Abstract
A practical access to four new halogen-substituted pyrrole building blocks was realized in two to five synthetic steps from commercially available starting materials. The target compounds were prepared on a 50 mg to 1 g scale, and their conversion to nanomolar inhibitors of bacterial DNA gyrase B was demonstrated for three of the prepared building blocks to showcase the usefulness of such chemical motifs in medicinal chemistry.
1. Introduction
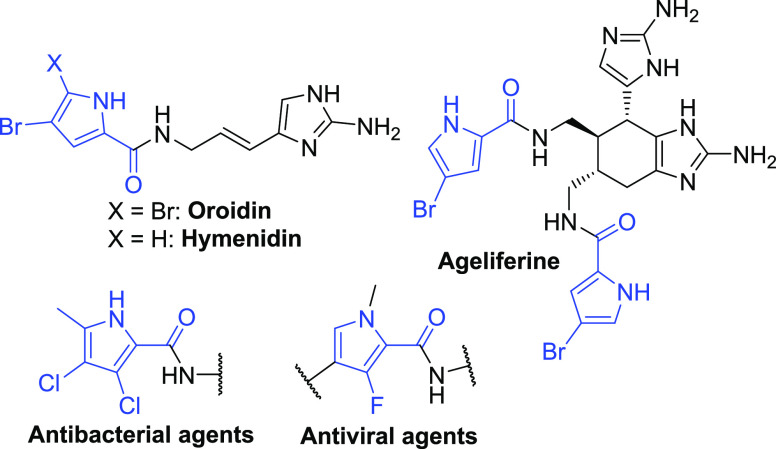
Halogen-substituted pyrrole-2-carboxamide is an integral molecular fragment of bioactive marine natural products as well as natural and synthetic anti-infectives (Figure 1). In particular, mono- and dibromopyrrole-2-carboxamide are found in oroidin1 and hymenidin,2 which are postulated precursors for structurally diverse mono- and oligomeric secondary metabolites involved in the chemical defense of Agelas marine sponges. A representative compound ageliferin3 features a complex multichiral scaffold.4 Furthermore, 3,4-dichloro-5-methyl-1H-pyrrole-2-carboxamide is a molecular fragment of natural5,6 and synthetic7 antibacterials, crucial for binding to the active site of bacterial topoisomerases, and the 3-fluoro-1H-pyrrole-2-carboxamide moiety is found in promising preclinical candidates, active against hepatitis B virus (Figure 1).8
Figure 1.
Representative natural products and bioactive compounds with a halogen-doped 1H-pyrrole-2-carboxamide fragment.
During our ongoing research in the field of dual bacterial DNA gyrase/topoisomerase IV inhibitors,9−15 a promising hit compound 1 (Figure 2) was identified, displaying low nanomolar inhibition of the target enzymes and broad-spectrum activity against gram-positive bacterial strains.16 Due to the compound’s high lipophilicity, its more polar analogues 2–5 were designed by varying the 3,4-dichloro-5-methyl-1H-pyrrole moiety, envisioning improved physical properties (c log P was calculated by ChemDraw) of the analogues while retaining the on-target activity (Figure 2).
Figure 2.
Design of antibacterial hit compound analogues with decreased lipophilicity.
With no preceding literature on the synthesis of the required pyrrole building blocks for the preparation of compounds 2–5, we report herein our synthetic endeavors, where the main goal was the timely delivery of at least 100 mg of the sample to be built into the bioactive molecules. The amide bond of the target compounds can be formed using pyrrole-2-carbonyl chloride or 2-trichloroacetylpyrrole; therefore, either would be an acceptable option.
2. Results and Discussion
The literature procedure for the synthesis of 4-chloro-5-methyl-1H-pyrrole-2-carboxylic acid involves chlorination of ethyl 5-methyl-1H-pyrrole-2-carboxylate using N-chlorosuccinimide at 0 °C and required in our hands laborious chromatographic separation of two barely resolved products.17 The practical synthesis of an alternative acylating agent 8 for the introduction of the same structural fragment was thus developed (Scheme 1). Trichloroacetylpyrrole 7 was prepared from pyrrole-2-carbaldehyde 6 employing the Wolff–Kishner reduction and Friedel–Crafts acylation.18 It was then directly monochlorinated using N-chlorosuccinimide at r.t. and the pure product 8 was obtained on the gram scale in 61% isolated yield after convenient crystallization from dichloromethane. Its structure was unambiguously determined by two-dimensional (2D) nuclear magnetic resonance (NMR) experiments (Supporting Information), showing that the electrophilic chlorination was selective for the position next to the electron-donating methyl substituent.
Scheme 1. Synthesis of 2-Trichloroacetyl-4-chloro-5-methyl-1H-pyrrole 8.
Reagents and conditions: (a) NH2NH2·H2O, ethylene glycol, 90 °C, 1 h, then KOH, 90 °C, 2.5 h (70% yield); (b) CCl3COCl, Et2O, r.t., 2 h (60% yield); and (c) N-chlorosuccinimide, dichloromethane, r.t., 4 h (61% yield).
Next, we targeted the 4-fluoro-substituted building block. The screening of different halogen exchange (“Halex”) conditions involving crown ether 18-C-6 and [2.2.2]cryptand, for the conversion of chloropyrrole 8 or ethyl 4-chloro-5-methylpyrrole-2-carboxylate to the corresponding arylfluorides, returned no hits.19 We thus resorted to electrophilic fluorination of ethyl 5-methyl-1H-pyrrole-2-carboxylate 9 (Scheme 2). Initial 0.5 mmol scale screening of the reaction conditions (Table S1 in the Supporting Information) revealed that Selectfluor-mediated fluorination20 outperformed the N-fluorobenzenesulfonimide (NFSI)-mediated Lewis acid-catalyzed fluorination,21 as the former resulted in somewhat cleaner conversions. When the fluorination was performed at 0 °C in a mixture of acetonitrile and acetic acid (Table S2, entries 11 and 12), the formation of target compound 10, accompanied by an acetoxy side product 11, was observed. Their structures were confirmed by single-crystal X-ray diffraction analysis (Figures S1 and S2 in the Supporting Information). Aiming for an efficient med–chem synthetic route, the reaction was performed on a 2 g scale (Scheme 2), delivering 10 in a consistent 4.5–6.5% yield after flash chromatography. Ester 10 was hydrolyzed to acid 12, requiring rather forcing conditions, and acyl chloride 13 was finally formed using oxalyl chloride in dichloromethane. It is noteworthy that acyl chloride formation using refluxing sulfonyl chloride or oxalyl chloride with the catalytic quantity of dimethylformamide (DMF) resulted in the formation of a significant amount of unidentified side products.
Scheme 2. Synthesis of 4-Fluoro-5-methyl-1H-pyrrole-2-carbonyl Chloride 13.
Reagents and conditions: (a) Selectfluor, MeCN/AcOH 5:1, 0 °C, 2 h (6.5% yield); (b) 10 M NaOH (aq), EtOH, 90 °C, 3 h (76% yield); and (c) oxalyl chloride, dichloromethane, r.t., overnight (quant. yield).
Ethyl 3-fluoro-1H-pyrrole-2-carboxylate 14 has recently become commercially available at a reasonable price because it is a key building block for a drug candidate against hepatitis B virus.22 This was a good starting point for the synthesis of 3-fluoro-5-methyl-1H-pyrrole-2-carboxylic acid 18 (Scheme 3). The Vilsmeier–Haack formylation of 14 gave at 68% conversion a 43:57 mixture of 4- and 5-formylated regioisomers 16 and 15, which were separated by flash chromatography. The regioisomers’ identity was assigned by 19F NMR as follows: 4-formyl isomer 16 features a singlet and 5-formyl isomer 15 features a doublet, 3JF,H = 4 Hz, confirming the presence of a vicinal proton. Moreover, the 13C NMR peak of the formyl carbon of 15 is a singlet and that of 16 is a doublet, 3JC,F = 2.8 Hz.
Scheme 3. Synthesis of 3-Fluoro-5-methyl-1H-pyrrole-2-carbonyl Chloride 19.
Reagents and conditions: (a) DMF, POCl3, 0 °C, 30 min, then 14, 90 °C, overnight (25% yield); (b) Zn, 4 M HCl/dioxane, r.t., 40 min (20% yield); (c) 10 M NaOH(aq), EtOH, 90 °C, 5 h (82% yield); and (d) oxalyl chloride, dichloromethane, r.t., overnight (quant. yield).
Based on the literature reports on the reduction of ester-containing formylpyrroles to methylpyrroles,23 we first attempted a BH3·THF-mediated reduction of 15–17, which in this case yielded the intermediate alcohol; no full reduction was observed even after several days of stirring with periodical addition of excess BH3·THF. Other literature reports on aldehyde-to-methyl reduction in the presence of ester include a two-step Mozingo protocol via thioketal.24 Aiming to secure a convenient one-pot procedure, we opted for the modified Clemmensen reduction.25 A dioxane-soluble [ZnCl2(dioxane)2] complex26 was prepared by treating zinc dust with anhydrous 4 M HCl in dioxane. This proved to be a very efficient and reasonably selective reduction medium, delivering 17 after 40 min at r.t. in 20% isolated yield. Optimization of the reaction conditions and elucidation of the mechanism is beyond the aim of this study; however, we speculate that a dioxane-soluble Zn(II) species forms a zinc–ylide intermediate more efficiently compared to the classical heterogeneous Clemmensen reduction (Zn/Hg/HCl/H2O), allowing the reaction to proceed at room temperature.27 The side products are essentially a result of the zinc–ylide reaction with other present electrophiles (ester, aldehyde, and arylfluoride). Using the conditions developed for the synthesis of 13, ester 17 was readily transformed to acyl chloride 19.
Ethyl 5-chloromethyl-3,4-dichloro-1H-pyrrole-2-carboxylate 21 was prepared from commercially available 20 according to the literature procedure (Scheme 4).18 After conversion to azide 22 by KI-mediated nucleophilic substitution, the reduction of 22 to amine 23 was first attempted via Pd/C-catalyzed hydrogenation. This resulted in significant side-product formation, possibly via the nucleophilic attack of amine 23 to the electrophilic methylene moiety of 22, and aryl dehalogenation, as apparent from the 1H NMR analysis of the crude reaction mixture. Avoiding the coexistence of amine and azide species during the reaction, we resorted to the milder Staudinger reduction,28 which furnished amine 23 in 78% isolated yield. Saponification to 24, followed by phthalimide protection in neat phthalic anhydride gave 25 with 41% yield over four steps from 21.
Scheme 4. Synthesis of 3,4-Dichloro-5-phthalimidomethyl-1H-pyrrole-2-carboxylic Acid 25.
Reagents and conditions: (a) SO2Cl2, CCl4, −5 °C, 5 h; (b) NaN3, KI (cat.), DMF, r.t., 1.5 h (95% yield); (c) PPh3, H2O/THF, r.t., 2.5 h (78% yield); (d) 10 M NaOH, EtOH, 90 °C, 4 h (quant. yield); and (e) phthalic anhydride, 130–180 °C, 1 h (55% yield).
To showcase the usefulness of the prepared building blocks in medicinal chemistry, the synthesis of compound 5, the analogue of antibacterial hit compound 1, was tackled (Scheme 5). After the smooth coupling of the acyl chloride, prepared from 25 in neat thionyl chloride, with the 2-aminobenzothiazole building block29 in refluxing toluene, the deprotection step required some special attention. The formation of stable hydrazinium salt 27 was observed during the phthalimide deprotection, arguably due to the electron-withdrawing character of dichloropyrrole, which increases the acidity of the neighboring amides. It was crucial to first reprotonate the nitrogens of 27 to achieve complete deprotection after refluxing in ethanol overnight. Alkaline hydrolysis of phthalic hydrazide salt 28 yielded phthalate salt 29 and the anion was readily exchanged to the chloride salt of 5 by trituration with methanolic HCl.
Scheme 5. Synthesis of DNA Gyrase B Inhibitor 5·HCl.
Reagents and conditions: (a) SOCl2, 75 °C, 1 h (quant. yield); (b) methyl 2-amino-4-benzyloxybenzo[d]thiazole-6-carboxylate, toluene, 130 °C, 24 h (81% yield); (c) NH2NH2·H2O, EtOH, 50 °C, 40 min; (d) HCl, MeOH, r.t., 15 min; (e) EtOH, 80 °C, 18 h (88% yield from 26); (f) 4 M KOH, EtOH, 50 °C, 24 h then 1 M HCl to pH = 9; and (g) HCl, MeOH, r.t. (55% yield from 28).
Antibacterial hit compound 1 (c log P = 5.8) inhibited Escherichia coli DNA gyrase with IC50 < 10 nM, and compound 5 (c log P = 2.0) inhibited the same enzyme with IC50 < 10 nM. Moreover, 5 exhibits activity against Staphylococcus aureus (ATCC29213) with a minimal inhibitory concentration of 1 μg/mL. This confirms the hypothesis that the single-digit nanomolar inhibitory on-target activity coupled to the antibacterial activity can be retained while significantly reducing the lipophilicity by the modification of the pyrrole moiety.
To explore the reactivity and bioactivity of the fluorinated pyrroles, two additional analogues of 1 were prepared (Scheme 6) and evaluated for their on-target and antibacterial activities. Thus, compounds 31 and 33 inhibited E. coli DNA gyrase with IC50 values of 32 and 150 nM, respectively, and possessed weak activity against S. aureus (ATCC29213) (31: MIC = 64 μg/mL; 33: MIC > 64 μg/mL).
Scheme 6. Synthesis of DNA Gyrase B Inhibitors 31 and 33.
Reagents and conditions: (a) methyl 2-amino-4-(1-phenylethoxy)benzo[d]thiazole-6-carboxylate, toluene, 130 °C, 24 h (57% yield); (b) MeOH, 2 M NaOH, 40 °C, 48 h; (c) tert-butyl 2-amino-4-(2-propyloxy)benzo[d]thiazole-6-carboxylate, toluene, 130 °C, 24 h (79% yield); and (d) CF3COOH, DCM, r.t., 24 h (98% yield).
3. Conclusions
In summary, practical synthetic routes to four new halogen-doped pyrrole building blocks were developed, delivering the target compounds in sufficient quantities for further elaboration. Moreover, the transformation of the building blocks to potent DNA gyrase B inhibitors was demonstrated. Such building blocks are polar alternatives to molecular fragments found in naturally occurring or natural-product-inspired bioactive compounds and are useful in hit-to-lead optimization.
4. Experimental Section
4.1. General
Reactions were conducted under an inert atmosphere using anhydrous solvents when required. Analytical thin-layer chromatography (TLC) was performed on silica gel 60 F254 plates. Flash column chromatography was performed using silica gel 60 (40–63 μm). Melting points were determined on a Kofler apparatus and are uncorrected. 1H NMR (400 MHz, internal Me4Si), 13C NMR (101 MHz, internal CDCl3 or DMSO-d6), and 19F NMR (376 MHz, external CCl3F) spectra were recorded on a Bruker AVANCE III 400 spectrometer (Bruker Corporation, Billerica, MA) in a DMSO-d6 or CDCl3 solution. HRMS analysis was performed on a VG Analytical Autospec Q mass spectrometer (Fisons, VG Analytical, Manchester, U.K.).
4.2. Synthetic Procedures
4.2.1. 2-Trichloroacetyl-4-chloro-5-methyl-1H-pyrrole (8)
A mixture of 2-trichloroacetyl-5-methyl-1H-pyrrole (2.14 g, 9.44 mmol), N-chlorosuccinimide (1.26 g, 9.44 mmol), and dichloromethane (9.0 mL) was stirred at r.t. overnight. The reaction mixture was partitioned between EtOAc (50 mL) and water (50 mL), and the organic layer was washed with water and brine, dried (Na2SO4), and concentrated. The residue was recrystallized from dichloromethane to get the title compound as white crystals (1.51 g, 61% yield). Mp 140–142 °C (DCM). 1H NMR (400 MHz, CDCl3) δ 10.37 (s, 1H), 7.31 (d, J = 2.9 Hz, 1H), 2.41 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 172.89, 137.01, 121.03, 119.78, 114.13, 94.87, 11.37. HRMS calcd for C7H4ONCl4 [M – H]− 257.90525, found 257.90521.
4.2.2. Ethyl 4-Fluoro-5-methyl-1H-pyrrole-2-carboxylate (10)
To a solution of ethyl 5-methyl-1H-pyrrole-2-carboxylate (2.00 g, 13.1 mmol) in acetonitrile (360 mL) and AcOH (72 mL) at 0 °C was added Selectfluor (9.25 g, 26.1 mmol), and the mixture was stirred at 0 °C for 2 h (full conversion by 1H NMR). The reaction mixture was partitioned between water (500 mL) and dichloromethane (500 mL), and the organic layer was washed with sat. NaHCO3 (aq) and brine, dried (Na2SO4), and concentrated to get the crude product. Crude products from two 2 g runs were combined and purified by flash chromatography, eluent hexane/EtOAc 4:1, to get the title compound (first eluting) as a colorless amorphous solid (288 mg, 6.5% yield). 1H NMR (400 MHz, CDCl3): δ 8.92 (bs, 1H), 6.54 (d, 1H, J = 4.0 Hz), 4.30 (q, 2H, J = 7.1 Hz), 2.24 (s, 3H), 1.34 (t, 3H, J = 7.1 Hz). 13C NMR (101 MHz, CDCl3) δ 161.3 (d, J = 3.3 Hz), 148.9 (d, J = 238.7 Hz), 117.6, 116.2 (d, J = 7.4 Hz), 102.3 (d, J = 15.6 Hz), 60.6, 14.6, 9.4 (d, J = 2.1 Hz). 19F NMR (376 MHz, CDCl3): δ −166.4. HRMS calcd for C8H11FNO2 [M + H]+ 172.0768, found 172.0769. This procedure was repeated several times, consistently yielding 4.5–6.5% of the title compound. A monocrystal suitable for single-crystal X-ray diffraction analysis was grown from dichloromethane/hexane.
4.2.3. Ethyl 4-Acetoxy-5-methyl-1H-pyrrole-2-carboxylate (11)
Ethyl 4-acetoxy-5-methyl-1H-pyrrole-2-carboxylate was isolated as a second eluting product (see purification of 10 above), a white amorphous solid. 1H NMR (400 MHz, CDCl3): δ 10.10 (s, 1H), 6.72 (d, J = 2.8 Hz, 1H), 4.29 (q, J = 7.1 Hz, 1H), 2.24 (s, 1H), 2.16 (s, 1H), 1.32 (t, J = 7.1 Hz, 1H). 13C NMR (101 MHz, CDCl3): δ 169.23, 161.46, 134.49, 123.33, 117.80, 108.22, 60.46, 20.85, 14.55, 10.16. A monocrystal suitable for single-crystal X-ray diffraction analysis was grown from dichloromethane/hexane.
4.2.4. 4-Fluoro-5-methyl-1H-pyrrole-2-carboxylic Acid (12)
A solution of the above ester 10 (280 mg, 1.07 mmol) in abs. EtOH (15 mL) and 10 M NaOH (3.0 mL) was stirred at 90 °C under an argon atmosphere for 3 h, and then the reaction mixture was concentrated under reduced pressure. The residue was acidified to pH = 3 by adding 4 M HCl, and the precipitate was collected, washed with water, and air-dried to get the title compound as a light brown amorphous solid (177 mg, 76% yield). 1H NMR (400 MHz, DMSO-d6) δ 12.25 (s, 1H), 11.51 (s, 1H), 6.45 (dd, J = 2.9, 1.1 Hz, 1H), 2.13 (s, 3H). 19F NMR (376 MHz, DMSO-d6) δ −167.66 to −167.70 (m). 13C NMR (101 MHz, DMSO-d6) δ 161.51 (d, J = 3.3 Hz), 147.77 (d, J = 235.0 Hz), 116.90 (d, J = 25.1 Hz), 116.15 (d, J = 7.3 Hz), 100.94 (d, J = 14.9 Hz), 8.70 (d, J = 2.2 Hz). HRMS calcd for C6H5O2NF [M – H]−142.03098, found 142.03001.
4.2.5. 4-Fluoro-5-methyl-1H-pyrrole-2-carbonyl Chloride (13)
A suspension of the above acid (75 mg, 0.52 mmol) in dry dichloromethane (5.2 mL) and oxalyl chloride (0.45 mL, 5.2 mmol) was stirred at r.t. overnight. The resulting clear solution was concentrated under reduced pressure to get the title compound as a light brown amorphous solid (quant. yield). 1H NMR (400 MHz, CDCl3) δ 8.66 (s, 1H), 6.83 (d, J = 2.8 Hz, 1H), 2.30 (s, 3H). 19F NMR shows no clearly identifiable peak.
4.2.6. Ethyl 3-Fluoro-5-formyl-1H-pyrrole-2-carboxylate (15) and Ethyl 3-Fluoro-4-formyl-1H-pyrrole-2-carboxylate (16)
To DMF (8.9 mL, 115 mmol) at 0 °C under Ar was added POCl3 (1.95 mL, 21.0 mmol). After stirring at 0 °C for 30 min, a solution of ethyl 3-fluoro-1H-pyrrole-2-carboxylate 14 (3.00 g, 19.1 mmol) in DMF (29 mL) was added, and the resulting solution was stirred at 90 °C overnight. The reaction mixture was cooled to r.t. and poured onto ice (100 mL), the pH was adjusted to 9 by adding 2 M NaOH (aq) and the product was extracted to Et2O (3 × 200 mL). The combined organic layers were washed with brine, dried (Na2SO4), and concentrated to get the crude product, containing 14, 5-formylated (15), and 4-formylated product (16) in a 42:34:24 ratio (by 1H NMR). The three compounds were separated by column chromatography, eluent dichloromethane, then hexane/EtOAc 2:1 to yield 14 (983 mg, a white amorphous solid), 15 (869 mg, 25% yield, an orange amorphous solid), and 16 (724 mg, a yellow amorphous solid).
Compound 15: 1H NMR (400 MHz, CDCl3) δ 9.88 (s, 1H), 9.42 (br s, 1H), 7.36 (app t, J = 3.9 Hz, 1H), 4.39 (q, J = 7.1 Hz, 2H), 1.39 (t, J = 7.1 Hz, 3H). 19F NMR (376 MHz, CDCl3) δ −150.04 (d, J = 4 Hz). 13C NMR (101 MHz, CDCl3) δ 183.57, 160.22 (d, J = 3.5 Hz), 152.93 (d, J = 269.6 Hz), 124.59 (d, J = 3.6 Hz), 115.00 (d, J = 8.5 Hz), 109.40 (d, J = 18.1 Hz), 61.48, 14.35. HRMS calcd for C8H9O3NF [M + H]+ 186.05610, found 186.05611.
Compound 16: 1H NMR (400 MHz, CDCl3) δ 9.59 (s, 1H), 9.47 (s, 1H), 6.71–6.54 (m, 1H), 4.41 (q, J = 7.1 Hz, 2H), 1.40 (t, J = 7.1 Hz, 3H). 19F NMR (376 MHz, CDCl3) δ −146.95. 13C NMR (101 MHz, CDCl3) δ 180.38 (d, J = 2.8 Hz), 159.67 (d, J = 3.9 Hz), 153.06 (d, J = 260.3 Hz), 129.65 (d, J = 4.4 Hz), 113.90 (d, J = 20.0 Hz), 105.40 (d, J = 14.2 Hz), 61.75, 14.32.
4.2.7. Ethyl 3-Fluoro-5-methyl-1H-pyrrole-2-carboxylate (17)
To a solution of the above aldehyde 15 (500 mg, 2.70 mmol) in 4 M HCl in dioxane (27 mL) was added Zn dust (1.78 g, 27.0 mmol) portionwise at room temperature over 2 min. The reaction is slightly exothermic and performs better if no external cooling is applied. After 40 min (full conversion by TLC), the reaction mixture was concentrated under reduced pressure and filtered through a plug of silica, eluting with EtOAc (200 mL), and concentrated to 5 mL, and the colorless crystals were filtered off and washed with EtOAc (1H NMR analysis revealed only a dioxane peak; it is likely a dioxane-soluble [ZnClx(dioxane)x] complex involved in the aldehyde reduction). The filtrate was diluted with EtOAc (200 mL), washed with water (2 × 100 mL), NaHCO3 (100 mL), and brine, dried (Na2SO4), and concentrated to get the crude product (486 mg), containing 30 mol % of the desired product (by 19F NMR). The pure 17 was isolated by flash chromatography on silica, eluent hexane/EtOAc 4:1 (first eluting compound) as a colorless oil containing residual EtOAc (by 1H NMR) (94 mg, 20% yield) and was used as such in the next step. 1H NMR (400 MHz, CDCl3) δ 8.32 (br s, 1H), 6.58 – 6.51 (m, 1H), 4.33 (q, J = 7.1 Hz, 2H), 2.02 (s, 3H), 1.36 (t, J = 7.1 Hz, 3H). 19F NMR (376 MHz, CDCl3) δ −154.53 (d, J = 4.7 Hz). HRMS calcd for C8H11O2NF [M + H]+ 172.07683, found 172.07625.
4.2.8. 3-Fluoro-5-methyl-1H-pyrrole-2-carboxylic Acid (18)
A solution of the above ester 17 (80 mg, 0.47 mmol) in abs. EtOH (3.5 mL) and 10 M NaOH (0.63 mL) was heated at 90 °C under Ar for 2 h, and then it was concentrated under reduced pressure. The oily residue was dissolved in water (2 mL), and 4 M HCl (1.6 mL) was added. The obtained precipitate was collected, washed with water, and air-dried to get the title compound as a white amorphous powder (55 mg, 82% yield). 1H NMR (400 MHz, DMSO-d6) δ 12.32 (s, 1H), 11.25 (s, 1H), 6.66 (app t, J = 4.4 Hz, 1H), 1.93 (s, 3H). 19F NMR (376 MHz, DMSO-d6) δ −156.45. 13C NMR (101 MHz, DMSO-d6) δ 160.49 (d, J = 3.5 Hz), 152.39 (d, J = 252.4 Hz), 119.46 (d, J = 6.4 Hz), 106.77 (d, J = 18.3 Hz), 105.46 (d, J = 13.9 Hz), 7.26. HRMS calcd. for C6H7O2NF [M + H]+ 144.04553, found 144.04552.
4.2.9. 3-Fluoro-5-methyl-1H-pyrrole-2-carbonyl Chloride (19)
A suspension of the above acid 18 (50 mg, 0.35 mmol) in dry dichloromethane (3.5 mL) and oxalyl chloride (0.3 mL, 3.5 mmol) was stirred at r.t. overnight. The resulting clear solution was concentrated under reduced pressure to get the title compound as a light brown amorphous solid (quant. yield). 1H NMR (400 MHz, CDCl3) δ 8.51 (br s, 1H), 6.81–6.76 (m, 1H), 2.04 (d, J = 0.9 Hz, 3H). 19F NMR showed no clearly identifiable peak.
4.2.10. Ethyl 5-(azidomethyl)-3,4-dichloro-1H-pyrrole-2-carboxylate (22)
To a solution of ethyl 5-(chloromethyl)-3,4-dichloro-1H-pyrrole-2-carboxylate 21 (2.00 g, 7.80 mmol) in DMF (16 mL) was added NaN3 (1.00 g, 15.6 mmol), followed by KI (130 mg, 0.78 mmol). The resulting suspension was vigorously stirred at r.t. for 1.5 h, and then it was poured into water (100 mL). The white precipitate was collected, washed with water, and air-dried to yield the title compound as a white amorphous powder (1.95 g, 95% yield). 1H NMR (400 MHz, CDCl3) δ 9.57 (s, 1H), 4.45 (s, 2H), 4.40 (q, J = 7.1 Hz, 2H), 1.41 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 160.08, 126.48, 118.30, 117.72, 113.26, 61.66, 45.22, 14.45. HRMS calcd for C8H7O2N4Cl2 [M – H]− 260.99515, found 260.99529.
4.2.11. Ethyl 5-(aminomethyl)-3,4-dichloro-1H-pyrrole-2-carboxylate (23)
To a solution of the above azide 22 (1.70 g, 6.46 mmol) in THF/H2O 10:1 (35 mL) was added PPh3 (3.39 g, 12.9 mmol) at r.t. The resulting amber solution was stirred at r.t. (caution: gas evolution) for 2.5 h, and then it was concentrated under reduced pressure. The oily residue was partitioned between EtOAc (200 mL) and 0.5 M HCl (300 mL). The water layer was brought to pH = 11 by adding 2 M NaOH. The precipitate was collected, washed with water, and dried in vacuo to yield the title compound as a white amorphous powder (1.20 g, 78%). 1H NMR (400 MHz, CDCl3) δ 9.87 (s, 1H), 4.36 (q, J = 7.1 Hz, 2H), 3.99 (s, 2H), 1.63 (s, 2H), 1.38 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 160.16, 133.16, 117.50, 116.76, 110.24, 61.02, 36.85, 14.49. HRMS calcd for C8H9O2N2Cl2 [M – H]− 235.00466, found 235.00459.
4.2.12. 5-(Aminomethyl)-3,4-dichloro-1H-pyrrole-2-carboxylic Acid (24)
A mixture of the above ester 23 (1.42 g, 6.00 mmol), abs. EtOH (60 mL), and 10 M NaOH (10.8 mL) was stirred at 90 °C under argon for 3 h. The reaction mixture was concentrated under reduced pressure, dissolved in water (20 mL), filtered through cotton, and cooled to 0 °C. Conc. HCl(aq) (7.5 mL) was added, followed by 2 M HCl to adjust the pH to 8. The precipitate was collected, washed with water, and air-dried to yield the title compound as a white amorphous powder (1.3 g, quant. yield). 1H NMR (400 MHz, DMSO-d6) δ 3.91 (s, 2H). HRMS calcd for C6H5O2N2Cl2 [M – H]− 206.97336, found 206.97342.
4.2.13. 5-(Phthalimidomethyl)-3,4-dichloro-1H-pyrrole-2-carboxylic Acid (25)
A homogeneous mixture of the above amino acid 24 (300 mg, 1.44 mmol) and powdered phthalic anhydride (213 mg, 1.44 mmol) in a 25 mL round-bottom flask was heated on an oil bath under a stream of argon from 150 to 180 °C for 15 min while stirring with a magnetic stirrer at 100 rpm and agitating the flask manually. The temperature was kept at 180 °C for 30 min during which the reaction mixture caked. After cooling to r.t., the crude product was triturated successively with dichloromethane, EtOAc, and 1 M HCl, washed with water, and air-dried to get the title compound as a light gray amorphous solid (270 mg, 55% yield). 1H NMR (400 MHz, DMSO-d6) δ 13.04 (s, 1H), 12.57 (s, 1H), 8.01–7.76 (m, 4H), 4.79 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 167.31, 160.04, 134.42, 131.83, 127.18, 123.14, 117.38, 114.98, 109.12, 33.00. HRMS calcd for C14H7O4N2Cl2 [M – H]− 336.97884, found 336.97929.
4.2.14. Methyl 4-(benzyloxy)-2-(3,4-dichloro-5-((1,3-dioxoisoindolin -2-yl)methyl)-1H-pyrrole-2-carboxamido)benzo[d]thiazole-6-carboxylate (26)
A suspension of the above carboxylic acid 25 (110 mg, 0.324 mmol) in SOCl2 (1 mL) was refluxed for 1 h and then concentrated under reduced pressure. To the solid residue were added methyl 2-amino-4-(benzyloxy)benzo[d]thiazole-6-carboxylate (102 mg, 0.324 mg) and normal grade toluene (6.5 mL), and the resulting suspension was refluxed overnight. After cooling to r.t., the precipitate was collected, washed with toluene, and air-dried to get the title compound as a gray amorphous solid (165 mg, 81% yield). 1H NMR (400 MHz, DMSO-d6) δ 12.44 (s, 1H), 12.39 (s, 1H), 8.30 (s, 1H), 7.95–7.85 (m, 4H), 7.61 (s, 1H), 7.55–7.50 (m, 2H), 7.46–7.34 (m, 3H), 5.30 (s, 2H), 4.82 (s, 2H), 3.88 (s, 3H). HRMS calcd for C30H19O6N4Cl2S [M – H]− 633.04078, found 633.04098.
4.2.15. (5-((4-(Benzyloxy)-6-(methoxycarbonyl)benzo[d]thiazol-2-yl)carbamoyl)-3,4-dichloro-1H-pyrrol-2-yl)methanaminium 1,4-Dioxo-3,4-dihydro-1H-phthalazin-2-ide (28)
To a suspension of 26 (100 mg, 0.157 mmol) in abs. EtOH (3.2 mL) was added 80% aqueous hydrazine hydrate (0.1 mL, 10 equiv). After stirring at 50 °C for 30 min, full conversion to 27 was achieved; 1H NMR (400 MHz, DMSO-d6) δ 8.66 (t, J = 5.6 Hz, 1H), 7.95 (d, J = 1.6 Hz, 1H), 7.56–7.52 (m, 2H), 7.51–7.45 (m, 4H), 7.45–7.39 (m, 3H), 7.37–7.31 (m, 1H), 5.29 (s, 2H), 4.43 (d, J = 5.6 Hz, 2H), 3.83 (s, 3H). The reaction mixture was concentrated under reduced pressure, suspended in MeOH (5 mL), treated with 37% HCl(aq) (three drops), and concentrated to get a white residue. 1H NMR (400 MHz, DMSO-d6) δ 12.50–12.36 (m, 2H), 11.41 (s, 1H), 10.63 (s, 2H), 9.10 (t, J = 5.4 Hz, 1H), 8.30 (d, J = 1.4 Hz, 1H), 7.76–7.71 (m, 1H), 7.67–7.58 (m, 3H), 7.57–7.48 (m, 3H), 7.47–7.41 (m, 2H), 7.40–7.35 (m, 1H), 5.33 (s, 2H), 4.46 (d, J = 5.3 Hz, 2H), 3.89 (s, 3H). This reprotonated compound was suspended in 96% EtOH and stirred at 80 °C overnight, and then the reaction mixture was concentrated and triturated with acetone to get the title compound 28 as a gray amorphous solid (90 mg, 88% yield). 1H NMR (400 MHz, DMSO-d6) δ 12.66 (s, 1H), 12.51 (s, 1H), 11.64 (s, 1H), 8.42–8.28 (m, 4H), 8.19–7.98 (m, 2H), 7.89 (dd, J = 5.9, 3.3 Hz, 2H), 7.64 (d, J = 1.5 Hz, 1H), 7.58–7.49 (m, 2H), 7.48–7.41 (m, 2H), 7.41–7.35 (m, 1H), 5.34 (s, 2H), 4.06 (q, J = 5.2 Hz, 2H), 3.89 (s, 3H). HRMS calcd for C22H19O4N4Cl2S [M]+ 505.04986, found 505.04879; calcd for C8H5O2N2 161.03565 [M]−, found 161.03471.
4.2.16. (5-((4-(Benzyloxy)-6-carboxybenzo[d]thiazol-2-yl)carbamo-yl)-3,4-dichloro-1H-pyrrol-2-yl)methanaminium 2-Carboxybenzoate (29)
A mixture of the above ester 28 (50 mg, 0.075 mmol), abs. EtOH (1.0 mL), and 4 M KOH (0.15 mL) was stirred at 80 °C for 2 h and then at 50 °C overnight. The reaction mixture was concentrated, water (1 mL) was added, and the solids were filtered off. The filtrate was brought to pH = 6 by adding 1 M HCl and cooled to 0 °C. The amorphous precipitate was collected, washed with water, and air-dried. 1H NMR (400 MHz, DMSO-d6) δ 8.18 (s, 1H), 8.08 (s, 2H), 7.96–7.82 (m, 3H), 7.60–7.50 (m, 3H), 7.48–7.31 (m, 3H), 5.30 (s, 2H), 3.94 (s, 2H), exchangeable protons were not observed.
4.2.17. (5-((4-(Benzyloxy)-6-carboxybenzo[d]thiazol-2-yl)carbamo-yl)-3,4-dichloro-1H-pyrrol-2-yl)methanaminium Chloride (5·HCl)
The above phthalate salt 29 was triturated with a fresh solution of HCl in MeOH (2 × 1 mL) (prepared by adding two drops of 37% HCl (aq) to absolute methanol (5 mL) (methanol was chosen as a solvent because of the known good solubility of phthalic acid in methanol)) to get the title compound as a brown amorphous powder (22 mg, 55% yield). 1H NMR (400 MHz, DMSO-d6) δ 13.05 (s, 1H), 12.64 (s, 1H), 12.47 (s, 1H), 8.39–8.20 (m, 4H), 7.64 (d, J = 1.3 Hz, 1H), 7.55 (d, J = 7.2 Hz, 2H), 7.47–7.42 (m, 2H), 7.41–7.36 (m, 1H), 5.33 (s, 2H), 4.07 (q, J = 5 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) representative peaks: δ 167.46, 137.04, 128.91, 128.76, 128.58, 127.47, 125.72, 116.82, 115.03, 112.68, 109.63, 70.58, 33.42. HRMS calcd for C21H17O4N4Cl2S [M – Cl]+ 491.0348, found 491.0334.
4.2.18. Methyl 2-(3-fluoro-5-methyl-1H-pyrrole-2-carboxamido)-4-(1-phenylethoxy)benzo[d]thiazole-6-carboxylate (30)
A suspension of 19 (56 mg, 0.35 mmol) and methyl 2-amino-4-(1-phenylethoxy)benzo[d]thiazole-6-carboxylate (114 mg, 0.349 mmol) in toluene (7 mL) was refluxed overnight, equipped with a CaCl2 tube. After cooling to r.t., the precipitate was collected and washed with toluene to get the title compound as a light gray amorphous powder (91 mg, 57% yield). 1H NMR (400 MHz, DMSO-d6) δ 12.23 (s, 1H), 11.37 (s, 1H), 8.17 (d, J = 1.5 Hz, 1H), 7.49–7.42 (m, 2H), 7.40–7.31 (m, 3H), 7.29–7.25 (m, 1H), 6.91 (app t, J = 4.2 Hz, 1H), 5.80 (q, J = 6.1 Hz, 1H), 3.80 (s, 3H), 1.99 (s, 3H), 1.64 (d, J = 6.3 Hz, 3H). 19F NMR (376 MHz, DMSO-d6) δ −153.97 (d, J = 4 Hz). HRMS calcd for C23H19O4N3FS 452.10858; found 452.10924 [M – H]−.
4.2.19. 2-(3-Fluoro-5-methyl-1H-pyrrole-2-carboxamido)-4-(1-phenylethoxy)benzo[d]thiazole-6-carboxylic Acid (31)
A solution of the above ester 30 (70 mg, 0.154 mmol) in MeOH (3.0 mL) and 2 M NaOH (0.40 mL) was stirred at 40 °C overnight. NaOH (2 M, 0.40 mL) was added and stirred another night, and then the reaction mixture was concentrated. The residue was suspended in water (2 mL), the pH was adjusted to 2 by adding 4 M HCl, and the precipitate was collected, washed with water, air-dried, and triturated with MeOH to get the title compound as a beige amorphous solid (55 mg, 81% yield). 1H NMR (400 MHz, DMSO-d6) δ 12.82 (s, 1H), 12.20 (s, 1H), 11.37 (s, 1H), 8.13 (s, 1H), 7.52–7.40 (m, 2H), 7.40–7.31 (m, 3H), 7.31–7.20 (m, 1H), 6.91 (app t, J = 4.2 Hz, 1H), 5.79 (q, J = 6.3 Hz, 1H), 1.99 (s, 3H), 1.64 (d, J = 6.3 Hz, 3H). 19F NMR (376 MHz, DMSO-d6) δ −154.06 (d, J = 4 Hz). 13C NMR (101 MHz, DMSO-d6) δ 166.92, 159.75, 156.78, 154.36, 151.82, 148.98, 142.57, 132.83, 128.61, 127.55, 126.31, 125.53, 120.98 (d, J = 5.4 Hz), 115.90, 110.44, 107.83 (d, J = 15.0 Hz), 106.18 (d, J = 13.7 Hz), 75.48, 24.33, 7.17. HRMS calcd for C22H17O4N3FS 438.09293; found 438.09338 [M – H]−.
4.2.20. tert-Butyl 2-(4-fluoro-5-methyl-1H-pyrrole-2-carboxamido)-4-isopropoxybenzo[d]thiazole-6-carboxylate (32)
A suspension of 13 (58 mg, 0.36 mmol) and methyl 2-amino-4-(2-propyloxy)benzo[d]thiazole-6-carboxylate (93 mg, 0.30 mmol) in toluene (7 mL) was stirred at 130 °C overnight. The gray amorphous precipitate was collected and washed with toluene. Yield: 79% (104 mg). 1H NMR (400 MHz, DMSO-d6) δ 12.66 (s, 1H), 11.85 (s, 1H), 8.13 (d, J = 1 Hz, 1H), 7.44 (d, J = 1 Hz, 1H), 7.26 (d, J = 2.3 Hz, 1H), 4.95–4.84 (m, 1H), 2.20 (s, 3H), 1.36 (d, J = 6.0 Hz, 6H). MS 432.2 [M – H]−.
4.2.21. 2-(4-Fluoro-5-methyl-1H-pyrrole-2-carboxamido)-4-isopropoxybenzo[d]thiazole-6-carboxylic Acid (33)
The above tert-butyl ester 32 (75 mg, 0.173 mmol) was suspended in dichloromethane (3 mL). Trifluoroacetic acid (0.13 mL, 1.73 mmol) was added and the suspension turned into a brown solution. The reaction mixture was stirred overnight at room temperature. The solvent was removed under reduced pressure and the residue was triturated with methanol to give 33 as an amorphous solid. Yield: 98% (64 mg). 1H NMR (400 MHz, DMSO-d6) δ 12.92 (s, 1H), 12.65 (s, 1H), 11.84 (s, 1H), 8.17 (d, J = 1.2 Hz, 1H), 7.48 (s, 1H), 7.26 (d, J = 2.7 Hz, 1H), 4.89 (hept, J = 6 Hz, 1H), 2.19 (s, 3H), 1.36 (d, J = 6 Hz, 6H). HRMS calcd for C17H17O4N3FS [M + H]+ 378.09183, found 378.09203.
4.3. Biological Assays
4.3.1. Determination of Inhibitory Activity on E. coli. DNA Gyrase
The supercoiling assay for the determination of IC50 values was performed according to previously reported procedures.30
Acknowledgments
Some of the research leading to these results was conducted as part of the ND4BB ENABLE Consortium and has received support from the Innovative Medicines Initiative Joint Undertaking under Grant No. 115583, resources of which comprise financial contributions from the European Union’s seventh framework program (FP7/2007-2013) and EFPIA companies’ in-kind contribution. We gratefully acknowledge the Slovenian Research Agency (Grant No. P1-0208), COST Action CA15135 MuTaLig, the French Agence Nationale pour la Recherche (ANR) (grant number ANR-17-CE07-0008-01, DEFIS), CNRS, and Universite′ de Strasbourg for financial support. The French Fluorine Network (GIS Fluor) is also acknowledged. T.G. is much grateful to the French Ministry of Education and Research for funding. The authors thank L. Karmazin and C. Bailly from the Service de radiocristallographie de la Fédération de Chimie Le Bel FR 2010 for the SCXRD analyses. Maja Frelih is acknowledged for the acquisition of HRMS spectra and Dr. Žiga Skok for performing the enzyme inhibition assay.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c00331.
Accession Codes
CCDC 2022198 and 2022199 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB21EZ, UK; fax: +44 1223 336033.
Author Present Address
∥ Faculty of Chemical Technology, Institute of Organic Chemistry and Technology, University of Pardubice, Studentská, 573, Pardubice 53210, Czech Republic
Author Present Address
⊥ Department of Life and Environmental Sciences—Drug section, University of Cagliari, via Ospedale 72, 09124 Cagliari, Italy
The authors declare no competing financial interest.
Supplementary Material
References
- Kovalerchik D.; Singh R. P.; Schlesinger P.; Mahajni A.; Shefer S.; Fridman M.; Ilan M.; Carmeli S. Bromopyrrole Alkaloids of the Sponge Agelas oroides Collected Near the Israeli Mediterranean Coastline. J. Nat. Prod. 2020, 83, 374–384. 10.1021/acs.jnatprod.9b00863. [DOI] [PubMed] [Google Scholar]
- de Souza R. T. M. P.; Freire V. F.; Gubiani J. R.; Ferreira R. O.; Trivella D. B. B.; Moraes F. C.; Paradas W. C.; Salgado L. T.; Pereira R. C.; Amado Filho G. M.; Ferreira A. G.; Williams D. E.; Andersen R. J.; Molinski T. F.; Berlinck R. G. S. Bromopyrrole Alkaloid Inhibitors of the Proteasome Isolated from a Dictyonella Sp. Marine Sponge Collected at the Amazon River Mouth. J. Nat. Prod. 2018, 81, 2296–2300. 10.1021/acs.jnatprod.8b00533. [DOI] [PubMed] [Google Scholar]
- Ma Z.; Wang X.; Wang X.; Rodriguez R. A.; Moore C. E.; Gao S.; Tan X.; Ma Y.; Rheingold A. L.; Baran P. S.; Chen C. Asymmetric Syntheses of Sceptrin and Massadine and Evidence for Biosynthetic Enantiodivergence. Science 2014, 346, 219–224. 10.1126/science.1255677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman A. E. Escaping from Flatland: Stereoconvergent Synthesis of 3-Dimensional Scaffolds via Ruthenium(II)-Catalyzed Noyori-Ikariya Transfer Hydrogenation. Chem. - Eur. J. 2021, 27, 39–53. 10.1002/chem.202002779. [DOI] [PubMed] [Google Scholar]
- Singh S. B. Discovery and Development of Kibdelomycin, a New Class of Broad-Spectrum Antibiotics Targeting the Clinically Proven Bacterial Type II Topoisomerase. Bioorg. Med. Chem. 2016, 24, 6291–6297. 10.1016/j.bmc.2016.04.043. [DOI] [PubMed] [Google Scholar]
- Sawa R.; Takahashi Y.; Hashizume H.; Sasaki K.; Ishizaki Y.; Umekita M.; Hatano M.; Abe H.; Watanabe T.; Kinoshita N.; Homma Y.; Hayashi C.; Inoue K.; Ohba S.; Masuda T.; Arakawa M.; Kobayashi Y.; Hamada M.; Igarashi M.; Adachi H.; Nishimura Y.; Akamatsu Y. Amycolamicin: A Novel Broad-Spectrum Antibiotic Inhibiting Bacterial Topoisomerase. Chem.–Eur. J. 2012, 18, 15772–15781. 10.1002/chem.201202645. [DOI] [PubMed] [Google Scholar]
- Basarab G. S.; Hill P. J.; Garner C. E.; Hull K.; Green O.; Sherer B. A.; Dangel P. B.; Manchester J. I.; Bist S.; Hauck S.; Zhou F.; Uria-Nickelsen M.; Illingworth R.; Alm R.; Rooney M.; Eakin A. E. Optimization of Pyrrolamide Topoisomerase II Inhibitors Toward Identification of an Antibacterial Clinical Candidate (AZD5099). J. Med. Chem. 2014, 57, 6060–6082. 10.1021/jm500462x. [DOI] [PubMed] [Google Scholar]
- Vandyck K.; HACHÉ G. Y. P.; Last S. J.; Gowan D. C. M.; Rombouts G.; Verschueren W. G.; Raboisson P. J.-M. B.. Sulphamoylpyrrolamide Derivatives and the Use Thereof as Medicaments for the Treatment of Hepatitis b. WO2014184350A1November 20,2014.
- Gjorgjieva M.; Tomašič T.; Kikelj D.; Mašič L. P. Benzothiazole-Based Compounds in Antibacterial Drug Discovery. Curr. Med. Chem. 2019, 25, 5218–5236. 10.2174/0929867324666171009103327. [DOI] [PubMed] [Google Scholar]
- Zidar N.; Macut H.; Tomašič T.; Mašič L. P.; Ilaš J.; Zega A.; Tammela P.; Kikelj D. New N -Phenyl-4,5-Dibromopyrrolamides as DNA Gyrase B Inhibitors. MedChemComm 2019, 10, 1007–1017. 10.1039/C9MD00224C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiz D. B.; Skok Ž.; Durcik M.; Tomašič T.; Mašič L. P.; Ilaš J.; Zega A.; Draskovits G.; Révész T.; Nyerges Á.; Pál C.; Cruz C. D.; Tammela P.; Žigon D.; Kikelj D.; Zidar N. An Optimised Series of Substituted N-Phenylpyrrolamides as DNA Gyrase B Inhibitors. Eur. J. Med. Chem. 2019, 167, 269–290. 10.1016/j.ejmech.2019.02.004. [DOI] [PubMed] [Google Scholar]
- Lamut A.; Cruz C. D.; Skok Ž.; Barančoková M.; Zidar N.; Zega A.; Mašič L. P.; Ilaš J.; Tammela P.; Kikelj D.; Tomašič T. Design, Synthesis and Biological Evaluation of Novel DNA Gyrase Inhibitors and Their Siderophore Mimic Conjugates. Bioorg. Chem. 2020, 95, 103550 10.1016/j.bioorg.2019.103550. [DOI] [PubMed] [Google Scholar]
- Lamut A.; Skok Ž.; Barančoková M.; Gutierrez L. J.; Cruz C. D.; Tammela P.; Draskovits G.; Szili P. É.; Nyerges Á.; Pál C.; Molek P.; Bratkovič T.; Ilaš J.; Zidar N.; Zega A.; Enriz R. D.; Kikelj D.; Tomašič T. Second-Generation 4,5,6,7-Tetrahydrobenzo[d]Thiazoles as Novel DNA Gyrase Inhibitors. Future Med. Chem. 2020, 12, 277–297. 10.4155/fmc-2019-0127. [DOI] [PubMed] [Google Scholar]
- Jakopin Ž.; Ilaš J.; Barančoková M.; Brvar M.; Tammela P.; Sollner Dolenc M.; Tomašič T.; Kikelj D. Discovery of Substituted Oxadiazoles as a Novel Scaffold for DNA Gyrase Inhibitors. Eur. J. Med. Chem. 2017, 130, 171–184. 10.1016/j.ejmech.2017.02.046. [DOI] [PubMed] [Google Scholar]
- Cotman A. E.; Trampuž M.; Brvar M.; Kikelj D.; Ilaš J.; Peterlin-Mašič L.; Montalvão S.; Tammela P.; Frlan R. Design, Synthesis, and Evaluation of Novel Tyrosine-Based DNA Gyrase B Inhibitors. Arch. Pharm. 2017, 350, 1700087 10.1002/ardp.201700087. [DOI] [PubMed] [Google Scholar]
- Nyerges A.; Tomašič T.; Durcik M.; Revesz T.; Szili P.; Draskovits G.; Bogar F.; Skok Ž.; Zidar N.; Ilaš J.; Zega A.; Kikelj D.; Daruka L.; Kintses B.; Vasarhelyi B.; Foldesi I.; Kata D.; Welin M.; Kimbung R.; Focht D.; Mašič L. P.; Pal C. Rational Design of Balanced Dual-Targeting Antibiotics with Limited Resistance. PLoS Biol. 2020, 18, e3000819 10.1371/journal.pbio.3000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer B. A.; Hull K.; Green O.; Basarab G.; Hauck S.; Hill P.; Loch J. T.; Mullen G.; Bist S.; Bryant J.; Boriack-Sjodin A.; Read J.; DeGrace N.; Uria-Nickelsen M.; Illingworth R. N.; Eakin A. E. Pyrrolamide DNA Gyrase Inhibitors: Optimization of Antibacterial Activity and Efficacy. Bioorg. Med. Chem. Lett. 2011, 21, 7416–7420. 10.1016/j.bmcl.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Basarab G. S.; Hill P.; Sherer B.; Zhou F.. 2- (Piperidin-1-Yl) -4-Heterocyclyl-Thiazole-5-Carboxylic Acid Derivatives against Bacterial Infections. WO2010067123A1June 17, 2010.
- Worrell B. T.; Hein J. E.; Fokin V. V. Halogen Exchange (Halex) Reaction of 5-Iodo-1,2,3-Triazoles: Synthesis and Applications of 5-Fluorotriazoles. Angew. Chem., Int. Ed. 2012, 51, 11791–11794. 10.1002/anie.201204979. [DOI] [PubMed] [Google Scholar]
- Heeran D.; Sandford G. Fluorination of Pyrrole Derivatives by Selectfluor. Tetrahedron 2016, 72, 2456–2463. 10.1016/j.tet.2016.03.067. [DOI] [Google Scholar]
- Zhang Y.; Shibatomi K.; Yamamoto H. Lewis Acid Catalyzed Highly Selective Halogenation of Aromatic Compounds. Synlett 2005, 2005, 2837–2842. 10.1055/s-2005-918919. [DOI] [Google Scholar]
- René A.; Quilan M.; Deng Y.; Cheng Y.; Teleha C. A.; Raboisson P.; Bonfanti J.-F.; Fortin J.; Charette André. B.; Pannecoucke X.; Poisson T.; Jubault P. Practical Synthesis of Ethyl 3-Fluoro-1-Pyrrole-2-Carboxylate: A Key Fragment of a Potent Drug Candidate against Hepatitis B Virus. Org. Process Res. Dev. 2020, 24, 792–801. 10.1021/acs.oprd.9b00382. [DOI] [Google Scholar]
- Smith K. M.; Minnetian O. M. Novel Porphyrins from Copper(II)-Mediated Cyclizations of 1’,8’-Dimethyl-A,C-Biladiene Salts: Mechanism of the Cyclization Reaction. J. Org. Chem. 1985, 50, 2073–2080. 10.1021/jo00212a014. [DOI] [Google Scholar]
- Carey F. A.; Sundberg R. J.. Advanced Organic Chemistry: Part B: Reaction and Synthesis; 5th ed.; Springer: US, 2007. [Google Scholar]
- Yamamura S.; Toda M.; Hirata Y. Modified Clemmensen Reduction: Cholestane. Org. Synth. 2003, 53, 86. 10.1002/0471264180.os053.20. [DOI] [Google Scholar]
- Boardman A.; Small R. W. H.; Worrall I. J. Structure of Catena-Dichloro-μ-(1,4-Dioxane-O,O’)-(1,4-Dioxane)Zinc(II),[Zn(C4H8O2)2Cl2]. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1983, 39, 1005–1007. 10.1107/S0108270183007179. [DOI] [Google Scholar]
- Brewster J. H. Reductions at Metal Surfaces. II. A Mechanism for the Clemmensen Reduction. J. Am. Chem. Soc. 1954, 76, 6364–6368. 10.1021/ja01653a035. [DOI] [Google Scholar]
- Staudinger H.; Meyer J. Ü. New Organic Compounds of Phosphorus. III. Phosphine-Methylene Derivatives and Phosphinimines. Helv. Chim. Acta 1919, 2, 635–646. 10.1002/hlca.19190020164. [DOI] [Google Scholar]
- Durcik M.; Toplak Ž.; Zidar N.; Ilaš J.; Zega A.; Kikelj D.; Mašič L. P.; Tomašič T. Efficient Synthesis of Hydroxy-Substituted 2-Aminobenzo[d]Thiazole-6-Carboxylic Acid Derivatives as New Building Blocks in Drug Discovery. ACS Omega 2020, 5, 8305–8311. 10.1021/acsomega.0c00768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidar N.; Tomašič T.; Macut H.; Sirc A.; Brvar M.; Montalvão S.; Tammela P.; Ilaš J.; Kikelj D. New N-Phenyl-4,5-Dibromopyrrolamides and N-Phenylindolamides as ATPase Inhibitors of DNA Gyrase. Eur. J. Med. Chem. 2016, 117, 197–211. 10.1016/j.ejmech.2016.03.079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.