Abstract
Background:
Scales to assess disability in multiple sclerosis (MS) rarely provide reliable data on actual global impairment. Upper limb dysfunction is usually overlooked, which has a negative effect on patient well-being. We sought to analyze associations among upper limb dexterity, lower limb speed, and Expanded Disability Status Scale (EDSS) score; the difference in upper limb dexterity between patients with EDSS scores less than 5 and 5 or greater; and the associations that upper limb dexterity, lower limb speed, and EDSS score have with health-related quality of life measurements and depression.
Methods:
A total of 140 adults with MS were evaluated using the Nine-Hole Peg Test, Timed 25-Foot Walk test, EDSS, Multiple Sclerosis International Quality of Life (MusiQoL) questionnaire, and Beck Depression Inventory. Thorough descriptive-analytical research was conducted using the Spearman correlation, multiple linear regression, and structural equation modeling.
Results:
Upper limb dexterity was more closely related to EDSS score than lower limb speed (r = 0.43 vs 0.29, R2 = 0.38) and was greatest in patients with EDSS scores less than 5 (P < .01). Moreover, upper limb dexterity was negatively associated with EDSS score and the MusiQoL questionnaire (rS = −0.557 to −0.321, P < .05). The correlation that depression has with upper limb dexterity loss was higher than the one it has with lower limb speed (0.098 vs 0.066, t > 1.96).
Conclusions:
Upper limb dexterity is associated with global disability, depression, and health-related quality of life. We advocate for the assessment of upper limb dexterity in patients with MS to adopt a better approach to their functional impairment.
Keywords: Disability, Multiple sclerosis (MS), Quality of life (QOL), Upper limb
Multiple sclerosis (MS) is a chronic and disabling disease that jeopardizes patient quality of life (QOL) by affecting their social, economic, and professional networks.1 This neurodegenerative and inflammatory disease of the central nervous system leads to the development of persistent neurologic deficit over time, with potential impact over many years of life.2
Motor impairment is the most striking sign of MS. Although lower limbs are the most frequently impaired, upper limbs are also usually affected by weakness or ataxia.3,4 Johansson et al5 reported that up to 76% of patients being studied for any type of incapacity had upper limb impairment, and in at least 50% of patients the severity of the dysfunction was moderate.
Several disability scales have been developed to measure the functional impact of different impairments. The Expanded Disability Status Scale (EDSS) has been widely accepted in the clinical field despite its understandable flaws,6 such as scores being highly oriented toward ambulation capacity and thus upper limb and other functions being underestimated.7 Previous reports have highlighted the importance of assessing upper limb dexterity to determine the therapeutic effects of different treatments in clinical trials.8 In this regard, Feys et al9 demonstrated that the Nine-Hole Peg Test (NHPT) detects progression over time and is sensitive to treatment. Solaro et al10 conducted a study to asses upper limb dysfunction using the NHPT and suggested that said test should be used with caution in MS settings, especially in patients with low or high disability levels.10
Furthermore, several authors11–13 have pointed out that upper limb dexterity is strongly associated with activities of daily living (ADLs) such as dressing, self-care, and bathing, thus affecting the patient’s independence14 and QOL.15 In this sense, Lamers et al12 found that general upper limb strength was the most important variable related to the capacity to perform ADLs. Besides general upper limb strength, other parameters, such as the active range of motion of wrist extension and tactile sensitivity of the thumb, were also associated with the capacity to perform ADLs. In addition, Cattaneo et al16 observed that hand dexterity was predominantly associated with participation in home activities, and participation restrictions were found to be more correlated with cognitive deficits.
Based on all this information we have mentioned, the present study aimed to address the usefulness of evaluating upper limb dexterity during neurologic routine consultations of patients with MS. The objectives were to 1) analyze the associations among upper limb dexterity, lower limb speed (gait speed), and EDSS score; 2) analyze the difference in upper limb dexterity between patients with EDSS scores less than 5 and 5 or greater; and 3) study the associations that upper limb dexterity, lower limb speed, and EDSS score have with both health-related QOL (HRQOL) measurements and depression, a variable strongly related to disability.5
Methods
To evaluate the usefulness of routine measuring of upper limb dexterity in medical practice, we designed a retrospective study performed in our MS clinic. This study was approved by the ethics committee of J.M. Ramos Mejía Hospital, and all participants signed an informed consent form authorizing us to use the information in their medical records. Patients aged 18 to 60 years who had a diagnosis of MS according to the McDonald criteria17 and whose upper limb dexterity had been evaluated were included. The exclusion criteria were a concomitant neurologic disorder other than MS, a history of alcoholism or drug abuse, a diagnosis of a psychiatric disorder, being in clinical relapse, or having received a corticosteroid treatment within 4 weeks before the study. All the measurements described in the following subsection were performed by an MS expert neurologist during a single visit.
Measurements
Lower Limb Assessment
The Timed 25-Foot Walk test (T25FW)18 is a walking speed measure. It is performed by registering the time it takes a patient to walk across a clearly marked 25-foot course as fast and safely as possible. If needed, assistive walking devices, such as canes or walkers, can be used during the test. The task is performed twice, and the average score is recorded. The raw score will be later analyzed in the present research.
Upper Limb Assessment
The NHPT19 measures dexterity in both hands by asking the patient to pick up nine pegs from a container and insert them into a nine-hole board. Participants must then remove the pegs as quickly as possible, placing them back into the container. The task is performed with the dominant arm first and then with the contralateral hand. This test comprises two trials for each hand, and scores are the average time of the four trials. The raw score will be analyzed later in the present research.
Global Disability Assessment
The EDSS20 addresses eight functional systems: pyramidal, cerebellar, brainstem, sensory, bowel and bladder, visual, mental, and miscellaneous. It ranges from best to worst performance, from 0 to 10 points, in half-point increments.
QOL Assessment
The Multiple Sclerosis International Quality of Life (MusiQoL) questionnaire21 is a multidimensional self-administered questionnaire validated in 14 languages. It is shorter and faster that other similar tools. Each item has a Likert-type scale ranging from 1 (never/nothing) to 5 (always/very much), with an option left for not applicable questions. The MusiQoL questionnaire comprises 31 questions in nine dimensions (subscales): ADLs (eight items), psychological well-being (four items), symptoms (four items), relationships with friends (three items), relationships with family (three items), sentimental and sexual life (two items), coping (two items), rejection (two items), and relationships with health care system (three items). The index score is calculated based on the mean subscale scores. All nine dimensions and the index score are linearly transformed and standardized on a scale from 0 to 100, where 0 indicates the worst possible level of QOL and 100 indicates the best level.
Depression Assessment
The Beck Depression Inventory II22 is also a self-administered questionnaire, with 21 items addressing the patient’s feelings and perceptions during the previous 2 weeks. Each item has a 4-point Likert-type scale. It is a well-known tool for screening and rating depression, with results ranging from better to worse, from 0 to 63 points. Based on the scores obtained in the test, the Beck Depression Inventory II provides the following classification to indicate a patient’s level of depression: without depression (score, 0–13), mild depression (score, 14–19), moderate (score, 20–28), and severe (score, 29–63), according to local regulations.
Statistical Analysis
To analyze the relationship that upper limb dexterity and lower limb speed have with EDSS score, we used a structural equation model with observed variables. To perform structural equation modeling in variables that do not meet the normality assumption, it is necessary to create an asymptotic covariance matrix. Therefore, we used the formula (k + 1) (k + 2)/2, with k representing the number of variables.23 We later evaluated the sample size by comparing it with the empirically used samples.24 We used the MLR robust estimator (software: OpenMx packages) because the used variables were continuous without multivariate normality.
Considering that the EDSS is strongly biased by ambulatory capacity, we performed a nonparametric comparison of upper limb function between patients with EDSS scores of 5 or greater and those with EDSS scores less than 5 using the Mann-Whitney U test. Considering a score of 5 as the cutoff point on the EDSS was decided by the clinical team. The decision was based on EDSS scores of 1.0 to 4.5 referring to patients with MS who are able to walk without any aid. Patients with EDSS scores of 5 or greater present impairments that vary from a marked alteration of gait resistance to needing help to walk or inability to walk.7
A structural equation model with observed variables was also used to analyze the relation that upper limb dexterity, lower limb speed, and EDSS score have with depression. Spearman correlations were used to assess the association of these variables with HRQOL measurements. Said evaluation comprised a sample of 52 patients, and the normality assumptions of these subpopulations were not met by all the variables. Finally, linear multiple regression was used for the analysis of the possible role of upper limb dexterity, lower limb speed, and the EDSS score as predictors of individual HRQOL measurements. Statistical analysis was performed using SPSS Statistics for Windows, version 20 (IBM Corp) and structural equation modeling with LISREL software, version 9.3 (Scientific Software International).
Results
A total of 140 patients were included. Their general characteristics are listed in Table 1.
Table 1.
Demographic and clinical characteristics of the 140 study patients
| Characteristic | Results |
|---|---|
| Age, y | 39.79 ± 12.74 |
| Schooling duration, y | 13.43 ± 3.44 |
| Female sex | 90 (64.3) |
| Disease duration, y | 10.16 ± 9.93 |
| EDSS score | 2.87 ± 1.78 [0–7] |
| NHPT time, s | 26.03 ± 9.01 |
| T25FW time, s | 7.23 ± 3.44 |
| BDI II score | 14.58 ± 11.15 |
| Depression severity (BDI II) | |
| Mild | 14.3% |
| Moderate | 15.1% |
| Severe | 13.5% |
| Clinical variant | |
| Relapsing-remitting | 131 |
| Secondary progressive | 4 |
| Primary progressive | 5 |
Note: Unless otherwise indicated, values are given as mean ± SD, mean ± SD [range], number (percentage), or number.
Abbreviations: BDI II, Beck Depression Inventory II; EDSS, Expanded Disability Status Scale; NHPT, Nine-Hole Peg Test; T25FW, Timed 25-Foot Walk test.
The global indicators of the structural equation model regarding the relationship that upper limb dexterity and lower limb speed have with EDSS score were adequate (Table 2). Standardized coefficients showed a closer association between upper limb dexterity and EDSS score compared with the association with lower limb speed (0.43 vs 0.29, P < .01). The R2 was 0.38, which is high considering that the EDSS measures multiple functional systems. All t values of the model were statistically significant, except for upper limb dexterity and lower limb speed correlations that, despite being high, did not reach significance (1.79 vs 1.96, P < .01) (Figure 1).
Table 2.
Structural equation modeling’s global indicators (N = 140)
| χ2/df (<3)a | χ2P value (>.05)a | NFI (>0.9)a | GFI (>0.9)a | RMSEA (<0.08)a | SRMR (<0.08)a |
|---|---|---|---|---|---|
| 2.38 | .09 | 0.955 | 0.998 | 0.028 | 0.036 |
Abbreviations: NFI, normed fit index; GFI, goodness of fit index, RMSEA, root mean square error of approximation; SRMR, standardized root mean square residual.
Brown.24
Figure 1.
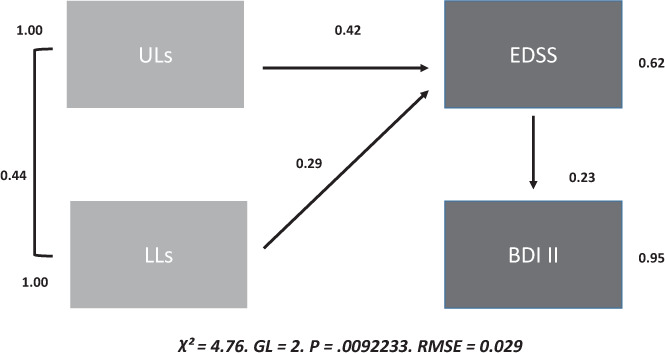
Structural equation modeling for upper limb (UL) dexterity, lower limb (LL) speed, global disability, and depression (N = 140)
BDI II, Beck Depression Inventory II; EDSS, Expanded Disability Status Scale; GL, generalized least squares; RMSE, root mean square error = 0.029.
When considering upper limb dexterity in patients with EDSS scores less than 5 (mean ± SD NHPT time, 24.21 ± 6.20 seconds) and 5 or greater (36.17 ± 14.36 seconds), patients in the latter group had a significantly (U = −0.34, P < .01) slower performance than those with EDSS scores less than 5, even considering the EDSS’s underestimation of upper limb dexterity in patients with scores of 5 or greater (given that it is not measured).
The subgroup of patients in which HRQOL was analyzed included 52 participants. Sixty-five percent were women with a mean ± SD age of 40.62 ± 14.71 years, schooling of 13.75 ± 3.33 years, disease evolution length of 12.37 ± 11.87, and EDSS score of 2.48 ± 1.65. No significant differences were found between the sample of 140 patients and the subsample of 52 patients (P > .05). The correlation analysis between upper limb dexterity, lower limb speed, and EDSS score and individual HRQOL measurements showed statistically significant negative correlations between all three variables and ADLs (rS = −0.557, −0.377, and −0.506, respectively, P < .01). Psychological well-being was negatively associated with upper limb dexterity (rS = −0.355, P < .05) and EDSS score (rS = −0.358, P < .05). The symptoms dimension was also negatively associated with upper limb dexterity (rS = −0.360, P < .01) and EDSS score (rS = −0.315, P < .05). The global index of QOL was associated only with upper limb dexterity (rS = −0.321, P < .05), and rejection was correlated only with lower limb speed (rS = −0.279, P < .05). See Table S1, which is published in the online version of this article at ijmsc.org.
Multiple linear regression using each of the dimensions of the MusiQoL questionnaire as dependent variables and upper limb dexterity, lower limb speed, and EDSS score as independent variables was significant only in terms of upper limb function with ADLs (R2 = 0.377, P = .042, typified β = −0.352). Upper limb dexterity accounted for 38% of the ADL variance, thus implying a moderate effect of upper limb dexterity in this dimension. Lower limb speed and EDSS score were not associated with any of the tested MusiQoL questionnaire dimensions.
Regarding upper limb dexterity, lower limb speed, EDSS score, and depression, the structural equation model found a statistically significant association (t value > 1.96, punctually = 2.52) between EDSS score and depression, with a standardized coefficient of 0.23. The indirect association (through EDSS score) with depression was significantly (t value > 1.96) higher for upper limb dexterity (0.43 × 0.23 = 0.098) than for lower limb speed (0.29 × 0.23 = 0.066). These results are shown in Figure 1.
Discussion
The findings from this study show the impact that upper limb dexterity has in patients with MS, as well as its association with disability measured by the EDSS. Although it is intuitive to think that upper limb functional indemnity is essential for ADLs, upper limb dexterity is not usually measured in routine neurologic consultations, at least in Argentina. The main objective was to thoroughly evaluate upper limb dexterity in patients with MS and, eventually, relate it to the patient’s QOL. Bearing this in mind, we decided to administer the NHPT, the most commonly used test in clinical trials for upper limb assessment and the only one with psychometric properties that have been duly studied.25 Remarkably, in our research, the strongest association among those variables was the one found between upper limb dexterity and EDSS score. Moreover, the results of the present study showed a positive association between upper limb dexterity and having an EDSS score of 5 or greater, even when upper limb dexterity does not influence the EDSS score beyond that point. Similar findings were reported by Cadavid et al,26 who described an association between upper limb dexterity (measured by NHPT) and having an EDSS score higher than 6.
Upper limb dexterity was strongly associated with worse HRQOL measurements in general and with psychological well-being and ADLs in particular. These findings are in line with those of other published studies.13–16 Bertoni et al11 concluded that loss of manual dexterity has been shown to be associated with decreased independence in ADLs and is probably due to a combination of tremor, sensory, and strength impairments. Cattaneo et al16 observed that hand dexterity was predominantly associated with participation in ADLs and that manual dexterity impairment was associated with restrictions on home activities. These authors emphasized the role of the upper limbs in activities such as dressing, bathing, or cooking. We also found associations between lower limb speed and HRQOL measurements, but only in ADLs and rejection. In the same way, Kohn et al27 observed decrements in HRQOL as patients perceived greater levels of reduction in their walking speed; however, other authors have not found such a relationship.28
Multivariate analysis in this sample allowed us to discover that the relationship that HRQOL measurements have with upper limb dexterity is stronger than the one they have with lower limb speed or EDSS score. Furthermore, through a linear regression analysis, upper limb function was found to be the only measure that predicted QOL, producing a moderate effect on ADLs. On the other hand, upper limb impairment was also associated with depression in a stronger way than lower limb speed. To our knowledge, this is the first time such a relationship has been reported.
The use of scales to assess disability rarely provides reliable data on the actual global impairment of the patient because each scale usually focuses on a certain aspect, dismissing others. Several authors have pointed out that the EDSS has several biases, which are particularly noticeable in progressive forms of MS.29 Therefore, having only this scale as a reference, it is possible to overlook a patient’s disability progression if it does not reach the point of walking capacity. In this sense, several studies have demonstrated that the Multiple Sclerosis Functional Composite scale (which includes the T25FW and the NHPT) has greater sensibility than the EDSS to detect progression of disability in patients with secondarily progressive MS.30 Similarly, Cadavid et al26 demonstrated that the NHPT was more sensitive than the EDSS (34.4% vs 24.7%) for identifying disability progression in a 2-year observation period in patients with MS of the secondarily progressive form. Remarkably, despite the lack of disability progression in progressive forms of MS when measured using the EDSS, some clinical trials have showed therapeutic benefits from different treatments when performing a subanalysis focused in upper limb dexterity (metrotexate31 interferon beta-1a32, rituximab,33 and natalizumab34).
In the present sample we found strong associations between upper limb dexterity and several clinical outcomes. Because this is seemingly the first time that this type of analysis has been performed in Argentina, we have no other local results to compare with the present findings. Nevertheless, and despite the sample size, we believe that the functional importance of upper limb dexterity should be acknowledged to gain a better understanding of the global disability of every patient.
All things considered, we believe that the assessment of a patient’s global disability cannot be delegated solely to the EDSS and that other scales should be incorporated in the routine neurologic controls. To our knowledge, the NHPT is the simplest, shortest, and better-validated test, as has also been widely reported in previous studies.35
It is important to consider that to generalize the results of this study, future research is needed to strengthen the data presented. This work presents some limitations, such as the sample size of the patients who had QOL evaluated and the lack of evaluation of cognitive aspects that contribute to MS-related disability. Likewise, it would be interesting to compare the performance of patients in different clinical forms, for which it would be necessary to expand the sample.
PRACTICE POINTS
Upper limb dexterity is associated with daily life activities and affects a patient’s quality of life.
Upper limb dexterity could be evaluated using the Nine-Hole Peg Test and should be considered for global disability assessment.
Supplementary Material
Footnotes
Financial Disclosures: Dr Alonso has received reimbursement for developing educational presentations, educational grants, and travel stipends from Biogen, Genzyme, Merck, Novartis, and Roche. Ms Eizaguirre has received reimbursement for educational grants from Novartis and Roche. Dr Cohen has received reimbursement for developing educational presentations, educational grants, and travel stipends from Genzyme, Novartis, and Roche. Dr Silva has received reimbursement for developing educational presentations, educational grants, consultation fees, and travel stipends from Biogen, Genzyme, Novartis, and Merck. Dr Pita has received reimbursement for developing educational presentations, educational grants, consultation fees, and travel stipends from Genzyme, Roche, and Biogen. Dr Vanotti has received reimbursement for developing educational presentations from Biogen Argentina and Novartis Argentina. Dr Garcea has received reimbursement for developing educational presentations, educational and research grants, consultations fees, and travel stipends from Biogen, Genzyme, and Merck. The other authors declare no conflicts of interest.
Funding/Support: None.
References
- 1.De Judicibus MA, McCabe MP. The impact of the financial costs of multiple sclerosis on quality of life. Int J Behav Med. 2007;14:3–11. doi: 10.1007/BF02999222. [DOI] [PubMed] [Google Scholar]
- 2.Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378:169–180. doi: 10.1056/NEJMra1401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonald I, Compston A. The symptoms and signs of multiple sclerosis. In: Compston A, Confavreaux C, Lassmann H, et al., editors. McAlpine’s Multiple Sclerosis. 4th ed. Elsevier; 2006. pp. 287–346. [Google Scholar]
- 4.Feys P, Duportail M, Kos D, Van Asch P, Ketelaer P. Validity of the TEMPA for the measurement of upper limb function in multiple sclerosis. Clin Rehabil. 2002;16:166–173. doi: 10.1191/0269215502cr471oa. [DOI] [PubMed] [Google Scholar]
- 5.Johansson S, Ytterberg C, Claesson IM et al. High concurrent presence of disability in multiple sclerosis: associations with perceived health. J Neurol. 2007;254:767–773. doi: 10.1007/s00415-006-0431-5. [DOI] [PubMed] [Google Scholar]
- 6.Izquierdo G, Ruiz-Peña JL. Clinical evaluation of multiple sclerosis: quantification by use of scales [in Spanish] Rev Neurol. 2003;36:145–152. [PubMed] [Google Scholar]
- 7.Sharrack B, Hughes RAC. Clinical scales for multiple sclerosis. J Neurol Sci. 1996;135:1–9. doi: 10.1016/0022-510x(95)00261-y. [DOI] [PubMed] [Google Scholar]
- 8.Giovannoni G, Cutter G, Sormani MP et al. Is multiple sclerosis a length-dependent central axonopathy? the case for therapeutic lag and the asynchronous progressive MS hypotheses. Mult Scler Relat Disord. 2017;12:70–78. doi: 10.1016/j.msard.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Feys P, Lamers I, Francis G et al. The Nine-Hole Peg Test as a manual dexterity performance measure for multiple sclerosis. Mult Scler. 2017;23:711–720. doi: 10.1177/1352458517690824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solaro C, Cattaneo D, Brichetto G et al. Clinical correlates of 9-hole peg test in a large population of people with multiple sclerosis. Mult Scler Relat Disord. 2019;30:1–8. doi: 10.1016/j.msard.2019.01.043. [DOI] [PubMed] [Google Scholar]
- 11.Bertoni R, Lamers I, Chen CC, Peter Feys P, Cattaneo D. Unilateral and bilateral upper limb dysfunction at body functions, activity and participation levels in people with multiple sclerosis. Mult Scler. 2015;21:1566–1574. doi: 10.1177/1352458514567553. [DOI] [PubMed] [Google Scholar]
- 12.Lamers I, Cattaneo D, Chen CC, Bertoni R, Van Wijmeersch B, Feys P. Associations of upper limb disability measures on different levels of the International Classification of Functioning, Disability and Health in people with multiple sclerosis. Phys Ther. 2015;95:65–75. doi: 10.2522/ptj.20130588. [DOI] [PubMed] [Google Scholar]
- 13.Kierkegaard M, Einarsson U, Gottberg K, von Koch L, Widén Holmqvist L. The relationship between walking, manual dexterity, cognition and activity/participation in persons with multiple sclerosis. Mult Scler. 2012;18:639–646. doi: 10.1177/1352458511426736. [DOI] [PubMed] [Google Scholar]
- 14.O’Hara L, Cadbury H, De SL, Ide L. Evaluation of the effectiveness of professionally guided self-care for people with multiple sclerosis living in the community: a randomized controlled trial. Clin Rehabil. 2002;16:119–128. doi: 10.1191/0269215502cr478oa. [DOI] [PubMed] [Google Scholar]
- 15.Yozbatiran N, Baskurt F, Baskurt Z, Ozakbas S, Idiman E. Motor assessment of upper extremity function and its relation with fatigue, cognitive function and quality of life in multiple sclerosis patients. J Neurol Sci. 2006;246:117–122. doi: 10.1016/j.jns.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Cattaneo D, Lamers I, Bertoni R, Feys P, Jonsdottir J. Participation restriction in people with multiple sclerosis: prevalence and correlations with cognitive, walking, balance, and upper limb impairments. Arch Phys Med Rehabil. 2017;98:1308–1315. doi: 10.1016/j.apmr.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 17.Polman C, Reingold S, Banwell B et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudick RA, Cutter G, Reingold S. The Multiple Sclerosis Functional Composite: a new clinical outcome measure for multiple sclerosis clinical trials. Mult Scler. 2002;8:359–365. doi: 10.1191/1352458502ms845oa. [DOI] [PubMed] [Google Scholar]
- 19.Mathiowetz V, Weber K, Kashman N, Volland G. Adult norms for the Nine Hole Peg Test of Finger Dexterity. Occup Ther J Res. 1995;5:24–38. [Google Scholar]
- 20.Kurtzke JF. Rating neurological impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 21.Simeoni M, Auquier P, Fernández O, et al. ; MusiQol Study Group Validation of the Multiple Sclerosis International Quality of Life questionnaire. Mult Scler. 2008;14:219–230. doi: 10.1177/1352458507080733. [DOI] [PubMed] [Google Scholar]
- 22.Brenlla ME, Rodríguez CM. Beck Depression Inventory-Spanish version for Argentina. In: Beck AT, Steer RA, Brown GK, editors. Beck Depression Inventory. 2nd ed. Paidós;; 2006. [Google Scholar]
- 23.Jöreskog K, Sörbom D. PRELIS 2 User’s Reference Guide. Scientific Software International; 1996. [Google Scholar]
- 24.Brown TA. Confirmatory Factor Analysis for Applied Research. Guilford Publications; 2014. [Google Scholar]
- 25.Naci H, Fleurence R, Birt J, Duhig A. The impact of increasing neurological disability of multiple sclerosis on health utilities: a systematic review of the literature. J Med Econ. 2010;13:78–89. doi: 10.3111/13696990903543085. [DOI] [PubMed] [Google Scholar]
- 26.Cadavid D, Cohen JA, Freedman MS et al. The EDSS-Plus, an improved endpoint for disability progression in secondary progressive multiple sclerosis. Mult Scler. 2017;23:94–105. doi: 10.1177/1352458516638941. [DOI] [PubMed] [Google Scholar]
- 27.Kohn CG, Baker WL, Sidovar MF, Coleman C. Walking speed and health-related quality of life in multiple sclerosis. Patient. 2014;7:55–61. doi: 10.1007/s40271-013-0028-x. [DOI] [PubMed] [Google Scholar]
- 28.Bethoux FA, Palfy DM, Plow MA. Correlates of the Timed 25 Foot Walk in a multiple sclerosis outpatient rehabilitation clinic. Int J Rehabil Res. 2016;39:134–139. doi: 10.1097/MRR.0000000000000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hobart J, Freeman J, Thompson A. Kurtzke scales revisited: the application of psychometric methods to clinical intuition. Brain. 2000;123:1027–1040. doi: 10.1093/brain/123.5.1027. [DOI] [PubMed] [Google Scholar]
- 30.Orbach R, Zhao Z, Wang YC, O’Neill G, Cadavid D. Comparison of disease activity in SPMS and PPMS in the context of multicenter clinical trials. PLoS One. 2012;7:e45409. doi: 10.1371/journal.pone.0045409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodkin DE, Rudick RA, Vander Brug Medendorp S et al. Low-dose (7.5 mg) oral methotrexate reduces the rate of progression in chronic progressive multiple sclerosis. Ann Neurol. 1995;37:30–40. doi: 10.1002/ana.410370108. [DOI] [PubMed] [Google Scholar]
- 32.Cohen JA, Cutter GR, Fischer JS, et al. ; IMPACT Investigators Benefit of interferon beta-1a on MSFC progression in secondary progressive MS. Neurology. 2002;59:679–687. doi: 10.1212/wnl.59.5.679. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, Waubant E, Cutter G, Wolinsky J, Leppert D. Composite end points to assess delay of disability progression by MS treatments. Mult Scler. 2014;20:1494–1501. doi: 10.1177/1352458514527180. [DOI] [PubMed] [Google Scholar]
- 34.Kapoor R, Ho PR, Campbell N, et al. ; ASCEND Investigators Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): a phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol. 2018;17:405–415. doi: 10.1016/S1474-4422(18)30069-3. [DOI] [PubMed] [Google Scholar]
- 35.Kraft GH, Amtmann D, Bennett SE et al. Assessment of upper extremity function in multiple sclerosis: review and opinion. Postgrad Med. 2014;126:102–108. doi: 10.3810/pgm.2014.09.2803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


