, B 2008, 130, 739–749. 10.1016/j.snb.2007.10.040. [DOI] [Google Scholar]
- Varmira K.; Abdi O.; Gholivand M. B.; Goicoechea H. C.; Jalalvand A. R. Intellectual Modifying a Bare Glassy Carbon Electrode to Fabricate a Novel and Ultrasensitive Electrochemical Biosensor: Application to Determination of Acrylamide in Food Samples. Talanta 2018, 176, 509–517. 10.1016/j.talanta.2017.08.069. [DOI] [PubMed] [Google Scholar]
- Tezcan F.; Erim F. B. On-Line Stacking Techniques for the Nonaqueous Capillary Electrophoretic Determination of Acrylamide in Processed Food. Anal. Chim. Acta 2008, 617, 196–199. 10.1016/j.aca.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Tan L.; Zie Q.; Yao S. Electrochemical and Spectroelectrochemical Studies on Pyridoxine Hydrochloride Using a Poly(Methylene Blue) Modified Electrode. Electroanalysis 2004, 16, 1592–1597. 10.1002/elan.200302993. [DOI] [Google Scholar]
- Sun X.; Ji J.; Jiang D.; Li X.; Zhang Y.; Li Z.; Wu Y. Development of a Novel Electrochemical Sensor Using Pheochromocytoma Cells and Its Assessment of Acrylamide Cytotoxicity. Biosens. Bioelectron. 2013, 44, 122–126. 10.1016/j.bios.2013.01.031. [DOI] [PubMed] [Google Scholar]
- Stobiecka A.; Radecka H.; Radecki J. Novel Voltammetric Biosensor for Determining Acrylamide in Food Samples. Biosens. Bioelectron. 2007, 22, 2165–2170. 10.1016/j.bios.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Silva N.; Gil D.; Karmali A.; Matos M. Biosensor for Acrylamide Based on an Ion-Selective Electrode Using Whole Cells of Pseudomonas Aeruginosa Containing Amidase Activity. Biocatal. Biotransformation 2009, 27, 143–151. 10.1080/10242420802604964. [DOI] [Google Scholar]
- Quan Y.; Chen M.; Zhan Y.; Zhang G. Development of an Enhanced Chemiluminescence ELISA for the Rapid Detection of Acrylamide in Food Products. J. Agric. Food Chem. 2011, 59, 6895–6899. 10.1021/jf200954w. [DOI] [PubMed] [Google Scholar]
- Pan M.; Liu K.; Yang J.; Hong L.; Xie X.; Wang S. Review of Research into the Determination of Acrylamide in Foods. Foods 2020, 9, 524 10.3390/foods9040524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paleologos E. K.; Kontominas M. G. Determination of Acrylamide and Methacrylamide by Normal Phase High Performance Liquid Chromatography and UV Detection. J. Chromatogr. A 2005, 1077, 128–135. 10.1016/j.chroma.2005.04.037. [DOI] [PubMed] [Google Scholar]
- Nemoto S.; Takatsuki S.; Sasaki K.; Maitani T. Determination of Acrylamide in Foods by GC/MS Using 13C-Labeled Acrylamide as an Internal Standard. J. Food Hyg. Soc. Japan 2002, 43, 371–376. 10.3358/shokueishi.43.371. [DOI] [PubMed] [Google Scholar]
- Li N.; Liu X.; Zhu J.; Zhou B.; Jing J.; Wang A.; Xu R.; Wen Z.; Shi X.; Guo S. Simple and Sensitive Detection of Acrylamide Based on Hemoglobin Immobilization in Carbon Ionic Liquid Paste Electrode. Food Control 2020, 109, 106764 10.1016/j.foodcont.2019.106764. [DOI] [Google Scholar]
- Liu X.; Mao L. G.; Wang Y. L.; Shi X. B.; Liu Y.; Yang Y.; He Z. Electrochemical Sensor Based on Imprinted Sol-Gel Polymer on Au NPs-MWCNTs-CS Modified Electrode for the Determination of Acrylamide. Food Anal. Methods 2016, 9, 114–121. 10.1007/s12161-015-0172-0. [DOI] [Google Scholar]
- Manière I.; Godard T.; Doerge D. R.; Churchwell M. I.; Guffroy M.; Laurentie M.; Poul J. M. DNA Damage and DNA Adduct Formation in Rat Tissues Following Oral Administration of Acrylamide. Mutat. Res., Genet. Toxicol. Environ. Mutagen. 2005, 580, 119–129. 10.1016/j.mrgentox.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Krajewska A.; Radecki J.; Radecka H. A Voltammetric Biosensor Based on Glassy Carbon Electrodes Modified with Single-Walled Carbon Nanotubes/Hemoglobin for Detection of Acrylamide in Water Extracts from Potato Crisps. Sensors 2008, 8, 5832–5844. 10.3390/s8095832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garabagiu S.; Mihailescu G. Simple Hemoglobin-Gold Nanoparticles Modified Electrode for the Amperometric Detection of Acrylamide. J. Electroanal. Chem. 2011, 659, 196–200. 10.1016/j.jelechem.2011.06.003. [DOI] [Google Scholar]
- Batra B.; Lata S.; Sharma M.; Pundir C. S. An Acrylamide Biosensor Based on Immobilization of Hemoglobin onto Multiwalled Carbon Nanotube/Copper Nanoparticles/Polyaniline Hybrid Film. Anal. Biochem. 2013, 433, 210–217. 10.1016/j.ab.2012.10.026. [DOI] [PubMed] [Google Scholar]
- Batra B.; Lata S.; Pundir C. S. Construction of an Improved Amperometric Acrylamide Biosensor Based on Hemoglobin Immobilized onto Carboxylated Multi-Walled Carbon Nanotubes/Iron Oxide Nanoparticles/Chitosan Composite Film. Bioprocess Biosyst. Eng. 2013, 36, 1591–1599. 10.1007/s00449-013-0931-5. [DOI] [PubMed] [Google Scholar]
- Li D.; Xu Y.; Zhang L.; Tong H. A Label-Free Electrochemical Biosensor for Acrylamide Based on DNA Immobilized on Graphene Oxide-Modified Glassy Carbon Electrode. Int. J. Electrochem. Sci. 2014, 9, 7217–7227. [Google Scholar]
- Huang S.; Lu S.; Huang C.; Sheng J.; Zhang L.; Su W.; Xiao Q. An Electrochemical Biosensor Based on Single-Stranded DNA Modified Gold Electrode for Acrylamide Determination. Sens. Actuators, B 2016, 224, 22–30. 10.1016/j.snb.2015.10.008. [DOI] [Google Scholar]
- Lin K. C.; Yin C. Y.; Chen S. M. An Electrochemical Biosensor for Determination of Hydrogen Peroxide Using Nanocomposite of Poly(Methylene Blue) and FAD Hybrid Film. Sens. Actuators, B 2011, 157, 202–210. 10.1016/j.snb.2011.03.050. [DOI] [Google Scholar]
- Giribabu K.; Suresh R.; Manigandan R.; Munusamy S.; Kumar S. P.; Muthamizh S.; Narayanan V. Nanomolar Determination of 4-Nitrophenol Based on a Poly(Methylene Blue)-Modified Glassy Carbon Electrode. Analyst 2013, 138, 5811–5818. 10.1039/c3an00941f. [DOI] [PubMed] [Google Scholar]
- Gorle D. B.; Kulandainathan M. A. Electrochemical Sensing of Dopamine at the Surface of a Dopamine Grafted Graphene Oxide/Poly(Methylene Blue) Composite Modified Electrode. RSC Adv. 2016, 6, 19982–19991. 10.1039/c5ra25541d. [DOI] [Google Scholar]
- Brett C. M. A.; Inzelt G.; Kertesz V. Poly(Methylene Blue) Modified Electrode Sensor for Haemoglobin. Anal. Chim. Acta 1999, 385, 119–123. 10.1016/S0003-2670(98)00808-3. [DOI] [Google Scholar]
- Marinho M. I. C.; Cabral M. F.; Mazo L. H. Is the Poly (Methylene Blue)-Modified Glassy Carbon Electrode an Adequate Electrode for the Simple Detection of Thiols and Amino Acid-Based Molecules?. J. Electroanal. Chem. 2012, 685, 8–14. 10.1016/j.jelechem.2012.08.023. [DOI] [Google Scholar]
- Topçu E.; Dağcı K.; Alanyalıoğlu M. Free-Standing Graphene/Poly(Methylene Blue)/AgNPs Composite Paper for Electrochemical Sensing of NADH. Electroanalysis 2016, 28, 2058–2069. 10.1002/elan.201600108. [DOI] [Google Scholar]
- Manasa G.; Mascarenhas R. J.; Satpati A. K.; D’Souza O. J.; Dhason A. Facile Preparation of Poly(Methylene Blue) Modified Carbon Paste Electrode for the Detection and Quantification of Catechin. Mater. Sci. Eng. C 2017, 73, 552–561. 10.1016/j.msec.2016.12.114. [DOI] [PubMed] [Google Scholar]
- Qu W.; Wu K.; Hu S. Voltammetric Determination of Pyridoxine (Vitamin B 6) by Use of a Chemically-Modified Glassy Carbon Electrode. J. Pharm. Biomed. Anal. 2004, 36, 631–635. 10.1016/j.jpba.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Barsan M. M.; Pinto E. M.; Brett C. M. A. Methylene Blue and Neutral Red Electropolymerisation on AuQCM and on Modified AuQCM Electrodes: An Electrochemical and Gravimetric Study. Phys. Chem. Chem. Phys. 2011, 13, 5462–5471. 10.1039/C1CP20418A. [DOI] [PubMed] [Google Scholar]
- Barsan M. M.; Pinto E. M.; Brett C. M. A. Electrosynthesis and Electrochemical Characterisation of Phenazine Polymers for Application in Biosensors. Electrochim. Acta 2008, 53, 3973–3982. 10.1016/j.electacta.2007.10.012. [DOI] [Google Scholar]
- Barsan M. M.; Pinto E. M.; Brett C. M. A. Methylene Blue and Neutral Red Electropolymerisation on AuQCM and on Modified AuQCM Electrodes: An Electrochemical and Gravimetric Study. Phys. Chem. Chem. Phys. 2011, 13, 5462–5471. 10.1039/C1CP20418A. [DOI] [PubMed] [Google Scholar]
- Atay N. Z.; Çalgan D.; Özakat E.; Varnali T. Acrylamide and Glycidamide Adducts of Guanine. J. Mol. Struct.: THEOCHEM 2005, 728, 249–251. 10.1016/j.theochem.2005.06.039. [DOI] [Google Scholar]
- González-Fuentes F. J.; Manríquez J.; Godínez L. A.; Escarpa A.; Mendoza S. Electrochemical Analysis of Acrylamide Using Screen-Printed Carboxylated Single-Walled Carbon Nanotube Electrodes. Electroanalysis 2014, 26, 1039–1044. 10.1002/elan.201300636. [DOI] [Google Scholar]
- Ciesarová Z.; Balasová V.; Kiss E.; Kolek E.; Šimko P.; Kováč M. Comparison of Two Methods for Acrylamide Determination and Dietary Intake of Acrylamide from Potato Crisps in Slovakia. Czech J. Food Sci. 2018, 22, S251–S254. 10.17221/10674-cjfs. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.










